Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Biomedical competency of bassanite (plaster of Paris) synthesized from waste Pila globosa shells
Md.
Sahadat Hossain
 a,
Md. Najem
Uddin
a,
Md. Najem
Uddin
 b and
Samina
Ahmed
b and
Samina
Ahmed
 *ab
*ab
aInstitute of Glass & Ceramic Research and Testing, Bangladesh Council of Scientific and Industrial Research (BCSIR), Dhaka-1205, Bangladesh. E-mail: shanta_samina@yahoo.com
bBCSIR Laboratories Dhaka, Bangladesh Council of Scientific and Industrial Research (BCSIR), Dhaka-1205, Bangladesh
First published on 13th February 2023
Abstract
This communication, for the first time to the best of our knowledge, describes the biocompatibility of bassanite synthesized from waste Pila globosa shells. The biocompatibility evaluation involved cytotoxicity and hemocompatibility assessments. Both assessments yielded very good results, making the synthesized bassanite a potential biomaterial that can be derived from waste bioresources.
The hemihydrate form of calcium sulfate, also widely known as bassanite and having the chemical formula CaSO4·0.5H20,1 can be used as bone cement and has been used since the 19th century in the medical field, especially for tissue replacement.2 An in vivo analysis of plaster of Paris with hydroxyapatite, which is another well-known biomaterial, also showed promising outcomes for its applicability in the biomedical field.3 Hence, the demand for compounds based on calcium sulfate is increasing day by day; and this increase is also due to its widespread applications, which include drug delivery, construction, bone regeneration, heritage conservation, and dental prosthetics.4 A few experimental and clinical trials of compounds based on calcium sulfate have shown encouraging results for the filling up of dead bone gaps.5,6 Calcium sulfate, however, has been reported to exhibit cytotoxic effects5 and complications after application7 in the human body. A clinical trial also found a severe inflammatory reaction having developed during the application of calcium sulfate.7 When calcium sulfate was used with other biomaterials such as tricalcium phosphate, many complications were experienced.8 Three patients out of 22 displayed laryngospasm, and tachyarrhythmia was found in one patient into whom calcium sulfated was injected.9
Thus, it is essential to assess the biomedical competency of plaster of Paris (bassanite) before deciding whether to apply it biomedically. In this research, we set out to assess the biomedical compatibility of bassanite synthesized from a bio-resource (Pila globosa).
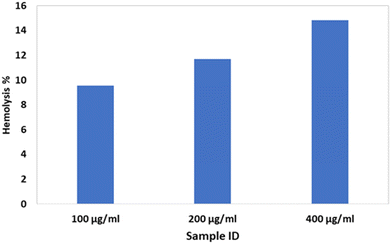
A hemolysis profile was monitored by assessing breakdown properties of RBCs during their contact with the bassanite samples. The hemolysis test was implemented as described earlier, and heparin was used as an anti-coagulant agent for blood.10 In short, physiological saline extract and water were chosen as the negative and positive controls, respectively. Various samples with different concentrations, specifically 100, 200, and 400 μg mL−1, were each incorporated into 0.3 mL of blood. The hemolysis percentage was estimated using the equation
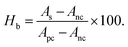 | (1) |
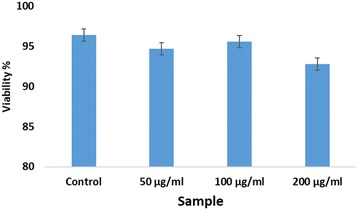
The trypan blue exclusion method was the protocol chosen for estimating the quantities of viable and non-viable cells of the African green monkey kidney (Vero) cell line (CLS 605372, Germany), following a previously described methodology.11,12 A concentration variation of bassanite was maintained (50, 100, and 200 μg mL−1) to identify the change in dead cells after 72 hours. This experiment was performed using an automatic cell counter (LUNA-II™, Analytikjena). The percentage of cells that were viable was computed using eqn (2).13
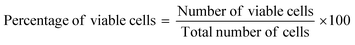 | (2) |
Bassanite was synthesized from a well-known bioresource, namely Pila globosa, and the characterization of the prepared samples was reported earlier.15 The biomedical applicability of the synthesized bassanite has not been reported yet to the best of our knowledge. The main concern involving biomaterials is their interaction with living cells. Fig. 1 shows photographs of the co-presence of bassanite and living cells under a microscope. After 72 hours, only a few cells were found to be dead, and the viability percentages are plotted in Fig. 2. More than 92% of the cells were observed to be alive, whether 50 μg mL−1 or 200 μg mL−1 of bassanite was applied. And, in fact, the control condition also killed a few cells. Thus, the bassanite derived from Pila globosa shells (plaster of Paris) was concluded to be biocompatible. Whether the doses were high or low, the synthesized materials can be categorized as non-cytotoxic biomaterials based on the ISO 10993-5 standard. The compatibility of any material with a biological system can be predicted by the ability of the material or chemicals generated from the material to destroy living cells.16 The synthesized materials did not exert any adverse effects on living cells, and thus can be considered for further applicability, in particular in biomedical fields.
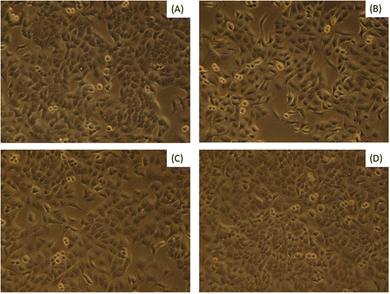 | ||
| Fig. 1 Cell viability assessments of synthesized bassanite samples at various concentrations: (A) control, (B) 50 μg mL−1, (C) 100 μg mL−1, and (D) 200 μg mL−1. | ||
In addition to subjecting the synthesized bassanite to the cytotoxicity test, we also monitored its hemolytic performance to further assess its biocompatibility. In general, a material that breaks down red blood cell (RBCs) would not be considered a biocompatible material. In the current work, this property was investigated by using a very high dose of the material, as high as 400 μg mL−1. With the augmentation of the sample dose, the hemolysis percentages were also amplified. Nevertheless, despite a high dose having been applied, none of the samples were deemed to be non-hemocompatible according to the ASTM standard. In general, according to this standard, a material is considered to be (i) highly hemocompatible if generating a hemolysis count of < 5%, (ii) hemocompatible if generating a hemolysis count of less than 10%, and (iii) non-hemocompatible if generating a hemolysis count of > 20%. Fig. 3 shows a plot of hemolysis counts generated by bassanite samples at various concentrations. The results indicated that bassanite at any of the tested concentrations would not adversely affect RBCs when applied in the biomedical field.
Antimicrobial properties of any biomaterial always carry extra benefits during application as well as after application. The antimicrobial character of bassanite was examined using the disc diffusion technique with E. coli and S. aureus as the standard Gram-positive and Gram-negative bacteria, respectively. The bassanite showed no antimicrobial activity (Fig. 4).
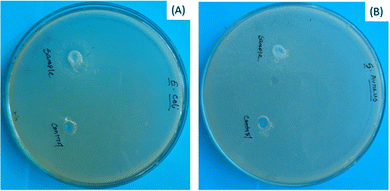 | ||
| Fig. 4 Antimicrobial performances of plaster of Paris, specifically against (A) E. coli and (B) S. aureus. | ||
In conclusion, the bassanite sample synthesized from waste Pila globosa shells can be safely applied as a biomaterial: the cytotoxicity and hemolysis assessments showed no adverse effects. But this material failed to show antimicrobial activity when testing it against E. coli and S. aureus. Based on this research, it is suggested that waste Pila globosa shells be chosen for the synthesis of bassanite/plaster of Paris when aiming for a biomedical application of this product.
Md. Sahadat Hossain conceived and designed the experiment, analysed the data, wrote the original manuscript and performed the experiments. Md. Najem Uddin executed the biocompatibility assessment. Samina Ahmed supervised the overall work and assisted in writing the manuscript.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are grateful to the Bangladesh Council of Scientific and Industrial Research (BCSIR) authority for financial support through the R&D project (ref. no. 39.02.0000.011.14.134.2021/900; Date: 30.12.2021). Md. Najem Uddin thanks the Pharmaceutical Research Division, BCSIR Laboratories Dhaka, for executing the biocompatibility assessment. The authors also thank Dr. Shirin Akter Jahan, PSO and Project Director, Strengthening Institute of Glass and Ceramics Research & Testing for sophisticated instrumental support.References
- C. E. Schrank, O. Gaede, T. Blach, K. C. M. Gioseffi, S. Mudie, N. Kirby, K. Regenauer-Lieb and A. P. Radliński, Commun. Mater., 2021, 2, 1–6 CrossRef.
- E. Hughes, T. Yanni, P. Jamshidi and L. M. Grover, Adv. Appl. Ceram., 2015, 114, 65–76 CrossRef CAS.
- N. G. Georgiade, J. Hanker, G. Ruff and S. Levin, Aesthetic Plast. Surg., 1993, 17, 85–92 CrossRef CAS PubMed.
- M. Burgos-Ruiz, G. Pelayo-Punzano, E. Ruiz-Agudo, K. Elert and C. Rodriguez-Navarro, Chem. Commun., 2021, 57, 7304–7307 RSC.
- M. A. Rauschmann, T. A. Wichelhaus, V. Stirnal, E. Dingeldein, L. Zichner, R. Schnettler and V. Alt, Biomaterials, 2005, 26, 2677–2684 CrossRef CAS PubMed.
- A. S. Coetzee, Arch. Otolaryngol., 1980, 106, 405–409 CrossRef CAS PubMed.
- D. Robinson, D. Alk, J. Sandbank, R. Farber and N. Halperin, Ann. Transplant., 1999, 4, 91–97 CAS.
- J. Friesenbichler, W. Maurer-Ertl, P. Sadoghi, U. Pirker-Fruehauf, K. Bodo and A. Leithner, Clin. Orthop. Relat. Res., 2014, 472, 976–982 CrossRef PubMed.
- L. Nystrom, R. Raw, J. Buckwalter and J. A. Morcuende, Iowa Orthop. J., 2008, 28, 81 Search PubMed.
- V. P. Padmanabhan, T. S. N. Sankara Narayanan, S. Sagadevan, M. E. Hoque and R. Kulandaivelu, New J. Chem., 2019, 43, 18484–18494 RSC.
- A. Basu, S. Kundu, A. Das, C. Basu, S. Bhayye, S. Das and A. Mukherjee, Mater. Adv., 2020, 1, 1142–1150 RSC.
- S. Sultana, M. S. Hossain, M. Mahmud, M. B. Mobarak, M. H. Kabir, N. Sharmin and S. Ahmed, RSC Adv., 2021, 11, 3686–3694 RSC.
- S. Stagnoli, M. A. Luna, C. C. Villa, F. Alustiza, A. Niebylski, F. Moyano, N. M. Correa and R. D. Falcone, RSC Adv., 2017, 7, 5372–5380 RSC.
- R. Augustine, H. N. Malik, D. K. Singhal, A. Mukherjee, D. Malakar, N. Kalarikkal and S. Thomas, J. Polym. Res., 2014, 21, 1–17 CrossRef CAS.
- M. S. Hossain and S. Ahmed, RSC Adv., 2022, 12, 25096–25105 RSC.
- N. Goonoo, A. Bhaw-Luximon and D. Jhurry, RSC Adv., 2014, 4, 31618–31642 RSC.
| This journal is © The Royal Society of Chemistry 2023 |