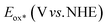DOI:
10.1039/D3MA00277B
(Paper)
Mater. Adv., 2023,
4, 3270-3284
Halogen functionalized D–A–D-type unsymmetrical squaraine dyes for dye-sensitized solar cells†
Received
2nd June 2023
, Accepted 26th June 2023
First published on 27th June 2023
Abstract
The development of sensitizers with the D–A–D architecture with an indoline donor and squaryl acceptor is attractive towards achieving the high-efficiency dye-sensitized solar cells (DSSCs) due to an intense absorption (ε > 105 M−1 cm−1) in the far-red region and dye aggregation behaviour on the mesoporous TiO2 surface, which broadens the light harvesting properties. To further enhance the photovoltaic parameters, features that help to enhance the charge injection and dye-regeneration processes must be built into the sensitizers, and the charge recombination must be diminished. Here, a series of squaraine dyes (ISQ) in which an alkyl-group-wrapped indoline donor functionalized with a halogen atom, which is separated from another indoline donor that contains an anchoring group, have been designed and synthesized. Photophysical, electrochemical and photovoltaic characterizations have been carried out to understand the effect of the halogen atom on their electronic, photo-excited and DSSC device properties. Photophysical characterization revealed the singlet nature of the photo-excited state despite the presence of a heavy atom such as iodine, bromine and chlorine in the ISQ dyes. The singlet excited state lifetime and the fluorescence quantum yield for the ISQ dyes were almost equal to or slightly higher than those of the ISQ-H dye. The molecular electrostatic potential obtained from DFT studies indicated the presence of a σ-hole in the halogen-functionalized ISQ dyes, which assists in the dye regeneration process through halogen bonding. DSSC devices were fabricated with iodolyte electrolytes in high (Z-150) and low (Z-50) redox concentrations in order to determine the importance and effect of halogen functionalization in squaraine dyes on the DSSC performance. Different photovoltaic performances were observed for the ISQ dyes when the devices were fabricated with the Z-50 and Z-150 iodolyte electrolytes. A maximum DSSC efficiency of 6.48% was achieved using SQS4 dye in Z-50 without CDCA, and an efficiency of 7.80% was achieved using ISQ-I dye in Z-150 with CDCA.
1. Introduction
In the context of third-generation photovoltaic devices, dye-sensitized solar cells (DSSCs) in particular offer avenues to modulate the charge-transfer dynamics across various interfaces via the design of the photoanode, dye, electrolyte and cathode materials for enhanced device performance.1–6 Synergistic integration of interfacial properties is required to achieve the required charge injection, dye-regeneration and transport processes while minimizing charge-recombination processes.3,6–9 Metal-free organic dyes with different architectures have been utilized to harvest visible and NIR photons for photocurrent generation, in addition to sensitizers based on the ruthenium–polypyridine and zinc–porphyrin complexes.10 Indeed, the cheap precursors, easy synthesis, and modular photophysical and electrochemical properties of metal-free organic dyes make them very attractive compared to metal-containing dyes.11–15 The aggregation of dyes on nano-crystalline TiO2 is a facile process due to the presence of periodical 5-coordinated Ti centres (in which a dye forms a covalent bond with the anchoring group) in the anatase 101 facet, which has been considered to be a detrimental factor for DSSC device performance.16–18 However, one of the design principles to control the aggregation of the dyes on the TiO2 surface is tailoring the hydrophobic alkyl groups in the sensitizer, which also helps in passivating/shielding the surface to minimize the charge recombination of electrons in TiO2 with oxidized electrolyte species.19–22 It is known that the dye regeneration kinetics affect the photovoltaic parameters such as VOC and JSC in DSSC performance.8,23 Hence, integrating the features to enhance the dye regeneration process in the dye is a very attractive and important aspect, in addition to the built-in electronic and steric factors of the sensitizers.24–27 Furthermore, in order to enhance the dye regeneration efficiency, the inclusion of triphenylamine-based additives in the electrolyte28 and functionalization of the dye with bulky 3D-triarylamine donors have been reported.29
Halogen bonding has been utilised in chemistry as well as in chemical biology due to its highly directional intermolecular interactions.30–32 In this regard, polarizable atoms such as the –Br or –I atom have been integrated with D–π–A based sensitizers in conjunction with an I−/I3− based electrolyte, as the non-covalent interaction between a halogen atom of the oxidised dye and the iodide ions in the electrolyte facilitate dye regeneration.26,27 Additionally, a pyridyl-donor-based D–π–A dye on TiO2 showed enhanced regeneration kinetics with a cobalt electrolyte for improved device performance by improving the interaction between the dye and the cobalt electrolyte through the pyridyl unit in the dye.33–35 Hence, integration of a functional moiety that helps to promote the dye-regeneration process by either halogen-bonding or halogen-bonding (σ-hole in the halogen-atom-containing dyes) with a Lewis base are very important to enhance the DSSC device JSC and efficiency.25,34 As there are only a few chromophores that harvest photons in the far-red and NIR regions of the solar spectrum, such as porphyrin- and phthalocyanine-based dyes, it is important to develop metal-free organic dyes for dye-sensitized solar cells. Squaraine dyes from polymethine chromophores absorb in the far-red region of the spectrum with a high extinction coefficient.36,37 An N-alkylated unsymmetrical squaraine dye exhibited a DSSC device efficiency of 4.5% (VOC = 603 mV and JSC = 10.5 mA cm−2).38 Extending the π-conjugation “outside the polymethine framework” with a naphthalene donor unit,39 dithienothiophene40 and thiophene41 π-spacers increased the DSSC device performance to 5.4% (VOC = 667 mV and JSC = 11.3 mA cm−2), 6.0% (VOC = 644 mV and JSC = 13.1 mA cm−2) and 6.74% (VOC = 642 mV and JSC = 14.8 mA cm−2), respectively. Additionally, the alkyl-group-wrapped squaraine dye SQS4 showed a device efficiency of 7.1% (VOC = 715 mV and JSC = 13.05 mA cm−2).21 Wrapping the squaraine dye with alkyl groups helps to control the aggregation of dyes as well as to passivate the TiO2 surface efficiently to enhance the JSC and VOC of the DSSC devices, respectively. Furthermore, to enhance the dye regeneration process with the D–A–D type squaraine dyes, a halogen-bonding interaction between the oxidized sensitizer and iodine ion in the electrolyte is envisaged. Hence, functionalization of the squaraine dyes with a halogen atom is designed in the present investigation. As it is reported in the literature that functionalizing the organic chromophores enhances the intersystem-crossing process,43,44 the excited state properties of halogen-functionalized squaraine dyes have been studied in detail.
Systematic photophysical, electrochemical, theoretical, and photovoltaic characterizations have been carried out to investigate the effect of the squaraine dye with halogen functionality on the photovoltaic performance. The structures of the halogen functionalized D–A–D-based ISQ dyes are provided in Fig. 1.
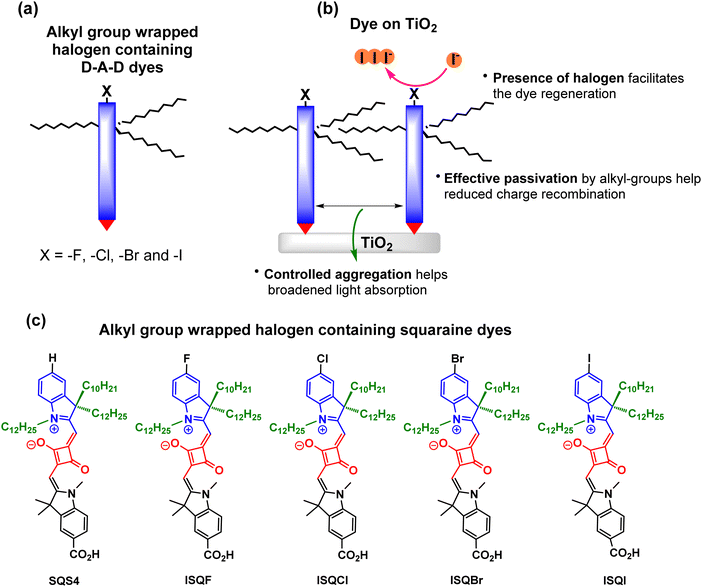 |
| | Fig. 1 (a) Schematic representation of the alkyl-group-wrapped halogen-containing dye and (b) the self-assembly of dyes on the TiO2 surface. (c) Structures of the targeted squaraine dyes. | |
2. Experimental section
Compounds 2, 4c, and 5 have been reported in the literature.21,42 Hydrazine derivatives 1a–d and 1-iodododecane are commercially available.
2.1. General procedure for the synthesis of 3-decyl-3-dodecyl-5-halo-2-methyl-3H-indoles, (3a–d)
Halogen-substituted phenylhydrazine hydrochloride (1) and 3-decylpentadecan-2-one (2) (1.3 equiv.) were dissolved in 10 mL of AcOH in a 50 mL round-bottom flask. The reaction mixture was refluxed under a nitrogen atmosphere for 24 h. The reaction mixture was cooled, the solvent was removed under reduced pressure, and the product was purified via column chromatography (100–200 mesh SiO2) using EtOAc and petroleum ether as the eluents.42
2.1.1. 3-Decyl-3-dodecyl-5-fluoro-2-methyl-3H-indole (3a).
Starting materials 1a (0.5 g, 3.07 mmol) and 3-decylpentadecan-2-one (1.46 g, 3.99 mmol, 1.3 equiv.), reaction time 24 h, column chromatography (SiO2, 100–200 mesh, 5% EtOAc and 95% pet. ether). Yield: 0.77 g, 54%. 1H NMR (CDCl3, 400 MHz): δ 7.43 (m, 1H), 6.97 (m, 1H), 6.89 (m, 1H), 2.19 (s, 3H), 1.86–1.68 (m, 4H), 1.29–1.10 (m, 30 H), 0.89–0.84 (m, 8H), 0.73–0.67 (m, 2H), 0.68–0.54 (m, 2H); 13C NMR (CDCl3, 100 MHz): δ 186.4, 161.2 (d, J = 244 Hz), 151.0, 144.4 (d, J = 9 Hz), 120.1 (d, J = 9 Hz), 114.0 (d, J = 24 Hz) 109.3 (d, J = 25 Hz), 63.2, 45.70, 36.9, 32.4, 31.8, 29.5, 29.7, 29.5, 29.2, 27.5, 23.5, 22.6, 15.9, 14.1. MALDI-TOF (m/z): [M]+ 457.288.
2.1.2. 3-Decyl-3-dodecyl-5-chloro-2-methyl-3H-indole (3b).
Starting materials (4-chlorophenyl)hydrazine hydrochloride (0.5 g, 2.79 mmol) and 3-decylpentadecan-2-one (1.33 g, 3.63 mmol, 1.3 equiv.), reaction time 24 h, column chromatography (SiO2, 100–200 mesh, 2% EtOAc and 98% pet. ether). Yield: 0.60 g, 45%. 1H NMR (CDCl3, 200 MHz): δ 7.42 (d, J = 8 Hz, 1H), 7.25 (dd, J = 8 Hz, 4 Hz, 1H), 7.15 (d, J = 4 Hz, 1H), 2.20 (s, 3H), 1.87–1.80 (m, 2H), 1.75–1.66 (m, 2H), 1.24–1.10 (m, 30 H), 0.89–0.84 (m, 8 H), 0.71–0.65 (m, 2H), 0.60–0.53 (m, 2H); 13C NMR (CDCl3 100 MHz): δ 187.2, 154.0, 144.7, 130.6, 124.1, 120.9, 118.9, 63.2, 45.7, 37.02, 32.4, 31.9, 29.8, 29.6, 29.5, 29.2, 27.5, 23.6, 22.7, 16.08, 14.2. MALDI-TOF (m/z): [M]+ 473.286.
2.1.3. 5-Bromo-3-decyl-3-dodecyl-2-methyl-3H-indole (3c).
Starting materials (4-bromophenyl)hydrazine hydrochloride (0.5 g, 2.23 mmol) and 3-decylpentadecan-2-one (1.06 g, 2.90 mmol, 1.3 equiv.), reaction time 24 h, column chromatography (SiO2, 100–200 mesh, 1% ethyl acetate and 99% pet. ether). Yield: 0.55 g, 47%. 1H NMR (CDCl3, 500 MHz): δ 7.43–7.25 (m, 3H), 2.19 (s, 3H), 1.86–1.81 (m, 2H), 1.72–1.65 (m, 2H) 1.24–1.11 (m, 30 H), 0.85–0.88 (m, 8H), 0.68 (m, 2H), 0.56 (m, 2H); 13C NMR (CDCl3 100 MHz): δ 187.1, 154.8, 145.0, 136.5, 130.7. 121.4, 63.2, 45.6, 36.9, 32.3, 31.8, 29.7, 29.6, 29.4, 29.1, 27.4, 23.5, 22.6, 16.0, 14.1. MALDI-TOF (m/z): [M + H]+ 518.288.
2.1.4. 3-Decyl-3-dodecyl-5-iodo-2-methyl-3H-indole (3d).
Starting materials 4-iodophenylhydrazine hydrochloride (0.34 g, 1.25 mmol) and 3-decylpentadecan-2-one (0.59 g, 1.63 mmol, 1.3 equiv.), reaction time 24 h, column chromatography (SiO2, 100–200 mesh, 2% EtOAc and 98% pet. ether). Yield: 0.29 g, 40%. 1H NMR (CDCl3, 400 MHz): δ 7.63 (dd J = 8 Hz, 4 Hz, 1H), 7.50 (d, J = 4 Hz, 1H), 7.28 (d, J = 8 Hz, 1H), 2.19 (s, 3H), 1.78–1.86 (m, 2H), 1.66–1.74 (m, 2H), 1.10–1.25 (m, 30H), 0.85–0.89 (m, 8H), 0.64–0.71 (m, 2H), 0.51–0.59 (m, 2H); 13C NMR (CDCl3 100 MHz): δ 195.3, 161.0 (d, J = 228 Hz), 141.3 (d, J = 9 Hz), 138.2, 117.7 (d, J = 10.6 Hz), 117.1 (d, J = 25 Hz), 111.4 (d, J = 25 Hz), 90.1, 63.2, 45.1, 36.9, 31.9, 29.8, 29.6, 29.5, 29.2, 23.5, 22.7, 16.1, 14.2. MALDI-TOF (m/z): [M + H]+ 566.302.
2.2. General procedure for the synthesis of halogen-substituted iodolium salts (4a–d)
1-Iodododecane (1.3 equiv.) and corresponding the 3-decyl-3-dodecyl-5-halo-2-methyl-3H-indole (3a–d) (1 equiv.) were dissolved in CH3CN (10 mL) in a 50 mL round-bottom flask and refluxed under a nitrogen atmosphere. The reaction mixture was cooled, and the solvent was evaporated under reduced pressure. The reaction mixture was washed with n-pentane (5 × 2 mL) to obtain the required compound as a brown-red viscous liquid.42
2.2.1. 3-Decyl-1,3-didodecyl-5-fluoro-2-methyl-3H-indol-1-ium (4a).
Starting materials 3a (0.63 g, 1.38 mmol) and 1-iodododecane (0.53 g, 1.80 mmol), reaction time 48 h. Yield: 0.72 g, 68%. 1H NMR (CDCl3, 400 MHz): δ 7.88 (m, 1H), 7.29 (m, 1H), 7.15 (m, 1H), 4.85 (t, J = 7.26 Hz, 2H), 3.12 (s, 3H), 2.06–2.04 (m, 2H), 1.71–1.77 (m, 2H), 1.07–1.19 (m, 54H), 0.81–0.78 (m, 9H), 0.59–0.61 (m, 2H); 13C NMR (CDCl3, 100 MHz): δ 195.4, 161.0 (d, J = 228 Hz), 141.3 (d, J = 9 Hz), 138.2, 120.0, 117.7 (d, J = 10 Hz), 117.1 (d, J = 25 Hz), 111.4 (d, J = 25 Hz), 63.8, 50.9, 37.2, 33.5, 31.8, 30.4, 29.5, 29.3, 28.5, 26.9, 24.08, 22.6, 17.5, 15.9, 14.1.
2.2.2. 5-Chloro-3-decyl-1,3-didodecyl-2-methyl-3H-indol-1-ium (4b).
Starting materials 3b (0.42 g, 0.56 mmol) and 1-iodododecane (0.22 g, 0.734 mmol), reaction time 48 h. Yield: 0.185 g, 71%. 1H NMR (CDCl3, 400 MHz): δ 7.83 (d, J = 8 Hz, 1H), 7.63 (dd, J = 8 Hz, 4 Hz, 1H), 7.47 (d, J = 4 Hz, 1H), 4.92 (t, J = 7.26 Hz, 2H), 3.19 (s, 3H), 1.80–1.84 (m, 4H), 1.10–1.26 (m, 52H), 0.84–0.88 (m, 9H), 0.66–0.70 (m, 2H), 0.55–0.58 (m, 2H); 13C NMR CDCl3 100 MHz): δ 195.9, 140.8, 140.6, 136.8, 130.2, 124.0, 116.9, 63.9, 63.2, 51.1, 45.6, 37.0, 33.6, 31.9. 29.7, 29.5, 29.3, 23.5, 22.7, 17.6, 16.1, 14.2.
2.2.3. 5-Bromo-3-decyl-1,3-didodecyl-2-methyl-3H-indol-1-ium (4c).
Starting materials 3c (0.56 g, 1.08 mmol) and 1-iodododecane (0.41 g, 1.40 mmol), reaction time 48 h. Yield: 0.62 g, 70%. 1H NMR (CDCl3, 400 MHz): δ 7.81 (m, 1H), 7.66–7.64 (m, 2H), 4.81 (t, J = 7.26 Hz, 2H), 3.05 (s, 3H), 2.12 (m, 4H), 1.91 (m, 2H), 1.17–1.25 (m, 52H), 1.85–0.88 (m, 9H), 0.69–0.72 (m, 2H); 13C NMR (CDCl3 100 MHz): δ 195.8, 141.3, 140.9, 133.0, 126.9, 124.6, 117.2, 63.8, 51.0, 45.3, 37.2, 33.5, 31.9, 30.5, 29.6, 29.4, 29.3, 28.5, 26.9, 24.1, 22.6, 14.1.
2.2.4. 3-Decyl-1,3-didodecyl-5-iodo-2-methyl-3H-indol-1-ium (4d).
Starting materials 3d (0.32 g, 0.56 mmol) and 1-iodododecane (0.21 g, 0.73 mmol), reaction time 48 h. Yield: 0.315 g, 75%. 1H NMR (CDCl3, 400 MHz): δ 8.0–7.98 (m, 2H), 7.84 (d, J = 4 Hz. 1H), 4.84 (t, J = 7.26, 2H), 3.18 (s, 3H), 1.78–1.83 (m, 4H) 1.10–1.26 (m, 57H), 0.85–1.13 (m, 4H), 0.67–0.72 (m, 2H), 0.53–0.65 (m, 2H); 13C NMR (CDCl3 100 MHz): δ 195.5, 141.0, 138.9, 132.7, 128.9, 125.4, 96.2, 63.8, 63.2, 37.3, 36.9, 33.5, 31.9, 29.6, 29.4, 29.3, 28.5, 24.1, 22.7, 22.6, 16.02, 14.1.
2.3. Synthesis of unsymmetrical ISQ dyes (ISQF, ISQCl, ISQBr and ISQI)
Compound 4a–d (1 equiv.) and 5 (1 equiv.) were dissolved in n-butanol and anhydrous PhMe (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 10 mL of each) in a 50 mL round-bottom flask equipped with a Dean–Stark apparatus and refluxed under an inert atmosphere for 24 h. The reaction mixture was cooled, the solvents were removed under reduced pressure and the products were purified using column chromatography (100–200 mesh SiO2, CH2Cl2:MeOH) to obtained the required dyes.21
1, 10 mL of each) in a 50 mL round-bottom flask equipped with a Dean–Stark apparatus and refluxed under an inert atmosphere for 24 h. The reaction mixture was cooled, the solvents were removed under reduced pressure and the products were purified using column chromatography (100–200 mesh SiO2, CH2Cl2:MeOH) to obtained the required dyes.21
2.3.1. (E)-4-((5-Carboxy-1,3,3-trimethyl-3H-indol-1-ium-2-yl)methylene)-2-(((Z)-3-decyl-1,3-didodecyl-5-fluoroindolin-2-ylidene)methyl)-3-oxocyclobut-1-en-1-olate (ISQF).
Starting materials 4a (0.49 g, 0.66 mmol) and 5 (0.20 g, 0.66 mmol), reaction time 24 h, column chromatography (SiO2, 100–200 mesh, from 1.5% MeOH and 98.5% CH2Cl2). Yield: 0.20 g, 32%. 1H NMR (CDCl3, 400 MHz): δ 8.12 (d, J = 8 Hz, 1H), 8.05 (s, 1H), 7.05 (d, J = 8 Hz, 2H), 6.98 (d, J = 8 Hz, 2H), 6.14 (s, 1H), 5.98 (s, 1H), 4.04 (b, 2H), 3.52 (s, 3H), 3.05 (b, 2H), 1.99–1.95 (m, 2H), 1.84 (s, 8H), 1.43–1.38 (m, 2H), 1.25–1.06 (m, 52H), 0.88–0.76 (m, 8H), 0.76 (b, 2H), 0.47 (b, 2H); 13C NMR (CDCl3, 100.61 MHz): δ 181.7, 176.5, 170.4, 168.8, 161.8, 159.4, 147.4, 141.8, 139.1, 131.2, 124.4, 124.0, 123.5, 114.6 (d, J = 24 Hz), 110.4, 110.3 (d, J = 25 Hz), 108.1, 88.6, 88.1, 59.6, 48.3, 44.4, 40.3, 35.0, 31.9, 29.6, 29.6, 29.5, 29.4, 29.3, 27.3, 24.1, 22.7, 22.7, 14.1; IR (cm−1): 2956, 2920, 2851, 1698, 1597, 1565, 1503, 1495, 1468, 1442, 1355, 1261, 1206, 1176, 1082, 1052, 935, 844, 812, 793, 772, 737, 711, 689; MALDI-TOF (m/z): [M]+ 921.799.
2.3.2. (E)-4-((5-Carboxy-1,3,3-trimethyl-3H-indol-1-ium-2-yl)methylene)-2-(((Z)-5-chloro-3-decyl-1,3-didodecylindolin-2-ylidene)methyl)-3-oxocyclobut-1-en-1-olate (ISQCl).
Starting materials 4b (0.4 g, 0.62 mmol) and 5 (0.19 g, 0.62 mmol), reaction time 24 h, column chromatography (SiO2, 100–200 mesh, from 1% MeOH and 99% CH2Cl2). Yield: 0.22 g, 36%. 1H NMR (CDCl3, 400 MHz): δ 8.12 (d, J = 8 Hz, 1H), 8.05 (s, 1H), 7.32 (d, J = 8 Hz, 2H), 7.0 (d, J = 8 Hz, 1H), 6.92 (d, J = 8 Hz, 1H), 6.15 (s, 1H), 6.01 (s, 1H), 4.02 (b, 2H), 3.54 (s, 3H), 3.04 (b, 2H), 1.99–1.96 (m, 2H), 1.84 (s, 8H), 1.43–1.38 (m, 2H), 1.26–1.06 (m, 53H), 0.89–0.81 (m, 8H), 0.74 (b, 2H), 0.48 (b, 2H); 13C NMR (CDCl3, 100.61 MHz): δ 182.4, 178.1, 170.4, 170.1, 169.2, 147.4, 142.6, 141.9, 131.3, 130.1, 127.1, 124.1, 122.8, 110.4, 108.2, 88.3, 59.3, 48.4, 40.1, 31.9, 29.7, 29.6, 29.6, 29.5, 29.4, 29.3, 27.3, 24.1, 22.7, 14.1; IR (cm−1): 2955, 2920, 2852, 1699, 1597, 1566, 1504, 1485, 1466, 1425, 1350, 1261, 1179, 1084, 1055, 934, 842, 801, 772, 739, 688, 666; MALDI-TOF (m/z): [M]+ 937.726 [M + H]+ and 939.53 [M + H + 2]+.
2.3.3. (E)-2-(((Z)-5-Bromo-3-decyl-1,3-didodecylindolin-2-ylidene)methyl)-4-((5-carboxy-1,3,3-trimethyl-3H-indol-1-ium-2-yl)methylene)-3-oxocyclobut-1-en-1-olate (ISQBr).
Starting materials 4c (0.77 g, 0.95 mmol) and 5 (0.3 g, 0.95 mmol), reaction time 24 h, column chromatography (SiO2, 100–200 mesh, from 1% MeOH and 99% CH2Cl2). Yield: 0.380 g, 40%. 1H NMR (CDCl3, 400 MHz): δ 8.13 (d, J = 8 Hz, 1H), 8.06 (s, 1H), 7.46 (d, J = 8 Hz, 1H), 7.41 (s, 1H), 7.0 (d, J = 8 Hz, 1H), 6.89 (d, J = 8 Hz, 1H), 6.16 (s, 1H), 6.02 (s, 1H), 4.02 (b, 2H), 3.54 (s, 3H), 3.03 (b, 2H), 1.98–1.93 (m, 2H), 1.84–1.78 (m, 8H), 1.42–1.38 (m, 2H), 1.24–1.06 (m, 52H), 0.89–0.81 (m, 9H), 0.74 (b, 2H), 0.48 (b, 2H); 13C NMR (CDCl3, 100.61 MHz): δ 181.8, 177.9, 172.1, 170.5, 169.7, 169.5, 147.3, 142.1, 141.9, 138.8, 131.3, 131.2, 130.8, 125.9, 125.6, 123.9, 117.5, 117.1, 110.9, 108.3, 88.3, 87.9, 59.3, 58.7, 48.4, 44.2, 40.3, 40.0, 31.9, 29.6, 29.5, 29.5, 29.3, 29.3, 27.2, 24.1, 22.1, 22.7, 22.6, 14.1; IR (cm−1): 2954, 2920, 2853, 1701, 1598, 1565, 1490, 1464, 1425, 1350, 1266, 1216, 1085, 1056, 938, 809, 737, 709, 676, 658; MALDI-TOF (m/z): [M]+ 981.663 [M + H]+ and 983.736 [M + H + 2]+.
2.3.4. (E)-4-((5-Carboxy-1,3,3-trimethyl-3H-indol-1-ium-2-yl)methylene)-2-(((Z)-3-decyl-1,3-didodecyl-5-iodoindolin-2-ylidene)methyl)-3-oxocyclobut-1-en-1-olate (ISQI).
Starting materials 4d (0.58 g, 0.79 mmol) and 5 (0.25 g, 0.79 mmol), reaction time 24 h, column chromatography (SiO2, 100–200 mesh, from 1.5% MeOH and 98.5% CH2Cl2). Yield: 0.250 g, 30%. 1H NMR (CDCl3, 400 MHz): δ 8.11 (d, J = 8 Hz, 1H), 8.06 (s, 1H), 7.65 (d, J = 8 Hz, 1H), 7.58 (s, 1H), 7.01 (d, J = 8 Hz, 1H), 6.79 (d, J = 8 Hz, 1H), 6.16 (s, 1H), 6.02 (s, 1H), 4.01 (b, 2H), 3.54 (s, 3H), 3.02 (b, 2H), 1.97–1.91 (m, 2H), 1.84 (s, 8H), 1.42–1.39 (m, 2H), 1.24–1.06 (m, 52H), 0.87–0.82 (m, 8H), 0.73 (b, 2H), 0.47 (b, 2H); 13C NMR (CDCl3, 100.61 MHz): δ 182.1, 175.8, 170.3, 170.1, 168.1, 147.3, 143.6, 141.5, 130.9, 127.6, 123.7, 122.2, 109.6, 107.8, 88.2, 87.8, 59.1, 58.9, 49.3, 47.9, 44.0, 39.7, 31.7. 31.6, 29.4, 29.3, 29.2, 29.1, 29.0, 23.8, 22.4, 21.7, 17.4, 13.9; IR (cm−1): 2957, 2921, 2852, 1701, 1596, 1568, 1487, 1465, 1422, 1350, 1267, 1214, 1088, 1056, 936, 795, 689, 654; MALDI-TOF (m/z): [M]+ 1029.701.
2.4. General methods and instrumentation
General methods, experimental section, and characterization techniques are provided in the ESI.†
2.5. Dye-sensitized solar cell fabrication
DSSC fabrication and characterization for the ISQ dyes have been provided in the ESI.†![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 22
22
3. Results and discussion
3.1. Synthesis of halogen-functionalized D–A–D-type unsymmetrical squaraine dyes
Synthesis of an unsymmetrical squaraine dye requires the condensation of a suitably functionalized indolium salt with the semisquaraic acid. The halo-substituted-4-hydrazinohydrochloride derivatives 1a–d were reacted with branched ketone 2 to give the corresponding halo-substituted indolines 3a–dvia Fischer indole synthesis. The indoline derivatives 3a–d were further reacted with 1-iodododecane to afford the corresponding branched-indolium salts 4a–d. Condensation of indolium salts 4a–d with the semisquaric acid 5 under the azeotropic removal of water afforded the required halogen-containing unsymmetrical squaraine dyes 6a–d (Scheme 1) with moderate yields (30–40%). The final dyes were dark-blue solids and solubilized well in dichloromethane, chloroform, ethanol, and DMSO. All the newly synthesized compounds were characterized using 1H-NMR, 13C-NMR, and IR spectroscopic methods and mass spectrometry.
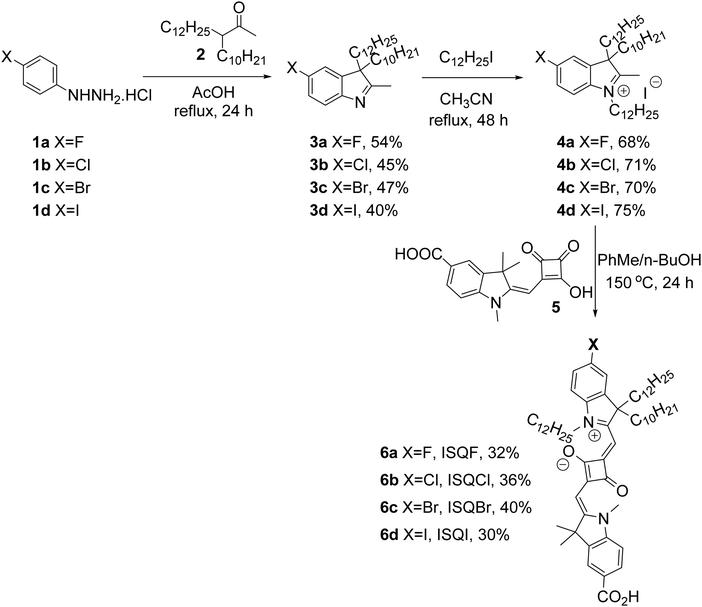 |
| | Scheme 1 Synthesis of halogen-containing (ISQF–ISQI) unsymmetrical squaraine dyes. | |
3.2. Photophysical and electrochemical properties
The UV-vis absorption and emission studies of the ISQ dyes, as well as the dye SQS4, were carried out in CH3CN/CHCl3 (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5%) solutions at room temperature (Table 1). The solution UV-vis absorption spectrum for the indoline-donor-based squaraine dyes contain a sharp and intense peak (ε > 105 M−1 cm−1) at 640–646 nm due to the π–π* transition, and a vibronic side peak located at a higher energy of 580 nm appeared as the shoulder. Normalized absorption and emission spectra for the ISQ and SQS4 dyes are displayed in Fig. 2a. A small red-shift was observed in the absorption band for the ISQCl, ISQBr, and ISQI dyes (λmax 645 and 646 nm) compared to the ISQF and SQS4 dyes (λmax 641 and 642 nm). A similar phenomenon can be observed in the emission of the ISQ dyes (650–656 nm) compared to the SQS4 dye (650 nm). The ISQ dyes showed molar extinction coefficients in the range of 2.1–3.1 × 105 M−1 cm−1. Furthermore, to investigate the aggregation behaviour of the ISQ dyes on the metal-oxide surface and the light harvesting properties, UV-vis characterization was carried out on a TiO2 surface. The dipping time of the electrodes was 5 min in a 0.1 mM dye solution prepared in MeCN: CHCl3 (95
5%) solutions at room temperature (Table 1). The solution UV-vis absorption spectrum for the indoline-donor-based squaraine dyes contain a sharp and intense peak (ε > 105 M−1 cm−1) at 640–646 nm due to the π–π* transition, and a vibronic side peak located at a higher energy of 580 nm appeared as the shoulder. Normalized absorption and emission spectra for the ISQ and SQS4 dyes are displayed in Fig. 2a. A small red-shift was observed in the absorption band for the ISQCl, ISQBr, and ISQI dyes (λmax 645 and 646 nm) compared to the ISQF and SQS4 dyes (λmax 641 and 642 nm). A similar phenomenon can be observed in the emission of the ISQ dyes (650–656 nm) compared to the SQS4 dye (650 nm). The ISQ dyes showed molar extinction coefficients in the range of 2.1–3.1 × 105 M−1 cm−1. Furthermore, to investigate the aggregation behaviour of the ISQ dyes on the metal-oxide surface and the light harvesting properties, UV-vis characterization was carried out on a TiO2 surface. The dipping time of the electrodes was 5 min in a 0.1 mM dye solution prepared in MeCN: CHCl3 (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) (Fig. 2c and Table 1). The ISQ and SQS4 dyes all showed red-shifted charge transfer (π–π*) peaks (646–658 nm), which correspond to the monomeric dye structure, with additional transition peaks for smaller aggregated structures between 605–610 nm compared to their solution spectra (643–649 nm) when the photoanode was sensitized for 5 min. In the presence of optically transparent co-adsorbent chenodeoxycholicacid (CDCA), competitive anchoring of CDCA on the metal-oxide surface reduces the dye–dye interaction, and hence, a reduction in the peak intensity at 605–610 nm was observed.(Fig. S10, ESI†). The light-harvesting efficiency (LHE) is one of the key factors in DSSCs; it represents the ability of the sensitizers to absorb a certain range of photons for a particular dye. The LHE of the ISQ and SQS4 dyes is displayed in Fig. 2d and Table 1 (sensitization time 12 h). The LHE properties of the ISQF, ISQCl, ISQBr, and ISQI dyes on the mesoporous TiO2 thin films showed broad absorbance between 500–720 nm. The spectral broadening at 60% LHE increased from SQS4 to ISQI (Δλ = 193, 192, 209, 204, and 211 nm). This indicates that the extent of dye–dye interactions on the TiO2 surface is almost the same for this set of dyes.
5) (Fig. 2c and Table 1). The ISQ and SQS4 dyes all showed red-shifted charge transfer (π–π*) peaks (646–658 nm), which correspond to the monomeric dye structure, with additional transition peaks for smaller aggregated structures between 605–610 nm compared to their solution spectra (643–649 nm) when the photoanode was sensitized for 5 min. In the presence of optically transparent co-adsorbent chenodeoxycholicacid (CDCA), competitive anchoring of CDCA on the metal-oxide surface reduces the dye–dye interaction, and hence, a reduction in the peak intensity at 605–610 nm was observed.(Fig. S10, ESI†). The light-harvesting efficiency (LHE) is one of the key factors in DSSCs; it represents the ability of the sensitizers to absorb a certain range of photons for a particular dye. The LHE of the ISQ and SQS4 dyes is displayed in Fig. 2d and Table 1 (sensitization time 12 h). The LHE properties of the ISQF, ISQCl, ISQBr, and ISQI dyes on the mesoporous TiO2 thin films showed broad absorbance between 500–720 nm. The spectral broadening at 60% LHE increased from SQS4 to ISQI (Δλ = 193, 192, 209, 204, and 211 nm). This indicates that the extent of dye–dye interactions on the TiO2 surface is almost the same for this set of dyes.
Table 1 Photophysical and electrochemical characterizations of SQS4, ISQF, ISQCl, ISQBr and ISQI dye at room temperature
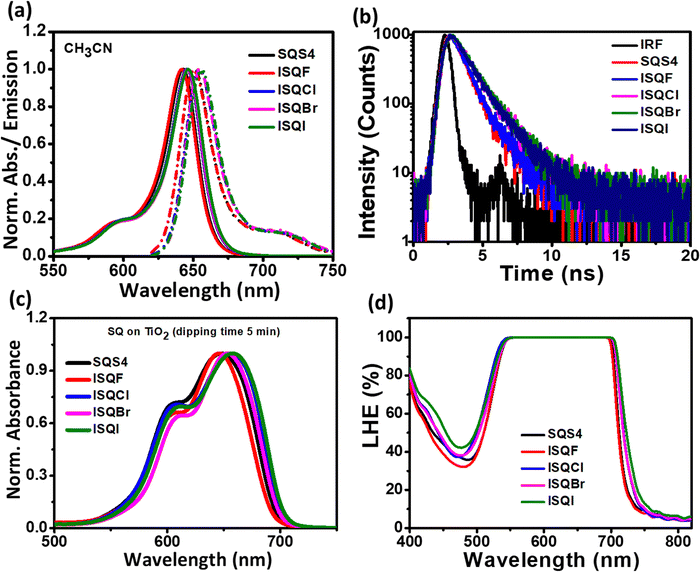 |
| | Fig. 2 (a) Normalized UV-vis and emission spectra of SQS4, ISQF, ISQCl, ISQBr, and ISQI in CH3CN/CHCl3 (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5%) solution. (b) TCSPC decay profile for the SQS4, ISQF, ISQCl, ISQBr, and ISQI dyes in dichloromethane solution; excitation wavelength was 635 nm, emission monitored at 650 nm. (c) UV-vis absorption spectra of SQS4, ISQF, ISQCl, ISQBr, and ISQI dyes on the TiO2 electrode. (d) LHE of SQS4, ISQF, ISQCl, ISQBr, and ISQI dyes on the TiO2 electrode (LHE = 1–10−A). 5%) solution. (b) TCSPC decay profile for the SQS4, ISQF, ISQCl, ISQBr, and ISQI dyes in dichloromethane solution; excitation wavelength was 635 nm, emission monitored at 650 nm. (c) UV-vis absorption spectra of SQS4, ISQF, ISQCl, ISQBr, and ISQI dyes on the TiO2 electrode. (d) LHE of SQS4, ISQF, ISQCl, ISQBr, and ISQI dyes on the TiO2 electrode (LHE = 1–10−A). | |
To investigate the photo-excited state properties of the ISQ dyes further, lifetime measurement using the time-correlated single-photon counting method (TCSPC) in dichloromethane (CH2Cl2) solutions was carried out (Fig. 2b, Table 2 and Table S1 in the ESI†). In general, the ground-state and the excited-state (S0–S1) electronic structures of D–A–D squaraine dyes are highly polarized, with the indoline moiety being an electron donor (D) and the central C4O2 unit being an electron acceptor (A). It is known that heavy-atom-functionalized organic chromophores undergo an efficient intersystem-crossing process to populate the triplet photo-excited state.43,44 Thiosquaraine dyes are well-known chromophores for the triplet state; in these dyes, the oxygen atoms of C4O2 are replaced with sulfur atoms to form C4S2, and are responsible for the enhanced intersystem crossing (ISC).45 Halogen atom incorporation outside the polymethine framework leads to enhanced fluorescence quantum yield, whereas, heavy atom functionalization within the polymethine framework leads to triplet-state formation.46ISQ dyes showed a biexponential lifetime with decay times τ1 in the range of 0.79–1.10 ns. Furthermore, a relative method was used with the reference DMASQ dye (quantum yield 0.42 in dichloromethane solution) for the quantum yield calculations of the ISQ and SQS4 dyes.47 No significant changes were observed in the quantum yield (0.28 to 0.31%) for the ISQ and SQS4 dyes. Hence, the charge injection takes place from the photoexcited singlet state of the ISQ dyes. As the heavy atoms are separated from the polymethine framework, spin–orbit coupling is not affected in these heavy-atom-containing ISQ dyes; hence, only the effect of halogen bonding is effectively considered for the dye regeneration.
Table 2 Fluorescence lifetimes of ISQ and SQS4 dyes in CH2Cl2 at room temperature
| Dye |
τ
1 (ns) |
a
1 (%) |
τ
2 (ns) |
a
2 (%) |
χ
2
|
Quantum yielda |
|
Using a relative method with DMASQ dye as a standard,47 the absorbance values for ISQ dyes and the standard dye; DMASQ has been used as follows: SQS4 (596 nm), ISQF (599 nm), ISQCl (593 nm), ISQBr (593 nm) and ISQI (590 nm).
|
|
SQS4
|
0.79 |
86 |
0.17 |
14 |
1.24 |
0.28 |
|
ISQF
|
0.91 |
93 |
0.25 |
7 |
1.16 |
0.34 |
|
ISQCl
|
1.13 |
80 |
0.21 |
20 |
1.10 |
0.35 |
|
ISQBr
|
1.07 |
53 |
0.16 |
47 |
1.13 |
0.32 |
|
ISQI
|
1.10 |
76 |
0.19 |
24 |
1.08 |
0.31 |
Cyclic voltammetry studies were carried out in anhydrous dichloromethane solution to measure the electrochemical characteristics of the ISQ dyes (Fig. 3a and Table 1). The HOMO energy levels of the ISQ dyes were calculated from the first oxidation onset value of the cyclic voltammograms, which was converted to NHE by adding 0.7 V.48 The ground state oxidation potential (Eox) values of 0.79, 0.83, 0.84 and 0.83 V have been obtained for ISQF, ISQCl, ISQBr, and ISQI dyes versus NHE, respectively. The Eox of the ISQ dyes was ∼0.4 V more positive than the redox function of the I−/I3− electrolyte (0.4 V vs NHE), which is favourable for the dye regeneration process. The excited state oxidation potential (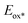 ) energy levels were found by subtracting the optical band gap (E0–0) from the Eox values, which were found between −1.12 and −1.06 V and were favourable for the electron injection process for the ISQ dyes to the conduction band of TiO2. The energy level diagrams of the SQS4, ISQF, ISQCl, ISQBr, and ISQI dyes with the DSSC device components are displayed in Fig. 3b.
) energy levels were found by subtracting the optical band gap (E0–0) from the Eox values, which were found between −1.12 and −1.06 V and were favourable for the electron injection process for the ISQ dyes to the conduction band of TiO2. The energy level diagrams of the SQS4, ISQF, ISQCl, ISQBr, and ISQI dyes with the DSSC device components are displayed in Fig. 3b.
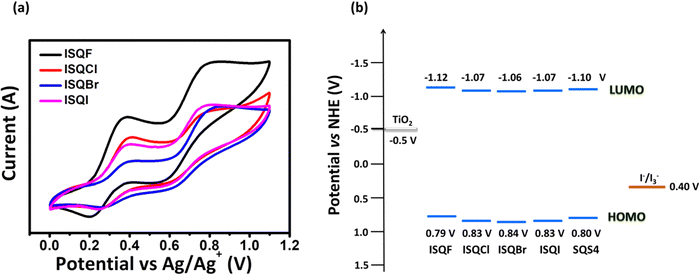 |
| | Fig. 3 (a) Cyclic voltammograms of the ISQF, ISQCl, ISQBr, and ISQI dyes. (b) Energy level diagram of the ISQF, ISQCl, ISQBr, and ISQI dyes along with the SQS4 dye and DSSC device components. | |
3.3. Theoretical calculations
Using DFT calculations, the ISQ dye structures were optimized at the B3LYP/6-311G** and B3LYP/LANL2DZ (for ISQBr and ISQI) level using Gaussian 09;49 the results showed that the inherent electronic structures, such as the HOMOs and LUMOs, were not affected by the presence of halogen atoms. The optimized structure for the ISQI dye and molecular electrostatic potential (MEP) mapped onto the van der Waals surface top view for the SQ-X and ISQ-X+ dyes are provided in Fig. 4 and Fig. 5, respectively. The detailed optimized structures and corresponding tables are provided in the Supplementary information (Fig. S12–S18, and Tables S3–S5, ESI†). The HOMO of the ISQ dyes was mainly distributed over the polymethine framework, with lower electron densities at the indoline donors, and the LUMO was distributed over the carboxylic-acid-containing donor site in addition to the polymethine framework; this feature promotes the unidirectional charge injection process upon photoexcitation. Furthermore, the electrostatic potential plots of the dyes showed the presence of a σ-hole in the bromine- and iodine-containing ISQ dyes. Additionally, the DFT studies of the oxidised ISQI dye showed the contribution of the halogen atoms to the HOMO−1 level, as well as the presence of a σ-hole in the oxidized dye as indicated by the molecular electrostatic potential. These studies support the presence halogen atoms in ISQBr and ISQI, which may promote the dye-regeneration process by forming halogen bonding with the iodolyte electrolyte.
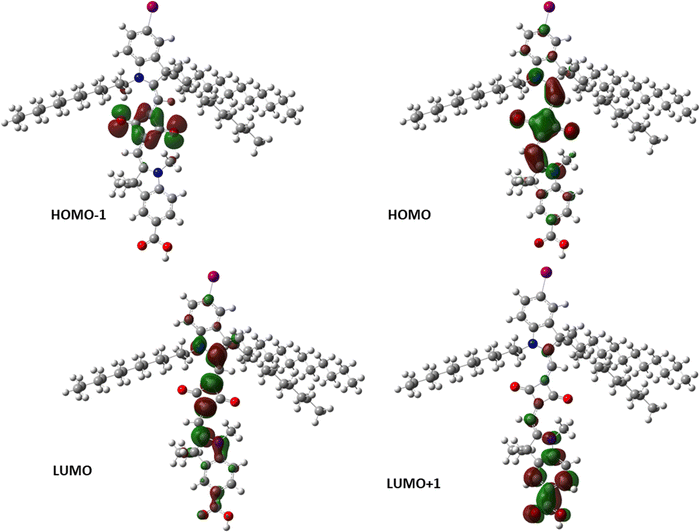 |
| | Fig. 4 DFT-optimized energy levels (HOMO, HOMO−1, LUMO, LUMO+1) of the ISQI dye. | |
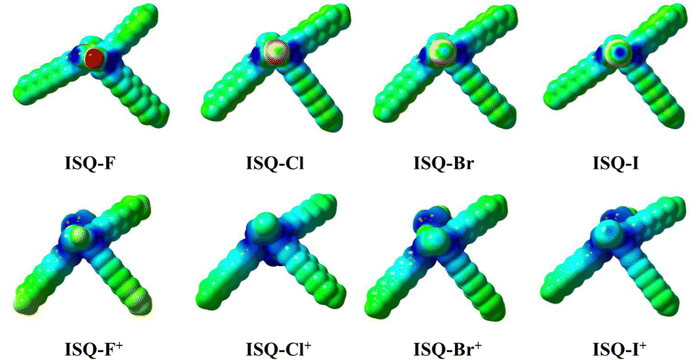 |
| | Fig. 5 Molecular electrostatic potential (MEP) mapped onto the van der Waals surfaces (top view) of the ISQ-X and ISQ-X+ dyes. The colour scale ( ) red to blue represents −0.02 to +0.02 kcal mol−1. ) red to blue represents −0.02 to +0.02 kcal mol−1. | |
The interaction energies (ΔE) of the ISQI dye with the iodide ions of the electrolyte in its neutral (ISQ-I) and oxidised (ISQ-I+) form were compared to that of the corresponding neutral and oxidised states of non-halogenated SQS4 dye (ISQ-H and ISQ-H+), respectively. The electrolyte interaction energy of the oxidised dye ISQ-I+ was found to be lower than that of ISQ-H+ by 10.71 kcal mol−1. When the interaction distance between the ISQ-I+ dye and I− was investigated, it was found to show a 0.45 Å contraction, while ISQ-H+ dye was found to show a 0.05 Å extension from the predicted van der Waals interaction distance (Fig. 6). Therefore, the calculations support the assumption that halogen bonding can exist between iodide and ISQ-I+, and this effect is much more pronounced compared to its non-halogenated counterpart ISQ-H+. Further analysis via a natural bond orbital (NBO) study showed that the halogen bonding between the ISQ-I dyes and I− corresponds to a Lewis acid–base adduct formed by a filled C–I σ bond to the empty π* orbitals of I−. The orbitals associated with halogen bonding for the ISQ-I+ and ISQ-H+ dyes, along with the interaction stabilisation energy (E2), are provided in Table 3. Interestingly, for neutral dyes, the ISQ-H–I− interaction is 6.06 kcal mol−1 lower than the ISQ-I–I− interaction, which is attributed to the 0.11 Å contraction in the van der Waals distance and an additional stabilisation between a lone pair on iodide and the empty C–X σ* orbital. The higher interaction energy of I−, contraction from the predicted van der Waals distance and higher stabilisation energy support the observation that better halogen bonding is observed in the dyes with heavier halogen substituents. Hybridisation data for selected orbitals for the donor–acceptor interaction with the I− ion are provided in Table S8 (ESI†).
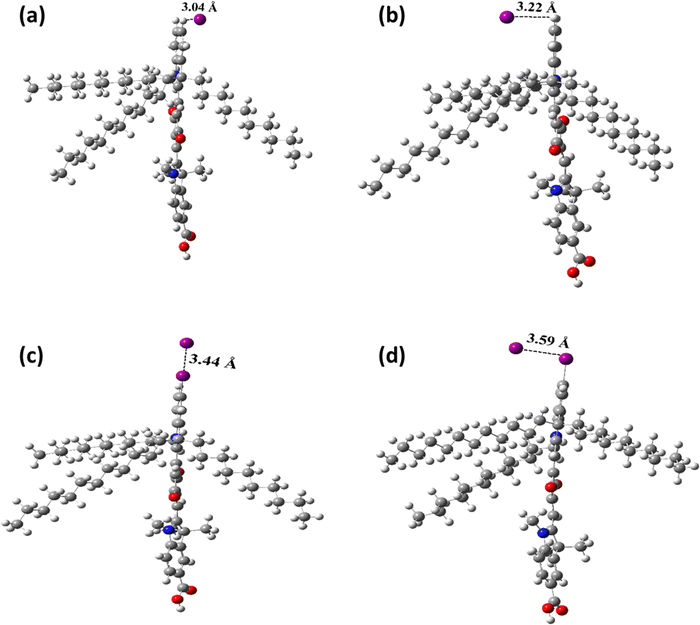 |
| | Fig. 6 Optimized structures of the ISQ–I− halogen–iodide interaction in (a) ISQ-H, (b) ISQ-H+, (c) ISQ-I and (d) ISQ-I+, with the dye–I− distances indicated. | |
Table 3 Calculated halogen–iodide distances, donor and acceptor with orbital contributions and stabilization energies (E2) of the halogen–iodide interaction as shown in Fig. 6a
|
ISQ dye |
ΔE (kJ mol−1) |
Dye–I− (Å) |
Donor |
Acceptor |
E
2 (kcal mol−1) |
|
van der Waals radii of I− (2.06 Å), H (1.09 Å) and I (1.98 Å); predicted van der Waals distance between ISQ-H–I− (3.15 Å) and ISQ-I–I− (4.04 Å). BD: Bonding orbital, LP: Lone-Pair orbital, and RY: Rydberg orbital; LP*, LP* and RY*: Virtual empty orbitals.
|
|
ISQ-H |
−22.71 |
3.04 |
BD (1) C4–H152 |
RY*(1) I157 |
4.74 |
| LP (2) I 157 |
BD*(1) C4–H152 |
0.13 |
| ISQ-I |
−16.64 |
3.44 |
BD (1) C4–I156 |
RY*(2) I157 |
2.39 |
| ISQ-H+ |
−64.13 |
3.22 |
BD (1) C4–H152 |
RY*(1) I157 |
10.46 |
| ISQ-I+ |
−74.84 |
3.59 |
BD (1) C4–I156 |
RY*(1) I157 |
51.19 |
3.4. Photovoltaic characterization
Photovoltaic characterizations of the halogen containing ISQ dyes were carried out with two iodolyte electrolytes (I−/I3−) and optimized using different concentrations of CDCA. As the IPCE is the product of light-harvesting efficiency (LHE), electron injection efficiency (φinj), charge collection efficiency (φcc), and dye regeneration efficiency (φreg) (eqn (1)),| | | IPCE(λ) = LHE(λ) × φinj × φcc × φreg | (1) |
The dye regeneration efficiency (φreg) depends on the concentration of the redox species as provided in the eqn (2) (where D = donor concentrations of (I−), kreg is the regeneration rate constant and kcr is the charge recombination rate constant). The DSSC devices were fabricated with two different concentrations of iodolyte (concentration of I3− was 50 mM for Z-50 and 150 mM for Z-150).
| | 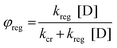 | (2) |
The photovoltaic parameters (VOC, JSC, ff, and η) are displayed in Fig. 7 and Table 4. The DSSC devices fabricated in iodolyte Z-50 without CDCA showed low JSC values and DSSC efficiencies for the ISQ dyes compared to that for SQS4 with a hydrogen atom. Use of iodolyte Z-150 as an electrolyte enhanced the JSC and decreased the VOC values for all the dyes in the absence of CDCA, and a maximum JSC of 13.30 mA cm−2 was achieved using the ISQI dye.9,50 The DSSC device fabricated with 3 equivalents of CDCA showed enhanced VOC, JSC, and efficiency for all the ISQ and SQS4 dyes in both electrolytes (iodolyte Z-50 and Z-150) compared to the device sensitized in the absence of CDCA. SQS4 dye in the absence of CDCA showed the maximum DSSC device efficiency compared to the ISQ dyes in both electrolyte systems (iodolyte Z-50 and Z-150). The ISQI dye with 3 equivalents of CDCA showed greater device efficiency compared to the other ISQ dyes and SQS4 dye in both iodolyte Z-50 and Z-150 electrolytes. Furthermore, it was observed that the ISQBr dye sensitized with CDCA showed enhanced DSSC efficiency in iodolyte Z-150 electrolyte compared to the non-halogenated SQS4 dye. The maximum DSSC device efficiency of 7.81% (with VOC of 692 mV, JSC of 15.06 mA cm−2) was achieved using ISQI dye in iodolyte Z-150 electrolyte when the ISQI dye was sensitized with 3 equivalents of CDCA. The ISQ dyes showed excellent IPCE responses from the visible and NIR regions. The enhanced IPCE for the ISQ dyes in iodolyte Z-150 electrolyte was the reason for the improved JSC compared to iodolyte Z-50 electrolyte. Additionally, a high-concentration electrolyte enhances the halogen bonding between the oxidized ISQBr and ISQI dyes on the TiO2 and I−; these interactions facilitate the dye-regeneration process, which in turn enhances the JSC of the devices.26,27 The low DSSC performance observed for ISQF and ISQCl compared to SQS4 may be due to the electronic factors of the fluoride and chloride atoms on the indoline unit, respectively. A graphical representation of the VOC and JSC values in the iodolyte electrolytes (Z-50 and Z-150) for the ISQ and SQS4 dyes in the presence and absence of CDCA is displayed in Fig. 8. The enhanced photocurrent generation with Z-150 compared to Z-50 indicates the interaction between the electrolyte and oxidized dye with halogen atoms compared to that for SQS4. The determination of dye regeneration rate constant would have provided more clear understanding. Furthermore, the amount of dye of adsorbed on the TiO2 surface was calculated and provided in the Supplementary information (Fig. S20 and Table S7, ESI†).
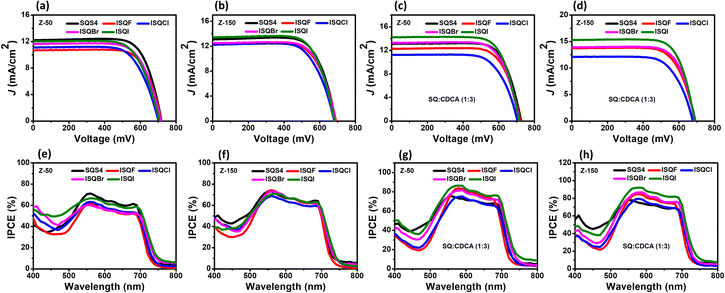 |
| | Fig. 7
I–V curves (a) and (c) in Z-50 and (b) and (d) in Z-150, and IPCE profiles in Z-50 (e) and (g) and in Z-150 (f) and (h) for the ISQ and SQS4 dyes. | |
Table 4 Photovoltaic performance of ISQ dyes along with SQS4 dye in Z-50 and Z-150a
| Dye |
CDCA (mM) |
V
OC (mV) Z-50/Z-150 |
J
SC (mA cm−2) Z-50/Z-150 |
ff (%) Z-50/Z-150 |
η (%) Z-50/Z-150 |
|
Concentration of I3− was 50 mM or 150 mM.
Concentrations: 0.25 M DMPII, 15 mM I2, 0.125 M NaI, 0.5 M TBP, 0.1 M GuSCN.
Concentrations: 0.50 M DMPII, 30 mM I2, 0.250 M NaI, 0.5 M TBP, 0.1 M GuSCN. DMPII = 1,2-dimethyl-3-propylimidazolium iodide, TBP = 4-tert-butylpyridine, and GuSCN = guanidinium thiocyanate.
|
|
SQS4
|
0 |
719 ± 2.75/690 ± 1.46 |
12.18 ± 0.09/12.99 ± 0.08 |
74 ± 1.13/72 ± 0.96 |
6.48 ± 0.05/6.47 ± 0.12 |
|
ISQF
|
0 |
718 ± 1.77/691 ± 1.17 |
10.68 ± 0.07/12.2 ± 0.19 |
73 ± 1.24/71 ± 1.11 |
5.60 ± 0.06/5.99 ± 0.18 |
|
ISQCl
|
0 |
702 ± 2.56/681 ± 2.25 |
11.10 ± 0.04/12.20 ± 0.03 |
70 ± 0.81/70 ± 0.95 |
5.45 ± 0.10/5.82 ± 0.10 |
|
ISQBr
|
0 |
715 ± 1.53/687 ± 2.87 |
11.62 ± 0.18/12.44 ± 0.12 |
71 ± 1.15/71 ± 1.32 |
5.90 ± 0.18/6.10 ± 0.20 |
|
ISQI
|
0 |
705 ± 1.72/678 ± 3.90 |
12.01 ± 0.13/13.42 ± 0.15 |
70 ± 1.21/71 ± 1 |
5.92 ± 0.16/6.46 ± 0.25 |
|
SQS4
|
0.3 |
726 ± 1.73/693 ± 3.29 |
13.05 ± 0.19/13.65 ± 0.10 |
75 ± 0.83/72 ± 1.36 |
7.12 ± 0.19/6.85 ± 0.24 |
|
ISQF
|
0.3 |
721 ± 2.92/692 ± 1.09 |
12.20 ± 0.12/13.65 ± 0.10 |
73 ± 0.76/71 ± 0.82 |
6.44 ± 0.15/6.72 ± 0.16 |
|
ISQCl
|
0.3 |
705 ± 2.75/678 ± 2.47 |
11.18 ± 0.20/12.07 ± 0.05 |
70 ± 1.02/69 ± 1.00 |
5.53 ± 0.20/5.62 ± 0.14 |
|
ISQBr
|
0.3 |
715 ± 1.76/690 ± 1.28 |
13.21 ± 0.22/13.83 ± 0.15 |
74 ± 0.68/74 ± 0.94 |
6.99 ± 0.20/7.07 ± 0.28 |
|
ISQI
|
0.3 |
710 ± 3.14/690 ± 2.13 |
14.19 ± 0.07/15.28 ± 0.05 |
73 ± 1/74 ± 1.20 |
7.35 ± 0.10/7.80 ± 0.09 |
|
ISQI
|
0.3 |
733 ± 1 |
10.61 ± 0.10 |
73 ± 0.88 |
5.67 ± 0.12 |
|
ISQI
|
0.3 |
709 ± 2 |
12.21 ± 0.07 |
68 ± 0.50 |
5.81 ± 0.10 |
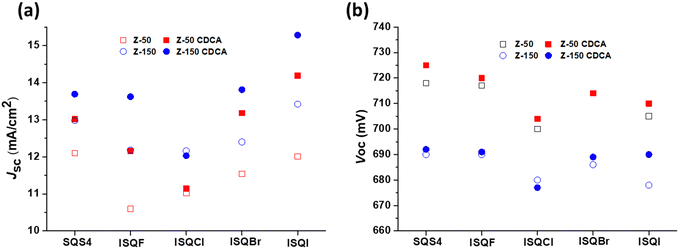 |
| | Fig. 8 Graphical representation of the (a) JSC and (b) VOC values of the halogen-containing ISQ dyes along with the SQS4 dye in iodolyte electrolyte (Z-50 and Z-150). | |
Photovoltaic characterizations of the ISQI dye with 0.3 mM of CDCA were carried out with different concentrations of I−/I3− electrolytes to evaluate the effect of iodolyte concentration on the DSSC performance. The photovoltaic parameters are provided in Fig. 9 and Table 4. The DSSC device fabricated with a low-concentration electrolyte (0.25 M DMPII) showed a relatively low JSC compared to those with the high-concentration electrolytes (0.50 and 0.75 M DMPII). The photovoltaic parameters for the different electrolyte concentrations are provided in the Supplementary information (Fig. S19 and Table S6, ESI†).
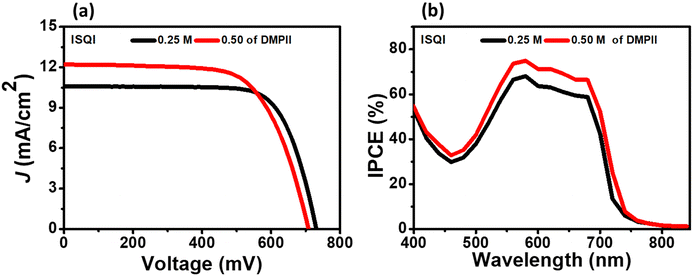 |
| | Fig. 9 (a) I–V and (b) IPCE curves of the ISQI dye with different concentration electrolytes. | |
Current transient experiments for the SQS4 and ISQI dyes were carried out by switching the light source on and off (for 20 s each) to investigate the effect of non-halogenated and halogenated squaraine dyes with different electrolyte concentrations on the regeneration efficiency of dyes (Fig. 10).51 Both SQS4 and ISQI dyes showed enhanced current with Z-150 iodolyte electrolyte compared to Z-50, which was further improved in the presence of CDCA for both dyes. Here, the ISQI dye showed high current responses at different sun intensities with both Z-50 and Z-150 iodolyte electrolytes compared to SQS4 dye, which clearly showed the importance of halogen-atom-containing squaraine dyes in achieving high DSSC JSC and efficiency. A combined current transient profile of the SQS4 and ISQI dyes with CDCA in Z-150 electrolyte is provided in Fig. 10c for better understanding. Furthermore, the regeneration efficiency (RE(%) = (Isat/Imax) × 100) of the SQS4 and ISQI dyes with CDCA at different sun intensities and electrolyte concentrations has been calculated (Fig. 10d), and ISQI showed the highest RE of 96.6% with Z-150 electrolyte compared to SQS4 at 1.0 sun light intensity. This improved regeneration efficiency can be explained by the interaction between the electrolyte and the iodine atom of ISQI. Furthermore, the zwitterionic structures of the squaraine dyes might be the reason for the low RE compared to neutral D–π–A dye with iodolyte electrolytes at different light intensities.51
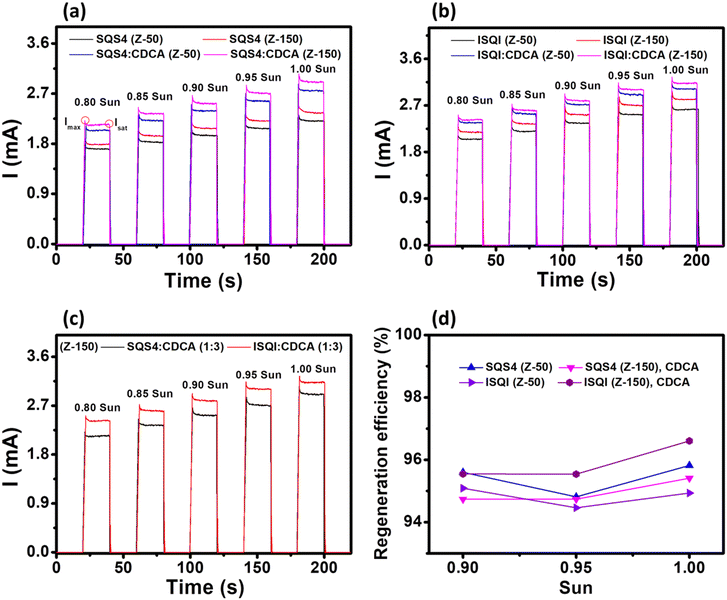 |
| | Fig. 10 Current transient profiles of (a) SQS4 and (b) and ISQI in different CDCA electrolyte concentrations. (c) SQS4 and ISQI with CDCA in Z-150 electrolyte. (d) Regeneration efficiency of SQS4 and ISQI dyes with Z-50 and Z-150 iodolyte electrolyte in the presence of CDCA at different sun intensities. | |
3.5. Electrochemical impedance spectroscopy (EIS)
To understand the charge recombination resistance (Rct), chemical capacitance (Cμ), and lifetime of electron (τ) at the dye–TiO2/electrolyte interface, EIS characterization of the halogen-containing ISQ dyes was carried out in the dark at different applied potentials with iodolyte electrolyte (Z-50 and Z-150).52 The EIS parameters optimized with CDCA are provided in Fig. 11 and Table 5, and the EIS study without CDCA is provided in Fig. S21 (ESI†). As the ISQ and SQS4 dyes have similar steric features, no significant changes are observed in the Rct, Cμ, and τ when the electrolyte used for device fabrication was iodolyte Z-50 or Z-150 for all the dyes (Table 5) with CDCA. The maximum of values of 7.12 Ohm, 0.445 mF, and 3.16 ms for Rct, Cμ, and τ were achieved for the ISQI dye with iodolyte Z-150 in the presence of CDCA, and are the reason for its high device efficiency compared to SQS4 and the other ISQ dyes. It is observed that better Rct parameters from the EIS studies were correlated with lower VOC of the DSSC devices with the Z-150 electrolyte. The introduction of any new functional group in the dye backbone changes the inherent properties of the dyes, such as dipole moment, which may affect the conduction band position of TiO2 and hence produce the variation in the obtained VOC of the devices, despite the increased Rct obtained from the EIS studies.
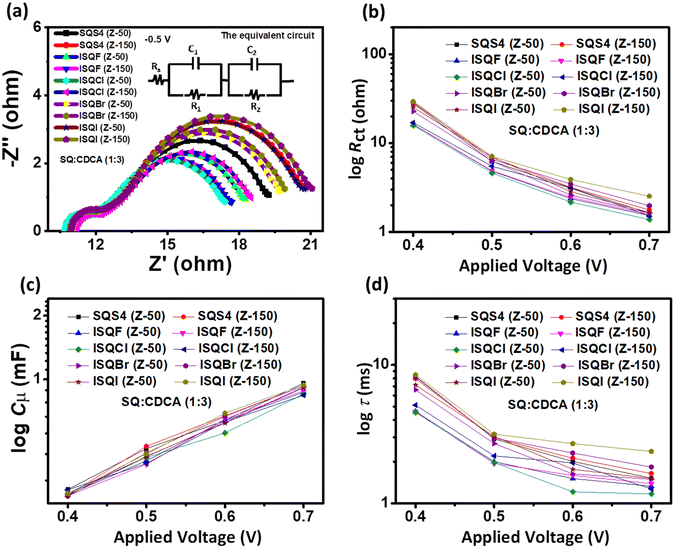 |
| | Fig. 11 (a) Nyquist plots, (b) Rct, (c) Cμ, and (d) τ of the ISQ and SQS4 dyes with iodolyte electrolyte (Z-50 and Z-150) in the presence of CDCA. | |
Table 5 EIS parameters of ISQ and SQS4 dyes in the dark with iodolyte electrolyte (Z-50 and Z-150) and an applied bias of 0.50 V
| Dye |
R
ct (Ohm) Z-50/Z-150 |
C
μ (mF) Z-50/Z-150 |
τ (ms) Z-50/Z-150 |
|
SQS4
|
6.00 ± 0.09/6.03 ± 0.24 |
0.461/0.468 |
2.72/2.82 |
SQS4:CDCA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
6.23 ± 0.10/6.93 ± 0.08 |
0.471/0.483 |
2.92/2.97 |
|
ISQF
|
4.40 ± 0.28/4.43 ± 0.25 |
0.392/0.41 |
1.72/1.81 |
ISQF:CDCA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
4.85 ± 0.25/4.89 ± 0.18 |
0.413/0.40 |
2.00/1.94 |
|
ISQCl
|
4.65 ± 0.19/6.01 ± 0.22 |
0.432/0.437 |
2.00/2.62 |
ISQCl:CDCA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
4.62± 0.15/5.50 ± 0.10 |
0.428/0.401 |
2.62/2.20 |
|
ISQBr
|
6.12 ± 0.08/5.70 ± 0.12 |
0.392/0.391 |
2.39/2.22 |
ISQBr:CDCA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
6.27 ± 0.20/6.58 ± 0.10 |
0.432/0.450 |
2.70/2.96 |
|
ISQI
|
6.10 ± 0.10/6.23 ± 0.19 |
0.413/0.418 |
2.51/2.60 |
ISQI:CDCA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
7.03 ± 0.22/7.12 ± 0.18 |
0.434/0.445 |
3.05/3.16 |
4. Conclusion
To achieve the advantages of halogen bonding in enhancing the JSC and efficiency of DSSCs, a series of halogen-atom-functionalized D–A–D based unsymmetrical squaraine dyes, ISQF, ISQCl, ISQBr and ISQI, have been synthesized and characterized for DSSC applications. UV-vis absorption and emission studies showed slight red-shifts when the H-atom was replaced with a halo-atom in the ISQ dyes. Additionally, fluorescence lifetime and quantum yield measurements revealed biexponential lifetimes with a decay time τ1 in the range of 0.79–1.10 ns, and the observed quantum yields of 0.28–0.31% did not show significant changes for the ISQ dyes. The DSSC devices for ISQ dyes were fabricated using iodolyte electrolytes (Z-50 and Z-150) and optimized with CDCA. The ISQ dyes showed varied photovoltaic characteristics with both electrolytes in the absence of CDCA. Sensitization of the ISQ dyes with 3 equivalents of CDCA showed enhanced photovoltaic parameters compared to those obtained without CDCA sensitization. The maximum DSSC device efficiency of 7.80% was achieved by the ISQI dye with a VOC of 690 mV and JSC of 15.28 mA cm−2 in iodolyte Z-150 electrolyte when the ISQI dye was sensitized with 3 equivalents of CDCA. The observed high DSSC device JSC and efficiency in Z-150 electrolyte for the ISQI dye was due to the presence of a σ-hole, which enhanced the interaction between the electrolyte and the dye on TiO2 and promoted the dye-regeneration process.
Author contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
Conflicts of interest
The authors declare no competing financial interest.
Acknowledgements
The financial support by SERB, India (CRG/2020/003105), and CSIR, India (NWP0054, CSIR-TAPSUN), are greatly acknowledged. I. S. N., A. K. S., A. M. and S. S. D. thank CSIR, New Delhi, India, for the research fellowships.
References
- O. Regan and M. Grätzel, Nature, 1991, 353, 737–740 CrossRef.
- A. Hagfeldt, G. Boschloo, L. Sun, L. Kloo and H. Pettersson, Chem. Rev., 2010, 110, 6595–6663 CrossRef CAS PubMed.
- J. M. Ji, H. Zhou and H. K. Kim, J. Mater. Chem. A, 2018, 6, 14518–14545 RSC.
- A. B. Muñoz-García, I. Benesperi, G. Boschloo, J. J. Concepcion, J. H. Delcamp, E. A. Gibson, G. J. Meyer, M. Pavone, H. Pettersson, A. Hagfeldt and M. Freitag, Chem. Soc. Rev., 2021, 50, 12450–12550 RSC.
- M. Ye, X. Wen, M. Wang, J. Iocozzia, N. Zhang, C. Lin and Z. Lin, Mater. Today, 2015, 18, 155–162 CrossRef CAS.
- J. Wu, Z. Lan, J. Lin, M. Huang, Y. Huang, L. Fan and G. Luo, Chem. Rev., 2015, 115, 2136–2173 CrossRef CAS PubMed.
- A. Listorti, B. O’Regan and J. R. Durrant, Chem. Mater., 2011, 23, 3381–3399 CrossRef CAS.
- A. Y. Anderson, P. R. F. Barnes, J. R. Durrant and B. C. O’regan, J. Phys. Chem. C, 2011, 115, 2439–2447 CrossRef CAS.
- S. Martiniani, A. Y. Anderson, C. H. Law, B. C. O’regan and C. Barolo, Chem. Commun., 2012, 48, 2406–2408 RSC.
- Q. Yu, Y. Wang, Z. Yi, N. Zu, J. Zhang, M. Zhang and P. Wang, ACS Nano, 2010, 4, 6032–6038 CrossRef CAS PubMed.
- J. Yang, F. Guo, J. Hua, X. Li, W. Wu, Y. Qu and H. Tian, J. Mater. Chem., 2012, 22, 24356 RSC.
- Y. Wu, M. Marszalek, S. M. Zakeeruddin, Q. Zhang, H. Tian, M. Grätzel and W. Zhu, Energy Environ. Sci., 2012, 5, 8261 RSC.
- Y. Jiao, F. Zhang, M. Grätzel and S. Meng, Adv. Funct. Mater., 2013, 23, 424–429 CrossRef CAS.
- J. Yang, P. Ganesan, J. Teuscher, T. Moehl, Y. J. Kim, C. Yi, P. Comte, K. Pei, T. W. Holcombe, M. K. Nazeeruddin, J. Hua, S. M. Zakeeruddin, H. Tian and M. Grätzel, J. Am. Chem. Soc., 2014, 136, 5722–5730 CrossRef CAS PubMed.
- N. Zhou, K. Prabakaran, B. Lee, S. H. Chang, B. Harutyunyan, P. Guo, M. R. Butler, A. Timalsina, M. J. Bedzyk, M. A. Ratner, S. Vegiraju, S. Yau, C. G. Wu, R. P. H. Chang, A. Facchetti, M. C. Chen and T. J. Marks, J. Am. Chem. Soc., 2015, 137, 4414–4423 CrossRef CAS PubMed.
- S. Alex, U. Santhosh and S. Das, J. Photochem. Photobiol., A, 2005, 172, 63–71 CrossRef CAS.
- K. R. Mulhern, M. R. Detty and D. F. Watson, J. Photochem. Photobiol., A, 2013, 264, 18–25 CrossRef CAS.
- G. De Miguel, M. Ziółek, M. Zitnan, J. A. Organero, S. S. Pandey, S. Hayase and A. Douhal, J. Phys. Chem. C, 2012, 116, 9379–9389 CrossRef CAS.
- A. Otsuka, K. Funabiki, N. Sugiyama, T. Yoshida, H. Minoura and M. Matsui, Chem. Lett., 2006, 35, 666–667 CrossRef CAS.
- A. Alagumalai, M. F. Munavvar, P. Vellimalai, M. C. Sil and J. Nithyanandhan, ACS Appl. Mater. Interfaces, 2016, 8, 35353–35367 CrossRef CAS PubMed.
- A. K. Singh, M. F. Mele Kavungathodi and J. Nithyanandhan, ACS Appl. Mater. Interfaces, 2020, 12, 2555–2565 CrossRef PubMed.
- A. K. Singh and J. Nithyanandhan, ACS Appl. Energy Mater., 2021, 4, 13932–13942 CrossRef CAS.
- T. Daeneke, A. J. Mozer, Y. Uemura, S. Makuta, M. Fekete, Y. Tachibana, N. Koumura, U. Bach and L. Spiccia, J. Am. Chem. Soc., 2012, 134, 16925–16928 CrossRef CAS PubMed.
- F. J. Malzner, S. Y. Brauchli, E. C. Constable, C. E. Housecroft and M. Neuburger, RSC Adv., 2014, 4, 48712–48723 RSC.
- C. Curiac, L. A. Hunt, M. A. Sabuj, Q. Li, A. Baumann, H. Cheema, Y. Zhang, N. Rai, N. I. Hammer and J. H. Delcamp, J. Phys. Chem. C, 2021, 125, 17647–17659 CrossRef CAS.
- S. J. C. Simon, F. G. L. Parlane, W. B. Swords, C. W. Kellett, C. Du, B. Lam, R. K. Dean, K. Hu, G. J. Meyer and C. P. Berlinguette, J. Am. Chem. Soc., 2016, 138, 10406–10409 CrossRef CAS PubMed.
- F. G. L. Parlane, C. Mustoe, C. W. Kellett, S. J. Simon, W. B. Swords, G. J. Meyer, P. Kennepohl and C. P. Berlinguette, Nat. Commun., 2017, 8, 1–7 CrossRef CAS PubMed.
- M. Ayaz, J. Khan Kasi, A. Khan Kasi, M. Bokhari and G. Boschloo, ACS Appl. Energy Mater., 2022, 5, 4240–4246 CrossRef CAS.
- L. Zhao, P. Wagner, J. E. Barnsley, T. M. Clarke, K. C. Gordon, S. Mori and A. J. Mozer, Chem. Sci., 2016, 7, 3506–3516 RSC.
- G. Cavallo, P. Metrangolo, R. Milani, T. Pilati, A. Priimagi, G. Resnati and G. Terraneo, Chem. Rev., 2016, 116, 2478–2601 CrossRef CAS PubMed.
- C. W. Kellett, P. Kennepohl and C. P. Berlinguette, Nat. Commun., 2020, 11, 1–8 CrossRef PubMed.
- W. B. Swords, S. J. C. Simon, F. G. L. Parlane, R. K. Dean, C. W. Kellett, K. Hu, G. J. Meyer and C. P. Berlinguette, Angew. Chem., Int. Ed., 2016, 55, 5956–5960 CrossRef CAS PubMed.
- D. Nugegoda, L. A. Hunt, A. Devdass, H. Cheema, R. C. Fortenberry, J. W. Jurss, N. I. Hammer and J. H. Delcamp, ACS Appl. Energy Mater., 2022, 5, 1516–1527 CrossRef CAS.
- J. M. Ji, H. J. Lee, H. Zhou, Y. K. Eom, C. H. Kim and H. K. Kim, ACS Appl. Mater. Interfaces, 2022, 14, 52745–52757 CrossRef CAS PubMed.
- A. L. Raithel, W. E. Meador, T. Y. Kim, R. J. Staples, J. H. Delcamp and T. W. Hamann, J. Am. Chem. Soc., 2023, 145, 1367–1377 CrossRef CAS PubMed.
- S. Khopkar and G. Shankarling, Dyes Pigm., 2019, 170, 107645 CrossRef CAS.
- J. He, Y. J. Jo, X. Sun, W. Qiao, J. Ok and T. Kim, Adv. Funct. Mater., 2009, 19, 2720–2727 CrossRef.
- J. H. Yum, P. Walter, S. Huber, D. Rentsch, T. Geiger, F. Nüesch, F. De Angelis, M. Grätzel and M. K. Nazeeruddin, J. Am. Chem. Soc., 2007, 129, 10320–10321 CrossRef CAS PubMed.
- T. Geiger, S. Kuster, J. H. Yum, S. J. Moon, M. K. Nazeeruddin, M. Grätzel and F. Nüesch, Adv. Funct. Mater., 2009, 19, 2720–2727 CrossRef CAS.
- F. M. Jradi, X. Kang, D. Oneil, G. Pajares, Y. A. Getmanenko, P. Szymanski, T. C. Parker, M. A. El-Sayed and S. R. Marder, Chem. Mater., 2015, 27, 2480–2487 CrossRef CAS.
- Y. Shi, R. B. M. Hill, J. H. Yum, A. Dualeh, S. Barlow, M. Grätzel, S. R. Marder and M. K. Nazeeruddin, Angew. Chem., Int. Ed., 2011, 50, 6619–6621 CrossRef CAS PubMed.
- A. K. Singh and J. Nithyanandhan, ACS Appl. Energy Mater., 2022, 5, 1858–1868 CrossRef CAS.
- W. Nau, ChemPhysChem, 2011, 12, 2496–2497 CrossRef CAS.
- J. Zhao, W. Wu, J. Sun and S. Guo, Chem. Soc. Rev., 2013, 42, 5323 RSC.
- D. Peceli, H. Hu, D. A. Fishman, S. Webster, O. V. Przhonska, V. V. Kurdyukov, Y. L. Slominsky, A. I. Tolmachev, A. D. Kachkovski, A. O. Gerasov, A. E. Masunov, D. J. Hagan and E. W. Van Stryland, J. Phys. Chem. A, 2013, 117, 2333–2346 CrossRef CAS PubMed.
- S. Webster, D. Peceli, H. Hu, L. A. Padilha, O. V. Przhonska, A. E. Masunov, A. O. Gerasov, A. D. Kachkovski, Y. L. Slominsky, A. I. Tolmachev, V. V. Kurdyukov, O. O. Viniychuk, E. Barrasso, R. Lepkowicz, D. J. Hagan and E. W. Van Stryland, J. Phys. Chem. Lett., 2010, 1, 2354–2360 CrossRef CAS.
- C. Cornelissen-Gude, W. Rettig and R. Lapouyade, J. Phys. Chem. A, 1997, 101, 9673–9677 CrossRef CAS.
- S. Aghazada, P. Gao, A. Yella, G. Marotta, T. Moehl, J. Teuscher, J. E. Moser, F. De Angelis, M. Grätzel and M. K. Nazeeruddin, Inorg. Chem., 2016, 55, 6653–6659 CrossRef CAS PubMed.
-
M. J. Frisch; G. W. Trucks; H. B. Schlegel; G. E. Scuseria; M. A. Robb; J. R. Cheeseman; G. Scalmani; V. Barone; G. A. Petersson; H. Nakatsuji; X. Li; M. Caricato; A. Marenich; J. Bloino; B. G. Janesko; R. Gomperts; B. Mennucci; H. P. Hratchian; J. V. Ortiz; A. F. Izmaylov; J. L. Sonnenberg; D. Williams-Young; F. Ding; F. Lipparini; F. Egidi; J. Goings; B. Peng; A. Petrone; T. Henderson; D. Ranasinghe; V. G. Zakrzewski; J. Gao; N. Rega; G. Zheng; W. Liang; M. Hada; M. Ehara; K. Toyota; R. Fukuda; J. Hasegawa; M. Ishida; T. Nakajima; Y. Honda; O. Kitao; H. Nakai; T. Vreven; K. Throssell; J. A. Montgomery.; J. E. Peralta; F. Ogliaro; M. Bearpark; J. J. Heyd; E. Brothers; K. N. Kudin; V. N. Staroverov; T. Keith; R. Kobayashi; J. Normand; K. Raghavachari; A. Rendell; J. C. Burant; S. S. Iyengar; J. Tomasi; M. Cossi; J. M. Millam; M. Klene; C. Adamo; R. Cammi; J. W. Ochterski; R. L. Martin; K. Morokuma; O. Farkas; J. B. Foresman and D. J. Fox, Gaussian 09, Gaussian, Inc., Wallingford CT, 2016 Search PubMed.
- Z. Yu, M. Gorlov, J. Nissfolk, G. Boschloo and L. Kloo, J. Phys. Chem. C, 2010, 114, 10612–10620 CrossRef CAS.
- S. C. Pradhan, A. Hagfeldt and S. Soman, J. Mater. Chem. A, 2018, 6, 22204–22214 RSC.
- Q. Wang, J.-E. Moser and M. Grätzel, J. Phys. Chem. B, 2005, 109, 14945–14953 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: General methods, DSSC device fabrication, 1H- and 13C-NMR, IR, DFT, EIS and dye-desorption study of ISQ dyes are provided in the ESI. See DOI: https://doi.org/10.1039/d3ma00277b |
|
| This journal is © The Royal Society of Chemistry 2023 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article ac,
Shivdeep Suresh
Deshmukh
ac,
Sailaja
Krishnamurty
*ac,
Kothandam
Krishnamoorthy
ac,
Shivdeep Suresh
Deshmukh
ac,
Sailaja
Krishnamurty
*ac,
Kothandam
Krishnamoorthy
 *bc and
Jayaraj
Nithyanandhan
*bc and
Jayaraj
Nithyanandhan
 *ac
*ac

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 10 mL of each) in a 50 mL round-bottom flask equipped with a Dean–Stark apparatus and refluxed under an inert atmosphere for 24 h. The reaction mixture was cooled, the solvents were removed under reduced pressure and the products were purified using column chromatography (100–200 mesh SiO2, CH2Cl2:MeOH) to obtained the required dyes.21
1, 10 mL of each) in a 50 mL round-bottom flask equipped with a Dean–Stark apparatus and refluxed under an inert atmosphere for 24 h. The reaction mixture was cooled, the solvents were removed under reduced pressure and the products were purified using column chromatography (100–200 mesh SiO2, CH2Cl2:MeOH) to obtained the required dyes.21
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 22
22
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5%) solutions at room temperature (Table 1). The solution UV-vis absorption spectrum for the indoline-donor-based squaraine dyes contain a sharp and intense peak (ε > 105 M−1 cm−1) at 640–646 nm due to the π–π* transition, and a vibronic side peak located at a higher energy of 580 nm appeared as the shoulder. Normalized absorption and emission spectra for the ISQ and SQS4 dyes are displayed in Fig. 2a. A small red-shift was observed in the absorption band for the ISQCl, ISQBr, and ISQI dyes (λmax 645 and 646 nm) compared to the ISQF and SQS4 dyes (λmax 641 and 642 nm). A similar phenomenon can be observed in the emission of the ISQ dyes (650–656 nm) compared to the SQS4 dye (650 nm). The ISQ dyes showed molar extinction coefficients in the range of 2.1–3.1 × 105 M−1 cm−1. Furthermore, to investigate the aggregation behaviour of the ISQ dyes on the metal-oxide surface and the light harvesting properties, UV-vis characterization was carried out on a TiO2 surface. The dipping time of the electrodes was 5 min in a 0.1 mM dye solution prepared in MeCN: CHCl3 (95
5%) solutions at room temperature (Table 1). The solution UV-vis absorption spectrum for the indoline-donor-based squaraine dyes contain a sharp and intense peak (ε > 105 M−1 cm−1) at 640–646 nm due to the π–π* transition, and a vibronic side peak located at a higher energy of 580 nm appeared as the shoulder. Normalized absorption and emission spectra for the ISQ and SQS4 dyes are displayed in Fig. 2a. A small red-shift was observed in the absorption band for the ISQCl, ISQBr, and ISQI dyes (λmax 645 and 646 nm) compared to the ISQF and SQS4 dyes (λmax 641 and 642 nm). A similar phenomenon can be observed in the emission of the ISQ dyes (650–656 nm) compared to the SQS4 dye (650 nm). The ISQ dyes showed molar extinction coefficients in the range of 2.1–3.1 × 105 M−1 cm−1. Furthermore, to investigate the aggregation behaviour of the ISQ dyes on the metal-oxide surface and the light harvesting properties, UV-vis characterization was carried out on a TiO2 surface. The dipping time of the electrodes was 5 min in a 0.1 mM dye solution prepared in MeCN: CHCl3 (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) (Fig. 2c and Table 1). The ISQ and SQS4 dyes all showed red-shifted charge transfer (π–π*) peaks (646–658 nm), which correspond to the monomeric dye structure, with additional transition peaks for smaller aggregated structures between 605–610 nm compared to their solution spectra (643–649 nm) when the photoanode was sensitized for 5 min. In the presence of optically transparent co-adsorbent chenodeoxycholicacid (CDCA), competitive anchoring of CDCA on the metal-oxide surface reduces the dye–dye interaction, and hence, a reduction in the peak intensity at 605–610 nm was observed.(Fig. S10, ESI†). The light-harvesting efficiency (LHE) is one of the key factors in DSSCs; it represents the ability of the sensitizers to absorb a certain range of photons for a particular dye. The LHE of the ISQ and SQS4 dyes is displayed in Fig. 2d and Table 1 (sensitization time 12 h). The LHE properties of the ISQF, ISQCl, ISQBr, and ISQI dyes on the mesoporous TiO2 thin films showed broad absorbance between 500–720 nm. The spectral broadening at 60% LHE increased from SQS4 to ISQI (Δλ = 193, 192, 209, 204, and 211 nm). This indicates that the extent of dye–dye interactions on the TiO2 surface is almost the same for this set of dyes.
5) (Fig. 2c and Table 1). The ISQ and SQS4 dyes all showed red-shifted charge transfer (π–π*) peaks (646–658 nm), which correspond to the monomeric dye structure, with additional transition peaks for smaller aggregated structures between 605–610 nm compared to their solution spectra (643–649 nm) when the photoanode was sensitized for 5 min. In the presence of optically transparent co-adsorbent chenodeoxycholicacid (CDCA), competitive anchoring of CDCA on the metal-oxide surface reduces the dye–dye interaction, and hence, a reduction in the peak intensity at 605–610 nm was observed.(Fig. S10, ESI†). The light-harvesting efficiency (LHE) is one of the key factors in DSSCs; it represents the ability of the sensitizers to absorb a certain range of photons for a particular dye. The LHE of the ISQ and SQS4 dyes is displayed in Fig. 2d and Table 1 (sensitization time 12 h). The LHE properties of the ISQF, ISQCl, ISQBr, and ISQI dyes on the mesoporous TiO2 thin films showed broad absorbance between 500–720 nm. The spectral broadening at 60% LHE increased from SQS4 to ISQI (Δλ = 193, 192, 209, 204, and 211 nm). This indicates that the extent of dye–dye interactions on the TiO2 surface is almost the same for this set of dyes.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5%) (excitation wavelength was 590 nm).
c Oxidation potential.
d
E
ox reference electrode of Ag/AgNO3 was used and was calibrated vs NHE via the addition of 0.7 V using ferrocene as an external standard.
e
E
0-0 optical band gap (from the intersect point of absorption and fluorescence, 1240/λ).
5%) (excitation wavelength was 590 nm).
c Oxidation potential.
d
E
ox reference electrode of Ag/AgNO3 was used and was calibrated vs NHE via the addition of 0.7 V using ferrocene as an external standard.
e
E
0-0 optical band gap (from the intersect point of absorption and fluorescence, 1240/λ).
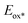 ) energy levels were found by subtracting the optical band gap (E0–0) from the Eox values, which were found between −1.12 and −1.06 V and were favourable for the electron injection process for the ISQ dyes to the conduction band of TiO2. The energy level diagrams of the SQS4, ISQF, ISQCl, ISQBr, and ISQI dyes with the DSSC device components are displayed in Fig. 3b.
) energy levels were found by subtracting the optical band gap (E0–0) from the Eox values, which were found between −1.12 and −1.06 V and were favourable for the electron injection process for the ISQ dyes to the conduction band of TiO2. The energy level diagrams of the SQS4, ISQF, ISQCl, ISQBr, and ISQI dyes with the DSSC device components are displayed in Fig. 3b.

 ) red to blue represents −0.02 to +0.02 kcal mol−1.
) red to blue represents −0.02 to +0.02 kcal mol−1.
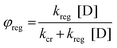



![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3)
3)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3)
3)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3)
3)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3)
3)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3)
3)