Open Access Article
Open Access ArticleWearable strain sensors: state-of-the-art and future applications
Ashish
Yadav
 *a,
Neha
Yadav
a,
Yongling
Wu
*a,
Neha
Yadav
a,
Yongling
Wu
 a,
Seeram
RamaKrishna
a,
Seeram
RamaKrishna
 b and
Zheng
Hongyu
b and
Zheng
Hongyu
 a
a
aCenter for Advanced Laser Manufacturing (CALM), Shandong University of Technology, Zibo, 255000, P. R. China. E-mail: ashish@sdut.edu.cn; zhenghongyu@sdut.edu.cn
bNanoscience and Nanotechnology Initiative, National University of Singapore, 10 Kent RidgeCrescent, 119260, Singapore
First published on 8th February 2023
Abstract
Wearable strain sensors have drawn massive awareness in various studies and industrial fields. The growing suitability and adaptability of such systems have increased the demand for developing instruments with improved aspects and packaging required to investigate bio-integrated gadgets, electronic skins, wearable wellness care techniques, and smooth robotics. Despite the outstanding performance, there are enormous challenges in the production of significant fundamental enrichments to the latest optical, mechanical, electrical, and sensing modalities and developing these for precise detection needs. Given all these demanding challenges, state-of-the-art wearable strain sensor technology is evolving towards a roadmap for exploring next-level generation innovations and breakthroughs in nanotechnology, and flexible strain sensors incorporate the virtues of stretchability, high detection power, and several other features. Skin-attachable wearable devices are developing as a tremendously attractive area due to their capable applications in the fields of artificial intelligence, human–machine systems, and healthcare tools. To understand and explore wearable strain sensors, this brief review describes aspects of flexible wearable strain sensing systems along with their challenges and prospects.
 Neha Yadav | Dr Neha Yadav obtained her bachelor*s degree in science and Master’s degree in Computer application. Now she is working on smart materials and Artificial Intelligence. |
1. Introduction
Wearable sensing technology1–5 has attracted significant attention and has swiftly transformed from a scientific idea to an extensive collection of traditional consumer and healthcare goods. This rapid evolution of wearable sensors may be credited to numerous features, e.g., comparatively low-cost prices and ergonomics implemented by advancements in the small sizes of electronic components, the vast demand and use of smartphones and associated gadgets, a rising human yearning for health-related awareness, and the unachieved medical requirements to continuously obtain correct and precise medical quality data from the patients.6–9 Despite the substantial preliminary success, a large amount of knowledge is still required to monitor exact information from the body. These requirements are still only partially met by the detecting modalities used in contemporary wearables,10–12 which are not specific. Besides, most wearable sensor products are still being manufactured based on outdated techniques, which have been accessible for decades. Even the most innovative wearables, such as continuous transdermal glucose monitoring equipment, leverage almost 30 years of advances in enzyme electrodes using simple and ultra-cheap finger-prick test strips of glucose.13 Transcutaneous blood glucose monitoring may be the only prevalent wearable sensor that measures the persistent observation of underlying explicit disease or infection.13–16Technology has been playing larger roles in our daily lives, which helps to meet some of the demands of modern living while also adding to others. To fulfill these needs, there are numerous opportunities in the textile business to increase the usefulness and performance of textiles. The emergence of intelligent nanotextiles17 will transform how we dress, how our houses are furnished, and how industrial materials are utilised. Expectations for textile performance have increased due to the impending revolution, and there is a high demand for “smart materials” that are more aware of their surroundings. A wide variety of uses for technical and functional textiles can be found in fields ranging from the military and security to individualized healthcare, cleanliness, and entertainment.18,19
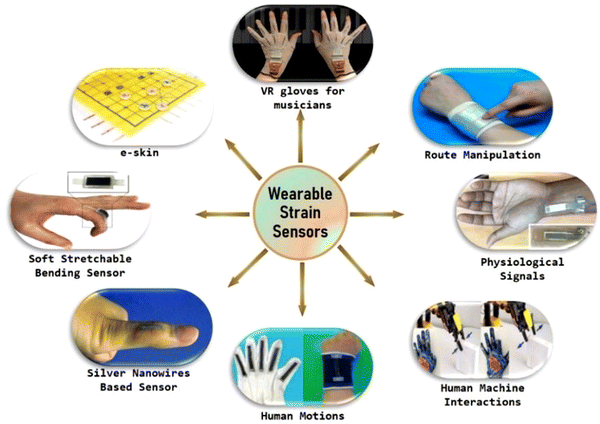
Doctors can diagnose the disease and monitor the wellness of the patients by using numerous outdated available diagnostic tools for nearly every analyte. Nevertheless, these tests require the drawing of blood from a patient's body because of their non-wearability and outdated bench-top assessment procedures.20–25 The main question of whether wearable sensor technology can evolve for the precise and more specific physiological phenomena, such as monitoring the health of a baby inside the mother's womb by the measurement of simple mechanical movements of the fetus, or the differentiation of a fatal seizure from just higher physical exertion,26–29 or to warn a sportsperson or a worker about their dangerous dehydration because of over-exercising or overwork by continuous measurement of their health analysis data, still needs answers.9,20,30 Usually, wearable electronic gadgets are fabricated using wearable non-toxic materials, smart sensors, modules and linkages, actuators, power sources, control and processing units, a user interface, software, and sophisticated algorithms for data collecting and decision-making, which are all components of wireless communication systems. As a result, the system processes data from factors including body temperature, blood pressure, the patient's level of exertion, and the concentrations of gases, different ions, and proteins in circulation to monitor the patient's physiological information. The smart sensors will be utilized in wearable electronic devices, which are surrounded by conducting electrodes. However, these constituent materials must be lightweight, highly flexible, ultrathin, stretchable, and with low moduli. Various state-of-the-art applications of wearable strain sensors are illustrated in Fig. 1.
In this brief review, our focus will be on wearable technologies that can extract data from the interior of the body, but without the need to implant a sensor within the body. Typical environmental sensors and limb-movement accelerometers are not in the scope of this review. This report focuses on the new borderline of wearable techniques and terms traditionally utilized by analytical chemists. In brief, this report describes the categorization of wearable strain sensors,31–33 the basic physics principles of the body-to-signal transduction method, the state of the art, unsettled challenges, and finally, comments on the prospects. The authors believe that the outcomes of this mini-review will serve as a basic text for beginners in the field of wearable sensors, and it will also be helpful to experienced researchers in wearables by expanding the knowledge of challenging sensing modalities and the vital obstacles that are being faced by wearable sensors and devices.
2. Historical viewpoint
There is a long history of wearable strain sensors. Some are briefly described herein. Wearables were first realized in the Apollo Space Program during the early 1960s, when scientists were well aware of the physical extremes of humans on this mission. They recognized the need for some specific non-invasive sensors30 to continuously monitor the health status of astronauts, as well as transmit real-time data back to earth. Wearable sensors fulfilled this job in a very efficient manner. These sensors included an electrocardiogram (Fig. 2(a)), and a heated thermistor to sense breathing by measuring the cooling due to inhaled and exhaled air movement from the mouth. Within very little time, pulse oximetry proved itself as an entirely safe and standard measurement technique during general anesthesia (Fig. 2(b)). There was also a biosensor probe for the precise measurement of glucose in the body (Fig. 2(c)).34 Wireless electrocardiogram (EKG) (Fig. 2(d)) heart rate monitors started as standard equipment in 1977. Wearable monitors first gained popularity in the early 1980s and started being used by the common man in the late 1980s.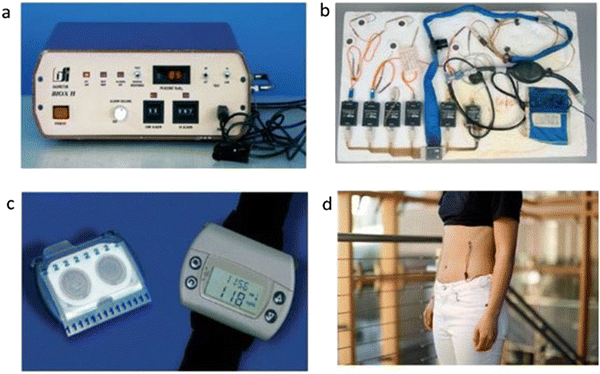 | ||
| Fig. 2 Some historical glimpses of wearable sensors: (a) pulse oximetry for wearing on the fingertip, (b) wearable sensors used during the Apollo Space Program, (c) an early wearable digital glucose sensor for diabetic patients, and (d) ‘Sport Tester PE2000’ heart rate monitor by Polar. (Adapted and reprinted with permissions,35 Copyright 2018, Royal Society of Chemistry). | ||
Leland Clark and Ann Lyons from the Cincinnati Children's hospital developed the first precise glucose enzyme sensing electrode in 1962. However, it took a long time to realize the non-invasive wearable sensor. Electro-osmotic flow, also known as reverse iontophoresis, involves generating interstitial fluid through paracellular pathways so that the negatively charged plasma membranes can promote a moving electro-osmotic sheath of Na+ ions. Cygnus Inc. was granted FDA approval for its GlucoWatch, which was a significant novel noninvasive approach to diabetes monitoring since diabetes can be deadly if glucose monitoring is not performed accurately. However, to date, non-invasive wearable chemical sensors are still unavailable as popular products36 (the broadly used transcutaneous glucose monitors are invasive and are not a part of this review).
Commercially available wearable sensors like watches by Fitbit and Apple and medical patches by Medtronic's SEEQ cardiac monitoring system are examples of the latest wearable strain sensors, which indicate the advancement of technology; the current wearable sensors consist of simple electrical and optical measurement components on the skin.
3. Wearable sensing mechanism
Inertial motion and plantar force sensors can empirically monitor the movement of the human body. The miniaturized size of these sensors makes them wearable. The measurement of linear acceleration, angular velocity, and the direction of human body movements allow inertial motion sensors to distinguish the postural sway of humans. Also, the measurement of the coefficient of performance (COP) path at the plantar surface of the foot and stance/swing time during walking enables plantar force sensors to perceive the postural sway and gait variability. Flexible and wearable electronics that can be implanted into clothing have recently drawn increasing interest.37,38 In such a flexible system, information on mechanical deformation has always been an important research topic.39–42 The gauge factor of a strain sensor is low to facilitate a higher strain sensing range. Further, the mechanical properties of fundamentally fragile materials are incompatible with those of textiles for integrated systems.Another approach is to fabricate sensing devices from soft and flexible materials. Composites of polymers and conductive fillers have been successfully demonstrated for this purpose. Such materials can sense mechanical strain by the electron tunneling effect between adjacent particles or by sensing the resistance change due to the opening and closing of micro-cracks by mechanical deformation.43–50 These schemes have shown more extensive strain sensing ranges with similar gauge factors compared to rigid materials. Furthermore, they can be integrated into textiles with low manufacturing costs. The conductive composites are usually obtained by mixing an insulating polymer with carbon black powders or carbon nanotubes. In general, these flexible strain sensors are fabricated based on thin-film technologies with limited placements and configurations.
4. Classification of wearable sensors
In this section of the review article, we discuss optical, mechanical, electrical, and chemical sensors. The primary body-to-signal transduction method for each sensing modality presented is also explained in brief, and the real-life devices and their practical illustrations are discussed. Newly developed wearable physical sensors have enough stretchability and flexibility to resist the deformations caused by human activities.51–53 For example, portable sensors measure physical factors in a patient's body condition, including the temperature of the body and skin, wrist, blood pressure, and skin fatigue. These aspects critically indicate the complete health of a person. With this measuring capability, physical sensors can measure these physical parameters and can electronically monitor the human–machine interface, skin, or overall patient health.54–574.1. Pressure sensors
Pressure ranges vary differently from one part or target of the body to another to measure the parameters. For wearable pressure sensors, pressure ranges can be divided into three different categories, namely a range of low pressure (100 kPa),58 which includes a person's weight or high-altitude atmospheric pressure.59 Diseases related to the eyes, heart, damaged vocal cords, etc., can be monitored by sensing the changes in some different types of pressure. The extensive studies on portable pressure sensors have opened a pathway for their utilization in personal health-related wellness and medical diagnostic instruments. Many sensing mechanisms, including piezoresistivity, piezoelectricity, and capacitance, can be used to convert physical stimuli into measurable electric signals. The basic principle of piezoelectric pressure sensors is founded on the piezoelectric effect, which is the change in the electrical charge that occurs in some specific solid materials as a result of the pressure applied on them. The following equations can indicate pressure-induced electricity:| Di = ε0εijσEj + dijσj and δI = SijEσj + dIjEj | (1) |
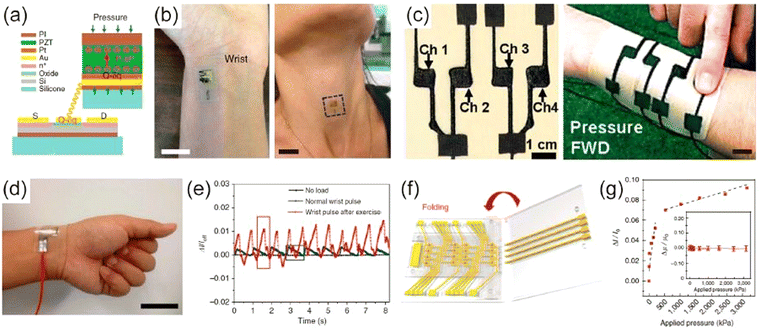 | ||
| Fig. 3 Wearable pressure sensing gadgets. (a) A schematic diagram of the cross-section of the pressure sensor and its contacts in an accompanying transistor. (b) The pressure sensor positioned on a wrist and neck to measure instantaneous variations in blood pressure70 (reprinted with permission 2014, Copyright, Nature Publishing Group (NPG)). (c) Printed pressure sensor mounted on a commercially available elastomeric patch. The arrays of the sensor consist of four channels of pressure sensors71 (reprinted with permission 2014, Copyright, John Wiley and Sons). (d) The skin-mounted sensor directly above the artery of the wrist (scale bar 3 cm). (e) The heartbeat is measured by sensing the physical force under healthy and exercising conditions72 (reprinted with permission 2014, Copyright, Nature Publishing Group (NPG)). (f) A picture representation of pressure-sensitive graphene FETs. (g) A plot of changes in normalized drain current versus pressure applied on the device73 (reprinted with permission 2014, Copyright, Nature Publishing Group (NPG)); (figure reproduced with permission,64 Copyright 2017, from MDPI, Polymer). | ||
The human skin can successfully adopt this wearable porous PSR pressure sensor for several applications, e.g., man–machine interface, sanitary healthcare observations, and remotely controlling robots (Fig. 3(c)). Researchers have not only developed pressure sensors based on the microstructure and porous structures, but they have also successfully developed piezoresistive pressure sensors based on several other materials.66–68 Gong et al. successfully fabricated a highly sensitive flexible pressure sensor by inserting gold nanowires (AuNWs) between two PDMS films.69 The Au-NW-incorporated flexible pressure sensors delivered real-time blood pressure monitoring with high precision and sensitivity, as shown in Fig. 3(d) and (e).
As is evident from the name, capacitors make up capacitive pressure sensors. Fundamentally, the magnitude of capacitance varies with the thickness of the layer of dielectric material. The capacitance C may be expressed by the relation C = ε0εr(A/d), where A is the overlapping area between the two plates, d is the thickness of the dielectric material, ε0 is the electrical permittivity of free space, and εr is the relative static electrical permittivity of the dielectric. As an inference, the capacitance increases while externally applied pressure reduces the thickness. To alter the pressure-dependent thickness, materials with small modulus values, such as polyurethane (PU), PDMS, and eco-flex are generally used. Sun et al. fabricated a pressure sensor comprised of a stretchable and transparent dielectric material crammed in between two flexible ion conductors.31,68 Owing to its transparency, stretchability, and better biocompatibility with the human body, this sensor can be applied in implantable or wearable electronic gadgets. Park et al. also developed a flexible and highly sensitive capacitive pressure sensor by raising an air gap between a single-walled carbon nanotube (SWCNT) film and a porous PDMS film.74 Nevertheless, capacitive pressure sensors have certain restrictions, such as slow response time and low sensitivity due to the small modulus of the elastomer used in the fabrication. The use of air as a dielectric layer improves sensitivity. To resolve this issue, Zang et al. successfully demonstrated a flexible pressure sensor with remarkably high sensitivity in the low-pressure region, which was constructed on a suspended FET gate electrode.75 It can be worn on the wrist and can monitor pulse waves. The pressure sensors described above are sensitive and can detect precise pressure intervals. However, a pressure sensor capable of detecting a wide range of pressures for diverse applications is always needed. Very recently, Shin et al. also demonstrated an alternative approach to fabricating a pressure sensor array64 filled with an air dielectric layer using folding panels (Fig. 3(f)). The sensor can be used successfully over a vast interval in tactile sensing, as shown in Fig. 3(g). All the results above have established that pressure sensing devices are arising as promising candidates for monitoring human movements, individual healthcare, and wellness.
4.2. Strain sensors
While a person moves, small and large deformations occur. Wearable strain sensors must be able to precisely detect and observe the movements of the targeted organs of the human body continuously, whether this movement is generated from the faint vibration of the vocal cords or the activities of the joints; therefore, there is a need for flexible strain sensors. Because of the flexibility requirements, the strain sensors must consist of a flexible and stretchable material, and this differentiates them from conventional silicon-based strain sensors.76 The constituent material of wearable strain sensors must be mechanically reliable, stretchable, sensitive, should have the property of hysteresis, and must produce a linear form of the output signal.77 The classification of wearable strain sensors is according to the working mechanism used for sensing. Piezoresistive strain sensors notice the deformations within the targeted body organ primarily by variations in the resistance. The following well-known equation may describe the change in the resistance of the materials used in piezoresistive strain sensors: R = ρ(l/A), where ρ is resistivity, l is the length, and A is the area of the cross-section of the constituent material used in the piezoresistive sensor. A and I are both geometrical factors, and the piezoresistivity of the used materials induces changes in ρ. When a metal or semiconductor is deformed, its interatomic spacing changes and as a result, its bandgap changes. This change is often expressed as the gauge factor, ΔR/R of strain sensors. Consequently, because of substantial changes in resistivity, semiconductors have a high gauge factor. Carbon black, zinc oxide (ZnO), and CNT are some examples of semiconducting materials for use as piezoresistive strain sensors.63,78–81 Roh et al. embedded single-walled carbon nanotubes in a conductive elastomer polyurethane-poly(3,4-ethylenedioxythiophene) polystyrene sulfonate (PU-PEDOT:PSS) to fabricate the strain sensor82 as shown in Fig. 4(a). This sensor exhibited a gauge factor of 62, stretchability of 100% strain, and 62% transmittance63,82 in the visible range (Fig. 4(b)). Furthermore, deformations may lead to changes in the resistance (Fig. 4(c)) as a result of disconnections in the network geometry or cracks by the applied force or pressure. CNTs, graphene flakes/films, and metal nanowires (mNWs) are some examples (Fig. 4(d)) of materials possessing a conductive network. While these materials are stretched or bent under external force, there may be the sliding of atoms or molecules within the network, which produces a measurable change in resistance.69,83–86 Frutiger et al. demonstrated a capacitive strain sensor87 analogous to fiber using silicon elastomer and conductive ionic fluid (Fig. 4(g) and (h)). The strain can also be detected using the property of the piezoelectric effect of some explicit materials.88 Various oxides have been used to develop a piezoelectric strain sensor.89–91 Recently, a flexible piezoelectric sensor based on a ZnSnO3 nano/microwire was fabricated successfully by Wu et al.; interestingly, the gauge factor of the sensor was 3740 at 0.35% strain.92 There is no requirement for an external power source for piezoelectric strain sensors as they are mostly self-powered. However, the main challenges are still stretchability and flexibility because of the unsuitable mechanical properties of piezoelectric substances.91,93,94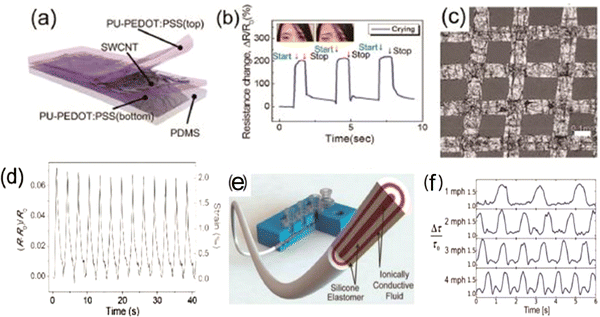 | ||
| Fig. 4 Some examples of wearable strain sensors: (a) schematic cross-sectional diagram of the strain sensor comprised of 3-layers stacked in a nanohybrid structure. (b) Time-dependent resistance variations ΔR/R0 of the sensors82 (reprinted with permission, American Chemical Society, Copyright 2015). (c) Optical micrograph of a graphene woven fabric's relative change of resistance. (d) Relative resistance variation between 0% and 0.2% strain96 (reprinted with permission, John Wiley & Sons, Copyright 2014). (e) Representation of the multicore–shell printing procedure for a fiber-type capacitive strain sensor. (f) Normalized decay-time response of the sensor (reprinted with permission John Wiley & Sons,87 Copyright 2015). | ||
4.3. Piezoresistive sensors
Several transduction mechanisms with capacitance, piezoresistivity, piezoelectricity, etc., are available for converting tactile stimuli into electrical signals.95,97–100 Every transduction method has its characteristic features (Fig. 5), which are presented in the paragraph below. Accordingly, R is the resistance of the conductive material, A is the conductor's cross-sectional area, L is its length, and ρ is its resistivity. Wearable electronics frequently exploit the piezoresistive effect. The variation of resistance with the applied pressure and its transduction into electrical signals is the foundation principle of piezoresistive sensors (Fig. 5(a)). These sensors have been studied intensively due to the fact of their remarkably intense pixel density, comparatively simple device structure, and protected read-out mechanism algorithms.101 The suitability of piezoresistive sensors over vast pressure ranges makes the precise measurement of large strains possible. The variation of contact resistance (Rc) between two materials is governed by the applied forces (F) and is the primary source of the change in the electrical signal.102 The resistance of a pressure sensor changes as a consequence of the pressure applied to the device. The power law, Rc ∼ F−1/2, confirms the remarkably high sensitivity of piezoresistive sensors in low-pressure regimes and their relatively wide operating ranges. Additionally, these devices have a fast response speed.103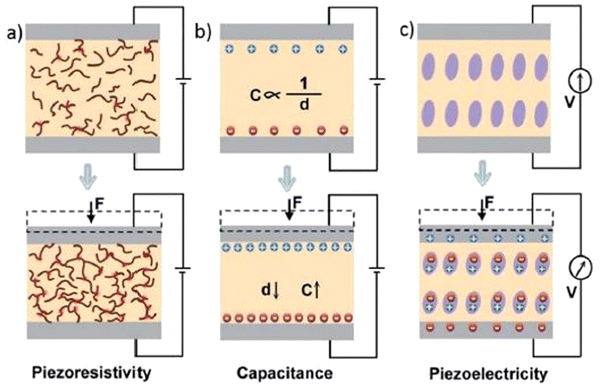 | ||
| Fig. 5 Schematic illustrations109 of transduction methods: (a) piezoresistivity, (b) capacitance, and (c) piezoelectricity. (Adapted and reprinted with permission, Copyright 2015, Royal Society of Chemistry). | ||
The term capacitance characterizes the ability of a body to store electrical charge. An applied pressure produces a deflection in the plate, followed by a change in the capacitance of the used parallel-plate capacitor (Fig. 5(b)). These changes don’t need to be linear and they may be of the order of several picofarads (pF). By changing the pressure-induced capacitance, the frequency of an oscillator can be controlled, or the coupling of an alternating current (AC) signal through a network can be varied. These sensors tend to exhibit low sensitivity due to the relatively small changes in the parallel plate capacitance.
The OFETs can be categorized among the most meticulously studied electronic components. A typical OFET structure comprises a semiconductor layer, a gate dielectric layer, source–drain electrodes, and a gate electrode. In a typical device structure, the gate dielectric layer is positioned between the gate electrode and the semiconductor layer. As a gate voltage is applied, the charge carriers gather at the interface of the dielectric/organic layer and establish a conductive channel and finally, current drifts from the source to drain electrodes while applying a source–drain bias.104–106
Piezoelectricity is also one of the frequently followed transduction methods to fabricate pressure sensors. Piezoelectricity denotes the generation of electrical charges in some specific solid materials as a result of the applied mechanical stress.107 It is the presence of electric dipole moments in solids that contributes to the generation of the piezoelectric effect (Fig. 5(c)). Piezoelectric sensors can be used in a wide range of applications for the detection of dynamic pressures such as sound vibrations, owing to their remarkably high sensitivity and ultrafast response time. Piezoelectric sensors are promising for the development of ultra-low-power-consuming or even self-powered pressure sensors.14,108
4.4. Wearable humidity sensors based on porous graphene:
One of the most important physiological markers that may be utilized to track a person's health and activity levels is respiration. The porous graphene network is flexible, light, and extremely conductive, thus making it a good candidate for a humidity sensor for respiration monitoring. Graphene oxide (GO), poly(3,4-ethylenedioxythiophene)-poly(styrene sulfonate) (PEDOT:PSS), and Ag colloids (AC) were employed to modify the porous graphene to improve the sensing performance. At various relative humidities (RH), the characteristics of porous-based graphene networks have been studied. The porous graphene sensors are capable of monitoring various breathing patterns, including mouth and nasal respiration, and normal and deep respiration. Additionally, the sensors record the signal changes before and after water consumption, demonstrating the capacity to monitor health processes. Furthermore, the humidity sensor showed the ability to detect physiological activities including skin moisture, and speaking and whistling rhythms, which could be promising for clinical respiration monitoring.109–1115. Promising state-of-the-art strain sensing applications
Due to the excellent flexibility and fitting exhibited by flexible stress sensing materials, they have great potential in many applications, especially in the monitoring of stress and stress distribution on surfaces with three-dimensional irregular curved structures. The chemical fabrication process for flexible (humidity) stress sensors based on porous graphene network has been shown, step-by-step in Fig. 6(a)–(g). The process is initiated with a nickel foam template, then a graphene layer is prepared on the foam using CVD, followed by acidic chemical etching to obtain a porous network, chemical modification of this porous network, and finally transferring the as-obtained porous network to a flexible PET substrate. Flexible stress-sensing materials are commonly used in applications with different types of stress regions.82,112–115 The applications include ultra-low stress (<1 Pa) signal monitoring such as sound capture; weak stress (<1 kPa) signal acquisition, such as ultra-sensitive electronic skin, and touchpads; low stress (<10 kPa) signal detection, including motion monitoring in daily activities; and medium pressure (<100 kPa) signal acquisition, which includes plantar pressure collection.116–119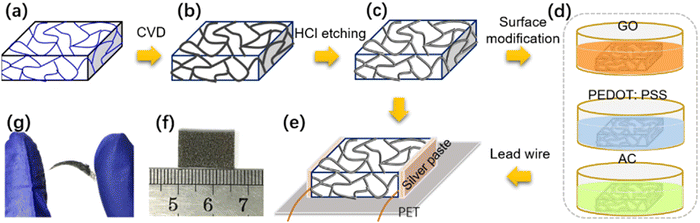 | ||
| Fig. 6 Detailed step-by-step diagram of the fabrication process for devices. (A) A template made of nickel foam. (b) Using the CVD process, a graphene layer was created on the nickel foam. (c) A porous graphene network was created by etching the nickel skeleton with a solution of hydrochloric acid. (d) To obtain the surface-modified samples, the porous graphene networks were submerged in GO, PEDOT: PSS, and AC solutions. (e) Copper wires were led out after the modified samples were put into a flexible PET substrate. (f) The graphene/nickel foam image. (g) The image of the porous graphene network sensor demonstrates its ability to bend and stretch. Adapted and reprinted with permission,113 Copyright 2018, Elsevier. | ||
The current applications of wearable, flexible stress sensors are mainly concentrated in the fields of human physiological signal monitoring, motion and gesture recognition, flexible touch switches, electronic skin, and robots, which are introduced briefly below.
5.1. Human physiological signal monitoring
The flexible wearable stress sensors can realize real-time and long-term evaluation and feedback of individual physiological data by avoiding the burden of heavy instruments and the storage of complicated cables. They can continuously perceive, acquire and transmit physiological signals such as the pulse and blood pressure of the human body without being affected by daily activities and can realize better personal medical treatment.76,77,120–123 Currently, researchers have designed and developed a variety of flexible sweat sensors (Fig. 7) that monitor the pulse, blood pressure, and respiratory rate. Bao et al. designed a flexible stress sensor (Fig. 8(a)) with a micro-hair structure124 that fits well on the surface of the human body and uses the micro-hair structure to adhere to the skin to monitor the depth. Wang et al. integrated the sensor with the breathing mask125 to form a device that can monitor the respiratory rate of the human body and can accurately observe the breathing process in different states of the human body in real-time (Fig. 8(b)). To observe the pulsation of blood vessels, Lee et al. prepared a bending-insensitive stress sensor126–130 based on graphene and carbon tube composites, which can continuously monitor the pressure on the artificial heart and blood vessel wall in real-time without being affected by activities such as human limb bending (Fig. 8(c)).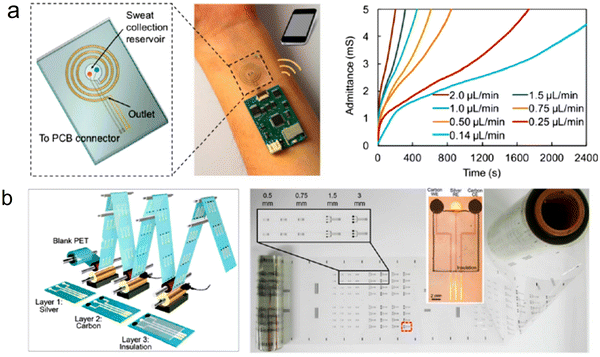 | ||
| Fig. 7 (a) Microfluidics-based sensing system for sweat sampling, sensing, and sweat rate analysis. Reprinted with permission, Copyright,109 2018 American Chemical Society. (b) Roll-to-roll gravure printing enabled mass production of high-performance flexible chemical sensors at low cost. Reproduced from ref.110 Copyright 2018 American Chemical Society. | ||
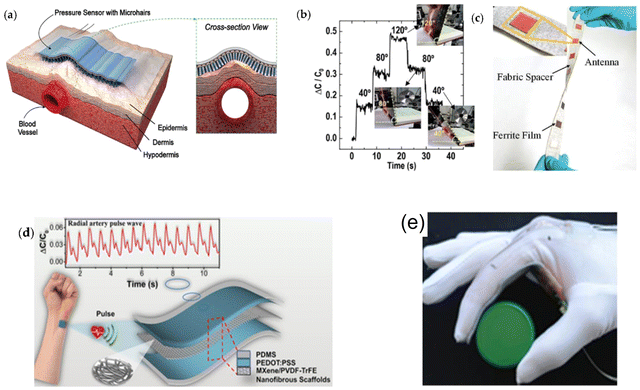 | ||
| Fig. 8 The applications of flexible stress sensors on (a) a pulse-detectable pressure sensor with a microhair structure (reprinted with permission from ref. 126. Copyright 2014 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim). (b) Capacitance response plot obtained from the wrinkled pressure sensor (reprinted with permission from ref. 127. Copyright 2017 Royal Society of Chemistry). (c) Textile-based flexible wireless pressure sensor (reprinted with permission from ref. 128. Copyright 2019 John Wiley and Sons). (d) Wearable capacitive pressure sensor based on MXene for reliable human physiological signals (reprinted with permission from ref. 129. Copyright 2020 American Chemical Society). (e) Textile glove with capacitive pressure sensors (reprinted with permission from ref. 130. Copyright 2015 John Wiley and Sons). | ||
5.2. Motion and gesture recognition
Statistical data shows that when humans communicate, the amount of information transmitted by language is about 7%, and expressions and physical movements express about 50% of the messages. Therefore, facial expression recognition and gesture recognition have gradually become research hotspots.131 A flexible and lightweight stress–strain sensor matrix can be placed on the face or different joints to analyze the expression and posture of the human body.132–134 It is safe to wear without any harmful effects, and the cost-effective mass production of such devices is also possible. Su et al. laced multiple flexible stress sensors135 on the forehead, eyebrows, nose, chin, and corners of the mouth for the multi-recognition of body expressions. Multi-channel linkage was used for smiles, laughter, surprise, sadness, fear, frustration, anger, and relaxation to identify the eight primary facial expressions. The same stress–strain sensor can also be integrated directly into the joints and muscles to detect movement of the joints or muscles136 of the human body. The realization of the detection function of the joint activity of the human body can be applied in the medical field, such as in physical therapy rehabilitation, and prostheses.Self-powered body motion skin sensors fabricated using triboelectric nanogenerator137 (TENGs) can be categorized into several groups based on the triboelectric materials138 used. The first type is built on materials that can be stretched, such as rubber and silicone elastomers. A stretchable rubber-based (SR-based) TENG was reported by Yin et al.,139 which makes use of the triboelectricity between a stretchable rubber and an aluminum (Al) film, as shown in Fig. 9(a)–(c). The SR-based TENG operates on a novel theoretical foundation. It causes in-plane charge separation between rubber and Al instead of rubber shifting in position, which results in the potential difference between the Al electrode and the ground. Fig. 9(d)–(f) illustrates the soft, stretchable triboelectric band described by the author, which is made of a rubber tube filled with physiological saline.
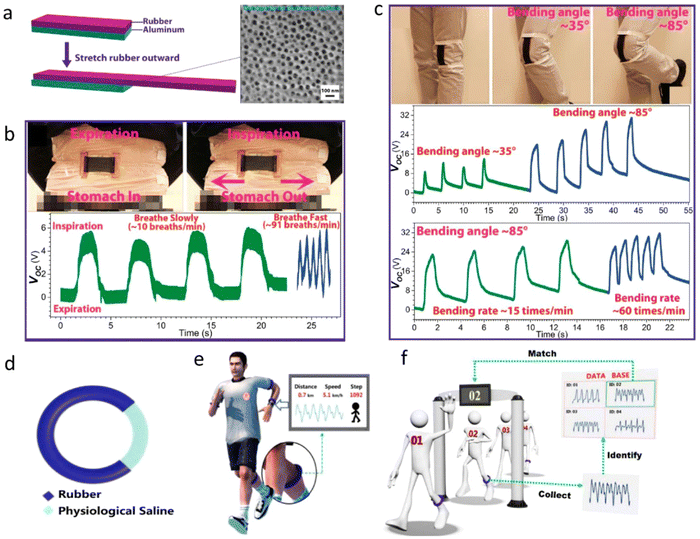 | ||
| Fig. 9 (a) Device structure of the stretchable rubber based on a triboelectric nanogenerator (TENG). (b) Images and electrical outputs of the TENG on the abdomen during expiration and inspiration. (c) Optical images of the device on the knee at different bending angles and voltage responses when bending the knee at different angles and different rates.139 (Reprinted with permission Copyright 2015, John Wiley & Sons). (d) The typical device structure of the TENG band. (e) Schematic illustration of the skin TENG band for body motion detection.140 (Reprinted with permission Copyright 2018, Elsevier). (f) Schematic illustration of the skin TENG band for identification. (Reprinted with permission Copyright 2018, Elsevier).140 | ||
5.3. Electronic skin and a flexible wearable touch switch
Electronic skin140–142 is a flexible sensor device that mimics the human skin's perception and collection of external stimulus signals. Its main feature is the ability to monitor the stress distribution at the pixel points in the 3D surface in real time.69,143–145 Electronic skin was prepared by Liu et al.146 using an elastic carbon sponge obtained by the carbonization of melamine, which utilizes inter-digitated electrodes to increase sensitivity. The ability of the material to contact the electrode147 results in stress distribution and the magnitude is determined by scanning the rate of change of resistance at each pixel in the sensor dot matrix. The case shown is a schematic diagram for detecting the areas and sizes of different chess pieces.Similar to electronic skin, after integration, the trajectory of the finger or other object moving on the sensor can be observed, and the stress between them can also be recorded by the sensor array so that it can be applied to the flexible touch-field,148–150 (Fig. 10(a)). There are integrated sensor components with different shapes on the wristband, and wireless manipulation of the wrist band for household appliances.151 They have been proven to be useful for Washable Electronic Textiles152 as Self-Powered Touch/Gesture Tribo-Sensors for Intelligent Human–Machine Interactions (Fig. 10(b)). Flexible touch panels may also achieve the remote control of the turning on and off of household appliances such as lamps, fans, and microwave ovens via signal amplifiers (Fig. 10(c)), relays, wireless transmission (Fig. 10) and receiving devices.153
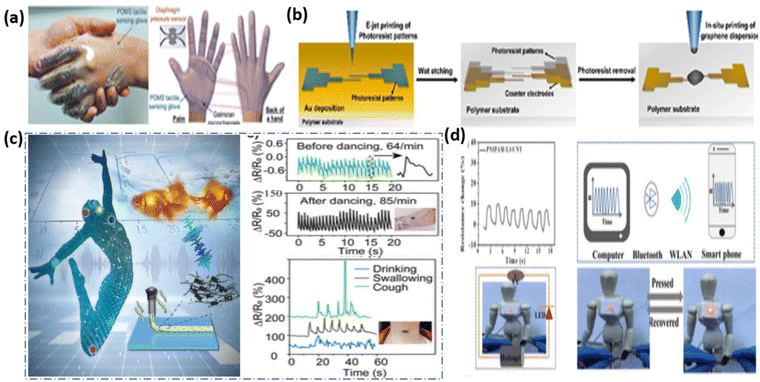 | ||
| Fig. 10 (a) Photograph of a hand-shake greeting after wearing the PDMS tactile sensing gloves (left); representative diagram of the PDMS tactile sensing glove154 (reprinted with permission Copyright 2017, John Wiley & Sons). (b) Representative diagram of the process of fabricating fully printed graphene photodetector gadgets using the mask-free direct-writing method155 (reprinted with permission Copyright 2021, Elsevier). (c) Flexible composite pressure sensors for underwater monitoring (left); pulse wave curves and response signals under different conditions.156 (Reprinted with permission Copyright 2022, American Chemical Society). (d) Photographs of the increased light intensity.157 (Reprinted with permission Copyright 2019, American Chemical Society). | ||
5.4. Robots and intelligent interaction
The stress sensor can accurately capture the fine movements of the human body or humanoid robots and can thus be applied to the field of robots and intelligent interactions.154–160 At present, the stress sensors used in robotics or intelligent interaction fields are mainly multi-channel work. Similar to expression recognition, the multi-channel sensors are used to monitor the stress and strain of different parts, and the action or posture of the object to be tested is judged in real-time.161–163Fig. 11(a)–(c) are the applications of robot motion recognition, somatosensory games, and wrist movement digitization.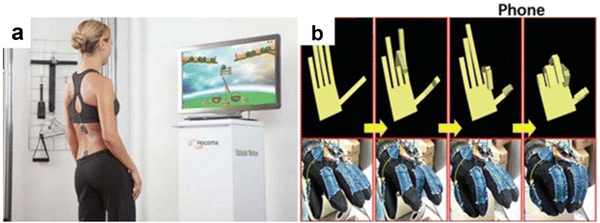 | ||
| Fig. 11 (a) Motion-sensing game with a wearable stress sensor system162 (reprinted with permission Copyright 2012, BioMed Central). (b) The capture and digitalization of hand motion with a smart glove integrated with sensors.163 (Reprinted with permission Copyright 2014, American Chemical Society). | ||
6. Challenges in wearable strain sensing technology
There has been profound progress in the clever use of flexible and stretchable wearable sensors in this field throughout the years. The latest achievements and continuous advancement of the technology have proved the sensing conductors to be promising candidates in numerous profound applications to detect the motion of the human body by utilizing the simple principle of the variation of resistance in terms of a measurable electric current under ambient distortions, and thus an all-new sector of consumer health care has emerged. Numerous sensing devices concerning health-centered applications, which were just a theoretical possibility, have turned into reality. However, lots of significant challenges still exist on the long path towards precision and more diverse applications. For the further development of more innovative and versatile devices, a robust understanding of the transduction mechanism is a must because it is the most critical part of enhancing the overall detection precision and further enhancement of the gain factor of the transduced signal obtained as a result of deformations. Recently, the resistance and hence the current variation in strain sensors were proved to be produced due to numerous mechanisms, including piezoresistivity, the connection–disconnection mechanism, geometry effect, and tunneling effect. The scientific community needs to focus on further sharpening the knowledge and the understanding of robust mechanical properties to develop highly intelligent sensors for monitoring the specifically targeted organs of the human body; failure to do this could result in worse situations for individuals.For the precise measurement of the data, a secure data read-write mechanism in severe circumstances is also of vital importance for real-world applications, along with a stable and diverse signal output.
7. Conclusions and outlooks
In this review, we have presented a compressive study on the recent progress of wearable strain sensors and advanced applications. The latest research outcomes of several papers in the field were considered to provide a glimpse of the state-of-the-art technology and to understand the physical phenomena behind the strain-responsive mechanisms of strain sensors at the microscale. Lots of conventional approaches, including several new ones such as disconnection between overlapped nanomaterials, crack proliferation in thin films, and the tunneling effect, have been used for the development of highly efficient flexible strain sensors.After speedy progress on wearable strain sensors in the previous few decades, incredible advancements have been achieved, thus making the use of pressure sensors in real-life electronic applications possible. The thriving development of organic electronics inspired the unprecedented advancement of flexible pressure sensors with sensitive response proficiencies. Ultrasensitive, stretchable, and flexible pressure sensors are currently emerging as ideal candidates for wearable wellness monitoring applications. These accomplishments and potential applications have enabled flexible pressure sensors to be a crucial component of upcoming electronics.
We strongly believe that stretchable, flexible strain sensors can serve as a vital component for several applications such as biomedicine, robotics, and entertainment sectors. However, high dynamic response with low overshoot and decay, a certain degree of hysteresis, robust and flexible packaging, and conformal attachment to the targeted organs of the human body still need to be achieved.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
Each contributor thanks the appropriate government for providing the necessary resources and facilities. We gratefully accept financial assistance from the Chinese National Natural Science Foundation.References
- S. F. Clarke and J. R. Foster, Br. J. Biomed. Sci., 2012, 69, 83–93 CrossRef CAS PubMed
.
- C. E. Sims and N. L. Allbritton, Lab Chip, 2007, 7, 423–440 RSC
.
- T. Matsunaga, M. Hosokawa, A. Arakaki, T. Taguchi, T. Mori, T. Tanaka and H. Takeyama, Anal. Chem., 2008, 80, 5139–5145 CrossRef CAS PubMed
.
- K. Klepárník and M. Horký, Electrophoresis, 2003, 24, 3778–3783 CrossRef PubMed
.
- E. Proksch, J. M. Brandner and J.-M. Jensen, Exp. Dermatol., 2008, 17, 1063–1072 CrossRef PubMed
.
- X. Liu, C. Tang, X. Du, S. Xiong, S. Xi, Y. Liu, X. Shen, Q. Zheng, Z. Wang, Y. Wu, A. Horner and J.-K. Kim, Mater. Horiz., 2017, 4, 477–486 RSC
.
- Y. Wang, A. X. Wang, Y. Wang, M. K. Chyu and Q.-M. Wang, Sens. Actuators, A, 2013, 199, 265–271 CrossRef CAS
.
- M. Abkarian, M. Faivre and H. A. Stone, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 538–542 CrossRef CAS PubMed
.
- M. L. Yarmush, A. Golberg, G. Serša, T. Kotnik and D. Miklavčič, Annu. Rev. Biomed. Eng., 2014, 16, 295–320 CrossRef CAS PubMed
.
- H.-B. Yao, J. Ge, C.-F. Wang, X. Wang, W. Hu, Z.-J. Zheng, Y. Ni and S.-H. Yu, Adv. Mater., 2013, 25, 6692–6698 CrossRef CAS PubMed
.
- L. Sheng, Y. Liang, L. Jiang, Q. Wang, T. Wei, L. Qu and Z. Fan, Adv. Funct. Mater., 2015, 25, 6545–6551 CrossRef CAS
.
- Y. Wang, C. Xie, D. Liu, X. Huang, J. Huo and S. Wang, ACS Appl. Mater. Interfaces, 2016, 8, 18652–18657 CrossRef CAS PubMed
.
- A. El-Laboudi, N. S. Oliver, A. Cass and D. Johnston, Diabetes Technol. Ther., 2013, 15, 101–115 CrossRef CAS PubMed
.
- Y. Zang, F. Zhang, C. Di and D. Zhu, Mater. Horiz., 2015, 2, 140–156 RSC
.
- S. Mitragotri, Adv. Drug Delivery Rev., 2013, 65, 100–103 CrossRef CAS PubMed
.
- P. Garstecki, I. Gitlin, W. DiLuzio, G. M. Whitesides, E. Kumacheva and H. A. Stone, Appl. Phys. Lett., 2004, 85, 2649–2651 CrossRef CAS
.
- S. Coyle, Y. Wu, K.-T. Lau, D. De Rossi, G. Wallace and D. Diamond, MRS Bull., 2007, 32, 434–442 CrossRef CAS
.
- W. Zhong, H. Jiang, L. Yang, A. Yadav, X. Ding, Y. Chen, M. Li, G. Sun and D. Wang, Polymers, 2019, 11, 1883 CrossRef CAS PubMed
.
- W. Zhong, X. Ding, W. Li, C. Shen, A. Yadav, Y. Chen, M. Bao, H. Jiang and D. Wang, Polymers, 2019, 11, 1289 CrossRef CAS PubMed
.
- J. Heikenfeld, A. Jajack, J. Rogers, P. Gutruf, L. Tian, T. Pan, R. Li, M. Khine, J. Kim, J. Wang and J. Kim, Lab Chip, 2018, 18, 217–248 RSC
.
- S. P. Nichols, A. Koh, W. L. Storm, J. H. Shin and M. H. Schoenfisch, Chem. Rev., 2013, 113, 2528–2549 CrossRef CAS PubMed
.
- X. Zhao, Q. Hua, R. Yu, Y. Zhang and C. Pan, Adv. Electron. Mater., 2015, 1, 1500142 CrossRef
.
- X. Liang and S. A. Boppart, IEEE Trans. Biomed. Eng., 2010, 57, 953–959 Search PubMed
.
- M. Amjadi, Y. J. Yoon and I. Park, Nanotechnology, 2015, 26, 375501 CrossRef PubMed
.
- X. Ye, Z. Yuan, H. Tai, W. Li, X. Du and Y. Jiang, J. Mater. Chem. C, 2017, 5, 7746–7752 RSC
.
- T. Li, H. Luo, L. Qin, X. Wang, Z. Xiong, H. Ding, Y. Gu, Z. Liu and T. Zhang, Small, 2016, 12, 5042–5048 CrossRef CAS PubMed
.
- J. Wang, J. Jiu, M. Nogi, T. Sugahara, S. Nagao, H. Koga, P. He and K. Suganuma, Nanoscale, 2015, 7, 2926–2932 RSC
.
- B. C.-K. Tee, A. Chortos, R. R. Dunn, G. Schwartz, E. Eason and Z. Bao, Adv. Funct. Mater., 2014, 24, 5427–5434 CrossRef CAS
.
- G. Schwartz, B. C.-K. Tee, J. Mei, A. L. Appleton, D. H. Kim, H. Wang and Z. Bao, Nat. Commun., 2013, 4, 1859 CrossRef PubMed
.
- J. Levin and H. Maibach, J. Controlled Release, 2005, 103, 291–299 CrossRef CAS PubMed
.
- H. Sun, Y. Bu, H. Liu, J. Wang, W. Yang, Q. Li, Z. Guo, C. Liu and C. Shen, Sci. Bull., 2022, 67, 1669–1678 CrossRef CAS PubMed
.
- D. Zhang, R. Yin, Y. Zheng, Q. Li, H. Liu, C. Liu and C. Shen, Chem. Eng. J., 2022, 438, 135587 CrossRef CAS
.
- Y. Bu, T. Shen, W. Yang, S. Yang, Y. Zhao, H. Liu, Y. Zheng, C. Liu and C. Shen, Sci. Bull., 2021, 66, 1849–1857 CrossRef CAS PubMed
.
- P. R. Miller, R. J. Narayan and R. Polsky, J. Mater. Chem. B, 2016, 4, 1379–1383 RSC
.
- J. Heikenfeld, A. Jajack, J. Rogers, P. Gutruf, L. Tian, T. Pan, R. Li, M. Khine, J. Kim, J. Wang and J. Kim, Lab Chip, 2018, 18, 217–248 RSC
.
- A. Abbosh, Sensors, 2019, 19, 1662 CrossRef PubMed
.
- Q. Li, R. Yin, D. Zhang, H. Liu, X. Chen, Y. Zheng, Z. Guo, C. Liu and C. Shen, J. Mater. Chem. A, 2020, 8, 21131–21141 RSC
.
- R. Yin, S. Yang, Q. Li, S. Zhang, H. Liu, J. Han, C. Liu and C. Shen, Sci. Bull., 2020, 65, 899–908 CrossRef CAS PubMed
.
- D.-Y. Khang, H. Jiang, Y. Huang and J. A. Rogers, Science, 2006, 311, 208–212 CrossRef CAS PubMed
.
- D.-H. Kim, J.-H. Ahn, W. M. Choi, H.-S. Kim, T.-H. Kim, J. Song, Y. Y. Huang, Z. Liu, C. Lu and J. A. Rogers, Science, 2008, 320, 507–511 CrossRef CAS PubMed
.
- Y. Huang, W. Dong, C. Zhu and L. Xiao, Complexity, 2018, 2018, e3016343 Search PubMed
.
- R. Bogue, Sens. Rev., 2015, 35, 321–328 CrossRef
.
- A. Nocke, S. Richter, M. Wolf and G. Gerlach, Procedia Chem., 2009, 1, 1151–1154 CrossRef CAS
.
-
M. Farooq and E. Sazonov, in Wearable Electronics Sensors: For Safe and Healthy Living, ed. S. C. Mukhopadhyay, Springer International Publishing, Cham, 2015, pp.221–239 Search PubMed
.
- M. Knite, V. Teteris, A. Kiploka and J. Kaupuzs, Sens. Actuators, A, 2004, 110, 142–149 CrossRef CAS
.
- T. Yamada, Y. Hayamizu, Y. Yamamoto, Y. Yomogida, A. Izadi-Najafabadi, D. N. Futaba and K. Hata, Nat. Nanotechnol., 2011, 6, 296–301 CrossRef CAS PubMed
.
- D. J. Lipomi, M. Vosgueritchian, B. C.-K. Tee, S. L. Hellstrom, J. A. Lee, C. H. Fox and Z. Bao, Nat. Nanotechnol., 2011, 6, 788–792 CrossRef CAS PubMed
.
- Y. Wang, H. Mi, Q. Zheng, H. Zhang, Z. Ma and S. Gong, J. Mater. Chem. C, 2016, 4, 460–467 RSC
.
- Alamusi, N. Hu, H. Fukunaga, S. Atobe, Y. Liu and J. Li, Sensors, 2011, 11, 10691–10723 CrossRef CAS PubMed
.
- C. Dagdeviren, Y. Su, P. Joe, R. Yona, Y. Liu, Y.-S. Kim, Y. Huang, A. R. Damadoran, J. Xia, L. W. Martin, Y. Huang and J. A. Rogers, Nat. Commun., 2014, 5, 4496 CrossRef CAS PubMed
.
- R. C. Webb, A. P. Bonifas, A. Behnaz, Y. Zhang, K. J. Yu, H. Cheng, M. Shi, Z. Bian, Z. Liu, Y.-S. Kim, W.-H. Yeo, J. S. Park, J. Song, Y. Li, Y. Huang, A. M. Gorbach and J. A. Rogers, Nat. Mater., 2013, 12, 938–944 CrossRef CAS PubMed
.
- M. Kaltenbrunner, T. Sekitani, J. Reeder, T. Yokota, K. Kuribara, T. Tokuhara, M. Drack, R. Schwödiauer, I. Graz, S. Bauer-Gogonea, S. Bauer and T. Someya, Nature, 2013, 499, 458–463 CrossRef CAS PubMed
.
- L. Y. Chen, B. C.-K. Tee, A. L. Chortos, G. Schwartz, V. Tse, D. J. Lipomi, H.-S. P. Wong, M. V. McConnell and Z. Bao, Nat. Commun., 2014, 5, 5028 CrossRef CAS PubMed
.
- S. Jung, J. H. Kim, J. Kim, S. Choi, J. Lee, I. Park, T. Hyeon and D.-H. Kim, Adv. Mater., 2014, 26, 4825–4830 CrossRef CAS PubMed
.
- W.-H. Yeo, Y.-S. Kim, J. Lee, A. Ameen, L. Shi, M. Li, S. Wang, R. Ma, S. H. Jin, Z. Kang, Y. Huang and J. A. Rogers, Adv. Mater., 2013, 25, 2773–2778 CrossRef CAS PubMed
.
- J. Park, Y. Lee, J. Hong, Y. Lee, M. Ha, Y. Jung, H. Lim, S. Y. Kim and H. Ko, ACS Nano, 2014, 8, 12020–12029 CrossRef CAS PubMed
.
- S. C. B. Mannsfeld, B. C.-K. Tee, R. M. Stoltenberg, C. V. H.-H. Chen, S. Barman, B. V. O. Muir, A. N. Sokolov, C. Reese and Z. Bao, Nat. Mater., 2010, 9, 859–864 CrossRef CAS PubMed
.
- Y. Wang, C. Zhu, R. Pfattner, H. Yan, L. Jin, S. Chen, F. Molina-Lopez, F. Lissel, J. Liu, N. I. Rabiah, Z. Chen, J. W. Chung, C. Linder, M. F. Toney, B. Murmann and Z. Bao, Sci. Adv., 2017, 3, e1602076 CrossRef PubMed
.
- X. Wang, T. Xu, S. Dong, S. Li, L. Yu, W. Guo, H. Jin, J. Luo, Z. Wu and J. M. King, RSC Adv., 2017, 7, 48461–48465 RSC
.
-
S. Bauer and S. Bauer-Gogonea, in Electromechanically Active Polymers: A Concise Reference, ed. F. Carpi, Springer International Publishing, Cham, 2016, pp.1–15 Search PubMed
.
- J. S. Lee, K.-Y. Shin, O. J. Cheong, J. H. Kim and J. Jang, Sci. Rep., 2015, 5, 7887 CrossRef CAS PubMed
.
- S.-W. Kim, Y. Lee, J. Park, S. Kim, H. Chae, H. Ko and J. J. Kim, Sensors, 2018, 18, 78 CrossRef PubMed
.
- D. Zaharie-Butucel, L. Digianantonio, C. Leordean, L. Ressier, S. Astilean and C. Farcau, Carbon, 2017, 113, 361–370 CrossRef CAS
.
- B. W. An, J. H. Shin, S.-Y. Kim, J. Kim, S. Ji, J. Park, Y. Lee, J. Jang, Y.-G. Park, E. Cho, S. Jo and J.-U. Park, Polymers, 2017, 9, 303 CrossRef PubMed
.
- G. Yu, J. Hu, J. Tan, Y. Gao, Y. Lu and F. Xuan, Nanotechnology, 2018, 29, 115502 CrossRef PubMed
.
- Y. Meng, H. Li, K. Wu, S. Zhang and L. Li, Polymers, 2018, 10, 587 CrossRef PubMed
.
- D. Kang, P. V. Pikhitsa, Y. W. Choi, C. Lee, S. S. Shin, L. Piao, B. Park, K.-Y. Suh, T. Kim and M. Choi, Nature, 2014, 516, 222–226 CrossRef CAS PubMed
.
- J.-Y. Sun, C. Keplinger, G. M. Whitesides and Z. Suo, Adv. Mater., 2014, 26, 7608–7614 CrossRef CAS PubMed
.
- S. Gong, D. T. H. Lai, B. Su, K. J. Si, Z. Ma, L. W. Yap, P. Guo and W. Cheng, Adv. Electron. Mater., 2015, 1, 1400063 CrossRef
.
- C. Dagdeviren, Y. Su, P. Joe, R. Yona, Y. Liu, Y.-S. Kim, Y. Huang, A. R. Damadoran, J. Xia, L. W. Martin, Y. Huang and J. A. Rogers, Nat. Commun., 2014, 5, 4496 CrossRef CAS PubMed
.
- S. Jung, J. H. Kim, J. Kim, S. Choi, J. Lee, I. Park, T. Hyeon and D.-H. Kim, Adv. Mater., 2014, 26, 4825–4830 CrossRef CAS PubMed
.
- S. Gong, W. Schwalb, Y. Wang, Y. Chen, Y. Tang, J. Si, B. Shirinzadeh and W. Cheng, Nat. Commun., 2014, 5, 3132 CrossRef PubMed
.
- S.-H. Shin, S. Ji, S. Choi, K.-H. Pyo, B. Wan An, J. Park, J. Kim, J.-Y. Kim, K.-S. Lee, S.-Y. Kwon, J. Heo, B.-G. Park and J.-U. Park, Nat. Commun., 2017, 8, 14950 CrossRef CAS PubMed
.
- S. Park, H. Kim, M. Vosgueritchian, S. Cheon, H. Kim, J. H. Koo, T. R. Kim, S. Lee, G. Schwartz, H. Chang and Z. Bao, Adv. Mater., 2014, 26, 7324–7332 CrossRef CAS PubMed
.
- Y. Zang, F. Zhang, D. Huang, X. Gao, C. Di and D. Zhu, Nat. Commun., 2015, 6, 6269 CrossRef CAS PubMed
.
- T. Q. Trung and N.-E. Lee, Adv. Mater., 2016, 28, 4338–4372 CrossRef CAS PubMed
.
- M. Amjadi, K.-U. Kyung, I. Park and M. Sitti, Adv. Funct. Mater., 2016, 26, 1678–1698 CrossRef CAS
.
- N. Lu, C. Lu, S. Yang and J. Rogers, Adv. Funct. Mater., 2012, 22, 4044–4050 CrossRef CAS
.
- C. Yan, J. Wang, W. Kang, M. Cui, X. Wang, C. Y. Foo, K. J. Chee and P. S. Lee, Adv. Mater., 2014, 26, 2022–2027 CrossRef CAS PubMed
.
- T. Lee, W. Lee, S.-W. Kim, J. J. Kim and B.-S. Kim, Adv. Funct. Mater., 2016, 26, 6206–6214 CrossRef CAS
.
- X. Xiao, L. Yuan, J. Zhong, T. Ding, Y. Liu, Z. Cai, Y. Rong, H. Han, J. Zhou and Z. L. Wang, Adv. Mater., 2011, 23, 5440–5444 CrossRef CAS PubMed
.
- E. Roh, B.-U. Hwang, D. Kim, B.-Y. Kim and N.-E. Lee, ACS Nano, 2015, 9, 6252–6261 CrossRef CAS PubMed
.
- C. S. Boland, U. Khan, C. Backes, A. O’Neill, J. McCauley, S. Duane, R. Shanker, Y. Liu, I. Jurewicz, A. B. Dalton and J. N. Coleman, ACS Nano, 2014, 8, 8819–8830 CrossRef CAS PubMed
.
- Y. Wang, T. Yang, J. Lao, R. Zhang, Y. Zhang, M. Zhu, X. Li, X. Zang, K. Wang, W. Yu, H. Jin, L. Wang and H. Zhu, Nano Res., 2015, 8, 1627–1636 CrossRef CAS
.
- B.-U. Hwang, J.-H. Lee, T. Q. Trung, E. Roh, D.-I. Kim, S.-W. Kim and N.-E. Lee, ACS Nano, 2015, 9, 8801–8810 CrossRef CAS PubMed
.
- B. Saha, S. Baek and J. Lee, ACS Appl. Mater. Interfaces, 2017, 9, 4658–4666 CrossRef CAS PubMed
.
- A. Frutiger, J. T. Muth, D. M. Vogt, Y. Mengüç, A. Campo, A. D. Valentine, C. J. Walsh and J. A. Lewis, Adv. Mater., 2015, 27, 2440–2446 CrossRef CAS PubMed
.
- Y. Tomimatsu, H. Takahashi, T. Kobayashi, K. Matsumoto, I. Shimoyama, T. Itoh and R. Maeda, J. Micromech. Microeng., 2013, 23, 125023 CrossRef
.
- J. Lei, B. Yin, Y. Qiu, H. Zhang, Y. Chang, Y. Luo, Y. Zhao, J. Ji and L. Hu, RSC Adv., 2015, 5, 59458–59462 RSC
.
- G. Poulin-Vittrant, C. Oshman, C. Opoku, A. S. Dahiya, N. Camara, D. Alquier, L.-P. T. H. Hue and M. Lethiecq, Phys. Procedia, 2015, 70, 909–913 CrossRef CAS
.
- J. Zhong, Q. Zhong, Q. Hu, N. Wu, W. Li, B. Wang, B. Hu and J. Zhou, Adv. Funct. Mater., 2015, 25, 1798–1803 CrossRef CAS
.
- J. M. Wu, C.-Y. Chen, Y. Zhang, K.-H. Chen, Y. Yang, Y. Hu, J.-H. He and Z. L. Wang, ACS Nano, 2012, 6, 4369–4374 CrossRef CAS PubMed
.
- Y. Wang, Y. Yu, X. Wei and F. Narita, Adv. Mater. Technol., 2021, 2200318 Search PubMed
.
- H. Høyer, M. Knaapila, J. Kjelstrup-Hansen and G. Helgesen, J. Appl. Phys., 2012, 112, 094324 CrossRef
.
- E. Roh, B.-U. Hwang, D. Kim, B.-Y. Kim and N.-E. Lee, ACS Nano, 2015, 9, 6252–6261 CrossRef CAS PubMed
.
- Y. Wang, L. Wang, T. Yang, X. Li, X. Zang, M. Zhu, K. Wang, D. Wu and H. Zhu, Adv. Funct. Mater., 2014, 24, 4666–4670 CrossRef CAS
.
- W. Choi, J. Lee, Y. Kyoung Yoo, S. Kang, J. Kim and J. Hoon Lee, Appl. Phys. Lett., 2014, 104, 123701 CrossRef
.
- H. C. Lim, B. Schulkin, M. J. Pulickal, S. Liu, R. Petrova, G. Thomas, S. Wagner, K. Sidhu and J. F. Federici, Sens. Actuators, A, 2005, 119, 332–335 CrossRef CAS
.
- G. Darlinski, U. Böttger, R. Waser, H. Klauk, M. Halik, U. Zschieschang, G. Schmid and C. Dehm, J. Appl. Phys., 2005, 97, 093708 CrossRef
.
- J. Zhou, Y. Gu, P. Fei, W. Mai, Y. Gao, R. Yang, G. Bao and Z. L. Wang, Nano Lett., 2008, 8, 3035–3040 CrossRef CAS PubMed
.
- L. Pan, A. Chortos, G. Yu, Y. Wang, S. Isaacson, R. Allen, Y. Shi, R. Dauskardt and Z. Bao, Nat. Commun., 2014, 5, 3002 CrossRef PubMed
.
- C.-L. Choong, M.-B. Shim, B.-S. Lee, S. Jeon, D.-S. Ko, T.-H. Kang, J. Bae, S. H. Lee, K.-E. Byun, J. Im, Y. J. Jeong, C. E. Park, J.-J. Park and U.-I. Chung, Adv. Mater., 2014, 26, 3451–3458 CrossRef CAS PubMed
.
- M. L. Hammock, A. Chortos, B. C.-K. Tee, J. B.-H. Tok and Z. Bao, Adv. Mater., 2013, 25, 5997–6038 CrossRef CAS PubMed
.
- Y. Guo, G. Yu and Y. Liu, Adv. Mater., 2010, 22, 4427–4447 CrossRef CAS PubMed
.
- Y. Zang, F. Zhang, D. Huang, C. Di, Q. Meng, X. Gao and D. Zhu, Adv. Mater., 2014, 26, 2862–2867 CrossRef CAS PubMed
.
- C. Di, Y. Liu, G. Yu and D. Zhu, Acc. Chem. Res., 2009, 42, 1573–1583 CrossRef CAS PubMed
.
- K.-I. Park, J. H. Son, G.-T. Hwang, C. K. Jeong, J. Ryu, M. Koo, I. Choi, S. H. Lee, M. Byun, Z. L. Wang and K. J. Lee, Adv. Mater., 2014, 26, 2514–2520 CrossRef CAS PubMed
.
- S. Xu, Y. Qin, C. Xu, Y. Wei, R. Yang and Z. L. Wang, Nat. Nanotechnol., 2010, 5, 366–373 CrossRef CAS PubMed
.
- H. Y. Y. Nyein, L.-C. Tai, Q. P. Ngo, M. Chao, G. Zhang, W. Gao, M. Bariya, J. Bullock, H. Kim, H. M. Fahad and A. Javey, A Wearable Microfluidic Sweat Sensing Patch for Dynamic Sweat Secretion Analysis, ACS Sens., 2018, 3, 944–952 CrossRef CAS PubMed
.
- M. Bariya, Z. Shahpar, H. Park, J. Sun, Y. Jung, W. Gao, H. N. N. Nyein, T. S. Liaw, L.-C. Tai, Q. P. Ngo, M. Chao, Y. Zhao, M. Hettick, G. Cho and A. Javey, Roll-to-Roll Gravure Printed Electrochemical Sensors for Wearable and Medical Devices, ACS Nano, 2018, 12, 6978–6987 CrossRef CAS PubMed
.
- A. dos Santos, E. Fortunato, R. Martins, H. Águas and R. Igreja, Sensors, 2020, 20, 4407 CrossRef CAS PubMed
.
- L. Lin, Y. Xie, S. Wang, W. Wu, S. Niu, X. Wen and Z. L. Wang, ACS Nano, 2013, 7, 8266–8274 CrossRef CAS PubMed
.
- X. He, Q. Liu, J. Wang and H. Chen, Front. Mater. Sci., 2019, 13, 305–313 CrossRef
.
- Y. Pang, J. Jian, T. Tu, Z. Yang, J. Ling, Y. Li, X. Wang, Y. Qiao, H. Tian, Y. Yang and T.-L. Ren, Biosens. Bioelectron., 2018, 116, 123–129 CrossRef CAS PubMed
.
- L.-C. Tai, T. S. Liaw, Y. Lin, H. Y. Y. Nyein, M. Bariya, W. Ji, M. Hettick, C. Zhao, J. Zhao, L. Hou, Z. Yuan, Z. Fan and A. Javey, Nano Lett., 2019, 19, 6346–6351 CrossRef CAS PubMed
.
- L. M. Castano and A. B. Flatau, Smart Mater. Struct., 2014, 23, 053001 CrossRef CAS
.
- D. T.-P. Fong and Y.-Y. Chan, Sensors, 2010, 10, 11556–11565 CrossRef PubMed
.
- S. C. Mukhopadhyay, IEEE Sens. J., 2015, 15, 1321–1330 Search PubMed
.
- A. H. Abdul Razak, A. Zayegh, R. K. Begg and Y. Wahab, Sensors, 2012, 12, 9884–9912 CrossRef PubMed
.
- J. R. Windmiller and J. Wang, Electroanalysis, 2013, 25, 29–46 CrossRef CAS
.
- X. Liu, B. Du, Y. Sun, M. Yu, Y. Yin, W. Tang, C. Chen, L. Sun, B. Yang, W. Cao and M. N. R. Ashfold, ACS Appl. Mater. Interfaces, 2016, 8, 16379–16385 CrossRef CAS PubMed
.
- Z. He, A. Elbaz, B. Gao, J. Zhang, E. Su and Z. Gu, Adv. Healthcare Mater., 2018, 7, 1701306 CrossRef PubMed
.
- Kenry, J. C. Yeo and C. T. Lim, Microsyst. Nanoeng., 2016, 2, 1–19 Search PubMed
.
- T. Wang, H. Yang, D. Qi, Z. Liu, P. Cai, H. Zhang and X. Chen, Small, 2018, 14, 1702933 CrossRef PubMed
.
- X. Wang, Y. Gu, Z. Xiong, Z. Cui and T. Zhang, Adv. Mater., 2014, 26, 1336–1342 CrossRef CAS PubMed
.
- C. Pang, J. H. Koo, A. Nguyen, J. M. Caves, M. G. Kim, A. Chortos, K. Kim, P. J. Wang, J. B. Tok and Z. Bao, Highly skin-conformal microhairy sensor for pulse signal amplification, Adv. Mater., 2015, 27, 634–640 CrossRef CAS PubMed
.
- S. Baek, H. Jang, S. Y. Kim, H. Jeong, S. Han, Y. Jang, D. H. Kim and H. S. Lee, Flexible piezocapacitive sensors based on wrinkled microstructures: Toward low-cost fabrication of pressure sensors over large areas, RSC Adv., 2017, 7, 39420–39426 RSC
.
- B. Nie, R. Huang, T. Yao, Y. Zhang, Y. Miao, C. Liu, J. Liu and X. Chen, Textile-Based Wireless Pressure Sensor Array for Human-Interactive Sensing, Adv. Funct. Mater., 2019, 29, 1808786 CrossRef
.
- S. Sharma, A. Chhetry, M. Sharifuzzaman, H. Yoon and J. Y. Park, Wearable Capacitive Pressure Sensor Based on MXene Composite Nanofibrous Scaffolds for Reliable Human Physiological Signal Acquisition, ACS Appl. Mater. Interfaces, 2020, 12, 22212–22224 CrossRef CAS PubMed
.
- A. P. Gerratt, H. O. Michaud and S. P. Lacour, Elastomeric Electronic Skin for Prosthetic Tactile Sensation, Adv. Funct. Mater., 2015, 25, 2287–2295 CrossRef CAS
.
- X. Guo, Y. Huang, Y. Zhao, L. Mao, L. Gao, W. Pan, Y. Zhang and P. Liu, Smart Mater. Struct., 2017, 26, 095017 CrossRef
.
- K. Muldoon, Y. Song, Z. Ahmad, X. Chen and M.-W. Chang, Micromachines, 2022, 13, 642 CrossRef PubMed
.
- G. Jia, H. Wang, L. Yan, X. Wang, R. Pei, T. Yan, Y. Zhao and X. Guo, Environ. Sci. Technol., 2005, 39, 1378–1383 CrossRef CAS PubMed
.
- D. Kwon, T.-I. Lee, J. Shim, S. Ryu, M. S. Kim, S. Kim, T.-S. Kim and I. Park, ACS Appl. Mater. Interfaces, 2016, 8, 16922–16931 CrossRef CAS PubMed
.
- M. Su, F. Li, S. Chen, Z. Huang, M. Qin, W. Li, X. Zhang and Y. Song, Adv. Mater., 2016, 28, 1369–1374 CrossRef CAS PubMed
.
- A. M. Almassri, W. Z. Wan Hasan, S. A. Ahmad, A. J. Ishak, A. M. Ghazali, D. N. Talib and C. Wada, J. Sens., 2015, 2015, e846487 Search PubMed
.
- R. Cao, X. Pu, X. Du, W. Yang, J. Wang, H. Guo, S. Zhao, Z. Yuan, C. Zhang, C. Li and Z. L. Wang, ACS Nano, 2018, 12, 5190–5196 CrossRef CAS PubMed
.
- F. Rahimi Sardo, A. Rayegani, A. Matin Nazar, M. Balaghiinaloo, M. Saberian, S. A. H. Mohsan, M. H. Alsharif and H.-S. Cho, Biosensors, 2022, 12, 697 CrossRef CAS PubMed
.
- F. Yi, L. Lin, S. Niu, P. K. Yang, Z. Wang, J. Chen, Y. Zhou, Y. Zi, J. Wang and Q. Liao,
et al.
, Adv. Funct. Mater., 2015, 25, 3688–3696 CrossRef CAS
.
- Y. Han, F. Yi, C. Jiang, K. Dai, Y. Xu, X. Wang and Z. You, Nano Energy, 2018, 56, 516–523 CrossRef
.
- J. Rao, Z. Chen, D. Zhao, Y. Yin, X. Wang and F. Yi, Sensors, 2019, 19, 2763 CrossRef CAS PubMed
.
- X. Wang, L. Dong, H. Zhang, R. Yu, C. Pan and Z. L. Wang, Adv. Sci., 2015, 2, 1500169 CrossRef PubMed
.
- W. Liu, N. Liu, Y. Yue, J. Rao, C. Luo, H. Zhang, C. Yang, J. Su, Z. Liu and Y. Gao, J. Mater. Chem. C, 2018, 6, 1451–1458 RSC
.
- C. G. Núñez, W. T. Navaraj, E. O. Polat and R. Dahiya, Adv. Funct. Mater., 2017, 27, 1606287 CrossRef
.
- S. Gong, D. T. H. Lai, Y. Wang, L. W. Yap, K. J. Si, Q. Shi, N. N. Jason, T. Sridhar, H. Uddin and W. Cheng, ACS Appl. Mater. Interfaces, 2015, 7, 19700–19708 CrossRef CAS PubMed
.
- Y. Liu, H. Wang, W. Zhao, M. Zhang, H. Qin and Y. Xie, Sensors, 2018, 18, 645 CrossRef PubMed
.
- J. Kim, W. J. da Silva, A. R. bin Mohd Yusoff and J. Jang, Sci. Rep., 2016, 6, 19813 CrossRef CAS PubMed
.
- Y. Li, B. Zhou, G. Zheng, X. Liu, T. Li, C. Yan, C. Cheng, K. Dai, C. Liu, C. Shen and Z. Guo, J. Mater. Chem. C, 2018, 6, 2258–2269 RSC
.
- S. J. Lim, J. H. Bae, S. J. Jang, J. Y. Lim and J. H. Ko, Fibers Polym., 2018, 19, 2622–2630 CrossRef
.
- C. Nam and D. Shin, Int. J. Distrib. Sens. Netw., 2018, 14, 1550147718767794 Search PubMed
.
- X. Liu, J. Miao, Q. Fan, W. Zhang, X. Zuo, M. Tian, S. Zhu, X. Zhang and L. Qu, Adv. Fiber Mater., 2022, 4, 361–389 CrossRef CAS
.
- H. Liu, Q. Li, Y. Bu, N. Zhang, C. Wang, C. Pan, L. Mi, Z. Guo, C. Liu and C. Shen, Nano Energy, 2019, 66, 104143 CrossRef CAS
.
- H. Jin, S. Jung, J. Kim, S. Heo, J. Lim, W. Park, H. Y. Chu, F. Bien and K. Park, Sci. Rep., 2017, 7, 10854 CrossRef PubMed
.
- Y. Gao, H. Ota, E. W. Schaler, K. Chen, A. Zhao, W. Gao, H. M. Fahad, Y. Leng, A. Zheng, F. Xiong, C. Zhang, L.-C. Tai, P. Zhao, R. S. Fearing and A. Javey, Adv. Mater., 2017, 29, 1701985 CrossRef PubMed
.
- W. Zou, H. Yu, P. Zhou, Y. Zhong, Y. Wang and L. Liu, Appl. Surf. Sci., 2021, 543, 148800 CrossRef CAS
.
- Y. Song, H. Dong, W. Liu, X. Fu, Z. Fu, P. Li, L. Chen, Z. Ahmad, J. Liu, X. Chen and M.-W. Chang, ACS Appl. Polym. Mater., 2022, 4, 868–878 CrossRef CAS
.
- Z. Deng, T. Hu, Q. Lei, J. He, P. X. Ma and B. Guo, ACS Appl. Mater. Interfaces, 2019, 11, 6796–6808 CrossRef CAS PubMed
.
-
S. Kagami, K. Nishiwaki, J. Kuffner, S. Thompson, J. Chestnutt, M. Stilman and P. Michel, in Robotics Research, ed. S. Thrun, R. Brooks and H. Durrant-Whyte, Springer, Berlin, Heidelberg, 2007, pp.103–117 Search PubMed
.
- H. Morishita, R. Fukui and T. Sato, in IEEE/RSJ International Conference on Intelligent Robots and Systems, 2002, vol. 2, pp. 1246–1251 vol.2.
- V. J. P. Amorim, M. C. Silva and R. A. R. Oliveira, Sensors, 2019, 19, 1904 CrossRef PubMed
.
- Z. He, W. Chen, B. Liang, C. Liu, L. Yang, D. Lu, Z. Mo, H. Zhu, Z. Tang and X. Gui, ACS Appl. Mater. Interfaces, 2018, 10, 12816–12823 CrossRef CAS PubMed
.
- S. Patel, H. Park, P. Bonato, L. Chan and M. Rodgers, J. Neuroeng. Rehabilitation, 2012, 9, 21 CrossRef PubMed
.
- M. Amjadi, A. Pichitpajongkit, S. Lee, S. Ryu and I. Park, ACS Nano, 2014, 8, 5154–5163 CrossRef CAS PubMed
.
| This journal is © The Royal Society of Chemistry 2023 |