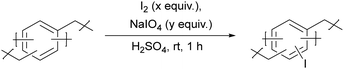Open Access Article
Open Access ArticleA facile synthesis of iodine-functionalized hypercrosslinked polymers†
Chanachon
Thiamsiri
,
Thanchanok
Ratvijitvech
and
Torsak
Luanphaisarnnont
 *
*
Department of Chemistry and Center of Excellence for Innovation in Chemistry, Faculty of Science, Mahidol University, 272 Rama VI Road, Ratchathewi, Bangkok 10400, Thailand. E-mail: torsak.lua@mahidol.ac.th
First published on 4th October 2023
Abstract
Hypervalent iodine compounds have gained significant attention for their applications in highly efficient and environmentally benign chemical transformations. Many homogeneous variants of hypervalent iodine compounds have been investigated; however, the development of the heterogeneous variants is still limited despite their potential applications as recyclable catalysts. In this study, we report a synthesis of novel hypercrosslinked polymers bearing iodine functional groups. These polymers were synthesized using an efficient iodination method, allowing iodine incorporation up to 1.96 mmol g−1. Various iodinated hypercrosslinked polymers, featuring electron-rich and electron-poor substituents, were synthesized with good yields and high iodine incorporation. The utility of these iodinated polymers as a heterogeneous catalyst was demonstrated in an alcohol oxidation, giving the corresponding ketone in high yields. This polymeric catalyst could be easily recovered through simple filtration and reused without any deterioration in the yields of the product.
Introduction
Development of green and efficient syntheses is an important challenge in chemistry. Catalysis offers a solution for more efficient and more environmentally friendly syntheses because catalysts can be used in a sub-stoichiometric amount; thereby, reducing the synthesis cost and waste. Heterogeneous catalysis for organic synthesis has recently gained a lot of attention because heterogeneous catalysts can be recovered and reused with a convenient and inexpensive operation such as simple filtration. Development of new heterogeneous catalysts is therefore important. One commonly used approach to develop heterogeneous catalysts is to functionalize the surface of a material to install reactive functionality necessary for catalysis.1Hypercrosslinked polymers (HCPs) are an important class of porous materials with extensive crosslinking in the polymer chains.2,3 The structural framework of HCPs generally has a highly porous structure and high surface area, making them suitable for various applications such as catalysis,4,5 gas storage,6–8 separation,9,10 adsorption,11 solid supports,12 and sensors.13 One important advantage of HCPs is their high porosity and high surface area, which allows high functional group incorporation on their surface. HCPs with various functional groups, such as sulfonic acid,14 carboxylic acid,15 amide,16 hydroxyl group,17 and amine18 have been developed. In addition to neutral HCPs, ionic HCPs have been constructed for various applications.19–21 However, iodine-functionalized HCPs have not been reported possibly because alkyl or aryl iodide functional groups could interfere with common polymerization methods to synthesize HCPs such as Friedel–Crafts reactions or cross-coupling reactions.
Iodine-functionalized aromatic compounds are an important structural motif in organic chemistry. One important application is its use as a precursor for hypervalent iodine chemistry. Due to their high efficiency, low toxicity, and easy handling, hypervalent iodines have been used for a wide range of chemical transformations, such as oxidation,22–24 oxidative addition,25 oxidative cleavage,23,26–30 oxidative rearrangement,31–37 and oxidative cyclization reactions.38,39
Previously, the development of homogeneous catalytic hypervalent iodines has been reported to make the reactions more environmentally friendly and cost-effective. An approach to develop a catalytic variant for hypervalent iodines is to use iodoarenes as a pre-catalyst and a stoichiometric amount of various oxidants to generate an iodine(I)/iodine(III) catalytic cycle. This approach has been used for various reactions such as C–H activation,40,41 oxidative decarboxylation,40 Hoffmann rearrangement,42 and oxidative cyclization.43
Although homogeneous hypervalent iodine chemistry has been developed, the development of a heterogeneous variant has still been limited. Only a few types of materials, such as silica44 and polystyrene44–51 have been used as a framework for heterogeneous hypervalent iodine chemistry. A direct iodination of polystyrene offered simple access to iodinated polystyrene, which has been shown to be an effective heterogeneous pre-catalyst for hypervalent iodine chemistry in many chemical reactions. This iodination method (using the I2/I2O5 iodination system and CCl4 as a solvent under refluxing condition) required harsh reaction conditions with a toxic solvent.49 We envisioned that the development of new iodinated polymers for heterogeneous hypervalent iodine chemistry may be accomplished with milder and greener reaction conditions (Fig. 1). Iodinated hypercrosslinked polymers are chosen because their polymeric framework is expected to have high surface area, high porosity, and high thermal and chemical stability. The framework can be conveniently modified with various functional groups (such as electron-rich or electron-poor substituents), which may allow access to hypervalent iodine with different oxidation potentials. Herein, we report a synthesis of novel iodinated hypercrosslinked polymers using mild iodination conditions and use the resulting iodinated polymers as a recyclable heterogeneous catalyst for alcohol oxidation.
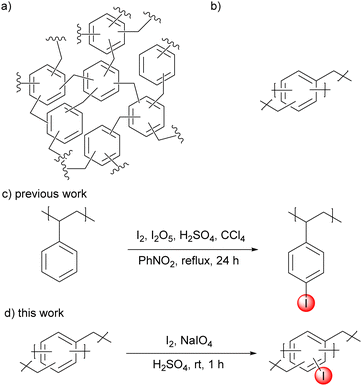 | ||
| Fig. 1 (a) Hypercrosslinked polymer (HCP), (b) a simplified structure of HCPs, (c) previously reported direct iodination of polystyrene and (d) this work. | ||
Results and discussion
A non-functionalized hypercrosslinked polymer (HCP-H) using a benzene monomer with a methylene linkage was chosen to be the model substrate for the iodination. The polymer was synthesized from the Friedel–Crafts reaction between benzene and dimethoxymethane using the reported procedure in the literature.52 A brown solid of HCP-H was obtained in a good yield (quantitative).To investigate the iodination conditions, three different iodination methods were tested with HCP-H to generate HCP-I (Table 1). The iodine content in the iodinated hypercrosslinked polymer was determined by a method based on a modified combustion53 and an indirect atomic absorption spectroscopy measurement.
| Entry | Condition | Yield (%) | Iodine content (mmol g−1) |
|---|---|---|---|
a Reaction conditions: HCP (5 mmol), I2 (130 mmol, 26 equiv.), KIO3 (20 mmol, 4 equiv.) in a mixed solvent (AcOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2SO4/50 H2SO4/50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 550 mL).
b Reaction conditions: HCP (5 mmol), KI (5 mmol, 1 equiv.), H2SO4 (7.5 mmol, 1.5 equiv.), 30% H2O2 (10 mmol, 2 equiv.) in MeOH (25 mL).
c Reaction conditions: HCP (5 mmol), I2 (4.3 mmol, 0.86 equiv.), NaIO4 (1.4 mmol, 0.28 equiv.) in 98% H2SO4 (15 mL). 1, 550 mL).
b Reaction conditions: HCP (5 mmol), KI (5 mmol, 1 equiv.), H2SO4 (7.5 mmol, 1.5 equiv.), 30% H2O2 (10 mmol, 2 equiv.) in MeOH (25 mL).
c Reaction conditions: HCP (5 mmol), I2 (4.3 mmol, 0.86 equiv.), NaIO4 (1.4 mmol, 0.28 equiv.) in 98% H2SO4 (15 mL).
|
|||
| 1a | I2/KIO3/H2SO4/AcOH/H2O, 80 °C, 10 h | 46 | 0.63 |
| 2b | KI/H2O2/H2SO4, MeOH, 60 °C, 24 h | 53 | 0.47 |
| 3c | I2/NaIO4/H2SO4, rt, 1 h | 58 | 0.97 |
A combination of I2/KIO3/H2SO4 gave the desired iodinated product in 46% yield with the iodine content of 0.63 mmol g−1 (entry 1). A system of KI/H2O2/H2SO4 gave the iodinated polymer in 53% yield with the iodine content of 0.47 mmol g−1 (entry 2). An I2/NaIO4/H2SO4 oxidative condition gave the iodinated polymer in the highest yield (58%) and the highest iodine content (0.97 mmol g−1) (entry 3). It should be noted that the iodination using I2/NaIO4/H2SO4 did not require heating, and the reaction time was significantly shorter. This condition was chosen for further optimization.
The equivalent of the iodinating agents was subsequently studied (Table 2). Increasing the amount of the reagent led to a higher iodine content in the polymer; however, increasing the amount beyond 2 equivalents did not significantly change the iodine content. The iodination condition with 2 equivalents of the iodinating agent gave the product in 65% yield and the iodine content of 1.96 mmol g−1. This condition was chosen as the optimal condition for further investigation.
| Entry | I2 (equiv.) | NaIO4 (equiv.) | Yielda (%) | Iodine content (mmol g−1) |
|---|---|---|---|---|
| a The yields were calculated from the stoichiometry based on the molecular weight of the expected structure of the product with an assumption that each aromatic ring was iodinated one time (see ESI). | ||||
| 1 | 0.43 | 0.14 | 58 | 0.97 |
| 2 | 0.54 | 0.17 | 44 | 1.33 |
| 3 | 0.64 | 0.21 | 64 | 1.53 |
| 4 | 0.75 | 0.24 | 67 | 1.57 |
| 5 | 0.86 | 0.28 | 65 | 1.96 |
| 6 | 1.29 | 0.42 | 71 | 2.07 |
With the optimal iodination conditions in hand, HCPs with different substituents were investigated to explore the possibility of using the method for a wider range of materials (Table 3). Alkyl substituents (methyl, ethyl, or isopropyl) did not affect the yields of the iodinated polymers, but the iodine content decreased to 0.90, 1.14, and 1.18 mmol g−1, respectively (entries 2–4). When the substituent was changed to an electron donating methoxy group, the iodine content was 1.05 mmol g−1 (entry 5). These results suggested that substrates with various substituents were well tolerated in this iodination method. The lower iodine content of the substituted polymers may result from the increasing steric demand of the substituents and/or the lower number of iodinating sites on the aromatic rings in the polymers. HCP with an electron withdrawing nitro group gave the iodinated product in 50% yield and the iodine content of 1.11 mmol g−1 (entry 6).54
| Entry | R | Yield (%) | Iodine content (mmol g−1) |
|---|---|---|---|
| a Reaction conditions: HCP (5 mmol), I2 (4.3 mmol, 0.86 equiv.), NaIO4 (1.4 mmol, 0.28 equiv.) in 98% H2SO4 (15 mL). | |||
| 1 | H (2a) | 65 | 1.96 |
| 2 | Me (2b) | 70 | 0.90 |
| 3 | Et (2c) | 67 | 1.14 |
| 4 | iPr (2d) | 70 | 1.18 |
| 5 | OMe (2e) | 58 | 1.05 |
| 6 | NO2 (2f) | 50 | 1.11 |
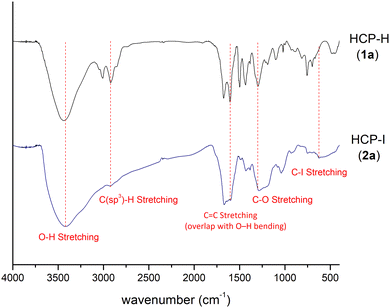
The chemical properties of the iodinated hypercrosslinked polymers were studied using IR spectroscopy (Fig. 2). The IR spectrum of HCP-I (2a) exhibited a new absorption at 624 cm−1, corresponding to the C–I stretching. This result suggested that the iodination condition successfully installed iodine functional groups onto the surface of the hypercrosslinked polymers. In addition, a comparison between the IR spectra of HCP-H (1a) and HCP-I (2a) showed no significant change in the peaks of the polymer framework: C–H stretching of the methylene bridge (2920 cm−1), overlapping of the peaks from C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching of the aromatic ring and O–H bending of water (1676 cm−1), and the C–O stretching of ether or alcohol possibly from the unreacted site of the crosslinker on the polymer (1297 cm−1). For HCPs with different substituents (2b–2f), C–I stretching is also observed (see ESI†).
C stretching of the aromatic ring and O–H bending of water (1676 cm−1), and the C–O stretching of ether or alcohol possibly from the unreacted site of the crosslinker on the polymer (1297 cm−1). For HCPs with different substituents (2b–2f), C–I stretching is also observed (see ESI†).
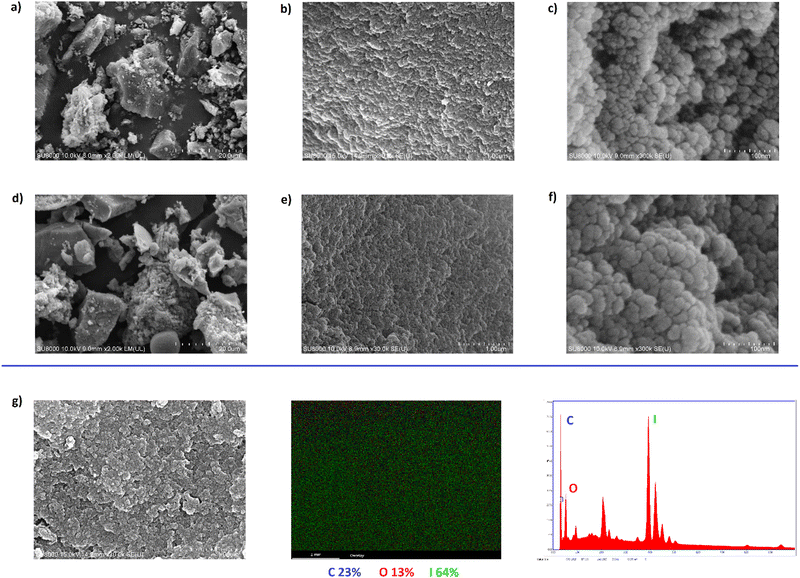
HCP-I (2a) was chosen for further characterization and utilization because it had the highest iodine content. The morphology of the HCPs was further studied using scanning electron microscopy (SEM). The SEM images showed that the morphology of HCP-H (Fig. 3(a)–(c)) and that of HCP-I (Fig. 3(d)–(f)) were similar. This result suggested that the polymer backbones of HCP-H and HCP-I were similar, which agreed well with the data from IR spectroscopy. In addition, SEM–EDX elemental mapping was performed to confirm the presence of iodine atoms on the surface of the iodinated polymers (Fig. 4(f)). The elemental mapping data indicated that carbon, oxygen, and iodine atoms were found on the surface of the polymer and that iodine atoms were distributed over the surface of the polymer.
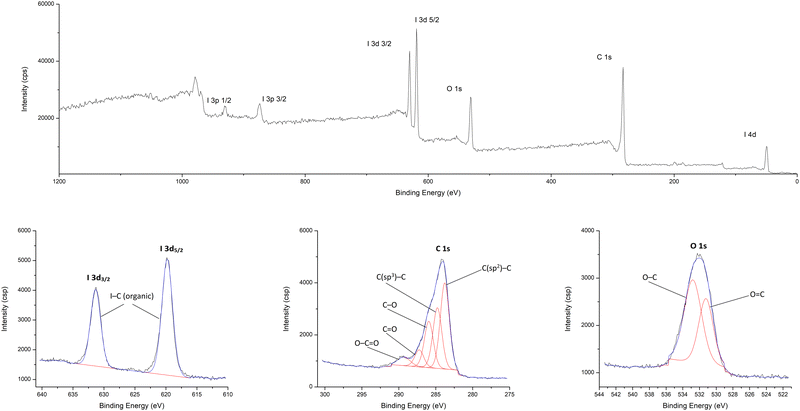
To study the chemical composition on the surface of HCP-I (2a), XPS spectra were measured (Fig. 4). The spectrum showed two major peaks located at binding energies of 619.8 and 631.4 eV, which were characteristic peaks of the I-3d spin–orbit doublet (I-3d5/2 and I-3d3/2, respectively) in an organic molecule.55 These peaks suggested the presence of a C(aromatic)–I bond on the HCP surface.56 The detection of C 1s peaks at 283.8, 284.8, 286.0, 287.3, and 289.3 eV described the chemical states of carbon atoms on the surface, which were corresponding to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, C–C, C–O, C
C, C–C, C–O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, and O–C
O, and O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds, respectively.57,58 The remaining oxygen atoms from the crosslinking reagent were confirmed by the presence of O 1s signals at 531.2 and 532.8 eV, which were corresponding to the organic O
O bonds, respectively.57,58 The remaining oxygen atoms from the crosslinking reagent were confirmed by the presence of O 1s signals at 531.2 and 532.8 eV, which were corresponding to the organic O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and O–C bonds, respectively.59 The presence of the carbonyl group might arise from the polymerization of HCP.60
C and O–C bonds, respectively.59 The presence of the carbonyl group might arise from the polymerization of HCP.60
The thermal stability of the HCP-I was investigated using TGA analysis (see ESI†). The TGA data showed 2 main ranges of weight loss. The range lower than 100 °C could be associated with the release of physically adsorbed water on the surface of the resin. The moisture adsorbed on the polymer was determined to be around 19%. The mass loss in the range of 300–500 °C was possibly due to the decomposition of the iodine functional group on the surface of the polymers and the breakdown of the polymeric backbone. The TGA result suggested that the novel iodinated polymer was thermally stable up to 300 °C.
To study the porosity property of HCP-I, N2 adsorption–desorption analysis at 77 K was performed. The data showed a type-IV isotherm with a significant hysteresis loop, similar to that of HCP-H, indicating that the iodination process did not significantly change or destroy the core structure of the polymers. The pattern of the isotherms revealed the presence of micropores and mesopores in the material.61 The adsorption isotherm (Fig. 5(a)) showed a steep nitrogen gas uptake at low relative pressure (P/P0 < 0.1), indicating the abundance of micropores. The adsorption isotherm also displayed a large rise in adsorption at higher relative pressure, which resulted from the presence of mesopores in the polymer. The HCP-I exhibited a high Brunauer–Emmett–Teller (BET) surface area of about 606 m2 g−1 with a total pore volume of approximately 0.20 cm3 g−1, slightly lower than the surface area of HCP-H of 858 m2 g−1 with a total pore volume of approximately 0.60 cm3 g−1. The lower surface area could be due to the installation of heavy iodine atoms. The pore size distribution, using nonlocal density functional theory (NLDFT) for the pillared clay model, showed the distribution of the pore width from 1.7 to 21 nm, with the most abundant pore width around 1.67 nm (Fig. 5(b)), suggesting the presence of both micropores and mesopores in the material. Therefore, HCP-I was classified as a hierarchically structured porous material, which normally has a high surface area, excellent accessibility to active sites, and enhanced mass transport and diffusion.
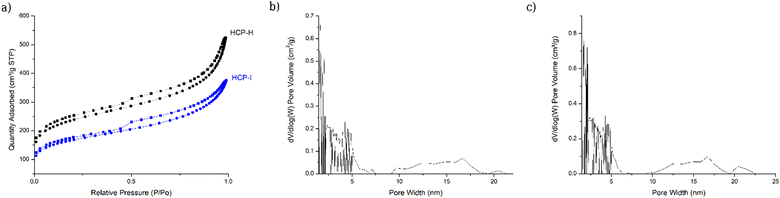 | ||
| Fig. 5 (a) Absorption isotherm of HCP-I and HCP-H, (b) pore size distribution of HCP-I and (c) pore size distribution of HCP-H. | ||
To illustrate the utility of the iodinated HCPs, HCP-I (2a) was used as a heterogeneous catalyst for an oxidation reaction of diphenylmethanol (Table 4). In the presence of the catalyst, the alcohol was effectively converted to the corresponding ketone in 80% yield (entry 3). In the absence of the catalyst, no reaction occurred (entry 1). When HCP-H (1a) was used as a catalyst, no product was obtained (entry 2). These results suggested that the iodine functional group was essential for the oxidation. The iodinated material could act as a precursor for the heterogeneous hypervalent iodine catalyst. A comparison between HCP-I and commercially available iodinated polystyrene (PS-I, entry 4) showed that under these reaction conditions, HCP-I had significantly better catalytic activity than PS-I.
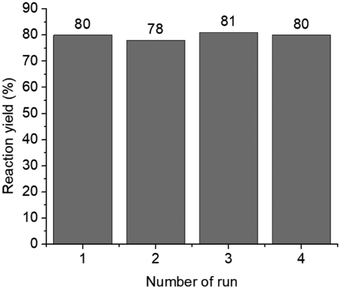
In addition, the reusability of the HCP-I catalyst in an oxidation of diphenylmethanol was investigated. After the HCP-I catalyst was used in the first run of the reaction, the catalyst was recovered by simple filtration, and the recovered catalyst was used in subsequent runs. The result showed that the catalyst can catalyze subsequent runs of the recycling experiment effectively with no erosion in the yields of the product (Fig. 6). This result showed high robustness and high efficiency of the iodinated hypercrosslinked polymers.
Conclusions
In summary, we have reported a synthesis of a novel material with iodine functional groups by functionalization of the surface of hypercrosslinked polymers. The method used mild reaction conditions without destroying the hypercrosslinkage in the polymers. Hypercrosslinked polymers with various substituents were iodinated under this method to give the iodinated polymers in high yields and high iodine incorporation. HCP-I was used as a heterogeneous catalyst for the oxidation of alcohols to give the corresponding ketone in high yields. The catalyst can be recovered by simple filtration and reused with no deterioration in the yield of the product. Further application of the iodinated hypercrosslinked polymers is under investigation and will be reported in due course.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by Mahidol University (Fundamental Fund: fiscal year 2023 by National Science Research and Innovation Fund (NSRF), FF-054/2566). C. T. is grateful for financial support through a scholarship from the Science Achievement Scholarship of Thailand (SAST). We thank Prof. Atitaya Siripinyanond for helpful discussion. We also thank Mr Watcharapon Prasitwatcharakorn and Mr Thitiporn Sangchai for experimental assistance. We are grateful for the support from the Center of Excellence for Innovation in Chemistry (PERCH-CIC, Ministry of Higher Education, Science, Research and Innovation), Thailand; the Department of Chemistry; and the Central Instrumental Facility (CIF), Faculty of Science, Mahidol University for the research facilities.Notes and references
- P. B. Jagadhane, N. C. Jadhav, O. P. Herlekar and V. N. Telvekar, Synth. Commun., 2015, 45, 2130–2134 CrossRef CAS.
- J.-X. Jiang and A. I. Cooper, in Functional Metal-Organic Frameworks: Gas Storage, Separation and Catalysis, ed. M. Schröder, Springer Berlin Heidelberg, Berlin, Heidelberg, 2010, pp.1–33 DOI:10.1007/128_2009_5.
- H. J. Mackintosh, P. M. Budd and N. B. McKeown, J. Mater. Chem., 2008, 18, 573–578 RSC.
- P. Pandey, O. K. Farha, A. M. Spokoyny, C. A. Mirkin, M. G. Kanatzidis, J. T. Hupp and S. T. Nguyen, J. Mater. Chem., 2011, 21, 1700–1703 RSC.
- T. Ratvijitvech, Polymers, 2022, 14, 2749 CrossRef CAS PubMed.
- S. Ren, M. J. Bojdys, R. Dawson, A. Laybourn, Y. Z. Khimyak, D. J. Adams and A. I. Cooper, Adv. Mater., 2012, 24, 2357–2361 CrossRef CAS PubMed.
- A. Thomas, P. Kuhn, J. Weber, M.-M. Titirici and M. Antonietti, Macromol. Rapid Commun., 2009, 30, 221–236 CrossRef CAS PubMed.
- T. Ben, H. Ren, S. Ma, D. Cao, J. Lan, X. Jing, W. Wang, J. Xu, F. Deng, J. M. Simmons, S. Qiu and G. Zhu, Angew. Chem., Int. Ed., 2009, 48, 9457–9460 CrossRef CAS PubMed.
- J. Weber and A. Thomas, J. Am. Chem. Soc., 2008, 130, 6334–6335 CrossRef CAS PubMed.
- N. A. Rakow, M. S. Wendland, J. E. Trend, R. J. Poirier, D. M. Paolucci, S. P. Maki, C. S. Lyons and M. J. Swierczek, Langmuir, 2010, 26, 3767–3770 CrossRef CAS PubMed.
- T. Ratvijitvech, J. Polym. Environ., 2020, 28, 2211–2218 CrossRef CAS.
- T. Ratvijitvech and S. Na Pombejra, Mater. Today Commun., 2021, 28, 102617 CrossRef CAS.
- O. K. Farha, A. M. Spokoyny, B. G. Hauser, Y.-S. Bae, S. E. Brown, R. Q. Snurr, C. A. Mirkin and J. T. Hupp, Chem. Mater., 2009, 21, 3033–3035 CrossRef CAS.
- S. K. Kundu and A. Bhaumik, ACS Sustainable Chem. Eng., 2015, 3, 1715–1723 CrossRef CAS.
- K.-Y. Yan, J.-Y. Chen, X.-Y. Li, Q. Wang and G.-C. Kuang, Macromol. Chem. Phys., 2020, 221, 1900553 CrossRef CAS.
- V. M. Suresh, S. Bonakala, H. S. Atreya, S. Balasubramanian and T. K. Maji, ACS Appl. Mater. Interfaces, 2014, 6, 4630–4637 CrossRef CAS PubMed.
- N. B. McKeown and P. M. Budd, Chem. Soc. Rev., 2006, 35, 675–683 RSC.
- A. Li, Q. Zhang, G. Zhang, J. Chen, Z. Fei and F. Liu, Chemosphere, 2002, 47, 981–989 CrossRef CAS PubMed.
- K. Wang, W. Cui, Z. Bian, Y. Liu, S. Jiang, Y. Zhou and J. Wang, Appl. Catal., B, 2021, 281, 119425 CrossRef CAS.
- D. Jia, L. Ma, Y. Wang, W. Zhang, J. Li, Y. Zhou and J. Wang, Chem. Eng. J., 2020, 390, 124652 CrossRef CAS.
- W. Zhang, F. Ma, L. Ma, Y. Zhou and J. Wang, ChemSusChem, 2020, 13, 341–350 CrossRef CAS PubMed.
- T. Dohi, N. Takenaga, A. Goto, A. Maruyama and Y. Kita, Org. Lett., 2007, 9, 3129–3132 CrossRef CAS PubMed.
- W. Zhong, J. Yang, X. Meng and Z. Li, J. Org. Chem., 2011, 76, 9997–10004 CrossRef CAS PubMed.
- T. Wirth, Angew. Chem., Int. Ed., 2005, 44, 3656–3665 CrossRef CAS PubMed.
- S. Schäfer and T. Wirth, Angew. Chem., Int. Ed., 2010, 49, 2786–2789 CrossRef PubMed.
- M. Çelik, C. Alp, B. Coşkun, M. S. Gültekin and M. Balci, Tetrahedron Lett., 2006, 47, 3659–3663 CrossRef.
- Y.-B. Kang and L. H. Gade, J. Am. Chem. Soc., 2011, 133, 3658–3667 CrossRef CAS PubMed.
- M. Fujita, M. Wakita and T. Sugimura, Chem. Commun., 2011, 47, 3983–3985 RSC.
- M. Fujita, H. Suzawa, T. Sugimura and T. Okuyama, Tetrahedron Lett., 2008, 49, 3326–3329 CrossRef CAS.
- M. Fujita, Y. Ookubo and T. Sugimura, Tetrahedron Lett., 2009, 50, 1298–1300 CrossRef CAS.
- M. Kirihara, S. Yokoyama, H. Kakuda and T. Momose, Tetrahedron, 1998, 54, 13943–13954 CrossRef CAS.
- M. Kirihara, T. Nishio, S. Yokoyama, H. Kakuda and T. Momose, Tetrahedron, 1999, 55, 2911–2926 CrossRef CAS.
- X. Li, Z. Xu, E. F. DiMauro and M. C. Kozlowski, Tetrahedron Lett., 2002, 43, 3747–3750 CrossRef CAS.
- L.-H. Zhang, J. C. Chung, T. D. Costello, I. Valvis, P. Ma, S. Kauffman and R. Ward, J. Org. Chem., 1997, 62, 2466–2470 CrossRef CAS PubMed.
- N. Okamoto, Y. Miwa, H. Minami, K. Takeda and R. Yanada, Angew. Chem., Int. Ed., 2009, 48, 9693–9696 CrossRef CAS PubMed.
- H. R. Khatri and J. Zhu, Chem. – Eur. J., 2012, 18, 12232–12236 CrossRef CAS PubMed.
- Z. Jia, E. Gálvez, R. M. Sebastián, R. Pleixats, Á. Álvarez-Larena, E. Martin, A. Vallribera and A. Shafir, Angew. Chem., Int. Ed., 2014, 53, 11298–11301 CrossRef CAS PubMed.
- J. Y. Cha, Y. Huang and T. R. R. Pettus, Angew. Chem., Int. Ed., 2009, 48, 9519–9521 CrossRef CAS PubMed.
- N. A. Braun, M. A. Ciufolini, K. Peters and E.-M. Peter, Tetrahedron Lett., 1998, 39, 4667–4670 CrossRef CAS.
- S. K. Alla, R. K. Kumar, P. Sadhu and T. Punniyamurthy, Org. Lett., 2013, 15, 1334–1337 CrossRef CAS PubMed.
- D. Liang, W. Yu, N. Nguyen, J. R. Deschamps, G. H. Imler, Y. Li, A. D. MacKerell, C. Jiang and F. Xue, J. Org. Chem., 2017, 82, 3589–3596 CrossRef CAS PubMed.
- M. Ngatimin, R. Frey, A. Levens, Y. Nakano, M. Kowalczyk, K. Konstas, O. E. Hutt and D. W. Lupton, Org. Lett., 2013, 15, 5858–5861 CrossRef CAS PubMed.
- K. Miyamoto, Y. Sakai, S. Goda and M. Ochiai, Chem. Commun., 2012, 48, 982–984 RSC.
- J.-M. Chen, X.-M. Zeng and V. V. Zhdankin, Synlett, 2010, 2771–2774 CrossRef CAS.
- H. Togo and K. Sakuratani, Synlett, 2002, 1966–1975 CrossRef CAS.
- J.-M. Chen, X.-M. Zeng, K. Middleton, M. S. Yusubov and V. V. Zhdankin, Synlett, 2011, 1613–1617 CrossRef CAS.
- S. V. Ley, A. W. Thomas and H. Finch, J. Chem. Soc., Perkin Trans. 1, 1999, 669–672, 10.1039/A809799B.
- S. Abe, K. Sakuratani and H. Togo, J. Org. Chem., 2001, 66, 6174–6177 CrossRef CAS PubMed.
- T. Dohi, M. Ito, K. Morimoto, Y. Minamitsuji, N. Takenaga and Y. Kita, Chem. Commun., 2007, 4152–4154, 10.1039/B708802G.
- J.-M. Chen, X.-M. Zeng, K. Middleton and V. V. Zhdankin, Tetrahedron Lett., 2011, 52, 1952–1955 CrossRef CAS.
- H. Tohma, H. Morioka, Y. Harayama, M. Hashizume and Y. Kita, Tetrahedron Lett., 2001, 42, 6899–6902 CrossRef CAS.
- R. Dawson, T. Ratvijitvech, M. Corker, A. Laybourn, Y. Z. Khimyak, A. I. Cooper and D. J. Adams, Polym. Chem., 2012, 3, 2034–2038 RSC.
- D. R. Burfield and S.-C. Ng, J. Chem. Educ., 1984, 61, 917 CrossRef CAS.
- HCP-NO2 (1f) was synthesized from HCP-H (1a) using nitration reaction (see ESI†).
- J. Raymakers, H. Krysova, A. Artemenko, J. Čermák, S. S. Nicley, P. Verstappen, S. Gielen, A. Kromka, K. Haenen, L. Kavan, W. Maes and B. Rezek, RSC Adv., 2018, 8, 33276–33290 RSC.
- H. F. Wang, X. Y. Deng, J. Wang, X. F. Gao, G. M. Xing, Z. J. Shi, Z. N. Gu, Y. F. Liu and Y. Zhao, Acta Phys. Chim. Sin., 2004, 20, 673 CAS.
- N. Dwivedi, R. J. Yeo, N. Satyanarayana, S. Kundu, S. Tripathy and C. S. Bhatia, Sci. Rep., 2015, 5, 7772 CrossRef CAS PubMed.
- R. E. Priestley, A. Mansfield, J. Bye, K. Deplanche, A. B. Jorge, D. Brett, L. E. Macaskie and S. Sharma, RSC Adv., 2015, 5, 84093–84103 RSC.
- A. V. Shchukarev and D. V. Korolkov, Cent. Eur. J. Chem., 2004, 2, 347–362 CAS.
- C. Wilson, M. J. Main, N. J. Cooper, M. E. Briggs, A. I. Cooper and D. J. Adams, Polym. Chem., 2017, 8, 1914–1922 RSC.
- R. Gomes and A. Bhaumik, J. Solid State Chem., 2015, 222, 7–11 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ma00316g |
| This journal is © The Royal Society of Chemistry 2023 |