Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Molecular engineered A–D–A–D–A organic electrode system for efficient supercapacitor applications†
Sudhir D.
Jagdale
ac,
Chepuri R. K.
Rao
*ac,
Sidhanath V.
Bhosale
 *ac and
Sheshanath V.
Bhosale
*ac and
Sheshanath V.
Bhosale
 *b
*b
aPolymers and Functional Materials Division, CSIR-Indian Institute of Chemical Technology, Hyderabad-500007, Telangana, India. E-mail: ramchepuri@iict.res.in; bhosale@iict.res.in
bDepartment of Chemistry, School of Chemical Sciences, Central University of Karnataka, Kadaganchi, Kalaburagi-585 367, Karnataka, India. E-mail: bsheshanath@gmail.com
cAcademy of Scientific and Innovative Research (AcSIR), Ghaziabad-201002, Uttar Pradesh, India
First published on 27th June 2023
Abstract
Pseudocapacitors (PSCs) play a key role in energy storage (ES) technology development today. PSCs offer higher energy density as compared to their inorganic counterparts. Moreover, as compared to battery systems, they also exhibit higher power density for a shorter duration of time. In the present investigation, we designed, synthesized and demonstrated a novel acceptor (A)–donor (D)–acceptor (A)–donor (D)–acceptor (A) molecular architecture comprising naphthalene-1,4,5,8-tetracarboxylic diimide (NDI), tryptophan (Trp) and dopamine (DP) organic components. The as-fabricated NDI-Trp-DP/graphite foil (GF) electrode material was employed for three-electrode supercapacitor (SC) and two-electrode symmetric supercapacitor (SSC) device fabrication. The NDI-Trp-DP/GF material exhibited pseudocapacitive behaviour with an excellent specific capacitance (Csp) of about 267.90 F g−1 at a scan rate of 5 mV s−1 (cyclic voltammetry, CV) and 323 F g−1 at a current density 0.5 A g−1 (galvanostatic charge–discharge, GCD) in a three-electrode SC and a Csp of 152 F g−1 at 0.5 A g−1 in two-electrode SSC device systems. The NDI-Trp-DP/GF electrode exhibits an excellent cycling stability of about 95.87% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 galvanostatic charging–discharging (GCD) cycles and 97.76% continuous GCD charge–discharge cycling coulombic efficiency. The enhanced Csp and cycling stability performance of the NDI-Trp-DP/GF electrode results from the reversible redox reactions of the organic subunits present in the molecule, faster ion diffusion, and improved mechanical and chemical stability. This novel A–D–A–D–A design offers an efficient way to improve the electrochemical performance of PSCs. The design of this molecular engineered architecture and its redox properties with excellent cycling stability will help to fabricate future PSC-based electronics.
000 galvanostatic charging–discharging (GCD) cycles and 97.76% continuous GCD charge–discharge cycling coulombic efficiency. The enhanced Csp and cycling stability performance of the NDI-Trp-DP/GF electrode results from the reversible redox reactions of the organic subunits present in the molecule, faster ion diffusion, and improved mechanical and chemical stability. This novel A–D–A–D–A design offers an efficient way to improve the electrochemical performance of PSCs. The design of this molecular engineered architecture and its redox properties with excellent cycling stability will help to fabricate future PSC-based electronics.
Introduction
The growing energy demand depends significantly on fossil fuels. The excessive utilization of natural resources is exerting ever-increasing pressure on the environment, causing climate change and forest fires. Therefore, to meet our energy demands, alternative energy resources such as sunlight, geothermal heat, seawater and wind are utilized.1 Energy harvesting from these renewable resources and their storage utilizing greener technologies are currently demanded.2 To provide more reliable, sustainable and environmentally friendly technologies with high energy and high power, research and development efforts towards small-scale energy storage (ES) devices, i.e., batteries and supercapacitors (SCs), are required.3 Batteries and SCs are utilized in hybrid electric vehicles, electronic devices such as mobiles, wearable electronics and flexible displays.4,5 Although rechargeable batteries exhibit higher energy density, they provide lower power density due to their slow charging–discharging rate and suffer from poor cycling stability.6,7 SCs have attracted the attention of researchers as ES devices because of their high power density, longer cycle life and fast charging–discharging rate.8,9 Therefore, SCs have the potential to meet the increasing energy demand of modern society. However, SCs exhibit some limitations, i.e., lower energy density than batteries, which in turn result in limited practical applications, especially in portable electric devices. Therefore, researchers have focused on the development of SCs with higher energy density, higher power density and ultra-long electrochemical stability. Such SC devices are essential for the development of cutting-edge technology products.10 SCs are classified into two major categories: (i) electric double-layer capacitors (EDLCs) and (ii) pseudocapacitors based on the electrochemical energy storage mechanism.11 EDLCs store energy through the adsorption/desorption mechanism at the interface between the electrode and electrolyte materials on the double layer. EDLCs exhibit very limited energy density and low electrochemical capacity.12 In comparison, pseudocapacitors displayed greater energy storage on the electrode surface through rapid reversible faradaic redox reactions.8,13 Therefore, PSCs are fabricated with high-energy-density electrodes, which can boost the specific capacitance (Csp).Graphene-based materials have been utilized for fabrication of EDLCs as well as PSCs. Herein, the functional groups of graphene-based materials are involved in reversible redox reactions, exhibiting pseudocapacitive properties.14 However, graphene-, CNT- and reduced-graphene-oxide-based materials are insufficient to achieve a high Csp.15 Therefore, pseudocapacitor materials include transition metal oxides,16 precious metal oxides, e.g., RuO217 and IrO2,18 and conducting polymers, i.e., polyaniline19 and polypyrrole.20 However, metal oxides and conducting polymers exhibited poor stability and low electrical conductivity.21 To overcome all these limitations, it is important to develop appropriate electrode materials with suitable redox subunits for improving the electrochemical performance of the next-generation low-cost PSCs. Electrode materials based on organic compounds are an attractive green alternative for the fabrication of PSCs.22–25 It is notable that organic electrode materials have underperformed compared with inorganic materials.26 To incorporate the desired physical, chemical and electrochemical properties: (i) higher power density, (ii) energy density, and (iii) rate capability, and (iv) longer cycle life, the design and synthesis of novel organic molecules are required. These properties can be achieved by incorporating multiple electronic reversible redox-active subunits in the organic molecular architecture.27–29 To achieve the highest Csp with a long cycle life and stability, the fabrication of PSCs based on organic electrode materials, both electron-accepting and electron-donating subunits in the molecular framework with a wide potential range are required.30 In addition, fast surface reactions can be enhanced by utilizing a π-conjugated molecular skeleton.31
Herein, we develop a NDI-Trp-DP (naphthalenediimide-tryptophan-dopamine) molecular architecture fabricated from the π-conjugated symmetric naphthalene-1,4,5,8-tetracarboxylic diimide (NDI), the amino acid tryptophan (Trp) and the neurotransmitter dopamine (DP). These subunits were selected based on their electron donor (D) and electron acceptor (A) properties. Generally, such A–D–A–D–A molecular architectures provide substantial delocalization of the π-electrons over the system, which in turn enhances the polarizability and energy-gap manipulation between the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO). Redox-active-molecular-scaffold-, donor–acceptor-based organic electrode systems have not been fully explored for energy storage applications.32 Such materials are more appealing, as one can tune the donor and acceptor redox properties by manipulating the chemical structure design for utilization in SCs.32 The donor and acceptor subunits in the molecular architecture could enhance the intramolecular charge transfer between the donor and acceptor, which in turn would lead to an enhancement in the operating voltage. Herein, the electron transfer between the organic electrode material and electrolyte can increase the performance of the SCs. NDIs are employed in various optoelectronic applications due to their electrochemical and optical properties and molecular stability.33 Moreover, NDI-based organic electrode materials have been utilized for energy storage applications.29,34,35 Tryptophan (Trp), an aromatic amino acid, is the most electron-rich π-system bearing an indole subunit, which can exhibit electron-donating properties.36,37 A redox-active tryptophan-based graphene quantum dot hybrid material was employed in supercapacitor applications.38 A tryptophan–picric acid complex was utilized for SC applications by Srinivasan et al.39 Very recently, Zaijun and co-workers employed RuO2-functionalized graphene quantum dots for flexible supercapacitor applications.40 Dopamine (DP) is a naturally occurring neurotransmitter molecule that plays an important role in the body.41 Recent studies have shown that DP can be efficiently utilized in energy storage applications.34b,42–46 Thus, in the design of NDI-Trp-DP, we considered three major principles: (i) A–D–A–D–A molecular architecture; (ii) the selection of multiple redox systems, which may enhance the potential window; and (iii) an aromatic π-conjugated surface involved in pseudocapacitive behaviour to increase the surface redox reactions on the organic molecular electrode through electrolyte or ion interactions, which in turn will enhance the rate capability. Here, for the first time, we demonstrate the utilization of an A–D–A–D–A molecular architecture as a redox-active organic electrode material in a PSC device. We fabricated electrodes comprising NDI-Trp-DP on a graphite foil (GF) surface. The as-fabricated electrode NDI-Trp-DP/GF electrode material exhibited excellent electrochemical properties with outstanding cycling stability. Real-world applications were demonstrated by constructing symmetric supercapacitor two-electrode systems (SSE) and employed as an energy storage device. The supercapacitor results in an SSE system, demonstrating the potential of the NDI-Trp-DP (A–D–A–D–A) systems in SC technologies.
Experimental section
Materials
1,4,5,8-Naphthalenetetracarboxylic dianhydride (NDA) (>95.0%) was purchased from TCI (Hyderabad), and tryptophan (≥98%) and dopamine hydrochloride were purchased from Sigma-Aldrich (Hyderabad) Pvt. Ltd, India. N,N-Dimethylformamide (DMF) (99.50%), methanol (99.50%), HCl (35–38%), sulphuric acid (H2SO4) (95.0–98%) and N-methyl-2-pyrrolidone (C5H9NO) (99.0%) were purchased from Finar (Ahmadabad/Mumbai), India Limited. Graphite foil (GF) was purchased from Falcon Graphite Industries, India, and carbon black (super p-conductive) (99.0+%) and polytetrafluoroethylene (PTFE) were procured from Alfa Aesar India.Structural characterizations
1H NMR and 13C NMR spectra were performed using a Bruker Avance-400 MHz spectrometer and 100 MHz spectrometer, respectively. FT-IR spectra were performed using a PerkinElmer Spectrum (Spectrum 100; PerkinElmer) instrument. MALDI-TOF experiments were performed using a Shimadzu Biotech Axima performance spectroscopic instrument. A Thermofisher Exactive Orbitrap instrument was used for HRMS measurements. TGA was performed using a Q-500 TGA under an N2 atmosphere. At a rate of 10 °C min−1, the samples were heated to 800 °C. The detailed morphology of the synthesized samples of NDI-Trp-DP were examined using FE-SEM (Quanta FEG 250).Electrochemical measurements
The electrochemical experiments (CV, GCD and EIS) were performed using an AUTOLAB (potentiostat and galvanostat, 320N, Netherlands) ZIVE LAB (MP 5) at room temperature. For the three-electrode device, NDI-Trp-DP/GF (working), platinum (Pt) wire (counter) and Ag/AgCl(sat. KCl) (reference) electrodes were used in aq. 1 M H2SO4 electrolyte. CV was carried out at different scan rates, i.e., 5, 10, 15, 20, 25, 30, and 35 mV s−1, and GCD was carried out at current densities of 0.5, 1, 2, 3, 4, 5, and 10 A g−1. EIS was measured at frequencies of 0.01 Hz to 100 kHz at 0 V bias conditions with a 10 mV AC sinus perturbation.Synthesis of (2S,2′S)-2,2′-(1,3,6,8-tetraoxo-1,3,6,8-tetrahydrobenzo[lmn] [3,8] phenanthroline-2,7-diyl)bis(3-(1-hydroxyindol-3-yl)propanoicacid) (NDI-Trp)47
1,4,5,8-Naphthalenetetracarboxylicdianhydride (1 g, 3.70 mmol) and L-tryptophan (1.52 g, 7.40 mmol) were suspended in 10 mL of dimethylformamide (DMF) in a 100 mL round bottom flask under an N2 atmosphere and stirred for 10 min at room temperature. Triethylamine (TEA) (0.5 mL) was then added. The reaction mixture was refluxed for 12 h. The completion of the reaction was monitored using TLC. The mixture was cooled to room temperature. Under reduced pressure, the DMF was removed. 200 mL of 2 N HCl was added with stirring for 1 h. The reaction mixture was filtered and washed three times with distilled water, and then dried under high vacuum at 100 °C to yield NDI-Trp (80%). FT-IR (γ, cm−1, KBr) 3410, 2934, 1710, 1640, 1343, 742; 1H NMR (400 MHz, DMSO-d6) δ ppm: 10.66 (2H, s), 8.60 (4H, s), 7.47 (2H, d, J = 7.9 Hz), 7.20 (2H, d, J = 8.1 Hz), 7.05 (2H, d, J = 2.3 Hz), 6.94 (2H, t, J = 7.5 Hz), 6.81 (2H, t, J = 7.9 Hz), 5.85 (2H, dd, J = 9.1, 5.5 Hz), 3.69 (2H, dd, J = 15, 5.3 Hz), 3.50 (2H, dd, J = 14.9, 9.2 Hz); 13C NMR (100 MHz, DMSO-d6): 171.0, 162.5, 136.3, 131.6, 127.5, 126.4, 126.1, 124.1, 121.2,118.7, 118.4, 11.7, 110.6, 54.8, 24.5; ESI-MS (m/z): 640.16 [M]+; HRMS: calcd for C36H25O8N4 (M + H)+: 641.1666, found 641.1695 (M + H)+; MALDI-TOF C36H24O8N4 = 640.16 [M]+, found 663.178 [M + Na]+.Synthesis of (2S,2′S)-2,2′-(1,3,6,8-tetraoxo-1,3,6,8-tetrahydrobenzo[lmn][3,8]phenanthroline-2,7-diyl)bis(N-(3,4-dihydroxyphenethyl)-3-(1-hydroxyindol-3-yl)propanamide) (NDI-Trp-DP)48a
The compound NDI-Trp (500 mg, 780.05 mmol), N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide (EDC) hydrochloride (448 mg, 2340.15 mmol) and 1-hydroxybenzotriazolehydrate (HOBT) (316 mg, 2340.15 mmol) were suspended in 15 mL dimethylformamide (DMF) in a 100 mL round bottom flask under a nitrogen atmosphere. The reaction mixture was then stirred for half an hour at 0 °C. Dopamine (DP) (358 mg, 2340.15 mmol) and N,N-diisopropylethylamine (DIPEA) (0.5 mL) were added to the reaction mixture under a nitrogen atmosphere and stirred for 48 h at room temperature. The completion of the reaction was monitored using TLC. After consumption of the starting material, the reaction mixture was allowed to cool at room temperature. Under reduced pressure, the DMF was removed, and the mixture was then poured into ice-cold water. Under vacuum, the obtained precipitate was filtered and washed with hot water (100 mL) followed by hot methanol (50 mL). The dark brown product (NDI-Trp-DP) was dried overnight at 100 °C. Yield: 77%; FT-IR (γ, cm−1, KBr) 3406, 2906, 1705, 1664, 1581, 1452, 1341, 748; 1H NMR (400 MHz, DMSO-d6) δ ppm: 10.57 (2H, s), 8.70 (4H, s), 8.54 (4H, s), 8.08 (2H, s),7.45 (2H, d, J = 7.8 Hz), 7.15 (2H, d, J = 7.9 Hz), 6.97–6.87(4H, m), 6.81 (2H, t, J = 7.4 Hz), 6.65–6.53 (4H, m), 6.41 (2H, d, J = 7.8 Hz), 5.76 (2H, dd, J = 9.4, 5.1 Hz), 3.84–3.65 (2H, m), 3.62–3.43 (4H, m), 3.18 (6H, s). 13C NMR (100 MHz, DMSO-d6): 168.6, 162.8, 145.5, 143.9, 136.3, 130.9, 127.6, 127.0, 126.8, 126.5, 123.9, 121.2, 119.7, 118.7, 118.4, 116.4, 15.9, 111.6, 110.8, 55.7, 41.7, 35.2, 23.9; ESI-MS (m/z): 911 [M]+; MALDI-TOF C52H43O10N6 = 911.303 [M]+; found 933.391 [M + Na]+; HRMS: calcd for C52H43O10N6 = 911.3035 [M]+, found 911.3067 [M]+.Cyclic voltammetry (CV)
The CV measurements were carried out utilizing a ZIVE LAB (MP 5) series potentiostat instrument. 0.1 M tetrabutylammonium hexafluorophosphate electrolyte was prepared in dimethylformamide (20 mL). A 3 mm glassy carbon (working) electrode, Ag/AgCl (sat. KCl) electrode48b,c,d as a reference and a Pt wire electrode as a counter were utilized. The CV measurements were recorded at a scan rate of 10 mV s−1 under a nitrogen atmosphere. 10 mg of NDI-Trp-DP was utilized for CV measurements. In the Ag/AgCl (sat KCl) aqueous electrode, we used a Luggin capillary for avoiding water interference.Electrode fabrications
In order to construct the working electrode, a slurry of NDI-Trp-DP, carbon black (super p-conductive) as the conductive material and polytetrafluoroethylene (PTFE) as a binder was used. The three components were mixed thoroughly by grinding in weight ratios of 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (14
10 (14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 mg). A homogeneous slurry was prepared by hand grinding using a mortar and pestle with N-methyl-2-pyrrolidone (NMP) as a solvent. The as-prepared slurry was precisely coated on a graphite foil (GF) with dimensions of 1 × 1 cm2. The electrode material was placed in a hot air oven at 80 °C for 12 h. The mass of the organic active material NDI-Trp-DP in the electrodes was maintained at nearly 2 mg.
2 mg). A homogeneous slurry was prepared by hand grinding using a mortar and pestle with N-methyl-2-pyrrolidone (NMP) as a solvent. The as-prepared slurry was precisely coated on a graphite foil (GF) with dimensions of 1 × 1 cm2. The electrode material was placed in a hot air oven at 80 °C for 12 h. The mass of the organic active material NDI-Trp-DP in the electrodes was maintained at nearly 2 mg.
Fabrication of symmetric supercapacitor device (NDI-Trp-DP/GF//NDI-Trp-DP/GF)
The symmetric NDI-Trp-DP/GF supercapacitor device was fabricated utilizing a Swagelok cell. The NDI-Trp-DP/GF electrodes were employed as a pair of electrodes, i.e., as the anode and cathode. A piece of filter paper (Whatman) was used as a separator. 1 M H2SO4 as an electrolyte was dropped between NDI-Trp-DP/GF/separator/NDI-Trp-DP/GF interface.Formulae used:
C sp from the three-electrode CV studies:49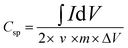 | (1) |
C sp from the three-electrode GCD investigation:49
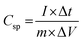 | (2) |
C sp from the two-electrode GCD studies:49
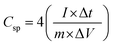 | (3) |
Energy density was estimated from the two-electrode GCD studies:49
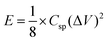 | (4) |
Power density was evaluated from the two-electrode GCD analysis:49
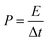 | (5) |
The coulombic efficiency of NDI-Trp-DP/GF//NDI-Trp-DP/GF device was analyzed using the equation50
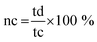 | (6) |
Results and discussion
Synthesis and characterization of NDI-Trp-DP
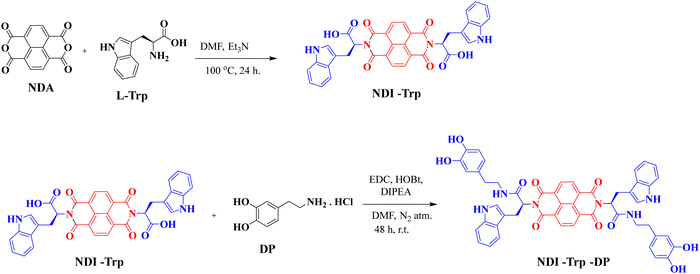
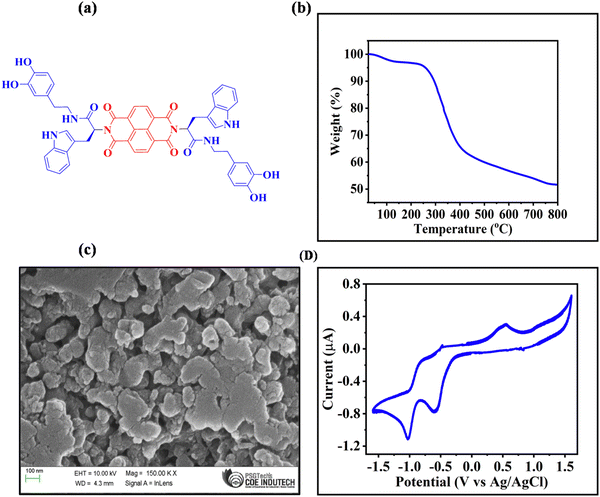
The synthesis of NDI-Trp-DP is represented in Scheme 1. NDI-Trp-DP was synthesized by adopting a multistep synthetic reaction strategy following a previously reported procedure.47,48NDI-Trp was obtained by reacting naphthalene dianhydride (NDA) with tryptophan (Trp) in DMF at 100 °C for 24 h based on a procedure reported in the literature.47NDI-Trp was further reacted with dopamine (DP) via an amide coupling reaction in the presence of EDC, HOBT and DIPEA in DMF for 48 h at room temperature to yield NDI-Trp-DP (Fig. 1a).48FT-IR, 1H and 13C NMR spectroscopy and HRMS spectrometry techniques were used to establish the chemical structure of NDI-Trp and NDI-Trp-DP (Fig. S1 to S10, ESI†). Thermogravimetric analysis (TGA) in a nitrogen atmosphere revealed that NDI-Trp-DP is thermally stable, showing 5% weight loss at about 300 °C (Fig. 1b). Field-emission scanning electron microscopy (FE-SEM) revealed that NDI-Trp-DP exhibited a 2D-sheet-like morphology (Fig. 1c). The cyclic voltammogram (CV) of NDI-Trp-DP in DMF/0.1 M tetrabutylammonium hexafluorophosphate was recorded in the −1.6 to 1.6 V potential window at a scan rate of 10 mV s−1 and exhibited two reversible redox peaks (Fig. 1d). Two reduction peaks of NDI-Trp-DP appeared at −0.58 and −1.03 V and were attributed to the NDI subunits,33 and two oxidation peaks were found at +0.55 and +1.07 V. These oxidation peaks are typical for Trp.51 The CV curve was utilized to calculate the onset oxidation (0.30 V) and reduction onset (−0.48 V) potential (vs. Ag/Ag+) of NDI-Trp-DP. The highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) were evaluated to be 4.70 eV and 3.92 eV from Eonsetox and Eonsetred using the formulas HOMO = −e [Eonsetox + 4.4] eV and LUMO = −e [Eonsetred + 4.4 eV], respectively. From the HOMO and LUMO energy levels, the calculated energy gap was found to be 0.78 eV, indicating that the NDI-Trp-DP material can be adopted for electrochemical energy storage applications.
Three-electrode system
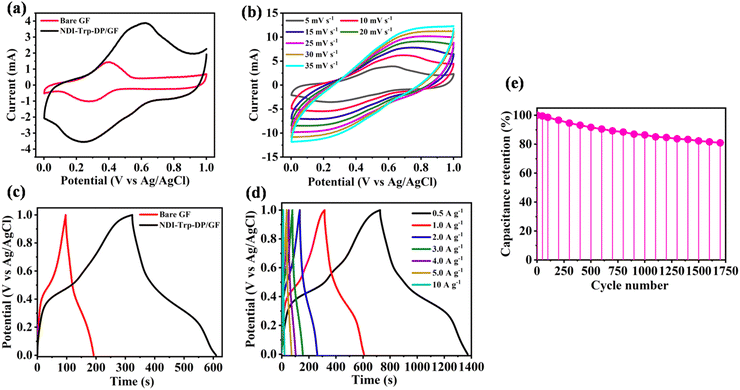
The as-prepared NDI-Trp-DP/GF was utilized as an electrode material for SC applications. The electrochemical properties of GF and NDI-Trp-DP/GF were studied using CV measurements in 1 M H2SO4 within the potential window of 0 to 1.0 V at 5 mV s−1 in the three-electrode system. The CV measurement results of GF and NDI-Trp-DP/GF are depicted in Fig. 2a. At 5 mV s−1, the CV curve of GF vs. Ag/AgCl exhibited a pair of strong redox peaks at 0.35 V (anodic) and 0.39 V (cathodic), which were ascribed to the faradaic reversible redox reactions. Thus, the CV curve shape of GF displays the pseudocapacitive behavior of the electrode and is totally different than that of the double-layer energy storage mechanism.52 In comparison, the CV curve of NDI-Trp-DP/GF displays a pair of well-resolved reversible redox peaks at about 0.43 V and 0.52 V, which were ascribed to the pseudocapacitive characteristics of the electrode material.53 These redox peaks are attributed to the oxidation and reduction of NDI-Trp-DP active subunits on the electrode surface. The faradaic reversible peaks are symmetric in nature, suggesting that the electrode material comprising NDI-Trp-DP/GF could exhibit outstanding electrochemical properties through pseudocapacitive behaviour.54Fig. 2b exhibits the CV measurement curves of the three-electrode SC device at scan rates of 5, 10, 15, 20, 25, 30 and 35 mV s−1 within the potential window of 0 to 1.0 V. The shapes of all the CV curves suggest pseudocapacitive characteristics. The Csp calculated from CV curve of NDI-Trp-DP/GF at 5 mV s−1 using eqn (1) (Experimental Section) was found to be 267.90 F g−1 (Fig. 2b and Table 1). As the scan rate increased from 5 to 35 mV s−1, the peak intensity of CV curve rapidly increased with small changes in the cathodic peak to the right and anodic peak to the left. The shifting of the cathodic and anodic peak towards more positive and negative values was ascribed to an enhancement of the internal diffusive resistance with increasing scan rate in pseudocapacitive materials.55 From Fig. 2b, we observed that the CV curve at a higher scan rate of about 35 mV s−1 retains its shape, indicating the capacitive behaviour of the NDI-Trp-DP. Moreover, as we moved from a lower scan rate of 5 mV s−1 to a higher one of 35 mV s−1, the estimated Csp was found to be 104.33 F g−1. The gradual decrease in Csp was ascribed to the slow redox reaction at high scan rate. At a scan rate of 35 mV s−1, NDI-Trp-DP/GF displays 35% specific capacitance retention, suggesting the feasibility of the NDI-Trp-DP/GF electrode material in supercapacitor applications (Table 1 and Fig. S11a, ESI†). GCD experiments were performed to examine the electrochemical properties of NDI-Trp-DP/GF in the three-electrode SC system. The GCD profiles of the GF (red curve) and NDI-Trp-DP/GF (black curve) electrodes in the potential window 0 to 1.0 V versus Ag/AgCl at 1 A g−1 current density were recorded and are depicted in Fig. 2c. The GCD curve area of NDI-Trp-DP/GF is higher than that of GF. Moreover, NDI-Trp-DP/GF exhibited an almost symmetrical charging and discharging GCD curve, suggesting that the electrode material had good pseudocapacitive behaviour due to faradaic redox reactions and electrochemical reversibility.56 The GCD curves recorded for NDI-Trp-DP/GF at current densities of 0.5, 1, 2, 3, 4, 5 and 10 A g−1 within the 0 to 1.0 V applied potential window at room temperature are presented in Fig. 2d. The Csp calculated using eqn (2) (Experimental Section) from the GCD curve of NDI-Trp-DP/GF at 0.5 A g−1 current density was 323 F g−1 (Fig. 2d and Table 1). The GCD data suggest that the NDI-Trp-DP/GF electrode-containing three-electrode SC exhibited the best charge storage capacity. With increasing the current density from 0.5 to 10 A g−1, the estimated Csp value of the NDI-Trp-DP/GF electrode decreased to 92.8 F g−1 (Fig. 2d and Table 1). At 10 A g−1 current density, a Csp retention of 28.48% was found (Table 1 and Fig. S11b, ESI†), indicating the feasibility of the NDI-Trp-DP/GF electrode material in SC applications. The cycling stability of the NDI-Trp-DP/GF electrode at 4 A g−1 over 1700 GCD cycles was examined, and the capacitance retention was found to be 80.95% (Fig. 2e). We performed the three-electrode-system experiments to confirm the reproducibility of the results using CV and GCD, and we found that the results were consistent in the presence of aqueous electrolyte and Ag/AgCl as a reference electrode (Fig. S12, ESI†). Thus, the NDI-Trp-DP/GF electrode material was found to be stable enough for long-term cycling performance. From the above three-electrode SC device results, it could be confirmed that increasing the number of redox subunits could improve the electrode conductivity and the exposure of high surface area could allow more active sites in contact with the electrolyte, which in turn enhanced the pseudocapacitive behaviour of the NDI-Trp-DP/GF electrode material. Moreover, the π-conjugated NDI surface exhibited π–π stacking interactions with the GF surface, which favours fast charging and discharging between the electrode and electrolyte interface. Thus, all these factors provided good capacitance retention of the SC at a higher current density of about 4 A g−1.| CV analysis | GCD analysis | ||
|---|---|---|---|
| Scan rate mV s−1 | Specific capacitance (F g−1) | Current density (A g−1) | Specific capacitance (F g−1) |
| 5 | 267.90 | 0.5 | 323 |
| 10 | 211.65 | 1 | 292 |
| 15 | 174.60 | 2 | 256 |
| 20 | 149.31 | 3 | 231 |
| 25 | 134.27 | 4 | 203.03 |
| 30 | 114.64 | 5 | 177 |
| 35 | 104.33 | 10 | 92.80 |
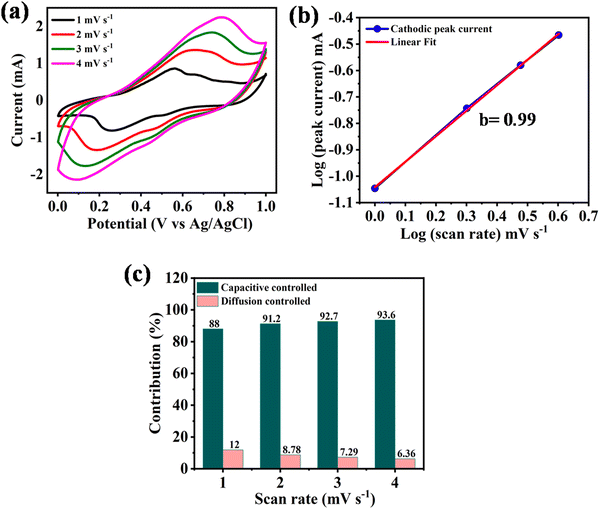
To further obtain insights into the electrochemical kinetics, the value of b at the reversible redox peaks (Fig. 3a) for NDI-Trp-DP/GF was estimated based on eqn (7)
| Ip = avb | (7) |
Two-electrode symmetric supercapacitor (SSC)
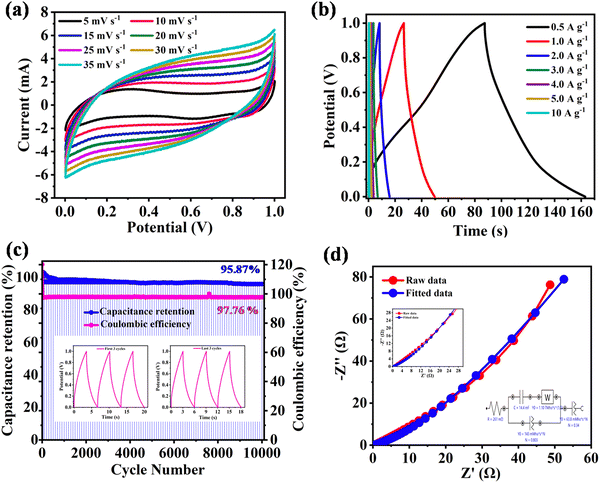
To examine the practical applicability of NDI-Trp-DP/GF, the symmetric supercapacitor (SSC) device NDI-Trp-DP/GF//NDI-Trp-DP/GF was fabricated in a two-electrode (Swagelok) setup using 1 M H2SO4 as an electrolyte. Herein, NDI-Trp-DP/GF was utilized as the anode as well as the cathode electrode material. The electrochemical performance of NDI-Trp-DP/GF in the two-electrode SSC was examined using CV, GCD and electrochemical impedance spectroscopy (EIS), as shown in Fig. 4a, b, c and d, respectively. The CV curves of the as-fabricated SSC utilizing NDI-Trp-DP/GF were investigated to estimate the potential window at a 10 mV s−1 scan rate (Fig. S13a, ESI†). The CV study demonstrates that the potential windows reached a maximum at a 0 to 1.3 V potential window for the examined SSC. Moreover, the GCD measurements of NDI-Trp-DP/GF//NDI-Trp-DP/GF SSC at 1 A g−1 current density were carried out by employing various potential windows, i.e., 0 to 0.8 V, 0 to 0.9 V, 0 to 1.0 V, 0 to 1.1 V, 0 to 1.2 V and 0 to 1.3 V (Fig. S13b, ESI†). To investigate the CV and GCD measurements by considering the aqueous electrolyte deposition, we selected 0 to 1.0 V potential windows for the examined SSC device. The CVs of the NDI-Trp-DP/GF//NDI-Trp-DP/GF SSC device configuration at scan rates of 5, 10, 15, 20, 25, 30 and 35 mV s−1 are shown in Fig. 4a. Fig. 4a indicated good electrochemical reversibility. At 5 mV s−1, the NDI-Trp-DP/GF electrode in SSC device displayed a reversible redox peak indicating the pseudocapacitive behaviour of the device.58 For the NDI-Trp-DP/GF//NDI-Trp-DP/GF SSC device, as the scan rate was increased from 5 to 35 mV s−1, an increase in the area under the CV curves was observed. At higher scan rates, a rectangular shape along, with faradaic peaks, were found for the CV curves. These observations indicate that at higher scan rates, comparably slow redox reactions were happening due to higher internal resistance.59 To calculate the Csp of NDI-Trp-DP/GF//NDI-Trp-DP/GF, GCD tests were conducted to explore the advantages of NDI-Trp-DP/GF as an electrode material for the SSC device. The obtained GCD results of the NDI-Trp-DP/GF//NDI-Trp-DP/GF SSC device at a constant potential window of about 0 to 1.0 V at 0.5, 1, 2, 3, 4, 5 and 10 A g−1 are depicted in Fig. 4b. The distorted shape of the GCD curves suggests the pseudocapacitive nature of the SSC device (Fig. 4b).60 Moreover, ion diffusion was slower and enhanced the resistance in the SSC device. From the GCD curve at 0.5 A g−1 current density, the estimated Csp using eqn (3) (Experimental Section) was found to be 152 F g−1 (Fig. 4b and Table 2). This is ascribed to the small IR drop at the 0.5 A g−1 current density, indicating the fast kinetics of the charge-storage process. The Csp of the SSC calculated from the GCD curves indicated the excellent electrochemical properties of the device. The estimated Csp for the two-electrode SSC system is higher than that of the reported organic electrode materials. Furthermore, as the current density increased from 0.5 A g−1 to 10 A g−1, the Csp of the NDI-Trp-DP/GF//NDI-Trp-DP/GF SSC device decreased gradually and reached Csp = 10 F g−1 at 10 A g−1 (Fig. 4b, Table 2 and Fig. S14, ESI†). More impressively, the NDI-Trp-DP/GF-based SSC device shows a capacitance retention rate of about 6.5% at an ultrahigh current density of 10 A g−1. This trend of decreasing Csp at higher current density is found in organic-electrode-material-based SC devices. The decreased Csp with increasing current density could be ascribed to the restricted ion migration and diffusion on the electrode surface.61 The cycling stability of the NDI-Trp-DP/GF was examined by using GCD measurement. The cycling stability results for the NDI-Trp-DP/GF//NDI-Trp-DP/GF SSC device are displayed in Fig. 4c. After 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 GCD cycles, an ultrahigh Csp retention of about 95.87% was found at 3 A g−1 current density, suggesting that the NDI-Trp-DP/GF electrode system be an ideal for real-world supercapacitor applications. This excellent cycling stability of the NDI-Trp-DP/GF electrode should be due to the presence of the conjugated π-system on the graphene foil surface, which provides π–π stacking interactions. This non-covalent interaction between the organic material and the GF surface leads to the enhancement of surface contact area between the electrode and the electrolyte.63
000 GCD cycles, an ultrahigh Csp retention of about 95.87% was found at 3 A g−1 current density, suggesting that the NDI-Trp-DP/GF electrode system be an ideal for real-world supercapacitor applications. This excellent cycling stability of the NDI-Trp-DP/GF electrode should be due to the presence of the conjugated π-system on the graphene foil surface, which provides π–π stacking interactions. This non-covalent interaction between the organic material and the GF surface leads to the enhancement of surface contact area between the electrode and the electrolyte.63
| GCD analysis | Specific energy (W h kg−1) | Specific power (W kg−1) | |
|---|---|---|---|
| Current density (A g−1) | Specific capacitance (F g−1) | ||
| 0.5 | 152 | 19 | 900 |
| 1 | 92 | 11.50 | 1789.11 |
| 2 | 60.48 | 7.56 | 3600 |
| 3 | 38.56 | 4.82 | 5405.60 |
| 4 | 27.77 | 3.41 | 7071.43 |
| 5 | 21.60 | 2.70 | 9000 |
| 10 | 10.00 | 1.25 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
Furthermore, for the NDI-Trp-DP/GF//NDI-Trp-DP/GF SSC device, the EIS data were analysed using Nyquist plots (Fig. 4d and Table 3). The experimental results data are well fitted by an electrical circuit (Fig. 4d, inset). Rs can obtained from the high-frequency region of the Nyquist plot, which is the combined internal resistance, the electrolyte ohmic resistance and the current collector intrinsic resistance. In the low-frequency region, the linearly vertical shape of the curve suggests fast diffusion of ions towards the electrode surface, enhancing the Csp of the NDI-Trp-DP/GF.64 The estimated value of Rs was found to be 0.261 Ω cm2; this lower Rs value indicated that the NDI-Trp-DP/GF electrode material possesses good rate capability. The double-layer capacitance Cdl was calculated and found to be 14.4 mF. The calculated constant phase elements Cpe1 and Cpe2 equivalent to capacitance were observed to be 0.340 F cm2 and 0.803 F cm2. The lower Cdl, Cpe1 and Cpe2 values indicated that the electrode material exhibited very low EDCL behavior.
| Parameter | R s (Ω cm2) | C dl (mF) | C pe1 (F cm2) | C pe2 (F cm2) |
|---|---|---|---|---|
| NDI-Trp-DP/GF//NDI-Trp-DP/GF | 0.261 | 14.4 | 0.340 | 0.803 |
A Ragone plot for evaluating the energy and power densities of the as-fabricated NDI-Trp-DP/GF//NDI-Trp-DP/GF SSC device was created and is displayed in Fig. 5. The energy density and power density of the SSC device were estimated using eqn (4) and (5) (Experimental Section), respectively. The obtained results are tabulated in Table 2. As displayed in Fig. 5, the NDI-Trp-DP/GF//NDI-Trp-DP/GF SSC device exhibited an energy density of 19 W h kg−1 at a power density of 900 W kg−1 at 0.5 A g−1. These were compared to the data for SCs reported in the literature, such as GH-DN//rGO-NDI,34 NDI-2DP/CP//NDI-2DP/CP,34 rGO-NDI-CN//rGO-NDI-CN,35 NQ-DP/CP//NQDP/CP,43 and P2P(NDI2ODOTh(NPV)//P2P(NDI2ODOTh(NPV).62 Moreover, at 10 A g−1 (higher current density), the estimated energy and power densities are 1.25 W h kg−1 and 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 W kg−1, respectively, which are comparable to those of SSC devices based on organic electrode materials. The high Csp and higher energy density are attributed to the higher redox surface and demonstrate their promising potential for utilization in modern energy storage devices.
000 W kg−1, respectively, which are comparable to those of SSC devices based on organic electrode materials. The high Csp and higher energy density are attributed to the higher redox surface and demonstrate their promising potential for utilization in modern energy storage devices.
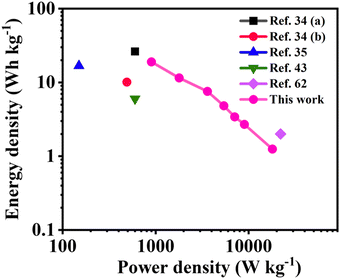 | ||
| Fig. 5 Ragone plot comparing NDI-Trp-DP/GF//NDI-Trp-DP/GF with reported SCs: GH-DN//rGO-NDI,34a NDI-2DP/CP//NDI-2DP/CP,34b rGO-NDI-CN//rGO-NDI-CN,35 NQ-DP/CP//NQ-DP/CP,43 and P2P(NDI2ODOTh(NPV)//P2P(NDI2ODOTh(NPV).62 | ||
Influence of different supercapacitor devices on Csp
It is well documented that the estimated Csp for an SC depends on the type of cell configuration.65 We employed an NDI-Trp-DP/GF-based organic electrode material for the fabrication of a three-electrode supercapacitor device and a two-electrode symmetric SC, and the Csp values were estimated using CV and GCD curves (Table 1, Fig. 2, and Table 2, Fig. 4, respectively). The calculated Csp values for the three-electrode and symmetric two-electrode SC devices were 323 F g−1 and 152 F g−1 at a current density of 0.5 A g−1. These results for the NDI-Pyr-DP/GF electrode in the two-electrode SSC cell set-up demonstrate the practical applicability of the material.Physical and chemical electrode stability
The morphology and “external” structural physical stability of the as-prepared NDI-Trp-DP/GF were examined using FE-SEM. FE-SEM images of the NDI-Trp-DP/GF electrode material before and after the GCD performance test are depicted in Fig. 6a and b, respectively. The FE-SEM image of the electrode before GCD testing was found to show a 3D rough crystalline surface (Fig. 6a). The granular rough surface came from the organic materials in combination with the binder and GF. After 10000 GCD cycles, the morphology of the electrode was found to be unaltered (Fig. 6b). The FE-SEM results indicate that the as-fabricated electrode is physically stable after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 GCD cycles. We presume that the physical stability of electrode could be responsible for the excellent cycling stability after 10
000 GCD cycles. We presume that the physical stability of electrode could be responsible for the excellent cycling stability after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 GCD cycles and retention of 95.87% Csp.
000 GCD cycles and retention of 95.87% Csp.
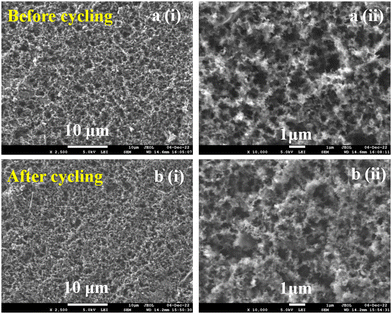 | ||
| Fig. 6 SEM images of the NDI-Trp-DP/GF electrode (a) (i–ii) before and (b) (i–ii) after the cyclic stability test. | ||
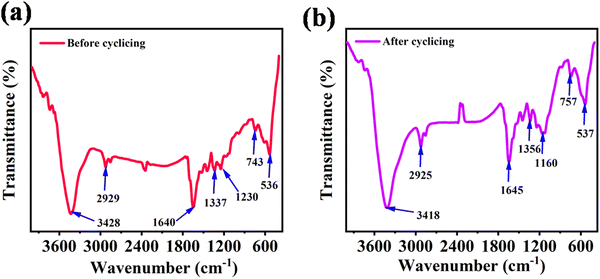
Fig. 7a and b display the FT-IR spectra of the as-fabricated electrode before and after the GCD performance test. Before cycling, the electrode material exhibited IR peaks at 3428, 2929, 1640, 1337, 1230, 743 and 536 cm−1. As shown in Fig. 7b, after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 GCD cycles, the characteristic peaks appeared at 3418, 2925, 1645, 1356, 1160, 757 and 537 cm−1. These results indicate that NDI-Trp-DP/GF can be tightly coated on the GF surface, which leads to chemical stability even after 10
000 GCD cycles, the characteristic peaks appeared at 3418, 2925, 1645, 1356, 1160, 757 and 537 cm−1. These results indicate that NDI-Trp-DP/GF can be tightly coated on the GF surface, which leads to chemical stability even after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 GCD cycles. Thus, the mechanical and chemical stability of the NDI-Trp-DP/GF electrode during charging and discharging resulted in prolonged cycling stability of the SSC cell architecture. These features of the NDI-Trp-DP/GF electrode seem suitable for its utilization in technological development.
000 GCD cycles. Thus, the mechanical and chemical stability of the NDI-Trp-DP/GF electrode during charging and discharging resulted in prolonged cycling stability of the SSC cell architecture. These features of the NDI-Trp-DP/GF electrode seem suitable for its utilization in technological development.
The electrochemical properties of the three-electrode SC system and two-electrode SSC device architecture fabricated utilizing the NDI-Trp-DP/GF organic electrode material indicate promise for energy storage applications. Moreover, the excellent cycling stability, high energy density and power density associated with the NDI-Trp-DP/GF electrode in the two-electrode SSC device make it a promising material for large-scale energy storage devices for electrical vehicles and flexible electronics applications.
Working mechanism of NDI-Trp-DP
The redox reaction mechanism of NDI-Trp-DP system is presented in Fig. 8. We presume that in presence of aqueous H2SO4 electrolyte, the reaction occurs in two steps: in the first step, the NDI imide carbonyl functional groups are protonated, yielding [NDI]˙−·H+, and in the second step, incorporation of another H+ into the imide carbonyl of the NDI ring system results in [NDI]˙−·2H+.32–34 Furthermore, the redox chemistry of tryptophan37–40 as well as dopamine41–46 with a two-electron transfer process are involved. Thus, one NDI ring system, two tryptophan and two dopamine molecular subunits participated, resulting in a ten-electron/proton reversible redox reaction process (Fig. 8).66–69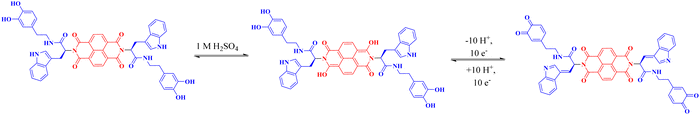 | ||
| Fig. 8 Schematic of the plausible proposed reversible redox mechanism of the NDI-Trp-DP molecular architecture. | ||
The results for the SSC based on the NDI-Trp-DP/GF//NDI-Trp-DP/GF electrode material will bring about advanced organic supercapacitors with higher energy density, long-term cycling stability and rate capability. Moreover, optimizing the redox behaviour of the organic electrode material can enhance the capacitance of the SC cells (Table 4).
| Sr. No | Electrode material | Electrolyte | Voltage (V) | Current density (A g−1) | C sp GCD (F g−1) | Energy density (W h kg−1) | Power density (W kg−1) | No. cycles @ current density (A g−1) | C sp Retention (%) | Ref No. |
|---|---|---|---|---|---|---|---|---|---|---|
| a Symmetric two-electrode system. b Asymmetric two-electrode system. c Three-electrode system. | ||||||||||
| 1 | P2P(NDI2ODOThCNPV)//P2P(NDI2ODOThCNPV) | 0.5 M H2SO4 | −0.7 to 0.5 | 0.5 | 124 | 2.0 | 22000 | 5000 @ 5 | 100a | 62 |
| 2 | BQ-DP//BQ-DP | 1 M H2SO4 | 0 to 1 | 9.5 | 416 | 13.88 | 22000 | 5000 @ 9.5 | 70.6a | 46 |
| 3 | NDI-2DP/CP//NDI-2DP/CP | 1 M H2SO4 | 0 to 1 | 0.5 | 73.1 | 10.1 | 490 | 4000 @ 3 | 97a | 34b |
| 4 | NQ-DP/CP//NQ-DP/CP | 1 M H2SO4 | 0 to 1.2 | 0.5 | 43.4 | 6.00 | 600 | 5000 @ 4 | 92a | 43 |
| 5 | GH-DN//rGO-NDI | 1 M H2SO4 | 0 to 1.6 | 1 | 111.3 | 26.3 | 600 | 8000 @ 3 | 83.4b | 34b |
| 6 | PDI-Py/GF//PDI-Py/GF | 1 M H2SO4 | −0.2 to 1.5 | 1 | 128 | 46 | 3060 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 @ 5 000 @ 5 |
95a | 63 |
| 7 | rGO/NDI-CN//rGO/NDI-CN | 1 M H2SO4 | 0 to 1.2 | 0.5 | 336 | 16.80 | 149.60 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 @ 10 000 @ 10 |
80c | 35 |
| 8 | NDI-Trp-DP/GF//NDI-Trp/GF | 1 M H2SO4 | 0 to 1 | 0.5 | 152 | 19 | 900 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 @ 3 000 @ 3 |
95.87a | Present work |
Conclusions
The supercapacitor applications of NDI-Trp-DP-deposited graphite foil electrodes have been shown using aqueous 1 M H2SO4 electrolyte. The three-electrode SC device based on the NDI-the Trp-DP/GF electrode material showed a maximum Csp of 267.90 F g−1 at 5 mV s−1 and 323 F g−1 at 0.5 A g−1 as examined using CV and GCD measurements vs Ag/AgCl in 1 M H2SO4 electrolyte, respectively. Moreover, the two-electrode symmetric SC device fabricated using NDI-Trp-DP/GF electrodes in 1 M H2SO4 exhibited an excellent Csp of 152 F g−1 at 0.5 A g−1. At 0.5 A g−1, the SSC device displayed a high energy density of 19 W h kg−1 at a power density of 900 W kg−1. The NDI-Trp-DP/GF electrode exhibits an excellent cycling stability of about 95.87% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 galvanostatic charging–discharging (GCD) cycles, and 97.76% continuous GCD charge–discharge cycle coulombic efficiency. The high performance of the NDI-Trp-DP/GF electrode material is attributed to the number of redox subunits in the molecular architecture and the chemical and physical stability of the electrode. The high performance of NDI-Trp-DP-based organic material is promising for its viable SC application. Thus, the use of organic materials from rich renewable sources with high performance in SCs will significantly reduce the cost of SC devices for real-world applications.
000 galvanostatic charging–discharging (GCD) cycles, and 97.76% continuous GCD charge–discharge cycle coulombic efficiency. The high performance of the NDI-Trp-DP/GF electrode material is attributed to the number of redox subunits in the molecular architecture and the chemical and physical stability of the electrode. The high performance of NDI-Trp-DP-based organic material is promising for its viable SC application. Thus, the use of organic materials from rich renewable sources with high performance in SCs will significantly reduce the cost of SC devices for real-world applications.
Data availability statement
Experimental data is available with reasonable request to the corresponding authors.Author contributions
Sudhir D. Jagdale: methodology, synthesis and characterization, device fabrication and measurement; Chepuri R. K. Rao: methodology, writing – review & editing; Sidhanath V. Bhosale: conceptualization, supervision, funding acquisition, writing – original draft, review & editing, investigation; Sheshanath V. Bhosale: conceptualization, resources, writing – review & editing.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
Sidhanath Vishwanath Bhosale (IICT) is grateful for financial support from BRNS under the project No.: 58/14/01/2020-BRNS/37047 and the Director, CSIR-IICT (MS No. IICT/Pubs./2023/066). Sheshanath Vishwanath Bhosale (GU) acknowledges University Grant Commission (UGC) Faculty Research Program, New Delhi, India (F.4-5(50-FRP) (IV-Cycle)/2017(BSR)) for an award of Professorship and also acknowledges Council of Scientific & Industrial Research (CSIR), India for providing support, code No. 02(0357)/19/EMR-II. Sudhir D. Jagdale is grateful for financial support through SRF from UGC, New Delhi.Notes and references
- O. Ellabban, H. Abu-Rub and F. Blaabjerg, Renewable Sustainable Energy Rev., 2014, 39, 748–764 CrossRef.
- N. Armaroli and V. Balzani, Energy Environ. Sci., 2011, 4, 3193–3222 RSC.
- (a) W.-J. Liu, H. Jiang and H.-Q. Yu, Energy Environ. Sci., 2019, 12, 1751–1779 RSC; (b) T. Liu, L. Zhang, B. Chemg and J. Yu, Adv. Energy Mater., 2019, 9, 1803900 CrossRef; (c) H. Jin, J. Li, Y. Yuan, J. Wang, J. Lu and S. Wang, Adv. Energy Mater., 2018, 8, 1801007 CrossRef.
- L. Yangyang, Y. Chenhui, T. Yapeng, T. Yi, Y. Xingtian and Q. J. Wenxiu, J. Power Sources, 2020, 450, 227694 CrossRef.
- R. Bondade, Y. Zhang, B. Wei, T. Gu, H. Chen and D. Ma, IEEE Trans. Ind. Electron., 2016, 63, 2850–2861 Search PubMed.
- S. Liu, L. Kang, J. Zhang, C. S. Jun and Y. Yamauchi, ACS Energy Lett., 2021, 6, 4127–4154 CrossRef CAS.
- W.-H. Li, W.-H. Deng, G.-E. Wang and G. Xu, Energy Chem., 2020, 2, 100029 CrossRef.
- M. Beidaghi and Y. Gogotsi, Energy Environ. Sci., 2014, 7, 867–884 RSC.
- P. Simon and Y. Gogotsi, Nat. Mater., 2008, 7, 845–854 CrossRef CAS PubMed.
- N. Kamboj, T. Purkait, M. Das, S. Sarkar, K. S. Hazra and R. S. Dey, Energy Environ. Sci., 2019, 12, 2507 RSC.
- J. Zhang, N. Kong, S. Uzun, A. Levitt, S. Seyedin, P. A. Lynch, S. Qin, M. Han, W. Yang, J. Liu, X. Wang, Y. Gogotsi and J. M. Razal, Adv. Mater., 2020, 32, 2001093 CrossRef CAS PubMed.
- G. Yury, Nature, 2014, 509, 568–569 CrossRef PubMed.
- P. K. Sharma, A. Arora and S. K. Tripathi, J. Energy Storage, 2019, 21, 801–825 CrossRef.
- A. González, E. Goikolea, J. A. Barrena and R. Mysyk, Renewable Sustainable Energy Rev., 2016, 58, 1189–1206 CrossRef.
- L. G. Beka, X. Li, X. Wang, C. Han and W. Liu, RSC Adv., 2019, 9, 26637–26645 RSC.
- P. Veerakumar, A. Sangili, S. Manavalan, P. Thanasekaran and K.-C. Lin, Ind. Eng. Chem. Res., 2020, 59, 6347–6374 CrossRef CAS.
- D. Majumdar, T. Maiyalagan and Z. Jiang, ChemElectroChem, 2019, 6, 4343–4372 CrossRef CAS.
- S. Korkmaz, F. Meydaneri Tezel and İ. A. Kariper, J. Alloys Compd., 2018, 754, 14–25 CrossRef CAS.
- A. Eftekhari, L. Li and Y. Yang, J. Power Sources, 2017, 347, 86–107 CrossRef CAS.
- R. B. Choudhary, S. Ansari and B. Purty, J. Energy Storage, 2020, 29, 101302 CrossRef.
- (a) J. R. Miller and P. Simon, Science, 2008, 321, 651–652 CrossRef CAS PubMed; (b) L. Wang, T. Wu, S. Du, M. Pei, W. Guo and S. Wei, RSC Adv., 2016, 6, 1004–1011 RSC.
- T. B. Schon, B. T. McAllister, P. F. Li and D. S. Seferos, Chem. Soc. Rev., 2016, 45, 6345–6404 RSC.
- M. Boota, C. Chen, M. Bécuwe, L. Miao and Y. Gogotsi, Energy Environ. Sci., 2016, 9, 2586–2594 RSC.
- J. Yang, P. Xiong, Y. Shi, P. Sun, Z. Wang, Z. Chen and Y. Xu, Adv. Funct. Mater., 2020, 30, 1909597 CrossRef CAS.
- R. Shi, C. Han, H. Duan, L. Xu, D. Zhou, H. Li, J. Li, F. Kang, B. Li and G. Wang, Adv. Energy Mater., 2018, 8, 1802088 CrossRef.
- S. K. Kim, J. Cho, J. S. Moore, H. S. Park and P. V. Braun, Adv. Funct. Mater., 2016, 26, 903–910 CrossRef CAS.
- P. Poizot, J. Gaubiher, S. Renault, L. Duois, Y. Liang and Y. Yao, Chem. Rev., 2020, 120, 6490–6557 CrossRef CAS PubMed.
- S. P. Ega and P. Srinivasan, J. Energy Storage, 2022, 47, 103700 CrossRef.
- M. R. Biradar, S. V. Bhosale, P. P. Morajkar and S. V. Bhosale, Fuel, 2022, 310, 122487 CrossRef CAS.
- A. M. Bryan, L. M. Lu, Y. Santino, S. Acharya and J. M. D’Arcy, Chem. Mater., 2016, 28, 5989–5998 CrossRef CAS.
- A. D. Adhikari, A. Morag, J. Seo, J. M. Kim and R. Jelinek, ChemSusChem, 2020, 13, 3230–3236 CrossRef PubMed.
- M. A. Kobaisi, S. V. Bhosale., K. Latham, A. M. Raynor and S. V. Bhosale, Chem. Rev., 2016, 116, 11685–11796 CrossRef CAS PubMed.
- S. V. Bhosale, M. A. Kobaisi, R. W. Jadhav, P. P. Morajkar, L. A. Jones and S. George, Chem. Soc. Rev., 2021, 50, 9845–9998 RSC.
- (a) F. Ma, Z. Hu, L. Jiao, X. Wang, Y. Yang, Z. Li and Y. He, Adv. Mater. Interfaces, 2021, 8, 2002161 CrossRef CAS; (b) M. R. Biradar, A. V. Salkar, P. P. Morajkar, S. V. Bhosale and S. V. Bhosale, New J. Chem., 2021, 45, 9346–9357 RSC; (c) C. Weng, X. Li, Z. Yang, H. Long, C. Lu, L. Dong, S. Zhao and L. A. Tan, Chem. Commun., 2022, 58, 6809–6812 RSC.
- A. B. Deshmukh, M. R. Biradar, M. D. Pawar, S. V. Bhosale and M. V. Shelke, J. Energy Storage, 2022, 56, 106036 CrossRef.
- J. C. Ma and D. A. Dougherty, Chem. Rev., 1997, 97, 1303–1324 CrossRef CAS PubMed.
- C. R. Hoopes, F. J. Garcia, A. M. Sarkar, N. J. Kuehl, D. T. Barkan, N. L. Collins, G. E. Meister, T. R. Bramhall, C.-H. Hsu, M. D. Jones, M. Schirle and M. T. Taylor, J. Am. Chem. Soc., 2022, 144, 6227–6236 CrossRef CAS PubMed.
- H. Wang, Y. Yang, X. Zhou, R. Li and Z. Li, New J. Chem., 2017, 41, 1110–1118 RSC.
- R. Srinivasan, E. Elaiyappillai, S. Gowri, A. Bella, A. B. Sathiyan and J. P. Meenatchia Merlin, Phys. Chem. Chem. Phys., 2019, 21, 11829–11838 RSC.
- L. Ruiyi, H. Keyang, Y. Yongqiang, Z. Haiyan and L. Zaijun, Chem. Eng. J., 2021, 426, 130893 CrossRef.
- A. Björklund and S. B. Dunnett, Trends Neurosci., 2007, 30, 194–202 CrossRef PubMed.
- G. Wang, X. Huang and P. Jiang, Sci. Rep., 2017, 7, 43071 CrossRef CAS PubMed.
- M. R. Biradar, A. V. Salkar, P. P. Morajkar, S. V. Bhosale and S. V. Bhosale, New J. Chem., 2021, 45, 5154–5164 RSC.
- V. Veeramani, R. Madhu, S.-M. Chen and M. Sivakumar, ACS Sustainable Chem. Eng., 2016, 4, 5013–5020 CrossRef CAS.
- P. Flouda, S. A. Shah, D. C. Lagoudas, M. J. Green and J. L. Lutkenhaus, Matter, 2019, 1, 1532–1546 CrossRef.
- S. P. Ega, M. R. Biradar, P. Srinivasan and S. V. Bhosale, Electrochim. Acta, 2020, 357, 136835 CrossRef CAS.
- S. P. Goskulwad, V. G. More, D. D. La, R. S. Bhosale, A. L. Puyad, S. V. Bhosale and S. V. Bhosale, Ind. J. Chem., 2019, 58B, 200–208 CAS.
- (a) W. Liyanage, P. W. Rubeo and B. L. Nilsson, Interface Focus, 2017, 7, 20160099 CrossRef PubMed; (b) C. Wiberg, F. Owusu, E. Wang and E. Ahlberg, Energy Technol., 2019, 7, 1900843 CrossRef CAS; (c) C. Liu, J. Wang, J. Li, R. Luo, J. Shen, X. Sun, W. Han and L. Wang, ACS Appl. Mater. Interfaces, 2015, 7, 18609–18617 CrossRef CAS PubMed; (d) S. D. Jagdale, C. R. K. Rao, S. V. Bhosale and S. V. Bhosale, J. Energy Storage., 2023, 66, 107482 CrossRef.
- (a) J. Yu, N. Fu, J. Zhao, R. Liu, F. Li, Y. Du and Z. Yang, ACS Omega, 2019, 4, 15904–15911 CrossRef CAS PubMed; (b) R. Velayuthama, R. Manikandana, J. Raj, A. M. Kale, C. Kaya, K. Palanisamye and B. C. Kim, J. Alloys Compd., 2021, 863, 158332 CrossRef.
- A. M. Kale, R. Manikandan, C. J. Raj, R. Velayutham, W.-J. Cho and B. C. Kim, Appl. Surf. Sci., 2021, 542, 148716 CrossRef CAS.
- A. Sangili, V. Vinothkumar, S.-M. Chen, P. Veerakumar, C.-W. Chang, I. P. Muthuselvam and K.-C. Lin, J. Phys. Chem. C, 2020, 124, 25821–25834 CrossRef CAS.
- J. Yan, Z. Fan, W. Sun, G. Ning, T. Wei, Q. Zhang, R. Zhang, L. Zhi and F. Wei, Adv. Funct. Mater., 2012, 22, 2632–2641 CrossRef CAS.
- B. Huskinson, M. P. Marshak, C. Suh, S. Er, M. R. Gerhardt, C. J. Galvin and M. J. Aziz, Nature, 2014, 505, 195–198 CrossRef CAS PubMed.
- D. D. Potphode, L. Sinha and P. M. Shirage, Appl. Surf. Sci., 2019, 469, 162–172 CrossRef CAS.
- K. K. Ramakrihnan, C. Nithya and R. Karvmbu, Nanoscale Adv., 2019, 1, 334–341 RSC.
- C. Molji, A. S. Aashish, K. Neethu and S. J. Devaki, J. Mater. Chem. A, 2017, 5, 16636–16645 RSC.
- H. Lindstrom, S. Sodergren, A. Solbrand, H. Rensmo, J. Hjelm, A. Hagfeldt and S. E. Lindquist, J. Phys. Chem. B, 1997, 101, 7717–7722 CrossRef.
- J. Wang, J. Polleux, J. Lim and B. Dunn, J. Phys. Chem. C, 2007, 111, 14925–14931 CrossRef CAS.
- Y. Jiang and J. Liu, Energy Environ. Mater., 2019, 2, 30–37 CrossRef.
- B. Pal, S. Yang, S. Ramesh, V. Thangadurai and R. Jose, Nanoscale Adv., 2019, 1, 3807–3835 RSC.
- H. Ma, H. Chen, Y. Hu, B. Yang, J. Feng, Y. Xu, Y. Sun, H. Cheng, C. Li, X. Yan and L. Qu, Energy Environ. Sci., 2022, 15, 1131–1143 RSC.
- S. Sharma, R. Soni, S. Kurugot and S. K. Asha, Macromolecules, 2018, 51, 954–965 CrossRef CAS.
- J. C. Rusell, V. A. Posey, J. Gray, R. May, D. A. Reed, H. Zhang, L. E. Marbella, M. L. Steigerwald, Y. Yang, X. Roy, C. Nuckolls and S. R. Peurifoy, Nat. Mater., 2021, 20, 1136–1141 CrossRef PubMed.
- B. K. Kim, S. Sy, A. Yu and J. Zhang, Electrochemical Supercapacitors for Energy Storage and Conversion, Wiley-VCH, 2015 Search PubMed.
- M. R. Biradar, A. M. Kale, B. C. Kim, S. V. Bhosale and S. V. Bhosale, Energy Technol., 2022, 10, 2200154 CrossRef CAS.
- V. S. Sapner, P. P. Chavan, R. V. Digraskar, S. S. Narwade, B. B. Mulik, S. M. Mali and B. R. Sathe, ChemElectroChem, 2018, 5, 3191–3197 CrossRef CAS.
- B. Song, J. Zhao, M. Wang, J. Mullavey, Y. Zhu, Z. Geng, D. Chen, Y. Ding, K.-S. Moon, M. Liu and C.-P. Wong, Nano Energy, 2017, 31, 183–193 CrossRef CAS.
- M. A. Azam, M. A. Azizan, N. S. A. Manaf, R. Izamshah and N. Mohamad, Adv. Sci. Eng. Med., 2014, 6, 1–4 CrossRef.
- H. Li, J. Wang, Q. Chub, Z. Wang, F. Zhanga and S. Wang, J. Power Sources, 2009, 190, 578–586 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ma00296a |
| This journal is © The Royal Society of Chemistry 2023 |