Open Access Article
Open Access ArticleRealizing fast Li-ion conduction of Li3PO4 solid electrolyte at low temperature by mechanochemical formation of lithium-containing dual-shells†
Shunqin
Zeng
ab,
Xiaoli
Ding
 *a,
Liqing
He
c,
Hai-Wen
Li
*a,
Liqing
He
c,
Hai-Wen
Li
 *c,
Qingan
Zhang
a and
Yongtao
Li
ad
*c,
Qingan
Zhang
a and
Yongtao
Li
ad
aSchool of Materials Science and Engineering & Low-Carbon New Materials Research Center, Anhui University of Technology, Maanshan 243002, China. E-mail: dingxiaoli@ahut.edu.cn
bHunan Engineering Laboratory for Preparation Technology of Polyvinyl Alcohol Fiber (PVA) Material, Huaihua University, Huaihua, 418000, China
cHefei General Machinery Research Institute, Hefei, Anhui 230031, P. R. China. E-mail: lihaiwen66@hotmail.com
dKey Laboratory of Green Fabrication and Surface Technology of Advanced Metal Materials of Ministry of Education, Anhui University of Technology, Maanshan 243002, China
First published on 12th June 2023
Abstract
Dual lithium-containing hydride/oxide shells are constructed by in situ mechano-induced assembly of Li3PO4 reacting with LiBH4. The ionic conductivity of the Li3PO4-based composite is promoted nearly 4 orders of magnitude, up to 0.04 mS cm−2 at 75 °C as compared to the pure one, possessing an electrochemical window of −0.2–5 V (vs. Li/Li+).
As an efficient energy storage device, rechargeable lithium-ion batteries (LIBs) play an important role in replacing fossil fuels that cause environmental pollution.1 Commercial liquid lithium-ion batteries are widely used in micro-electronic products, hybrid electric vehicles, grid energy conversion storage systems and other energy storage fields.2–4 However, they can lead to safety issues as they use volatile liquid electrolytes, which are flammable and explosive.5,6 At present, it is urgent to develop safe and reliable batteries with high storage energy density, long cycle life and low price. To solve the above-mentioned problem, all-solid-state batteries have been designed where high-capacity lithium metal acts as the anode and the organic liquid electrolytes are replaced by safe and stable solid electrolytes (SSEs).7,8 The key issues of the electrolyte of all-solid-state batteries depend on the employment of new electrolyte materials, possessing high ion conductivity, and good electrochemical and mechanical stability at room temperature.9,10
So far, extensive research has been conducted on SSEs covering polymer materials and inorganic materials.11,12 Among them, the typical conductive polymers are polyoxyethylene (PEO),13 polyacrylonitrile (PAN),14 polymethyl methacrylate (PMMA)15 and polyvinylidene difluoroethylene (PVDF),16 and the typical inorganic conductive materials include glass/ceramic,17 garnets,18 sulfides,19 perovskites20 and hydrides.21 Despite extensive research and significant progress in solid electrolytes over the past few decades, there are currently no commercially available battery solutions to meet the requirements of production and daily life.22,23 The main challenge at present is to develop new solid electrolytes with facile preparation processes and excellent electrochemical performance, possessing high lithium ion conductivity.24
As a typical glassy material, Li3PO4 takes advantages of low cost, easy preparation, environmental inertness, and negligible electron conductivity.25–27 Therefore, it has been widely applied in improving the electro-chemical properties of lithium-rich electrode materials28 and inhibiting the growth of lithium dendrites in lithium metal batteries.29 However, the application of Li3PO4 in solid electrolytes is barely reported because of its much lower conductivity of 10−10 S cm−1 at room temperature (RT).25
Herein, we propose the technique of surficial modification of Li3PO4 particles by introducing lithium borohydride (LiBH4) to achieve high ionic conductivity and good electro-chemical properties. The LiBH4 as a fast-ion conductive solid electrolyte was first reported by Matsuo et al., which shows good electro-chemical stability and good interface compatibility with electrode materials.30,31 It was found that the prepared LiBH4/Li3PO4 composite had a double-coated structure, namely, LiBO2 as the intermediate layer to prevent further reaction between LiBH4 and Li3PO4, and the amorphous LiBH4 as an outer layer to provide a continuous ionic conductivity network among Li3PO4 particles to synergistically improve their ionic conductivity. The composites presented an ionic conductivity up to 0.04 mS cm−2 at 75 °C, which is nearly 4 orders of magnitude higher than that of pure Li3PO4. Moreover, the solid electrolyte of LiBH4/Li3PO4 composites exhibited excellent electro-chemical properties, such as a wide electro-chemical window (5 V vs. Li/Li+) and negligible number of electron migrations. These results provide new possibilities for developing suitable SSEs with excellent ionic conductivity.
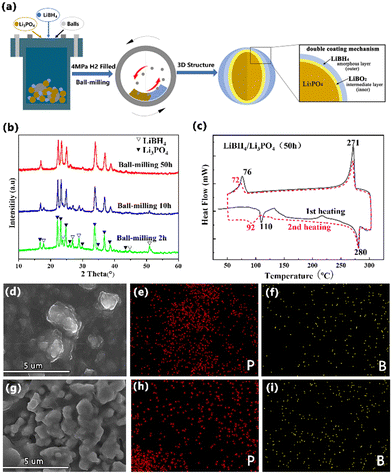
The lithium-contained dual shells of Li3PO4 electrolyte were assembled by one-step ball milling (Fig. 1a). According to the X-ray diffraction (XRD) patterns in Fig. 1b, the diffraction peaks of Li3PO4 keep stable while the peak strength of LiBH4 gradually weakens with the increase of the milling time from 2 h to 50 h, indicating that the grain size becomes refined. The thermal stability of the 50 wt% LiBH4/Li3PO4 composite as a model was further studied by in situ differential calorimetry. From (Fig. 1c and Fig. S1a, ESI†), it can be seen that endothermic and exothermic peaks are observed on each differential scanning calorimeter (DSC). The endothermic peak at about 110 °C and the exothermic peak at ∼76 °C correspond to the phase transition temperature of LiBH4 from Pnma to P63mc,30 while the exothermic peak observed at ∼280 °C and the endothermic peak observed at ∼270 °C represent its melting and re-crystallization process. The second cycle of differential scanning calorimeter exhibits a similar tendency though a slight shift in temperature curve. These results confirm that the LiBH4/Li3PO4 composites are thermally stable at a temperature range of 308–573 K, which would help the all-solid-state battery maintain stability in a high-temperature environment. Fig. 1(d–i) shows the scanning electron microscope (SEM) images and Energy Dispersive Spectrometer (EDS) mapping of the 50 wt% LiBH4/Li3PO4 composites with the milling time of 2 h and 50 h, respectively. It can be seen that the introduction of LiBH4 caused obvious agglomeration and adhesion of the particles. On magnified observation, we found that some of the sample particles exhibited an encapsulated morphology, which may be due to the formation of a low Young's modulus LiBH4 covering layer around high Young's modulus Li3PO4 particles.32,33 This is further confirmed by the EDS mappings of highly dispersed B and P elements.
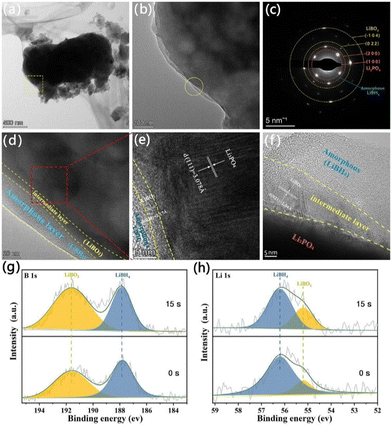
To gain a deeper understanding of the sample micro-structure, transmission electron microscope (TEM) and X-ray photoelectron spectroscopy (XPS) surface sputtering techniques were conducted. From the TEM image and the corresponding selected electron diffraction pattern (Fig. 2a–d), it can be seen that the sample has a dual-layer structure, where the amorphous coating corresponds to LiBH4, the lattice fringes correspond to the (010) intermediate layer LiBO2 and the inter-planar spacing of 0.3078 nm belongs to the (111) crystal plane of the Li3PO4 core, respectively (Fig. 2d and e). Fig. 2(g and h) show the XPS depth profiling plots of elements B and Li in the samples before and after sputtering, respectively. The peaks located at 187.8 eV and 191.7 eV in the high-resolution B 1s XPS spectrum before sputtering correspond to the B–H bonds34 in LiBH4 and the B–O bonds in LiBO2,35 respectively, and the relative intensity of the characteristic peaks of LiBO2 in the B1s spectrum is further enhanced after 15 s sputtering. The high-resolution Li 1s characteristic peaks corresponding to LiBH4 (∼56.2 eV) and LiBO2 (∼55.2 eV) also appear in the XPS spectrum,33 and the relative content of LiBO2 and the relative content of LiBH4 gradually decrease after sputtering for 15 s. Combining the TEM and XPS results, we found that the cladding layer formed around the Li3PO4 particles is a bilayer structure composed of LiBH4 as the outer layer and LiBO2 as the intermediate layer. LiBH4 as an amorphous provides more lithium-ion transmission channels and continuous ion-guided circuits for the Li3PO4, while LiBO2 as the intermediate layer plays a role in preventing LiBH4 and Li3PO4 from continuous reaction in the ball-milling process. Therefore, the formation of the cladding layer not only improves the ionic conductivity of Li3PO4 but also enhance their cold-working formability.
In order to determine the Li+ ion conductivity, the as-milled LiBH4/Li3PO4 composite was cold pressed into pellets at room temperature and then held for 8 h subjected to electrochemical impedance spectra (EIS) examination. It could be found that the longer the ball grinding time, the smaller the impedance of the sample at room temperature (Fig. 3a). The ion conductivity curves of the sample with different milling times are shown in Fig. 3b. Compared with the 50 wt% LiBH4/Li3PO4 composite material ground for 2 h at room temperature, the ion conductivity of the 50 wt% LiBH4/Li3PO4 composite material ground for 50 h increased by nearly an order of magnitude. In addition, the activation energy (Ea) of lithium-ion mobility can be quantitatively analyzed by linear fitting of the data in Fig. 3b by using Arrhenius eqn (1):31
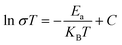 | (1) |
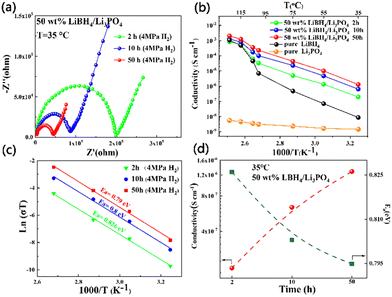 | ||
| Fig. 3 (a)–(c) Ion transport characteristics of the 50 wt% LiBH4/Li3PO4 composite under different ball milling times; and (d) the relationship between ionic conductivity and milling time. | ||
The direct current polarization curve of SUS|50 wt% LiBH4/Li3PO4|SUS symmetrical batteries was measured at 105 °C. As shown in Fig. 4a, the discharge current drops sharply after applying a 10 mV step voltage and then tends to stabilize. The electron conductivity (σe) of the sample is calculated using eqn (2):31
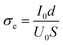 | (2) |
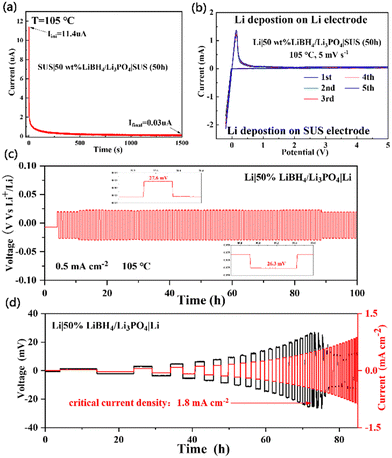 | ||
| Fig. 4 (a) DC polarization curve, (b) cyclic voltammetry curve, (c) constant current cycle curve and (d) stepped current density galvanostatic cycle of the 50 wt% LiBH4/Li3PO4 composite. | ||
The cycling performance of the 50 wt% LiBH4/Li3PO4 composite solid electrolytes and the inhibition ability of lithium dendrites were further evaluated. At 105 °C, we applied to the Li|50wt% LiBH4/Li3PO4|Li symmetrical battery a constant current of 0.5 mA/cm−2 and a step current density of 0.1 mA cm−2 as the starting current and 0.1 mA cm−2 as the step length. As is shown in Fig. 4c, the battery has no significant voltage fluctuations within 100 cycles, which proves that the 50 wt% LiBH4/Li3PO4 composite solid electrolyte has good electro-chemical cycling stability after ball-milling for 50 h. In addition, the limited current density of the Li|50wt% LiBH4/Li3PO4|Li battery is determined to be 1.8 mA cm−2 (Fig. 4d), which corresponds to the critical current density for lithium dendrite formation and the above results indicate that the stable interphase between Li and LiBH4/Li3PO4 can effectively suppress the growth of Li dendrites. This remarkable cycling performance can be attributed to two factors including the stable surface contact among particles due to the filling of the amorphous outer layer of LiBH4 providing a continuous ionic conductive network between Li3PO4 particles and the extremely low electronic conductivity that effectively suppresses the growth of Li dendrites.36
In summary, we developed an effective strategy to improve the lithium-ion conductivity of the Li3PO4-based solid-state electrolyte, namely, the LiBH4/Li3PO4 solid-state electrolyte constructed by in-situ mechano-chemical reaction, which exhibits a bilayer structure: (i) the formed LiBO2 as an intermediate layer will prevent further reactions between LiBH4 and Li3PO4, (ii) the amorphous outer layer of LiBH4 can provide a continuous ionic conductive network between Li3PO4 particles. The LiBH4/Li3PO4 (50 wt%) composite exhibits an excellent ion conductivity of 0.04 m S cm−2 at 75 °C. This is mainly due to the amorphous LiBH4 outer layer providing more lithium ion transfer channels and fine particles resulting in lower energy interfaces for lithium ion conduction. In addition, the LiBH4/Li3PO4 electrolyte also exhibits a wide electro-chemical stability window, excellent cycling stability and strong lithium dendrite inhibition. These findings can provide an alternative route to improve the ion conductivity of solid electrolytes in all-solid-state batteries.
This work was financially supported by the Key Program for International S&T Cooperation Projects of China (No. 2017YFE0124300), National Natural Science Foundation of China (No. 51971002, 52171205, 52101249 and 52171197), Scientific Research Foundation of Anhui Provincial Education Department (No. KJ2021A0360, 2108085QE191), Anhui Provincial Natural Science Foundation for Excellent Youth Scholars (No. 2108085Y16) and Scientific Research Foundation of Hunan Provincial Education Department (No. 22B0769).
Conflicts of interest
There are no conflicts to declare.Notes and references
- S. Chu and A. Majumdar, Nature, 2012, 488, 294–303 CrossRef CAS PubMed; M. Armand and J. M. Tarascon, Nature, 2008, 451, 652–657 CrossRef PubMed.
- Y. Kato, S. Hori, T. Saito, K. Suzuki, M. Hirayama, A. Mitsui, M. Yonemura, H. Iba and R. Kanno, Nat. Energy, 2016, 1, 16030 CrossRef CAS; J. W. Choi and D. Aurbach, Nat. Rev. Mater., 2016, 1, 16013 CrossRef.
- N. Kamaya, K. Homma, Y. Yamakawa, M. Hirayama, R. Kanno, M. Yonemura, T. Kamiyama, Y. Kato, S. Hama, K. Kawamoto and A. Mitsui, Nat. Mater., 2011, 10, 682–686 CrossRef CAS PubMed.
- Z. Yang, J. Zhang, M. C. W. Kintner-meyer, X. Lu, D. Choi, J. P. Lemmon and J. Liu, Chem. Rev., 2011, 111, 3577–3613 CrossRef CAS PubMed.
- Q. Wang, L. Jiang, Y. Yu and J. Sun, Nano Energy, 2019, 55, 93–114 CrossRef CAS; Y. Zhao, K. Zheng and X. Sun, Joule, 2018, 2, 2583–2604 CrossRef.
- S. Mao, Q. Wu, F. Ma, T. Wu and Y. Lu, Chem. Commun., 2021, 57, 840–857 RSC.
- Z. Piao, P. Xiao, R. Luo, J. Ma, R. Gao, C. Li, J. Tan, K. Yu, G. Zhou and H. Cheng, Adv. Mater., 2022, 34, 2108400 CrossRef CAS PubMed.
- Q. Zhao, S. Stalin, C. Z. Zhao and L. A. Archer, Nat. Rev. Mater., 2020, 5, 229–252 CrossRef CAS.
- Y. Xiao, Y. Wang, S.-H. Bo, J. Kim, L. J. Miara and G. Ceder, Nat. Rev. Mater., 2020, 5, 105–126 CrossRef CAS.
- X. Chen, C. Zhao, Y. Yao, H. Liu and Q. Zhang, Chemistry, 2019, 5, 74–96 CrossRef.
- X. Liu, X. Li, H. Li and H. Wu, Chem. – Eur. J., 2018, 24, 18293–18306 CrossRef CAS PubMed.
- T. Zhang, W. He, W. Zhang, T. Wang and P. Li, Chem. Sci., 2020, 11, 8686–8707 RSC.
- Q. Zhou, J. Ma, S. Dong, X. Li and G. Cui, Adv. Mater., 2019, 31, 1902029 CrossRef CAS PubMed.
- Y. Shuai, Z. Zhang, K. Chen, J. Lou and Y. Wang, Chem. Commun., 2019, 55, 2376–2379 RSC.
- S. Venkatesan, I. P. Liu, J. Lin, M. Tsai, H. Teng and Y. Lee, J. Mater. Chem. A, 2018, 6, 100856 RSC.
- Y. Shan, L. Li, X. Chen, S. Fan, H. Yang and Y. Jiang, ACS Energy Lett., 2022, 7, 2289–2296 CrossRef CAS.
- X. Chi, Y. Zhang, F. Hao, S. Kmiec, H. Dong, R. Xu, K. Zhao, Q. Ai, T. Terlier, L. Wang, L. Zhao, L. Guo, J. Lou, H. Xin, S. W. Martin and Y. Yao, Nat. Commun., 2022, 13, 2854 CrossRef CAS PubMed.
- R. Murugan, V. Thangadurai and W. Weppner, Angew. Chem., Int. Ed., 2007, 46, 7778–7781 CrossRef CAS PubMed; J. Zhang, C. Wang, M. Zheng, M. Ye, H. Zhai, J. Li, G. Tan, X. Tang and X. Sun, Nano Energy, 2022, 102, 107672 CrossRef.
- Y. Lee, J. Jeong, H. Lee, M. Kim, D. Han, H. Kim, J. Yuk, K. Nam, K. Chung, H. Jung and S. Yu, ACS Energy Lett., 2022, 7, 171–179 CrossRef CAS; J. Xu, Y. Li, P. Lu, W. Yan, M. Yang, H. Li, L. Chen and F. Wu, Adv. Mater., 2021, 13, 2102348 Search PubMed; P. Lu, L. Li, S. Wang, J. Xu, J. Peng, W. Yan, Q. Wang, H. Li, L. Chen and F. Wu, Adv. Mater., 2021, 33, 210096 Search PubMed.
- Q. Zhang, N. Schmidt, J. Lan, W. Kim and G. Cao, Chem. Commun., 2014, 50, 5593–5596 RSC.
- H. Liu, Z. Ren, X. Zhang, J. Hu, M. Gao, H. Pan and Y. Liu, Chem. Mater., 2019, 32, 671–678 CrossRef.
- J. Zhang, X. Zang, H. Wen, T. Dong, J. Chai, Y. Li, B. Chen, J. Zhao, S. Dong, J. Ma, L. Yue, Z. Liu, X. Guo, G. Cui and L. Chen, J. Mater. Chem. A, 2017, 5, 4940–4948 RSC.
- R. Chen, Q. Li, X. Yu, L. Chen and H. Li, Chem. Rev., 2020, 120, 6820–6877 CrossRef CAS PubMed.
- E. Quartarone and P. Mustarelli, Chem. Soc. Rev., 2011, 40, 2525–2540 RSC.
- F. Lu, Y. Pang, M. Zhu, F. Han, J. Yang, F. Fang, D. Sun, S. Zheng and C. Wang, Adv. Funct. Mater., 2019, 29, 1809219 CrossRef.
- E. Kartini, M. Nakamura, M. Arai, Y. Inamura, K. Nakajima, T. Maksum, W. Honggowiranto and T. Y. S. P. Putra, Solid State Ion, 2014, 262, 833–836 CrossRef CAS.
- L. D. Prayogi, M. Faisal, E. Kartini, W. Honggowiranto and C. Supardi, AIP Conf. Proc., 2016, 1710, 030047 CrossRef; M. B. Bechir and A. B. Rhaiem, Phys. E, 2021, 130, 114686 CrossRef.
- B. Xu, W. Li, H. Duan, H. Wang, Y. Guo, H. Li and H. Liu, J. Power Sources, 2017, 354, 68–73 CrossRef CAS.
- N. D. Lepley, N. A. W. Holzwarth and Y. A. Du, Phys. Rev. B: Condens. Matter Mater. Phys., 2013, 88, 104103 CrossRef.
- H. Maekawa, M. Matsuo, H. Takamura, M. Ando, Y. Noda, T. Karahashi and S. Orimo, J. Am. Chem. Soc., 2009, 131(3), 894–895 CrossRef CAS PubMed.
- Z. Wu, K. Wang, W. Sun, Z. Li, Z. Ma, Y. Zhu, Y. Zou and Y. Zhang, Adv. Funct. Mater., 2022, 2205677 CrossRef CAS.
- N. Li, Y. Yin, C. Yang and Y. Guo, Adv. Mater., 2016, 28(9), 1853–1858 CrossRef CAS PubMed.
- Y. Gao, S. Sun, X. Zhang, Y. Liu, J. Hu, Z. Huang and M. Gao, Adv. Funct. Mater., 2021, 31(15), 2009692 CrossRef CAS.
- E. Deprez, M. A. Muñoz-Márquez, M. C. Jimenez de Haro, F. J. Palomares, F. Soria, M. Dornheim, R. Bormann and A. Fernández, J. Appl. Phys., 2011, 109(1), 014913 CrossRef.
- D. A. Hensley and S. H. Garofalini, Appl. Surf. Sci., 1994, 81(3), 331–339 CrossRef CAS.
- C. Tsai, V. Roddatis, C. V. Chandran, Q. Ma, S. Uhlenbruck, M. Bram, P. Heitjans and O. Guillon, ACS Appl. Mater. Interfaces, 2016, 8(16), 10617 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, DSC curves, EIS, Arrhenius diagram and specific surface area for the LiBH4/Li3PO4 composite. See DOI: https://doi.org/10.1039/d3ma00125c |
| This journal is © The Royal Society of Chemistry 2023 |