Open Access Article
Open Access ArticleAdvances of nanoworms in diagnosis, treatment, and theranostics
Kadambari
Borse
and
Pravin
Shende
 *
*
Shobhaben Pratapbhai Patel School of Pharmacy and Technology Management, SVKM's NMIMS, Mumbai, India. E-mail: pravin.shende@nmims.edu
First published on 30th August 2023
Abstract
Engineered nanoparticles offer potential applications in the biomedical field, such as drug delivery and magnetic resonance imaging; however, they exhibit poor hemocompatibility. Thus, elongated nanoparticles known as nanoworms with a length of 30 nm have received considerable attention in various applications such as tissue engineering, microfluidics, biosensors, and drug delivery. Synthesizing with different metals, polymers, and biological molecules, nanoworms are used as templates for inorganic nanoparticles, superstructure building blocks, synthetic dendritic cells for immunotherapy, temperature-responsive gels for medical purposes, and traditional nanocarriers for drug delivery. Nanoworms demonstrate significant characteristic benefits over spherical counterparts, such as higher surface area, resembling the extracellular matrix of human cells, availability of numerous attachment points, increased likelihood of effective delivery to biological targets, prolonged circulatory half-life, and clear imaging. Considering nanoworms as an isolated research area, this review article focuses on providing an overview, as well as discussing the advantages, disadvantages, and applications of nanoworms, specifically in the diagnosis, treatment, and theranostics of detrimental diseases such as COVID-19, autoimmune disorders, cancer, bacterial infections, and atherosclerosis. Since the risks, benefits, and wide range of uses of nanoworms remain to be explored, substantial research is beneficial to investigate the development and applicability of their use in the future in a variety of fields, including medicine, electronics, and materials science.
1. Introduction
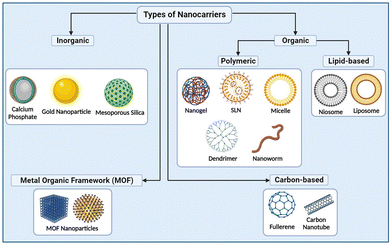
Nanomedicine uses tiny materials such as biocompatible nanoparticles (NPs) and nanorobots for a wide range of practices, like diagnosis, evaluation, surveillance, mitigation, and intervention of different diseases like cancer, Alzheimer's disease, cardiovascular disorders, etc.1,2Among the variety of nanomaterials like liposomes, niosomes, ethosomes, transfersomes, nanorods, and nanotubes, newly discovered nanoworms (NWs) are attracting a lot of attention in applications such as tissue engineering, microfluidics, biosensors, and drug delivery.3,4Fig. 1 shows the different types of nanocarriers for targeting drugs in the body. NWs are a type of nanomaterial characterized by elongated cylindrical structures measuring 30 nm in diameter and are composed of a variety of different materials, including metals,5,6 polymers,7,8 and biological molecules.9,10 NWs are used as templates for inorganic NPs, superstructure building blocks, synthetic dendritic cells for immunotherapy, temperature-responsive gels for medical use, and traditional nanocarriers for drug delivery. The small dimension of NWs allows them to easily navigate through the body and reach remote sites unlike traditional medical treatments, making them ideal for targeted drug delivery and medical procedures. Thus, NWs have the potential to improve the diagnosis, treatment, and theranostics (combination of therapy and diagnostics) of various diseases like cancer, neurological conditions, COVID-19, etc.
NWs can transport imaging agents such as NPs or fluorescent dyes to visualize specific cells or tissues, greatly improving the accuracy of diagnostic imaging and enabling earlier detection of diseases like cancer.11–13 NWs are used in treatment to distribute drugs directly to diseased cells, avoiding healthy cells, thereby increasing the effectiveness and decreasing the side effects of chemotherapy such as hair loss, and decreased immunity.14 Additionally, medical procedures, such as the delivery of gene therapy or removal of plaque from blood vessels, are also carried out using NWs.15 Engineered NWs are used for theranostics, to improve the efficiency and effectiveness of medical care. NWs can recognize cancer cells and deliver chemotherapy drugs to target cells in the same process. Hence, NWs are a great isolated area of research as they overcome the drawbacks and offer significant advantages over the currently available nanomaterials like nanobulges,16 nanospheres,17 nanoflowers,18etc. as shown in Fig. 2. Although NWs are employed in diagnostic imaging, targeted drug delivery, medical procedures, and theranostics, their further study and development are still in their infancy, and therefore it is essential to carefully consider the potential risks and benefits before utilizing NWs extensively in therapeutic settings.
Advantages:
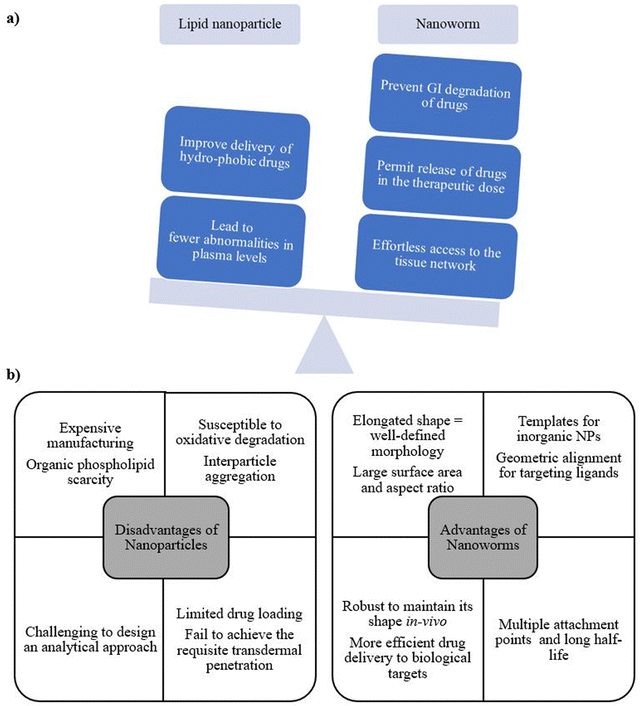
1. The geometric alignment of cores and the elongated shape of NWs provide not only a well-defined morphology but also a variety of desirable physical properties, including high surface area and large aspect ratio (in the range of 75 to 100), thus offering two main advantages over spherical equivalents (liposomes, niosomes, transferosomes, etc.).10
2. NWs travel through the bloodstream without being significantly hindered by the immune system and target tumors like small anti-cancer missiles because of the shape and the polymer coating on their surfaces. As a result, the worms are able to circulate for up to 24 h in the system.19
3. To enable the delivery of drugs to specific malignancies, organs, and other locations in the body, researchers are currently devising approaches to coat NW exteriors with different chemical “zip codes”. The zip codes enhance the capacity of NWs to deliver drugs directly to tumors, thereby increasing drug efficacy.20
4. NWs reduce the adverse effects of harmful anti-cancer medications and improve the identification of tumor and abnormal lymph by limiting their exposure to healthy tissues.21
5. Owing to their iron-oxide composition, NWs are observed clearly in diagnostic equipment, in particular magnetic resonance imagining (MRI), or scanners used to locate tumours. In MRI scans, the NWs’ extremely bright appearance is due to the superparamagnetism of the iron oxide used. Hence, it is simpler to detect microscopic tumours, thus aiding medical professionals in diagnosing cancer at an earlier stage of the illness.22
6. NWs are more favourable than pre-existing NP systems in terms of kinetics, stability, and half-life, thus demonstrating them as one of the best delivery systems for all kinds of diseases.
Disadvantages:
1. The manufacturing and disposal of NWs result in negative environmental impacts. The materials and chemicals used in the production process are harmful to the environment and potentially to human health. Thus, the disposal of NWs can also be challenging, as they accumulate in soil and water sources and potentially cause harm to wildlife.23,24
2. The concern about the potential unintended consequences of using NWs in medical applications is still unclear. Although NWs are useful in targeted drug delivery and disease diagnosis, the long-term effects of introducing these tiny devices into the body are not fully explored. NWs still pose the risk of causing unforeseen side effects, such as triggering an immune response or disrupting natural biological processes.25
2. Applications
2.1. Diagnosis
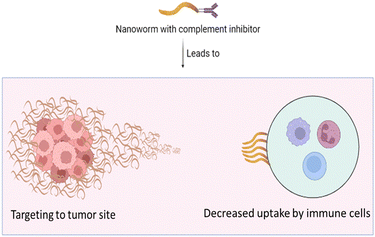
C3 opsonization is similarly reduced by cross-connecting hydrogelation technique of SPIO NW surfaces after treatment with epichlorohydrin, resulting in in vivo reduction of utilization by mice leukocytes by 70% in mouse serum. Interestingly, these cross-connected particles are not able to demonstrate a fall in C3 opsonization in the case of human serum, yet they did show a noteworthy decline of about 60% in human leukocyte take-up, as shown in Fig. 3. The take-up of cross-connected NPs is less significantly slowed by the presence of EDTA. Thus, these discoveries reveal the divergence between species in recognition of NPs using the complement system and assimilation by leukocytes, as well as how inhibitors of the supplement elective route and NP surface coating improve human hemocompatibility. These outcomes give significant knowledge of the mechanisms of hemocompatibility of nanomedicines for therapeutic purposes.41
2.2. NW-based therapies
The resulting NWs were expressed in E. coli and cryogenic transmission electron microscopy and light scattering technique were used to examine the efficacy of NWs in NHL. In two B-cell NHL cell lines, the NWs conjugated to CD20 performed apoptosis more effectively than rituximab alone. Rituximab exhibited a concentration-dependent reduction in the feasibility of SUDHL7 cells, resulting in a 4.8 μM IC50. By staining with annexin V/propidium iodide and transferase dutp nick-end label, late-stage apoptosis induction was individually identified at the appropriate time and a similar Fv ratio of 1.5 mg ml−1 was used to compare all combinations.50,51 Using the staining agent annexin V/propidium iodide, NWs significantly increased timely apoptosis (P = 0.0005) and improved programmed cell death in each of the CD20 cell lines in comparison to rituximab alone (P = 0.006). Moreover, in a NHL xenograft model, CD20 NWs effectively reduced the incidence of back cancer compared to rituximab.52 Thus, according to the findings, CD20 NWs are a convincing persuader of programmed cell death for the treatment of NHL and can potentially overcome the limitations of rituximab in vitro.7
To overcome these drawbacks, researchers developed biodegradable self-assembling NWs by the direct hydration (DH) technique using PEG–PCLgTMC and PTMC-Q.57 The DH method is a significant advance in achieving regulated self-assembly under more biocompatible conditions and avoids the use of hazardous organic solvents. Hydrophobic self-association served as the driving force leading to amphiphilic copolymer self-assembly of drug-loaded NVs produced by DH. BCPs like PEGPTMC served as the fundamental building block and yielded highly pure units able to eliminate the need for refinement prior to use in both in vitro and in vivo settings.58,59 Chemical signals like thermally responsive, pH-responsive, or ionic subunits were incorporated in a systematic manner to control BCP assembly, resulting in smart nanostructures, owing to their sensitivity towards changes in the surroundings.60 Controlling BCP self-assembly by coordinating amphipathic and controlled interactions with ions in solution was a successful technique for controlling particle morphology.61 The alteration in ionic strength of NaCl (0 to 400 × 10−3 M) used to hydrate TerP22-Q BCPs helped the globular elongated micelles switch to NWs. Compared to globular micelles, NWs displayed a 2-fold enhancement in affinity to cancer biological membrane due to the high aspect ratio and interaction between the NWs and the cell membrane as demonstrated by the low IC50 data of HepG2 cells (micelles = 207 μg mL−1, NWs = 27.9 μg mL−1). A significant number of anionic phospholipids in the cell membrane revealed pronounced toxicity for cancer cells, while cationic polymer aggregates were selective for cancer cells, supporting their therapeutic promise. Also, enhanced infiltration into 3D multicellular spheroids including melanoma and fit fibroblasts (HeLa![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3T3 = 1
3T3 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) was achieved due to the unusual elongated form. Thus, the study revealed the potential of NWs for high drug loading capacity of cargo drugs, selective delivery to cancerous tissues and promoting penetration of drugs into cells in anticancer drug delivery.62
5) was achieved due to the unusual elongated form. Thus, the study revealed the potential of NWs for high drug loading capacity of cargo drugs, selective delivery to cancerous tissues and promoting penetration of drugs into cells in anticancer drug delivery.62
2.3. Theranostics
To eliminate the drawbacks, researchers developed A192, an ELP linked to an anti-CD99 scFv for the targeted distribution of cytotoxic agents to AML cells, offering a potentially safer and more effective treatment option for patients. A192 is a human-origin product from tropoelastin designed and tested for survival while CD99 neutralizes cell motility and is the initiator of cellular apoptosis. The combination of A192 and CD99scFv significantly produced stable NWs after 72 h at 37 °C. αCD99A192 demonstrated positive limits for CD99+ cells but not for CD99293T cells across a multitude of AML cell lines. CD99A192 also reduced viability and improved PCD in AML cell lines, thus demonstrating excellent anti-leukemic effects in vitro as well as in vivo.
One of the advantages of associating A192 with CD99-scFv is the significant recycling of the coupled protein (34 mg L−1) in sample extract followed by bacterial lysis.74,75 ELP and CD99-scFv worked together to remove scFv and detoxify proteins, balancing dynamic NWs and enhancing the CD99-scFv PK profile. As a prospective treatment for AML, the stated investigation revealed a powerful anti-leukemic potency with the additional benefits of ideal colloidal stability as well as a longer PK half-life.14
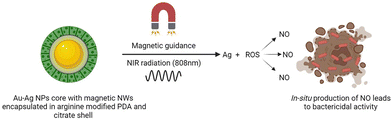
Magnetic NWs of Fe2O3 encapsulated in a shell of arginine-modified polydopamine (PDA) with a core of Au–Ag NPs (CitAug NP) capped with citrate were developed to exhibit photothermal repair effects and were remotely controlled by a magnetic field to transport them to the target site. Nitric oxide (NO) is an indigenous volatile component that harms healthy tissues, potentially destroying bacteria by damaging DNA and blocking its repair.81 NO released in situ worked synergistically with Ag particles and reactive oxygen species to eliminate bacterial contamination in vivo, enhancing hyperthermia and bactericidal tendencies of Ag particles, as shown in Fig. 4. With a biocompatible nature and magnetic properties, the production of NIR-triggered NO in situ is thus a smart and promising NP delivery system to address bacterial infections in the body.82
To support the benefits of AFe/AuAg@PDA NWs, the antibacterial efficacy with and without NIR laser light was researched using Staphylococcus aureus and Escherichia coli. The turbidity method and optical thickness measurements at 600 nm revealed the effectiveness of NWs in reducing the development of malignancy in both the strains independently of the NIR irradiation. However, the blend of high temperature and Ag particles was not able to kill bacterial cells. Also, without NIR laser light, the restraint productivity of NWs (150 g mL−1) was only 60% and 63.6% for Escherichia coli. On the other hand, NWs displayed a definite bactericidal effect (less than 10% of the microorganisms were precipitated in Kori and Staphylococcus aureus) with NIR laser light.83 From the results it is evident that magnetic field can precisely control the operation and mobility of magnetostrictive nanosystems to accomplish aggregation and deep tissue infiltration due to the superior biological compatibility,22,84 demonstrating the effectiveness of magnetically controlled NPs for next-generation personalized medicine.85,86 Hence, the stated study revealed the first magnetically guided distribution of NWs for the treatment of antibiotic-resistant bacteria without the use of antibiotics utilizing in situ synthesized NO gas, demonstrating promising importance for use in commercial gas therapy.87
In physiological fluids, the NWs displayed a restricted size distribution and were easily observed using photoacoustic and CT dual-mode imaging.101 To evaluate the ability of Pt-PEG NWs to sensitize cancer cells to radiation, a colonization experiment was conducted in vitro. X-Ray-induced DNA damage was assessed using a phosphohistone-H2AX mouse monoclonal antibody and a [4′,6-diamidino-2-phenlyindole] (DAPI) label.102 Balb/c mice were inoculated with 4T1 cells and treated with NWs for 4 h, then irradiated with X-rays. After injecting cancer-carrying mice with NWs (16 mg kg−1), the major tissues and organs were harvested and liquefied in a blend containing HCl, HNO3 and HClO4 at a high temperature (2 °C) and scanned with a laser system. The 1064 nm laser has a higher residual power density than the 808 nm laser and can penetrate up to 0.9 mm of pork slice, and thus is beneficial for imaging and treatment of deep cancers like basal-cell carcinoma, gastric cancer, oropharyngeal cancer, etc.
The combination of platinum NWs (with excellent biocompatibility and photothermal killing effect) and laser irradiation is proven to cause thermal necrotic cell death in 4T1 breast cancer cells. NWs in PBS were used as CT contrast agents and showed strong CT signals in cross-sections and reconstructed 3D images. The group receiving laser, X-ray irradiation and NWs showed the strongest antitumor activity and completely suppressed tumor growth. Additionally, studies showed that after injection with NWs into healthy mice, blood levels returned to normal 7 days later and the daily stress response of NWs displayed no long-term damage. Photothermal effects in vivo were studied using polyethylene glycol (PEG) NWs with laser irradiation, and the tumor sample was stained with a hypoxic probe consisting of an anti-CD31 antibody and DAPI. The co-administration of Pt-PEG NWs with a combination of a 1064 nm laser and X-rays resulted in a significant reduction in tumor size in mice within 14 days of treatment. The Pt-PEG NWs were prepared using a simple and reproducible process, making them an ideal photothermal agent in the second NIR optical window, as well as a radiosensitizer with remarkable stability in physiological solutions. Hence, the findings suggest the use of platinum NWs in combination with laser irradiation for cancer treatment and imaging applications.101
3. Conclusion
As tiny, biologically inspired robots, NWs possess the potential to revolutionize medicine by navigating through the human body and delivering drugs or performing surgeries with unprecedented precision and efficiency. NWs are a promising choice for improving drug delivery systems for malignant and inflammatory diseases, due to the small size, high surface area, ability to evade physiological elimination, capability to recognize tumours through superparamagnetism, and improved kinetics, stability and shelf-life attributes. NWs find immense applications in diagnostic methods such as MRI and ELISA for more accurate diagnosis of disease, in therapy for efficient delivery systems for a given target with minimum systemic adverse effects and in theranostics for the delivery of siRNA using dendriworms, combating focal bacterial infection, and treating atherosclerosis and deadly diseases like AML. Recent advancements in NWs include surface-coated IONWs for early complement opsonization, hybrid protein–polymer NWs for apoptosis of diseased cells, IONWs for tumor suppression in breast cancer and platinum NWs for multimodal imaging-guided cancer therapy. NWs are still in an early stage of development and are not fully explored, and much research is required to completely realize the applicability of NWs in the future. Future developments in this area will lead to a new generation of revolutionary nano-biomaterials designed to enhance patient quality of life.Author contributions
All the authors contributed equally. Dr Pravin Shende: conceptualization and supervision; Ms Kadambari Borse: data curation, writing – original draft preparation, reviewing and editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
I would like to thank Dr Pravin Shende for patient instruction, passionate support, and proofreading of the review article.References
- J.-H. Park, G. Von Maltzahn, L. Zhang, M. P. Schwartz, E. Ruoslahti, S. N. Bhatia, et al., Magnetic Iron Oxide Nanoworms for Tumor Targeting and Imaging. Available from: https://www.advmat.de.
- J. K. Patra, G. Das, L. F. Fraceto, E. V. R. Campos, M. D. P. Rodriguez-Torres and L. S. Acosta-Torres, et al., Nano based drug delivery systems: recent developments and future prospects, J. Nanobiotechnol., 2018, 16(1), 1–33 CrossRef PubMed . Available from: https://jnanobiotechnology.biomedcentral.com/articles/10.1186/s12951-018-0392-8.
- J. K. Patra and K. H. Baek, Green Nanobiotechnology: Factors Affecting Synthesis and Characterization Techniques, J. Nanomater., 2014, 417305 Search PubMed.
- Review: nanoparticles in delivery of cardiovascular drugs – PubMed [Internet]. [cited 2022 Jul 22]. Available from: https://pubmed.ncbi.nlm.nih.gov/17604260/.
- S. M. Kim, T. Lee, Y. G. Gil, G. H. Kim, C. Park and H. Jang, et al., Fabrication of Bioprobe Self-Assembled on Au–Te Nanoworm Structure for SERS Biosensor, Mater, 2020, 13(14), 3234 CrossRef CAS PubMed . Available from: https://www.mdpi.com/1996-1944/13/14/3234/htm.
- V. Perumal, U. Hashim, S. C. B. Gopinath, R. Haarindraprasad, P. Poopalan and W. W. Liu, et al., A new nano-worm structure from gold-nanoparticle mediated random curving of zinc oxide nanorods, Biosens. Bioelectron., 2016, 78, 14–22 CrossRef CAS PubMed . Available from: https://pubmed.ncbi.nlm.nih.gov/26584078/.
- S. R. Aluri, P. Shi, J. A. Gustafson, W. Wang, Y. A. Lin and H. Cui, et al., A hybrid protein-polymer nanoworm potentiates apoptosis better than a monoclonal antibody, ACS Nano, 2014, 8(3), 2064–2076 CrossRef CAS PubMed.
- N. P. Truong, J. F. Quinn, M. R. Whittaker and T. P. Davis, Polymeric filomicelles and nanoworms: Two decades of synthesis and application, Polym. Chem., 2016, 7(26), 4295–4312 RSC.
- Z. Jia, V. A. Bobrin, N. P. Truong, M. Gillard and M. J. Monteiro, Multifunctional nanoworms and nanorods through a one-step aqueous dispersion polymerization, J. Am. Chem. Soc., 2014, 136(16), 5824–5827, DOI:10.1021/ja500092m.
- M. S. Hossain, J. Ji, C. J. Lynch, M. Guzman, S. Nangia and D. Mozhdehi, Adaptive Recombinant Nanoworms from Genetically Encodable Star Amphiphiles, Biomacromolecules, 2022, 23(3), 863–876, DOI:10.1021/acs.biomac.1c01314.
- J. H. Park, G. Von Maltzahn, L. Zhang, M. P. Schwartz, E. Ruoslahti and S. N. Bhatia, et al., Magnetic Iron Oxide Nanoworms for Tumor Targeting and Imaging, Adv. Mater., 2008, 20(9), 1630 CrossRef CAS PubMed.
- Y. J. Xu, L. Dong, Y. Lu, L. C. Zhang, D. An and H. L. Gao, et al., Magnetic hydroxyapatite nanoworms for magnetic resonance diagnosis of acute hepatic injury, Nanoscale, 2016, 8(3), 1684–1690 RSC . Available from: https://rsc.66557.net/en/content/articlehtml/2016/nr/c5nr07023f.
- G. Wang, S. Inturi, N. J. Serkova, S. Merkulov, K. McCrae and S. E. Russek, et al., High-relaxivity superparamagnetic iron oxide nanoworms with decreased immune recognition and long-circulating properties, ACS Nano, 2014, 8(12), 12437–12449 CrossRef CAS PubMed.
- V. P. Vaikari, M. Park, L. Keossayan, J. A. MacKay and H. Alachkar, Anti-CD99 scFv-ELP nanoworms for the treatment of acute myeloid leukemia, Nanomed.: Nanotechnol. Biol. Med., 2020, 29, 102236, DOI:10.1016/j.nano.2020.102236.
- A. Agrawal, D. H. Min, N. Singh, H. Zhu, A. Birjiniuk and G. Von Maltzahn, et al., Functional delivery of siRNA in mice using dendriworms, ACS Nano, 2009, 3(9), 2495–2504, DOI:10.1021/nn900201e.
- P. Shende and A. Mondal, Nanobulges: A Duplex Nanosystem for Multidimensional Applications, Curr. Nanosci., 2020, 16(5), 668–675 CrossRef CAS.
- M. Sardesai and P. Shende, Engineering of Nanospheres Dispersed Microneedle System for Antihypertensive Action, Curr. Drug Delivery, 2020, 17(9), 776–786 CrossRef CAS PubMed.
- P. Shende, P. Kasture and R. S. Gaud, Nanoflowers: the future trend of nanotechnology for multi-applications, Artif. Cells, Nanomed., Biotechnol., 2018, 46, 413–422, DOI:10.1080/2169140120181428812.
- Nanoworms Target Tumors|MIT Technology Review [Internet]. [cited 2022 Oct 1]. Available from: https://www.technologyreview.com/2008/05/14/269730/nanoworms-target-tumors/.
- S. S. Timur, D. Yöyen-Ermiş, G. Esendağlı, S. Yonat, U. Horzum and G. Esendağlı, et al., Efficacy of a novel LyP-1-containing self-microemulsifying drug delivery system (SMEDDS) for active targeting to breast cancer, Eur. J. Pharm. Biopharm., 2019, 136, 138–146 CrossRef CAS PubMed.
- Researchers target tumors with tiny “nanoworms” [Internet]. [cited 2023 Feb 14]. Available from: https://phys.org/news/2008-05-tumors-tiny-nanoworms.html.
- Y. Guo, Y. Ran, Z. Wang, J. Cheng, Y. Cao and C. Yang, et al., Magnetic-responsive and targeted cancer nanotheranostics by PA/MR bimodal imaging-guided photothermally triggered immunotherapy, Biomaterials, 2019, 219, 119370 CrossRef CAS PubMed . Available from: https://pubmed.ncbi.nlm.nih.gov/31357006/.
- P. C. Ray, H. Yu and P. P. Fu, Toxicity and Environmental Risks of Nanomaterials: Challenges and Future Needs, J. Environ. Sci. Health, Part C: Environ. Carcinog. Ecotoxicol. Rev., 2009, 27(1), 1 CrossRef CAS PubMed.
- S. A. Bradford, C. Shen, H. Kim, R. J. Letcher, J. Rinklebe and Y. S. Ok, et al., Environmental applications and risks of nanomaterials: An introduction to CREST publications during 2018–2021, Crit. Rev. Environ. Sci. Technol., 2021, 52(21), 3753–3762, DOI:10.1080/1064338920212020425.
- W. H. De Jong and P. J. A. Borm, Drug delivery and nanoparticles:applications and hazards, Int. J. Nanomedicine, 2008, 3(2), 133–149 CrossRef CAS PubMed . Available from: https://pubmed.ncbi.nlm.nih.gov/18686775/.
- J. H. Park, G. Von Maltzahn, L. Zhang, A. M. Derfus, D. Simberg and T. J. Harris, et al., Systematic Surface Engineering of Magnetic Nanoworms for in vivo Tumor Targeting, Small, 2009, 5(6), 694 CrossRef CAS PubMed.
- A. D. Warren, G. A. Kwong, D. K. Wood, K. Y. Lin and S. N. Bhatia, Point-of-care diagnostics for noncommunicable diseases using synthetic urinary biomarkers and paper microfluidics, Proc. Natl. Acad. Sci. U. S. A., 2014, 111(10), 3671–3676, DOI:10.1073/pnas.1314651111.
- G. A. Kwong, G. Von Maltzahn, G. Murugappan, O. Abudayyeh, S. Mo and I. A. Papayannopoulos, et al., Mass-encoded synthetic biomarkers for multiplexed urinary monitoring of disease, Nat. Biotechnol., 2012, 31(1), 63–70 CrossRef PubMed . Available from: https://www.nature.com/articles/nbt.2464.
- K. Y. Lin, G. A. Kwong, A. D. Warren, D. K. Wood and S. N. Bhatia, Nanoparticles that sense thrombin activity as synthetic urinary biomarkers of thrombosis, ACS Nano, 2013, 7(10), 9001–9009, DOI:10.1021/nn403550c.
- N. Arndt, H. D. N. Tran, R. Zhang, Z. P. Xu and H. T. Ta, Different Approaches to Develop Nanosensors for Diagnosis of Diseases, Adv. Sci., 2020, 7(24), 2001476, DOI:10.1002/advs.202001476.
- T. Muniraj, P. A. Jamidar and H. R. Aslanian, Pancreatic cancer: a comprehensive review and update, Disease-a-Month, 2013, 59(11), 368–402 CrossRef PubMed . Available from: https://pubmed.ncbi.nlm.nih.gov/24183261/.
- G. Kostenich, N. Livnah, T. A. Bonasera, T. Yechezkel, Y. Salitra and P. Litman, et al., Targeting small-cell lung cancer with novel fluorescent analogs of somatostatin, Lung Cancer, 2005, 50(3), 319–328 CrossRef PubMed . Available from: https://pubmed.ncbi.nlm.nih.gov/16159681/.
- A. Louie, Multimodality imaging probes: Design and challenges, Chem. Rev., 2010, 110(5), 3146–3195, DOI:10.1021/cr9003538.
- S. Yu and C. M. Chow, Carboxyl group (–CO2H) functionalized ferrimagnetic iron oxide nanoparticles for potential bio-applications, J. Mater. Chem., 2004, 14(18), 2781–2786 RSC . Available from: https://rsc.66557.net/en/content/articlehtml/2004/jm/b404964k.
- Y. Ahmadi, G. Kostenich, M. Oron-Herman, W. Wadsak, M. Mitterhauser and A. Orenstein, et al., In vivo magnetic resonance imaging of pancreatic tumors using iron oxide nanoworms targeted with PTR86 peptide, Colloids Surf., B, 2017, 158, 423–430, DOI:10.1016/j.colsurfb.2017.06.051.
- L. C. Hull, D. Farrell and P. Grodzinski, Highlights of recent developments and trends in cancer nanotechnology research-view from NCI Alliance for Nanotechnology in Cancer, Biotechnol. Adv., 2014, 32(4), 666–678 CrossRef CAS PubMed . Available from: https://pubmed.ncbi.nlm.nih.gov/23948249/.
- P. Grodzinski and D. Farrell, Future opportunities in cancer nanotechnology-NCI strategic workshop report, Cancer Res., 2014, 74(5), 1307–1310 CrossRef CAS PubMed . Available from: https://pubmed.ncbi.nlm.nih.gov/24413533/.
- K. Y. Helmy, K. J. Katschke, N. N. Gorgani, N. M. Kljavin, J. M. Elliott and L. Diehl, et al., CRIg: a macrophage complement receptor required for phagocytosis of circulating pathogens, Cell, 2006, 124(5), 915–927 CrossRef CAS PubMed . Available from: https://pubmed.ncbi.nlm.nih.gov/16530040/.
- P. R. Taylor, L. Martinez-Pomares, M. Stacey, H. H. Lin, G. D. Brown and S. Gordon, Macrophage receptors and immune recognition, Annu. Rev. Immunol., 2005, 23, 901–944 CrossRef CAS PubMed . Available from: https://pubmed.ncbi.nlm.nih.gov/15771589/.
- D. Ricklin, G. Hajishengallis, K. Yang and J. D. Lambris, Complement: a key system for immune surveillance and homeostasis, Nat. Immunol., 2010, 11(9), 785–797 CrossRef CAS PubMed . Available from: https://pubmed.ncbi.nlm.nih.gov/20720586/.
- S. Inturi, G. Wang, F. Chen, N. K. Banda, V. M. Holers and L. P. Wu, et al., Modulatory Role of Surface Coating of Superparamagnetic Iron Oxide Nanoworms in Complement Opsonization and Leukocyte Uptake, ACS Nano, 2015, 9(11), 10758–10768 CrossRef CAS PubMed.
- R. L. Brutchey and D. E. Morse, Silicattein and the translation of its molecular mechanism of biosilicification into low temperature nanomaterial synthesis, Chem. Rev., 2008, 108(11), 4915–4934 CrossRef CAS PubMed.
- P. J. M. Smeets, K. R. Cho, R. G. E. Kempen, N. A. J. M. Sommerdijk and J. J. De Yoreo, Calcium carbonate nucleation driven by ion binding in a biomimetic matrix revealed by in situ electron microscopy, Nat. Mater., 2015, 14(4), 394–399 CrossRef CAS PubMed . Available from: https://pubmed.ncbi.nlm.nih.gov/25622001/.
- Y. Y. Kim, A. S. Schenk, J. Ihli, A. N. Kulak, N. B. J. Hetherington and C. C. Tang, et al., A critical analysis of calcium carbonate mesocrystals, Nat. Commun., 2014, 5, 4341 CrossRef CAS PubMed . Available from: https://pubmed.ncbi.nlm.nih.gov/25014563/.
- SEER Cancer Statistics Review, 1975–2010 – Previous Version - SEER Cancer Statistics Review [Internet]. [cited 2022 Sep 2]. Available from: https://seer.cancer.gov/archive/csr/1975_2010/.
- (PDF) Improved tumor targeting with chemically cross-linked recombinant antibody fragments [Internet]. [cited 2022 Sep 2]. Available from: https://www.researchgate.net/publication/15243703_Improved_tumor_targeting_with_chemically_cross-linked_recombinant_antibody_fragments.
- N. E. Weisser and J. C. Hall, Applications of single-chain variable fragment antibodies in therapeutics and diagnostics, Biotechnol. Adv., 2009, 27(4), 502–520 CrossRef CAS PubMed . Available from: https://pubmed.ncbi.nlm.nih.gov/19374944/.
- A. L. Nelson, Antibody fragments: hope and hype, MAbs, 2010, 2(1), 77–83 CrossRef PubMed . Available from: https://pubmed.ncbi.nlm.nih.gov/20093855/.
- X. Qian, X. H. Peng, D. O. Ansari, Q. Yin-Goen, G. Z. Chen and D. M. Shin, et al., In vivo tumor targeting and spectroscopic detection with surface-enhanced Raman nanoparticle tags, Nat. Biotechnol., 2008, 26(1), 83–90 CrossRef CAS PubMed . Available from: https://pubmed.ncbi.nlm.nih.gov/18157119/.
- N. Zhang, L. A. Khawli, P. Hu and A. L. Epstein, Lym-1-induced apoptosis of non-Hodgkin's lymphomas produces regression of transplanted tumors, Cancer Biother. Radiopharm., 2007, 22(3), 342–356 CrossRef CAS PubMed . Available from: https://pubmed.ncbi.nlm.nih.gov/17651040/.
- E. Tobin, G. L. DeNardo, N. Zhang, A. L. Epstein, C. Liu and S. DeNardo, Combination immunotherapy with anti-CD20 and anti-HLA-DR monoclonal antibodies induces synergistic anti-lymphoma effects in human lymphoma cell lines, Leuk. Lymphoma, 2007, 48(5), 944–956 CrossRef CAS PubMed . Available from: https://pubmed.ncbi.nlm.nih.gov/17487739/.
- N. Zhang, L. A. Khawli, P. Hu and A. L. Epstein, Generation of rituximab polymer may cause hyper-cross-linking-induced apoptosis in non-Hodgkin's lymphomas, Clin. Cancer Res., 2005, 11(16), 5971–5980 CrossRef CAS PubMed . Available from: https://pubmed.ncbi.nlm.nih.gov/16115941/.
- J. Shao, M. Abdelghani, G. Shen, S. Cao, D. S. Williams and J. C. M. Van Hest, Erythrocyte Membrane Modified Janus Polymeric Motors for Thrombus Therapy, ACS Nano, 2018, 12(5), 4877–4885, DOI:10.1021/acsnano.8b01772.
- Z. Li, J. Ma, N. S. Lee and K. L. Wooley, Dynamic cylindrical assembly of triblock copolymers by a hierarchical process of covalent and supramolecular interactions, J. Am. Chem. Soc., 2011, 133(5), 1228–1231, DOI:10.1021/ja109191z.
- J. R. Lovett, N. J. Warren, L. P. D. Ratcliffe, M. K. Kocik and S. P. Armes, pH-Responsive Non-Ionic Diblock Copolymers: Ionization of Carboxylic Acid End-Groups Induces an Order–Order Morphological Transition, Angew. Chem., Int. Ed., 2015, 54(4), 1279–1283, DOI:10.1002/anie.201409799.
- F. H. Schacher, P. A. Rupar and I. Manners, Functional Block Copolymers: Nanostructured Materials with Emerging Applications, Angew. Chem., Int. Ed., 2012, 51(32), 7898–7921, DOI:10.1002/anie.201200310.
- L. M. Van Oppen, L. K. Abdelmohsen, S. E. Van Emst-De Vries, P. L. Welzen, D. A. Wilson and J. A. Smeitink, et al., Biodegradable synthetic organelles demonstrate ROS shielding in human-complex-I-deficient fibroblasts, ACS Cent. Sci., 2018, 4(7), 917–928 CrossRef CAS . Available from: https://research.tue.nl/en/publications/biodegradable-synthetic-organelles-demonstrate-ros-shielding-in-h.
- D. Xu, Q. Ran, Y. Xiang, L. Jiang, B. M. Smith and F. Bou-Abdallah, et al., Toward Hemocompatible Self-assembling Antimicrobial Nanofibers: Understanding the Synergistic Effect of Supramolecular Structure and PEGylation on Hemocompatibility, RSC Adv., 2016, 6(19), 15911 RSC.
- B. Chen, W. Le, Y. Wang, Z. Li, D. Wang and L. Ren, et al., Targeting negative surface charges of cancer cells by multifunctional nanoprobes, Theranostics, 2016, 6(11), 1887–1898 CrossRef CAS PubMed.
- K. Han, J. Zhang, W. Zhang, S. Wang, L. Xu and C. Zhang, et al., Tumor-Triggered Geometrical Shape Switch of Chimeric Peptide for Enhanced in Vivo Tumor Internalization and Photodynamic Therapy., ACS Nano, 2017, 11(3), 3178–3188, DOI:10.1021/acsnano.7b00216.
- C. Kinnear, T. L. Moore, L. Rodriguez-Lorenzo, B. Rothen-Rutishauser and A. Petri-Fink, Form Follows Function: Nanoparticle Shape and Its Implications for Nanomedicine, Chem. Rev., 2017, 117(17), 11476–11521 CrossRef CAS PubMed . Available from: https://pubmed.ncbi.nlm.nih.gov/28862437/.
- S. Cao, J. Shao, Y. Xia, H. Che, Z. Zhong and F. Meng, et al., Molecular Programming of Biodegradable Nanoworms via Ionically Induced Morphology Switch toward Asymmetric Therapeutic Carriers, Small, 2019, 15(38), 1901849 CrossRef PubMed . Available from: https://onlinelibrary.wiley.com/doi/full/10.1002/smll.201901849.
- L. Agemy, D. Friedmann-Morvinski, V. Ramana Kotamraju, L. Roth, K. N. Sugahara and O. M. Girard, et al., Targeted nanoparticle enhanced proapoptotic peptide as potential therapy for glioblastoma, Proc. Natl. Acad. Sci. U. S. A., 2011, 108(42), 17450–17455, DOI:10.1073/pnas.1114518108.
- K. Fujimori, D. G. Covell, J. E. Fletcher and J. N. Weinstein, A modeling analysis of monoclonal antibody percolation through tumors: a binding-site barrier, J. Nucl. Med., 1990, 31(7), 1191–1198 CAS . Available from: https://europepmc.org/article/med/2362198.
- E. Ruoslahti, Tumor penetrating peptides for improved drug delivery, Adv. Drug Delivery Rev., 2017, 110–111, 3 CrossRef CAS PubMed.
- X. Wu, G. Yu, D. Lindner, S. M. Brady-Kalnay, Q. Zhang and Z.-R. Lu, Peptide targeted high-resolution molecular imaging of prostate cancer with MRI, Am. J. Nucl. Med. Mol. Imaging, 2014, 4(6), 525 Search PubMed.
- S. Sharma, V. R. Kotamraju, T. Mölder, A. Tobi, T. Teesalu and E. Ruoslahti, Tumor-Penetrating Nanosystem Strongly Suppresses Breast Tumor Growth, Nano Lett., 2017, 17(3), 1356–1364 CrossRef CAS PubMed.
- V. A. Bobrin, S. P. Chen, C. F. Grandes Reyes, B. Sun, C. K. Ng and Y. Kim, et al., Water-Borne Nanocoating for Rapid Inactivation of SARS-CoV-2 and Other Viruses, ACS Nano, 2021, 15(9), 14915–14927 CrossRef CAS PubMed . Available from: https://pubmed.ncbi.nlm.nih.gov/34423970/.
- H. Döhner, E. Estey, D. Grimwade, S. Amadori, F. R. Appelbaum and T. Büchner, et al., Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel, Blood, 2017, 129(4), 424–447 CrossRef PubMed . Available from: https://ashpublications.org/blood/article/129/4/424/36196/Diagnosis-and-management-of-AML-in-adults-2017-ELN.
- V. P. Vaikari, Y. Du, S. Wu, T. Zhang, K. Metzeler and A. M. N. Batcha, et al., Clinical and preclinical characterization of CD99 isoforms in acute myeloid leukemia, Haematologica, 2020, 105(4), 999–1012 CrossRef CAS PubMed . Available from: https://pubmed.ncbi.nlm.nih.gov/31371417/.
- A. Skerra and A. Plückthun, Assembly of a functional immunoglobulin Fv fragment in Escherichia coli, Science, 1988, 240(4855), 1038–1041 CrossRef CAS PubMed . Available from: https://pubmed.ncbi.nlm.nih.gov/3285470/.
- M. Hutt, A. Färber-Schwarz, F. Unverdorben, F. Richter and R. E. Kontermann, Plasma half-life extension of small recombinant antibodies by fusion to immunoglobulin-binding domains, J. Biol. Chem., 2012, 287(7), 4462–4469 CrossRef CAS PubMed . Available from: https://pubmed.ncbi.nlm.nih.gov/22147690/.
- A. Hayhurst and W. J. Harris, Escherichia coli skp chaperone coexpression improves solubility and phage display of single-chain antibody fragments, Protein Expression Purif., 1999, 15(3), 336–343 CrossRef CAS PubMed . Available from: https://pubmed.ncbi.nlm.nih.gov/10092493/.
- M. Sabaty, S. Grosse, G. Adryanczyk, S. Boiry, F. Biaso and P. Arnoux, et al., Detrimental effect of the 6 His C-terminal tag on YedY enzymatic activity and influence of the TAT signal sequence on YedY synthesis, BMC Biochem., 2013, 14(1), 28 CrossRef CAS PubMed.
- T. Christensen, M. Amiram, S. Dagher, K. Trabbic-Carlson, M. F. Shamji and L. A. Setton, et al., Fusion order controls expression level and activity of elastin-like polypeptide fusion proteins, Protein Sci., 2009, 18(7), 1377–1387 CrossRef CAS PubMed . Available from: https://pubmed.ncbi.nlm.nih.gov/19533768/.
- Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report: 2021 [Internet]. [cited 2022 Sep 4]. Available from: https://www.who.int/publications/i/item/9789240027336.
- Y. Gao, J. Wang, M. Chai, X. Li, Y. Deng and Q. Jin, et al., Size and Charge Adaptive Clustered Nanoparticles Targeting the Biofilm Microenvironment for Chronic Lung Infection Management, ACS Nano, 2020, 14(5), 5686–5699 CrossRef CAS PubMed . Available from: https://pubmed.ncbi.nlm.nih.gov/32320228/.
- Y. Qiao, J. He, W. Chen, Y. Yu, W. Li and Z. Du, et al., Light-Activatable Synergistic Therapy of Drug-Resistant Bacteria-Infected Cutaneous Chronic Wounds and Nonhealing Keratitis by Cupriferous Hollow Nanoshells, ACS Nano, 2020, 14(3), 3299–3315 CrossRef CAS PubMed . Available from: https://pubs.acs.org/doi/abs/10.1021/acsnano.9b08930.
- J. Wu, F. Li, X. Hu, J. Lu, X. Sun and J. Gao, et al., Responsive Assembly of Silver Nanoclusters with a Biofilm Locally Amplified Bactericidal Effect to Enhance Treatments against Multi-Drug-Resistant Bacterial Infections, ACS Cent. Sci., 2019, 5(8), 1366–1376, DOI:10.1021/acscentsci.9b00359.
- X. Pang, Q. Xiao, Y. Cheng, E. Ren, L. Lian and Y. Zhang, et al., Bacteria-Responsive Nanoliposomes as Smart Sonotheranostics for Multidrug Resistant Bacterial Infections, ACS Nano, 2019, 13(2), 2427–2438 CAS . Available from: https://pubmed.ncbi.nlm.nih.gov/30657302/.
- M. Li, J. Li, J. Chen, Y. Liu, X. Cheng and F. Yang, et al., Platelet Membrane Biomimetic Magnetic Nanocarriers for Targeted Delivery and in Situ Generation of Nitric Oxide in Early Ischemic Stroke, ACS Nano, 2020, 14(2), 2024–2035, DOI:10.1021/acsnano.9b08587.
- C. Tong, X. Zhong, Y. Yang, X. Liu, G. Zhong and C. Xiao, et al., PB@PDA@Ag nanosystem for synergistically eradicating MRSA and accelerating diabetic wound healing assisted with laser irradiation, Biomaterials, 2020, 243, 119936 CrossRef CAS PubMed.
- C. Mao, Y. Xiang, X. Liu, Z. Cui, X. Yang and K. W. K. Yeung, et al., Photo-Inspired Antibacterial Activity and Wound Healing Acceleration by Hydrogel Embedded with Ag/Ag@AgCl/ZnO Nanostructures, ACS Nano, 2017, 11(9), 9010–9021 CrossRef CAS PubMed . Available from: https://pubmed.ncbi.nlm.nih.gov/28825807/.
- L. Xie, X. Pang, X. Yan, Q. Dai, H. Lin and J. Ye, et al., Photoacoustic Imaging-Trackable Magnetic Microswimmers for Pathogenic Bacterial Infection Treatment, ACS Nano, 2020, 14(3), 2880–2893, DOI:10.1021/acsnano.9b06731.
- C. Gao, Y. Wang, Z. Ye, Z. Lin, X. Ma and Q. He, Biomedical Micro-/Nanomotors: From Overcoming Biological Barriers to In Vivo Imaging, Adv. Mater., 2021, 33(6), 2000512, DOI:10.1002/adma.202000512.
- P. Erkoc, I. C. Yasa, H. Ceylan, O. Yasa, Y. Alapan and M. Sitti, Mobile Microrobots for Active Therapeutic Delivery, Adv. Ther., 2019, 2(1), 1800064, DOI:10.1002/adtp.201800064.
- B. Lu, E. Hu, R. Xie, K. Yu, F. Lu and R. Bao, et al., Magnetically Guided Nanoworms for Precise Delivery to Enhance in Situ Production of Nitric Oxide to Combat Focal Bacterial Infection in Vivo, ACS Appl. Mater. Interfaces, 2021, 13(19), 22225–22239 CrossRef CAS PubMed.
- Y. Yang, J. Shi, T. Tanaka and M. Nogami, Self-assembled silver nanochains for surface-enhanced Raman scattering, Langmuir, 2007, 23(24), 12042–12047, DOI:10.1021/la701610s.
- Z. Yin, Y. Wang, C. Song, L. Zheng, N. Ma and X. Liu, et al., Hybrid Au-Ag Nanostructures for Enhanced Plasmon-Driven Catalytic Selective Hydrogenation through Visible Light Irradiation and Surface-Enhanced Raman Scattering, J. Am. Chem. Soc., 2018, 140(3), 864–867, DOI:10.1021/jacs.7b11293.
- D. Solis, B. Willingham, S. L. Nauert, L. S. Slaughter, J. Olson and P. Swanglap, et al., Electromagnetic energy transport in nanoparticle chains via dark plasmon modes, Nano Lett., 2012, 12(3), 1349–1353, DOI:10.1021/nl2039327.
- Y. S. Li, B. F. Chen, X. J. Li, W. K. Zhang and H. B. Tang, Cytotoxicity of Polyaniline Nanomaterial on Rat Celiac Macrophages In Vitro, PLoS One, 2014, 9(9), e107361, DOI:10.1371/journal.pone.0107361.
- H. Chen, L. Shao, Q. Li and J. Wang, Gold nanorods and their plasmonic properties, Chem. Soc. Rev., 2013, 42(7), 2679–2724 RSC . Available from: https://rsc.66557.net/en/content/articlehtml/2013/cs/c2cs35367a.
- Z. Yin, W. Zhang, Q. Fu, H. Yue, W. Wei and P. Tang, et al., Construction of stable chainlike Au nanostructures via silica coating and exploration for potential photothermal therapy, Small, 2014, 10(18), 3619–3624 CrossRef CAS PubMed . Available from: https://pubmed.ncbi.nlm.nih.gov/24861373/.
- C. K. K. Choi, Y. T. E. Chiu, X. Zhuo, Y. Liu, C. Y. Pak and X. Liu, et al., Dopamine-Mediated Assembly of Citrate-Capped Plasmonic Nanoparticles into Stable Core-Shell Nanoworms for Intracellular Applications, ACS Nano, 2019, 13(5), 5864–5884 CrossRef CAS PubMed.
- F. Islami, A. G. Sauer, K. D. Miller, R. L. Siegel and S. A. Fedewa, et al., Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States, Ca-Cancer J. Clin., 2018, 68(1), 31–54, DOI:10.3322/caac.21440.
- C. Sawyers, Targeted cancer therapy, Nature, 2004, 432(7015), 294–297 CrossRef CAS PubMed . Available from: https://pubmed.ncbi.nlm.nih.gov/15549090/.
- Y. Chen, L. Cheng, Z. Dong, Y. Chao, H. Lei and H. Zhao, et al., Degradable Vanadium Disulfide Nanostructures with Unique Optical and Magnetic Functions for Cancer Theranostics, Angew. Chem., 2017, 129(42), 13171–13176, DOI:10.1002/ange.201707128.
- D. Jaque, L. Martínez Maestro, B. Del Rosal, P. Haro-Gonzalez, A. Benayas and J. L. Plaza, et al., Nanoparticles for photothermal therapies, Nanoscale, 2014, 6(16), 9494–9530 RSC . Available from: https://rsc.66557.net/en/content/articlehtml/2014/nr/c4nr00708e.
- P. Zhang, L. Wang, C. Rodriguez-Aguayo, Y. Yuan, B. G. Debeb and D. Chen, et al., miR-205 acts as a tumour radiosensitizer by targeting ZEB1 and Ubc13, Nat. Commun., 2014, 5, 5671 CrossRef CAS PubMed . Available from: https://pubmed.ncbi.nlm.nih.gov/25476932/.
- V. E. Zannella, A. D. Pra, H. Muaddi, T. D. McKee, S. Stapleton and J. Sykes, et al., Reprogramming metabolism with metformin improves tumor oxygenation and radiotherapy response, Clin. Cancer Res., 2013, 19(24), 6741–6750 CrossRef CAS PubMed . Available from: https://pubmed.ncbi.nlm.nih.gov/24141625/.
- Q. Ma, L. Cheng, F. Gong, Z. Dong, C. Liang and M. Wang, et al., Platinum nanoworms for imaging-guided combined cancer therapy in the second near-infrared window, J. Mater. Chem. B, 2018, 6(31), 5069–5079 RSC.
- E. Porcel, S. Liehn, H. Remita, N. Usami, K. Kobayashi and Y. Furusawa, et al., Platinum nanoparticles: a promising material for future cancer therapy?, Nanotechnology, 2010, 21(8), 85103 CrossRef PubMed . Available from: https://pubmed.ncbi.nlm.nih.gov/20101074/.
- G. Ma, Q. Xue, J. Zhu, X. Zhang, X. Wang and H. Yao, et al., Ultrafine Rh nanocrystals decorated ultrathin NiO nanosheets for urea electro-oxidation. undefined, Appl. Catal., B, 2020, 15, 265 Search PubMed.
- L. Falbo, C. G. Visconti, L. Lietti and J. Szanyi, The effect of CO on CO2 methanation over Ru/Al2O3 catalysts: a combined steady-state reactivity and transient DRIFT spectroscopy study, Appl. Catal., B, 2019, 256, 117791 CrossRef CAS.
- K. Bhunia, M. Chandra and D. Pradhan, Exposed Facets-Dependent Catalytic Properties of Nanocrystals: Noble Metals (Pd, Pt, and Au) and Oxides of First Row d-Block Elements, J. Nanosci. Nanotechnol., 2019, 19(1), 332–355 CrossRef CAS PubMed . Available from: https://pubmed.ncbi.nlm.nih.gov/30327041/.
- J. Zhou, K. T. Shum, J. C. Burnett and J. J. Rossi, Nanoparticle-Based Delivery of RNAi Therapeutics: Progress and Challenges, Pharmaceuticals, 2013, 6(1), 85 CrossRef CAS PubMed.
- K. Liu, A. Wang and T. Zhang, Recent advances in preferential oxidation of co reaction over platinum group metal catalysts, ACS Catal., 2012, 2(6), 1165–1178, DOI:10.1021/cs200418w.
- H. Guan, J. Lin, B. Qiao, X. Yang, L. Li and S. Miao, et al., Catalytically Active Rh Sub-Nanoclusters on TiO2 for CO Oxidation at Cryogenic Temperatures, Angew. Chem., Int. Ed., 2016, 55(8), 2820–2824 CrossRef CAS PubMed . Available from: https://pubmed.ncbi.nlm.nih.gov/26797803/.
| This journal is © The Royal Society of Chemistry 2023 |