Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Acoustofluidics – changing paradigm in tissue engineering, therapeutics development, and biosensing
Reza
Rasouli
a,
Karina Martinez
Villegas
a and
Maryam
Tabrizian
 *ab
*ab
aDepartment of Biomedical Engineering, Faculty of Medicine and Health Sciences, McGill University, Montreal, Quebec, Canada. E-mail: maryam.tabrizian@mcgill.ca
bFaculty of Dental Medicine and Oral Health Sciences, McGill University, Montreal, Quebec, Canada
First published on 20th February 2023
Abstract
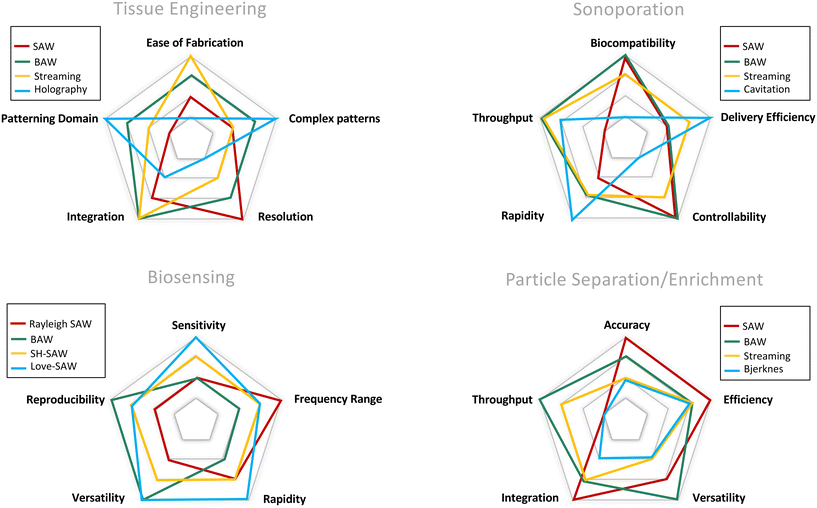
For more than 70 years, acoustic waves have been used to screen, diagnose, and treat patients in hundreds of medical devices. The biocompatible nature of acoustic waves, their non-invasive and contactless operation, and their compatibility with wide visualization techniques are just a few of the many features that lead to the clinical success of sound-powered devices. The development of microelectromechanical systems and fabrication technologies in the past two decades reignited the spark of acoustics in the discovery of unique microscale bio applications. Acoustofluidics, the combination of acoustic waves and fluid mechanics in the nano and micro-realm, allowed researchers to access high-resolution and controllable manipulation and sensing tools for particle separation, isolation and enrichment, patterning of cells and bioparticles, fluid handling, and point of care biosensing strategies. This versatility and attractiveness of acoustofluidics have led to the rapid expansion of platforms and methods, making it also challenging for users to select the best acoustic technology. Depending on the setup, acoustic devices can offer a diverse level of biocompatibility, throughput, versatility, and sensitivity, where each of these considerations can become the design priority based on the application. In this paper, we aim to overview the recent advancements of acoustofluidics in the multifaceted fields of regenerative medicine, therapeutic development, and diagnosis and provide researchers with the necessary information needed to choose the best-suited acoustic technology for their application. Moreover, the effect of acoustofluidic systems on phenotypic behavior of living organisms are investigated. The review starts with a brief explanation of acoustofluidic principles, the different working mechanisms, and the advantages or challenges of commonly used platforms based on the state-of-the-art design features of acoustofluidic technologies. Finally, we present an outlook of potential trends, the areas to be explored, and the challenges that need to be overcome in developing acoustofluidic platforms that can echo the clinical success of conventional ultrasound-based devices.
Reza Rasouli received his Ph.D. from McGill University in Biological and Biomedical Engineering. His Ph.D. thesis focuses on the application of acoustofluidic platforms for the manipulation of bioparticles and biofluids. In his research, he developed various acoustic platforms based on boundary-driven acoustic systems and surface acoustic waves for precise microstream handling in applications such as nanoparticle synthesis and spheroids formation. |
Karina Martinez Villegas is a master's student in the Department of Biological and Biomedical Engineering at McGill University with a background in Biomedical Mechanical engineering. She has been working in the past three years in the field of acoustofluidics, particularly with Surface Acoustic Waves (SAW)-based platforms, for cell patterning and cell characterization for tissue engineering applications. |
Maryam Tabrizian is a full Professor at the Biomedical Engineering Department and Canada Research Chair holder in Regenerative Medicine and Nanomedicine. She is fellow of Guggenheim Foundation in Biomedical Science, the Biomaterials Science & Engineering, Royal Society of Canada-Academy of Science, and Academy of Health Science for her contribution to the field of Biomedical Engineering and Biomedical Sciences. She was the founding director of the Centre for Biorecognition and Biosensors from 2003 to 2011 and is currently the Editor-in-Chief of Materials (MDPI ISSN 1996–1944). Maryam Tabrizian is the author of over 250 peer-reviewed papers. Her research program is built on four main pillars: 1. development of biomimetic materials promoting angiogenesis and osteogenesis, 2. nanoplexes with targeting and tracking/imaging abilities for tissue and cell imaging, 3. real-time monitoring of molecular and cellular events at biointerfaces, and 4. Lab-on a-chip devices for bacterial detection, bioparticle synthesis and sorting. |
1 Introduction
Acoustic waves have a long history in biomedical sciences and engineering with a wide range of applications from diagnostics and imaging techniques to shock wave lithotripsy using high-energy sound waves. The development of microelectromechanical systems (MEMS) and miniaturized technologies enabled the use of acoustics in minuscule, confined, and well-controlled microfluidic domains referred to as acoustofluidics. The miniaturization of acoustic platforms not only allows for the precise manipulation and analysis of cells, particles, and biomolecules from nanometers to millimeters scales, but also introduces numerous phenomena unique to the micro realm.1,2 The wide range of working frequencies (from kilohertz to gigahertz), efficient delivery of energy, confined and controlled domain, and the various acoustic phenomena that can be independently chosen and tuned, make this technology suitable for a diverse gamut of applications.In the biomedical field, acoustofluidics has attracted high interest due to its contactless and label-free nature, its capability to easily modify the energy and frequency of waves to preserve high biocompatibility, and its flexible working media in contrast to electrophoresis or optical tweezers.3–6 Moreover, miniaturized acoustic platforms allow to generate uniform and well-controlled acoustic pressure profiles using gentle and precise acoustic forces on sensitive bio-samples, thus minimizing uninhibited heating and mechanical stresses.6 Another distinctive feature of acoustic systems is their ability to be employed as both actuators, for particle and fluid manipulation, and as sensors, to detect bioparticles and biomolecules. This versatility emerges from the capability of piezoelectrics to convert energy between mechanical vibrations and electric signals in both directions, through direct and inverse piezoelectric effects.7
To move and manipulate objects using acoustic energy, known as acoustophoresis, acoustic waves exert energy on the objects mainly in the form of acoustic radiation forces (ARFs), and/or Stokes' drag forces initiated from acoustic streaming. One advantage of acoustic methods for particle manipulation is that every object that shows a mismatch in density and/or compressibility with its surrounding medium is acoustically visible to the system, without labeling. The acoustic contrast, together with the particle size, determines the magnitude of the acoustic force exerted on the particles and it is subsequently used for accurate and sensitive separation, label-free sorting, enrichment, and patterning of cells, bacteria, and nanoparticles. In addition, these precise and controllable acoustic forces on submicron to millimeter-scale particles, combined with their compatibility with complex biofluids such as sputum and blood, make acoustic manipulation a versatile and powerful tool for handling clinical specimens.
The non-invasive nature of acoustic tweezers can be employed to gently move and pattern single or arrays of bioparticles with a micro-scale precision to study cell–cell communications and to assemble complex cell architectures for tissue engineering.8–27 Furthermore, by introducing hydrogels before patterning cells, tissue constructs can be preserved in vitro for long-term culture after the removal of the acoustic field and can be further implanted in vivo.13
The interaction of acoustic waves with objects reciprocally impacts the characteristics of the waves too, causing detectable shifts in their amplitude, phase, and propagation velocity. These changes in the wave features can be measured and used as a sensing strategy to detect pathogenic species, quantify proteins and DNA, or even divulge information about the change in physical properties of cells such as viscoelasticity or stiffness. Portable and compact acoustic sensors can be instrumental for the detection of pathogens such as Ebola, Influenza A, and HIV with higher accuracy than the standard polymerase chain reaction (PCR) method.28–31 Another advantage of these acoustic sensors is their ability to be integrated with smartphones since the radiofrequency input and output of SAW-sensing facilitates the electronic readout.32 These unique features coupled with the fast detection time, establish acoustofluidics as a promising and powerful sensing technology for point of care testing.33
Apart from acoustophoresis and biosensing, acoustofluidic platforms have shown enormous potential in both developing therapeutic agents and also as an efficient method of drug delivery, vaccination, and gene transfection.34–38 Moreover, acoustofluidics can be further exploited to produce functional mechanical trigger to induce biological phenotypes in samples in unique ways: from enhancing wound healing to inducing concussions to worms for brain injury research!39–41
With the expansion of the acoustofluidic applications, valuable papers gathered and reviewed the advancement in each application such as acoustic separation,42,43 acoustic tweezers,5 active actuation,44 cavitation,45 sound-based mechanobiology,46 and technical aspects.47 Here, we aim to review some of the less discussed, yet emerging applications where acoustofluidics not only has offered superior performances but also has introduced features that are unique to acoustic systems. In this paper, we review the recent advancements in acoustofluidic technologies for diagnostic purposes, tissue engineering, drug synthesis and delivery, fundamental biology studies, bioparticle separation, and sorting. We briefly introduce the physics behind the acoustic phenomena to fathom the mechanism of operation and then put the spotlight on the exciting developments of acoustofluidics in recent years with an application-based perspective. The capacities, working conditions, application-specific considerations, and challenges of state-of-the-art acoustic techniques are described and compared to assist researchers in adopting the best-suited acoustofluidic strategy for their application.
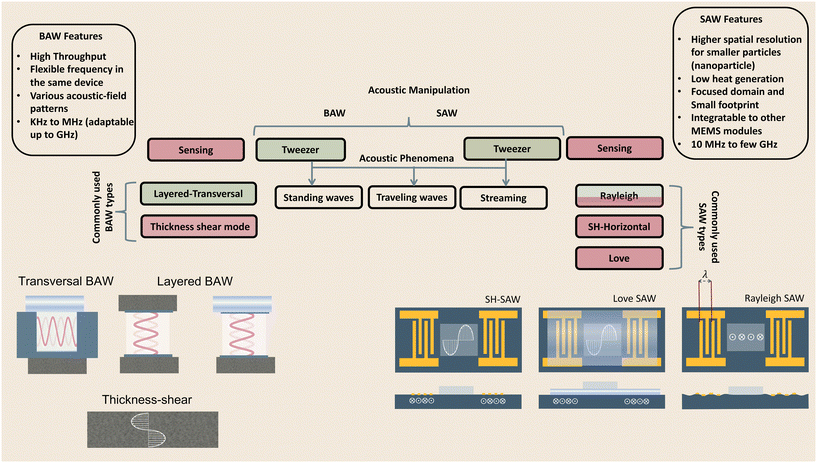
2 Physics and acoustic excitation methods
The generation of acoustic waves in on-chip platforms generally starts by introducing an alternating electric current (AC) to a piezoelectric material. The electrical field modifies the polarization of the dielectric material, in a process known as the converse piezoelectric effect. The electrical field is transduced into mechanical oscillations, and subsequently, into acoustic waves which carry the mechanical energy and momentum by compression and rarefication through the medium.48 The path of propagation in the medium within a piezoelectric slab classifies acoustic waves into two general types: surface acoustic waves (SAWs), which as the name suggests, propagate on the surface plane of the material, and bulk acoustic waves (BAWs), which travel throughout the bulk of the material. Although this general classification is based on the wave path, BAWs and SAWs show more fundamental differences including the selection of the piezoelectric material, the fabrication process, the operating conditions, and the nature of waves interacting with objects.2.1 Bulk acoustic waves
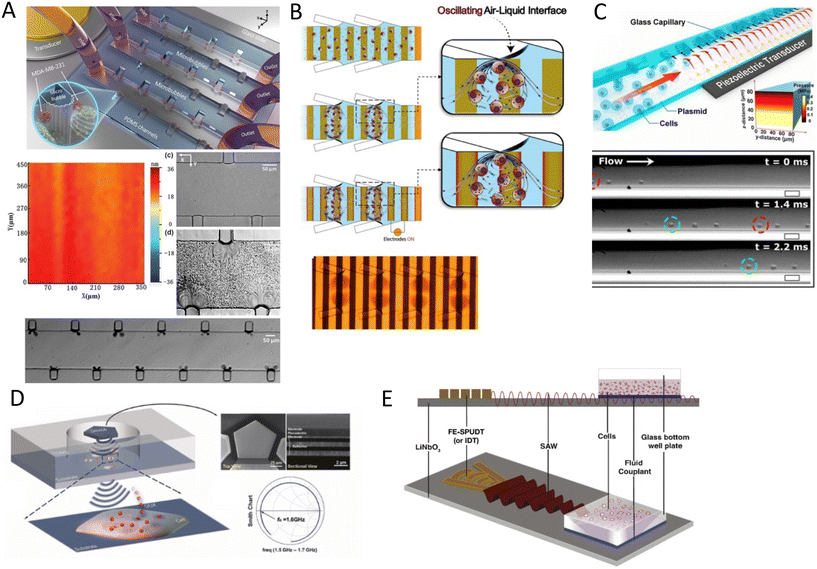
Bulk acoustic waves are a well-developed technology with decades of history in numerous biomedical applications, many of which are currently commercialized, such as the quartz crystal microbalance (QCM) biosensor. BAWs in microfluidic devices are typically produced by thickness or transverse vibrations of the piezoelectric transducers adhered in the vicinity of the fluid channel (Fig. 1). The piezoelectric material in BAWs is usually piezoceramics, such as lead zirconate titanate (PZT). By tailoring the position, configuration, and the number of piezo elements, and their interaction with intermediate layers, one can induce various acoustic phenomena, including standing acoustic waves, traveling acoustic waves, and different types of microstreaming. Generally, the operational frequency of these devices is lower than 10 megahertz (MHz), corresponding to wavelengths (λ > 100 μm) that are significantly larger than many biological particles, i.e. cells, biomolecules, and cellular vesicles. Therefore, the manipulation precision of individual particles can be lower in BAW compared to SAW, however, they can handle bigger clusters of particles and work with high flow rates, making them suitable for scale-up applications.2.2 Surface acoustic waves
Microfluidic SAW-based devices are fabricated by patterning interdigitated electrodes on highly efficient piezoelectric substrates, for instance, quartz crystals and lithium niobate (LibNO3). Upon activation, each interdigitated transducer (IDT) conducts an electrical signal to generate mechanical oscillations from its fingerprint, which subsequently propagates as surface acoustic waves (Fig. 1).49 When the wave from one IDT finger reaches the wave from an adjacent finger, it experiences a constructive interference, and the amplitude of the wave increases. In SAW systems, most of the acoustic energy is confined between the surface of the substrate to one wavelength below the surface.50 This localization of the acoustic energy at the surface leads to confined active regions in SAW devices and minimizes the power consumption compared to that of BAWs.5,51 SAW devices typically operate in the megahertz (MHz) to lower-end gigahertz (GHz) frequencies which corresponds to micron-order wavelengths. Hence, SAW devices are usually equipped with a high spatial resolution for the manipulation of single cells, and micron-to-nano-sized bioparticles. Since these piezoelectric substrates are biologically inert, the microchannel can be placed in direct contact with the substrate; however, the heat generation should be monitored.51The most explored SAW type is Rayleigh waves, which can efficiently leak into the liquids in contact with the propagation path. Due to this strong liquid coupling, these leaky waves are almost ubiquitously used for the manipulation of biofluids and suspended particles.33,52 Typically, the standard piezoelectric substrate to create leaky waves is a 127.86° Y-rotated, X-propagating lithium niobate, although the use of zinc oxide, aluminum nitride, and quartz have been also reported.49 For sensing applications, shear horizontal surface acoustic waves (SH-SAW) are the gold standard. The horizontal direction of vibration (Fig. 1) in this wave type reduces the leakage of acoustic energy into the media and preserves the signal strength. Hence, SH-SAW offers a higher signal-to-noise ratio compared to Rayleigh waves and is more commonly used for sensing in liquid environments. The most frequently used piezoelectric substrates for SH-SAW include 36° YX, 41° YX, 64° YX LiNbO3, lithium tantalite, and ST-cut quartz.33,52 Love mode wave is another SAW wave type for biosensing which is similar to SH-SAW with a waveguide layer on the propagation path (Fig. 1). The waveguides, typically SiO2 layers, have a smaller shear rate compared to that of the substrate and can further inhibit leakage into the media. This leads to the concentration of the acoustic energy at the surface, rendering Love-based devices highly sensitive to physical changes such as mass and viscosity for better sensing in both gas and liquid environments.33,53
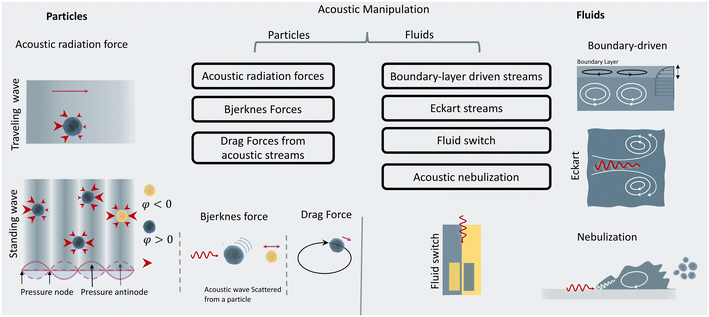
2.3 Acoustic phenomena
When objects are exposed to acoustic waves, they show distinctive absorption, reflection, and scattering behaviors that lead to various interesting phenomena. In fluids, acoustic forces can create different types of microstreams. On the other hand, for particles, bubbles, and droplets the main acoustic forces are acoustic radiation forces (ARF), Bjerknes forces, and drag forces induced by acoustic streams. These acoustic phenomena are introduced and briefly discussed in the following sections with a focus on the functional concepts and mechanisms. For readers interested in the theory of acoustics, we recommend the insightful articles by Friend's and Bruus' group.54,55Eckart streaming. The origin of the Eckart streaming is the viscous attenuation of the acoustic energy during the propagation of the waves in the bulk of a fluid.56 As the acoustic beam travels away from the source, the energy of the waves dissipates at a rate proportional to the square of their frequency, thus generating an acoustic pressure gradient along the direction of propagation (Fig. 2). In microfluidic channels, this phenomenon usually leads to circulatory streams. For noticeable Eckart streaming to happen, the length of the bulk fluid medium must be in the order of the acoustic attenuation length, which usually occurs in high-frequency SAW devices.56,57
Boundary-driven streaming. This streaming, also known as boundary-layer-driven streaming, is the result of the acoustic energy dissipation in a thin boundary layer around oscillatory solid–liquid or gas–liquid interfaces.58,59 The steep change of velocity from zero (on a no-slip interface) to a free-field value within a thin domain, transforms acoustic energy into a strong streaming flow in the confined boundary layer. The primary stream generates an outer counter-rotating vortex (Fig. 2). This method is commonly employed in microfluidic platforms by embedding oscillatory sharp edges,60 bubbles,45 the combination of both,34 and even channel walls.61
Acoustic streaming-induced drag forces (ASF). ASF is an indirect force of acoustic waves on particles that stems from vortices in their surrounding fluid. The acoustic streaming and their resultant drag forces can manipulate the trajectory of suspended cells or droplets. When a suspended particle with initial velocity (Vp) becomes exposed to a streaming velocity field (Vs), it experiences a Stokes' drag force, which realigns its direction of motion to the streamline of the vortices. The Stokes' drag forces are calculatable by:
| Fdrag = 6πηR(Vs − Vp) | (1) |
Primary acoustic radiation force. When acoustic waves face a change in the acoustic properties of their path, such as encountering immersed particles or interfaces of different media, they experience absorption, refraction, and scattering. The change in acoustic momentum creates a net body force on the particles or fluid interface, known as the acoustic radiation force. In general, the acoustic radiation force is comprised of two components: acoustic gradient forces and scattering forces.†62
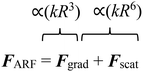 | (2) |
The gradient forces, as the name suggests, stem from the gradient of the acoustic pressure and it is proportional to R3, while the scattering component is proportional to R6. In small particles, the scattering force becomes negligible compared to the gradient pressure force, except when the acoustic field is relatively uniform in space, such as in traveling plane waves.
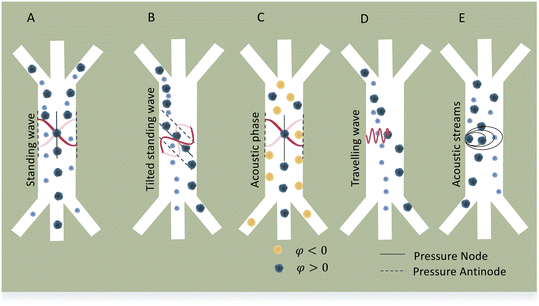
Standing acoustic waves create a strong ARF on particles by increasing the gradient force factor in eqn (2). This increase in the gradient pressure force is due to standing waves periodically dividing the acoustic domain into regions of low and high pressure that push particles and droplets to either pressure nodes or antinodes (Fig. 1). Standing acoustic waves in BAWs can be constructed through the interference of waves from two facing acoustic sources with the same frequency or through an incident wave from a single source and its reflection by an acoustic reflector. On the other hand, standing surface acoustic waves (SSAW) are usually generated by two or more even pairs of counter-facing IDTs. The acoustic contrast factor of particles, known by:6
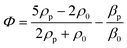 | (3) |
Traveling acoustic waves can be generated in either BAW devices, by using a single piezo ceramic, or SAW devices, by depositing one set of IDTs that propagate waves to the microchannel. By neglecting the gradient component in eqn (2), the ARF reduces to a scattering force with a magnitude that scales with R6. The scattering forces continuously push particles in the direction of the beam propagation, causing particles to migrate away from the acoustic source.
Secondary acoustic radiation forces. The scattering of primary acoustic waves from particles or bubbles creates a net force on their adjacent particles. This force is also known as the secondary Bjerknes force and can create mutual particle–particle attraction or repulsion depending on the nature of the particles. The intensity of the secondary acoustic radiation force decays by an increase in the distance of particles and is also frequency-dependent, with forces usually peaking when the particle diameter approaches half to one wavelength (d/λ ≈ 0.5–1).63,64
3 Acoustic cell patterning for tissue engineering
The interplay of primary and secondary radiation forces, and acoustic streaming forces creates various acoustic phenomena that allow flexible and versatile patterning of cells, while its gentle and non-contact nature preserves cell viability and functionality, rendering it a suitable tool for tissue engineering.65–67 Reconstructing the physical architecture of the native tissue is one of the key aspects of tissue engineering.68 Patterning cells is instrumental for analyzing cell–cell interactions and collective cell behavior such as network formations and neurite guidance, angiogenesis, cardiomyocyte beating, and myofibrillogenesis.13,14,69,70 Acoustic radiation forces are more commonly used to pattern cells into 2D or 3D constructs wherein suspended cells can be actively guided and accurately organized in pressure nodes or antinodes, based on their compressibility and density properties. These reposing sites can be designed to create various spatial patterns, which can remain fixed over time or dynamically reconfigured by changing the frequency or by shifting the phase.71The two main steps in tissue engineering are often divided into the acoustic organization of the cells into the pattern of interest, followed by a step to preserve the cell pattern to establish cell–cell connections and for cell patterns to mature into tissues.71 The preserving step, i.e., maintaining the patterned architecture over the tissue development period, is highly dependent on the cell type, cell–cell affinity, and cell-environment interactions. Cells with higher affinity rapidly form a strong arrangement dictated by the acoustic pressure node design, while low-affinity cells can easily migrate from their initial position.23 To overcome this issue, hydrogels are often used to better maintain the cell patterns over time.
3.1 Single pressure node patterning
The linear arrangement of cells is seen in many native tissues and plays a vital role in cell and tissue functionality. For instance, cardiac, endothelial, and fibroblast cells tend to spontaneously organize in linear patterns of elongated cell structures to induce cell–cell interconnections and enhance their capability to withstand contractile and tensile loads in tissues.72,73 ARFs in SSAW devices have been widely reported to effectively guide cells and particles in parallel lines and nodes within seconds by activating one or two pairs of IDTs (Fig. 3B).74 For instance, SSAWs were used to pattern HEK293T, endothelial (HMVEC), and glioma (U87) cells in pressure nodes and lines to study the gap junctional dye transfer dynamics of homotypic and heterotypic patterns.75 This method allowed to successfully quantify the average dye transfer rates for all three cell types, showing an increase in intensity signal over time as an indicator of stronger cell–cell interconnection. Taking a step further, epithelial cancer cells (HeLa) and endothelial cells (HMVEC-d) were linearly patterned using SSAWs to study the cell migration of cancer cells with a preserved cell viability after 24 h (>99%). By tuning the phase shift, sequential patterns of heterotypic cell lines were generated to study the cell mobility in monoculture, random co-culture, and acoustic co-culture in real-time to study the events during cancer metastasis.76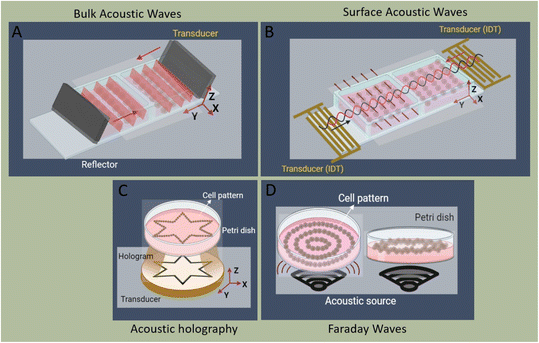 | ||
| Fig. 3 Schematic of cell patterning modes, including A) bulk acoustic waves, B) surface acoustic waves, C) acoustic holography, and D) Faraday waves. | ||
Despite the rapid acoustic linear cell patterning, one of the main challenges of tissue engineering is the preservation of the cell pattern over time for tissue maturation. One approach for maintaining the cell pattern is to transform the free-moving cells to an adherent state. Under the presence of SSAWs, cells can form linear patterns with a gap above the substrate, avoiding surface contact. Upon the removal of acoustic waves, cells were shown to be gently gravity-deposited on a collagen-treated surface, allowing for the pattern to be maintained.75 However, this scaffold-free approach to preserving cell patterns can be time-consuming (>1 h) and the patterns can easily be deformed. As a solution, scaffolds have been proposed to maintain the cell pattern over time. The formation of functional collateral cylindroid for ischemia therapy was performed by acoustically patterning endothelial cells (HUVECs) and human adipose-derived stem cells (hASC) in a catechol-conjugated hyaluronic acid hydrogel (Fig. 4B).13 SSAW patterned structures exhibited higher secretion of angiogenic (VEGF) and anti-inflammatory cytokines (IL-10) factors for up to 7 days prior to transplantation into a mouse model. Another solution to retain acoustically defined patterns is to polymerize the cells and their surrounding hydrogel after SAW exposure. Photocurable polymers, including PEGDA and GelMA, were used to create patterns of HeLa, MC3T3-E1, and P12 Adh cells in capillary tubes using a pair of IDTs,17 and slanted-finger interdigital transducers (SFITs) for the nodal alignment of cardiomyocytes in GelMA (<10 s).14 Acoustically patterned cardiac cells demonstrated beating activity after 5–7 days with high cell viability (90%).
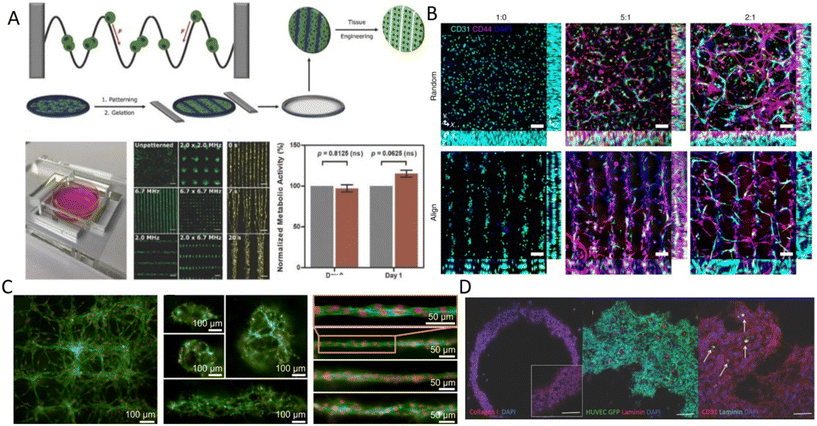 | ||
Fig. 4 Acoustic cell patterning for tissue engineering. A) Myoblasts patterning using BAWs with controlled gelation showing pattern preservation over time (scale bar = 200 μm).70 B) Microvessels in hindlimb muscle via SSAW patterning of HUVECs and hADSCs at different HUVEC/hADSC ratios (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, 5 0, 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2 1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (scale bar = 100 μm).13 C) SSAW-patterned fibroblasts in fibrin gels with multiple 3D microscale cellular structures forming network (left), cages (center), and unidirectional bundles (right) after 30 hours.11 D) Fibroblast spheroids patterning in ring shapes using Faraday waves showing vascularization (scale bar = 500 μm).87 Reprinted with permission. 1) (scale bar = 100 μm).13 C) SSAW-patterned fibroblasts in fibrin gels with multiple 3D microscale cellular structures forming network (left), cages (center), and unidirectional bundles (right) after 30 hours.11 D) Fibroblast spheroids patterning in ring shapes using Faraday waves showing vascularization (scale bar = 500 μm).87 Reprinted with permission. | ||
Linear patterning via BAWs (Fig. 3A) has also been vastly used, for instance, to study angiogenesis. ARFs in the form of standing waves were shown to induce cell banding patterns for HUVECs in 3D collagen-based hydrogels for up to 10 days.24 Acoustically patterned HUVECs resulted in lumen-containing networks throughout the hydrogel on day 10. Acoustophoresis patterning and high-frequency ultrasound imaging tools have also been integrated to study the vascularization of constructs with defined microvessel size and orientation.77 One particular prospect of acoustic manipulation of cells is their combination with 3D bioprinting. Acoustic waves were actuated in a bioprinter nozzle to align C2C12 cells and HUVECs in the center of a GelMA fibrin hydrogel, narrowing the cell distribution to 5% of the bioink width and thus enhancing the orientation control and the elongation of the cells in the printing direction.25 In a similar manner, but using human osteosarcoma cells (MG63) and hASCs, linear cell patterns were successfully produced in an alginate-CaCl2 solution as a bioink for acoustic printing with high cell viability (>80%).78 Linear patterning has also been reported to recreate muscle fibers and enhance the ability of cells to withstand tensile loads.70 C2C12 myoblasts cells were suspended and acoustically patterned in GelMA, where cells showed enhanced myofibrillogenesis with aligned bundles of myotubes after 7 days (Fig. 4A).70
3.2 Multiple pressure nodes
In addition to linear patterns, complex geometries of cellular arrangements can be attained using acoustofluidics. BAW-based devices have been more often reported for patterning cells and particles into various geometries due to their larger acoustic domain compared to SAWs. For instance, a heptagonal acoustic chamber with 7 transducers could dynamically pattern particles, MDCK cells, and microbubbles in linear and hexagonal geometries by controlling the number, position, and phase-shifting of activated transducers.8,79 In a similar heptagonal platform, C2C12 and Schwann cells were patterned to form neural network interconnections and study the outgrowth of neurites, which was largely governed by the orientation of the initial pattern.9,80 ARFs in BAWs also allowed studying the forces for cell adhesion and cell organization, which influence cell functionality. Rat C6 cells were patterned in a hexagonal arrangement, showing an increased concentration of adhesion molecules NCAM and N-cadherin at the cell–cell interfaces after 8 minutes of BAW induction.22As discussed in the previous section, cell patterns can be preserved over time by suspending cells in a hydrogel. As an example, NIH 3T3 fibroblasts were embedded in fibrin gels and were acoustically patterned using SSAW with different nodal configurations. After culturing the samples for 30 hours, different geometries were formed based on their initial patterning, including unidirectional bundles, a network structure, and a cage-like structure due to cell migration and growth (Fig. 4C).11 Moreover, acoustic radiation forces can levitate cells in multiple parallel horizontal planes to form 3D constructs. Examples have been shown for the development of multilayer brain-like architectures with human embryonic stem cell-derived neuro-progenitors using fibrin hydrogels,81 and sheet-like assemblies of epithelial and fibroblasts as a polarized epithelial barrier for up to 14 days.82 Furthermore, Cohen and colleagues applied standing traps for scaled neuronal patterning and demonstrated directed neuronal growth of DRG neurons due to cellular organization, for up to 6 days.19
One limitation that should be considered with SAW devices compared to BAWs, is that their operating frequency is usually predetermined by the IDT geometry. As a solution, a dynamic cell mechanism patterning can be performed, using slanted-finger IDT (SFITs).83 SFITs allow changing the distance between pressure nodal lines, and hence the dynamic adjustment of cell alignments. Circular slanted-finger interdigital transducers (CSFITs) were also reported to dynamically manipulate particles using multi-tone excitation signals.84 Another approach to create more dynamic geometries is using waveguides, consisting of structures mounted on top of the piezoelectric element. Circular, rectangular, and triangular acoustic waveguides have been studied for guiding particles to the reference waveguide shape.85 Similarly, but using a Petri dish as the waveguide coupled to a PZT piezo, PC12 cells were successfully patterned in concentric circles.19
3.3 Faraday standing waves and acoustic holography
Faraday waves occur at the surface of a fluid that is placed on a vertically vibrating plate and can take various complex shapes to pattern microparticles and cells (Fig. 3D). The patterns of Faraday waves are determined by the nature of vibration (frequency, amplitude, waveform), container geometries (boundary condition), and fluid properties (viscosity, density, surface tension).86 As an example, a bio-tunable acoustic node assembly using Faraday standing waves was used to form cell spheroids (>104) within seconds. Both homogenous and heterogeneous tissue constructs were formed using primary rat hepatocytes, fibroblasts, and HUVECs, where the formation of bile canaliculi, hepatic gap junctions, and extracellular matrix was observed (Fig. 4D).87 Faraday wave manipulation has also been used to induce vascularization, where HUVECs and hMSC spheroids were acoustically formed in fibrin and GelMA hydrogels inside Petri dishes and angiogenesis was recorded after 5 days.88 The hydrodynamic drag force, generated by Faraday waves was also used to pattern fibroblasts (NIH 3T3) into a ring shape, which was then immobilized by a cross-linkable alginate hydrogel after 72 h.89 Furthermore, Faraday waves have also been used to pattern pluripotent stem cell cardiomyocytes (hiPSC-CMs) in 3D aggregates via fibrin hydrogels, showing high levels of contractile stress, beating frequency, and contraction–relaxation rates.90 Faraday waves have also been reported to pattern cardiomyocytes as programmable functional biorobots rings. The contraction and relaxation cycles of cardiomyocyte rings can act as self-powered biorobots to induce synchronous locomotion and mechanical actuation.91Another method for creating complex cell patterns is the use of acoustic holography. In order to generate compound patterns by aforementioned methods, multiple acoustic sources must be carefully coordinated to superimpose waves which becomes excessively challenging for the arrangement of a large number of cells into complex topographies, as it requires thousands of transmitters. Static holograms are a smart solution wherein the phase information of the desired structure is encoded in a 3D-printed plate that is mounted on a single transducer and can generate more complex acoustic fields (Fig. 3C).92 This method was exploited to custom-design acoustic fields for colon cancer cells (HCT-116) in a collagen-based solution.93 Patterned cells showed high viability rate, where cells exhibited F-actin-based protrusions, indicating good cell growth and cell thriving within the matrix after 1 week. This method can thus add a new dimension to the large-scale patterning of complex shapes. However, as it stands, its resolution and cell–cell connections appear to be inefficient in comparison to SAW and BAW approaches. Using leaky surface acoustic wave holography can overcome the frequency and resolution limits of previous holographic techniques to control three-dimensional acoustic beams at the microscale region.11
3.4 Acoustofluidics for spheroids formation
In addition to linear and multiple nodal cell patterning, significant work on 3D spheroid formation via acoustophoresis has been conducted. Standing Bulk Acoustic Waves have been effectively used to aggregate cells into spheroids,23 including embryonic mouse brain cells using photocurable GelMA to model Alzheimer's disease,26 HepG2 in alginate/CaCl2 showing high viability for up to 10 days,94 RBCs and HepG2 using different transducer geometries,95 HepG2 spheroids showing great viability up to 3 weeks,96 and core-shell ovarian cancer (OVCAR-8) cells for therapeutic studies and tumor interactions.97SAWs have also been exploited to form spheroids by modulating pressure nodes for controlled cellular aggregation. In an SSAW platform, size controllable HepG2 and HEK293 aggregates were formed with a smooth spheroid surface after 24 h and high proliferation and viability for up to 7 days.98 Similarly, 3D multicellular human mononuclear leukemia spheroids (THP-1) were acoustically formed within pressure nodes using a photocurable GelMA hydrogel. These spheroids showed faithful resemblance to cancer models where the cell aggregate activity was inversely proportional to the drug concentration, with a lower sensitivity to drug toxicity in comparison to monolayers (Fig. 5A),99 and with high throughputs of 6000 spheroids.15 Single SSAW fields have also been shown to generate mono-sized spheroids by coupling capillary tubes to the piezoelectric substrate along the wave propagation direction.17,100,101 In this method, cells are first guided by acoustic pressure nodal arrays and then self-assembled to form spheroids. This mechanism was tested for HeLa cells in polymerized hydrogels (PEGDA and GelMA) to create spheroid embedded fibers ready for transplantation (Fig. 5B).17
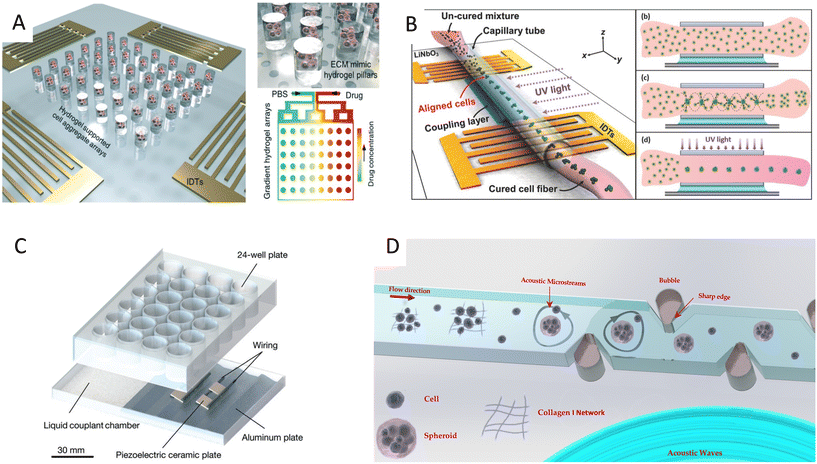 | ||
| Fig. 5 Cell spheroid formation methods using acoustofluidics. A) Acoustic assembly showing multicellular aggregates encapsulated in the ECM mimic hydrogel pillars (left, top right) and convection–diffusion based gradient drug fluid generating microfluidic system at different drug environments (bottom right).99 B) SSAW-based spheroid formation platform consisting of a polyethylene tube coupled to a parallel IDT setup with a water-coupling layer (left), where cells seeded in a cross-linkable hydrogel are patterned and UV crosslinked (right).17 C) Acoustic streaming-based cell agglomeration platform consisting of a fluid coupling layer (left) to transmit acoustic waves to a 24-well plate (right) (scale bar = 30 mm).102 D) Schematic illustration of acoustic formation platform using boundary-driven acoustic streams, where cells encompassed by collagen fibrils are trapped and reshaped into spheroids.103 Reprinted with permission. | ||
Although less explored, acoustic streaming can be also used for spheroid formation. The hydrodynamic drag forces induced by acoustic microstreaming via a piezo element coupled to a microwell channel can agglomerate cells in the center bottom of the wells, forming spherical and compact spheroids (Fig. 5C).102 A similar approach, albeit in a SAW setting, used 30 MHz focused surface acoustic waves to create microstreaming for BT-474 cell aggregation in well plates.16 Furthermore, our group recently reported the rapid formation of cell spheroids in acoustic streams as build blocks for tissue engineering. We used boundary-driven acoustic streams to trap cells, adhere them with collagen, and release them in a continuous flow, all in less than 10 seconds. Using collagen as a natural extracellular matrix (ECM) component allowed to attach multiple cell lines including MDA-MB-231 and MCF-7 spheroids without relying on their ability to secret ECM (Table 1). This approach was compatible with multicellular spheroids as well as cell–particle composite spheroids, and also showed the merging ability of spheroid building blocks to form tissue constructs (Fig. 5D).103
| Acoustic phenomena | Acoustic parameters | Cell/particle | Supporting material; culture time |
|---|---|---|---|
| SSAW | 12.65 MHz, 1.5 W cm−2 | 10 μm beads, HeLa S3, MC3T3-E1, PC12 Adh cells | PEGDA, GelMA17 |
| λ = 300 μm, 24.85 dBm | MCF-7, PS, fluorescent PS microspheres | Collagen type I; 1 day104 | |
| 3.4, 4.6, 6.4 MHz, −7 to −12 dBm | Neonatal rat ventricular cardiomyocytes | GelMA; 7 days14 | |
| 1.0, 3.4, 5.4, 7.5 MHz | NIH 3 T3 fibroblast | Fibrin gels; 30 hours105 | |
| 13.928 MHz | hADSCs, HUVECs, hiPSC-ECSs | HA-CA catechol-conjugated hyaluronic acid; 28 days13 | |
| BAW | 2.46 ± 0.5 MHz (1 kHz step), 15 Vpp | HepG2, A498, ACHN, LUTC-2 | 2% agarose hydrogel; 2 days23 |
| 0.5–2.0 MHz, 0–0.2 MPa | HUVEC24,77 and HMVEC-D77 | Collagen type I; 10 days24,77 | |
| 877 kHz;25 6.7 MHz, 20 Vpp70 | C2C12 myoblasts25,70 and HUVECs25 | GelMA + fibrin; 14 days25 and type I collagen, GelMa, agarose; 7 days70 | |
| 0.71–2 MHz, 100 and 200 m Vpp | Human adipose derived stem cells | Alginate; 4 days78 | |
| 340–680 kHz, 5 V | HUES 64 human embryonic stem cells | Fibrin; 30 days81 | |
| 1.95–2.12 MH, 4 Vpp | 16HBE14o- epithelial cell and MRC5 fibroblast | Scaffold-free; 14 days82 | |
| SSAW + SBAW | 19.4 MHz, 3–20 Vpp (SSAW) and 1.14 MHz, 10 Vpp (BAW) | 2 μm PS beads, DRG and PC12 | Collagen type I; 6 days19 |
| Acoustic streaming | 16.1 KHz, 10 Vpp | MCF-7, MDA-MB231 | Collagen type I103 |
| Faraday waves | 80–157 Hz, 0.5–3 g | TCP particles, GFP-HUVECs and human mesenchymal stem cells | GelMA and fibrin gel; 5 days88 |
4 Acoustofluidics for therapeutic applications
Acoustically formed tissue structures, such as tumoroids, are valuable drug screening models to investigate the response to chemotherapeutics such as Gemcitabine on pancreatic cancer cells (Panc02) and 5-fluorouracil (5-FU) on HepG2 spheroids.15,98 However, the role of acoustofluidics in drug development is not limited to the model's fabrication. Various phenomena caused by acoustofluidics, such as acoustic streaming and mixing, sonoporation, nebulization, and acoustic forces are shown to be powerful tools for the therapeutic development, from the initial drug synthesis and nanocarrier fabrication to the delivery of active agents to the target tissue using acoustic energy.4.1 Drug development and nanoparticle synthesis
One of the recent applications of acoustofluidics in drug development is the synthesis of nanoparticles, as drug nanocarriers. Nanoparticles have been increasingly employed for delivering therapeutic agents such as chemotherapeutics and vaccines. These nanocarriers can reduce the toxicity and immune response by protecting the cargo from opsonization and subsequent sequestration by the phagocyte system. This protective mechanism, combined with targeted delivery and controlled release, can help the nanocarriers to release their cargo more effectively at the target site of interest and reduce their overall toxicity.106Our group developed a boundary-driven acoustic streaming platform for the synthesis of liposomes and PLGA- PEG nanoparticles, two FDA-approved drug carriers. A combination of oscillatory bubbles and sharp-edges induced strong microstreams in the channels to control and accelerate the mixing of precursors and antisolvents. The mixing time generally governs the nucleation rate and the size of the nanoparticles. Therefore, controlling the mixing time allowed us to tune the size of the nanoparticles which is the key physical characteristic for their transportation in the body and the target delivery efficiency. Nanoparticle tracking analysis (NTA) results also showed a surge in the number of the produced nanoparticles using this acoustic streaming method, due to the obviation of aggregates (Fig. 6A).34 Microstreams raised from the acoustic actuation of sharp edges were also used to synthesize various types of organic and inorganic nanoparticles, such as DNA/lipid complexes, polymeric, and chitosan nanoparticles.107 The size of nanoparticles was shown to decrease by increasing the number of sharp edges. In another mixing mechanism, a resonating membrane was embedded in a microfluidic platform with multiple edges to induce acoustic microstreams. The microstreams were used to facilitate and accelerate the mixing time for the synthesis of budesonide nanodrugs, a poorly soluble asthma medication.108 Among various proposed designs, the 8-point star shape system showed the highest throughput of 8 ml min−1 with a star shape mixer for the production of Budesonide nanodrugs and DNA nanoparticles (Fig. 6B).109 A similar star shape acoustic mixer was also used for generating protein nanoparticles CA-P114, in which the size could be tuned by controlling the strength and time of mixing.110
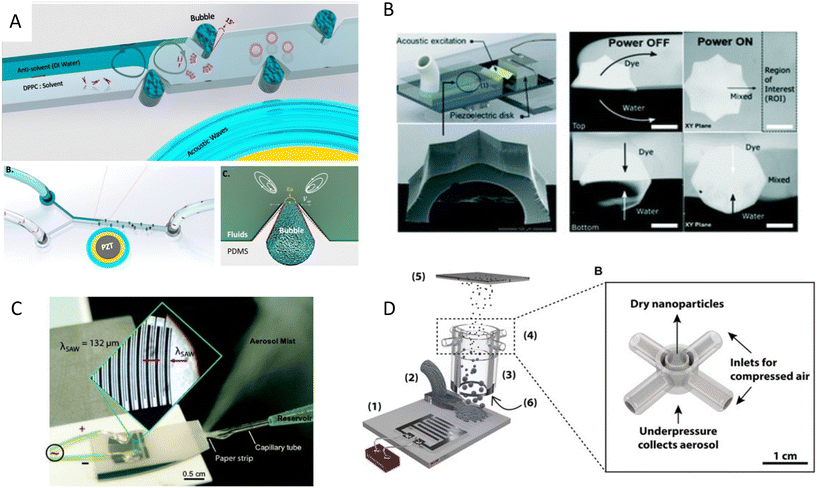 | ||
| Fig. 6 Mechanism of nanoparticle synthesis using acoustic waves A) strong boundary-driven microstreams are generated by the combination of oscillatory sharp-edges and bubbles to accelerate the mixing for controlled synthesis of PLGA and liposome nanoparticles.34 B) Acoustic microstreams induced by multiple edges system used for the synthesis of budesonide nanodrugs and DNA nanoparticles.109 C) SAW nebulization device for the synthesis of multilayer nanocarriers with encapsulated plasmid DNA.112 D) SAW nebulization device with a gas control unit which introduces reactive gases to atomized airborne drops for the synthesis of amorphous CaCO3 nanoparticles.119 Reprinted with permission. | ||
In addition to microstreams, SAWs can be exploited for nanoparticle synthesis, albeit through a mechanism known as acoustic nebulization. SAW atomizers, first introduced by Kurosawa,111 generally consist of a set of IDTs and a nozzle, channel, or paper wicking for the controlled dispensing of liquid at the propagation path of waves. When Rayleigh waves reach the liquid, the waves leak into the medium and deliver the acoustic energy to the fluid (33% approximately). The high-energy leaky SAW can cause destabilizing capillary waves at the interface of liquid and air, where droplets create fine aerosols (Fig. 6C).112–114 The size of the aerosols can be tuned within the range of 0.1 and 30 μm by tailoring the acoustic frequency, fluid flow rate, and liquid characteristics, such as the surface tension and viscosity.114–116
Furthermore, the lower power requirements and higher operating frequencies in SAW devices lead to higher biocompatibility of these systems, as opposed to the mechanical and cavitation-based aerosolization methods, which tend to cause damage to the structure and functionality of biomolecules during aerosolization. The preserved biocompatibility along with the control on the particle's size, higher delivery percentage,36 and the miniaturization of the system, render SAW a potential platform for the generation of micro/nano-sized drugs and also as a delivery method, particularly for the pulmonary system.117,37
Friend's and Yeo's groups have extensively investigated the use of high-frequency SAWs for nanoparticle generation and drug delivery purposes. They also studied the effects of SAW nebulization on the integrity and functionality of various shear-sensitive bioagents.118,35 Upon atomization, the solvent content of the aerosols evaporates in flight and leaves behind the polymeric or protein nanoparticles to solidify. Friend et al.113 used this mechanism to synthesize nanoparticles of poly-ε-caprolactone (PCL), a biocompatible and biodegradable polymer used for controlled release drug delivery. They further used this evaporative technique to produce solid protein nanoparticles of BSA and insulin and showed that the size of nanoparticles can be controlled by tuning the initial protein concentration in the solvent.113 In another work, a SAW atomizer was coupled with a drying unit to control the kinetics of crystallization through the rate of drying, where reactive gases were introduced for chemical modification of drops while in flight. As a proof of concept, CO2-enriched air was introduced to initiate the reaction with airborne drops of Ca(OH)2 and synthesized amorphous CaCO3 nanoparticles (Fig. 6D).119
One unique capacity of this nanoparticle generation method is the fabrication of multilayer polyelectrolyte nanocarriers. Qi et al.112 could successfully synthesize layer-by-layer coated nanoparticles of positively charged chitosan or polyethyleneimine and negatively charged carboxymethyl cellulose. In their method, they collected the condensed nanoparticles of the first layer in an oppositely charged polymer solution to form the second layer. Through the repetition of this atomizing-suspension cycle, nanoparticles with up to 8 alternating layers were produced, with a controlled drug release profile. These nanoparticles were used to encapsulate plasmid DNA and showed the capacity for transfection (gene delivery) of human mesenchymal progenitor cells and COS-7 cells.112
4.2 Drug delivery
Acoustic nebulization not only could be used for synthesizing therapeutic carriers, but it can act as means to deliver therapeutic cargo, including drugs, RNA, proteins, and even cells. The facile, low-energy, and biocompatible aerosol formation method via acoustic atomization has a vast potential for the delivery of drugs to the pulmonary system. In this non-invasive approach, aerosols between 1 and 5 μm diameters can penetrate and deposit in the lower pulmonary tract and alveoli, where the large surface area and network of blood vessels facilitate the drug uptake.120 Moreover, the high frequency and lower power requirement of SAW atomization methods significantly minimizes the large shear stress and cavitational damages of bigger molecules, thus being ideal for the manipulation of shear-sensitive bioagents, proteins, and DNA.37,35SAW atomization was also shown to be an efficient inhalation therapy method, which can directly deposit 70 to 80% of the short-acting b2 agonist salbutamol (asthmatic steroid) in the lungs, used for the treatment of asthma.117 In another study,113 a microdroplet containing bovine serum albumin (BSA) was produced as a proof of concept to show the compatibility of the SAW atomization with proteins. Furthermore, this technique was used to synthesize peptide-laden aerosols of size model anti-mycobacterial peptides for pulmonary peptide delivery.121 Interestingly, 70% of the aerosols were in a favorable size range for deep lung penetration with a 90% recovery rate, while retaining their integrity and anti-mycobacterial activity.121 Another antimicrobial application was shown by producing plasma-activated aerosols for surface disinfection. SAW atomized plasma-treated could induce high oxidative stresses on bacteria which led to a 96% reduction in E. coli colonies.122 This method was also used for the pulmonary delivery of antibiotic alternatives, phage K, and lysostaphin, to target Staphylococcus aureus with minimal losses in antimicrobial activity (Fig. 7A).123 Rajapaksa et al.36 proved the ability of the SAW nebulization method to address some of the challenges in pulmonary gene therapy and vaccination (Fig. 7B). The plasmid DNA encoding virus surface hemagglutinin protein (influenza A, human hemagglutinin H1N1) was delivered through intratracheal instillation to successfully immunize rats and sheep. The results showed that atomization did not harm the integrity of the plasmids nor hindered the vaccine to promote protective antibodies.
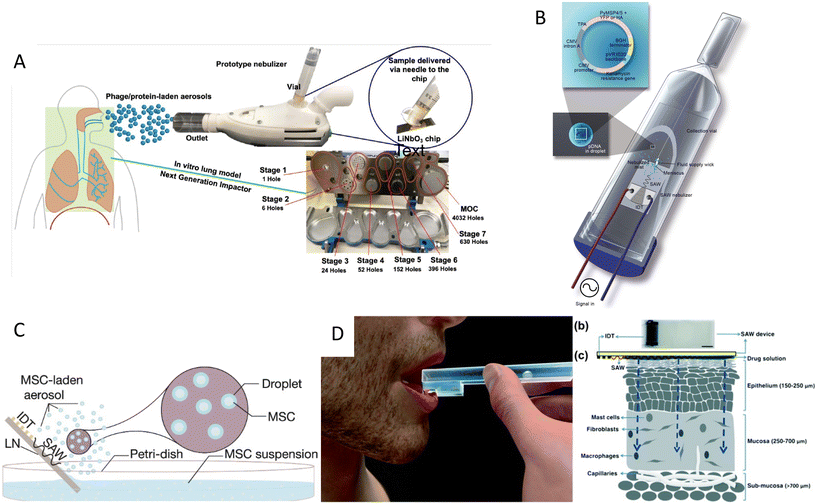 | ||
| Fig. 7 Acoustic devices for drug delivery. A) Acoustic nebulization for pulmonary drug delivery of aerosols.123 B) Acoustic nebulization for pulmonary plasmid delivery.36 C) Stem cell delivery by acoustic nebulization.124 D) Acoustic waves for permeabilization of tissue and inducing localized immune response.125 Reprinted with permission. | ||
A very novel application of biocompatible SAW nebulizers could be in the promising field of stem cell therapy. Cell therapy can be an alternative to chemotherapy for progressive respiratory system diseases. However, the susceptibility of stem cells to stresses during nebulization such as shear, cavitation, and heat, hinders their direct delivery to the lungs. Alhasan and coworkers studied the cell viability and functionality under acoustic nebulization with an optimal 1.5 W driving power with cell viability of up to 86.0%. They showed that the metabolic rate, proliferation, gene expression, and protein expression after SAW nebulization did not show significant differences compared to untreated cells, confirming the feasibility of this approach for pulmonary stem cell therapy (Fig. 7C).124
The ability of acoustofluidics as a drug-delivery method is not restricted to nebulization and the pulmonary system. Ramesan et al.125 used SAW to permeabilize the mucosal layer to enhance the delivery of small and large molecular therapeutic agents as an efficient route for vaccine administration. The low penetration depth of SAW waves proved to be useful for inducing a local immune response, allowing to transport the cargo through the mucus lining and epithelial barriers into immunocyte-rich regions. Moreover, this method avoids the cargo passing into the deeper vascularized submucosal regions in which the agent would be taken up by the circulatory system and thus diminishing the immunity (Fig. 7D).125 Despite this clever use of lower penetration depth in high-frequency acoustic waves, the limited penetration renders this mechanism more applicable for in vitro or ex vivo applications rather than in vivo. One of the growing applications of acoustic waves, especially with high-frequency SAW is to acoustically mediate the transport of biomolecules, siRNA, nanoparticles, DNA, and membrane-impermeable dyes or nanoparticles inside the cell.
The principal mechanism of acoustic-mediated cargo delivery into cells is sonoporation, which is the biophysical disruption of the cells' phospholipid membrane under ultrasound energy. When acoustic pressure waves encounter cells or tissues, they promote both the opening at the cellular junctions, as well as the poration of the cell membrane.126 Sonoporation has particularly been of interest as a nonviral transfection method to lower the toxicity and immunogenicity of viral transfection.127Microbubbles are the focus of many ultrasound sonoporation methods, as the expansion and contraction of microbubbles under acoustic waves are shown to induce cell permeability and facilitate the entry of therapeutics into the cells.128 If the variation in acoustic pressure is strong enough, it can lead to the collapse of the bubble. This phenomenon is known as inertial cavitation, which creates a shock wave and high shear stresses. Qiu showed that acoustic cavitation could induce pores with diameters from 100 nm to 1.25 μm in cells. The higher acoustic pressure or longer treatment, the larger the pore size, leading to also a higher permeability and better transfection efficiency; nonetheless, at the expense of cell viability.127,129,130
Working with the uncontrolled and unpredictable nature of inertial cavitation is challenging as it leads to excessive cell and DNA damage, as well as the production of free radicals and reactive oxygen species and the generation of oxidative stresses.126,131 Various proven alternatives have been introduced to avoid the micro-jetting forces from inertial cavitation such as stable cavitation. In stable cavitation, the acoustic pressure is controlled to make sure that the expansion and contraction do not lead to the collapse of the bubble. This oscillation can be used directly for infiltrating the cell membrane by pulling and pushing the plasma,132 or indirectly by generating acoustic microstreams. Although the flow field induced in steadily oscillating bubbles has a generally lower velocity than that of micro-jetting, the continuous shear stress and Stokes' drag force on cells can be sufficient to reduce the micro-viscosity of the lipid bilayer and effectively disrupt the membrane, while remaining controllable and safe for cells.57,133
For its part, Meng et al.134 used an array of monosized bubbles, sequestered in the sidewalls of their microfluidic channel to induce sonoporation (Fig. 8A). The microbubbles oscillate stably and created a pair of counter-rotating microstreams. The infused cells were trapped by the drag force from vortices and the secondary acoustic radiation force at the bubble surface, where the shear stress permeabilized MDA-MB-231 cells to allow propidium iodide (PI) to pass through the membrane. It would be of interest to see the performance of this sonoporation system for bigger molecules as well as for the transfection with mRNA or plasmids. Recently, a versatile and high throughput intracellular delivery was proposed by integrating hundreds of oscillating lateral cavities with an interdigitated electrodes array in a platform named acoustic–electric shear orbiting poration (AESOP).135 In this two-step strategy, cells were initially trapped in the acoustic microstreams where the mechanical shear stress induced nanopores in cell membranes while the electric field from electrodes expanded the nanopores (Fig. 8B). AESOP system showed the delivery of molecules from <1 kDa to 2 MDa into both adherent and suspension cells, with over 90% delivery efficiency, >80% cell viability, and remarkable throughput of 1 million cells per min per chip.
 | ||
| Fig. 8 Acoustic platforms for sonoporation and gene delivery to cells and tissue. A) Sonoporation by acoustic steams from an array of oscillatory bubbles.134 B) The combination of acoustic microstreams by bubbles and electroporation for gene delivery.135 C) BAW-based microfluidic device for high-throughput shear stress-sonoporation by the combination of microstreaming and acoustic radiation forces that push cells towards opposite capillary walls.137 D) High-frequency bulk-based nano-electromechanical device for delivery of eGFP plasmid DNA and doxorubicin through hypersonic membrane poration and acoustic streaming.139 E) Focused TSAW for delivering siRNA into nonadherent cells.38 Reprinted with permission. | ||
In addition to bubble-based mechanisms, direct interaction of acoustic waves without bubbles can also induce sonoporation, especially using high frequencies.127,133 For instance, standing waves can be an option to permeabilize the cell membrane in the absence of bubbles. The viability of cells with and without cavitation was compared and the cavitation-free method showed higher viability rates. This platform was further used to enhance the intracellular delivery of drugs such as doxorubicin, apigenin, and luteolin to cardiac myoblast cells.136 Belling et al.137 introduced a BAW-based microfluidic device for high throughput sonoporation. Their platform consisted of a square glass microcapillary attached to a piezoelectric operating at 3.3 MHz. Under this acoustic field, cells experienced acoustic microstreaming and acoustic radiation forces that thrust cells towards opposite capillary walls, inducing shear stress-sonoporation (Fig. 8C). The platform showed an efficient gene delivery with a nuclear membrane rupture at a clinically-relevant rate of 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells min−1, thus promising a non-viral transfection method for gene-modification treatments.137
000 cells min−1, thus promising a non-viral transfection method for gene-modification treatments.137
One concurring challenge is that the frequency in these bulk platforms is not high enough to completely obviate the possibility of cavitation. Belling et al.137 mentioned their system may not categorically suppress cavitation. As a solution to avoid the risk of cavitation, Zhang et al.138 used a bulk-based nano-electromechanical device for achieving a hypersound (≈GHz) regimen (Fig. 8D). They proved that both eGFP plasmid DNA and doxorubicin can be delivered through transient nanopores created in the cell membrane by the combination of hypersonic poration and acoustic streaming, with high viability and internalization efficiency.138 One reason for the high viability of high-frequency molecular delivery devices may lie in the mechanism of cell permeabilization. In high-frequency platforms, unlike with cavitational shock waves, the disruption of the lipid membrane is a temporal and rapidly healing mechanism.37,139 Hence, high frequencies systems such as SAW platforms can be pertinent technologies for efficient sonoporation.
Under SAWs, the openings in the membrane facilitate intracellular delivery through the cytosol in the absence of endocytosis. This lower power method allows the exogenous biomolecules to be uniformly distributed in the cytosol, escape endosomal recycling, and it also increases their chance of reaching the nuclei while retaining the integrity of both cells and cargo during exposure.37,140 One of the reasons for the maintained integrity is the nature of exposure, which involves continuous and low-amplitude waves with higher frequencies compared to short and fierce pulses.38 In this methods, when the transmembrane delivery is achieved and upon the removal of the acoustic excitation, the lipid membrane instantaneously reorganizes into its native structure to keep cells healthy. The high biocompatibility and delivery efficiency can be used for applications such as cell transfection in the emerging field of chimeric antigen receptor (CAR) T-cell therapy.37,140 The short exposure of cells to SAWs with frequencies above 10 MHz was also shown to facilitate homogenous internalization of gold nanoparticles by two- and six-fold increments after 30 s and 10 min exposure times, respectively. Successful cellular uptake of the fluorescently labeled Dextran and small interfering RNA (siRNA) was reported with cell viability of over 97%. The acoustically-transfected HeLa cells, with GAPDH silencing RNA, showed a two-fold knockdown in the gene expression and protein levels of the target enzyme.140 In a follow-up study, the same group exploited the use of focused TSAW to deliver siRNA into nonadherent Jurkat and HuT 78 cells which are particularly challenging to transfect (Fig. 8E). The efficiency of this acoustofection technique was comparable to that of the standard nucleofection in achieving a 2-fold gene knockdown, however, with superior cell viability of over 91%, as opposed to 76% in nucleofection (Table 2).38
| Application | Delivery mechanism/acoustic parameters | Nanoparticle and drug agents (target)/cell type (cargo) | Delivery route | Uptake/results advantages/shortcomings |
|---|---|---|---|---|
| Drug delivery | SAW nebulization (20–30 MHz, 1 W) | pVR1020-PyMSP4/5, pVR1020-YFP; H1N1 (pDNA encoding influenza virus protein) in droplets36 | Intratracheal instillation (in vivo, mice) and pulmonary inhalation (in vivo, sheep) | >90% of pDNA integrity preserved, YFP expression after 24 h |
| SAW nebulization (20 MHz, 1–1.5 W) | B2 agonist salbutamol–octanol (for asthma) in droplets (2.84 ± 0.14 μm)117 | In vitro pulmonary model (60 L min−1 airflow) | 70–80% delivery to lung model | |
| SAW (HYDRA) nebulization (10 MHz, 1.3 mL min−1) | Myoviridae (phage K) and a lytic enzyme (lysostaphin) (for staphylococcus aureus) (1–5 μm aerosols)123 | In vitro pulmonary model ‘next generation cascade impactor’ | >90% recovery rate | |
| SAW permeabilization (17–55 MHz, 100 mV) | Molecular therapeutic agents (fluorescein, FITC-dextran, FITC-albumin)125 | Mucosa of a porcine buccal model | >95% penetration rate in mucosal layer | |
| Stem cell therapy | SAW nebulization (30 MHz, 1.5–3 W, 350 μL min−1) | MSCs encapsulated in droplets (13.5 ± 0.5 μm)124 | Lung delivery | 86 ± 4.2% cell viability |
| Transfection & drug delivery | Inertial cavitation (1 MHz, 5–60 s (sonication)) | MCF-7 (PEI, DNA complex, pIRES2-eGFP)129 | Sonoporation | 32.0 ± 3.9% DNA transfection efficiency, 80 ± 2.3% cell viability |
| Microstreaming and electroporation) (10–30 kHz, 12.5–35 Vmax) | HeLa, K562, Jurkat (plasmid DNA135 encoding Cas9 protein and sgRNA) | >80% delivery efficiency | ||
| >20% gene knockout | ||||
| >80% cell viability | ||||
| Microstreaming (107 kHz, 30–90 s) | MDA-MB-231 (PI)134 | 96.6 ± 1.74% sonoporation efficiency, 97.9 ± 1.26% cell viability | ||
| BAW (with and without cavitation) (2.27 MHz, 3.2–40 Vpp) | H9c2 (doxorubicin, apigenin, luteolin)136 | Cavitation-free showed higher cell viability cytotoxicity | ||
| >40% (doxorubicin) | ||||
| >50% (apigenin) | ||||
| >90% (luteolin) | ||||
| BAW (3.3 MHz, 0.48 ± 0.04 MPa, 40 Vpp) | Jurkat, PBMCs, CD34 + HSPCs (Cy3-DNA, eGFP-expressing plasmid)137 | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per min delivery throughput 000 cells per min delivery throughput |
||
| >80% cell viability | ||||
| TSAW (Rayleigh wave) (10–30 MHz, 10 Vpp, 10 min (sonoporation)) | Jurkat and HuT78 (siRNA)38 | 2-fold gene knockdown | ||
| >91% viability | ||||
| TSAW (10 MHz, 2 W, 0.5–10 min (sonoporation)) | HEK293T and HeLa (Au NP-FITC tagged, dextran, Cy3-labelled siRNA, GAPDH)140 | >6-fold increase of NP concentration | ||
| 2-Fold knockdown in gene expression and protein levels | ||||
| >97% cell viability |
5 Acoustofluidics as a functional force for investigating phenotypes in biological organisms
Acoustic waves can have a complex functional influence on cells and tissues which requires extensive investigation. Sonoporation, as discussed in the previous section, is one of these functional effects of acoustics that leads to the disruption of the cell's membrane and nuclei. However, the interaction of acoustic waves with biological organisms is not limited to cell membrane rupture. Although many effects have been studied in conventional USW systems, the MEMS-specific wave modes and operating frequencies can also introduce unique and interesting effects on cell behavior, structure, and phenotypes. Moreover, miniaturization allows to focus or amplify the acoustic effects without drawbacks in large-scale systems such as self-heating and cavitation by offering a well-controlled domain.141Martinez Villegas et al.142 demonstrated the enhancement of the osteogenic differentiation and proliferation of adipose-derived stem cells embedded into a photocurable hydrogel via acoustic patterning using a frequency of 13.11 MHz (SSAW). Furthermore, Devendran et al.143 scrutinized the phenotypes of four distinct human and mouse cell lines after exposure to 48.5 MHz (SAW). The viability, nuclear morphology, and proliferation rates after exposure remained comparable. However, they noted less cell attachment and spreading for mesenchymal stromal cells and mouse osteosarcoma. More interestingly, SAWs increased the metabolic activity in human keratinocytes and mouse fibroblasts. They hypothesized that the increased metabolic activity might be due to the acoustophoretic forces pushing cells to the side channels, which could protect them from shear stresses by background flow. However, their results confirmed that the acoustic excitation directly increased the cell's metabolic activity (Fig. 9A).143 As suggested by this study, the SAW effect on cells can be very cell-type dependent. Moreover, since the acoustic radiation forces are often accompanied by other acoustic effects such as acoustic microstreaming, one of these acoustic phenomena might be the dominant effect depending on the experimental conditions. For instance, the change in the proliferation rate that was not observed in the previous study was reported in another study using a similar frequency of 48.8 MHz, but in a streaming-dominant system.144 The circulation of the culture media by SAW-driven acoustic streaming on a Petri-dish, coupled to a lithium niobate substrate by a layer of PDMS, showed a 36% increase in the rate of cell proliferation.
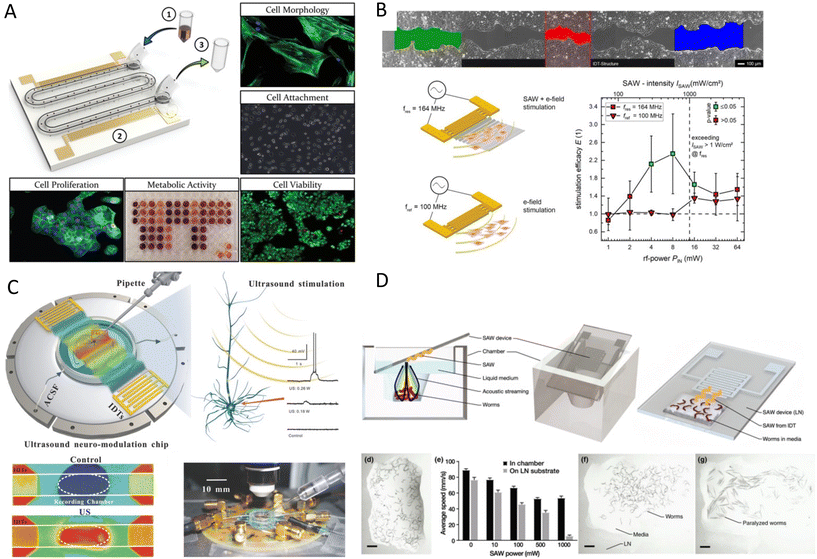 | ||
| Fig. 9 Acoustic settings to investigate the effect of the wave as functional mechanical stimuli on A) cell's viability, morphology, metabolic activity and proliferation,143 B) cell migration pattern and wound-healing properties,147 C) neuromodulation and stimulation of neurons,155,158 and D) the effect of acoustic waves on sensory neurons of C. elegans which induced short-term memory loss and brain injury.41 Reprinted with permission. | ||
One other interesting effect was reported by Brugger et al.145 who patterned primary neuron cells in an SSAW platform and investigated the cell growth and cell adhesion direction after acoustic exposure. They observed that the neurite outgrowth was preferentially aligned to the axis of the SSAW pressure nodal lines,145 which suggests the possibility of the cytoskeleton or the ECM realignment by acoustic waves.141 The mechanical stimulation of cells by ultrasound waves in low frequency and conventional systems has shown various effects such as the increase in production of IL-8, VEGF, and bFG, with a 112% surge in collagen synthesis and a 35–52% increase in the proliferation rate of fibroblasts and osteoblasts.146
Stamp et al.39 scrutinized the mechanical stimulations, along with electrical triggers that might be present in SAW platforms. They used their platform to promote cell growth and to direct the migration of Saos-2 cells in an acoustic path for tissue stimulation, showing a 17% increase in the healing rate of an in vitro artificial wound.39 The same group, in a more in-depth study, explored the effects of different acoustic parameters such as frequency, power, and wave modes on the proliferation rate and ROS levels for various cell types. Cells were exposed to SAWs and electrode-generated electrical fields, activated by similar radiofrequency signals, to distinguish the mechanical effects from the electrical stimulation (Fig. 9B). Cells exposed to piezo mechanical SAWs exhibited a marked increase in growth rate and a 135 ± 85% surge in wound-healing speed, while the electrical-only stimulation did not show a significant effect. By excluding the electrical effects, vibration and its mechanical stimulation on cells were pinpointed as the root cause for enhanced wound-healing and overall proliferation. Moreover, no SAW-induced ROS was observed for power levels ≤64 mW.147 SAW has been also reported as an effective stimulus for wound healing by promoting tissue oxygenation in ischemic feet. Human patients treated with a commercial SAW Patch device (NanoVibronix), experienced an increase in oxygen saturation and an overall reduction in pain.40
Additionally, acoustic waves are an interesting topic for studying mechanical cues in stem cell.126 Low-intensity ultrasound stimulation is associated with enhanced differentiation and production of growth factors in neural stem/progenitor cells,148 while acoustic biophysical stimuli have been employed to reprogram human dermal fibroblasts into extra multipotent cells.149 Acoustic waves have been shown to mechanically stimulate human articular chondrocytes aggregates, where tunable biomechanical forces led to the development of engineered human cartilage constructs in vitro with mechanical properties comparable to those of native human cartilage.150
One recent finding concerning the functional effects of acoustic waves on cells is the enhancement of exosome generation. After 10 min of low-intensity acoustic waves exposure to cancer cell lines, the exosome generation was increased by 1.7-fold.151 The high exosome production was attributed to increased calcium ion (Ca2+) levels after acoustic exposure, which subsequently triggered a pathway that regulates exosome production.
The change in ion profile by acoustic waves is reported in other studies too. Membrane proteins, including ion channels under acoustic waves, experience mechanical vibrations that can alter the conformation of their active state. Some of these mechanosensitive proteins are calcium, potassium, and sodium ion channel families which can translate mechanical stimuli into biochemical signals, known as mechanotransduction.152 These ion channels therefore can act as acoustically activated nano-valves which have been particularly a subject of interest in neuromodulation, where an altered ion profile in neurons is the hallmark of neurological disorders.153 The activation of ion channels and evoking action potentials are fundamental in the study of neuronal circuits and their functionality.141
Ye et al.154 transfected rat hippocampal neurons to express a mechanosensitive channel of large conductance (MscL), and used a 30 MHz SAW system to activate the channel. The MscL in the membrane sensitized the cells to an ultrasound stimulus of 0.25 MPa. While acoustically activated, the open MscL gate allows dyes to pass into the cytoplasm which can be a pathway for drug delivery. Moreover, the MscL-expressing cells showed faithful spike trains in response to acoustic pulses up to 5 Hz with millisecond accuracy. It was also reported that SSAW acoustic neurostimulation can modulate the kinetics of native mechanosensitive ion channels such as sodium and potassium in rat hippocampal slices.155,156 In doing so, they could control the ion efflux, activate and regulate the shape and rate of spikes, and could study the effects of acoustic waves on neurons' excitability and firing thresholds, as they are the minimum current neurons needed to produce an action potential (Fig. 9C).
The characteristics of acoustic signals such as intensity, duration, continuous or pulsed nature, and frequency of pulses are also influential in promoting or suppressing neuronal activities.156,157 TSAW was reported to trigger the neuronal behavior of C. elegans under a single-shot and short acoustic pulse.158 It was observed that the mechanical stimulation of USW can reverse the locomotion behavior in C. elegans, as 85.29% ± 6.17% of them started to move backward after a 6.4 ms pulse. The analysis of the calcium profile in the worms showed an elevated concentration of Ca2+ after acoustic excitation in a type of sensory neuron that is triggered in face of danger or stress.159,160 This hypothesis suggests that it is possible to directly activate sensory neurons by acoustic waves. In another study, Miansari et al.41 exposed C. elegans to SAW for an extended duration of 10 s to induce traumatic brain Injury (Fig. 9D). The continuous exposure, although with less acoustic pressure than that of the pulsed, caused temporary paralysis of the worms and reduced chemotaxis learning and short-term memory loss. The effects of SAW on C. elegans in both studies were tightly connected with the mechanical vibrations on the cell membrane by the acoustic waves. This connection is mentioned in many functional acoustic waves studies and suggests that the vibrational stimulus has a dominant effect (Table 3).
| Wave mode | Phenotype/phenomena study | Target species | Acoustic parameters | Outcomes |
|---|---|---|---|---|
| SAW | Enhanced proliferation and osteogenic differentiation | Adipose derived stem cells in methacrylated collagen142 | 13.11 MHz, 10–40 Vpp | >85% cell viability, >30% increase in metabolic activity, >80% increase in alkaline phosphatase activity, increase in osteocalcin expression |
| Cell adhesion and morphology143 and cell proliferation144 | MSCs, MG63, L929 and HaCaT143 and U-937144 | 48.5–48.8 MHz, 400–800 mV | Increased of metabolic activity in HaCaT and L929, change in morphology in MG63 and MSCs143 | |
| >36% increase in cell proliferation144 | ||||
| Cell growth and direct migration | Saos-239 | 159 MHz, 4 mW | >15% increased migration | |
| Cell migration and proliferation rate and ROS production | MDCK-II, SaOs-2, T-Rex-293147 | 164 MHz, 1–16 mW | 135 ± 85% wound healing increase | |
| Wound healing by tissue oxygenation | Ischemic feet patients40 | 96 kHz for 30 min | Increase in oxygen saturation, pain levels dropped | |
| Ion channel activation155 and neuromodulation156 | CA1155,156 and CA3155 | 27.38–30 MHz, 0.12–0.45 MPa | 20% calcein release in I92L-expressing neurons | |
| 30% increase of neuronal excitability155 | ||||
| 13% stimulation of outward potassium currents156 | ||||
| Epilepsy treatment161 | Human epilepsy brain samples161 | 28 MHz, 0.13 MPa | 65% inhibition of epileptiform activities161 | |
| Exosome generation | U87-MG, A549151 | 10 MHz, 10 min exposure | 1.7 and 10 fold increase of exosome generation and concentration | |
| 95% viability | ||||
| BAW | Cell proliferation, collagen, and NCP synthesis, and cytokine production of interleukin | Gingival fibroblasts, mandibular osteoblasts, monocytes146 | 1–3 MHz (pulsed) and 45 kHz (continuous) | 35–52% increase in cell proliferation in fibroblasts and osteoblasts, 112% increase in collagen and NCP synthesis, VEGF production is stimulated by all, IL-8 and bFGF production was enhanced by osteoblasts |
| Neurite outgrowth and cell differentiation | NSPCs spheroids148 | 560–1138 kHz, 40 kPa | Attachment and differentiation of NSPCs | |
| Increase in calcium ion influx by dual-frequency ultrasound |
6 Acoustic bio-characterization and sensing
The double capacity of acoustic systems as both actuators and sensors renders them unique in offering various characterization and biosensing techniques. In the first part of this section, we discuss the different acoustic actuation methods for the characterization of biosamples, specifically the mechanical properties of cells, such as compressibility and adhesion. In the second part, acoustic biosensors for the characterization of cells, proteins, and other biomolecules are reviewed. Finally, we discuss the combination of the acoustic actuators and biosensors for enhancing the sensing sensitivity through addressing the biofouling issues such as non-specific binding and low mixing.6.1 Acoustic Mechanotyping
Cell mechanical properties may serve as biomarkers due to their correlation to various diseases, such as malaria, sickle cell anemia, atherosclerosis, cerebral edema, stroke, and cancer.162–166 Acoustofluidics provides an alternative to conventional atomic force microscopy (AFM) and optical tweezers (OT) for cell analyses with higher throughput (>10 cells per s), contactless nature, and versatility to work in various media.Xie and colleagues167 used an acoustic oscillating bubble to deform cells suspended in an acoustic streaming field to quantify cell deformability. This mechanical biomarker was measured in different cells, including HeLa, HEK, and HUVEC in a single experiment. The deformability of each subpopulation in a mixed and heterogeneous cell sample was measured by using both fluorescent markers and mechanical biomarkers. Recently, Läubli et al.168 integrated an acoustic manipulation device with a micro-force sensor to rotate plant species and microorganisms and measure their mechanical properties at different regions of interest. In their model, acoustic radiation forces and acoustic streaming were used to manipulate single L. longiflorum (lily) pollen grains and C. elegans and measure the displacement at different regions and depths of the specimen.
Acoustofluidics can be also used to measure cell compressibility based on the acoustic radiation force. A particle/cell exposed to an acoustic field experiences an acoustic radiation force which is dependent on acoustic contrast. By determining all medium and wave properties, ARF can be calculated using cell trajectories in the acoustic fields. Hartono et al.169 recorded cell trajectories in a BAW field and obtained the compressibility of different cell types, which was correlated with a cancer biomarker. For its part, Wang et al.170 investigated the use of BAW-based acoustofluidics for measuring single-cell biophysical properties in cancer cell lines and immortalized cancer cells as biomarkers for cancer staging and prognosis. Cells with similar diameters were successfully classified based on their deflection to the acoustic field, where cells with a higher acoustic contrast factor experienced a higher ARF and hence, bigger deflection. Furthermore, acoustic systems can be also easily integrated to other modules as preparation step, for instance acoustic forces are proposed to align cells in pressure nodes to measure their impedance with the second module of a planar electrode impedance cytometer.171,172
The strength of single molecule adhesion has also been characterized with acoustofluidic methods, known as acoustic force spectroscopy (AFS). In this technique, cells and molecules that are in contact with a microfluidic surface are levitated using BAWs. Kamsma and colleagues introduced a single-cell AFS using acoustic waves to exert forces up to 1nN to cells in parallel.173 AFS has also been used to evaluate the binding and interactions of protein-DNA174–176 with forces up to 120 pN. ARF has also shown exceptional capacity as a high-throughput force spectroscopy method to quantify intercellular adhesion forces. The controllable and reversible acoustic radiation forces are successfully employed to pair and separate hundreds of cells in repeatable cycles to precisely measure cell affinity. Such platforms can be invaluable tools for the study of cell affinity, particularly for the development of CAR T cell therapies and the detection of the most responsive natural killer cells in the treatment of cancer.177
6.2 Acoustic biosensors
Acoustic waves have a long history of application as biosensors, one well-known example is the commercialized quartz crystal microbalance (QCM) and its dissipation version (QCM-D). The QCM-D is a BAW method that can measure mass/density changes, viscosity, and elastic modulus of biological samples inside the liquid medium with a pulsed excitation of 5–20 MHz.178 In recent years and with the advancements in MEMS manufacturing, the majority of the latest studies have been shifted from QCM-D to SAW biosensors. The wide range of operating frequencies of SAWs allows for easy customization to detect various biological samples, with higher frequencies yielding better sensitivity for smaller samples, such as biomolecules. Moreover, the confined and well-controlled detection region, low power consumption, and the capability of surface modification are some of the additional advantages of SAWs that render them appealing for biosensing applications.33 The main sensing mechanism of SAW biosensors is somehow similar to that of QCM, in that the decay in frequency and the amplitude of acoustic waves are quantified for the detection of various biomolecules and biospecies (Fig. 10).179This sensing zone can either be functionalized with probe molecules to bind to the floating target biomolecules and immobilize them for sensing, or it can be directly used for biosensing species, such as cells and tissues. As the acoustic waves, emanated from the input IDT, travel on the surface and reach the sensing region, they interact with the target species adsorbed on the surface, causing a change in the mass loading which is detectable by shifts in the SAW phase, resonant frequency, and amplitude.33,113,7 When the output acoustic waves are received in the readout IDT, they re-transduce into electric signals for deciphering the measured changes in the acoustic waves into the concentration of target biological species or other sensing targets, such as the viscoelasticity of the species.118 Alternatively, IDTs can be added to the delay line to act as resonators and minimize the scattering of acoustic waves and improve the signal power and performance.118 Other strategies such as coating the delay line with waveguides are also commonly used in biosensors to minimize the signal loss while allowing a wide range of operating frequencies. Here, we briefly discuss the potential of acoustics for biosensing cells, biomolecules, and bioparticles.
In addition to cell quantification, acoustic sensors can measure the mechanical properties of cells, such as stiffness and viscoelasticity. An extensive library of papers used QCM-D to measure these important physical properties of cells, which can divulge fundamental information about their physiological behavior and phenotypes.184 More recently, SAW-based sensors have been reported for measuring the cell mechanical response with higher sensitivity. A Love-mode SAW sensor was reported to measure the contractile properties of forces in HL-1 cardiomyocytes.184 They seeded 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 HL-1 cardiomyocytes cells in the detection regions where their contraction forces and stiffness were modulated by treating the cells with verapamil, which reduces the contraction forces and stiffness in the cells, and isoprenaline which acts reversely. By recording the phase and amplitude shifts, the changes in contractile properties could be correlated to the amplitude shift. Embedded microcavities on the SAW interrogation region could also be used to characterize the single-cell stiffness of MCF7, MDA-MB-231, SKBR3, and JJ012 cell lines.185 In this method, a single cell was placed in the microcavity, and the phase shift was compared to that of the microcavity filled with only media to calculate the velocity differential, and subsequently, the stiffness modulus of the trapped cell (Fig. 11A).
000 HL-1 cardiomyocytes cells in the detection regions where their contraction forces and stiffness were modulated by treating the cells with verapamil, which reduces the contraction forces and stiffness in the cells, and isoprenaline which acts reversely. By recording the phase and amplitude shifts, the changes in contractile properties could be correlated to the amplitude shift. Embedded microcavities on the SAW interrogation region could also be used to characterize the single-cell stiffness of MCF7, MDA-MB-231, SKBR3, and JJ012 cell lines.185 In this method, a single cell was placed in the microcavity, and the phase shift was compared to that of the microcavity filled with only media to calculate the velocity differential, and subsequently, the stiffness modulus of the trapped cell (Fig. 11A).
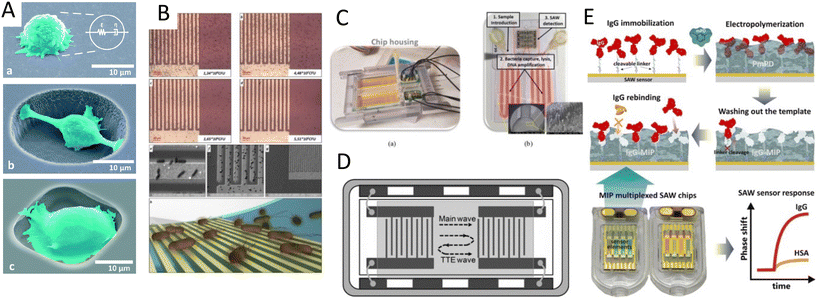 | ||
| Fig. 11 Acoustic settings for various biosensing strategies: A) embedded microcavities on a SAW platform to characterize the single-cell stiffness,185 B) a conformable SAW immunosensor for the detection of E. coli bacteria,186 C) integrated LOC device that can capture, lyse, and detect bacteria in separate modules for rapid detection of foodborne pathogens,189 D) a SAW sensor with three-fold path line for sensitive detection of cardiac disease biomarker,193 and E) a protein detection platform using imprinted polymers as synthetic probes.194 Reprinted with permission. | ||
The high sensitivity of SAW sensors renders them instrumental for the detection of pathogenic cells such as bacteria. Lamanna and colleagues proposed a conformable SAW immunosensor for the detection of E. coli bacteria where they grafted E. coli binding antibodies on the sensing zone and measured the change in mass loading caused by the bacterial adhesion. Their platform was based on the deposition of a thin film of piezoelectric aluminum nitride (AlN) on a recyclable polyethylene naphthalate substrate. They compared the detection limit of identical IDT designs and piezoelectric (AlN) mounted on flexible PET substrate, to create Lamb wave modes and on a hard silicon wafer to create Rayleigh wave modes. A superior detection limit was attained being 6.54 × 105 CFU (colony-forming units) mL−1 on PET substrate compared to 1.04 × 106 CFU mL−1 on silicon (Fig. 11B).186 The above paper is a showcase of direct bacteria detection, however, most studies in this area used the DNA equivalent of bacteria as the model for detection.
In addition, a similar mechanism has been specifically used to model bacterial detection, consisting of a shear horizontal surface acoustic wave (SH-SAW) to detect a food pathogenic strain (E. coli, O157![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H7).187 A specific DNA sequence from E. coli O157
H7).187 A specific DNA sequence from E. coli O157![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H7 was immobilized on the sensing region as the probe of ssDNA, allowing to measure the frequency shift (Δf) upon their hybridization with the complementary DNA with a detection sensitivity of 0.6439 × 10−9 m/0.1 kHz.187 A Love wave biosensor was also reported for the detection of Salmonella bacteria. The ratio of the amplitude change over phase shift (ΔA/Δph) was monitored during the binding of the target DNA to the Salmonella DNA template that was immobilized in the sensing zone and reported a detection limit of 100 bacteria cell equivalents per sample.188 In a recent study, an LoC sensing platform that utilized SAW was employed for rapid detection of foodborne pathogens such as Bacillus cereus, Salmonella, E. coli, and Listeria (Fig. 11C). This integrated device could capture and lyse bacteria in a separate module with an antibody functionalized surface, amplify the DNA isothermally, and finally introduce the amplified DNA to the SAW module for detection.189
H7 was immobilized on the sensing region as the probe of ssDNA, allowing to measure the frequency shift (Δf) upon their hybridization with the complementary DNA with a detection sensitivity of 0.6439 × 10−9 m/0.1 kHz.187 A Love wave biosensor was also reported for the detection of Salmonella bacteria. The ratio of the amplitude change over phase shift (ΔA/Δph) was monitored during the binding of the target DNA to the Salmonella DNA template that was immobilized in the sensing zone and reported a detection limit of 100 bacteria cell equivalents per sample.188 In a recent study, an LoC sensing platform that utilized SAW was employed for rapid detection of foodborne pathogens such as Bacillus cereus, Salmonella, E. coli, and Listeria (Fig. 11C). This integrated device could capture and lyse bacteria in a separate module with an antibody functionalized surface, amplify the DNA isothermally, and finally introduce the amplified DNA to the SAW module for detection.189
SAW biosensors have also been shown to detect DNA mutations in clinical samples. Liu et al. conjugated graphene oxide in a SAW-based sensor whereon they immobilized the probe DNA for monitoring DNA mutations. 136 clinical samples were used to measure the DNA hybridization with various targets by recording the phase shift in SAW signals. An identification specificity of 88.6% and a sensitivity of 100% for CC and CT genotype, and 96.2% and 98.0% for diagnosing CT and TT genotype, was recorded respectively.190 To enhance the detection limit of sequence-specific DNA in blood serum, nanoparticle-DNA complexes can be incorporated to amplify the signal of their SAW biosensor.191 After the initial hybridization of the DNA target with the DNA probe, the hybridized duplex was exposed to DNA polymerase enzymes that elongated the target protein, creating an extended ssDNA where the Ag+ specific sequence can hybridize. Finally, the complex was exposed to a reduction agent in which the silver ions nucleated and synthesized the silver nanoparticles, showing that the nanoparticles can significantly increase the mass loading, amplify the signal, and enhance the detection limit by 3 orders of magnitude.
An alternative to corresponding binding molecules used as probes was proposed by Tretjakov et al.,194 where an imprinted polymer was used to create synthetic receptors. As Fig. 11E shows, after immobilizing IgG, the polymer was deposited on the substrate to form an ultrathin film via electrosynthesis. After washing out the cleavable IgG, its shape remained imprinted on the surface and acted as a template for IgG detection for up to four regeneration cycles. The recognition efficiency of the imprinted surface for IgG was 4 times higher compared to the interfering IgA, and 10 times higher compared to Human serum albumin (HSA).194
The capacity of SAW sensors as a rapid and accurate PoC sensor for the detection of the Ebola virus, a relevant interest and necessity due to recent viral outbreaks, has been also demonstrated. By functionalizing the surface with an anti-Ebola virus antibody, inactivated and fragmented Ebola virus could be efficiently captured. A detection limit of 1.9 × 104 PFU (plaque-forming unit) mL−1 of the virus before inactivation was reported, being lower than that using standard PCR tests. Moreover, this platform was powered by batteries to make it portable, and also showed a reduction of detection time with a sensing time of 5–10 min.28 Influenza A virus was also shown to be captured and detected by the immobilization of anti-H1N1 HA antibodies on the SAW platform with a detection limit of 1 ng mL−1.29
Furthermore, the potential of SAW sensors as rapid detection chips for clinical applications was effectively proven by Gray et al.30,31 In their design, the authors functionalized detection channels using anti-HIV antibodies (anti-p24 or anti-gp41) through inkjet printing, and they engineered the chip to require only 6 μL of blood plasma. Samples from 31 patients with HIV and 102 healthy volunteers were tested using this device, showing a remarkable 100% for both sensitivity and detection specificity. Moreover, all the positive results were read within 60 s of sample injection, paving the groundwork for their followed smartphone-connected diagnostic platform (Table 4).30,31
| Detection target | Target details | Wave mode (substrate type); frequency/wavelength | Accuracy/detection limit sensitivity |
|---|---|---|---|
| Cells | MCF-7 (human breast cancer cell)180 | Leaky SAW (36° YX-LiTaO3); 100 MHz | 32 cells per mL |
| A549 (human lung adenocarcinoma tumoroids)181 | SH-SAW (36° Y-cut LiTaO3 with ZnO coating); 14.041 MHz | 3000–50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 in 100 μL 000 in 100 μL |
|
| HL-1 cardiomyocytes184 | SH-SAW (quartz crystal); λ = 28 μm | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per sensor 000 cells per sensor |
|
| E. coli 186 | Lamb wave SAW (polyethylene naphthalate); 500 MHz | LoD 6.54 × 105 CFU mL−1 | |
| DNA detection | DNA182 | SAW (LiNbO3); 6.4GHz | 6.7 × 10−16 g cm−2 Hz−1 |
E. coli O157![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H7 DNA187 H7 DNA187 |
SH-SAW (64° Y-cut LiNbO3); 386 MHz | 0.6439 nM/0.1 kHz | |
| Salmonella enterica, DNA binding188 | Love wave SAW (ST-cut quartz); 267–298 kHz | <100 bacterial cell equivalents | |
| Typhimurium cells, DNA amplification189 | Love wave SAW (ST quartz); 155 MHz | 1–5 cells in 25 mL of milk | |
| DNA mutation190 | SAW (graphine oxide) | 98.0% sensitivity and 96.2% specificity | |
| Protein detection | Rabbit immunoglobulin (antigen)192 | Love wave SH-SAW (ST-cut quartz); 163 MHz | 602 ng cm−2 |
| Mass sensitivity of 38 m2 kg−1 | |||
| Cardiac troponin193 | Triple transit echo TTE SAW (36° YX-cut LiTaO3); 200 MHz | Detection limit of 24.3 pg mL−1 | |
| IgA and HAS194 | Love wave SAW (IgG-MIP ultrathin films); 100–500 MHz | Selectivity factors of 0.3 and 0.09 for IgA and HSA | |
| Exosomes and viruses | Exosomes (HepG2)195 | SAW (silicon dioxide guiding layer SAW instruments GmbH) | 1.1 × 103 particles per mL exosomes |
| Ebola virus28 | SH-SAW (36° Y-cut LiTaO3); 80 and 400 MHz | 1.9 x 104 PFU mL−1 | |
| Influenza A virus (H1N1)29 | Love wave SH-SAW (41° YX LiNbO3); 120 MHz | Detection limit of 1 ng mL−1 | |
| HIV30,31 | SH-SAW (quartz crystal 36° Y-cut and 90° X-propagation); 251.5 MHz | 100% sensitivity for anti-gp41. 64.5% sensitivity for anti-p24 |
6.3 Acoustic waves for enhancing sensitivity limitations
One of the main challenges in biosensing assays which are based on the immobilization of the capture probe, such as ELISA and surface plasmon resonance (SPR), is the low signal-to-noise ratio, which weakens the sensing performance. Two of the factors that are key in low signal-to-noise scenarios are the inefficient binding between the probe and target molecules, which causes a low signal, and non-specific adsorption of parasitic molecules, interfering with the target signal.196 Acoustic waves have been used to address these biofouling issues by improving the target binding kinetics through acoustic mixing, and also by breaking off the unwanted molecules from the surface through acoustic vibrations and streaming.Renaudin et al.197 integrated a Rayleigh-mode SAW platform with an SPR sensing system to induce acoustic streaming and enhance the binding kinetics of an avidin-biotin assay (Fig. 12A). The acoustic streams led to effective mixing on the SPR prism and showed a 5-fold acceleration of binding kinetics. Despite these results, they reported interference in signal due to heat generated by the SAW platform.197 Another SAW-enhanced SPR sensor was proposed to discern the streaming contribution from the heating effect by varying the SAW parameters and monitoring the temperature and streaming velocity (Fig. 12B). By doing so, the saturation time for polyethylene and avidin adsorption was accelerated by 82% and 24%, respectively.198
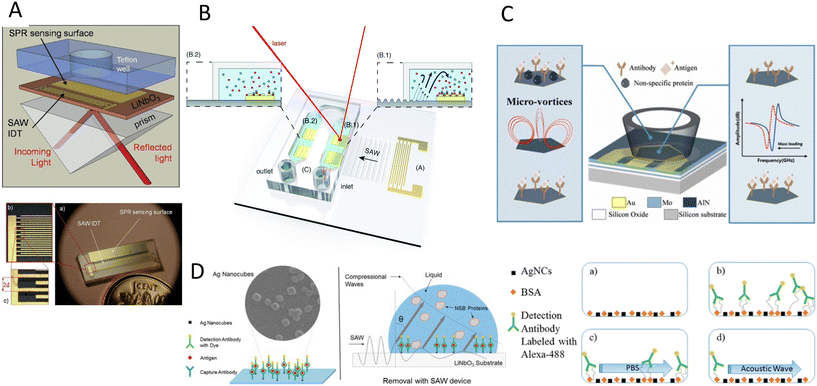 | ||
| Fig. 12 Acoustic waves for improving sensitivity and limiting biofouling: A)197 and B)198 integration of SAW mixers with SPR sensing systems to enhance the binding kinetics. C) The hydrodynamic forces from acoustic microvortices are used to wash off the loosely surface-bound non-specific proteins.200 D) SAW is used for both enhancing the mixing and removing non-specific protein bindings, showing significantly better performance over chemical rinsing.202 Reprinted with permission. | ||
Many studies have also been dedicated to investigating the ability of acoustic waves to break off non-specific bindings. The target biomolecules, especially in clinical samples, are immersed in a cocktail of other biological molecules which can induce noise signals in label-free biosensing applications. Meyer et al.199 used a thickness-shear mode resonator to clean non-target proteins from the patterned protein array, in a non-destructive manner. Upon the penetration of shear waves, the mechanical stress decreased the activation energy for the desorption of nonspecifically bound species, leading to an 85% drop in fluorescent intensity of the non-sensing area and 77% for the sensing area.199 Another method investigated a high-frequency resonator to create controllable acoustic microstreams and was used to wash off the loosely surface-bound proteins by vortex-induced hydrodynamic forces. A human IgG antibody was immobilized on their platform showing a 58.3% drop in fluorescence intensity of the nonspecific mouse IgG antigen, while the reduction for specific human IgG was only 12.1% after hypersonic treatment (Fig. 12C).200
A similar streaming approach for dissociating non-specifically bound (NSB) proteins was reported, albeit with a Rayleigh-mode SAW setting. A section of the delay path was treated with a goat anti-mouse IgG antibody as the sensing zone while the edges were coated with unlabeled BSA as the non-sensing area. The surface was then exposed to a fluorescently labeled IgG antigen for specific binding and also an additional labeled BSA for nonspecific adsorption. Upon exposure to SAW, the fluorescent intensity of BSA and the antigen in non-sensing zones dropped markedly; whereas in the sensing zone, only BSA showed significant desorption, with the specific antigen-receptor interaction remaining exclusively.201
Furthermore, a Rayleigh SAW combined with plasmonic silver nanocubes was shown to lower the detection limit for immunofluorescence assays to <1 ng mL−1 of the antigen. In this platform BSA and 50 nm silver nanocubes were immobilized on the sensor surface and carcinoembryonic capture antibody was incubated above the nanocubes. After adding a carcinoembryonic antigen, the fluorescently-labeled detection antibody was introduced to complete the sandwich structure. Both effects of acoustic waves improved sensing through enhanced mixing and prevention of nonspecific protein adsorption. The mixing induced by acoustic streaming reduced the antibody/antigen incubation time to 1/6th of the acoustic-absent setup. Moreover, removing the nonspecific protein adsorption using SAW was significantly more effective compared to the technique of chemical rinsing, thus leading to an overall stronger fluorescence signal (Fig. 12D).202
Merging both SAW-based sensing and biofouling alleviation mechanisms can be a very promising and efficient biosensing strategy. However, one should cautiously consider the difference in wave modes and the piezoelectric substrate used in each application. As mentioned earlier, the wave mode suitable for biosensing is SH-SAW and Love, which limit the damping of the wave in the liquid medium. However, for biofouling removal, the leakage of the waves is necessary to induce vortices, and therefore, substrates that support Rayleigh-SAWs are required.
To overcome this obstacle, substrates like LiTaO3 and LGS can be used to generate both wave modes by changing the direction of the wave propagation. A set of IDTs can be embedded on these substrates in the direction in which shear vertical waves are generated to create microstreams for inducing mixing and cleaning the surface, while the IDTs in the direction that activate shear horizontal waves can be used for the biosensing.203,204 Given this efficient integrability, there is an increasing interest for SAW-based sensors to combine these two features to increase the biosensing specificity and detection limits.
7 Acoustofluidics for particle separation, sorting, and enrichment
The label-free and biocompatible nature of acoustic forces render them as one of the most powerful tools for the separation, enrichment, and sorting of bioparticles. These systems, also called acoustic tweezers, can manipulate bioparticles without disrupting the surrounding fluid and separate them based on the size or acoustic contrast factor. One of the unique features of the acoustic tweezers is the ability to tailor the size sensitivity of the system by different acoustic phenomena. Acoustofluidics offers various force relations by producing hydrodynamic acoustic streaming (F ∝ R), standing fields (F ∝ R3), traveling fields (F ∝ R6), or the combination of these forces on a single particle.7.1 Acoustofluidic particle separation
ARF in standing waves stands out for particle separation due to their high controllability, relatively large magnitude, and its third power relationship with the particle's radius (R3), in which a small increase in the particle size leads to significantly stronger ARFs. Stronger ARFs in larger particles can overcome the fluid resistance due to drag force. Therefore, bigger particles can travel faster and longer distances towards low acoustic pressure nodes, while the movement of smaller particles is dominated by drag forces of background streamlines. This difference in the reposing site of particles, based on their size, has been extensively used in both BAW and SAW modes for particle separation (Fig. 13).51The use of standing BAWs (SBAWs) have been reported for separating MCF-7 cancer cells from red blood cells (RBC),205 prostate cancer cells from white blood cells with flow rates up to 100 μl min−1,206 and platelets from undiluted whole blood with over 85% platelet recovery, and flow rates of 10 mL min−1.207 SBAW were also used to separate different sized polystyrene particles (2, 5, 8, 10 μm) by increasing the source input power from ∼0.5 to ∼2.0 W.208 In a similar manner, standing SAWs (SSAWs) can separate particles by creating a single pressure node in the channel center, and relocate particles based on their size (Fig. 13A).209 This approach was used for the separation of platelets from whole blood with a purity of 98%,210 water in oil droplets with a sorting rate of 222 droplets per second,211 and extracellular microvesicles and 200 nm nanoparticles.212
Despite the great potential in applications, the isolation and enrichment of target nanosized organisms with this method is challenging. The difference in scaling order in the nanoparticles (ARF ∝ R3, Fd ∝ R) results in the dominance of Drag forces in the nano realm. Therefore, for the ARFs to dominate the manipulation of particles, the frequency of acoustic waves should be in the high MHz-to-GHz range, a feature typically exclusive to SAWs. SSAWs were successfully used to separate 500 nm from 100 nm particles,213 and exosomes (<300 nm) from a mixture of extracellular vesicles with >90% separation yields.212,214
The maximum travel distance of particles in standing waves is the spatial distance between pressure nodes and antinodes (λ/4). This distance in SAW devices which usually have a high frequency of operation can be very short, limiting their sensitivity and efficiency of separation.42 Hence, by tilting the SSAW direction, such that the pressure nodal lines stand at an angle to the flow direction (optimally 15°), the normal displacement of the particles can be increased by ∼10 times, while the trajectory of smaller particles is still dominated by the flow field (Fig. 13B).215 This mechanism was adopted by various groups to separate breast cancer cells from leukocytes with a purity of 84% and throughput of 2 μL min−1,215 and for the isolation of low concentrations of cancer cells (∼100 cells per mL) from white blood cells with an 83% recovery.216
In addition to the size, the acoustic contrast factor (Φ), associated with the compressibility and density of a particle and its contrast to the surrounding medium, can also be used to separate particles by standing acoustic waves.217 A particle with a higher density mismatch has a larger Φ value, and thus experiences a larger ARF. This concept can be particularly used when mixed particles are indistinguishable by their size. Furthermore, the acoustic contrast factor can have a negative value when the density of the particle is below that of the medium. As such, the density of the medium can be modulated, such that it divides the relative contrast factor of particles into negative (−Φ) and positive (+Φ), where in the presence of ARFs, two particles can move in opposite directions from one another, i.e., antinodes (−Φ) and nodes (+Φ) (Fig. 13C). To exploit this method, cesium chloride was added to the working media to reach a density of 1.16 g cm−3 and separate 3 μm polystyrene particles (1.05 g cm−3) from PMMA particles (1.22 g cm−3) of the same size.208 Similarly, a high acoustic impedance-to-viscosity ratio medium (OptiPrep™ density gradient medium) was used to increase the acoustic impedance of the medium. Cells were injected in normal media near the sidewalls where the pressure antinode was located, and high-contrast media was infused in the central channel where the pressure node sits. This allowed to effectively quantify the acoustic impedance of various cell types, including monocytes, lymphocytes, neutrophils, and cancer cells.218 In another study, RBCs were sorted in a BAW device by guiding RBCs (+Φ) towards the pressure nodes while lipid microemboli (−Φ) were located at the anti-pressure nodes, with a 100% efficiency.219
The other acoustophoretic method is traveling surface acoustic waves (TSAW), which can be a double-edged sword for particle separation. On one hand, its R6 relation results in smaller magnitudes of ARF compared to that of SSAW, on the other hand, the R6 relation renders it more sensitive to the size of particles. Another interesting feature of TSAW is the migration distance of particles, which unlike SSAWs, is not limited to the node-antinode distance. Instead, TSAW applies a unidirectional force on particles, continuously pushing them away from the source (IDT). Therefore, particles travel longer lateral distances allowing them to be sorted more efficiently (Fig. 13D). Straight TSAWs have been used to guide the direction of water droplets in oil and polyacrylamide particles,220 and slanted TSAW were used to sort human keratinocytes, mice fibroblasts, and melanoma cells.221 TSAW has also been used in combination with functionalized microparticles to separate target-protein complexes which have bigger diameter unbound particles.222 To compensate for the lower force magnitude, the acoustic field in TSAW is usually limited to a small, focused domain by using focused-IDTs (FIDT), used for the continuous separation of 3 μm and 10 μm particles with a 100% efficiency.223 SSAW and TSAW can also be combined in a multi-module platform to enhance separation (Fig. 14A).224
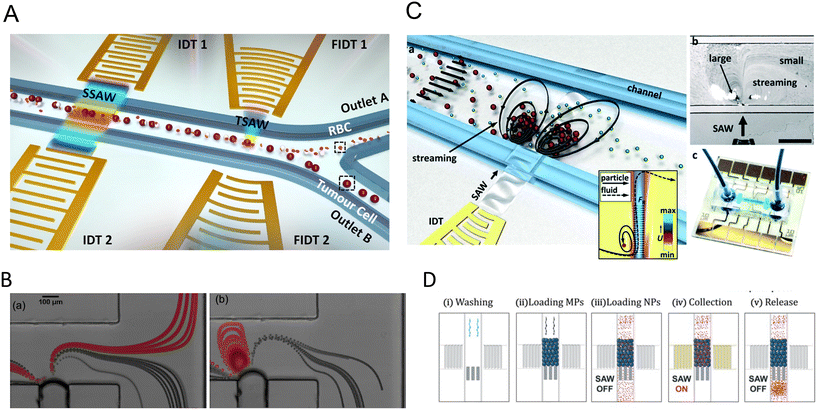 | ||
| Fig. 14 Acoustophoresis mechanisms for separation, isolation, and enrichment of particles. A) Double stage acoustic system using SSAW for alignment and TSAW for separation,224 B) particle separation by microstreams from oscillatory bubbles,227 C) particle separation by microstreams from focused TSAW.225 D) Exosome enrichment by Bjerknes forces.238 Reprinted with permission. | ||
Another efficient separation method is the use of acoustic streaming. Acoustically-induced microvortices are advantageous due to their high controllability, rapid formation, facile tuning, precise location, and the ability to scale up for high-throughput applications.225 When particles approach the acoustic field, microstreaming can deflect the trajectory of the particles based on their size. Larger particles move to the center of rotation, being trapped in closed streamlines, while smaller particles can pass through the vortex (Fig. 13E). By using oscillating microbubbles, the selective capturing and release of different sized particles was attained with a resolution of 1 μm.226 Bubbles can be used at the neck of bifurcated channels to deflect the trajectory of particles from the streamline and thus be collected at the outlets (Fig. 14B).227,228 Lateral cavity acoustic transducers (LCATs) with oscillatory bubbles at the sides of a channel, could efficiently separate platelets, red, and white blood cells from undiluted whole blood,229,230 with high throughput (50 × 106 cells per min).231 Moreover, acoustic streaming can be coupled to ARFs in SAW platforms. As an example, an acoustic beam was employed as a virtual filter membrane to separate 8 μm from 5 μm particles,232 2 μm from 1 μm particles, and MDA-MB-231 cancer cell lines from a mixture with red blood cells (Fig. 14C).225
The dominance of drag forces in sub-micron particles encourages the use of acoustic streaming-induced drag forces to separate and enrich nanoparticles. Using SAW-based acoustic streams, the enrichment of particles with sizes of 300 nm,233 87 nm,234 and even 80 nm were shown.235
Another interesting nanoparticle enrichment approach is using Bjerknes forces. In the presence of acoustic waves, microparticles scatter acoustic energy and emanate secondary ARF (or Bjerknes force), which can attract nanoscale particles and vesicles near the microparticles for filtration and enrichment. This technique was explored in both SAW and BAW devices for trapping E. coli bacteria and 110 nm diameter polymeric nanoparticles with a 95% efficiency,236 enriching extracellular vesicles (EVs) from cell culture media, urine, and blood plasma,237 and the enrichment of 100 nm particles by increasing the input power (Fig. 14D).238 The overview of acoustic modes, parameters, throughput, and efficiency of recent acoustophoretic systems for particle and cell manipulation is presented in Table 5.
| Acoustic mode | Acoustic parameters | Particles/cells | Throughput/efficiency |
|---|---|---|---|
| SBAW (primary ARF) | 2 MHz, 0.5–2.0 W; 0.36, 0.13, 0.04 mL min−1 | PS particles (2, 5, 8, 10 μm), RBC208 | ∼96% PS recovery, ∼92% RBC recovery |
| 4.91 MHz and 10 V (pre-alignment); 1.99 MHz and 0–12 V (actuation); 100 μL min−1 | PS particles (5, 7 μm), MCF-7, RBC, DU-145, WBC205 | 99.3 ± 0.3% PS, 91.8 ± 1.0% MCF-7, 84.1 ± 2.1% DU145 recovery | |
| 10 Vpp, 0.3 ml min−1 | Lipid particles (5 μm), RBC219 | 100% separation efficiency, 70% recover (RBC) >80% of lipid particles removed | |
| SSAW (primary ARF) | 12.6 MHz, (30-160 mW), 2 μL min−1 | Fluorescent PS beads (4.17 μm and 0.87 μm)209 | 90% recovery (4.17 μm) and 80% (0.87 μm) |
| 13.0168 MHz, 23.8–26.7 dBm, 0.8 μL min−1 (particles), 0.5 μL min−1 (cells) | Fluorescent PS particles (2 μm and 5.86 μm); E. Coli, PBMCs239 | 95.65% purity (E. coli/PBMCs) | |
| 410–463 mW, 0.25–5.0 μL min−1 | RBC, platelets, whole blood210 | 99% RBC clearance ratio, 98% purity of platelets, 2.7 × 104 cells per s | |
| 10.02, 10.20, 10.35, 10.46, 10.60 MHz, 48 Vpp, 0.2–8.0 μL min−1 | Picoliter water-in-oil droplets211 | 222 droplets per s | |
| 38.5 MHz, 0.5 W, 2.8 mm s−1 (particles), 1.5 W, 2 mm s−1 (eMVs) | PS beads (190 nm and 1000 nm), microvesicles (<200 nm)212 | >90% recovery rate particles, >80% for exosomes, >90% for larger MVs | |
| taSSAW (primary ARF) | 19.40 to 19.60 MHz (15°-tilted), 20–40 Vpp, 1–5 μl min−1 | PS beads (9.77 μm and 0.87 μm), MCF-7, WBC215 | >98% recovery (9.77/0.87 μm) |
| >97% purity (WBC/MCF-7) | |||
| 39.4 MHz, 45 Vpp, 4–8 μL min−1 | PS particles (110 nm, 5 μm), exosomes, MV (50–600 nm) from whole blood214 | 99% recovery rate | |
| 98.4% purity (EVs) with 99.999% blood cell removal rate | |||
| Acoustic streaming | 381 MHz, 68–200 mW, 0.5–8 μL min−1 | Particles (1 μm and 2 μm), MDA-MB-231, RBC225 | >98% capture efficiency at v* = 112 2 um particles |
| 32 kHz, 20–27 Vpp, 3 μL min−1 | K562, PS beads (10 μm)228 | 800 cells per s, 99% sorted cells, 94.5 ± 4.7 cell viability | |
| 15–50 kHz, 70 Vrms, 1.33 mm s−1 | PS particles (radius, 1, 2.5, 5 μm)226 | 1 μm resolution | |
| 49.8 KHz, 2.75–6.5 Vpp, 27.5 μL min−1 | PS particles (7.32, 25 μm), DU-145, WBCs, RBCs, monocytes231 | >50 × 106 min−1, 90% efficiency, 77 × enrichment, 91.7% purity (DU-145) | |
| 2.5–5.0 Vpp, 25 μL min−1 | Platelets, RBCs and WBCs, MCF-7, SKBR-3229 | 100% trapping efficiency, MCF7, 200× enrichment ratio | |
| SAW (Bjerknes) | 48.8 MHz (λ = 80 μm), 18.3 V (0.12 W mm−2), 12.2 μL min−1 | PS particles (100, 300 nm)240 | 86.3% separation efficiency |
| 663 MHz, 251 mW, 0.45 μL min−1 | PS particles (300, 500 and 100 nm)233 | 84 ± 9% capture of 300 nm | |
| 55–85 MHz, 12 dBm power, 1 μL h−1 flow rate | Particles (100 nm, 190 nm, 500 nm)238 | 97% collection efficiency (500 nm) | |
| BAW (Bjerknes) | 4 MHz, 17 Vpp, 10 μL min−1 | PS particles (110 nm, 490 nm), E. coli236 | 95 ± 3% capture efficiency |
| 4 MHz, 10 Vpp, 15 μL min−1 | PS particles (0.1–1 μm), EVs (30–500 nm)241 | 81.7% trapping efficiency (1 μm), 9.3% CV |
7.2 Cell sorting
Sorting in definition differs from the separation process, as it involves the active detection of targeted particles or cells, usually through fluorescence-activated cell sorting (FACS).1 Acoustic forces in particle sorting have been used in two roles: a) to pre-focus particles in lines to facilitate the detection of particles, and b) as the active pushing mechanism to move the particles that are signaled by the detection system.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 s−1 (Fig. 15A),246,247 and 100
000 s−1 (Fig. 15A),246,247 and 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 s−1 with high flow rates of up to 10 mL min−1.248
000 s−1 with high flow rates of up to 10 mL min−1.248
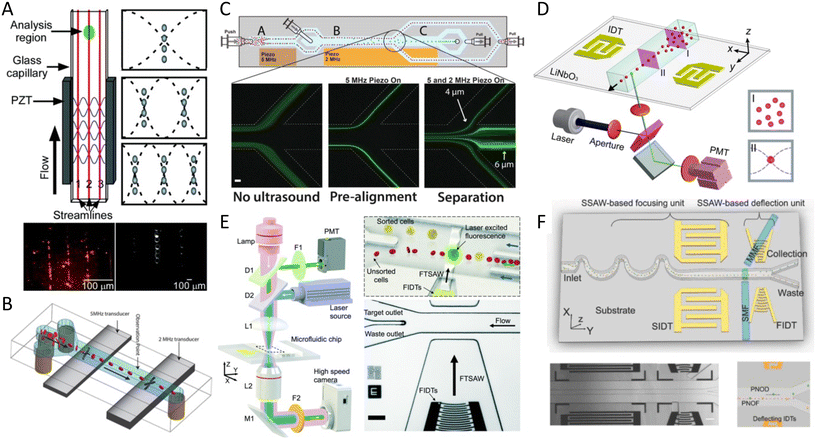 | ||
| Fig. 15 Acoustic cell sorting mechanisms including cell focusing (pre-alignment) and cell separation (deflection). A) BAW-based multi-node acoustic focusing in a rectangular glass capillary showing the location of pressure nodes (λ/2, λ, and 3/2 λ, respectively) (top) and optical micrographs showing two sizes of PS particles focused into 3 nodes (scale bar = 100 μm).247 B) Schematic illustration of BAW-based 2D-rotation cell focusing by the activation of two different frequency transducers of 5 MHz and 2 MHz for focusing and orientation, respectively.254 C) Schematic image of BAW-based acoustic chip for separation showing the laminated sample with no ultrasound (left), pre-aligned particles (center) with 5 MHz piezo ON, and separation of the pre-aligned 4 μm and 6 μm particles with 5 MHz and 2 MHz piezo elements (scale bar is 100 μm).252 D) Schematic image of SSAW-based microfluidic cytometer with an integrated LIF detection system, before (I) and after (II) SSAW focusing.253 E) Schematic image of SAW-based FACS platform showing optical set-up for fluorescence interrogation and high-speed imaging (left) and illustration of the FACS process in the sorting chip with focused interdigital transducer and micrograph of the acoustic sorting chip showing the focused and sorting regions (scale bar is 100 μm).255 F) SSAW-based FACS chip schematic (top) with a microscopic image of focusing and deflection units (bottom left) and illustration of the path of pressure nodes of deflected (PNOD) particles and pressure nodes of focused (PNOF) particles (bottom right) (scale bar = 200 μm).256 Reprinted with permission. | ||
BAW-based focusing systems have also shown their applications for high throughput platforms such as the “Imaging FlowCytobot” with resolutions up to 1 μm.249,250 Integrating a time-encoded pulsed laser to an embedded acoustic actuator was reported to move high acoustic contrast factor particles towards the center of the channel with flow rates up to 1000 μL h−1 and a throughput of 2000 cells per s.251 Furthermore, by using two different frequency transducers, simultaneous separation and enrichment of cancer cells were attained for direct on-chip tumor cell identification and counting (Fig. 15C).252 SSAW-based flow cytometry has also been shown in a simple design using one pair of IDTs, wherein particles were focused into pressure nodes and directed to an integrated laser-induced fluorescence (LIF) set up with less than 10% variation coefficient for 1000 beads per s (Fig. 15D).253
In addition to focusing cells on pressure lines, acoustic forces can also be used for locating cells in the camera's focus plane to enhance the quality of the focusing system as well as the imaging throughput. A BAW device was integrated with an imaging flow cytometer to manipulate particles in a single flat layer, allowing for low noise CMOS camera and high resolution with throughputs of 208![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 beads per s and 60
000 beads per s and 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per s at 104 mm s−1. This method can eliminate the error of not counting cells shadowed in vertical lines by another cell. Apart from cell focusing, acoustic waves can also control the 2D orientation of non-spherical particles. Two transducers with different excitation frequencies which correspond to the horizontal or vertical alignment of red blood cells were embedded in a single platform and could reorient 87.8 ± 3.8% and 98.7 ± 0.3% of red blood cells vertically and horizontally respectively (Fig. 15B).254,257 Acoustofluidic systems can also rotate cells and particles and capture planar images at different angles of rotation. For instance, the actuation of air microbubbles trapped in sidewall microcavities allowed the gradual and controlled rotation of GFP-expressed C. elegans and L. longiflorum flowers reducing the problem of single plane overlapping even at high rotation speeds (300 rpms).258,259 A SAW-based system was also used to produce a single acoustic vortex in millimeter scale for rapid rotation of model organisms such as multispectral imaging and 3D reconstruction of zebrafish larvae. The single vortex system could provide a more stable and controllable fluid motion for precise angular alignment of the zebrafish, leading to better visualization.260
000 cells per s at 104 mm s−1. This method can eliminate the error of not counting cells shadowed in vertical lines by another cell. Apart from cell focusing, acoustic waves can also control the 2D orientation of non-spherical particles. Two transducers with different excitation frequencies which correspond to the horizontal or vertical alignment of red blood cells were embedded in a single platform and could reorient 87.8 ± 3.8% and 98.7 ± 0.3% of red blood cells vertically and horizontally respectively (Fig. 15B).254,257 Acoustofluidic systems can also rotate cells and particles and capture planar images at different angles of rotation. For instance, the actuation of air microbubbles trapped in sidewall microcavities allowed the gradual and controlled rotation of GFP-expressed C. elegans and L. longiflorum flowers reducing the problem of single plane overlapping even at high rotation speeds (300 rpms).258,259 A SAW-based system was also used to produce a single acoustic vortex in millimeter scale for rapid rotation of model organisms such as multispectral imaging and 3D reconstruction of zebrafish larvae. The single vortex system could provide a more stable and controllable fluid motion for precise angular alignment of the zebrafish, leading to better visualization.260
In addition to SSAWs, TSAWs have also been proposed to drive cells into desired channels for fluorescent cell analysis. One application of TSAW emerged from its marriage with a fluorescence interrogation system and a high-speed imaging camera for FACS, where the width of acoustic beams was reduced to ∼50 μm to sort fluorescent MCF-7 cells from diluted blood with a 86% purity (Fig. 15E).255 Another clever mechanism combined TSAW with a multilayer feature consisting of a flow-focusing nozzle and a slanted groove to improve the sorting efficiency by a vertical component of the refracted acoustic wave with a purity rate up to 92%.265
8 Summary
To summarize, we compare the performance and potentials of the most widely used acoustic mechanisms for tissue engineering, therapeutics development and delivery, biosensing, cell separation, sorting, and enrichment. Moreover, to better distinguish the performance of direct BAW and SAW radiation forces from the secondary phenomena such as Bjerknes forces and streaming, they are categorized separately, although Bjerknes forces and streaming can occur in these systems too.Tissue engineering
Acoustofluidics offers biocompatible and rapid methods to pattern cells for tissue engineering purposes with a diverse range of resolution and complexity. To help with adopting the best-suited strategy, we ranked the capacity of each acoustic technique in tissue engineering based on a) cost and facility of fabrication, b) resolution, c) ability to pattern complex structures, d) the size of printing domain, and e) the integrability with other tissue engineering modules such as 3D printers (Fig. 16). To obtain complex geometries, acoustic holography is the leading choice, and it also offers a large patterning domain. However, their poor resolution and complicated fabrication processes should be considered. Moreover, the integrability of the platform with tissue engineering systems necessitates dynamic holograms, which require sophisticated systems to locally block sound waves with bubbles. SAW systems have the highest resolution, enabling the pattern at the single-cell level, but they offer limited-size domains and architectures. Nevertheless, the controllable SAW systems, particularly systems with variable frequencies, have enormous potential to be integrated with stereolithography-based printers to pattern complex and precise tissue geometries. Acoustic streaming systems are easily integrable with other tissue engineering systems such as 3D printers and their resolution can easily be tuned, however, the geometries they produce are mostly restricted to spheroids and ring shapes. So far, BAW systems are a more established technique for tissue engineering applications and offer a sizable patterning domain, satisfactory resolution in most applications, a dynamic frequency for producing various geometries, and easy integration with tissue engineering systems.Therapeutics development and delivery
Acoustic synthesis of therapeutic agents and nanoparticles is mostly done with two methods of acoustic microstreaming and nebulization. Both of these methods show remarkable levels of compatibility with biological components and versatility for the production of a diverse range of therapeutics. Acoustic streaming methods generally show higher throughput and easier retrieval of the output products while nebulization lays the groundwork for novel reactions and processes during formation such as layer-by-layer coating. Moreover, nebulization proved to be an efficient mechanism for drug delivery through the respiratory system.For transfection and drug delivery to cells by sonoporation, the performance of different techniques are compared based on a) throughput of cells that can be treated b) biocompatibility to both cells and the bioagents c) delivery efficiency, d) rapidity of sonoporation, and e) controllability and uniformity of forces on each cell (Fig. 16). Cavitation-based techniques are fast approach with high delivery efficiency while their spontaneous nature renders them uncontrollable and harmful to cells. SAW and BAW systems offer a more controllable force field, but their delivery efficiency needs to be further improved. BAW systems can handle outstanding throughputs while high-frequency SAW systems offer excellent biocompatibility. Continuous microstreams are reported to have high delivery efficiency while maintaining cell viability and offering high throughput. The controllable shear forces, present in acoustic microstream, are one of the main mechanisms of membrane rupture. In fact, even some of the SAW and BAW systems acknowledge the presence of the coexisting microstreams as the root cause of sonoporation.38 Moreover, the integrability of microstreams with other methods such as electroporation can ensure uniform exposure to forces and enhanced efficiency.135
Acoustically induced phenotypes in biological organisms
The overview of studies shows that both SAW and BAW systems can enhance the growth and proliferation of cells without harming cell viability. Moreover, acoustic waves are shown to trigger and direct differentiation in stem cells and modulate the ion flux in numerous cell types including neurons. The performance comparison of the SAW and BAW for each effect cannot be conclusive due to the cell-type dependency and also variation of the dominant acoustic phenomena even in similar platforms. For instance, in many of the reviewed papers, the accompanying streaming was suggested as a primary effect. This indicates that apart from frequency and wave mode, other parameters that can influence the balance of various acoustic phenomena should be standardized before concluding the effects of acoustic waves.266 Time of exposure, the amplitude of the driving voltage, and the chamber setting and geometry can also influence the dominant acoustic phenomenon and should be carefully considered. Moreover, the property of media such as viscosity and density are other important factors in determining acoustic radiation forces, dissipation of waves, and microstreaming.Biosensing
The criteria chosen for comparison of acoustic biosensors are a) sensitivity b) reproducibility of data c) range of available frequencies for each method d) rapidity of sensing, and e) versatility of each strategy in sensing biological components in liquids, gases, and complex or corrosive buffers (Fig. 16). The BAW methods such as QCM-D are reported with generally high reproducibility of data and the versatility for detection of various biological samples and suspended bioparticles in liquids due to their more established technology, but have lower sensitivity compared to many SAW systems. Rayleigh waves have a wide range of frequencies and are excellent for integration with fouling removal mechanisms and preparation steps, however, their leaky nature leads to low reproducibility and energy loss in liquids. SH-SAW and Love waves both offer a similarly wide range of frequency, rapid detection, and facile integrability. The waveguide layer in Love waves allows for better sensitivity and also the versatility to operate in corrosive materials. However, to ensure the reproducibility of the data, adverse interference effects should be carefully monitored and further measures for removing the interference might be necessary.Particle separation/enrichment
The performance of acoustic tweezers for particle separation, sorting, and enrichment are discussed based on a) the accuracy and resolution of differentiation b) efficiency, c) throughput, d) the ability for integration to downstream or upstream modules, and e) the versatility for distinguishing particles based on physical characteristics (Fig. 16). The BAW method is appropriate for high-throughput requirements and offers excellent versatility by offering separation based on both size and acoustic contrast factor and compatibility with complex biological buffers. For applications requiring higher resolution and smaller thresholds, SAW systems are the superior choice, however, it should be noted that they usually operate in lower throughputs. Both SAW and BAW offer high separation efficiency and are integratable as upstream modules in micro total analysis systems, but their functionality diminishes in the nano realm, particularly in BAW systems. Therefore, in applications focusing on nanoscale particle separation and sorting, Bjerknes' strategies can be a very promising alternative. Acoustic streaming offers continuous flow and high throughput separation, easily integratable into multimodule systems. The streaming-based separation is however less size sensitive compared to the ARF-based system, due to the linear relationship of the drag forces with size. However, since this linear relationship leads to the dominance of drag forces in the nanoscale, acoustic streaming can be a promising choice for the enrichment and separation of nanoparticles.9 Future trend and outlook
In this review, we have provided an overview of the most frequently studied acoustofluidic concepts and forces in both SAW and BAW-based devices, their resulting acoustic phenomena for sensing and manipulation of bioparticles and biofluids, as well as their application in biomedical sciences. The most recent advances of acoustofluidics in biological settings as actuators, sensors, or both, were presented and discussed, including cell patterning for tissue engineering, therapeutic synthesis and delivery, acoustofluidics as a functional force, bioparticle separation and enrichment, and biosensing applications.The miniaturization of acoustofluidics platforms opens a new venue for studying the physiological and physicochemical effects of acoustic waves in biological samples within a confined microscale domain with a uniform, precise, and controllable exposure to acoustic pressure. Moreover, acoustofluidics offer a wide frequency range, various waveforms, acoustic wave transmission settings, and a variety of acoustic forces and phenomena that can be tailored for miscellaneous experiment requirements, from cell separation, bioparticle isolation, and patterning to acoustic sensing. The unique features of miniatured acoustic systems open myriads of new applications with higher versatility in biotechnology applications compared to conventional ultrasound systems. Miniaturized systems can also potentially offer favorable and well-established functionalities of conventional ultrasound systems while increasing their usability by having enhanced and localized energy delivery, a controlled environment, low amplitude, and continuous wave exposure.
It would be particularly remarkable to investigate the capabilities of acoustofluidic in recreating the conventional ultrasound effects for inducing cytoskeletal vibration and reorganization of the cell structure,267,268 altering cell body morphology and contractility,269,270 studying the change in adhesion and migration, enhancing the degradation of cell scaffolds in tissues,271 enhancing drug delivery by ECM loosening in tumors,272 opening of the blood–brain barrier,273 and preventing toxin protein aggregation in neurodegenerative diseases.274 Hence, a great body of work is remained to be done both to translate the classic ultrasound effects at a microscale level as well as to investigate the change in the aforementioned effects when they are being translated to MEMS settings as well as their implications.
One of the most anticipated directions of acoustofluidics is in the emerging field of tissue engineering where acoustic devices have shown enormous compatibility and potential to be integrated with 3D bioprinters. The continuous and rapid formation of tissue structures such as cellular spheroids, linearly aligned cells within hydrogel fibers, and novel holographic patterning lays the groundwork for recapitulating complex tissue architectures to advance regenerative medicine.93,103,275
Another possibility that MEMS-based acoustic devices offer, owing to the biocompatible and flexible materials, is to either be implanted inside the body or as wearable devices to deliver continuous, low-amplitude, localized and high-frequency acoustic waves with minimal energy decay. The integration of MEMS devices with electronic devices is also another potential area for acoustic devices where the implanted devices can be combined with wireless activation, charging, sensing, and continuous monitoring.276,277
New trends advocate for the integration of low-cost and portable imaging systems, such as smartphones, with acoustic micro-imaging techniques to revolutionize the field of PoC sensing.278 As an example, smartphones can be integrated to acoustofluidics platforms for point of care testing,279 where the digital input and output of acoustic biosensors facilitate both the activation and the electronic readout. Moreover, the integration of acoustic biosensing and biofouling reduction mechanisms is one of the unique and promising potentials of acoustofluidics to develop rapid and precise biosensing strategies.
As observed, there is a wide range of opportunities in the field for both the manipulation and sensing of bioparticles using acoustofluidics. To transfer the acoustic devices to clinical applications, one challenge is the numerous parameters and boundary-conditions that influence the performance of the acoustofluidic devices. The miscellaneous working conditions can significantly compromise data reproducibility. Therefore, the standardization of devices and simplification of design is required to ensure robust, yet experimentally economical platforms.266 By cleverly selecting the wave mode, acoustic excitation method, chamber design, and materials, biomedical researchers can exploit a myriad of applications and benefit from the versatility of these platforms for single-cell analysis, development of therapeutic mechanisms, replicating tissue architectures, and the detection and quantification of small particles such as viruses, exosomes, proteins, and DNA. All these potentials prove that this field can contribute significantly to fundamental biological studies, tissue engineering, clinical sample handling, drug development, biosensing, as well as point of care devices.
Author contributions
The manuscript was written through contributions of all authors.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to acknowledge Natural Science and Engineering Council of Canada for their financial support through Discovery Grant and CREATE in Continuous Flow Synthesis.References
- A. Lenshof, C. Johannesson, M. Evander, J. Nilsson and T. Laurell, Acoustic Cell Manipulation, in Microsystems and Nanosystems, 2017, pp. 129–173, DOI:10.1007/978-3-319-44139-9_5.
- G. Destgeer and H. J. Sung, Recent advances in microfluidic actuation and micro-object manipulation via surface acoustic waves, Lab Chip, 2015, 15, 2722–2738 RSC.
- R. Pethig, Dielectrophoresis: Using Inhomogeneous AC Electrical Fields to Separate and Manipulate Cells, Crit. Rev. Biotechnol., 1996, 16, 331–348 CrossRef.
- M. P. MacDonald, G. C. Spalding and K. Dholakia, Microfluidic sorting in an optical lattice, Nature, 2003, 426(6965), 421–424 CrossRef CAS PubMed.
- A. Ozcelik, et al. Acoustic tweezers for the life sciences, Nat. Methods, 2018, 15, 1021–1028 CrossRef CAS PubMed.
- Y. Xie, H. Bachman and T. J. Huang, Acoustofluidic methods in cell analysis, TrAC, Trends Anal. Chem., 2019, 117, 280–290 CrossRef CAS PubMed.
- Y. Gao, A. K. Fajrial, T. Yang and X. Ding, Emerging on-chip surface acoustic wave technology for small biomaterials manipulation and characterization, Biomater. Sci., 2021, 9, 1574–1582 RSC.
- A. L. Bernassau, F. Gesellchen, P. G. A. MacPherson, M. Riehle and D. R. S. Cumming, Direct patterning of mammalian cells in an ultrasonic heptagon stencil, Biomed. Microdevices, 2012, 14, 559–564 CrossRef CAS PubMed.
- F. Gesellchen, A. L. Bernassau, T. Déjardin, D. R. S. Cumming and M. O. Riehle, Cell patterning with a heptagon acoustic tweezer-application in neurite guidance, Lab Chip, 2014, 14, 2266–2275 RSC.
- X. Tao, et al. 3D patterning/manipulating microparticles and yeast cells using ZnO/Si thin film surface acoustic waves, Sens. Actuators, B, 2019, 299, 126991 CrossRef CAS.
- Z. Tian, Z. Wang, P. Zhang, T. D. Naquin, J. Mai, Y. Wu, S. Yang, Y. Gu, H. Bachman, Y. Liang and Z. Yu, Generating multifunctional acoustic tweezers in Petri dishes for contactless, precise manipulation of bioparticles, Sci. Adv., 2020, 6(37), eabb0494 CrossRef CAS PubMed.
- X. Ding, et al. Surface acoustic wave microfluidics, Lab Chip, 2013, 13, 3626 RSC.
- B. Kang, et al. High-resolution acoustophoretic 3D cell patterning to construct functional collateral cylindroids for ischemia therapy, Nat. Commun., 2018, 9, 5402 CrossRef CAS PubMed.
- S. M. Naseer, et al. Surface acoustic waves induced micropatterning of cells in gelatin methacryloyl (GelMA) hydrogels, Biofabrication, 2017, 9, 015020 CrossRef PubMed.
- B. Chen, et al. High-throughput acoustofluidic fabrication of tumor spheroids, Lab Chip, 2019, 19, 1755–1763 RSC.
- L. Alhasan, et al. Rapid Enhancement of Cellular Spheroid Assembly by Acoustically Driven Microcentrifugation, ACS Biomater. Sci. Eng., 2016, 2, 1013–1022 CrossRef CAS PubMed.
- J. P. Lata, et al. Surface Acoustic Waves Grant Superior Spatial Control of Cells Embedded in Hydrogel Fibers, Adv. Mater., 2016, 28, 8632–8638 CrossRef CAS PubMed.
- J. Zhang, L. Meng, F. Cai, H. Zheng and C. R. P. Courtney, Multi-scale patterning of microparticles using a combination of surface acoustic waves and ultrasonic bulk waves, Appl. Phys. Lett., 2014, 104, 224103 CrossRef.
- S. Cohen, et al. Large-scale acoustic-driven neuronal patterning and directed outgrowth, Sci. Rep., 2020, 10, 1–11 CrossRef PubMed.
- C. Imashiro, et al. Cell Patterning Method on a Clinically Ubiquitous Culture Dish Using Acoustic Pressure Generated from Resonance Vibration of a Disk-Shaped Ultrasonic Transducer, IEEE Trans. Biomed. Eng., 2019, 66, 111–118 Search PubMed.
- W. T. Coakley, et al. Cell-cell contact and membrane spreading in an ultrasound trap, Colloids Surf., B, 2004, 34, 221–230 CrossRef CAS PubMed.
- D. Bazou, G. A. Foster, J. R. Ralphs and W. T. Coakley, Molecular adhesion development in a neural cell monolayer forming in an ultrasound trap, Mol. Membr. Biol., 2005, 22, 229–240 CrossRef CAS PubMed.
- K. Olofsson, et al. Acoustic formation of multicellular tumor spheroids enabling on-chip functional and structural imaging, Lab Chip, 2018, 18, 2466–2476 RSC.
- K. A. Garvin, D. Dalecki and D. C. Hocking, Vascularization of Three-Dimensional Collagen Hydrogels Using Ultrasound Standing Wave Fields, Ultrasound Med. Biol., 2011, 37, 1853–1864 CrossRef PubMed.
- Y. Sriphutkiat, S. Kasetsirikul, D. Ketpun and Y. Zhou, Cell alignment and accumulation using acoustic nozzle for bioprinting, Sci. Rep., 2019, 9, 1–12 CrossRef CAS PubMed.
- H. Cai, et al. Acoustofluidic assembly of 3D neurospheroids to model Alzheimer's disease, Analyst, 2020, 145, 6243–6253 RSC.
- S. Li, et al. Standing Surface Acoustic Wave Based Cell Coculture, Anal. Chem., 2014, 86, 9853–9859 CrossRef CAS PubMed.
- J. Baca, V. Severns, D. Lovato, D. Branch and R. Larson, Rapid Detection of Ebola Virus with a Reagent-Free, Point-of-Care Biosensor, Sensors, 2015, 15, 8605–8614 CrossRef CAS PubMed.
- Y. Jiang, et al. SAW sensor for Influenza A virus detection enabled with efficient surface functionalization, Sens. Actuators, B, 2015, 209, 78–84 CrossRef CAS.
- E. R. Gray, et al. Ultra-rapid, sensitive and specific digital diagnosis of HIV with a dual-channel SAW biosensor in a pilot clinical study, NPJ Digit. Med., 2018, 1, 35 CrossRef PubMed.
- V. Turbé, et al. Towards an ultra-rapid smartphone- connected test for infectious diseases, Sci. Rep., 2017, 7, 11971 CrossRef PubMed.
- V. Turbé, et al. Towards an ultra-rapid smartphone- connected test for infectious diseases, Sci. Rep., 2017, 7, 11971 CrossRef PubMed.
- S. I. Zida, Y. Lin and Y. L. Khung, Current Trends on Surface Acoustic Wave Biosensors, Adv. Mater. Technol., 2021, 6, 2001018 CrossRef.
- M. R. Rasouli and M. Tabrizian, An ultra-rapid acoustic micromixer for synthesis of organic nanoparticles, Lab Chip, 2019, 19, 3316–3325 RSC.
- A. Rajapaksa, A. Qi, L. Y. Yeo, R. Coppel and J. R. Friend, Enabling practical surface acoustic wave nebulizer drug delivery via amplitude modulation, Lab Chip, 2014, 14, 1858–1865 RSC.
- A. E. Rajapaksa, et al. Effective pulmonary delivery of an aerosolized plasmid DNA vaccine via surface acoustic wave nebulization, Respir. Res., 2014, 15, 60 CrossRef PubMed.
- A. R. Rezk, H. Ahmed, S. Ramesan and L. Y. Yeo, High Frequency Sonoprocessing: A New Field of Cavitation-Free Acoustic Materials Synthesis, Processing, and Manipulation, Adv. Sci., 2021, 8, 2001983 CrossRef CAS PubMed.
- S. Ramesan, et al. Acoustofection: High-Frequency Vibrational Membrane Permeabilization for Intracellular siRNA Delivery into Nonadherent Cells, ACS Appl. Bio Mater., 2021, 4, 2781–2789 CrossRef CAS PubMed.
- M. E. Stamp, M. S. Brugger, A. Wixforth and C. Westerhausen, Acoustotaxis – in vitro stimulation in a wound healing assay employing surface acoustic waves, Biomater. Sci., 2016, 4(7), 1092–1099 RSC.
- J. I. Rosenblum, M. I. Gazes and N. Greenberg, Surface acoustic wave patch therapy affects tissue oxygenation in ischemic feet, Wounds, 2014, 26, 301–305 Search PubMed.
- M. Miansari, et al. Inducing Mild Traumatic Brain Injury in C. elegans via Cavitation-Free Surface Acoustic Wave-Driven Ultrasonic Irradiation, Sci. Rep., 2019, 9, 1–11 CrossRef CAS PubMed.
- P. Li and T. J. Huang, Applications of Acoustofluidics in Bioanalytical Chemistry, Anal. Chem., 2019, 91, 757–767 CrossRef CAS PubMed.
- M. Wu, et al. Acoustofluidic separation of cells and particles, Microsyst. Nanoeng., 2019, 5, 32 CrossRef PubMed.
- S. Mohanty, I. S. M. Khalil and S. Misra, Contactless acoustic micro/nano manipulation: a paradigm for next generation applications in life sciences, Proc. R. Soc. A, 2020, 476, 20200621 CrossRef PubMed.
- A. Hashmi, G. Yu, M. Reilly-Collette, G. Heiman and J. Xu, Oscillating bubbles: a versatile tool for lab on a chip applications, Lab Chip, 2012, 12, 4216 RSC.
- J. Rufo, P. Zhang, R. Zhong, L. P. Lee and T. J. Huang, A sound approach to advancing healthcare systems: the future of biomedical acoustics, Nat. Commun., 2022, 131(13), 1–8 Search PubMed.
- L. Y. Yeo and J. R. Friend, Surface Acoustic Wave Microfluidics, Annu. Rev. Fluid Mech., 2014, 46, 379–406 CrossRef.
- J. Pons, Emerging Actuator Technologies. Emerging Actuator Technologies: A Micromechatronic Approach, John Wiley & Sons, Ltd, 2005, DOI:10.1002/0470091991.
- L. Y. Yeo and J. R. Friend, Surface acoustic wave microfluidics, Annu. Rev. Fluid Mech., 2014, 46(1), 379–406 CrossRef.
- H. Ahmed, S. Ramesan, L. Lee, A. R. Rezk and L. Y. Yeo, On-Chip Generation of Vortical Flows for Microfluidic Centrifugation, Small, 2020, 16, 1903605 CrossRef CAS PubMed.
- L. Meng, et al. Acoustic tweezers, J. Phys. D: Appl. Phys., 2019, 52, 273001 CrossRef CAS.
- J. Zhang, Q. Wu, X. Zhang, H. Wan and P. Wang, Acoustic Transducer and Its Applications in Biosensors, in Handbook of Cell Biosensors, Springer International Publishing, 2021, pp. 1–19, DOI:10.1007/978-3-319-47405-2_65-1.
- D. B. B. Go, M. Z. Z. Atashbar, Z. Ramshani, H.-C. Chang and P. D. Degree, Surface acoustic wave devices for chemical sensing and microfluidics: a review and perspective, Anal. Methods, 2017, 9, 4112–4134 RSC.
- J. Friend and L. Y. Yeo, Microscale acoustofluidics: Microfluidics driven via acoustics and ultrasonics, Rev. Mod. Phys., 2011, 83, 647–704 CrossRef.
- H. Bruus, Acoustofluidics 10: Scaling laws in acoustophoresis, Lab Chip, 2012, 12, 1578–1586 RSC.
- C. Eckart, Vortices and Streams Caused by Sound Waves, Phys. Rev., 1948, 73, 68–76 CrossRef.
- M. Wiklund, R. Green and M. Ohlin, Acoustofluidics 14: Applications of acoustic streaming in microfluidic devices, Lab Chip, 2012, 12(14), 2438–2451 RSC.
- W. L. Nyborg, Acoustic Streaming near a Boundary, J. Acoust. Soc. Am., 1958, 30, 329–339 CrossRef.
- J. Lei, P. Glynne-Jones and M. Hill, Comparing methods for the modelling of boundary-driven streaming in acoustofluidic devices, Microfluid. Nanofluid., 2017, 21, 23 CrossRef PubMed.
- I. Leibacher, P. Hahn and J. Dual, Acoustophoretic cell and particle trapping on microfluidic sharp edges, Microfluid. Nanofluid., 2015, 19, 923–933 CrossRef CAS.
- R. Manasseh, Acoustic Bubbles, Acoustic Streaming, and Cavitation Microstreaming, in Handbook of Ultrasonics and Sonochemistry, Springer, Singapore, 2016, pp. 33–68, DOI:10.1007/978-981-287-278-4_5.
- B. W. Drinkwater, Dynamic-field devices for the ultrasonic manipulation of microparticles, Lab Chip, 2014, 16(13), 2360–2375 RSC.
- R. Mettin, I. Akhatov, U. Parlitz, C. Ohl and W. Lauterborn, Bjerknes forces between small cavitation bubbles in a strong acoustic field, Phys. Rev. E: Stat. Phys., Plasmas, Fluids, Relat. Interdiscip. Top., 1997, 56, 2924–2931 CrossRef CAS.
- R. Habibi, V. He, S. Ghavamian, A. de Marco, T.-H. Lee and M.-I. Aguilar, et al. Exosometrapping and enrichment using a sound wave activated nano-sieve (SWANS), Lab Chip, 2020, 20(19), 3633–3643 RSC.
- D. J. Collins, et al. Self-Aligned Acoustofluidic Particle Focusing and Patterning in Microfluidic Channels from Channel-Based Acoustic Waveguides, Phys. Rev. Lett., 2018, 120, 074502 CrossRef CAS PubMed.
- A. G. Guex, N. Di Marzio, D. Eglin, M. Alini and T. Serra, The waves that make the pattern: a review on acoustic manipulation in biomedical research, Mater. Today Bio, 2021, 10, 100110 CrossRef CAS PubMed.
- J. P. K. Armstrong and M. M. Stevens, Using Remote Fields for Complex Tissue Engineering, Trends Biotechnol., 2020, 38, 254–263 CrossRef CAS PubMed.
- D. Dalecki and D. C. Hocking, Advancing Ultrasound Technologies for Tissue Engineering, in Handbook of Ultrasonics and Sonochemistry, Springer, Singapore, 2015, pp. 1–26, DOI:10.1007/978-981-287-470-2_28-1.
- Z. Wang, et al. Single-cell patterning technology for biological applications, Biomicrofluidics, 2019, 13, 061502 CrossRef PubMed.
- J. P. K. Armstrong, et al. Engineering Anisotropic Muscle Tissue using Acoustic Cell Patterning, Adv. Mater., 2018, 30, 1802649 CrossRef PubMed.
- K. Olofsson, B. Hammarström and M. Wiklund, Ultrasonic Based Tissue Modelling and Engineering, Micromachines, 2018, 9, 594 CrossRef PubMed.
- J. W. Buikema, P. Van Der Meer, J. P. G. Sluijter and I. J. Domian, Engineering Myocardial Tissue: The Convergence of Stem Cells Biology and Tissue Engineering Technology, Stem Cells, 2013, 31, 2587 CrossRef CAS PubMed.
- R. L. Mauck, et al. Engineering on the Straight and Narrow: The Mechanics of Nanofibrous Assemblies for Fiber-Reinforced Tissue Regeneration, Tissue Eng., Part B, 2009, 15, 171 CrossRef CAS PubMed.
- J. Shi, et al. Acoustic tweezers: patterning cells and microparticles using standing surface acoustic waves (SSAW), Lab Chip, 2009, 9, 2890 RSC.
- F. Guo, et al. Controlling cell–cell interactions using surface acoustic waves, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 43–48 CrossRef CAS PubMed.
- S. Li, et al. Standing Surface Acoustic Wave Based Cell Coculture, Anal. Chem., 2014, 86, 9853–9859 CrossRef CAS PubMed.
- E. S. Comeau, D. C. Hocking and D. Dalecki, Ultrasound patterning technologies for studying vascular morphogenesis in 3D, J. Cell Sci., 2017, 130, 232–242 CAS.
- P. Chansoria, L. K. Narayanan, K. Schuchard and R. Shirwaiker, Ultrasound-assisted biofabrication and bioprinting of preferentially aligned three-dimensional cellular constructs, Biofabrication, 2019, 11, 035015 CrossRef CAS PubMed.
- A. L. Bernassau, P. G. A. MacPherson, J. Beeley, B. W. Drinkwater and D. R. S. Cumming, Patterning of microspheres and microbubbles in an acoustic tweezers, Biomed. Microdevices, 2013, 15, 289–297 CrossRef CAS PubMed.
- A. Gladkov, et al. Design of Cultured Neuron Networks in vitro with Predefined Connectivity Using Asymmetric Microfluidic Channels, Sci. Rep., 2017, 7, 1–14 CrossRef CAS PubMed.
- C. Bouyer, et al. A Bio-Acoustic Levitational (BAL) Assembly Method for Engineering of Multilayered, 3D Brain-Like Constructs, Using Human Embryonic Stem Cell Derived Neuro-Progenitors, Adv. Mater., 2016, 28, 161–167 CrossRef CAS PubMed.
- A. Tait, et al. Engineering multi-layered tissue constructs using acoustic levitation, Sci. Rep., 2019, 9, 9789 CrossRef PubMed.
- X. Ding, et al. Tunable patterning of microparticles and cells using standing surface acoustic waves, Lab Chip, 2012, 12, 2491–2497 RSC.
- P. Kang, et al. Acoustic tweezers based on circular, slanted-finger interdigital transducers for dynamic manipulation of micro-objects, Lab Chip, 2020, 20, 987–994 RSC.
- Y. Bian, et al. Acoustofluidic waveguides for localized control of acoustic wavefront in microfluidics, Microfluid. Nanofluid., 2017, 21, 132 CrossRef PubMed.
- P. Chen and J. Viñals, Amplitude equation and pattern selection in Faraday waves, Phys. Rev. E: Stat. Phys., Plasmas, Fluids, Relat. Interdiscip. Top., 1999, 60, 559–570 CrossRef CAS PubMed.
- P. Chen, S. Güven, O. B. Usta, M. L. Yarmush and U. Demirci, Biotunable Acoustic Node Assembly of Organoids, Adv. Healthcare Mater., 2015, 4, 1937–1943 CrossRef CAS PubMed.
- D. Petta, et al. Sound-induced morphogenesis of multicellular systems for rapid orchestration of vascular networks, Biofabrication, 2020, 13, 15004 CrossRef PubMed.
- T. Ren, P. Chen, L. Gu, M. G. Ogut and U. Demirci, Soft Ring-Shaped Cellu-Robots with Simultaneous Locomotion in Batches, Adv. Mater., 2020, 32, 1905713 CrossRef CAS PubMed.
- P. Chen, et al. Bioacoustic-enabled patterning of human iPSC-derived cardiomyocytes into 3D cardiac tissue, Biomaterials, 2017, 131, 47–57 CrossRef PubMed.
- J. Wang, et al. Acoustic Fabrication of Living Cardiomyocyte-based Hybrid Biorobots, ACS Nano, 2022, 16, 10219–10230 CrossRef CAS PubMed.
- K. Melde, A. G. Mark, T. Qiu and P. Fischer, Holograms for acoustics, Nature, 2016, 537, 518–522 CrossRef CAS PubMed.
- Z. Ma, et al. Acoustic Holographic Cell Patterning in a Biocompatible Hydrogel, Adv. Mater., 2020, 32, 1904181 CrossRef CAS PubMed.
- D. Bazou, W. T. Coakley, A. J. Hayes and S. K. Jackson, Long-term viability and proliferation of alginate-encapsulated 3-D HepG2 aggregates formed in an ultrasound trap, Toxicol. In Vitro, 2008, 22, 1321–1331 CrossRef CAS PubMed.
- L. A. Kuznetsova, D. Bazou, G. O. Edwards and W. T. Coakley, Multiple three-dimensional mammalian cell aggregates formed away from solid substrata in ultrasound standing waves, Biotechnol. Prog., 2009, 25, 834–841 CrossRef CAS PubMed.
- J. Liu, et al. Functional three-dimensional HepG2 aggregate cultures generated from an ultrasound trap: Comparison with HepG2 spheroids, J. Cell. Biochem., 2007, 102, 1180–1189 CrossRef CAS PubMed.
- K. Olofsson, V. Carannante, M. Takai, B. Önfelt and M. Wiklund, Ultrasound-Based Scaffold-Free Core-Shell Multicellular Tumor Spheroid Formation, Micromachines, 2021, 12, 329 CrossRef PubMed.
- K. Chen, et al. Rapid formation of size-controllable multicellular spheroids via 3D acoustic tweezers, Lab Chip, 2016, 16, 2636–2643 RSC.
- X. Hu, et al. On-chip hydrogel arrays individually encapsulating acoustic formed multicellular aggregates for high throughput drug testing, Lab Chip, 2020, 20, 2228–2236 RSC.
- Y. Wu, et al. Acoustic assembly of cell spheroids in disposable capillaries, Nanotechnology, 2018, 29, 504006 CrossRef PubMed.
- F. Guo, et al. Precise Manipulation and Patterning of Protein Crystals for Macromolecular Crystallography Using Surface Acoustic Waves, Small, 2015, 11, 2733–2737 CrossRef CAS PubMed.
- Y. Kurashina, K. Takemura and J. Friend, Cell agglomeration in the wells of a 24-well plate using acoustic streaming, Lab Chip, 2017, 17, 876–886 RSC.
- R. Rasouli and M. Tabrizian, Rapid Formation of Multicellular Spheroids in Boundary-Driven Acoustic Microstreams, Small, 2021, 17, 2101931 CrossRef CAS PubMed.
- T. D. Nguyen, et al. Large-Scale Fabrication of 3D Scaffold-Based Patterns of Microparticles and Breast Cancer Cells using Reusable Acoustofluidic Device, Adv. Eng. Mater., 2021, 23, 2001377 CrossRef CAS.
- Z. Tian, S. Yang, P. H. Huang, Z. Wang, P. Zhang, Y. Gu, H. Bachman, C. Chen, M. Wu, Y. Xie and T. J. Huang, Wave number–spiral acoustic tweezers for dynamic and reconfigurable manipulation of particles and cells, Sci. Adv., 2019, 5(5), eaau6062 CrossRef CAS PubMed.
- E. Blanco, H. Shen and M. Ferrari, Principles of nanoparticle design for overcoming biological barriers to drug delivery, Nat. Biotechnol., 2015, 33, 941–951 CrossRef CAS PubMed.
- P. Huang, et al. Acoustofluidic Synthesis of Particulate Nanomaterials, Adv. Sci., 2019, 6, 1900913 CrossRef CAS PubMed.
- N. H. A. Le, et al. Acoustically enhanced microfluidic mixer to synthesize highly uniform nanodrugs without the addition of stabilizers, Int. J. Nanomed., 2018, 13, 1353–1359 CrossRef CAS PubMed.
- N. H. Le, H. Deng, C. Devendran, N. Akhtar, X. Ma, C. Pouton, H. K. Chan, A. Neild and T. Alan, Ultrafast star-shaped acoustic micromixer for high throughput nanoparticle synthesis, Lab Chip, 2020, 20(3), 582–591 RSC.
- A. Pourabed, et al. High throughput acoustic microfluidic mixer controls self-assembly of protein nanoparticles with tuneable sizes, J. Colloid Interface Sci., 2021, 585, 229–236 CrossRef CAS PubMed.
- M. Kurosawa, T. Watanabe, A. Futami and T. Higuchi, Surface acoustic wave atomizer, Sens. Actuators, A, 1995, 50, 69–74 CrossRef CAS.
- A. Qi, et al. Template-free Synthesis and Encapsulation Technique for Layer-by-Layer Polymer Nanocarrier Fabrication, ACS Nano, 2011, 5, 9583–9591 CrossRef CAS PubMed.
- M. Alvarez, J. Friend and L. Y. Yeo, Rapid generation of protein aerosols and nanoparticles via surface acoustic wave atomization, Nanotechnology, 2008, 19, 455103 CrossRef PubMed.
- A. Qi, J. R. Friend and L. Y. Yeo, Investigation of SAW atomization, in Proceedings - IEEE Ultrasonics Symposium, IEEE, 2009, pp. 787–790, DOI:10.1109/ULTSYM.2009.5441556.
- D. Van Assche, et al. Gradient acoustic focusing of sub-micron particles for separation of bacteria from blood lysate, Sci. Rep., 2020, 10, 1–13 CrossRef PubMed.
- A. Winkler, S. M. M. Harazim, S. B. B. Menzel and H. Schmidt, SAW-based fluid atomization using mass-producible chip devices, Lab Chip, 2015, 15, 3793–3799 RSC.
- A. Qi, et al. Miniature inhalation therapy platform using surface acoustic wave microfluidic atomization, Lab Chip, 2009, 9, 2184 RSC.
- D. B. Go, M. Z. Atashbar, Z. Ramshani and H. C. Chang, Surface acoustic wave devices for chemical sensing and microfluidics: a review and perspective, Anal. Methods, 2017, 9(28), 4112–4134 RSC.
- M. Steinacher, H. Du, D. Gilbert and E. Amstad, Production of Additive-Free Amorphous Nanoparticles with a SAW-Based Microfluidic Spray-Dryer, Adv. Mater. Technol., 2019, 4, 1800665 CrossRef.
- P. Barnes, New treatments for chronic obstructive pulmonary disease, Curr. Opin. Pharmacol., 2001, 1, 217–222 CrossRef CAS PubMed.
- Y. Wang, A. R. Rezk, J. S. Khara, L. Y. Yeo and P. L. R. Ee, Stability and efficacy of synthetic cationic antimicrobial peptides nebulized using high frequency acoustic waves, Biomicrofluidics, 2016, 10, 034115 CrossRef PubMed.
- K. S. Wong, W. T. Lim, C. W. Ooi, L. Y. Yeo and M. K. Tan, In situ generation of plasma-activated aerosols via surface acoustic wave nebulization for portable spray-based surface bacterial inactivation, Lab Chip, 2020, 20(10), 1856–1868 RSC.
- S. Marqus, et al. High frequency acoustic nebulization for pulmonary delivery of antibiotic alternatives against Staphylococcus aureus, Eur. J. Pharm. Biopharm., 2020, 151, 181–188 CrossRef CAS PubMed.
- L. Alhasan, A. Qi, A. R. Rezk, L. Y. Yeo and P. P. Y. Chan, Integrative Biology Assessment of the potential of a high frequency acoustomicrofluidic nebulisation platform for inhaled stem cell therapy †, This J. is Cite this, Integr. Biol., 2016, 8, 12 CrossRef PubMed.
- S. Ramesan, A. R. Rezk and L. Y. Yeo, High frequency acoustic permeabilisation of drugs through tissue for localised mucosal delivery, Lab Chip, 2018, 18, 3272–3284 RSC.
- Y. Li, Z. Chen and S. Ge, Sonoporation: Underlying Mechanisms and Applications in Cellular Regulation, BIO Integr., 2021, 2, 29–36 CrossRef CAS.
- M. P. Stewart, R. Langer and K. F. Jensen, Intracellular delivery by membrane disruption: Mechanisms, strategies, and concepts, Chem. Rev., 2018, 118, 7409–7531 CrossRef CAS PubMed.
- M. Tomizawa, et al. Sonoporation: Gene transfer using ultrasound, World J. Methodol., 2013, 3, 39–44 CrossRef PubMed.
- Y. Qiu, C. Zhang, J. Tu and D. Zhang, Microbubble-induced sonoporation involved in ultrasound-mediated DNA transfection in vitro at low acoustic pressures, J. Biomech., 2012, 45, 1339–1345 CrossRef PubMed.
- M. A. Hassan, et al. Modulation control over ultrasound-mediated gene delivery: Evaluating the importance of standing waves, J. Controlled Release, 2010, 141, 70–76 CrossRef CAS PubMed.
- D. Morshedi Rad, et al. A Comprehensive Review on Intracellular Delivery, Adv. Mater., 2021, 33, 2005363 CrossRef CAS PubMed.
- A. van Wamel, et al. Vibrating microbubbles poking individual cells: Drug transfer into cells via sonoporation, J. Controlled Release, 2006, 112, 149–155 CrossRef CAS PubMed.
- Z. Fan, R. E. Kumon and C. X. Deng, Mechanisms of microbubble-facilitated sonoporation for drug and gene delivery, Ther. Delivery, 2014, 5, 467–486 CrossRef CAS PubMed.
- L. Meng, et al. Sonoporation of Cells by a Parallel Stable Cavitation Microbubble Array, Adv. Sci., 2019, 6, 1900557 CrossRef PubMed.
- M. Aghaamoo, et al. High-Throughput and Dosage-Controlled Intracellular Delivery of Large Cargos by an Acoustic-Electric Micro-Vortices Platform, Adv. Sci., 2022, 9, 2102021 CrossRef CAS PubMed.
- D. Carugo, et al. Contrast agent-free sonoporation: The use of an ultrasonic standing wave microfluidic system for the delivery of pharmaceutical agents, Biomicrofluidics, 2011, 5, 044108 CrossRef PubMed.
- J. N. Belling, et al. Acoustofluidic sonoporation for gene delivery to human hematopoietic stem and progenitor cells, Proc. Natl. Acad. Sci. U. S. A., 2020, 117, 10976–10982 CrossRef CAS PubMed.
- Z. Zhang, et al. Hypersonic Poration: A New Versatile Cell Poration Method to Enhance Cellular Uptake Using a Piezoelectric Nano-Electromechanical Device, Small, 2017, 13, 1602962 CrossRef PubMed.
- T. Reusch, et al. Collective lipid bilayer dynamics excited by surface acoustic waves, Phys. Rev. Lett., 2014, 113, 118102 CrossRef CAS PubMed.
- S. Ramesan, A. R. Rezk, C. Dekiwadia, C. Cortez-Jugo and L. Y. Yeo, Acoustically-mediated intracellular delivery †, Nanoscale, 2018, 10, 13165 RSC.
- D. Peng, et al. Mechanisms and Applications of Neuromodulation Using Surface Acoustic Waves—A Mini-Review, Front. Neurosci., 2021, 15, 27 Search PubMed.
- K. Martinez Villegas, R. Rasouli and M. Tabrizian, Enhancing metabolic activity and differentiation potential in adipose mesenchymal stem cells via high-resolution surface-acoustic-wave contactless patterning, Microsyst. Nanoeng., 2022, 8, 79 CrossRef CAS PubMed.
- C. Devendran, J. Carthew, J. E. Frith and A. Neild, Cell Adhesion, Morphology, and Metabolism Variation via Acoustic Exposure within Microfluidic Cell Handling Systems, Adv. Sci., 2019, 6, 1902326 CrossRef CAS PubMed.
- G. Greco, et al. Surface-Acoustic-Wave (SAW)-Driven Device for Dynamic Cell Cultures, Anal. Chem., 2018, 90, 7450–7457 CrossRef CAS PubMed.
- M. S. Brugger, et al. Orchestrating cells on a chip: Employing surface acoustic waves towards the formation of neural networks, Phys. Rev. E, 2018, 98, 012411 CrossRef CAS PubMed.
- N. Doan, P. Reher, S. Meghji and M. Harris, In vitro effects of therapeutic ultrasound on cell proliferation, protein synthesis, and cytokine production by human fibroblasts, osteoblasts, and monocytes, Kouqiang Hemian Waike Zazhi, 1999, 57, 409–419 CAS.
- M. S. Brugger, et al. Vibration enhanced cell growth induced by surface acoustic waves as in vitro wound-healing model, Proc. Natl. Acad. Sci. U. S. A., 2020, 117, 31603–31613 CrossRef CAS PubMed.
- I.-C. Lee, H.-J. Wu and H.-L. Liu, Dual-Frequency Ultrasound Induces Neural Stem/Progenitor Cell Differentiation and Growth Factor Utilization by Enhancing Stable Cavitation, ACS Chem. Neurosci., 2019, 10, 1452–1461 CrossRef CAS PubMed.
- Y. S. Lee, et al. An ultra-effective method of generating extramultipotent cells from human fibroblasts by ultrasound, Biomaterials, 2017, 143, 65–78 CrossRef CAS PubMed.
- U. S. Jonnalagadda, et al. Acoustically modulated biomechanical stimulation for human cartilage tissue engineering, Lab Chip, 2018, 18, 473–485 RSC.
- L. A. Ambattu, et al. High frequency acoustic cell stimulation promotes exosome generation regulated by a calcium-dependent mechanism, Commun. Biol., 2020, 3, 1–9 CrossRef PubMed.
- S. S. Ranade, R. Syeda and A. Patapoutian, Mechanically Activated Ion Channels, Neuron, 2015, 87, 1162–1179 CrossRef CAS PubMed.
- P. Kumar, D. Kumar, S. K. Jha, N. K. Jha and R. K. Ambasta, Ion Channels in Neurological Disorders. in Advances in Protein Chemistry and Structural Biology, Academic Press Inc., 2016, vol. 103, pp. 97–136 Search PubMed.
- J. Ye, et al. Ultrasonic Control of Neural Activity through Activation of the Mechanosensitive Channel MscL, Nano Lett., 2018, 18, 4148–4155 CrossRef CAS PubMed.
- Z. Lin, et al. On-Chip Ultrasound Modulation of Pyramidal Neuronal Activity in Hippocampal Slices, Adv. Biosyst., 2018, 2, 1800041 CrossRef.
- Z. Lin, X. Huang, W. Zhou, W. Zhang, Y. Liu, T. Bian, L. Niu, L. Meng and Y. Guo, Ultrasound stimulation modulates voltage-gated potassium currents associated with action potential shape in hippocampal CA1 pyramidal neurons, Front. Pharmacol., 2019, 10, 544 CrossRef CAS PubMed.
- Y. Pan, et al. Mechanogenetics for the remote and noninvasive control of cancer immunotherapy, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, 992–997 CrossRef CAS PubMed.
- W. Zhou, J. Wang, K. Wang, B. Huang, L. Niu, F. Li, F. Cai, Y. Chen, X. Liu, X. Zhang and H. Chen, Ultrasound neuro-modulation chip: activation of sensory neurons in Caenorhabditis elegans by surface acoustic waves, Lab Chip, 2017, 17(10), 1725–1731 RSC.
- M. A. Hilliard, et al. Cellular response and adaptation to chemical repellents, In vivo imaging of C. elegans ASH neurons, EMBO J., 2005, 24, 63–72 CrossRef CAS PubMed.
- E. Gourgou and N. Chronis, Chemically induced oxidative stress affects ASH neuronal function and behavior in C. elegans, Sci. Rep., 2016, 6, 38147 CrossRef CAS PubMed.
- Z. Lin, et al. Non-invasive ultrasonic neuromodulation of neuronal excitability for treatment of epilepsy, Theranostics, 2020, 10, 5514–5526 CrossRef CAS PubMed.
- A. Adamo, et al. Microfluidics-based assessment of cell deformability, Anal. Chem., 2012, 84, 6438–6443 CrossRef CAS PubMed.
- W. H. Grover, et al. Measuring single-cell density, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 10992–10996 CrossRef CAS PubMed.
- S. E. Cross, Y. S. Jin, J. Rao and J. K. Gimzewski, Nanomechanical analysis of cells from cancer patients, Nat. Nanotechnol., 2007, 2, 780–783 CrossRef CAS PubMed.
- S. Suresh, Biomechanics and biophysics of cancer cells, Acta Biomater., 2007, 3, 413–438 CrossRef PubMed.
- G. Y. H. Lee and C. T. Lim, Biomechanics approaches to studying human diseases, Trends Biotechnol., 2007, 25, 111–118 CrossRef CAS PubMed.
- Y. Xie, et al. Probing Cell Deformability via Acoustically Actuated Bubbles, Small, 2016, 12, 902–910 CrossRef CAS PubMed.
- N. F. Läubli, et al. 3D mechanical characterization of single cells and small organisms using acoustic manipulation and force microscopy, Nat. Commun., 2021, 12, 1–11 CrossRef PubMed.
- D. Hartono, Y. Liu, P. L. Tan, X. Y. Then, L. Y. Yung and K. M. Lim, On-chip measurements of cell compressibility via acoustic radiation, Lab Chip, 2011, 11(23), 4072–4080 RSC.
- H. Wang, et al. A continuous-flow acoustofluidic cytometer for single-cell mechanotyping, Lab Chip, 2019, 19, 387–393 RSC.
- C. Grenvall, C. Antfolk, C. Z. Bisgaard and T. Laurell, Two-dimensional acoustic particle focusing enables sheathless chip Coulter counter with planar electrode configuration, Lab Chip, 2014, 14, 4629–4637 RSC.
- C. Grenvall, C. Magnusson, H. Lilja and T. Laurell, Concurrent Isolation of Lymphocytes and Granulocytes Using Prefocused Free Flow Acoustophoresis, Anal. Chem., 2015, 87, 5596–5604 CrossRef CAS PubMed.
- D. Kamsma, et al. Single-Cell Acoustic Force Spectroscopy: Resolving Kinetics and Strength of T Cell Adhesion to Fibronectin, Cell Rep., 2018, 24, 3008–3016 CrossRef CAS PubMed.
- D. Kamsma, R. Creyghton, G. Sitters, G. J. L. Wuite and E. J. G. Peterman, Tuning the Music: Acoustic Force Spectroscopy (AFS) 2.0, Methods, 2016, 105, 26–33 CrossRef CAS PubMed.
- G. Sitters, et al. Acoustic force spectroscopy, Nat. Methods, 2014, 12, 47–50 CrossRef PubMed.
- R. Sorkin, et al. Probing cellular mechanics with acoustic force spectroscopy, Mol. Biol. Cell, 2018, 29, 2005–2011 CrossRef CAS PubMed.
- S. Yang, et al. Harmonic acoustics for dynamic and selective particle manipulation, Nat. Mater., 2022, 215(21), 540–546 CrossRef PubMed.
- J. K. Luo, Y. Q. Fu and W. I. Milne, Acoustic wave based microfluidic and lab-on-chip, Modeling and Measurement Methods for Acoustic Waves and for Acoustic Microdevices, IntechOpen, 2013 Search PubMed.
- M.-I. Rocha-Gaso, C. March-Iborra, Á. Montoya-Baides and A. Arnau-Vives, Surface Generated Acoustic Wave Biosensors for the Detection of Pathogens: A Review, Sensors, 2009, 9, 5740–5769 CrossRef CAS PubMed.
- K. Chang, et al. Label-free and high-sensitive detection of human breast cancer cells by aptamer-based leaky surface acoustic wave biosensor array, Biosens. Bioelectron., 2014, 60, 318–324 CrossRef CAS PubMed.
- T. Wang, et al. Surface Acoustic Waves (SAW)-Based Biosensing for Quantification of Cell Growth in 2D and 3D Cultures, Sensors, 2015, 15, 32045–32055 CrossRef CAS PubMed.
- H. L. H.-L. Cai, et al. A third-order mode high frequency biosensor with atomic resolution, Biosens. Bioelectron., 2015, 71, 261–268 CrossRef CAS PubMed.
- J. Zhang, et al. Recent advances in acoustic wave biosensors for the detection of disease-related biomarkers: A review, Anal. Chim. Acta, 2021, 1164, 338321 CrossRef CAS PubMed.
- X. Wei, J. Zhang, L. Zhuang, H. Wan and P. Wang, A Novel Surface Acoustic Wave Biosensor for Real-Time Monitoring of Cell Contractile Properties, ECS Meeting Abstracts, 2020, MA2020-01, 1970 CrossRef.
- S. U. Senveli, et al. A surface acoustic wave biosensor for interrogation of single tumour cells in microcavities, Lab Chip, 2016, 16, 163–171 RSC.
- L. Lamanna, F. Rizzi, V. R. Bhethanabotla and M. De Vittorio, Conformable surface acoustic wave biosensor for E-coli fabricated on PEN plastic film, Biosens. Bioelectron., 2020, 163, 112164 CrossRef CAS PubMed.
- S. T. Ten, et al. Highly sensitive Escherichia coli shear horizontal surface acoustic wave biosensor with silicon dioxide nanostructures, Biosens. Bioelectron., 2017, 93, 146–154 CrossRef CAS PubMed.
- A. Kordas, et al. Rapid Salmonella detection using an acoustic wave device combined with the RCA isothermal DNA amplification method, Sens. Bio-Sens. Res., 2016, 11, 121–127 CrossRef.
- K. Tsougeni, et al. Lab-on-Chip platform and protocol for rapid foodborne pathogen detection comprising on-chip cell capture, lysis, DNA amplification and surface-acoustic-wave detection, Sens. Actuators, B, 2020, 320, 128345 CrossRef CAS.
- X. Liu, J.-Y. Wang, X.-B. Mao, Y. Ning and G.-J. Zhang, Single-Shot Analytical Assay Based on Graphene-Oxide-Modified Surface Acoustic Wave Biosensor for Detection of Single-Nucleotide Polymorphisms, Anal. Chem., 2015, 87, 9352–9359 CrossRef CAS PubMed.
- Y. Zhang, F. Yang, Z. Sun, Y. T. Li and G. J. Zhang, A surface acoustic wave biosensor synergizing DNA-mediated: In situ silver nanoparticle growth for a highly specific and signal-amplified nucleic acid assay, Analyst, 2017, 142, 3468–3476 RSC.
- D. Matatagui, et al. Comparison of two types of acoustic biosensors to detect immunoreactions: Love-wave sensor working in dynamic mode and QCM working in static mode, Sens. Actuators, B, 2013, 189, 123–129 CrossRef CAS.
- Y.-S. Choi, J. Lee, Y. Lee, J. Kwak and S. Suk Lee, Increase in detection sensitivity of surface acoustic wave biosensor using triple transit echo wave, Appl. Phys. Lett., 2018, 113, 083702 CrossRef.
- A. Tretjakov, V. Syritski, J. Reut, R. Boroznjak and A. Öpik, Molecularly imprinted polymer film interfaced with Surface Acoustic Wave technology as a sensing platform for label-free protein detection, Anal. Chim. Acta, 2016, 902, 182–188 CrossRef CAS PubMed.
- C. C. Wang, et al. AuNP-Amplified Surface Acoustic Wave Sensor for the Quantification of Exosomes, ACS Sens., 2020, 5, 362–369 CrossRef CAS PubMed.
- Y. Huang, P. K. Das and V. R. Bhethanabotla, Surface acoustic waves in biosensing applications, Sensors and Actuators Reports, 2021, 3, 100041 CrossRef.
- A. Renaudin, V. Chabot, E. Grondin, V. Aimez and P. G. Charette, Integrated active mixing and biosensing using surface acoustic waves (SAW) and surface plasmon resonance (SPR) on a common substrate, Lab Chip, 2010, 10, 111–115 RSC.
- A. Sonato, M. Agostini, G. Ruffato, E. Gazzola, D. Liuni, G. Greco, M. Travagliati, M. Cecchini and F. Romanato, A surface acoustic wave (SAW)-enhanced grating-coupling phase-interrogation surface plasmon resonance (SPR) microfluidic biosensor, Lab Chip, 2016, 16(7), 1224–1233 RSC.
- G. D. G. D. Meyer, et al. Nonspecific binding removal from protein microarrays using thickness shear mode resonators, IEEE Sens. J., 2006, 6, 254–261 CAS.
- S. Pan, et al. Biofouling Removal and Protein Detection Using a Hypersonic Resonator, ACS Sens., 2017, 2, 1175–1183 CrossRef CAS PubMed.
- S. Cular, D. W. Branch, V. R. Bhethanabotla, G. D. Meyer and H. G. Craighead, Removal of Nonspecifically Bound Proteins on Microarrays Using Surface Acoustic Waves, IEEE Sens. J., 2008, 8, 314–320 CAS.
- J. Liu, S. Li and V. R. Bhethanabotla, Integrating Metal-Enhanced Fluorescence and Surface Acoustic Waves for Sensitive and Rapid Quantification of Cancer Biomarkers from Real Matrices, ACS Sens., 2018, 3, 222–229 CrossRef CAS PubMed.
- S. Cular, V. R. Bhethanabotla and D. W. Branch, Simultaneous surface manipulation and sensing in a biosensor using a hexagonal saw device. in AIChE Annual Meeting, Conference Proceedings, 2006 Search PubMed.
- R. Singh, S. K. R. S. Sankaranarayanan and V. R. Bhethanabotla, Cite as, Appl. Phys. Lett., 2009, 94, 263503 CrossRef.
- M. Antfolk, C. Magnusson, P. Augustsson, H. Lilja and T. Laurell, Acoustofluidic, Label-Free Separation and Simultaneous Concentration of Rare Tumor Cells from White Blood Cells, Anal. Chem., 2015, 87, 9322–9328 CrossRef CAS PubMed.
- M. Antfolk, C. Antfolk, H. Lilja, T. Laurell and P. Augustsson, A single inlet two-stage acoustophoresis chip enabling tumor cell enrichment from white blood cells, Lab Chip, 2015, 15, 2102–2109 RSC.
- Y. Chen, et al. High-throughput acoustic separation of platelets from whole blood, Lab Chip, 2016, 16, 3466–3472 RSC.
- F. Petersson, L. Åberg, A.-M. Swärd-Nilsson and T. Laurell, Free Flow Acoustophoresis: Microfluidic-Based Mode of Particle and Cell Separation, Anal. Chem., 2007, 79, 5117–5123 CrossRef CAS PubMed.
- J. Shi, H. Huang, Z. Stratton, Y. Huang and T. J. Huang, Continuous particle separation in a microfluidic channel via standing surface acoustic waves (SSAW), Lab Chip, 2009, 9, 3354 RSC.
- J. Nam, H. Lim, D. Kim and S. Shin, Separation of platelets from whole blood using standing surface acoustic waves in a microchannel, Lab Chip, 2011, 11, 3361 RSC.
- S. Li, et al. An On-Chip, Multichannel Droplet Sorter Using Standing Surface Acoustic Waves, Anal. Chem., 2013, 85, 5468–5474 CrossRef CAS PubMed.
- K. Lee, et al. Acoustic Purification of Extracellular Microvesicles, ACS Nano, 2015, 9, 2321–2327 CrossRef CAS PubMed.
- M. Wu, et al. Acoustic Separation of Nanoparticles in Continuous Flow, Adv. Funct. Mater., 2017, 27, 1606039 CrossRef PubMed.
- M. Wu, et al. Isolation of exosomes from whole blood by integrating acoustics and microfluidics, Proc. Natl. Acad. Sci. U. S. A., 2017, 114, 10584–10589 CrossRef CAS PubMed.
- X. Ding, et al. Cell separation using tilted-angle standing surface acoustic waves, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 12992–12997 CrossRef CAS PubMed.
- P. Li, et al. Acoustic separation of circulating tumor cells, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 4970–4975 CrossRef CAS PubMed.
- H. Bruus, Acoustofluidics 7: The acoustic radiation force on small particles, Lab Chip, 2012, 12, 1014 RSC.
- P. Augustsson, J. T. Karlsen, H.-W. Su, H. Bruus and J. Voldman, Iso-acoustic focusing of cells for size-insensitive acousto-mechanical phenotyping, Nat. Commun., 2016, 7, 11556 CrossRef CAS PubMed.
- F. Petersson, A. Nilsson, C. Holm, H. Jönsson and T. Laurell, Continuous separation of lipid particles from erythrocytes by means of laminar flow and acoustic standing wave forces, Lab Chip, 2005, 5, 20–22 RSC.
- T. Franke, A. R. Abate, D. A. Weitz and A. Wixforth, Surface acoustic wave (SAW) directed droplet flow in microfluidics for PDMS devices, Lab Chip, 2009, 9, 2625 RSC.
- T. Franke, S. Braunmüller, L. Schmid, A. Wixforth and D. A. Weitz, Surface acoustic wave actuated cell sorting (SAWACS), Lab Chip, 2010, 10, 789 RSC.
- R. Ahmad, et al. Acoustic Wave-Driven Functionalized Particles for Aptamer-Based Target Biomolecule Separation, Anal. Chem., 2017, 89, 13313–13319 CrossRef CAS PubMed.
- G. Destgeer, B. H. Ha, J. H. Jung and H. J. Sung, Submicron separation of microspheres via travelling surface acoustic waves, Lab Chip, 2014, 14, 4665–4672 RSC.
- K. Wang, et al. Sorting of tumour cells in a microfluidic device by multi-stage surface acoustic waves, Sens. Actuators, B, 2018, 258, 1174–1183 CrossRef CAS.
- D. J. J. Collins, et al. Selective particle and cell capture in a continuous flow using micro-vortex acoustic streaming, Lab Chip, 2017, 17, 1769–1777 RSC.
- C. Wang, S. V. Jalikop and S. Hilgenfeldt, Efficient manipulation of microparticles in bubble streaming flows, Biomicrofluidics, 2012, 6, 012801 CrossRef PubMed.
- R. Thameem, B. Rallabandi and S. Hilgenfeldt, Particle migration and sorting in microbubble streaming flows, Biomicrofluidics, 2016, 10, 014124 CrossRef PubMed.
- M. V. Patel, A. R. Tovar and A. P. Lee, Lateral cavity acoustic transducer as an on-chip cell/particle microfluidic switch, Lab Chip, 2012, 12, 139–145 RSC.
- N. Garg, et al. Whole-blood sorting, enrichment and in situ immunolabeling of cellular subsets using acoustic microstreaming, Microsyst. Nanoeng., 2018, 4, 17085 CrossRef CAS.
- M. V. Patel, I. A. Nanayakkara, M. G. Simon and A. P. Lee, Cavity-induced microstreaming for simultaneous on-chip pumping and size-based separation of cells and particles, Lab Chip, 2014, 14, 3860 RSC.
- N. Nivedita, N. Garg, A. P. Lee and I. Papautsky, A high throughput microfluidic platform for size-selective enrichment of cell populations in tissue and blood samples, Analyst, 2017, 142(14), 2558–2569 RSC.
- A. Fakhfouri, C. Devendran, D. J. J. Collins, Y. Ai and A. Neild, Virtual membrane for filtration of particles using surface acoustic waves (SAW), Lab Chip, 2016, 16, 3515–3523 RSC.
- D. J. Collins, Z. Ma, J. Han and Y. Ai, Continuous micro-vortex-based nanoparticle manipulation via focused surface acoustic waves, Lab Chip, 2017, 17, 91–103 RSC.
- W. Cui, L. Mu, X. Duan, W. Pang and M. A. Reed, A polarized nonlinear optical response in a topological insulator Bi 2 Se 3-Au nanoantenna hybrid-structure for all-optical switching Trapping of sub-100 nm nanoparticles using gigahertz acoustofluidic tweezers for biosensing applications†, Nanoscale, 2019, 11, 14625 RSC.
- Z. Mao, et al. Enriching Nanoparticles via Acoustofluidics, ACS Nano, 2017, 11, 603–612 CrossRef CAS PubMed.
- B. Hammarström, T. Laurell and J. Nilsson, Seed particle-enabled acoustic trapping of bacteria and nanoparticles in continuous flow systems, Lab Chip, 2012, 12, 4296 RSC.
- A. Ku, et al. Acoustic Enrichment of Extracellular Vesicles from Biological Fluids, Anal. Chem., 2018, 90, 8011–8019 CrossRef CAS PubMed.
- R. Habibi and A. Neild, Sound wave activated nano-sieve (SWANS) for enrichment of nanoparticles, Lab Chip, 2019, 19, 3032–3044 RSC.
- Y. Ai, C. K. Sanders and B. L. Marrone, Separation of Escherichia coli Bacteria from Peripheral Blood Mononuclear Cells Using Standing Surface Acoustic Waves, Anal. Chem., 2013, 85, 9126–9134 CrossRef CAS PubMed.
- P. Sehgal and B. J. Kirby, Separation of 300 and 100 nm Particles in Fabry–Perot Acoustofluidic Resonators, Anal. Chem., 2017, 89, 12192–12200 CrossRef CAS PubMed.
- A. Ku, et al. Acoustic Enrichment of Extracellular Vesicles from Biological Fluids, Anal. Chem., 2018, 90, 8011–8019 CrossRef CAS PubMed.
- J. Shi, et al. Three-dimensional continuous particle focusing in a microfluidic channel via standing surface acoustic waves (SSAW), Lab Chip, 2011, 11, 2319–2324 RSC.
- X. Xuan, J. Zhu and C. Church, Particle focusing in microfluidic devices, Microfluid. Nanofluid., 2010, 9, 1–16 CrossRef.
- T. Zhang, Z. Y. Hong, S. Y. Tang, W. Li, D. W. Inglis, Y. Hosokawa, Y. Yalikun and M. Li, Focusing of sub-micrometer particles in microfluidic devices, Lab Chip, 2020, 20(1), 35–53 RSC.
- G. Goddard, J. C. Martin, S. W. Graves and G. Kaduchak, Ultrasonic particle-concentration for sheathless focusing of particles for analysis in a flow cytometer, Cytometry, Part A, 2006, 69, 66–74 CrossRef PubMed.
- P. P. A. Suthanthiraraj, et al. One-dimensional acoustic standing waves in rectangular channels for flow cytometry, Methods, 2012, 57, 259–271 CrossRef PubMed.
- M. E. Piyasena, et al. Multinode Acoustic Focusing for Parallel Flow Cytometry, Anal. Chem., 2012, 84, 1831–1839 CrossRef CAS PubMed.
- D. M. Kalb, et al. Line-Focused Optical Excitation of Parallel Acoustic Focused Sample Streams for High Volumetric and Analytical Rate Flow Cytometry, Anal. Chem., 2017, 89, 9967–9975 CrossRef CAS PubMed.
- R. J. Olson, A. Shalapyonok, D. J. Kalb, S. W. Graves and H. M. Sosik, Imaging FlowCytobot modified for high throughput by in-line acoustic focusing of sample particles, Limnol. Oceanogr.: Methods, 2017, 15, 867–874 CrossRef.
- B. S. Lambert, R. J. Olson and H. M. Sosik, A fluorescence-activated cell sorting subsystem for the imaging flowcytobot, Limnol. Oceanogr.: Methods, 2017, 15, 94–102 CrossRef.
- W. Zhao, et al. A high-throughput label-free time-stretch acoustofluidic imaging cytometer for single-cell mechanotyping, Microfluid. Nanofluid., 2020, 24, 88 CrossRef CAS.
- M. Antfolk, S. H. Kim, S. Koizumi, T. Fujii and T. Laurell, Label-free single-cell separation and imaging of cancer cells using an integrated microfluidic system, Sci. Rep., 2017, 7, 1–12 CrossRef PubMed.
- Y. Chen, A. A. Nawaz, Y. Zhao, P. H. Huang, J. P. McCoy, S. J. Levine, L. Wang and T. J. Huang, Standing surface acoustic wave (SSAW)-based microfluidic cytometer, Lab Chip, 2014, 14(5), 916–923 RSC.
- O. Jakobsson, M. Antfolk and T. Laurell, Continuous Flow Two-Dimensional Acoustic Orientation of Nonspherical Cells, Anal. Chem., 2014, 86, 6111–6114 CrossRef CAS PubMed.
- Z. Ma, Y. Zhou, D. J. Collins and Y. Ai, Fluorescence activated cell sorting via a focused traveling surface acoustic beam, Lab Chip, 2017, 17(18), 3176–3185 RSC.
- L. Ren, et al. Standing Surface Acoustic Wave (SSAW)-Based Fluorescence-Activated Cell Sorter, Small, 2018, 14, 1801996 CrossRef PubMed.
- R. Zmijan, et al. High throughput imaging cytometer with acoustic focussing, RSC Adv., 2015, 5, 83206–83216 RSC.
- D. Ahmed, A. Ozcelik, N. Bojanala, N. Nama, A. Upadhyay, Y. Chen, W. Hanna-Rose and T. J. Huang, Rotational manipulation of single cells and organisms using acoustic waves, Nat. Commun., 2016, 7(1), 1 CrossRef PubMed.
- N. F. Läubli, et al. 3D Manipulation and Imaging of Plant Cells using Acoustically Activated Microbubbles, Small Methods, 2019, 3, 1800527 CrossRef.
- C. Chen, et al. Acoustofluidic rotational tweezing enables high-speed contactless morphological phenotyping of zebrafish larvae, Nat. Commun., 2021, 121(12), 1–13 CAS.
- L. Johansson, F. Nikolajeff, S. Johansson and S. Thorslund, On-Chip Fluorescence-Activated Cell Sorting by an Integrated Miniaturized Ultrasonic Transducer, Anal. Chem., 2009, 81, 5188–5196 CrossRef CAS PubMed.
- O. Jakobsson, C. Grenvall, M. Nordin, M. Evander and T. Laurell, Acoustic actuated fluorescence activated sorting of microparticles, Lab Chip, 2014, 14(11), 1943–1950 RSC.
- A. A. Nawaz, et al. Acoustofluidic Fluorescence Activated Cell Sorter, Anal. Chem., 2015, 87, 12051–12058 CrossRef CAS PubMed.
- X. Huang, et al. A Surface Acoustic Wave Pumped Lensless Microfluidic Imaging System for Flowing Cell Detection and Counting, IEEE Trans. Biomed. Circuits Syst., 2017, 11, 1478–1487 Search PubMed.
- W. L. Ung, K. Mutafopulos, P. Spink, R. W. Rambach, T. Franke and D. A. Weitz, Enhanced surface acoustic wave cell sorting by 3D microfluidic-chip design, Lab Chip, 2017, 17(23), 4059–4069 RSC.
- J. Rufo, F. Cai, J. Friend, M. Wiklund and T. J. Huang, Acoustofluidics for biomedical applications, Nat. Rev. Methods Primers, 2022, 21(2), 1–21 Search PubMed.
- S. Hameroff, et al. Transcranial ultrasound (TUS) effects on mental states: A pilot study, Brain Stimul., 2013, 6, 409–415 CrossRef PubMed.
- M. Samandari, K. Abrinia, M. Mokhtari-Dizaji and A. Tamayol, Ultrasound induced strain cytoskeleton rearrangement: An experimental and simulation study, J. Biomech., 2017, 60, 39–47 CrossRef PubMed.
- Y. Hu, W. Zhong, J. M. F. Wan and A. C. H. Yu, Ultrasound can Modulate Neuronal Development: Impact on Neurite Growth and Cell Body Morphology, Ultrasound Med. Biol., 2013, 39, 915–925 CrossRef PubMed.
- Z. Fan, et al. Acoustic tweezing cytometry for live-cell subcellular modulation of intracellular cytoskeleton contractility, Sci. Rep., 2013, 3, 2176 CrossRef PubMed.
- M. K. DeBari, et al. Therapeutic Ultrasound Triggered Silk Fibroin Scaffold Degradation, Adv. Healthcare Mater., 2021, 10, 2100048 CrossRef CAS PubMed.
- S. Lee, et al. Extracellular matrix remodeling in vivo for enhancing tumor-targeting efficiency of nanoparticle drug carriers using the pulsed high intensity focused ultrasound, J. Controlled Release, 2017, 263, 68–78 CrossRef CAS PubMed.
- T. Mainprize, et al. Blood-Brain Barrier Opening in Primary Brain Tumors with Non-invasive MR-Guided Focused Ultrasound: A Clinical Safety and Feasibility Study, Sci. Rep., 2019, 9, 321 CrossRef PubMed.
- G. Leinenga, C. Langton, R. Nisbet and J. Götz, Ultrasound treatment of neurological diseases-current and emerging applications, Nat. Rev. Neurol., 2016, 12, 161–174 CrossRef PubMed.
- D. V. Deshmukh, et al. Continuous Production of Acoustically Patterned Cells Within Hydrogel Fibers for Musculoskeletal Tissue Engineering, Adv. Funct. Mater., 2022, 32, 2113038 CrossRef CAS.
- L. Zou, C. McLeod and M. R. Bahmanyar, Wireless Interrogation of Implantable SAW Sensors, IEEE Trans. Biomed. Eng., 2020, 67, 1409–1417 Search PubMed.
- O. H. Murphy, et al. Continuous in vivo blood pressure measurements using a fully implantable wireless SAW sensor, Biomed. Microdevices, 2013, 15, 737–749 CrossRef PubMed.
- A. Ozcan, Mobile phones democratize and cultivate next-generation imaging, diagnostics and measurement tools, Lab Chip, 2014, 14, 3187–3194 RSC.
- L. Zhang, Z. Tian, H. Bachman, P. Zhang and T. J. Huang, A Cell-Phone-Based Acoustofluidic Platform for Quantitative Point-of-Care Testing, ACS Nano, 2020, 14, 3159–3169 CrossRef CAS PubMed.
- D. Baresch, J.-L. Thomas and R. Marchiano, Observation of a Single-Beam Gradient Force Acoustical Trap for Elastic Particles: Acoustical Tweezers, Phys. Rev. Lett., 2016, 116, 024301 CrossRef PubMed.
Footnote |
† Baresch et al.280 considered only the monopole and dipole terms in the diffracted field, then derived the analytical ARF based on the generalized Lorenz–Mie theory with the following form: where: where: 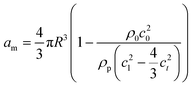 and and 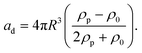 |
| This journal is © The Royal Society of Chemistry 2023 |