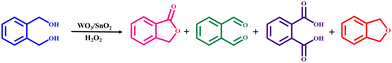Open Access Article
Open Access ArticleAdvanced metal oxide-based nanocatalysts for the oxidative synthesis of fine chemicals
Rakesh Kumar
Sharma
 *a,
Rakeshwar
Bandichhor
b,
Vishwesh
Mishra
c,
Shivani
Sharma
d,
Sneha
Yadav
a,
Shilpa
Mehta
d,
Bhavya
Arora
a,
Pooja
Rana
a,
Sriparna
Dutta
a and
Kanika
Solanki
a
*a,
Rakeshwar
Bandichhor
b,
Vishwesh
Mishra
c,
Shivani
Sharma
d,
Sneha
Yadav
a,
Shilpa
Mehta
d,
Bhavya
Arora
a,
Pooja
Rana
a,
Sriparna
Dutta
a and
Kanika
Solanki
a
aGreen Chemistry Network Centre, Department of Chemistry, University of Delhi, New Delhi-110007, India. E-mail: rksharmagreenchem@hotmail.com; Fax: +91-011-27666250; Tel: +91-011-276666250
bDr. Reddy's Laboratories, Limited, Innovation Plaza, IPDO Bachupally, Hyderabad, 500090, India
cDepartment of Chemistry, University of Delhi, Delhi-110007, India
dDepartment of Chemistry, Ramjas College, University of Delhi, Delhi-110007, India
First published on 27th February 2023
Abstract
The development of nanocatalysis has ushered in a new era in chemistry by promoting the design of new catalytic production processes in organic synthesis with utmost level of waste minimization by conforming to green chemistry principles. Consequently, significant advancement has been made towards the generation of highly active and selective metal oxide-based materials with tunable properties that can be controlled at the nano-scale. A thorough literature survey revealed that these functional nano-architectures are endowed with exceptional potential to boost industrially significant oxidation processes in terms of activity, selectivity, product yield, atom economy, recoverability and reusability. This review aims to combine all the scattered literature pertaining to the applicability of diverse metal oxide nanocatalysts in the manufacturing of fine chemicals, which can serve as a guide for the scientific community working in this area. In the first section, we present an in-depth understanding on the fundamental concepts of metal oxide-based nanoarchitectures, emphasizing their role in expediting practically important oxidation processes. Subsequently, we present the state-of-art progress on various synthetic methodologies and functionalization strategies adopted by different research groups to acquire strategic vision of essential requisites for the design of nanoengineered metal oxides. Furthermore, an in-depth discussion on surface coating agents and functionalization strategies, which are key structural aspects for manufacturing nanohybrid catalysts with enhanced activity and selectivity, will also be covered. Finally, we summarize all the research work conducted on the utilization of surface-engineered metal oxide-based nanomaterials in accelerating oxidation reactions including alkane oxidation, alkene oxidation, arene oxidation, alcohol oxidation, amine oxidation and thiol oxidation. Further, it is envisioned that this review will give new directions to nanocatalysis research by providing clues for the judicious design of next-generation catalytic nanomaterials, which will not only improve industrial production but also protect the environment and human health.
Introduction
Nanotechnology, a fast-evolving discipline, is often hailed as an innovative route for the design of next-generation raw materials, resulting in remarkable features useful in an array of scientific and industrial areas.1,2 In fact, it has dramatically fostered the development of the manufacturing sector worldwide by opening new avenues towards cleaner and sustainable catalytic protocols for industrial chemical processes in an economically competitive manner. Considering this, the scientific community is focused on cutting-edge research to unlock the potential of engineered nanostructures in the field of catalysis, originating from the precise control of the design of materials owing to their tunable physico-chemical properties.3 Indeed, the integration of nanoscience and catalysis is considered a versatile amalgamated stratagem that can bridge the gap between homogeneous and heterogeneous catalysts through the construction of nanometer-scale architectures having unprecedented catalytic efficacy due to the blend of the prominent facets of both catalysts.4,5 By exploiting the technological advances delivered by the heterogenization approach, there have been numerous breakthrough innovations in novel catalytic materials based on distinct metal oxides to address all the formidable challenges often encountered during the practical application of their bulk counterparts.6 Driven by the astonishing progress, pioneering researchers and materials science engineers have devoted their efforts to exploring green innovative synthetic strategies for the judicious design of several metal oxides nanocatalysts using iron oxide,7 silica,8,9 alumina,10 halloysite,11 copper oxide,12 zinc oxide,13 titania,14etc. as the support matrix. These engineered oxide based nanocatalytic systems significantly contribute to shaping the sustainable future of modern society given that they provide a myriad of opportunities such as low energy consumption, economic viability, little environmental impact, enhanced activity and selectivity together with facile recovery.15 Several studies proved that designed nano-scale metal oxide-based catalytic materials show remarkable potential to speed up previous and current oxidative transformations, which are the core of modern organic synthesis, given that they significantly contribute to the large-scale production of fine chemicals, intermediates and monomers for various polymers.16–19 Our literature survey revealed that these oxidation reactions are often achieved by adding stoichiometric amounts of strong oxidants such as chromium(IV) reagents, permanganate, tert-butyl hydroperoxide, selenium oxide, ruthenium(VIII) oxide, nitric acid, and oxygen.20–22 Unfortunately, the scope of the aforementioned oxidant-mediated protocols is limited given that these oxidizing agents are either toxic or unstable under atmospheric conditions, nonspecific in action, and produce undesirable side products. Thus, to improve the environmental footprint of chemical production processes, many research groups have developed another complementary pathway involving the utilization of hydrogen peroxide as a green oxidant, which is gradually replacing some well-established processes due to its excellent oxygen atom efficiency, low cost and production of water as the only by-product.23–27 However, despite these exciting opportunities, there are still several challenges ranging from the scaling up to final commercialization of these new-generation nanocatalysts, which need to be addressed. Hence, to overcome the predominant obstacles, we attempt to combine all the scattered literature reports pertaining to nanoscale catalysts in a single reference, which will serve as a guide for material chemists and researchers in the field of oxidative organic synthesis.The prime objective of this review is to shed light on the studies related to the fabrication of heterogeneous nanocatalysts based on different oxides nanostructures for boosting industrially significant oxidation processes. This review provides comprehensive coverage of the state-of-art progress on various synthetic methodologies adopted by different research groups together with an in-depth discussion on surface coating agents and functionalization strategies to acquire strategic vision on key structural aspects for manufacturing nanohybrid catalysts with enhanced activity and selectivity. Subsequently, we present a detailed discussion on the application of surface-engineered metal oxide-based nanomaterials in accelerating various oxidation reactions such as alkane oxidation, alkene oxidation, arene oxidation, alcohol oxidation, amine oxidation and thiol oxidation (Fig. 1). Also, we critically discuss the opportunities and challenges associated with emerging H2O2-based oxidation processes. Finally, we conclude with a summary and future perspective on new innovative directions towards the development of selective oxidation catalysts, highlighting the points needs to be addressed for their practical implications at the pilot and industrial scale.
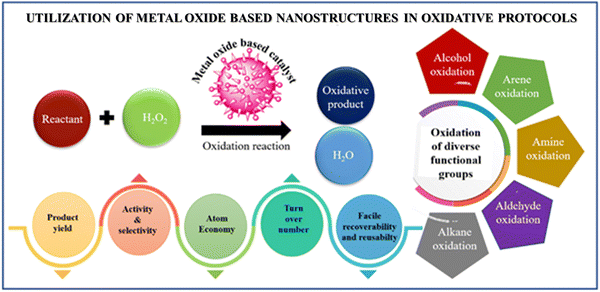 | ||
| Fig. 1 Schematic representation of the application of metal oxide-based nanostructures in catalytic protocols. | ||
It is worth mentioning that no review has been published to date that presents a compilation of the vast and scattered literature of heterogeneous nanocatalysts based on different oxide nanostructures for boosting industrially significant oxidation processes in a comprehensible manner. Finally, it is anticipated that this review will give new directions to nanocatalysis research by providing clues for the judicious design of next-generation benign catalytic technologies, which will not only improve industrial manufacturing but also assist in alleviating environmental challenges for sustainability in the near future.
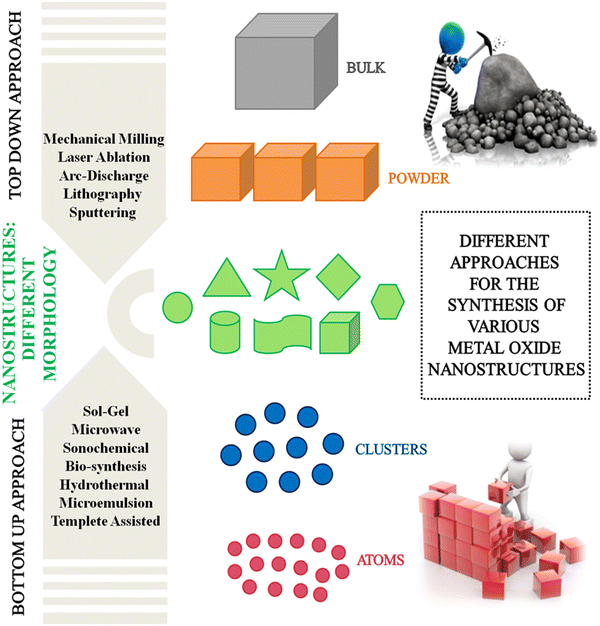
Synthetic strategies for the development of various metal oxide nanostructures
The choice of the synthetic route utilized for the formulation of nanoscience-inspired metal oxide-based materials plays a key role in achieving desired features including size, dispersity, morphology and crystal structure. The tuning of physicochemical properties at the nanometer level imparts distinct characteristics to the developed material according to a particular application. A comprehensive literature survey revealed that a myriad of different studies has been carried out on the synthesis of metal oxides, which can be mainly categorized as top-down and bottom-up methods (Fig. 2). The former includes mechanochemical synthesis, spray pyrolysis, laser-assisted deposition, atomic-layer deposition, ion implantation, sputtering and etching. For instance, Zhang and Dai and their research group utilized a different solid-state mechanochemical nanocasting approach for the synthesis of a series of highly porous metal oxides such as ZrO2, Fe2O3, CeO2, CuO, La2O3, CuOx–CeOy and CuOx–CoOy–CeOz, manifesting record-breaking surface areas.28 To obtain the desired nanoporous metal oxides, a ball-milling process utilizing commercial porous silica as the template precursor was employed, followed by calcination and subsequent removal of silica with NaOH. Likewise, Botta and co-workers synthesized iron oxide nanoparticles using three different reactive systems involving different oxidation states of precursor iron salts and mean sizes in the range of 8–10 nm.29 Flame spray pyrolysis is an advanced technique for the preparation of thermally stable and high surface area nanoparticles, and even their large-scale manufacture. In this case, Smirniotis et al. reported the fabrication of a series of MgO nanoparticles using a flame aerosol method, which were further utilized for CO2 sorption at lower temperatures.30 In another report, monodisperse and crystalline SnSe nanospheres were synthesized following a spray pyrolysis approach, which were subsequently transformed into SnO2 nanospheres through a post-treatment oxidation process at 600 °C in air relying on Kirkendall diffusion.31 CuO nanoparticles were also reported to be synthesized using the dc reactive magnetron sputtering technique at the low temperature of −194 °C. The synthesized nanoparticles were found to be highly pure with a narrow size distribution, average crystallite size ranging from 6–13 nm and high surface area.32 Camacho-Lopez et al. delineated the preparation of molybdenum oxide (MoOx) nanoparticles through the laser ablation of solids in liquids technique. This technique employed an Nd:YAG (neodymium-doped yttrium aluminium garnet) laser for the fabrication of MoOx nanoparticles with nanosecond pulses in deionized water without the use of any additives or surfactants.33 Likewise, Kupracz and co-workers synthesized a mixture of FeO, Fe3O4 and Fe2O3 nanoparticles via the pulsed laser ablation technique and further decorated on anodized TiO2 nanotubes, which subsequently exhibited enhanced activity towards the oxygen evolution reaction.34 Recently, an ion implantation method was employed by Gentils and co-workers for the synthesis of highly pure Fe–Cr alloy nanoparticles. The developed protocol involved the consecutive implantation of Al and O ions in the Fe–Cr alloy nanoparticles at room temperature.35 Alternatively, the bottom-up route includes sol–gel, sonochemical, photochemical solvothermal synthesis and thermal decomposition as the most commonly employed techniques for the synthesis of metal oxide nanoparticles. Kim and co-workers reported a template-free sonochemical reduction route for the transformation of potassium permanganate into α- and δ-MnO2 phase with two different reaction times (15 min and 30 min) using polyethylene glycol as the reducing agent.36 It was concluded that the spherical α-MnO2 nanoparticles synthesized after 30 min reaction and having uniformly distributed mesopores played a significant role as an electrode material for supercapacitor applications. In another report, a sol–gel method assisted with ultrasonic irradiation was utilized by Torres-Martínez et al. for the fabrication of ZnO nanoparticles with high surface area and low particle size, which were found to have a profound impact on the photocatalytic H2 production reaction without the use of any sacrificial agents.37 A prolific attempt in this direction was made by Lee and co-workers, wherein they synthesized hydrophilic superparamagnetic Fe3O4 nanoclusters via a one-pot solvothermal strategy.38 The gram-scale synthesis of spherical Fe3O4 nanoparticles having a uniform size of 200 nm was carried out through the reduction of FeCl3 with ethylene glycol at 200 °C in the presence of sodium acetate and trisodium citrate as the alkali source and stabilizing agent, respectively. Recently, a thermal decomposition technique was employed for the preparation of size- and shape-selective Co3O4 nanoparticles through the decomposition of [Co(acac)2] or [Co(NO3)2] in different solvents (ethanol and water) and capping agents (oleylamine or hexamethylenediamine), followed by calcination in air.39 The developed methodology led to the production of spherical, cubic, octahedral and plate-like spinel nanoparticles of Co3O4 with a size in the range of 8 to 90 nm. The sol–gel process, which converts molecular precursors into a polymeric oxide network through hydrolysis and subsequent condensation reaction, is a versatile methodology for the one-pot synthesis of size- and shape-controlled porous nanostructures. In this context, a method for the cost-effective and well-controlled sol–gel synthesis of thorn-like ZnO nanoparticles was reported by Aliev et al.40 The synthetic methodology involved the mechanical stirring of zinc acetate, NaOH and CTAB as the capping agent in bi-distilled water, which after a few minutes of agitation turned milky, indicating the generation of ZnO nanoparticles, and their antimicrobial activity was further evaluated against Gram-positive, Gram-negative bacteria and fungi. Along this line, a base-catalyzed sol–gel methodology accompanied with solvent-driven self-assembly process was employed for the fabrication of Mn3O4 and CuO nanoparticles.41 The employed synthesis route facilely generated hexagonal, ribbon- or irregular-shaped Mn3O4 nanoparticles having an average diameter of 100–200 nm using different solvent systems such as water, water/ethanol and water/toluene. On the contrary, the same sol–gel process produced nanoporous thin sheets, nanoparticles, rods and ribbon-shaped CuO nanostructures in different solvents. In another publication, Joshi et al. delineated a photochemical process for the preparation of ZnO nanoparticles having a size of around 12 nm in an aqueous solvent mixture of alkaline alcohol-acetone under UV light and devoid of any capping agent.42 Similarly, Mukasyan and research group developed a template-assisted combustion route for the fabrication of ultra-small α-Fe2O3 nanoparticles (size below 5 nm) and narrow particle size distribution within a very short reaction duration.43 In this protocol, SBA-15 was employed as the template together with iron (Fe(NO3)3) and ammonium nitrate as oxidizers and glycine as fuel.Recent advancements in the synthesis of nanoparticles have raised huge concern over chemical and physical methods given that they are not only exorbitant and labour intensive but also pose high risks to the environment due to the usage of toxic chemicals. Accordingly, greener biogenic approaches for the synthesis of nanoparticles have gained immense recognition in the past few years owing to their cost-effectiveness and environmentally benign routes. Wide arrays of biological entities are readily available such as extract from plants, plant products and microorganisms such as fungi, yeast, and bacteria, which are most commonly employed for the efficient production of non-toxic nano-sized metal oxide nanoparticles. Significant research has been conducted in this arena. A prolific attempt in this direction was made by Kim and co-workers, wherein they synthesized CuO nanoparticles from leaf extract of Psidium guajava, functioning as a reducing agent and stabilizing agent.44 The employed protocol led to the production of spherical mono-dispersed nanoparticles with an average size in the range of 2–6 nm, whose efficacy was further tested in the photocatalytic degradation of Nile blue and reactive yellow dyes. Another report highlighted an eco-friendly route, wherein a biogenic source such as marine brown algae Sargassum wightii was employed as a reducing agent for the preparation of magnesium oxide nanoparticles.45 The synthesized crystalline MgO NPs having an FCC structure and average size of 68.06 nm demonstrated potent antibacterial and antifungal activity towards pathogens together with good photodegradation efficacy towards methylene blue. Along similar lines, Stella et al. judiciously utilized immature fruit extract of Cocos nucifera as a reducing and stabilizing agent for the synthesis of three metal oxide nanoparticles, namely, CuO, NiO and Fe2O3, whose catalytic efficiency was further evaluated in the catalytic reduction of aromatic aldehydes using ammonium formate as the hydrogen donor.46 The catalytic studies revealed the superiority of NiO nanoparticles given that they led to the formation of the corresponding alcohols in excellent yields under mild reaction conditions compared to the other synthesized nanoparticles. A comparative analysis of all the synthetic approaches is provided in Table 1.
| Methodology | Conditions | Advantages | Limitations | Ref. |
|---|---|---|---|---|
| Sol–gel | • Wet chemical method | • Narrow particle size distribution | • Requires costly precursors | 47 |
| • Use of Molecular precursor | • Uniform nanostructures | • Sometimes necessitates high temperature | ||
| • Hydrolysis/alcoholysis | ||||
| Microwave | • Metal salt/precursor | • Rapid formation | • Extreme conditions | 48 |
| • Microwave heating | • High Repetition rate | |||
| • Ostwald ripening | ||||
| Sono-chemical | • Requires ultrasonic wave source | • Very quick synthesis | • Sometimes surfactants require | 49 |
| • Comparatively low temperature ≈ 60 °C | • Precise control over shape and size | • Costly and toxic precursors | ||
| Hydrothermal | • Use of autoclave | • Uniform size and shape | • High temperature and pressure | 50 |
| • High temperature and inorganic metal salts | • Ease of tenability | |||
| Microemulsion | • Utilizes surfactants | • Narrow size distribution | • Use of harmful substances | 51 |
| • Room temperature | • Regular shape and morphology | |||
| • Organic solvents or auxiliaries | ||||
| Template assisted | • Inorganic precursor | • High reproducibility | • Needs a template | 52 |
| • A template | ||||
| • Self-assembly of template precursor | • Controllable structure, size and morphology | • High temperature | ||
| • Condensation and removal of template | ||||
| Bio-synthesis | • Use of plant extracts | • Environmentally benign synthesis | • Longer time duration | 53 |
| • Varying temperature (100–250 °C) | • Poor control over size and shape | |||
| Mechano-chemical synthesis | • Pulverisation of precursors by ball milling | • Simple operation | • High purity is questionable | 54 |
| • Does not require organic solvents and high temperature | • Ease of scaling up | • Low crystallinity | ||
| Laser ablation | • Use of laser beam in liquid | • High purity | • Expensive | 55 |
| • Self-standing and remote control | • Ease of manipulation | • Needs specific set up and arrangements | ||
| Arc discharge | • Generation of NPs in both gas and liquid medium | • Higher generation rate | • Requires special reactors and other arrangements | 56 |
| • Generation of arc between the electrodes (dielectric medium) | • Tunable morphology | |||
| • Economical | ||||
| Lithography | • Preparation of colloidal crystal mask | • Homogenous NPs | • Multistep process | 57 |
| • Disposition of desired material through mask | • Precisely controlled morphology | • Expensive | ||
| • Removal of mask | ||||
| Sputtering | • Bombardment of energetic particles on the target | • Versatile approach | • Severe conditions | 58 |
| • Atoms from target are driven off | • NPs formed have same composition as target source | • Costly | ||
| • Deposition on a substrate |
Distinct morphologies of metal oxide architectures for tailoring nanoscale physico-chemical features
In the past few years, rapid advancements in materials science have enabled the development of novel structural and morphological metal oxide-based nanostructures with more exposed reactive crystal planes. Metal oxide nanomaterials of diverse shapes and dimensions have been reported to date (Fig. 3).8,59–65 For instance, metal oxide nanomaterials possessing a spherical shape are categorized as 0D nanomaterials. 1D nanomaterials are comprised of nanotubes, nanorods, nanowires and nanobelts, while nanosheets and nanofilms are characterized as 2D nanomaterials. 3D nanomaterials including core–shell and multi-nanolayers are advanced high-performance architectures. It has been perceived that the size and morphological features of these nanomaterials significantly affect their physico-chemical properties. Diverse crystal facets are accompanied with a difference in geometric structure and electronic state, which ultimately play a vital role in enhancing the catalytic performance of metal oxide nanomaterials.66–68 Extensive studies by various research groups demonstrated the critical role of anisotropic morphology in determining the activity and selectivity of the catalysts at the nanometer scale through the selective exposure of a large fraction of reactive facets or active sites.69,70 Thus, it is anticipated that the idea of morphology/plane-dependent nanocatalysis can bridge the gap between surface science and materials science by rendering potential routes for tuning the rate of reactions, extent of reactivity and formation of major products. It is worth highlighting here that Pathak et al. fabricated CuO nanostructures with different morphologies via the chemical precipitation technique, wherein Cu2+ was effectively stabilized after complexation with various organic acids including acetic acid, citric acid and tartaric acid.71 CuO nanostructures possessing various morphologies such as rod, spherical, star and flower were facilely obtained through this route and further successfully characterized using XRD, HRTEM, FTIR, DLS and zeta potential measurements. The catalytic performance of the resulting nanomaterials was investigated in the NaBH4-assisted reduction of 4-nitrophenol. Among the various morphologies, the star-shaped CuO exhibited the highest catalytic activity, which was further ascribed to its high specific surface area, positive surface charge and high indexed facet. Metal oxide nanoparticles as support materials have also been found to regulate the catalytic performance of the resulting nanocomposites. The great relevance of morphology-modulated properties stimulated Zhou and co-workers to synthesize Pd nanoparticle-loaded {100}-faceted CeO2 nanocubes, {111}-faceted CeO2 nanooctahedrons and [{100}{111}]-faceted CeO2 nanorods for HCHO oxidation to CO2.72 Besides exhibiting immense potential in catalysis, these metal oxide nanoarchitectures possessing varying morphologies have also demonstrated considerable applicability in diverse fields. For instance, Boada and co-workers recently reported the fabrication of superparamagnetic iron oxide nanoparticles in situ co-precipitated in commercial cellulose sponge and further utilized them as an adsorbent for As(III).73 Another research group explored the photocatalytic efficiency of NiO nanobelts in the degradation of organic pollutants such as rhodamine B (RhB), methyl orange (MO), methylene blue (MB) and crystal violet (CV).74 The few micrometer-long cubic crystalline NiO nanobelts possessing bandgap of 4.07 eV resulted in good degradation of 79.1%, 82.75%, 76.7% and 89% of MO, MB, CV and RhB after 140 min under a xenon lamp. Likewise, various morphologies of ZnO such as nanorods, nanosheets and nanoparticles were coupled with polyaniline (PANI) to yield ZnO/PANI nanocomposites whose potential was further investigated towards NH3 sensing at room temperature.75 The morphology exhibited a significant effect on the sensing application and amongst them, the ZnO nanosheets with vapor-phase polymerized aniline showed a good sensing performance, high response, excellent selectivity and appreciable repeatability.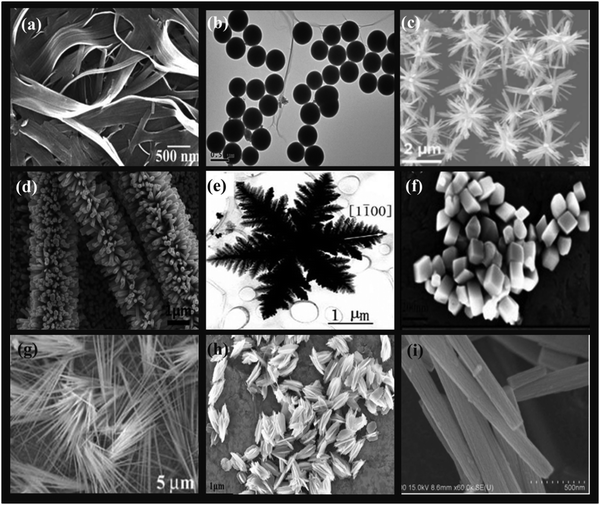 | ||
| Fig. 3 Diverse morphologies of metal oxide nanomaterials: (a) Nanobelts. Reproduced with permission from ref. 59, Copyright 2007, the American Chemical Society. (b) Nanospheres. Reproduced with permission from ref. 8, Copyright 2014, The Royal Society of Chemistry. (c) Nanoflowers. Reproduced with permission from ref. 60, Copyright 2017, the American Chemical Society. (d) Nanorods. Reproduced with permission from ref. 61, Copyright 2014, the American Chemical Society. (e) Snowflakes. Reproduced with permission from ref. 62, Copyright 2006, Elsevier, B.V. (f) Distorted cubes. Reproduced with permission from ref. 63, Copyright 2015, Springer Nature. (g) Nanowires. Reproduced with permission from ref. 64, Copyright 2002, the American Chemical Society. (h) Nanohusks. Reproduced with permission from ref. 63, Copyright 2015, Springer Nature. (i) Nanotubes. Reproduced with permission from ref. 65, Copyright 2013, The Royal Society of Chemistry. | ||
Recent reports have highlighted that metal oxide nanowires possessing good electrical properties, high dielectric constant and wide bandgaps have emerged as appealing materials towards sensing applications.76 It is worth mentioning here that Pinna and research group demonstrated the gas-sensing response of one-dimensional SnO2/NiO core–shell nanowires towards hydrogen at various temperatures.77 The overall composite was prepared through a two-step process, wherein single-crystalline SnO2 nanowires were first formed via the vapor–liquid–solid deposition method, and thereafter decorated with a polycrystalline NiO shell through atomic-layer deposition. The high surface to volume ratio of the SnO2 nanowires together with the optimized and conformal NiO shell layer resulted in a remarkable performance towards hydrogen sensing. Xia et al. reported a hydrothermal method for the synthesis of δ-MnO2 nanosheets, which exhibited an excellent supercapacitor performance with high specific capacitance of 411 F g−1 at a scan rate of 5 mV s−1 and 332.7 F g−1 at a current density of 0.5 A g−1.78 The remarkable performance of the developed δ-MnO2 was attributed to its porous nanosheets structure, which further enhanced the specific surface area, increased the number of active sites and shortened the ion diffusion distance in the δ-MnO2 electrodes. In recent years, metal oxide nanoparticles have become a popular choice for developing antibacterial active materials owing to their unique electronic configuration and physicochemical properties.79 In this case, Sundrarajan M. and co-workers fabricated titanium dioxide (TiO2) nanoparticles using Morinda citrifolia extract and studied their inhibitory activities against human pathogens including Staphylococcus aureus, Escherichia coli, Bacillus subtilis, Pseudomonas aeruginosa, Candida albicans and Aspergillus niger.80 The results revealed that the TiO2 nanoparticles demonstrated good antimicrobial activity against a broad spectrum of Gram-positive bacteria.
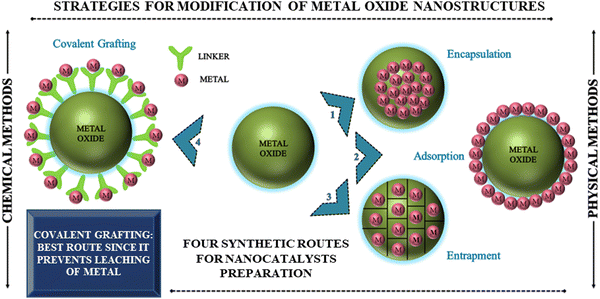
Surface modification of metal oxide nanostructures: way towards smart design of advanced nanocatalytic systems
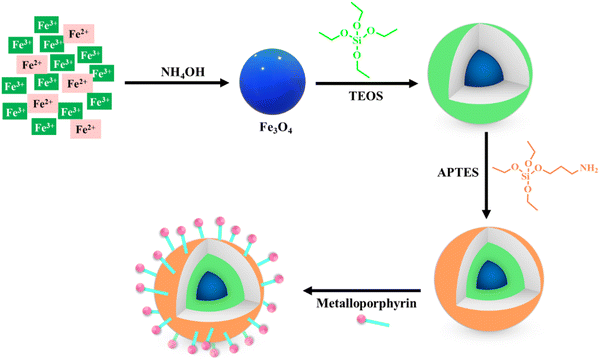
The synthesis of metal oxide nanostructure is often followed by further surface modification to support the active metal species, which can effectively enable recyclability, whilst retaining the metal catalytic efficacy. However, some studies also report the coating of the developed oxide nanomaterials to prevent them from agglomeration using different polymers prior to their functionalization, as shown in Fig. 4. Considering the substantial benefits of coating agents, it is evident from our literature survey that these agents not only prevent the aggregation of metal oxides but also impart active sites to a large extent for further tethering with functionalizing moieties, which actively participate in enhancing the catalytic efficacy of the resultant nanostructured matrices.81 For instance, the synergistic effect of sulfamic acid and amino-functionalized ferrite nanoparticles in catalyzing the one-pot synthesis of α-amino nitriles (up to 98%) was revealed by Kassaee et al.82 The reusability test showed that the catalyst could sustain its efficacy for at least four runs. In another report, Chen and co-workers investigated the catalytic potency of sulfonic acid-functionalized Fe3O4@C magnetic nanoparticles with a size of about 190 nm in the condensation of benzaldehyde and ethylene glycol under ambient reaction conditions.83 Likewise, sulfonated-mercaptopropionic acid-immobilized magnetic nanoparticles (Fe3O4/SMPA) manifested stupendous catalytic performance to yield 97% of 3,4-dihydropyrimidin-2(1H)-ones in a solvent-free environment.84 The optimization studies revealed that both the Lewis acidity (SO3H) and Brönsted acidity (Fe3+) of Fe3O4/SMPA effectively contributed to facilitating the formation of the desired product. Recently, Awasthi et al. outlined the fabrication of magnetically retrievable amine-functionalized silica-coated ferrite nanoparticles (NH2@SiO2@Fe3O4 NPs) and their catalytic efficacy in the multicomponent synthesis of bioactive 2-amino-4H-benzo[b]pyrans in good to excellent yields.85To make nano-oxides suitable candidates for catalytic applications, modification through certain specific functional groups can be achieved by physical or covalent tethering of existing homogeneous active sites on diverse solid support nanostructured matrices such as ferrite, nano-silica, and alumina.86–92
The physical modification strategy involves two ways, where one is the inclusion of a modifier in the pores of a solid support matrix and the other is the electrostatic interaction between them. However, surfactant molecules or macromolecules are mainly incorporated in metal oxide nanostructures by electrostatic interactions.93 For example, Zhu and group developed a nanofiller for polylactide matrices by modifying the surface of silica with oleic acid.94 Alternatively, a poly(L-lactic acid)/TiO2 nanocomposite film was fabricated by incorporating carboxylic acid and long-chain alkyl amine-modified TiO2 nanoparticles in a poly(L-lactic acid) matrix by Nakayama et al.95 Besides, the concept of physical adsorption of metal nanoparticles on the surface of metal oxides was illustrated in the work presented by Saire-Saire et al., which demonstrated the fabrication of a nanoengineered material composed of gold nanoparticles decorated on the surface of magnetic cobalt ferrite (Scheme 1).96 Their study revealed that the developed nanocatalyst exhibited an excellent performance not only in the reduction of 4-nitrophenol but also in the selective oxidation of dimethylphenylsilane to dimethylphenylsilanol. To date, several reports have been published involving hybrid catalyst preparation using a similar synthetic route. Although this strategy is an important advancement, it is not considered to be suitable from an industrial perspective due to the associated metal leaching problem given that the anchoring of the active species on the oxide support is based on weak physical forces. This serious metal contamination issue prompted the scientific community to direct their effort towards alternative approaches, i.e., covalent modification of metal oxide surfaces, which can effectively prevent leaching as a result of the much stronger interactions between the modifiers and support matrix.97 In this context, there are reports in the literature on the utilization of different modifying agents and grafting polymers to chemically modify the surface of diverse metal oxide nanostructures. Some of the reported modifiers include silane coupling agents, phosphonates, carboxylates, catechols, alkenes/alkynes, and amines.98 However, the functionalizing agents most often used for the surface modification of metal oxides are silanes and phosphonates, prompting us to provide a detailed discussion on these modifiers (Table 2).
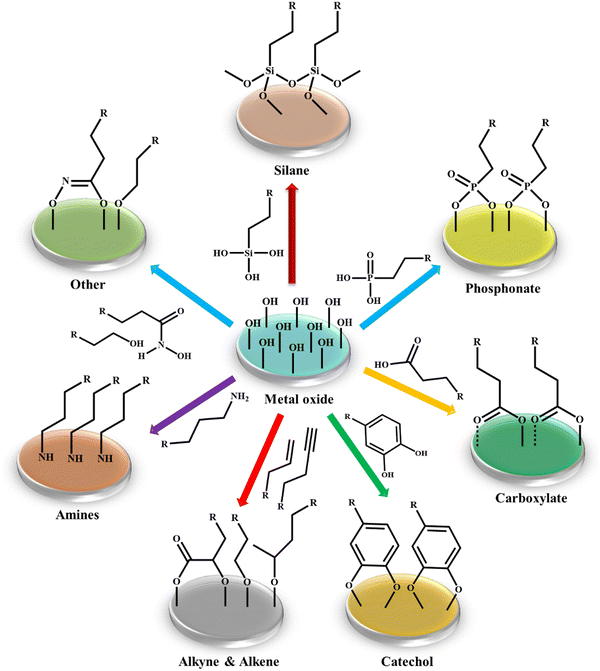 | ||
| Scheme 1 Most frequently employed functionalizing agents for modifying the surface of metal oxide nanoparticles. | ||
| S. no | Name of modifiers | Abbrev. | Structure | Ref. |
|---|---|---|---|---|
| Silane Coupling Agents | ||||
| 1. | 3-Methacryloxypropyltrimethoxysilane | MPS | CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CH3) C(CH3) |
99–101 |
| COO(CH2)3Si(OCH3)3 | ||||
| 2. | Triethoxyvinylsilane | TVS | CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHSi(OCH2CH3)3 CHSi(OCH2CH3)3 |
102–106 |
| 3. | Tris(2-methoxyethoxy)(vinyl)silane | TMEVS | CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHSi(OCH2CH2OCH3)3 CHSi(OCH2CH2OCH3)3 |
107 and 108 |
| 4. | 3-Aminopropyltrimethoxysilane | APTMS | NH2(CH2)3Si(OCH3)3 | 109 |
| 5. | 3-Aminopropyltriethoxysilane | APTES | NH2(CH2)3Si(OCH2CH3)3 | 110 |
| 6. | 3-Mercaptopropyltrimethoxysilane | MPTMS | HS(CH2)3Si(OCH3)3 | 101 |
| 7. | Hexadecyltrimethoxysilane | HDTMS | CH3(CH2)15Si(OCH3)3 | 101 |
| 8. | Bis[3-(triethoxysilyl)propyl]tetrasulfide | BTESPT | (CH3CH2O)3Si(CH2)3(S)4(CH2)3Si (OCH2CH3)3 | 108 |
| Phosphonates | ||||
| 9. | Aminotris(methylene phosphonic acid) | ATMP | N(CH2PO3H2)3 | 111 |
| 10. | Bis(hexamethylene-triamine)-penta(methylene phosphonic acid) | BHMTPMPA | H2PO3CH2-N-[(CH2)6-N(CH2PO3H2)2]2 | 111 |
| 11. | Phenyl phosphonic acid | PPA | C6H5–PO3H2 | 112 |
| 12. | Ethylenediamine tetrakis(methylene phosphonic acid) | EDTMP | (CH2)2-[N(CH2PO3H2)2]2 | 113 |
| 13. | 1-Hydroxyethane-1,1-diphosphonic acid | HEDP | OHCH3C–(PO3H2)2 | 113 |
| 14. | Benzene-1,3,5-triphosphonic acid | BTP | C6H3(PO3H2)3 | 114 |
| 15. | 3,5-Diphosphonobenzoic acid | BDP | HOOC–C6H3–(PO3H2)2 | 115 |
| 16. | 5-Phosphonoisophthalic acid | C6H3(COOH)2(PO3H2) | 115 | |
| 17. | 1,4-Phenylenediphosphonic acid | BDPA | C6H4(PO3H2)2 | 116 |
| 18. | Phosphorous acid | H3PO3 | 117 | |
| Carboxylates | ||||
| 19. | Lauric acid | LAU | CH3–(CH2)10COOH | 118 |
| 20. | Succinic acid | SA | HOOC–CH2–CH2–COOH | 119 |
| 21. | Stearic acid | SA | CH3–(CH2)16–COOH | 120 |
| 22. | Photopolymerisable 10-undecynylphosphonic acid | 10UCYPA | H2PO3–(CH2)9–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH CH |
121 |
| 23. | Citric acid | CA | HOC(CH2–COOH)2 | 122 |
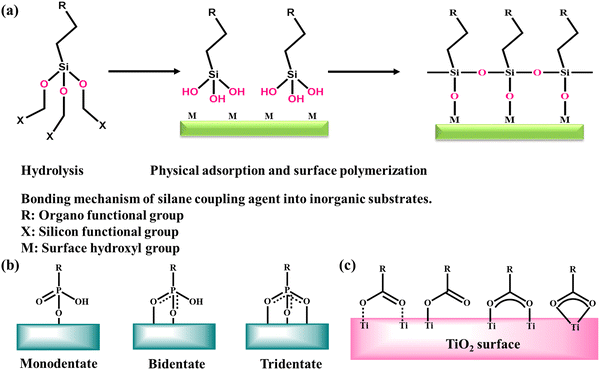
Silane coupling agents
The most common functionalizing entities preferentially chosen by several researchers working in this area are silane coupling agents, which are endowed with hydrolyzable and organo-functional end groups, further allowing the surface immobilization of the desired molecules.123,124 One of the main advantages of silanes is that they can bear numerous functionalities, for example, amino, cyano, carboxylic acid, and epoxy groups. This method is applicable to numerous metal oxides such as SiO2, Al2O3, TiO2, SnO2, ZrO2, and V2O5.125 The general representation of these linkers is RSiX3, where X is any organo-functional end group including chloro, ethoxy or methoxy groups, which react with the hydroxyl groups of oxide nanostructures, and R is an alkyl chain having a variety of functionalities and is chosen to meet the requirements of the polymer (Fig. 5a).126 Several research groups reported functionalization using different silylating agents as a facile route to construct hybrid nano design catalytic systems.Phosphonates
Surface modification of a distinct metal oxide nanostructures by phosphonate groups from either by P–OH, P–OR (R1/4 alkyl) or P–O−-containing precursors leads to the formation of M–O–P bonds. For example, if phosphonic acid is used as the precursor for the surface modification of TiO2 nanoparticles, then it will form Ti–O–P covalent bonds.126,127 Alternatively, the functionalization of titania through silane coupling agents will create Ti–O–Si bonds, which are very unstable toward hydrolysis. Another important difference from silanes is that phosphonates only undergo reactions with surface OH groups without undergoing homocondensation, and hence P–O–P bonds will not form. Various metal oxides nanostructures can easily be functionalized with phosphonates, which include alumina, stannia, zirconia, and magnetite, and also easily functionalized with phosphonate groups.128–130 The different modes of anchoring of a phosphonate group on a metal oxide surface, i.e., mono-, bi-, and tridentate, are well illustrated in Fig. 5b. A recent investigation of the binding of (11-hydroxyundecyl)phosphonic acid or (12-carboxydodecyl)phosphonic acid on an SnO2 surface by solid-state 31P NMR showed the bi- and tridentate attachment of the phosphonate ligands.131 Besides, multidentate attachment is another stabilizing factor for modified nanoparticles.132 In the case of the bifunctional (11-hydroxyundecyl)phosphonic acid, it is interesting to note that the phosphonate group and not the carboxylate group was bonded to the stannia surface.Carboxylates
Carboxylate ligands, in particular fatty acids, have also been utilized for the surface modification of metal oxide nanostructures (Fig. 5c). For instance, Zhang et al. employed all-trans-retinoic acid as a carboxylate modifier for the surface modification of titania nanoparticles with various controlled sizes.133 This study unveiled the size-dependent surface binding forms, indicating that adsorption decreases with a decrease in the size of the developed nanoparticle, while the percentage of chemisorption forms increases. Recently, Jia and co-workers designed chemoselective catalysts by modifying the surface of manganese oxide nanoparticles with carboxylate materials to tune their ammoxidation selectivity during the reaction of hydroxyaldehyde.134 The results of their study showed that the developed catalyst enhanced the selectivity for hydroxynitrile from 0 to 92%. Further, it was also reported that the product distribution of dinitrile and hydroxynitrile could be altered by simply varying the amount of carboxylate modifier.Nature of active sites of metal oxide-based catalysts
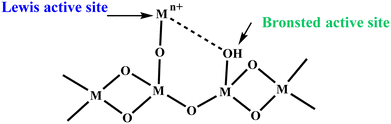
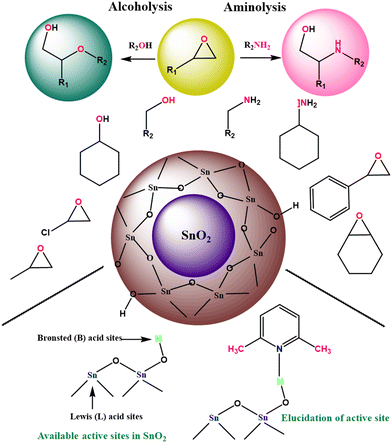
Metal oxide nanostructures invariably find applications in nanocatalysis owing to their array of fascinating properties and tunable structure, boosting numerous chemical conversions.16 The surface of metal oxides is generally terminated by oxide O2− because of its larger size compared to Mn+ cations, eventually leading to the loss of symmetry and coordination of Mn+ cations at the surface.135,136 Overall, the surface of metal oxides possesses oxide ions, coordinatively unsaturated cations (Mn+) as Lewis acid sites and cations terminated by –OH groups, which act as Brønsted acid sites (Scheme 2). Besides, at the nanoscale level, the surface of metal oxides may have different defect sites such as corners, edges and other crystalline discontinuities, which also play key role in expediting the catalytic performance in particular organic transformations.For instance, Pandian Manjunathan et al. utilized tin oxide with Brønsted acidic –OH sites and Lewis acidic Sn4+ sites (Scheme 3) in ring-opening reactions.137 Further, it was established that the developed catalyst showed an excellent performance with high activity and selectivity for different epoxides with alcohols and amines. Besides, studies with 2,6-lutidine were conducted to identify the active sites, which revealed that both the Lewis and Brønsted acid sites accelerate the reaction but the major input is from the Brønsted sites. Similarly, Liu et al. reported the preparation of a CoFe2O4@hollow@mesoporous TiO2 catalyst for the selective oxidation of styrene.138 It was observed that styrene oxide and benzoic acid were selectively obtained upon the use of oxygen and hydrogen peroxide as the oxidant, respectively.
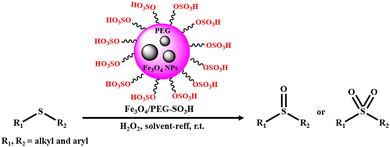
Indeed, it is evident from the literature that surface-engineered metal oxide nanocomposites having Brønsted acid (proton donating) and Lewis acid (electron withdrawing) sites are nowadays more often employed in industrial catalysis. In this context, Mirfakhraei and co-workers demonstrated the facile synthesis of sulfonated-polyethylene glycol-coated Fe3O4 (Fe3O4/PEG-SO3H), followed by its utilization as an effective nanocatalyst for the selective oxidation of sulfides to sulfoxides or sulfones using hydrogen peroxide as a green oxidant (Scheme 4).139
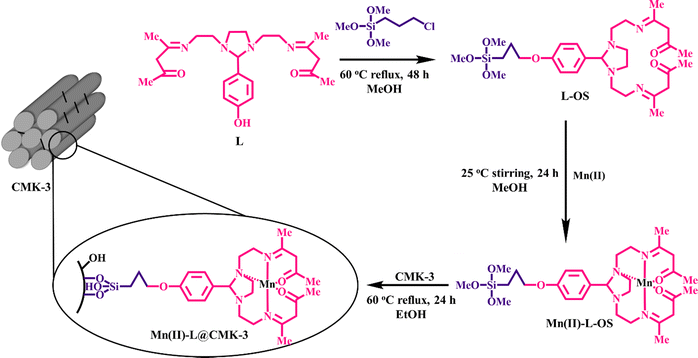
Ghorbani-Choghamarani et al. fabricated Lewis acid nanocatalytic materials by immobilizing Ni, Co, Cr, Zn or Cd complexes on Fe3O4 magnetic nanoparticles for the oxidation of sulfides and oxidative coupling of thiols (Scheme 5).140 The developed nanostructured catalytic system successfully afforded a wide range of aromatic and aliphatic sulfides and thiols with diverse functional groups.
These nanoengineered metal oxide materials represent model well-defined catalysts for fundamental studies and investigation of the structure–activity relationship in oxidation reactions.141 This can be attributed to the following facts: (1) the total number of active centres can be controlled during the development of these catalytic systems, (2) the determination of the number of exposed catalytic active sites allows quantitative calculation of the turnover frequencies for oxidation studies, (3) the influence of different metal oxide supports and distinct ligands on the reaction kinetics can be examined and (4) the molecular and electronic structures of surface-modified metal oxides can be studied in detail under all environmental conditions with the help of several physico-chemical characterization techniques. Subsequently, this information is very fruitful in the establishment of structure–activity relationships, which further facilitate the judicious design of advanced nanostructured catalytic architectures. In this context, a study by Kouotou and group revealed the synthesis of a series of cobalt ferrite thin films, which were then comprehensively characterized to gain insight into their structure, surface and morphological properties.142 It was further observed that Fe substitution by Co in the mixed oxide systems strongly influenced their catalytic performance towards CO oxidation. Besides, the catalytic activity of the Co–Fe oxides with bare α-Fe2O3 was also compared, which proved that Co–Fe oxides are better catalytic systems than the latter.
Improvising catalytic conversions utilizing nanoscale-engineered metal oxide-based materials
For instance, Cavaleiro et al. reported the fabrication of silica NP-supported iron(III)-containing polyoxometalates (POMs), i.e., Keggin-type α-[PW11FeIII(H2O)O39]4− and sandwich-type tungstophosphate B-α-[(PW9O34)2FeIII4(H2O)2]6− through the alkaline hydrolysis of TEOS using a reverse micelle and sol–gel approach involving Triton X-100 as the surfactant, 1-octanol as the co-surfactant and cyclohexane as the organic phase.148 The catalytic activity of the synthesized nanocomposites was investigated in the epoxidation of geraniol using H2O2 as the oxidizing agent. The experimental studies revealed the preferential epoxidation of geraniol at the 2,3-position to produce 2,3-epoxygeraniol in major amounts together with the formation of minor products such as 6,7-epoxide and diepoxide. Between the two fabricated nanomaterials, the α-[PW11FeIII(H2O)O39]4− demonstrated high geraniol conversion (96%) and good regioselectivity (88–91%) towards 2,3-epoxygeraniol after 3 h of reaction in comparison to the B-α-[(PW9O34)2FeIII4(H2O)2]6−, which exhibited 81% conversion even after 5 h.
In continuation of work focused on the development of immobilized catalysts for oxidation reactions, Karimi and co-workers delineated the synthesis of tungstate ions immobilized on periodic mesoporous organosilica (PMO) with an imidazolium ionic liquid framework (WO4−@PMO-IL) as an effective catalytic system for the H2O2-mediated selective oxidation of alcohols.149 To accomplish the desired goal, the catalyst was fabricated via step-by-step assembly, wherein the first step involved the preparation of the ionic liquid BTMSPI precursor by the addition of CPTMS to a suspension of sodium imidazolide and refluxing until a biphasic mixture of toluene and ionic liquid was obtained, and the former was removed to yield pure BTMSPI. The next step involved the hydrolysis and co-condensation of ionic liquid-based imidazolium cations (BTMSPI) and tetramethoxyorthosilicate (TMOS) in Pluronic P123 under acidic conditions in dry methanol with hydrothermal treatment at 100 °C for 72 h to generate PMO-IL. The final step involved the successive functionalization of PMO-IL by the replacement of the chloride ions with tungstate ions via a facile ion exchange approach. The resulting material was successfully utilized for the transformation of primary aromatic or aliphatic alcohols to aldehydes and secondary aromatic or aliphatic alcohols to ketones (Table 3). However, despite the fact that secondary aliphatic alcohols exhibit lower reactivity, even open chain and cyclic aliphatic substrates were converted into ketones with good yields under the optimized reaction conditions. Further, the catalyst was easily recovered from the reaction media and reused for six consecutive runs without showing any significant decrease in catalytic activity and selectivity.
Recently, Pineda et al. synthesized metal nanoparticles (Cu, W, Mo and Nb) supported on a silica matrix through a one-pot microwave-aided approach, which were denoted as M-MINT (M = metal supported and MINT = microwave-induced nanotubes), and subsequently utilized in the H2O2-assisted wet chemical oxidation of isoeugenol to vanillin.150 The size of the metal nanoparticles was found to be in the range of 2 to 52 nm and the overall material revealed a high surface area together with a pore size in the mesopore range. Catalytic studies were performed in a parallel reaction station Carrousel Reaction Station TM using acetonitrile as the solvent for 6 h at 80 °C. Amongst the various metal nanoparticles supported on silica, Cu-MINT demonstrated superior selectivity (88%) towards vanillin formation with isoeugenol conversion of 58 mol%.
Another publication reported the synthesis of iron oxide nanoparticles supported on two different aluminosilicates, namely, Al-MCM-41 and Al-SBA-15, via the microwave-assisted route.151 The catalytic performance of the resulting composites was evaluated in the H2O2-mediated aqueous selective oxidation of alcohols to the corresponding carbonyl motifs under both conventional and microwave heating. Quite astonishingly, the use of Fe/Al-SBA-15 resulted in slightly superior conversion towards the targeted products in comparison to Fe/Al-MCM-41, which was further attributed to the difference in the acidity or the difference in interactions between Fe and Al in both materials. Using Fe/Al-SBA-15 as the catalytic entity, the protocol was further explored for a range of aromatic and cyclic alcohols, furnishing the desired products in good to excellent yields. Besides, the magnetic characteristics arising due to the presence of iron oxide nanoparticles in the catalyst facilitated its easy removal from the reaction media via an external magnet, which was reused for multiple cycles and maintained 90% of its initial activity even after five cycles.
Nanostructured spinel oxide materials have gained significant attention not only in the field of materials science but also as heterogeneous catalysts because of their intriguing magnetic and optical properties.152–156 It is worth mentioning here that Vijaya and co-workers synthesized a CoAl2O4 normal spinel structure in which Co2+ is accommodated in the tetrahedral sites, while Al3+ occupies the octahedral positions.157 For comparative analysis, CoAl2O4 nanoparticles were prepared using both conventional hydrothermal treatment and microwave-assisted technique utilizing Sesamum (S. indicum L.) plant extract as the reducing and stabilizing agent. Spectroscopic and microscopic techniques revealed that CoAl2O4 synthesized through the microwave technique had a large surface area and fine crystallites with a narrow size distribution, ultimately paving the way towards improved catalytic performance in the selective oxidation of benzyl alcohol to benzaldehyde (up to 94% yield) using H2O2 as the oxidizing agent. The catalytic studies divulged the selective formation of benzaldehyde from benzyl alcohol when acetonitrile was employed as the solvent together with H2O2. The authors proposed a plausible mechanism for this observation, wherein acetonitrile activates H2O2 by forming a perhydroxyl anion (OOH−), and it nucleophilically attacks nitrile to form a peroxycarboximidic acid intermediate, which is an O2 transfer agent. The introduction of O2 produces coordinated perhydroxyl anion (Co–OOH*) species, which confirms the interaction of the phenyl ring and OH groups of benzyl alcohol with the Co metal of CoAl2O4. The subsequent regeneration of the active sites through the action of the oxidant with the catalyst leads to the desorption of the product molecule.
In fact, nowadays, the interesting feature of ferrite has transformed the creative outlook of researchers and motivated them towards crafting magnetically responsive catalysts. Working in this direction, Beller reported the use of unsupported or free nano-γ-Fe2O3 as an efficient and recyclable catalyst for the selective oxidation of alcohols and olefins to the corresponding carbonyl compounds in the presence of H2O2 as the oxidant of choice.158 High selectivity together with good conversion of the desired products was obtained with these nano-sized iron oxide particles with a size in the range of 20–50 nm. Furthermore, the presence of a thin carbon layer on the iron oxide nanoparticles resulted in the high stability of the catalyst during the course of the organic transformations. Likewise, another research group reported the synthesis of magnetic Fe3O4 nanoparticles using a co-precipitation method as a catalytic material for the oxidation of primary and secondary benzylic alcohols and aliphatic alcohols to corresponding carbonyl compounds.159 The reaction was carried out using Fe3O4 as the catalytic entity in a greener solvent-water using H2O2 as the oxidant at 50 °C for a specific period to produce the desired products in good to excellent yields. Continuing on the path of utilizing the benefits of the magnetic field-assisted catalyst separation strategy via the incorporation of magnetic nanoparticles (MNPs) as the core component, Bhat and co-workers prepared a nickel hydroxide-coated Fe3O4 nanocatalyst for the selective liquid-phase-assisted oxidation of alcohols utilizing H2O2 as the oxidant.160 The microscopy studies revealed that Fe3O4@APTES@Ni(OH)2 is a combination of spherical and rod-shaped nanoparticles having a smooth surface with a crystallite size of around 40 nm and 130 nm, respectively. The synthesized nanocatalyst exhibited a remarkable catalytic performance in the oxidation of alcohols, i.e., conversion rate of up to 98% in the case of phenylethanol. This enhanced catalytic efficacy was further attributed to the large surface area of the nanoparticles, which delivered abundant coordination sites and surface vacancies during the course of the transformation. Further, the adsorbed nickel hydroxide acted as a Bronsted base and worked synergistically with the Lewis acidic (Fe) center of the nanocatalyst, thereby promoting the quick deprotonation of the alcohols to yield the corresponding carbonyls in good yields.
With the aim to design a magnetically retrievable nanocatalyst, the same research group fabricated a nickel hydroxide-functionalized spinel AFe2O4 (A = Mn and Ni) nanocatalyst for the oxidation of aromatic alcohols to carbonyl motifs in the presence of H2O2 as the oxidizing agent.161 The motive to devote attention towards soft ferromagnetic spinel ferrites is associated with their inherent features including high dispersion, low toxicity, excellent stability, and low oxidation potential values. For accomplishing the desired goal, firstly, MnFe2O4 and NiFe2O4 nanoparticles were synthesized via the co-precipitation method, and further functionalized with APTES in toluene with continuous stirring at 80 °C for 6 h to form aminosilane-modified magnetic nanoparticles. Subsequently, the prepared aminosilane-modified magnetic nanoparticles were stirred with NiCl2 under basic conditions at r.t. for 24 h to produce nickel hydroxide-functionalized MnFe2O4 (10–20 nm) and NiFe2O4 (20–60 nm) nanoparticles. Between the two fabricated nanomaterials, the superior rate of alcohol conversion was observed in the case of nickel hydroxide-functionalized NiFe2O4 nanoparticles. The substrate scope of the developed nanocatalysts was explored for a diverse range of aromatic and aliphatic alcohols under the optimized reaction conditions. Furthermore, the recycling studies divulged that the nanomagnetic catalysts could be efficiently reused for five consecutive runs without showing any significant loss in their catalytic activity.
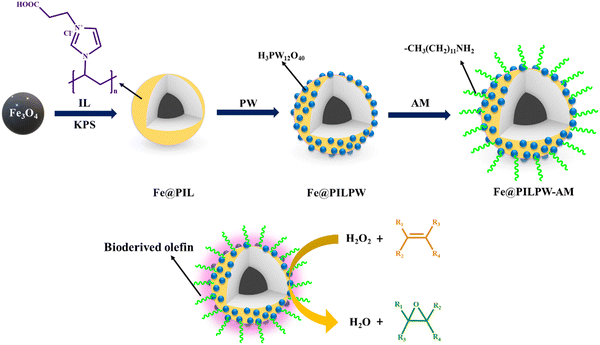
Pourjavadi and research group fabricated a magnetically retrievable catalyst, wherein Fe3O4 nanoparticles were entrapped in a tungstate-functionalized multi-layered cross-linked poly(ionic liquid) matrix.162 The catalyst was fabricated in a stepwise manner, wherein the synthesis of Fe3O4 nanoparticles via the co-precipitation method represents the first step towards achieving the desired goal. Subsequently, the resulting magnetic Fe3O4 nanoparticles were coated with silica and surface-modified using 3-(trimethoxysilyl)propylmethacrylate (MPS) to generate MNP@MPS. Then, MNP@MPS was covalently attached on the highly cross-linked poly(ionic liquid) possessing tungstate ions through the free radical initiation polymerization of the ionic liquid monomer to form the desired MNP@PILW. The catalytic potential of the resulting material was evaluated in the oxidation of a broad array of organic substrates ranging from alcohols and olefins to sulfides in the presence of H2O2 as the oxidizing agent. Furthermore, the high stability and durability of the catalyst was also evident from the recycling studies, which showed reusability up to ten cycles without any significant deterioration in catalytic efficacy.
Another research group led by Zamani et al. delineated the preparation of Cr(III)-containing Fe3O4/polyacrylonitrile-ethylenediamine nanocomposite as an effective catalyst for the selective oxidation of aromatic and aliphatic alcohols using H2O2 as the oxidant under mild reaction conditions.163 The catalyst was fabricated through a series of steps, wherein the synthesis of polyacrylonitrile (PAN)-coated Fe3O4 nanoparticles through in situ polymerization using benzoyl peroxide as the initiator was the key step. The prime idea behind the use of iron oxide as the core material was in view of its phenomenal capability to undergo ready separation from the reaction mixture using only an external magnet. The next step involved the functionalization of Fe3O4-PAN with ethylenediamine, onto which chromium was finally immobilized by stirring under an inert atmosphere at room temperature. The synthesized catalyst led to the generation of a library of corresponding aldehyde moieties in high yields together with good selectivity within a short reaction time.
Similarly, another report by Maleki et al. highlighted the use of a hybrid chromium(VI) magnetic nanocomposite, i.e., Fe3O4@SiO2@PPh3@Cr2O72−, for the oxidation of benzyl alcohol to benzaldehyde, cyclohexanol to cyclohexanone and epoxidation of cyclohexene using H2O2 under ultrasonic conditions at room temperature.164
Chattopadhyay and co-workers synthesized and characterized water-soluble Fe(III) and Mn(III) Schiff base complexes immobilized on dopamine-functionalized Fe3O4 nanoparticles possessing a CTAB micelle structure, i.e., CTAB/Fe3O4@dopa@ML (M = Fe or Mn, L = cyclohexane-1,2-diylbis(azanylylidene)bis(methanylylidene)bis(2,4-diXphenol, where X = Cl, Br, and I) (Scheme 6).165 The synthesized nanomaterials served as efficient catalysts for the oxidation of a diverse range of alcohols using H2O2 as a green oxidant at room temperature. Density functional theory and spectroscopic studies revealed the crucial role of the generated hydroperoxo intermediates in the reaction. Besides, the magnetic characteristics of the synthesized nanocatalyst permitted its facile removal from the reaction media via an external magnet and it could be reused for five successive runs.
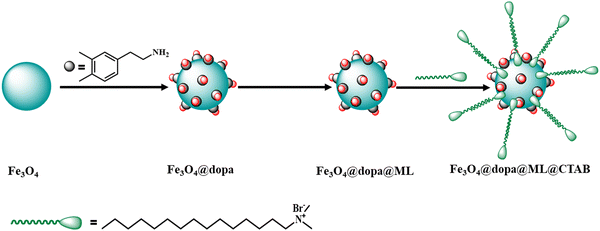 | ||
| Scheme 6 Schematic representation of the synthesis of bilayer CTAB/Fe3O4@dopa@FeL (CHCAT-1) and CTAB/Fe3O4@dopa@MnL (CHCAT-2). | ||
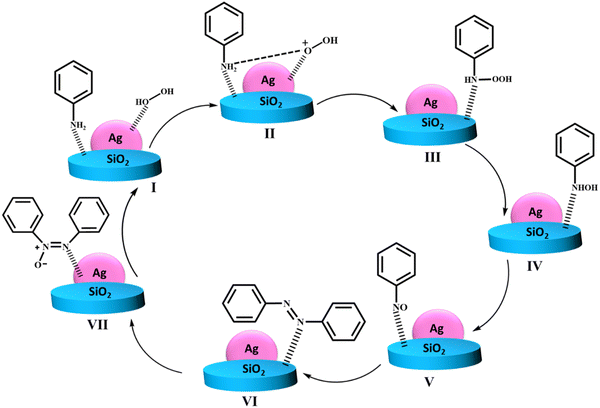
In recent years, owing to their unique catalytic properties, supported gold nanomaterials have been widely explored for executing a broad array of organic transformations. In this case, Cao et al. investigated the catalytic potential of supported Au nanomaterials such as Au/C, Au/Fe2O3, Au/Al2O3, Au/TiO2 (World Gold Council), Au/CeO2 and Au/TiO2 (Mintek) for the oxidation of various activated and non-activated alcohols using H2O2 as the oxidizing agent.166 Amongst the aforementioned catalysts, the H2O2–Au/TiO2 system demonstrated the highest conversion rate of 99% for 1-phenylethanol in aqueous media at 90 °C. The scope of the H2O2–Au/TiO2 system was also scrutinized for structurally diverse alcohols, which revealed that primary alcohols were oxidized to the corresponding carboxylic acids, while secondary alcohols to ketones. It is worthy to note that the H2O2–Au/TiO2 system was also capable of oxidizing even primary aliphatic alcohols, which are considered to be the most inactive alcohol species in the absence of a base. Another remarkable aspect of this report was the hetero-gram-scale clean oxidation of alcohols to the corresponding carboxylic acids. The authors further highlighted the role of H2O2 as the hydrogen scavenger, wherein H2O2 abstracts the α-hydrogen of the substrate to generate an Au–alcoholate complex, which is further attacked by another molecule of H2O2 to produce Au-hydroperoxide, ultimately forming the desired carbonyl products through the β-elimination pathway. Another publication by Jain and co-workers reported the use of nano zinc oxide particles as catalysts for the oxidation of aromatic alcohols to carbonyl motifs using H2O2 as an environmentally benign oxidant and dimethyl carbonate as the reaction medium.167 ZnO2 nanocrystals with a size ranging from 50–150 nm were synthesized via a facile hydrothermal route. The synthesized nanocatalyst successfully afforded a broad range of carbonyl compounds through the oxidation of alcohols in excellent yields. Furthermore, the spent zinc oxide (ZnO) was readily recovered from reaction media through filtration and treated with aq. H2O2 at 150 °C to yield ZnO2, which was used for further recycling studies. Jérôme et al. reported the fabrication of CuO nanoleaves through an unconventional sonochemical route, and subsequently evaluated their catalytic efficacy in the base-free aq. oxidation of glycerol to dicarboxylic acids.168 This protocol was surfactant and template free, involved an in situ pseudo-calcination step, and also credibly reduced the reaction time (synthesis time of 5 min). It is worth mentioning here that copper was chosen as the active metal of choice due to its abundance, low cost, low toxicity and exceptional performance. The microscopy analysis revealed that the CuO nanoleaves were comprised of numerous small particles, whose assembly resulted in the formation of the overall leaf-like morphology (Fig. 6). Further, using H2O2 as the oxidant of choice, glycerol selectively produced oxalic acid (56%) and tartronic acid (22%) in an overall yield of 78%. Besides, the DFT studies highlighted the synergistic role of CuO and H2O2 in achieving this high conversion rate, and also in maintaining the high stability of the catalyst.
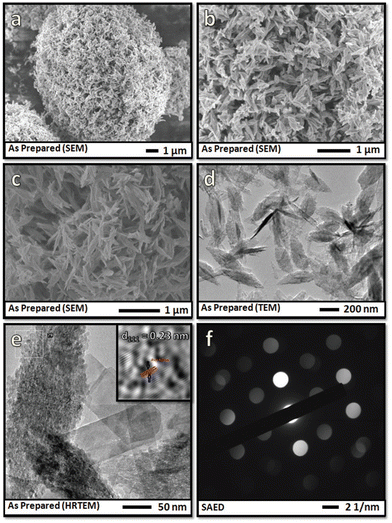 | ||
| Fig. 6 (a) Low- and (b) high-magnification SEM images of CuO nanoleaves synthesized by low-frequency ultrasound and (c) SEM images of CuO nanoleaves synthesized by the conventional method. (d) Low-magnification TEM image, (e) high-magnification TEM image, and (f) SAED pattern of CuO nanoleaves. Reproduced with permission from ref. 168. Copyright 2018, The Royal Society of Chemistry. | ||
Continuing the trend of developing supported catalysts for the oxidation of alcohols, Naeimi and his competent team of researchers came up with the idea of designing an MoO3/copper Schiff base complex for the green oxidation of alcohols.169 For the fabrication of the desired catalyst, MoO3 nanoparticles were first synthesized using Sesbania sesban plant extract. Simultaneously, the Schiff base ligand was prepared through the condensation of vanillin with 9-aminoacridine and its complexation with copper generated a copper Schiff base complex, which was eventually mixed with the MoO3 nanoparticles via ultrasonication to form the anticipated nanocomposite. The developed nanocatalyst successfully converted diverse alcohol substrates to the corresponding aldehydes or ketones with a high conversion rate and good selectivity. The same research group prepared a manganese oxide nanoparticle-supported copper complex nanocomposite (Mn3O4/CuL) via the sonochemical route and evaluated its catalytic potential in the oxidation of a broad array of primary and secondary alcohols using H2O2 as the oxidant under solvent-free conditions.170
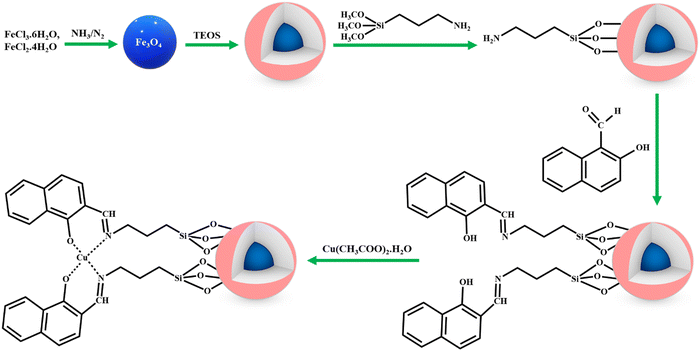
Recently, Amini and co-workers reported the use of ultra-small iron oxide hydroxide (FeOOH) having a size of <10 nm as the catalyst for the H2O2 assisted oxidation of a series of alcohols, sulfides and alkenes.171
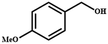
The selective oxidation of vanillyl alcohol to vanillin is a key transformation for the production of a wide array of pharmaceuticals. Accordingly, Valange et al. prepared spinel Co3O4 nanoparticles via a controlled homogeneous co-precipitation approach, and further explored their potential in catalyzing the oxidation of vanillyl alcohol to vanillin using H2O2 as the oxidant and water as the reaction medium under ultrasonication.172 In an attempt to study the influence of the reaction parameters on the concerned oxidation reaction, it was also performed under conventional heating conditions. The experimental results demonstrated the high efficiency of ultrasonication in comparison to traditional heating conditions (15 min in comparison to 60 min), conversion (38% vs. 32%) and selectivity (50% vs. 22%). The aforementioned results were further ascribed to the in situ generated chemical effects under ultrasonic conditions, i.e., the formation of hydroxyl radicals together with the enhanced mass transfer phenomenon between the Co3O4 catalyst and the organic substrate. Similarly, Hamid and research group fabricated a mesoporous, highly crystalline Cu–Ti composite as a heterogeneous catalyst for the liquid-phase oxidation of vanillyl alcohol.173 This composite was synthesized through a modified solution method-based approach, wherein copper acetate, titanium(IV) isopropoxide and trifluoroacetic acid were first mixed in methanol for 4 h. In the next step, the solvent was extracted by applying pressure under vacuum and the obtained solid was ground and calcined in air at 500 °C for 6 h to yield the desired composite catalyst. The synthesized catalyst exhibited a conversion rate of 66% together with 71% selectivity towards vanillin formation from vanillyl alcohol, which was further attributed to the synergistic effect between Cu and Ti of the Cu3TiO4–TiO2 mixed metal oxide catalyst.
Rabbani and co-workers synthesized a magnetically recyclable TCPP/Zn–Fe2O4@ZnO catalyst for the H2O2-mediated oxidation of primary and secondary alcohols.174 To synthesize the desired catalyst, Zn–Fe2O4 nanoparticles were first prepared through the hydrothermal route. In the next step, Zn–Fe2O4@ZnO was synthesized by dispersing the as-synthesized Zn–Fe2O4 nanoparticles in water together with zinc acetate and ammonia, and finally stirring at 120 °C for 3 h. In the final step, 5,10,15,20-meso-tetrakis(4-carboxyphenyl)porphyrin was immobilized on Zn-Fe2O4@ZnO via the co-precipitation approach. The resulting catalyst demonstrated high activity and selectivity (up to 100%) in the concerned oxidation reaction. Besides, the catalyst was magnetic in nature and could be facilely recovered from the reaction medium and reused for four consecutive runs without showing any appreciable loss in its catalytic performance.
The selective oxidation of 1,2-benzenedimethanol to generate phthalides is an important transformation for the production of dyes, pharmaceuticals, etc. A prolific attempt in this direction was made by Dai and co-workers, wherein a series of WO3/SnO2 composites was synthesized through the co-precipitation-impregnation approach, followed by calcination at varying temperatures, and subsequently utilized in the preparation of phthalides from 1,2-benzenedimethanol using H2O2 as the oxidizing agent (Scheme 7).175 The experimental studies revealed that the optimized calcination temperature for the support and catalyst, which resulted in an enhanced catalytic performance, was 1023 K and 823 K, respectively. The authors further highlighted that the high dispersion of tungsten species together with the existence of a few W6+ ions in the SnO2 lattice was beneficial for achieving the optimal catalytic activity in the oxidation reaction. Thus, the calcination temperature was found to have a profound influence on the overall catalytic activity of WO3/SnO2.
Thiol oxidation
The oxidation of thiols to disulphides or sulphoxides is a key preparative step in organic synthesis, which renders the production of several other significant sulphur-based moieties and related cyclic analogues such as disulphide S-oxides, disulphide S-dioxides and sulfonic acids possessing remarkable biological activities. Considerable work has been done by various research groups in this arena. It is worth mentioning here that Rajabi and co-workers fabricated a magnetically retrievable catalyst comprised of iron oxide nanoparticles stabilized on SBA-15 for carrying out the aqueous oxidation of sulfides to sulfoxides.176 The catalytic activity of the synthesized composite was investigated using methylphenyl sulfide as the model substrate in the presence of H2O2. Further, different experimental parameters such as amount of catalyst, polarity of solvent, oxidant dosage, temperature and time were studied to achieve the best reaction conditions. Interestingly, it was found that by using 1 mol% of catalyst, 0.5 mmol H2O2 and water as the solvent, high product yield was attained in just 120 min at room temperature. The other fascinating attributes of the developed protocol include broader substrate scope, high stability of composite with negligible leaching of active species, facile magnetic separation, and excellent reusability for ten successive runs.Rostami and Atashkar were the first to synthesize a chiral oxo-vanadium (+)-pseudoephedrine complex supported on Fe3O4 magnetic nanoparticles (VO(pseudoephedrine)@MNPs), and further utilized it as a catalyst in the chemoselective oxidation of sulfides to sulfoxides.177 The synthesized nanocomposite was systematically characterized via UV-Vis spectroscopy, SEM, XRD, TGA, FT-IR, VSM, EDX and AFM (Fig. 7). Further, experimental investigations revealed that VO(pseudoephedrine)@MNPs provided a high product yield (85–97%) with 20–27% enantiomeric excess under solvent-free conditions. Moreover, a diverse range of sulfides bearing different substituents were subjected to oxidation under the optimized conditions, furnishing good to excellent yields in shorter reaction times.
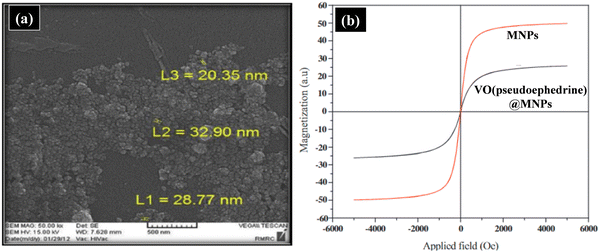 | ||
| Fig. 7 (a) SEM image of VO(pseudoephedrine)@MNPs and (b) VSM analysis curve comparing the magnetization of MNPs and VO(pseudoephedrine)@MNPs. Reproduced with permission from ref. 177. Copyright 2015, Elsevier B.V. | ||
Another study by Kuwahara et al. reported the successful conversion of sulfides to sulfoxides in the presence of a newly designed yolk–shell nanostructured Pd@TSS catalyst and H2O2 as the oxidant.178 A facile interfacial self-assembly approach was utilized for the fabrication of a titanosilicate (TSS) yolk–shell nanoarchitecture, which was composed of active Pd NPs inside a hollow cavity, while Ti sites were dispersed in a silica shell region. Compared to the traditional palladium homogenous catalyst, the designed Pd@TSS possessed higher catalytic efficacy and afforded excellent product yield. The impressive catalytic performance was accredited to the yolk–shell structure, which was responsible for the fast diffusion of H2O2 to the neighbouring active metal sites. Working along similar lines, Zhao and research group designed a greener route for the selective oxidation of sulfides via the design of a monoprotonated divanadium-substituted phosphotungstate ([γ-HPV2W10O40]4−)/H2O2 catalytic system.179 Using this catalyst, a wide range of sulfoxides was obtained with high turnover number and excellent conversion percentage. Closer inspection of the catalytic mechanism revealed that initially H2O2 attacks [γ-HPV2W10O40]4−, leading to the formation of μ-hydroxo-μ-hydroperoxo species. In the next step, the hydroperoxo species reversibly dehydrates to produce active vanadium oxygen entities, which further react with sulfides and form the corresponding sulfoxides. Notably, the attractive feature of the aforementioned protocol is the utilization of water as the solvent, thereby eliminating the need for harsh organic solvents.
Among discrete sulphur containing compounds, disulphides have gained much prominence as a key intermediate in various industrial, pharmaceutical, food and drug delivery systems. The reason behind their high significance is their characteristic S–S bond, which plays an important role in stabilizing the protein structure and can be easily regulated during the reactions. Thus, considering the significant benefits of disulphides, Rajabi et al. successfully fabricated a heterogeneous catalyst by decorating/incorporating Fe NPs on the surface of SBA-15 and employed it for the chemo-selective synthesis of disulphides.180 The reaction was performed using thiophenol as the reactant, which was dissolved in acetonitrile, and to initiate the reaction, an optimum amount of FeNPs@SBA-15 and H2O2 was added to the reaction mixture. It was then allowed to stir for 20 min at refluxing temperature, which eventually generated the desired disulphides. The special features of this protocol such as simple work-up procedure, mild reaction conditions, high selectivity, greater functional group tolerance, facile recoverability, excellent reusability, and shorter reaction time make a better alternative for conducting the reaction in contrast to the reported methodologies. Similarly, Sabet and co-workers described the fabrication of magnetically separable sulfonic acid-coated γ-Fe2O3 NPs.181 The synthesis of the nanocomposite was carried out in a stepwise manner. Initially, a simple co-precipitation method was employed to synthesize the γ-Fe2O3 NPs, and in the next step, chlorosulfonic acid reacted with the prepared magnetic NPs, which ultimately afforded the γ-Fe2O3–SO3H nanocomposites. Further, the catalytic performance of the γ-Fe2O3–SO3H nanocomposite was explored in the H2O2-mediated oxidation of thiols. Through a deeper analysis of the experimental results, it was found that the γ-Fe2O3–SO3H nanocomposite showed superior catalytic activity with promising product yield only in the short time of 60 s.
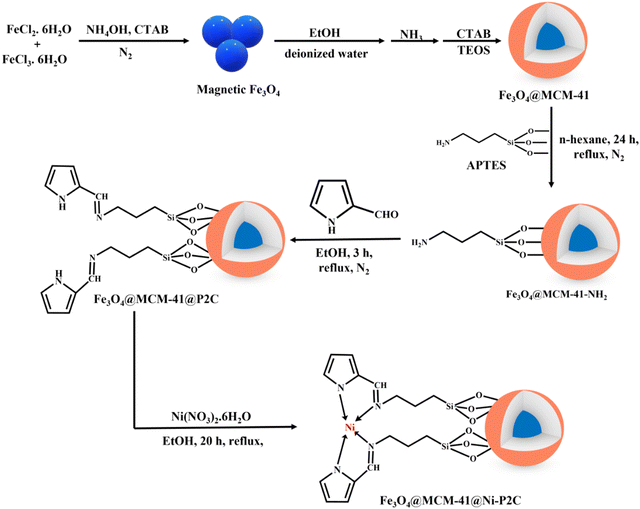
Kulkarni et al. delineated the fabrication of a novel, durable and recyclable NiFe2O4 nanocatalyst, which exhibited a splendid catalytic performance in the conversion of thiols and sulphides using H2O2 as the oxidant.182 The newly fabricated nanocomposite was well characterized via XRD, TEM, SEM, EDX and AAS and it was found that NiFe2O4 possessed a cubic morphology with an average size ranging from 14 to 20 nm. Further, the authors shed light on the reaction mechanism, revealing the formation of a redox couple between the two metal centres (Ni and Fe), which activates H2O2 to generate active ˙OH species. Further, the ˙OH species attacks the thiols and sulphides, ultimately forming the corresponding disulphides and sulfoxides, respectively. Besides, due to its ferrimagnetic nature, NiFe2O4 could be easily separated from the reaction mixture and reused for five successive runs without showing any appreciable loss in its activity. Similarly, Ghorbani-Choghamarani et al. fabricated metal (Cr, Zn, Cd, Co, Ni)-salen complexes immobilized on APTES-functionalized Fe3O4 for the oxidation of thiols and sulphides using H2O2.140 Among the synthesised catalysts, Co-salen-MNPs and Ni-salen-MNPs demonstrated the highest catalytic activity with excellent product yield at 35 °C under solvent-free conditions. Further, to broaden the scope of this protocol, the reaction was conducted using a variety of alkyl, aryl, dialkyl, cyclic sulfides and aromatic, aliphatic, benzylic or heteroaromatic thiols possessing different functionalities. The results indicated that the reaction proceeded satisfactorily without the formation of by-products, signifying the proficiency of adopted methodology. Nikoorazm et al. proposed the idea of designing a novel and reusable nickel Schiff base complex grafted on Fe3O4@MCM-41 nanocatalyst for the oxidation of sulfides to sulfoxides and thiols to disulfides (Scheme 8).183 As indicated by the SEM and TEM analysis, Fe3O4@MCM-41@Ni-P2C consisted of uniform spherical particles with a size in the nanometre range. Moreover, to obtain the best reaction profile, various parameters such as amount of oxidant, catalyst dosage and nature of solvent were studied. Simple work-up procedure, mild reaction conditions, green oxidant, high product yield, short reaction times and broad substrate scope were some of its notable advantages, making this protocol economically viable for large-scale production.
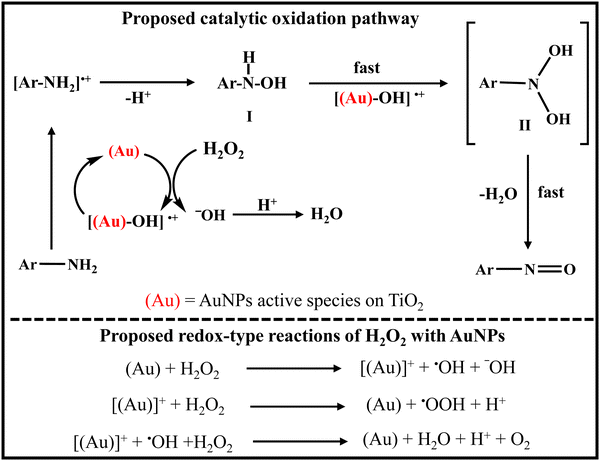
Oxidation of amines
The oxidation of aromatic amine motifs to O-containing compounds such as nitroso, hydroxylamine, and oxime compounds and their reduction to azo/azoxy derivatives are two significant sustainable chemical processes that have been widely used for the generation of dyes, pigments, pharmaceuticals, food additives, analytical reagents, etc. Owing to the broad and versatile application of azo and azoxy derivatives, various researchers have successfully carried out their reduction using reducing agents. However, the developed processes involve harsh reaction conditions, and generally are accompanied with hazardous by-products. Thus, to avoid these environmental issues, it is necessary to develop greener protocols. Based on this and in a quest to obtain promising results for the oxidation of aniline to azoxybenzene, Bal and co-workers employed the synergistic effect of two different metals (Cu and Cr) by fabricating copper chromite spinel nanoparticles (NPs).184 The catalyst preparation route involved the use of the anionic surfactant cetyltrimethylammonium chloride (CTAC) as a templating agent, which was added to the solution of metal ion salts, and then kept under solvothermal conditions at 200 °C for 18 h. Further, the calcination of obtained green fluffy material in a tubular furnace at 700 °C resulted in the formation of the desired spinel catalyst. The prudently constructed material successfully yielded suitable azoxybenzenes with outstanding selectivity (92%) and excellent conversion (78%) at 70 °C compared to the conventional and commercial catalysts. The underlying reason for this marvellous catalytic potency was accredited to the high surface/volume ratio and large accessible surfaces of the catalyst toward the reactant molecules. Besides, high functional group tolerance, broad substrate scope, exceptional yields and mild reaction conditions were some of the intriguing features of the developed protocol. One year later, the same research group reported a method for the facile synthesis of Ag/WO3via the sequential anchoring of Ag NPs on the surface of WO3 to produce azoxybenzenes from amino groups.185 The determination of the morphological and structural attributes of the catalyst was carried out using various techniques such as SEM, TEM, XRD, BET, XPS and TGA. After a deeper analysis, it was found that 0.10 g catalyst, 10 mL acetonitrile and 2.15 g of H2O2 at 25 °C were best reaction conditions, which oxidized 1 g of aniline, thereby yielding 99% of azoxybenzene. Moreover, the authors shed light on the significant role played by Ag nanoparticles in generating highly reactive peroxide (˙OOH) species, which initially attacked the N–H bond of aniline to form the phenyl hydroxyl amine intermediate (Scheme 9). In the next step, the intermediate further oxidized and ultimately produced azoxybenzene under similar conditions. Therefore, the findings led to the conclusion that due to the nanosize effect of the silver nanoparticles, Ag/WO3 exhibited incredible catalytic potency in comparison to bare Ag and WO3.Similarly, a study aimed at the selective oxidation of aniline was conducted by Cheng et al., wherein molybdenum oxide [N(C4H9)4]2[Mo6O19] was utilized as the catalyst for the reaction in the presence of H2O2 as an environmentally benign oxidant.186 Furthermore, to determine the best reaction conditions, various parameters such as influence of solvent, temperature, time, amount of catalyst and oxidant were investigated. The experimental investigations revealed that in the absence of catalyst, no desired product was formed, indicating the proficiency of the catalyst in the reaction system. Moreover, it was observed that the reaction failed to occur under an O2 atmosphere given that it was not reactive enough to activate the [N(C4H9)4]2[Mo6O19] composites, thereby signifying the importance of H2O2 in conducting the process. Under the optimized reaction conditions, the catalytic potency of the designed [N(C4H9)4]2[Mo6O19] composite was found to be exceptionally remarkable with high selectivity and excellent yield.
Besides yielding azoxybenzenes, the oxidation of amines with hydrogen peroxide also leads to the formation of nitroso compounds, which occupy a significant place in the field of discrete biological metabolic processes and synthetic chemical transformations, for instance, nitroso aldol cycloadditions, ene reactions, synthesis of indoles and azoarenes. In this direction, considerable efforts have been made by Lykakis and research team, wherein they successfully fabricated a novel, stable and recyclable Au/TiO2 nanocatalyst for the oxidation of amines.187 For this, the authors employed a facile deposition–precipitation method, wherein the surface of TiO2 was loaded with Au NPs. The synthesized Au/TiO2 displayed astonishing catalytic activity, which is higher than that of P25 and Hombikat. Moreover, deep insight into the mechanistic studies revealed that Au played a pivotal role in activating H2O2, which in situ generated highly reactive [Au–OH]˙+ species to trigger the concerned reaction (Scheme 10). Besides, the catalytic potency of Au/TiO2 was exploited for executing the oxidation of diverse aromatic amines, revealing promising outcomes. Based on these encouraging findings, the designed catalytic system was found to be highly chemoselective for obtaining nitroso motifs even for large-scale industrial synthesis.
Pyridine N-oxide, which is considered an active biological heterocyclic compound, has also been gaining immense popularity in the pharmaceutical sector. The benefits offered by this moiety are not just limited to its biological significance but also as an auxiliary agent, protecting group, oxidant and ligand for metal complexes in various fields. Considering this, a prolific attempt was made by Rajagopal et al., wherein the authors prepared an Ru(PVP)/c-Al2O3 composite by anchoring small-sized PVP-stabilised Ru NPs on the surface of Al2O3.188 During the preparation of the catalyst, PVP served as a suitable capping and stabilizing agent, which controlled the size and shape of the particle and protected them from aggregating in the system. The microscopy studies revealed that the Ru NPs possessed a spherical shape and uniform in size with an average diameter of 5–6 nm. This Ru(PVP)/c-Al2O3 nanocomposite exhibited unprecedented catalytic efficiency towards amine oxidation, yielding 99% of the desired product. Further, the authors also explored the scope and generality of the designed catalytic system by performing the reaction with a wide variety of tertiary amines, resulting in the product in good to excellent yield. Amine-bearing electron-donating groups reacted rapidly and afforded good yields of the corresponding desired product. However, reactants containing electron-withdrawing groups (–COOH, –Br and –CN) deactivated the basicity and nucleophilicity of the amine groups and lower yields were obtained in these cases.
In a recent work, Kidwai and Bhardwaj highlighted the significance of a newly designed heterogeneous nanocrystalline TiO2 catalyst in the transformation of amines into the corresponding oximes.189 Notably, the experimental analysis revealed an interesting fact that with only 10 mol% of nano-TiO2 and 1 mmol of benzylamine, 84% of the desired benzyloxime was produced in a short time of 6 h. The main reason for this marvellous efficacy is accredited to the higher surface area to volume ratio of nano-TiO2, which offered a larger number of active accessible sites in contrast to the traditional bulk TiO2. Over the past few decades, substantial progress has been made in designing efficient heterogeneous catalysts exhibiting tremendous potential for oxidation reactions. In this regard, Chu and group succeeded in developing a competent catalytic system, wherein the synergistic benefits of V2O5 and H2O2 were exploited for the oxidation benzyl amine to imines.190 For this, an adequate amount of V2O5 was initially added to a flask containing 1 equivalent of benzyl amine, followed by the addition of 3 equivalent of 30% of H2O2 to the above-mentioned reaction mixture and kept under constant stirring for 3.5 h. After completion of reaction, the yellow solid pure crystalline product was obtained, which required no further purification. It is worth mentioning that the nanometer size, smaller pores and abundant active sites were responsible for boosting the catalytic efficacy. One of the fascinating attributes of this protocol is that water was utilized as a green solvent, thereby eliminating the need for harsh organic solvents. Jain et al. proposed the idea of fabricating PEG@Fe3O4, which was eventually used for the oxidative cyanation of tertiary amines in the presence of H2O2.191 The utilization of PEG proved to be beneficial in the synthesis of the catalyst given that it endowed Fe3O4 NPs with high dispersibility and stability. The characterization of the designed nanocomposite was conducted using various physicochemical techniques such as SEM, TEM, XRD, UV-vis-NIR, FTIR, TGA, EDX and BET analysis. The microscopy studies revealed that the catalyst was spherical in nature, having a mean size of 10–25 nm. Due to the unique structural and morphological attributes of the designed PEG@Fe3O4, it demonstrated splendid activity towards the oxidation reaction. Besides, due to its magnetic nature, the catalyst was easily retrieved using an external magnet and recyclable for up to six consecutive runs without showing any significant loss in its activity.
Recent advancements in the arena of discrete cubic ferrite spinels have attracted interest from various researchers as suitable support materials in heterogeneous catalytic systems. In this case, Moradi-Shoeili et al. delineated the fabrication of metal ferrite nanocrystallites, MFe2O4 (M = Mn, Co, Ni, and Zn) through a facile co-precipitation method and utilized them for the oxidation of o-phenylenediamine (OPD) to 2,3-diaminophenazine with H2O2.192 Various physico-chemical studies revealed that the synthesized nanomaterials exhibited a spherical morphology with a size in the range of 20–40 nm. Moreover, a series of catalytic control experiments was carried out to obtain the best reaction conditions. According to the thorough assessment of the experimental results, it can be concluded that amongst the synthesized nanocomposites, MnFe2O4 demonstrated the highest catalytic activity.
Alkane oxidation
The selective conversion of alkanes to more valuable oxidized compounds is one of the most noteworthy transformations employed in industry, which generates countless motifs such as alcohols, aldehydes, and carboxylic acids of fundamental interest. However, the C–H bond is highly inert, and therefore these reactions are carried out under harsh temperature and pressure conditions. Thus, to overcome the problems associated with traditional protocols, chemists are relentlessly working towards developing greener and more sustainable approaches for these reactions. Diverse research groups are actively working in this direction. The idea of designing synergistically coupled tungsten–vanadia oxides (WO3/V2O5) for the liquid-phase oxidation of cyclohexane was executed well by Makgwane et al.193 On comparing individual oxides, the enhanced catalytic performance of the WO3/V2O5 composites was accredited to the redox and structural modifications. This protocol was found to be green and sustainable given that the reaction proceeded in H2O2 at room temperature. Additionally, the nanostructured WO3/V2O5 catalyst exhibited tremendous potential in generating free-radical oxy-functionalization, which afforded 90% conversion in just 6 h. Moreover, the catalytic efficacy of the nanocomposites was efficient after their reused for four cycles. Likewise, Bal and group devoted significant efforts to the synthesis of W(VI) oxide-supported Cu(II) nanoclusters with a size in the range of 35–65 nm.194 In this report, a promising hydrothermal approach was adopted for the preparation of Cu/WO3 nanoparticles, which were employed for the oxidation of cyclohexane to form adipic acid under the influence of H2O2. A plausible mechanistic route for the concerned reaction is outlined in Scheme 11. Further, the synthesized Cu/WO3 nanocatalyst presented 88% selectivity towards adipic acid at 70 °C. Besides, the catalyst possessed remarkable reusability for up to four consecutive runs without showing any appreciable loss in its activity.Further, Bordoloi et al. developed a strategy for the facile synthesis of WOx-SBA-15-based Ag nanoparticles for the direct oxidation of cyclohexane to adipic acid under ambient conditions.195 In this methodology, both WO3 and metallic Ag nanoparticles were incorporated in the mesoporous SBA-15 framework, as evidenced by the reduction in its pore size from 1.18 ccg−1 to 0.84–1.07 ccg−1. DFT studies revealed that the activation energy decreased to 1.96 eV in the case of Ag–W complex, and thus highlighted the significance of Ag-WOx-SBA-15 in promoting the selective oxidation of cyclohexanes. The catalyst showed high activity and selectivity in the presence of H2O2, which facilitated 89.6% conversion and ≥99% selectivity of adipic acid within a period of 16 h.
In another report, diverse silica-coated magnetite nanoparticle-supported catalysts [Fe3O4@SiO2-M2+, M = Mn2+, Co2+, Cu2+ and Zn2+] were synthesized by Martins and co-workers, which were employed in the oxidation of cyclohexane to KA oil using hydrogen peroxide as an oxidant under solvent-free conditions (Scheme 12).196 It was noteworthy that among the catalytic materials, Fe3O4@SiO2-Mn2+ and Fe3O4@SiO2-Cu2+ presented highest yield of oxygenated products (cyclohexanone and cyclohexanol, 24% and 21%, respectively) after 2 h of reaction at 80 °C. This protocol employed economically viable catalysts given that Fe3O4@SiO2-M2+ could be isolated from the reaction media with the aid of a readily available external magnet and efficiently reused for five successive runs with no appreciable loss in activity. Simple work-up procedure, cost-effective metal precursors, solvent-free approach and use of microwave irradiation for heating the reaction mixture are the additional salient features of this methodology, making this protocol sustainable and worthwhile.
One year later, Crombie and co-workers adopted an economically favourable route for the production of cyclohexane and cyclohexanol (KA oil) catalyzed by bi-functional Pd-based catalyst via the in situ generation of H2O2 from molecular H2 and O2.197 It was observed that the concentration of KA oil was improved by incorporating V in a Pd-supported catalyst. Besides, the in situ-produced H2O2 has immense benefits over the commercially utilized H2O2 given that it involves lower reaction temperatures.
To date, the oxidation of toluene is a challenge for researchers given that the available methods generate toxic chlorinated by-products, which are harmful to the environment. In this regard, Altaf et al. adopted an alternative greener and chlorine-free pathway to oxidize toluene to benzaldehyde and benzyl alcohol in the presence of MnMoO4 as catalytic entity and H2O2 as the oxidant at 80 °C.198 Here, the MnMoO4 nanocatalyst was synthesized via the solvothermal approach (Scheme 13), which was then exploited in the oxidation of toluene via C–H activation and it was observed that 40.62% of toluene was converted to the desired products after 18 h of reaction using 0.06 g of catalyst. The high conversion percentage of toluene and efficient selectivity towards benzaldehyde and benzyl alcohol highlighted the remarkable catalytic performance of MnMoO4 under mild reaction conditions. In addition, the catalyst could be efficiently reused for six cycles to transform toluene to the desired products with turnover frequencies in the range of 1.94–1.01 s−1.
 | ||
| Scheme 13 (a) Illustration depicting the fabrication of MnMoO4-based nanomaterial and (b) catalytic application of developed MnMoO4-based nanomaterial in the oxidation of toluene. | ||
Oxidation of alkenes
The oxidation of alkenes has acquired significance in the field of organic synthesis given that this oxidative transformation allows the conversion of carbon–carbon double (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) bonds into carbon oxygen (C–O) bonds, leading to the synthesis of valuable oxygen-containing chemicals (mostly alcohols or epoxides), which have widespread utility in the chemical sector. It is well known that oxidation can be used for versatile applications including epoxidation, dihydroxylation and carboxylation. However, traditional oxidation methods employ KMnO4 or other oxidizing agents such as OsO4–NaIO4, which do not comply with the principles of green and sustainable synthesis given that they lead to the generation of toxic by-products. Thus, the search for highly active and selective catalysts that can help in fulfilling the desirable attributes of sustainable catalytic oxidation is ongoing.
C) bonds into carbon oxygen (C–O) bonds, leading to the synthesis of valuable oxygen-containing chemicals (mostly alcohols or epoxides), which have widespread utility in the chemical sector. It is well known that oxidation can be used for versatile applications including epoxidation, dihydroxylation and carboxylation. However, traditional oxidation methods employ KMnO4 or other oxidizing agents such as OsO4–NaIO4, which do not comply with the principles of green and sustainable synthesis given that they lead to the generation of toxic by-products. Thus, the search for highly active and selective catalysts that can help in fulfilling the desirable attributes of sustainable catalytic oxidation is ongoing.
Inspired by the prospects offered by H2O2-mediated oxidations, Tong and co-workers designed hollow spheres of Mg–Cu ferrite (Mg0.5Cu0.5 Fe2O4) using carbon spheres as the template with the aid of a combination of approaches including sol–gel autocombustion, hydrothermal synthesis and co-precipitation for achieving the oxidation of styrene in the presence of H2O2 as the oxidant.199 For the preparation of the catalyst, the metal ions were first adsorbed on the surface of carbon spheres, and subsequently the carbon cores were eliminated via calcination in air, which resulted in the formation of the expected hollow spheres (Fig. 8). The hollow structure endowed the resultant material with a high surface area, as evidenced through BET analysis, and consequently enhanced catalytic activity (in terms of high turnover number and selectivity up to 75.2% for benzaldehyde). Additionally, the catalyst offered advantages of facile separation and remarkable reusability for up to six consecutive runs.
 | ||
| Fig. 8 (A) Schematic illustration of the preparation of Cu–Mg ferrite hollow nanospheres and (B) TEM images of (a) carbon spheres; (b and c) MCFO-1; (d) MCFO-2; (e) MCFO-3; and (f) MCFO-4 (MCFO-1 are Mg0.5Cu0.5Fe2O4 ferrite hollow spheres, while MCFO-2, MCFO-3 and MCFO-4 are Mg0.5Cu0.5Fe2O4 ferrite nanopowder synthesized via sol–gel auto-combustion, hydrothermal and co-precipitation route). Reproduced with permission from ref. 199. Copyright 2014, Elsevier B.V. | ||
On similar grounds, Rayati and co-researchers developed metalloporphyrin-based magnetic nanocatalysts comprised of meso-tetrakis(4-carboxyphenyl) porphyrinatoiron(III) chloride (Fe(TCPP)Cl) and meso-tetrakis(4-carboxyphenyl) porphyrinatomanganese(III) acetate (Mn(TCPP)OAc) discretely immobilized on an amine-functionalized silica-coated magnetite support (ASMNPs), as depicted in Scheme 14.200 The catalyst worked exceptionally well in oxidizing a wide range of alkenes with 100% conversion and 100% selectivity. The strategic design involved the basic principles of co-ordination chemistry, wherein metal–ligand complexes are usually tethered on the surface of a support material via co-ordinate/covalent linkages, which is named “covalent immobilization” and remains the preferred choice in the design of catalysts given that it avoids the problems associated with leaching typically encountered in the case of supports comprised of dispersed/physically adsorbed metal species. The durability of the catalyst was proven through a leaching test, and the results indicated that negligible leaching occurred, as evidenced through AAS. Thus, undeniably, the stability of the metalloporphyrins can be improved by immobilizing them on a support material.
Working along similar lines, Afshari et al. synthesized a cobalt salophen complex, which was supported on the surface of imidazole-functionalized magnetic nanoparticles (MNPs), and then utilized for the oxidation of alkenes (Scheme 15).201 A cobalt-based complex was targeted as the transition metal active complex owing to the ability of Co(II) to act a good oxygen transfer reagent, while MNPs as the core material not only offered facile catalytic separation but also displayed the added benefits of a high specific surface area together with the ability to avoid pore diffusion by allowing the catalytically active species to be distributed on only their outer surface. In the typical list of MNPs, spinel ferrite CoFe2O4 NPs were chosen because of their high magnetic anisotropy, notable magnetic saturation and outstanding chemical stability. The cobalt ferrite NPs were imparted special stability via encapsulation in a silica shell, besides acting as a good coating agent, also offered further prospects of surface functionalization owing to the presence of surface silanol groups, which were covalently tethered with a functionalizing agent/linker. An illustration of the catalyst preparation is provided in Scheme 11. The TEM micrograph revealed that the NPs were spherical in shape and possessed a size of less than 30 nm. The as-synthesized Co(salophen)-imid-Si@Si-MNPs (0.1 g) exhibited excellent efficiency in catalyzing the oxidation of alkenes (α-methylstyrene,4-methoxystyrene,4-methylstyrene,4-nitrostyrene,cyclohexene and 1-octene) using aqueous 30% H2O2, offering yields of up to 95% and recyclability in up to five continual runs.
 | ||
| Scheme 15 (a) Schematic representation of the formation of Co(salophen)-imid-Si@Si-MNP catalyst and (b) its application in catalyzing the oxidation of alkenes. | ||
Similarly, using the covalent immobilization approach, a transition metal complex (Mn Schiff base) was supported on different SiO2 nanomatrices (MCM-41, SBA-15 and carbon nanomaterials, i.e., CMK-3), leading to the formation of L@MCM-41-MnII, L@SBA-15-MnII and L@CMK-3-MnII for expediting the alkene epoxidation by Mavrogiorgou et al.202 Two different strategies were adopted for the general synthesis of the catalysts, involving two steps (ligand grafting and metalation and single-step method (ligand grafting and metalation accomplished in one-pot in one step (Scheme 16).
As highlighted in Scheme 17, demonstrating the synthesis of a CMK-3-supported Schiff base complex, a one-step covalent grafting methodology was adopted to obtain the desired catalyst. The resulting single-site heterogeneous catalysts exhibited excellent activity and selectivity in the formation of epoxides with the aid of H2O2 as the oxidant source and CH3COONH4 as the additive. In fact, the highest turnover frequency (TOF) of 634 h−1 was achieved with L@MCM-41-MnII and MnII-L@CMK-3 (Fig. 9).
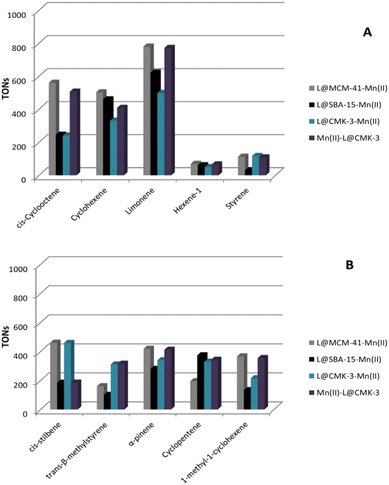 | ||
Fig. 9 (A and B) TOFs for alkene epoxidation catalyzed by L@MCM-41-MnII, L@SBA-15-MnII, L@CMK-3-MnII and MnII-L@CMK-3 in the presence of H2O2. Conditions: [catalyst![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O2 H2O2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH3COONH4 CH3COONH4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) substrate] = [1 substrate] = [1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2000 2000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000 1000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000]; catalyst = 1 mol in 0.85 mL CH3COCH3 1000]; catalyst = 1 mol in 0.85 mL CH3COCH3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH3OH (0.45 CH3OH (0.45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.40). Reproduced with permission from ref. 202. Copyright 2016, Elsevier B.V. 0.40). Reproduced with permission from ref. 202. Copyright 2016, Elsevier B.V. | ||
Lashanizadegan and team reported the preparation of a silica-coated magnetite (SiOs@Fe3O4 NP)-supported Cu(II) Schiff base complex (Scheme 18), which showed excellent efficiency for catalyzing the oxidation of diverse olefins (especially styrene and norbornene with 100% and 79% conversion, respectively).203 The average size of the nanoparticles, as evidenced through SEM and TEM, was found to be 27.35 nm. The striking attributes of this catalytic protocol included the use of a greener oxidant, shorter reaction time, good reusability of the catalyst, high conversion and decent selectivity.
Impressed by the high catalytic activity of surface-engineered CoFe2O4 NPs in the oxidation of alkenes, Liu et al. developed a magnetically separable yolk shell-structured CoFe2O4@hollow@mesoporous TiO2, and subsequently utilized it for the selective oxidation of styrenes (Scheme 19).138 The catalyst was prepared in a stepwise manner beginning from the synthesis of CoFe2O4 nanoparticles via a facile co-precipitation technique, followed by the coating of these NPs with silica using a modified Stober's method. The next step involved the introduction of a TiO2 layer using titanium tetraisopropanolate as the precursor, and finally etching of the intermediate silica layer.
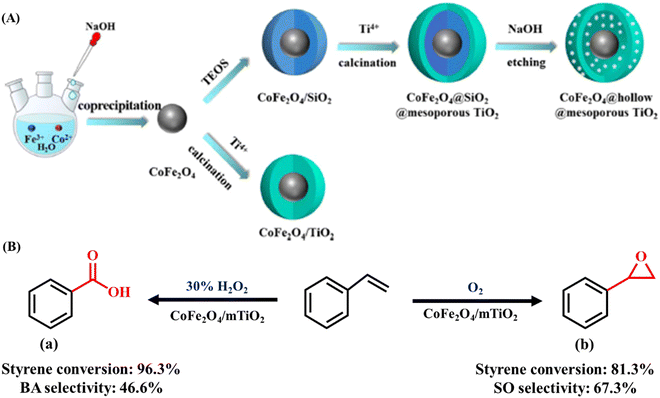 | ||
| Scheme 19 (A) Procedure for the synthesis of CoFe2O4@hollow@mesoporous TiO2 yolk–shell structure and CoFe2O4/TiO2 core–shell structure and (B) application in styrene conversion. Reproduced with permission from ref. 138. Copyright 2020, the American Chemical Society. | ||
Thus far, the spinel ferrites fabricated for the purpose of oxidizing alkenes saw a new interesting addition with the design and development of rare earth Ce and Nd”-doped spinel nickel ferrite (Ce–NiFe2O4 and Nd–NiFe2O4, respectively) via a sol–gel synthetic route.204 The route for the preparation of the NiFe2O4 NPs involved the dissolution of stoichiometric amounts of iron nitrate and nickel nitrate hexahydrate in water, followed by stirring at r.t., to which specific amounts of tartaric acid and polyethylene glycol (PEG) were added and the obtained sol was subjected to heating at 70 °C. For the synthesis of the Ce- and Nd-doped NPs, a similar strategy was adopted with the addition of Ce(NO3)3·6H2O/Nd(NO3)3·6H2O, respectively. The size of the bare and doped NPs was found to be in the range of 7–10 nm. Although the catalysts showed good conversion and selectivity in the presence of H2O2, TBHP could also be employed as the oxidant in the case of some of the substrates.
Lately, earth abundant metals have witnessed a radical revolution in the field of catalysis science and technology with their ability to replace the precious and toxic metals that were employed for catalyzing a number of important transformations for decades. Amongst the various earth abundant-based metal oxides synthesized thus far, MnO has seen expansive interest in its utility. One example was provided by Najafpour et al., where they synthesized nano-sized MnO using an aqueous solution of manganese nitrate and stirring it, followed by heating at 100 °C, which could achieve the epoxidation of both aromatic and non-aromatic olefins, displaying both selectivity and conversion up to 100%.205 Subsequently, the same team of researchers deposited nano-sized gold and silver on the surface of the active MnO layer and succeeded in obtaining enhanced catalytic activity of MnO towards the epoxidation of olefins using H2O2 and NaHCO3.206 Mechanistically, the catalyst worked by co-ordination of HCO4− to Mn present on the surface of MnO, followed by the transfer of an oxygen atom from the intermediate to olefin, which generated the epoxide.
Ghosh et al. designed a nanostructured silver-supported tungsten oxide (WO3) catalyst, which was comprised of metallic Ag NPs with a size of approx. 5 nm, which were supported on WO3 nanorods possessing a diameter in the range of 40–60 nm.207 The HR-TEM image of the catalyst demonstrated the one-dimensional rod-like morphology of WO3, which possessed an average width of ∼50 nm with uniformly dispersed silver particles having an average size of nearly ∼5 nm. This supported nanocatalyst effectively catalyzed the oxidation of cyclohexene to form adipic acid with a conversion of >99%, approx. 94% selectivity and notable TOF value of 12 h−1. The synthesis of adipic acid was attempted primarily considering its significance in the manufacture of a wide range of industrial products including nylon 6,6 plasticizers.
Tuning the morphology of a nanosized material using controlled synthetic pathways can result in tremendous boosting of its catalytic activity in oxidative transformations, which was proven by Sun and co-workers.208 By making proficient use of the sol–gel methodology, this team of researchers smartly designed titanium–zirconium mixed oxide spheres and titanium oxide cubes, which worked exceptionally well in the oxidation of alkenes. The focus of the synthesis was to design a material that possesses a new texture, which was achieved via the doping of either zirconium or L(+) or (−) sodium tartarate in a sol–gel. The Kirkendall effect was exploited in this process, which brought about a different texture as a result in the difference in the rate of diffusion of zirconium and titanium. The alternative use of tartarate was to induce chirality and bring about modification in the structure. Consequently, the catalytic oxidation of R(+) limonene could be carried out with good efficiency using H2O2 as the oxidant and 2 mol% of the Ti catalyst, showing up to 45% conversion, whereas the oxidative transformation of (−)-α-pinene could be accomplished with 100% conversion using a combination of the Ti catalyst (TIS) and H2O2 with higher diastereoselectivity of epoxide.
To achieve high selectivity of oxygenated products, tailoring the surface properties of catalysts by controlling their morphology has emerged as a rational solution, as already discussed in the example above. Likewise, Paul et al. also utilized a similar concept of structure-guided/morphology controlled catalytic activity and designed highly competent and robust 3-D octahedron NiCo2O4 spinel NPs using a one-pot poly(vinylpyrrolidone)-assisted hydrothermal route, which could successfully convert styrene into styrene oxide with 67% selectivity with the use of H2O2 as the oxidizing agent.209 The nanoparticles possessed a truncated octahedron shape with uniform size in the range of 20–30 nm, as indicated by the SEM micrograph. Furthermore, evidence of the existence of the NiCo2O4 spinel NPs in a pure phase was attained by EXAFS analysis. Additionally, the true heterogeneous nature of the catalyst was experimentally examined and proven by Sheldon's hot filtration test.
Another interesting example of improving the catalytic performance in styrene oxidation through morphology control was provided by Wang et al., where they designed supported cobalt oxide nanoparticles using a simple hydrothermal methodology.210 The morphology of the catalysts could be tuned by changing the synthetic process parameters such as time and temperature and the catalytic activity showed a significant difference with a change in the catalysts. As revealed by the SEM images displayed in Fig. 10, the silica-supported cobalt nanospheres possessed a mean diameter of 200 nm, which maintained their morphology even after calcination at 150 °C. With a increase rise in the calcination temperature to about 550 °C, the integrity of the nanostructure remained intact. However, calcination at 650 °C resulted in the formation of an egg-yolk structure (comprised of bundles of nanocrystals with structure confined by supported silica channels). Amongst the catalysts, Co3O4-550 showed the highest activity and selectivity to generate the desired epoxide (Table 4).
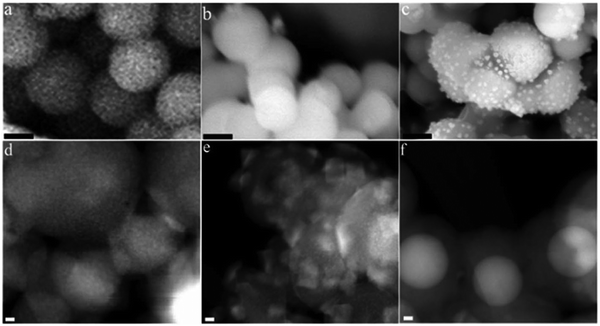 | ||
| Fig. 10 SEM images of (a) cobalt nanospheres before calcination, (b) Co3O4-150, and (c) Co3O4-550. STEM images (d) Co3O4-150, (e) Co3O4-550 and (f) Co3O4-650. Reproduced with permission from ref. 210. Copyright 2016, The Royal Society of Chemistry. | ||
| Particle size calculated from XRD | Selectivity (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Catalyst | [Co]b (wt%) | Before reaction | After reaction | Conv. (%) | Styrene epoxide | Benzaldehyde | Other product | TOFc | BET (m2 g−1) |
| a Reaction conditions: catalyst weight = 30 mg. b Determined by ICP-AES. c mol (C8H8 converted) mol(Co)−1 h−1. d The peak is too weak to do the calculation from the X-ray line broadening. | |||||||||
| Co3O4-150 | 9.1 | N/Ad> | N/Ad | 12 | 80 | 15 | 5 | 3.3 | 15 |
| Co3O4-550 | 8.4 | 11 | 12 | 97 | 91 | 5 | 4 | 28.3 | 46 |
| Co3O4-650 | 8.1 | 70 | 73 | 27 | 96 | 2 | 2 | 8.2 | 42 |
The objective of achieving unprecedented selectivity for the oxidation of styrene and its derivatives was also fulfilled by Rajabi and co-workers, who developed iron oxide NPs and supported them on a mesoporous aluminosilicate matrix.211 The catalyst was prepared by functionalizing SBA-15 with aminopropyl motifs to acquire NH2-functionalized SBA-15 and the ensuing step involved the deposition of iron oxide NPs, which was done using iron(III) nitrate nonahydrate as the precursor. The desirable feature of this catalytic protocol is that with the use of only 0.5 mol% of the active catalyst, remarkable yields of the targeted aldehyde products (>90%) and an exceptionally high oxidant efficiency (>95%) could be obtained. The supported catalyst showed good proficiency in its recovery, reuse as well as stability, showing no metal leaching.
In the quest to design a new promising class of transition metal-free oxide-based catalysts for the epoxidation of alkenes, Lueangchaichaweng along and team developed gallium oxide nanoparticles, which were embedded and stabilized in a mesoporous silica matrix.212 By fabricating the silica-supported gallium oxide NPs, they combined the intrinsic advantages of the high surface area to volume ratio of the nanoparticles and the defined pore size aspects of the mesoporous matrix material. Typically, the synthesis of the Ga2O3 NPs was conducted by the dissolution of gallium trichloride in a mixture of ethylene–glycol water, followed by the dropwise addition of NaOH, and after continuous stirring the contents were transferred to an autoclave. Thereafter, a series of mpGa2O3-x gallia/silica composites was prepared using a liquid-handling robotic workstation (programmed by Gemini software). For the preparation of the mesoporous silica-supported NPs, the templating solution was prepared via the addition of CTAB (a structure-directing reagent) to a mixture of water and ammonium hydroxide, followed by the stirring of the reaction contents in a closed bottle. In the following step, a suspension of Ga2O3 NPs in ethanol was prepared, which was subjected to ultrasonic irradiation for about 30 min, and subsequently stirred for some more time with the addition of tetraethyl orthosilicate (TEOS). The morphological analysis of the prepared material using TEM revealed that the Ga2O3 NPs possessed a narrow size distribution ranging between 1.5 to 3.0 nm (Fig. 11). Amongst the library of mesoporous composites synthesized, that containing 80% and 90% of gallium oxide nanoparticles rendered the best catalytic performance in terms of activity and selectivity (>99%) (Table 5).
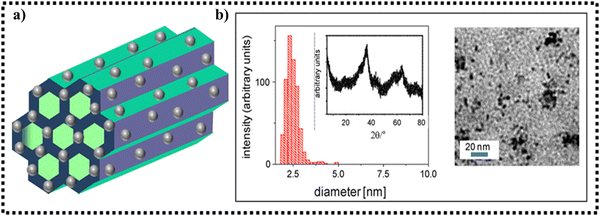 | ||
| Fig. 11 (a) Mesoporous composites of gallium oxide nanoparticles and silica. (b) Characterisation of Ga2O3-NP by DLS (left), wide-angle XRD (insert) and TEM (right). Reproduced with permission from ref. 212. Copyright 2013, Elsevier B.V. | ||
| Entry | Alkene | Reaction time (h) | Temperature (°C) | Epoxide yield (%) | Epoxide selectivity (%) |
|---|---|---|---|---|---|
| 1 | Cyclooctene | 4 | 80 | 25 | >99 |
| 2 | Cyclooctene | 6 | 80 | 34 | >99 |
| 3 | Cyclohexene | 6 | 70 | 10 | 90 |
| 4 | 1-Octene | 6 | 80 | 10 | 91 |
| 5 | Styrene | 4 | 80 | 7 | 49 |
| 6 | Styrene | 6 | 80 | 10 | 50 |
Polyoxometalates (POMs), as a class of celebrated transition metal oxygen clusters, have also received wide acceptance in the field of catalysis, and particularly proven promising for the H2O2-based epoxidation of olefins. However, the recovery of homogeneous POMs remains a challenge for scientists, thus witnessing an increase in the design of recoverable POMs, wherein a recoverable support matrix is chosen for the purpose of attaching the POMs. However, despite the attempts to engineer POMs through their immobilization on diverse support materials, and their subsequent use for the oxidation of alkenes, their low reaction rates, poor catalyst recovery ratio and leaching of the active catalytic species hinder their large-scale industrial applicability. Moreover, the epoxidation of bio-derived olefins, which form important vegetable oils using supported catalysts, has also been rarely attempted owing to the high viscosity and hydrophobicity associated with these type of olefins, which prevent the efficient mass transfer in the liquid–solid biphasic reaction medium. With the aim to overcome the shortcomings associated with supported POMs and considering the need to develop competent catalysts for the preparation of bio-derived olefins, the synthesis of an amphiphilic composite comprised of a magnetic core in addition to a dodecyl amine-modified polyoxometalate-paired poly-(ionic liquid) shell (Fe@PILPW-AM) was reported by Leng et al.213 The steps involved in the synthesis of the catalyst are depicted in Scheme 20. This catalyst could catalyse the epoxidation of bioderived olefins in the presence of H2O2 with extraordinarily high conversion and selectivity. The high activity of Fe@PILPW-AM was attributed to its amphiphilic nature and the possible intramolecular charge transfer between the polyanion (PILPW) and the amino group.
Oxidation of arenes
The production of high-value-added commodity chemicals at the industrial and laboratory scaled is a rapidly growing field of chemistry. Owing to the increasing demand for pharmaceuticals, perfumery, agrochemicals and clean technologies, the synthesis of resins, alcohols, esters and aldehydes is gaining considerable attention. Nevertheless, the direct synthesis of many of them is restricted due to the cumbersome work-up procedures, generation of by-products and use of toxic and expensive reagents. Thus, to overcome aforesaid issues and satisfy the economic and environmental requirements, the design and fabrication of recyclable, efficient, easily separable metal oxide-based heterogeneous catalysts has become one of the most active areas of research. In this regard, several efforts have been dedicated to catalysing the oxidation of arenes under mild conditions, which are summarized below.Arab and team prepared Fe3O4 supported on nanoporous carbon (Fe3O4/CMK-3) via the impregnation route, which was subsequently used for the oxidation of benzene to phenol.214 The superior activity and high selectivity of the developed catalyst were attributed to the generation of Fe3O4 on CMK-3 and uptake of excess hydroxyl radicals by CMK-3, respectively. Interestingly, the defect sites in CMK-3 were primarily responsible for the exceptional activity of the catalyst. The effect of different variables such as temperature, volume of H2O2, catalyst dosage and reaction time on the performance of the catalyst was scrutinized. In this direct hydroxylation process, 18% conversion with 92% phenol selectivity and 8.7 h−1 TOF was observed. The recyclability of the synthesized material was also analyzed, which indicated a slight decrease in its catalytic activity after completion of four runs.
Inspired by the versatility of bimetallic metal oxides, Acharyya and coworkers reported a facile hydrothermal, green and economical viable protocol to develop CuCr2O4 spinel NPs with an average size of 20–50 nm and applied them in the one-pot hydroxylation of benzene.215 The tetragonally distorted normal spinel structure and presence of copper in these NPs make them highly active for the selective oxidation of arenes. Investigations on temperature, reaction time, substrate to oxidant ratio, and solvent were carried out to ascertain the best outcomes. The reusability of the catalyst was also analyzed, which evinced that it could be recycled for more than 5 runs without any significant loss in its performance. These heterogeneous oxide NPs converted 72.5% benzene to the anticipated product with 94% selectivity at 80 °C and have potential to be explored for large-scale applications.
Moreover, considering the potential of three-dimensional (3D) nanoarchitectures in catalysis and extending their research work, this group designed raspberry-like CuCr2O4 spinel NPs via the hydrothermal route using cetyltrimethylammonium bromide (CTAB) as a cationic surfactant in a simple, low temperature and water-based pathway.216 The evolution of the desired morphology involved two stages, in which initially the NPs were produced, and then they self-assembled. Their unique surface properties and other features were thoroughly characterized using different physicochemical techniques, which suggested that the spinel phase was comprised of nanoparticles with a size in the range of 20 to 50 nm (Fig. 12). In the presence of H2O2, these raspberry-shape structures demonstrated remarkable catalytic activity at 80 °C for the oxidation of benzene to phenol with 68.5% conversion and 95% phenol selectivity. Further, no leaching was observed for up to 10 successive cycles, confirming the true heterogeneous nature of this 3D material.
 | ||
| Fig. 12 SEM images of (a and b) uncalcined and (c–e) calcined CuCr2O4 raspberry-like fresh and (f) spent catalyst (after 10 recycles). Reproduced with permission from ref. 216. Copyright 2014, the American Chemical Society. | ||
Proceeding on the same track, another methodology was described by Acharyya and team to generate spherical spinel CuCr2O4 NPs, wherein cetyl alcohol was employed as a soft template to form spinel structures.217 Here, the cationic surfactant served as a growth promoter and modifier, which controlled the particle size of the copper chromite NPs. The diameter of the NPs was found to be in the range of 20 to 40 nm. The studies illustrated that these nanostructures not only catalyzed the selective hydroxylation of benzene but also of other aromatic alkanes. With 67% conversion and 94% phenol selectivity at 75 °C, benzene was converted into phenol. The catalyst could be reused for more than five consecutive runs owing to its good stability and recyclability, thus making it a promising candidate for industrial purposes.
Considering the benefits of mixed nanostructured metal oxides, Makgwane and Ray fabricated a highly efficient and magnetically responsive copper–iron oxide (Cu–Fe) catalyst and successfully utilized it for the hydroxylation of benzene to phenol under benign reaction conditions.218 Their studies revealed that compared to the corresponding single nano metal oxide, the mixed binary catalyst displayed better activity and excellent performance. Various parameters such as solvent, reaction temperature, duration and amount of H2O2 were optimized to achieve the best results. It was also observed that the calcination temperature during the synthesis of the desired nanomaterial also a played a critical role in enhancing the activity of the catalyst. Calcination at 400 °C induced structural phase and redox transformations in the mixed CuFe catalyst, and consequently it efficaciously oxidized benzene to phenol with 44% conversion and 91% phenol selectivity in a sustainable manner.
On similar grounds, a novel and cost-effective method was adopted to synthesize nano Ag/ZnO using Zn(NO3)2·6H2O, AgNO3 and urea.219 The loading of Ag on ZnO was calculated by inductively coupled plasma (ICP) spectroscopy. Experimental exploration revealed that the ZnO-supported sliver proficiently catalyzed the oxidation of arenes with the aid of H2O2 under solvent-free conditions. The Ag-doped ZnO catalyst actively participated in the reaction, facilitated the oxidation of benzylic C–H bonds and gave the products in good to excellent yields.
Considering the benefits associated with vanadium-based materials, which are commonly known for their highly efficient oxidation of inert hydrocarbons, Wanna et al. delineated the fabrication of vanadium nanorods (Vnr) by using VCl3, acetonitrile and 35% H2O2.220 The synthesized Vnr were implemented for the selective oxidation of benzene to phenol in CH3CN using H2O2 and pyrazine-2-carboxylic acid (PCA) as the oxidant and co-catalyst, respectively (Scheme 21). The mechanistic studies disclosed that PCA assisted in the oxidation process by ameliorating the catalytic activity of Vnr with 87–99% phenol selectivity. This procedure was also successfully extended for the oxidation of toluene and benzyl alcohols. Due to the heterogeneity, high efficacy and flexibility of Vnr, the developed approach was proven to be a better alternative to existing methods.
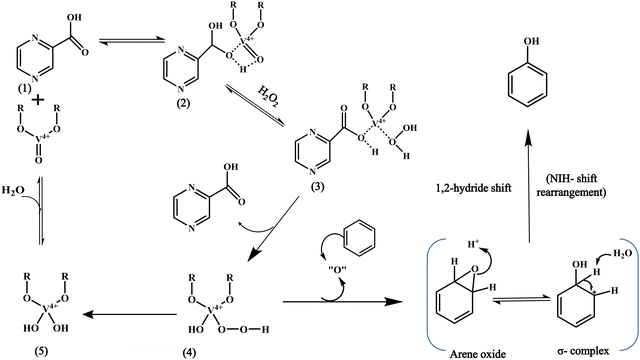 | ||
| Scheme 21 Proposed reaction mechanism for the formation of vanadium–PCA complex in H2O2(aq)–CH3CN system (R = bulk VOx). | ||
Recently, a novel recyclable catalyst was developed for the liquid-phase oxidation of ethyl substituted benzene to acetophenone, in which CuO NPs were impregnated on activated red mud (CuO_AARM).221 The analytical studies disclosed that the CuO NPs were largely scattered on the support material and played a crucial role in the progress of the reaction. Insights into the mechanistic pathway exposed that the process was driven by the generation of hydroperoxyl radicals and followed pseudo-first-order kinetics (Scheme 22). The synergism between the active components of red mud and ultra-small CuO NPs facilitated the reaction with a conversion efficiency of 86% together with 74% selectivity. The hydrothermally synthesized heterogeneous catalyst exhibited good stability and reusability.
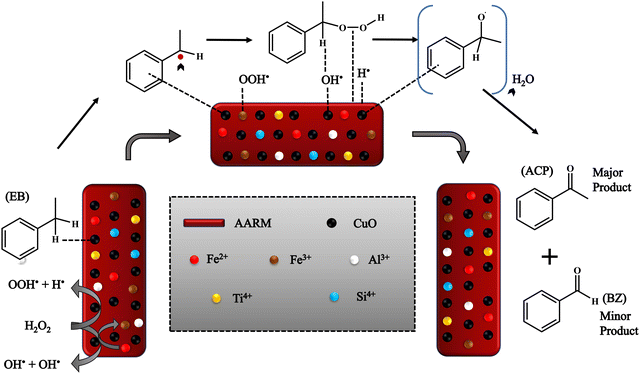 | ||
| Scheme 22 Surface-catalysed oxidation of ethyl benzene to acetophenone, with mechanism showing the cooperative effects of the presence of various metals in the CuO AARM catalyst. Reproduced with permission from ref. 221. Copyright 2021, The Royal Society of Chemistry. | ||
Conclusion
Over the past few decades, nanocatalysis has witnessed explosive growth, significantly contributing to shaping the sustainable future of modern society given that it provides a great array of opportunities such as lower energy consumption, economic viability, less environmental impact, industrial applications and sustainable development. The present review aimed to compile all the research work conducted on the utilization of surface-engineered metal oxide-based nanomaterials in expediting oxidation reactions, which is of paramount importance in manufacturing fine chemicals. The previous sections clearly demonstrated that nano-scale engineered metal oxide-based architectures have facilitated the large-scale production of an array of commodity products by speeding up a myriad of oxidative transformations. In fact, the use of hydrogen peroxide as an oxidant has also been proven to be a great asset in improvising industrially significant existing oxidative protocols for the greening of industrial manufacturing processes to tackle the significant environmental and economic issues.To date, there is no review in the literature that presents a compilation of the vast and scattered literature of heterogeneous nanocatalysis based on different oxide nanostructures in a comprehensible manner. All the presented information will be fruitful for researchers, academicians and industrial personnel to conceptualize the idea of a new generation of catalytic materials for sustainable chemical processes. Hence, it can bridge the gap between different research groups focused on designing distinct metal oxide-based nanostructures across the globe by providing vast knowledge in a single reference.
Despite the fabulous advancements, there is still room for great improvements in several areas, which further necessitate extra efforts to cover the remaining gaps. The focus of the future research must be directed towards designing multifunctional materials, addressing the complexity of catalysts, understanding their structure–activity relationships and mechanistic aspects, developing computational approaches and exploring the catalytic effect of different morphologies. An existing challenge is also obtaining identical nanostructures, where despite the fact that a variety of methods has been explored and provide prospects of controlling the size and morphology of NPs, developing a reliable strategy for the modulation of their texture, composition and electronic structure is crucial for various catalytic applications, especially electrocatalytic reactions. Another issue that confronts scientists is understanding the dynamics of the catalytic nanostructures given that they have a propensity to undergo structural and compositional changes during the course of the reaction. Henceforth, a correlation between the static and dynamic structure of the active catalytic centers is important. Also, emphasis is required in understanding the mechanisms at the nanoscale, which can be facilitated by the use of sophisticated and advanced computational tools. Undeniably, fruitful collaborations between theoretical and experimental chemists involving molecular modeling studies and reaction intermediate studies can have impressive effects in this regard. Further, researchers need to devote efforts towards the utilization of greener synthetic strategies that employ continuous flow microwave reactors and other advanced reactors for obtaining supported catalysts on a large scale. This certainly calls for further investigation in testing the efficacy of reactors for the production of a wide variety of supported nanocatalysts possessing durable properties. A number of gaps remain to be resolved towards the development of green and sustainable catalytic processes, which can be achieved only through united efforts of chemists, material engineers and other relevant stakeholders.
It is envisaged that this review will not only give directions to the upcoming scientific generation to decide their future work-plans in this field and improve on-going research programs but also offers benchmark reference for upcoming scientific generation, industrialists and academicians to construct next-generation benign catalytic technologies ranging from scaling up to final commercialization.
Conflicts of interest
There are no conflicts to declare.References
- S. Mitchell, R. Qin, N. Zheng and J. Pérez-Ramírez, Nat. Nanotechnol., 2021, 16, 129–139 CrossRef CAS PubMed.
- K. Pushparaj, W.-C. Liu, A. Meyyazhagan, A. Orlacchio, M. Pappusamy, C. Vadivalagan, A. A. Robert, V. A. Arumugam, H. Kamyab and J. J. Klemeš, Energy, 2022, 240, 122732 CrossRef CAS.
- M. Muzzio, J. Li, Z. Yin, I. M. Delahunty, J. Xie and S. Sun, Nanoscale, 2019, 11, 18946–18967 RSC.
- Y. Dai, Y. Wang, B. Liu and Y. Yang, Small, 2015, 11, 268–289 CrossRef CAS PubMed.
- R. K. Sharma, S. Yadav, S. Dutta, H. B. Kale, I. R. Warkad, R. Zbořil, R. S. Varma and M. B. Gawande, Chem. Soc. Rev., 2021, 50, 11293–11380 RSC.
- R. K. Sharma, S. Sharma, S. Dutta, R. Zboril and M. B. Gawande, Green Chem., 2015, 17, 3207–3230 RSC.
- M. B. Gawande, Y. Monga, R. Zboril and R. K. Sharma, Coord. Chem. Rev., 2015, 288, 118–143 CrossRef CAS.
- R. K. Sharma and S. Sharma, Dalton Trans., 2014, 43, 1292–1304 RSC.
- R. K. Sharma, S. Sharma and G. Gaba, RSC Adv., 2014, 4, 49198–49211 RSC.
- C. Xu, Q. Li, Q. Zhang, K. Li, H. Yin and S. Zhou, ACS Appl. Nano Mater., 2019, 2, 5086–5095 CrossRef CAS.
- S. Sadjadi, M. Akbari, B. Léger, E. Monflier and M. M. Heravi, ACS Sustainable Chem. Eng., 2019, 7, 6720–6731 CrossRef CAS.
- S. Dey and G. C. Dhal, Mater. Today Chem., 2020, 17, 100282 CrossRef CAS.
- S. H. Khan and B. Pathak, Environ. Nanotechnol., Monit. Manage., 2020, 13, 100290 Search PubMed.
- J. Zhang, H. Wang, L. Wang, S. Ali, C. Wang, L. Wang, X. Meng, B. Li, D. S. Su and F.-S. Xiao, J. Am. Chem. Soc., 2019, 141, 2975–2983 CrossRef CAS PubMed.
- R. K. Sharma, S. Dutta, S. Sharma, R. Zboril, R. S. Varma and M. B. Gawande, Green Chem., 2016, 18, 3184–3209 RSC.
- A. Akbari, M. Amini, A. Tarassoli, B. Eftekhari-Sis, N. Ghasemian and E. Jabbari, Nano-Struc. Nano-Objects, 2018, 14, 19–48 CrossRef CAS.
- G. Zhao, F. Yang, Z. Chen, Q. Liu, Y. Ji, Y. Zhang, Z. Niu, J. Mao, X. Bao and P. Hu, Nat. Commun., 2017, 8, 1–8 CrossRef CAS PubMed.
- R. Medhi, M. D. Marquez and T. R. Lee, ACS Appl. Nano Mater., 2020, 3, 6156–6185 CrossRef CAS.
- D. Wang and D. Astruc, Chem. Soc. Rev., 2017, 46, 816–854 RSC.
- Y. Ishii, S. Sakaguchi and T. Iwahama, Adv. Synth. Catal., 2001, 343, 393–427 CrossRef CAS.
- S. Wolfe and C. F. Ingold, J. Am. Chem. Soc., 1983, 105, 7755–7757 CrossRef CAS.
- J. Muzart, Chem. Rev., 1992, 92, 113–140 CrossRef CAS.
- M. Hu, W. Wu and H. Jiang, ChemSusChem, 2019, 12, 2911–2935 CrossRef CAS PubMed.
- G. De Faveri, G. Ilyashenko and M. Watkinson, Chem. Soc. Rev., 2011, 40, 1722–1760 RSC.
- R. Noyori, M. Aoki and K. Sato, Chem. Commun., 2003, 1977–1986 RSC.
- Q. Xing, Z. Hao, J. Hou, G. Li, Z. Gao, J. Gou, C. Li and B. Yu, J. Org. Chem., 2021, 86, 9563–9586 CrossRef CAS PubMed.
- D. Hâncu, J. Green and E. J. Beckman, Acc. Chem. Res., 2002, 35, 757–764 CrossRef PubMed.
- W. Xiao, S. Yang, P. Zhang, P. Li, P. Wu, M. Li, N. Chen, K. Jie, C. Huang and N. Zhang, Chem. Mater., 2018, 30, 2924–2929 CrossRef CAS.
- P. A. C. Bedoya, P. M. Botta, P. G. Bercoff and M. A. Fanovich, J. Alloys Compd., 2021, 860, 157892 CrossRef.
- T. Boningari, S. N. R. Inturi, V. I. Manousiouthakis and P. G. Smirniotis, Ind. Eng. Chem. Res., 2018, 57, 9054–9061 CrossRef CAS.
- G. D. Park, J. H. Kim and Y. C. Kang, Nanoscale, 2018, 10, 13531–13538 RSC.
- M. Verma, V. Kumar and A. Katoch, Mater. Sci. Semicond. Process., 2018, 76, 55–60 CrossRef CAS.
- N. Zamora-Romero, M. A. Camacho-Lopez, A. R. Vilchis-Nestor, V. H. Castrejon-Sanchez, G. Aguilar, S. Camacho-Lopez and M. Camacho-Lopez, Mater. Chem. Phys., 2020, 240, 122163 CrossRef CAS.
- P. Kupracz, E. Coy, K. Grochowska, J. Karczewski, J. Rysz and K. Siuzdak, Appl. Surf. Sci., 2020, 530, 147097 CrossRef CAS.
- C. Zheng, A. Gentils, J. Ribis, V. Borodin, L. Delauche and B. Arnal, Nucl. Instrum. Methods Phys. Res. B: Beam Interact. Mater. At., 2017, 409, 333–337 CrossRef CAS.
- S. Sankar, A. I. Inamdar, H. Im, S. Lee and D. Y. Kim, Ceram. Int., 2018, 44, 17514–17521 CrossRef CAS.
- E. Luévano-Hipólito and L. Torres-Martínez, Mater. Sci. Eng. B, 2017, 226, 223–233 CrossRef.
- J. Kim, V. T. Tran, S. Oh, C.-S. Kim, J. C. Hong, S. Kim, Y.-S. Joo, S. Mun, M.-H. Kim and J.-W. Jung, ACS Appl. Mater. Interfaces, 2018, 10, 41935–41946 CrossRef CAS PubMed.
- S. Saddeler, U. Hagemann and S. Schulz, Inorg. Chem., 2020, 59, 10013–10024 CrossRef CAS PubMed.
- M. F. Khan, A. H. Ansari, M. Hameedullah, E. Ahmad, F. M. Husain, Q. Zia, U. Baig, M. R. Zaheer, M. M. Alam and A. M. Khan, Sci. Rep., 2016, 6, 1–12 CrossRef PubMed.
- R. Yarbrough, K. Davis, S. Dawood and H. Rathnayake, RSC Adv., 2020, 10, 14134–14146 RSC.
- R. Joshi, Mater., 2018, 2, 104–110 Search PubMed.
- K. V. Manukyan, Y.-S. Chen, S. Rouvimov, P. Li, X. Li, S. Dong, X. Liu, J. K. Furdyna, A. Orlov and G. H. Bernstein, J. Phys. Chem. C, 2014, 118, 16264–16271 CrossRef CAS.
- J. Singh, V. Kumar, K.-H. Kim and M. Rawat, Environ. Res., 2019, 177, 108569 CrossRef CAS PubMed.
- A. Pugazhendhi, R. Prabhu, K. Muruganantham, R. Shanmuganathan and S. Natarajan, J. Photochem. Photobiol., B, 2019, 190, 86–97 CrossRef CAS PubMed.
- A. Muthuvinothini and S. Stella, Process Biochem., 2019, 77, 48–56 CrossRef CAS.
- M. Parashar, V. K. Shukla and R. Singh, J. Mater. Sci.: Mater. Electron., 2020, 31, 3729–3749 CrossRef CAS.
- N. P. Herring, A. B. Panda, K. AbouZeid, S. H. Almahoudi, C. R. Olson, A. Patel and M. El-Shall, Metal Oxide Nanomaterials for Chemical Sensors, 2013, 245–284 Search PubMed.
- N. Pokhrel, P. K. Vabbina and N. Pala, Ultrason. Sonochem., 2016, 29, 104–128 CrossRef CAS PubMed.
- A. Nandagudi, S. H. Nagarajarao, M. S. Santosh, B. M. Basavaraja, S. J. Malode, R. J. Mascarenhas and N. P. Shetti, Mater. Today Sustain., 2022, 100214 CrossRef.
- A. K. Ganguli, A. Ganguly and S. Vaidya, Chem. Soc. Rev., 2010, 39, 474–485 RSC.
- A. Kaur, B. Bajaj, A. Kaushik, A. Saini and D. Sud, Mater. Sci. Eng. B, 2022, 286, 116005 CrossRef CAS.
- J. Jeevanandam, Y. S. Chan and M. K. Danquah, ChemBioEng Rev., 2016, 3, 55–67 CrossRef.
- T. Tsuzuki, Commun. Chem., 2021, 4, 143 CrossRef CAS PubMed.
- V. Amendola, D. Amans, Y. Ishikawa, N. Koshizaki, S. Scirè, G. Compagnini, S. Reichenberger and S. Barcikowski, Chem. – Eur. J., 2020, 26, 9206–9242 CrossRef CAS PubMed.
- T. Bharat, S. Mondal, H. Gupta, P. Singh and A. Das, Mater. Today: Proc., 2019, 11, 767–775 CAS.
- P. Colson, C. Henrist and R. Cloots, J. Nanomater., 2013, 2013, 21 Search PubMed.
- A. V. Rane, K. Kanny, V. Abitha and S. Thomas, Synthesis of inorganic nanomaterials, Elsevier, 2018, pp. 121–139 Search PubMed.
- S. Shi, M. Cao, X. He and H. Xie, Cryst. Growth Des., 2007, 7, 1893–1897 CrossRef CAS.
- Z. Bao, Y. Yuan, C. Leng, L. Li, K. Zhao and Z. Sun, ACS Appl. Mater. Interfaces, 2017, 9, 16417–16425 CrossRef CAS PubMed.
- T. Guo, Y. Luo, Y. Zhang, Y.-H. Lin and C.-W. Nan, Cryst. Growth Des., 2014, 14, 2329–2334 CrossRef CAS.
- C. Jia, Y. Cheng, F. Bao, D. Chen and Y. Wang, J. Cryst. Growth, 2006, 294, 353–357 CrossRef CAS.
- F. N. Sayed and V. Polshettiwar, Sci. Rep., 2015, 5, 1–14 Search PubMed.
- L. Huang, H. Wang, Z. Wang, A. Mitra, D. Zhao and Y. Yan, Chem. Mater., 2002, 14, 876–880 CrossRef CAS.
- J. Li, X. Liu, Q. Han, X. Yao and X. Wang, J. Mater. Chem. A, 2013, 1, 1246–1253 RSC.
- Y. Li and W. Shen, Chem. Soc. Rev., 2014, 43, 1543–1574 RSC.
- X. Xie and W. Shen, Nanoscale, 2009, 1, 50–60 RSC.
- H.-X. Mai, L.-D. Sun, Y.-W. Zhang, R. Si, W. Feng, H.-P. Zhang, H.-C. Liu and C.-H. Yan, J. Phys. Chem., 2005, 109, 24380–24385 CrossRef CAS PubMed.
- Y. Zheng, Y. Cheng, Y. Wang, F. Bao, L. Zhou, X. Wei, Y. Zhang and Q. Zheng, J. Phys. Chem. B, 2006, 110, 3093–3097 CrossRef CAS PubMed.
- B. M. Choudary, R. S. Mulukutla and K. J. Klabunde, J. Am. Chem. Soc., 2003, 125, 2020–2021 CrossRef CAS PubMed.
- S. Konar, H. Kalita, N. Puvvada, S. Tantubay, M. K. Mahto, S. Biswas and A. Pathak, J. Catal., 2016, 336, 11–22 CrossRef CAS.
- H. Tan, J. Wang, S. Yu and K. Zhou, Environ. Sci. Technol., 2015, 49, 8675–8682 CrossRef CAS PubMed.
- X.-Y. Lou, R. Boada, L. Simonelli and M. Valiente, J. Colloid Interface Sci., 2022, 614, 460–467 CrossRef CAS PubMed.
- N. Kitchamsetti, M. S. Ramteke, S. R. Rondiya, S. R. Mulani, M. S. Patil, R. W. Cross, N. Y. Dzade and R. S. Devan, J. Alloys Compd., 2021, 855, 157337 CrossRef CAS.
- Y. Li, M. Jiao, H. Zhao and M. Yang, Sens. Actuators, B, 2018, 264, 285–295 CrossRef CAS.
- E. Comini, Mater. Today Adv., 2020, 7, 100099 CrossRef.
- M. H. Raza, N. Kaur, E. Comini and N. Pinna, ACS Appl. Mater. Interfaces, 2020, 12, 4594–4606 CrossRef CAS PubMed.
- A. Xia, W. Yu, J. Yi, G. Tan, H. Ren and C. Liu, J. Electroanal. Chem., 2019, 839, 25–31 CrossRef CAS.
- B. Abebe, E. A. Zereffa, A. Tadesse and H. Murthy, Nanoscale Res. Lett., 2020, 15, 1–19 CrossRef PubMed.
- M. Sundrarajan, K. Bama, M. Bhavani, S. Jegatheeswaran, S. Ambika, A. Sangili, P. Nithya and R. Sumathi, J. Photochem. Photobiol. B: Biology, 2017, 171, 117–124 CrossRef CAS PubMed.
- J. Rahimi, S. S. Mirmohammadi and A. Maleki, Front. Chem. Sci. Eng., 2021, 15, 1008–1020 CrossRef CAS.
- M. Z. Kassaee, H. Masrouri and F. Movahedi, Appl. Catal. A: Gen., 2011, 395, 28–33 CrossRef CAS.
- F.-C. Zheng, Q.-W. Chen, L. Hu, N. Yan and X.-K. Kong, Dalton Trans., 2014, 43, 1220–1227 RSC.
- F. Zamani, S. M. Hosseini and S. Kianpour, Solid State Sci., 2013, 26, 139–143 CrossRef CAS.
- P. Singh, P. Yadav, A. Mishra and S. K. Awasthi, ACS Omega, 2020, 5, 4223–4232 CrossRef CAS PubMed.
- J. He, S. Yang and A. Riisager, Catal. Sci. Technol., 2018, 8, 790–797 RSC.
- R. Eisavi and K. Naseri, RSC Adv., 2021, 11, 13061–13076 RSC.
- N. Ž. Knežević, B. G. Trewyn and V. S.-Y. Lin, Chem. Commun., 2011, 47, 2817–2819 RSC.
- A. Maleki, Z. Hajizadeh and P. Salehi, Sci. Rep., 2019, 9, 1–8 CrossRef CAS PubMed.
- S. Yu, H. J. Yun, D. M. Lee and J. Yi, J. Mater. Chem., 2012, 22, 12629–12635 RSC.
- H. Hagiwara, T. Nakamura, T. Hoshi and T. Suzuki, Green Chem., 2011, 13, 1133–1137 RSC.
- R. Tandon, N. Tandon and S. M. Patil, RSC Adv., 2021, 11, 29333–29353 RSC.
- X. Li, O. Niitsoo and A. Couzis, J. Colloid Interface Sci., 2013, 394, 26–35 CrossRef CAS PubMed.
- A. Zhu, H. Diao, Q. Rong and A. Cai, J. Appl. Polym. Sci., 2010, 116, 2866–2873 CAS.
- N. Nakayama and T. Hayashi, Polym. Degrad. Stab., 2007, 92, 1255–1264 CrossRef CAS.
- S. Saire-Saire, E. C. Barbosa, D. Garcia, L. H. Andrade, S. Garcia-Segura, P. H. Camargo and H. Alarcon, RSC Adv., 2019, 9, 22116–22123 RSC.
- Y.-m Kim, S. Farrah and R. H. Baney, Int. J. Antimicrob. Agents, 2007, 29, 217–222 CrossRef CAS PubMed.
- S. P. Pujari, L. Scheres, A. T. Marcelis and H. Zuilhof, Angew. Chem., Int. Ed., 2014, 53, 6322–6356 CrossRef CAS PubMed.
- K. Sever, M. Sarikanat, Y. Seki, G. Erkan and Ü. H. Erdoğan, J. Compos. Mater., 2010, 44, 1913–1924 CrossRef CAS.
- H. Kim, K. Okubo, T. Fujii and K. Takemura, J. Adhes. Sci. Technol., 2013, 27, 1348–1358 CrossRef CAS.
- M. Abdelmouleh, S. Boufi, M. N. Belgacem, A. Dufresne and A. Gandini, J. Appl. Polym. Sci., 2005, 98, 974–984 CrossRef CAS.
- L. A. Pothan, J. George and S. Thomas, Compos. Interfaces, 2002, 9, 335–353 CrossRef CAS.
- L. A. Pothan and S. Thomas, Compos. Sci. Technol., 2003, 63, 1231–1240 CrossRef CAS.
- L. A. Pothan, S. Thomas and G. Groeninckx, Compos. Part A Appl. Sci., 2006, 37, 1260–1269 CrossRef.
- H. Shah, B. Srinivasulu and S. Shit, Int. J. Sci. Eng. Technol., 2012, 1, 86–90 Search PubMed.
- M. Joshy, L. Mathew and R. Joseph, Int. J. Polym. Mater., 2008, 58, 2–20 CrossRef.
- P. Sreekumar, S. P. Thomas, J. Marc Saiter, K. Joseph, G. Unnikrishnan and S. Thomas, Compos. A Appl. Sci. Manuf, 2009, 40, 1777–1784 CrossRef.
- P. K. Kushwaha and R. Kumar, Polym. Plast. Technol. Eng., 2009, 49, 45–52 CrossRef.
- M. A. Sawpan, K. L. Pickering and A. Fernyhough, Compos. A Appl. Sci. Manuf., 2012, 43, 519–526 CrossRef CAS.
- K. O. Reddy, M. Shukla, C. U. Maheswari and A. V. Rajulu, J. Compos. Mater., 2012, 46, 2987–2998 CrossRef.
- T.-Y. Ma, X.-J. Zhang and Z.-Y. Yuan, J. Phys. Chem. C, 2009, 113, 12854–12862 CrossRef CAS.
- X. Wei and X. Shi, J. Phys. Chem. C, 2014, 118, 4213–4221 CrossRef CAS.
- Y.-P. Zhu, Y.-L. Liu, T.-Z. Ren and Z.-Y. Yuan, Nanoscale, 2014, 6, 6627–6636 RSC.
- K. Lv, J. Han, C.-T. Yang, C.-M. Cheng, Y.-M. Luo and X.-L. Wang, Chem. Eng. J., 2016, 302, 368–376 CrossRef CAS.
- V. Luca, J. J. Tejada, D. Vega, G. Arrachart and C. Rey, Inorg. Chem., 2016, 55, 7928–7943 CrossRef CAS PubMed.
- R. Silbernagel, C. H. Martin and A. Clearfield, Inorg. Chem., 2016, 55, 1651–1656 CrossRef CAS PubMed.
- R. Silbernagel, T. C. Shehee, C. H. Martin, D. T. Hobbs and A. Clearfield, Chem. Mater., 2016, 28, 2254–2259 CrossRef CAS.
- D. J. Miller, L. Sun, M. Walzak, N. Mclntyre, D. Chvedov and A. Rosenfeld, Surf. Interface Anal., 2003, 35, 463–476 CrossRef CAS.
- P. Taheri, J. Wielant, T. Hauffman, J. Flores, F. Hannour, J. De Wit, J. Mol and H. Terryn, Electrochim. Acta, 2011, 56, 1904–1911 CrossRef CAS.
- T. Bauer, T. Schmaltz, T. Lenz, M. Halik, B. Meyer and T. Clark, ACS Appl. Mater. Interfaces, 2013, 5, 6073–6080 CrossRef CAS PubMed.
- F. Cheng, E. Verrelli, F. A. Alharthi, S. Das, T. D. Anthopoulos, K. T. Lai, N. T. Kemp, M. O'Neill and S. M. Kelly, RSC Adv., 2020, 10, 25540–25546 RSC.
- L. M. Bishop, J. C. Yeager, X. Chen, J. N. Wheeler, M. D. Torelli, M. C. Benson, S. D. Burke, J. A. Pedersen and R. J. Hamers, Langmuir, 2012, 28, 1322–1329 CrossRef CAS PubMed.
- S. E. Denmark and N. S. Werner, J. Am. Chem. Soc., 2008, 130, 16382–16393 CrossRef CAS PubMed.
- S. M. Sieburth and W. Mu, J. Org. Chem., 1993, 58, 7584–7586 CrossRef CAS.
- A. Ulman, Chem. Rev., 1996, 96, 1533–1554 CrossRef CAS PubMed.
- A. Glaser, J. Foisner, H. Hoffmann and G. Friedbacher, Langmuir, 2004, 20, 5599–5604 CrossRef CAS PubMed.
- P. H. Mutin, G. Guerrero and A. Vioux, J. Mater. Chem., 2005, 15, 3761–3768 RSC.
- E. Hoque, J. DeRose, G. Kulik, P. Hoffmann, H. Mathieu and B. Bhushan, J. Phys. Chem. B, 2006, 110, 10855–10861 CrossRef CAS PubMed.
- M. A. White, J. A. Johnson, J. T. Koberstein and N. J. Turro, J. Am. Chem. Soc., 2006, 128, 11356–11357 CrossRef CAS PubMed.
- L. Forget, F. Wilwers, J. Delhalle and Z. Mekhalif, Appl. Surf. Sci., 2003, 205, 44–55 CrossRef CAS.
- G. P. Holland, R. Sharma, J. O. Agola, S. Amin, V. C. Solomon, P. Singh, D. A. Buttry and J. L. Yarger, Chem. Mater., 2007, 19, 2519–2526 CrossRef CAS.
- G. Guerrero, P. Mutin and A. Vioux, Chem. Mater., 2001, 13, 4367–4373 CrossRef CAS.
- Q.-L. Zhang, L.-C. Du, Y.-X. Weng, L. Wang, H.-Y. Chen and J.-Q. Li, J. Phys. Chem. B, 2004, 108, 15077–15083 CrossRef CAS.
- X. Jia, J. Ma, F. Xia, Y. Xu, J. Gao and J. Xu, Nat. Commun., 2018, 9, 1–7 CrossRef CAS PubMed.
- K. Wilson and J. H. Clark, Pure Appl. Chem., 2000, 72, 1313–1319 CrossRef CAS.
- C. Vogt and B. M. Weckhuysen, Nat. Rev. Chem., 2022, 6, 89–111 CrossRef.
- P. Manjunathan, V. Prasanna and G. V. Shanbhag, Sci. Rep., 2021, 11, 15718 CrossRef CAS PubMed.
- L. Liu, W. He, Z. Fang, Z. Yang, K. Guo and Z. Wang, Ind. Eng. Chem. Res., 2020, 59, 19938–19951 CrossRef CAS.
- S. Mirfakhraei, M. Hekmati, F. H. Eshbala and H. Veisi, New J. Chem., 2018, 42, 1757–1761 RSC.
- A. Ghorbani-Choghamarani, Z. Darvishnejad and B. Tahmasbi, Inorg. Chim. Acta, 2015, 435, 223–231 CrossRef CAS.
- A. Zheng, S. Li, S.-B. Liu and F. Deng, Acc. Chem. Res., 2016, 49, 655–663 CrossRef CAS PubMed.
- P. M. Kouotou, H. Vieker, Z. Tian, P. T. Ngamou, A. El Kasmi, A. Beyer, A. Gölzhäuser and K. Kohse-Höinghaus, Catal. Sci. Technol., 2014, 4, 3359–3367 RSC.
- T. Mallat and A. Baiker, Chem. Rev., 2004, 104, 3037–3058 CrossRef CAS PubMed.
- C. Parmeggiani, C. Matassini and F. Cardona, Green Chem., 2017, 19, 2030–2050 RSC.
- D. Wang, P. Wang, S. Wang, Y.-H. Chen, H. Zhang and A. Lei, Nat. Commun., 2019, 10, 1–8 CrossRef PubMed.
- S. E. Davis, M. S. Ide and R. J. Davis, Green Chem., 2013, 15, 17–45 RSC.
- I. Tamiolakis, I. N. Lykakis, A. P. Katsoulidis, C. D. Malliakas and G. S. Armatas, J. Mater. Chem., 2012, 22, 6919–6927 RSC.
- J. L. Sousa, I. C. Santos, M. M. Simões, J. A. Cavaleiro, H. I. Nogueira and A. M. Cavaleiro, Catal. Commun., 2011, 12, 459–463 CrossRef CAS.
- B. Karimi, F. B. Rostami, M. Khorasani, D. Elhamifar and H. Vali, Tetrahedron, 2014, 70, 6114–6119 CrossRef CAS.
- P. Garcia-Albar, N. Lazaro, Z. A. ALOthman, A. A. Romero, R. Luque and A. Pineda, Mol. Catal., 2021, 506, 111537 CrossRef CAS.
- F. Rajabi, A. Pineda, S. Naserian, A. M. Balu, R. Luque and A. A. Romero, Green Chem., 2013, 15, 1232–1237 RSC.
- R. K. Sharma, S. Yadav, S. Sharma, S. Dutta and A. Sharma, ACS Omega, 2018, 3, 15100–15111 CrossRef CAS PubMed.
- S. Yadav, S. Sharma, S. Dutta, A. Sharma, A. Adholeya and R. K. Sharma, Inorg. Chem., 2020, 59, 8334–8344 CrossRef CAS PubMed.
- S. Yadav, R. Dixit, S. Sharma, S. Dutta, B. Arora, P. Rana, B. Kaushik, A. Adholeya, M. B. Gawande and R. K. Sharma, Mater. Chem. Front., 2021, 5, 7343–7355 RSC.
- S. Yadav, R. Dixit, S. Sharma, S. Dutta, B. Arora, P. Rana, B. Kaushik, K. Solanki and R. K. Sharma, New J. Chem., 2022, 46, 10829–10843 RSC.
- S. Yadav, R. Dixit, S. Sharma, S. Dutta, K. Solanki and R. K. Sharma, Mater. Adv., 2021, 2, 2153–2187 RSC.
- C. Ragupathi, J. J. Vijaya, S. Narayanan, S. Jesudoss and L. J. Kennedy, Ceram. Int., 2015, 41, 2069–2080 CrossRef CAS.
- F. Shi, M. K. Tse, M.-M. Pohl, J. Radnik, A. Brückner, S. Zhang and M. Beller, J. Mol. Catal. A, 2008, 292, 28–35 CrossRef CAS.
- F. Sadri, A. Ramazani, A. Massoudi, M. Khoobi, R. Tarasi, A. Shafiee, V. Azizkhani, L. Dolatyari and S. W. Joo, Green Chem. Lett. Rev., 2014, 7, 257–264 CrossRef CAS.
- P. B. Bhat and B. R. Bhat, New J. Chem., 2015, 39, 273–278 RSC.
- P. B. Bhat and B. R. Bhat, Appl. Nanosci., 2016, 6, 425–435 CrossRef CAS.
- A. Pourjavadi, S. H. Hosseini, F. M. Moghaddam, B. K. Foroushani and C. Bennett, Green Chem., 2013, 15, 2913–2919 RSC.
- A. Heidarnezhad and F. Zamani, Catal. Commun., 2015, 60, 105–109 CrossRef CAS.
- A. Maleki, R. Rahimi and S. Maleki, Environ. Chem. Lett., 2016, 14, 195–199 CrossRef CAS.
- A. Chakraborty, T. Chakraborty, M. I. Menendez and T. Chattopadhyay, ACS Omega, 2019, 4, 11558–11565 CrossRef CAS PubMed.
- J. Ni, W.-J. Yu, L. He, H. Sun, Y. Cao, H.-Y. He and K.-N. Fan, Green Chem., 2009, 11, 756–759 RSC.
- S. Verma and S. L. Jain, Inorg. Chem. Front., 2014, 1, 534–539 RSC.
- P. N. Amaniampong, Q. T. Trinh, J. J. Varghese, R. Behling, S. Valange, S. H. Mushrif and F. Jérôme, Green Chem., 2018, 20, 2730–2741 RSC.
- A. Naeimi, M. Honarmand and A. Sedri, Ultrason. Sonochem., 2019, 50, 331–338 CrossRef CAS PubMed.
- A. Naeimi, S. Abbasspour and S. A. Torabizadeh, Artificial Cells, Nanomed. Biotechnol., 2020, 48, 560–571 CrossRef CAS PubMed.
- M. Amini, Y. Mousazade, Z. Zand, M. Bagherzadeh and M. M. Najafpour, Sci. Rep., 2021, 11, 1–10 CrossRef PubMed.
- R. Behling, G. Chatel and S. Valange, Ultrason. Sonochem., 2017, 36, 27–35 CrossRef CAS PubMed.
- S. Saha, S. B. Abd Hamid and T. H. Ali, Appl. Surf. Sci., 2017, 394, 205–218 CrossRef CAS.
- M. Heidari-Golafzani, M. Rabbani, R. Rahimi and A. Azad, RSC Adv., 2015, 5, 99640–99645 RSC.
- Z. Zhang, Q. Zhu, J. Ding, X. Liu and W.-L. Dai, Appl. Catal. A: Gen., 2014, 482, 171–178 CrossRef CAS.
- F. Rajabi, S. Naserian, A. Primo and R. Luque, Adv. Synth. Catal., 2011, 353, 2060–2066 CrossRef CAS.
- A. Rostami and B. Atashkar, J. Mol. Catal. A: Chem., 2015, 398, 170–176 CrossRef CAS.
- Y. Kuwahara, T. Ando, H. Kango and H. Yamashita, Chem. – Eur. J., 2017, 23, 380–389 CrossRef CAS PubMed.
- W. Zhao, C. Yang, H. Sun, Z. Cheng and T. Chang, New J. Chem., 2018, 42, 19349–19352 RSC.
- F. Rajabi, T. Kakeshpour and M. R. Saidi, Catal. Commun., 2013, 40, 13–17 CrossRef CAS.
- A. Sabet, E. Kolvari, N. koukabi, A. Fakhraee, M. Ramezanpour and G. Bahmannia, J. Sulfur Chem., 2015, 36, 300–307 CrossRef CAS.
- A. M. Kulkarni, U. V. Desai, K. S. Pandit, M. A. Kulkarni and P. P. Wadgaonkar, RSC Adv., 2014, 4, 36702–36707 RSC.
- M. Nikoorazm, F. Ghorbani, A. Ghorbani-Choghamarani and Z. Erfani, Phosphorus, Sulfur Silicon Relat. Elem., 2018, 193, 552–561 CrossRef CAS.
- S. S. Acharyya, S. Ghosh and R. Bal, ACS Sustainable Chem. Eng., 2014, 2, 584–589 CrossRef CAS.
- S. Ghosh, S. S. Acharyya, T. Sasaki and R. Bal, Green Chem., 2015, 17, 1867–1876 RSC.
- S. Han, Y. Cheng, S. Liu, C. Tao, A. Wang, W. Wei, H. Yu and Y. Wei, Angew. Chem., Int. Ed., 2021, 133, 6452–6455 CrossRef.
- S. Fountoulaki, P. L. Gkizis, T. S. Symeonidis, E. Kaminioti, A. Karina, I. Tamiolakis, G. S. Armatas and I. N. Lykakis, Adv. Synth. Catal., 2016, 358, 1500–1508 CrossRef CAS.
- P. Veerakumar, S. Balakumar, M. Velayudham, K.-L. Lu and S. Rajagopal, Catal. Sci. Technol., 2012, 2, 1140–1145 RSC.
- M. Kidwai and S. Bhardwaj, Synth. Commun., 2011, 41, 2655–2662 CrossRef CAS.
- G. Chu and C. Li, Org. Biomol. Chem., 2010, 8, 4716–4719 RSC.
- V. Panwar, P. Kumar, A. Bansal, S. S. Ray and S. L. Jain, Appl. Catal. A Gen., 2015, 498, 25–31 CrossRef CAS.
- F. Vetr, Z. Moradi-Shoeili and S. Özkar, Appl. Organomet. Chem., 2018, 32, e4465 CrossRef.
- P. R. Makgwane and S. Ray, Catal. Commun., 2014, 54, 118–123 CrossRef CAS.
- S. S. Acharyya, S. Ghosh and R. Bal, Green Chem., 2015, 17, 3490–3499 RSC.
- R. Goyal, B. Sarkar, S. Sameer, A. Bag and A. Bordoloi, ACS Appl. Nano Mater., 2019, 2, 5989–5999 CrossRef CAS.
- N. M. Martins, A. J. Pombeiro and L. M. Martins, Catal. Commun., 2019, 125, 15–20 CrossRef CAS.
- C. M. Crombie, R. J. Lewis, D. Kovačič, D. J. Morgan, T. J. Slater, T. E. Davies, J. Edwards, M. S. Skjøth-Rasmussen and G. J. Hutchings, Catal. Lett., 2021, 151, 2762–2774 CrossRef CAS.
- H. Shoukat, A. A. Altaf, M. Hamayun, S. Ullah, S. Kausar, M. Hamza, S. Muhammad, A. Badshah, N. Rasool and M. Imran, ACS Omega, 2021, 6, 19606–19615 CrossRef CAS PubMed.
- J. Tong, X. Cai, H. Wang and Q. Zhang, Mater. Res. Bull., 2014, 55, 205–211 CrossRef CAS.
- S. Rayati, D. Moradi and F. Nejabat, New J. Chem., 2020, 44, 19385–19392 RSC.
- M. Afshari, M. Gorjizadeh, S. Nazari and M. Naseh, J. Magn. Magn. Mater., 2014, 363, 13–17 CrossRef CAS.
- A. Mavrogiorgou, M. Baikousi, V. Costas, E. Mouzourakis, Y. Deligiannakis, M. Karakassides and M. Louloudi, J. Mol. Catal. A: Chem., 2016, 413, 40–55 CrossRef CAS.
- M. Lashanizadegan, Z. Gorgannejad and M. Sarkheil, Inorg. Chem. Commun., 2021, 125, 108373 CrossRef CAS.
- M. S. S. Adam, A. M. Hafez and M. M. Khalaf, J. Iran. Chem. Soc., 2020, 17, 3237–3250 CrossRef CAS.
- M. M. Najafpour, F. Rahimi, M. Amini, S. Nayeri and M. Bagherzadeh, Dalton Trans., 2012, 41, 11026–11031 RSC.
- M. M. Najafpour, M. Amini, D. J. Sedigh, F. Rahimi and M. Bagherzadeh, RSC Adv., 2013, 3, 24069–24074 RSC.
- S. Ghosh, S. S. Acharyya, S. Adak, L. S. Konathala, T. Sasaki and R. Bal, Green Chem., 2014, 16, 2826–2834 RSC.
- B. Huang, C. Fan, C. Pan, A. Zheng, X. Ma, Y. Li, J. Zhang and Y. Sun, Powder Technol., 2017, 315, 258–269 CrossRef CAS.
- B. Paul, S. K. Sharma, R. Khatun, S. Adak, G. Singh, V. Joshi, M. K. Poddar, A. Bordoloi, T. Sasaki and R. Bal, Ind. Eng. Chem. Res., 2019, 58, 18168–18177 CrossRef CAS.
- Z. Wang, X. Hou, J. Shen and T. Li, RSC Adv., 2016, 6, 89503–89509 RSC.
- F. Rajabi, N. Karimi, M. R. Saidi, A. Primo, R. S. Varma and R. Luque, Adv. Synth. Catal., 2012, 354, 1707–1711 CrossRef CAS.
- W. Lueangchaichaweng, L. Li, Q.-Y. Wang, B.-L. Su, C. Aprile and P. P. Pescarmona, Catal. Today, 2013, 203, 66–75 CrossRef CAS.
- Y. Leng, J. Zhao, P. Jiang and J. Wang, ACS Appl. Mater. Interfaces, 2014, 6, 5947–5954 CrossRef CAS PubMed.
- A. Pezhman, A. Badiei, A. Koolivand and G. M. Ziarani, Chin. J. Catal., 2011, 32, 258–263 CrossRef.
- S. S. Acharyya, S. Ghosh, S. Adak, T. Sasaki and R. Bal, Catal. Sci. Technol., 2014, 4, 4232–4241 RSC.
- S. S. Acharyya, S. Ghosh and R. Bal, ACS Appl. Mater. Interfaces, 2014, 6, 14451–14459 CrossRef CAS PubMed.
- S. S. Acharyya, S. Ghosh, N. Siddiqui, L. S. Konathala and R. Bal, RSC Adv., 2015, 5, 4838–4843 RSC.
- P. R. Makgwane and S. S. Ray, J. Mol. Catal. A: Chem., 2015, 398, 149–157 CrossRef CAS.
- M. Hosseini-Sarvari, T. Ataee-Kachouei and F. Moeini, RSC Adv., 2015, 5, 9050–9056 RSC.
- W. H. Wanna, D. Janmanchi, N. Thiyagarajan, R. Ramu, Y.-F. Tsai, C.-W. Pao and S. S.-F. Yu, New J. Chem., 2019, 43, 17819–17830 RSC.
- S. Mishra, S. W. Sangma, R. Bal and R. K. Dey, New J. Chem., 2021, 45, 13070–13079 RSC.
| This journal is © The Royal Society of Chemistry 2023 |