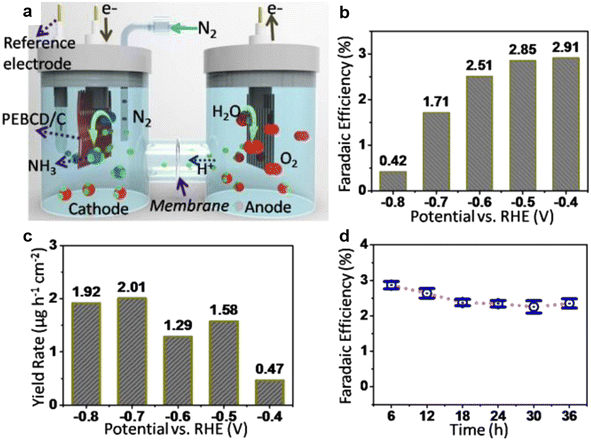
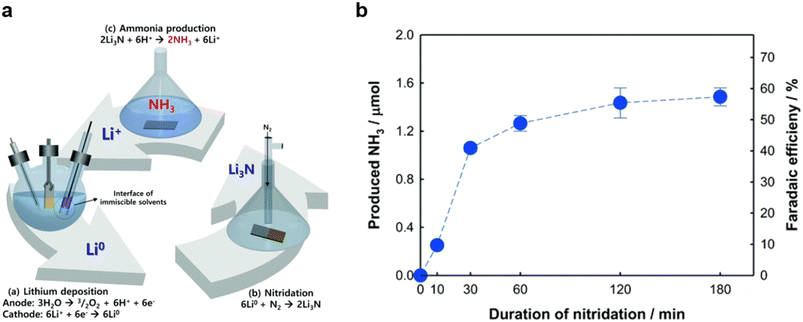
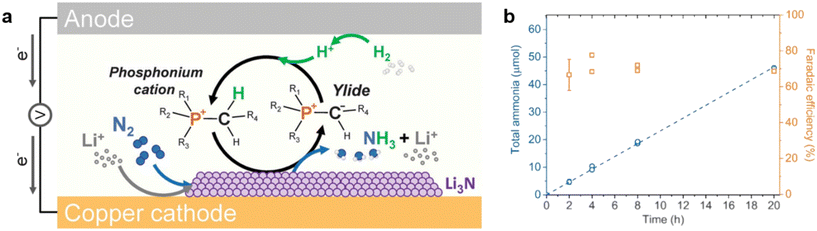
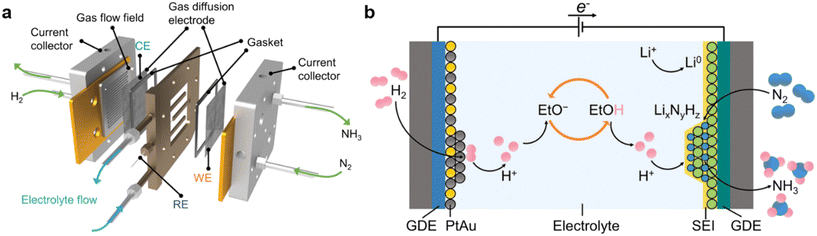
Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Correction: Lithium-mediated electrochemical dinitrogen reduction reaction
Muhammad Saqlain
Iqbal
b,
Yukun
Ruan
a,
Ramsha
Iftikhar
c,
Faiza Zahid
Khan
d,
Weixiang
Li
a,
Leiduan
Hao
*a,
Alex W.
Robertson
e,
Gianluca
Percoco
f and
Zhenyu
Sun
*a
aState Key Laboratory of Organic–Inorganic Composites, College of Chemical Engineering, Beijing University of Chemical Technology, Beijing 100029, P. R. China. E-mail: haold@buct.edu.cn; sunzy@mail.buct.edu.cn
bDepartment of Electrical and Information Engineering, Polytechnic University of Bari, Via E. Orabona 4, 70125 Bari, Italy
cSchool of Chemistry, University of New South Wales, 2033 Sydney, Australia
dInstitute of Chemistry, Rheinische Friedrich-Wilhelms-Universität Bonn, 53113 Bonn, Germany
eDepartment of Physics, University of Warwick, Coventry CV4 7AL, UK
fDepartment of Mechanics, Mathematics and Management, Polytechnic University of Bari, Via E. Orabona 4, 70125 Bari, Italy
First published on 19th May 2023
Abstract
Correction for ‘Lithium-mediated electrochemical dinitrogen reduction reaction’ by Muhammad Saqlain Iqbal et al., Ind. Chem. Mater., 2023, DOI: https://doi.org/10.1039/D3IM00006K.
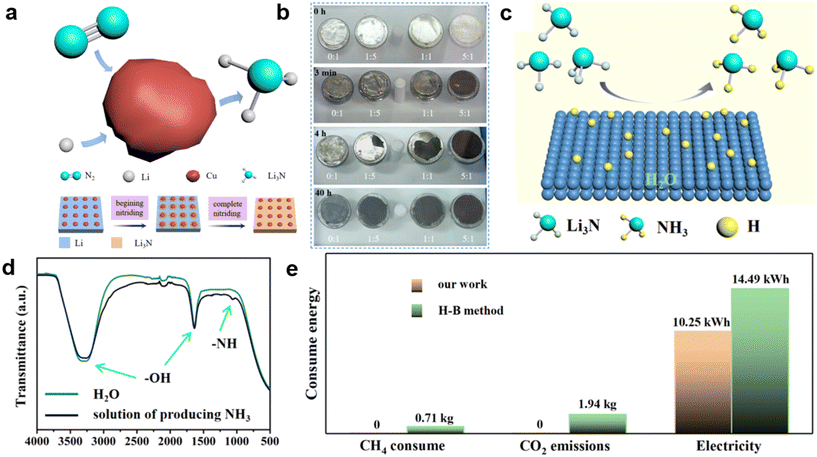
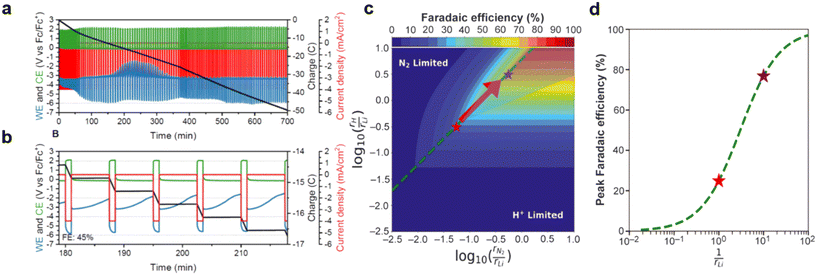
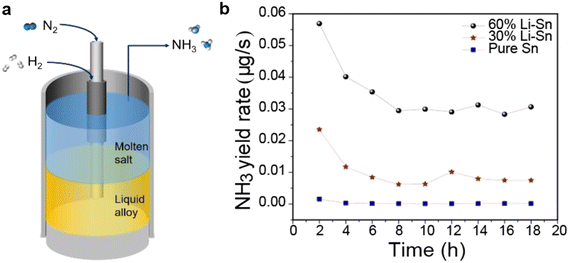
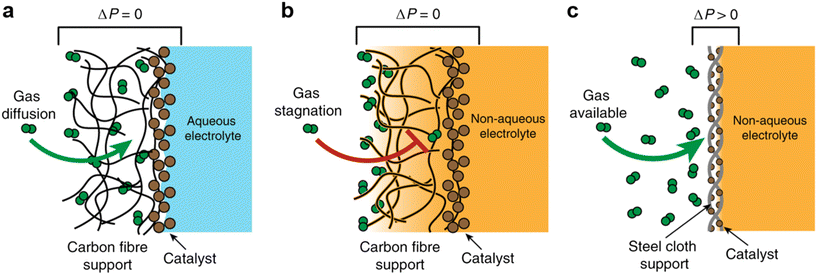
The authors regret that the incorrect permissions were provided in the figure captions of Fig. 1–15 in the original article. The correct versions of the figures, including the updated permissions, are shown below.
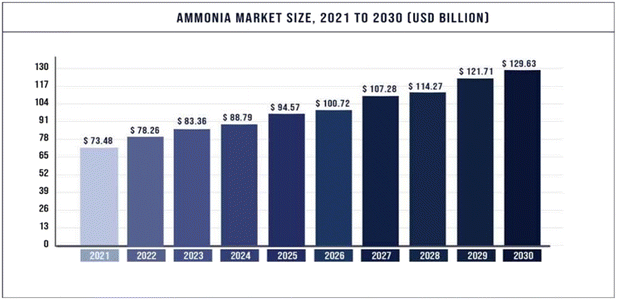 | ||
| Fig. 1 Projected global ammonia demand growth from 2021 to 2030.2 Copyright 2023, Precedence Research. | ||
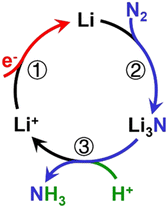 | ||
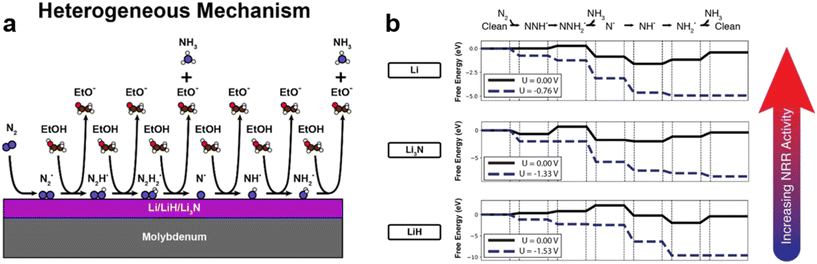
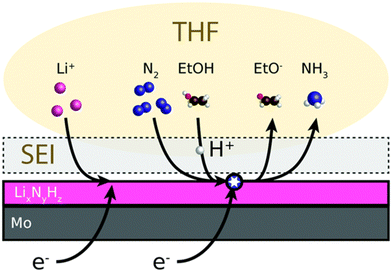
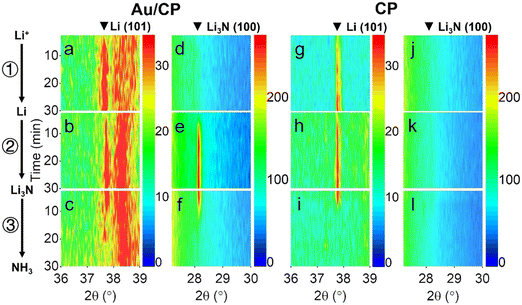
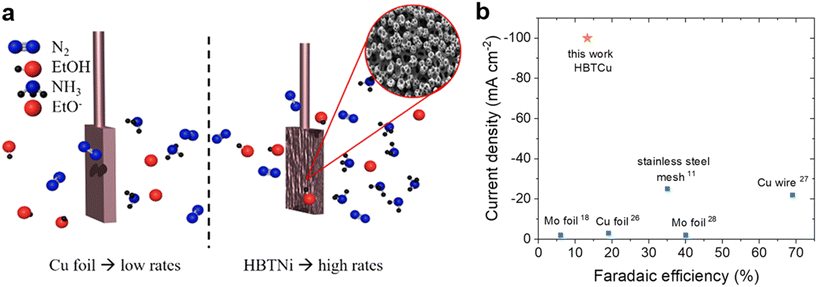
| Fig. 2 Mechanism of catalytic recycling of lithium intermediates. Reproduced with permission.23 Copyright 2021, Wiley-VCH. | ||
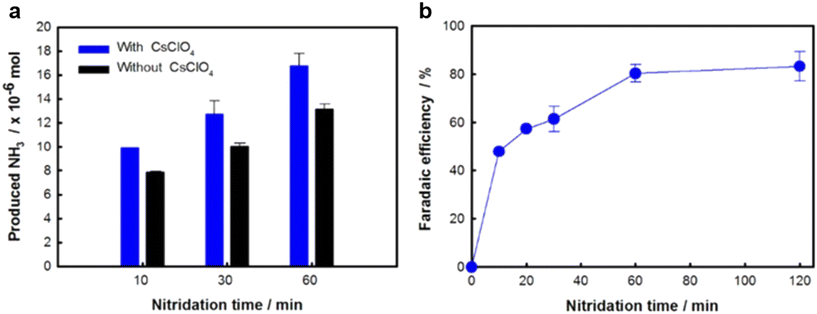 | ||
| Fig. 12 (a) NH3 yield and (b) NH3 FE in the presence and absence of 0.03 M CsClO4 at 220 °C over time. Reproduced with permission.8 Copyright 2018, IOP Publishing. | ||
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
| This journal is © Institute of Process Engineering of CAS 2023 |