Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Fabrication of nanoparticle assemblies with a controlled number of constituent nanoparticles by membrane emulsification using anodic porous alumina
Takashi
Yanagishita
 *a,
Kantaro
Yuda
a,
Toshiaki
Kondo
b and
Hideki
Masuda
a
*a,
Kantaro
Yuda
a,
Toshiaki
Kondo
b and
Hideki
Masuda
a
aDepartment of Applied Chemistry, Tokyo Metropolitan University, 1-1 Minamiosawa, Hachioji, Tokyo 192-0397, Japan
bDepartment of Mechanical System Engineering, Aichi University of Technology, 50-2 Manori, Nishihasama-cho, Gamagori, Aichi 443-0047, Japan. E-mail: yanagish@tmu.ac.jp
First published on 30th August 2023
Abstract
Nanoparticle assemblies with a controlled number of constituent nanoparticles were fabricated by membrane emulsification using an aqueous solution containing silica or Au nanoparticles as dispersed phases. For membrane emulsification, ideally ordered anodic porous alumina with extremely uniform-sized pores was used as an emulsification membrane to control the droplet size precisely. The nanoparticle assemblies were formed when droplets containing nanoparticles obtained by membrane emulsification dried up through solubilization in the continuous phase. In this process, the number of constituent nanoparticles in the assemblies could be controlled by changing the concentration of nanoparticles in the dispersed phase and the pore size of anodic porous alumina. Optimizing the concentration of nanoparticles in the dispersed phase and the pore size of anodic porous alumina also confirmed the preferential formation of dimers and trimers of nanoparticles. The Au nanoparticle assemblies obtained by this process could be applied to substrates for enhanced Raman scattering because of the enhanced photoelectric field formed between Au particles.
Introduction
Fine particle assemblies, which are aggregates of submicrometer- to nanometer-scale particles, are expected to be applied to various functional devices, including photonic crystals and sensors, owing to their unique geometrical structures.1–14 In particular, Au nanoparticle assemblies have potential applications in optical devices because their optical properties depend on the number of particles.15–17 For example, Au nanoparticle assemblies can be used as substrates for surface-enhanced Raman scattering measurement owing to the extremely strong enhancement of the incident electric field formed in small gaps between Au nanoparticles.18,19 If the formation of Au nanoparticle assemblies with a controlled number of nanoparticles becomes possible, it can be applied to various functional devices because the optical properties can be controlled by changing the number of Au nanoparticles. Several methods have been reported on the preparation of particle assemblies with a controlled number of particles, including the template process, microfluidic methods, and separation methods by centrifugation or chromatography.20–23 For example, it was reported that particle assemblies could be formed by trapping particles in depressions formed on a substrate surface that serves as a template. In this process, the number of particles constituting an assembly can be controlled by changing the depression size formed on template substrates. However, this method is time-consuming because it requires the use of capillary force of the solution to trap the particles in the depressions on the substrate, making it difficult to fabricate assemblies efficiently. A method to fabricate particle assemblies using microfluidic channels has also been reported. However, fabricating multiple arrays of microfluidic channels is difficult and problematic in terms of fabrication efficiency. On the other hand, as an efficient method to produce particle assemblies, it has been reported that particle assemblies with a random number of constituent particles are produced in advance, and then the assemblies with a uniform number of constituent particles are separated and purified using techniques such as centrifugal separation. However, this method has the problem that the number of particles in the resulting assemblies cannot be accurately controlled because of the difficulty of separation. A method that enables the efficient fabrication of assemblies with a controlled number of particles has not been established.A possible method of producing assemblies of a controlled number of particles is to use droplets of a suspension with a definite concentration of particles. Through the formation of a droplet with a diameter of several tens of micrometers containing fine particles using a single syringe, particle assemblies can be formed by drying.5 In this process, the uniformity of droplet sizes is essential for the control of the number of particles within the droplet. To form nanometer-scale assemblies, the droplet size should be reduced. The relationship between droplet size and particle assembly size is important because if the droplet size is too large compared to the size of the particle assembly to be fabricated, multiple assemblies may be formed from a single droplet. Therefore, we believe it is important to reduce the droplet size when fabricating fine assemblies using nanoparticles. In addition, the use of multiple nozzles is desirable for the efficient preparation of the assemblies. We have been investigating membrane emulsification using ordered anodic porous alumina for the preparation of monodisperse droplets with controlled size on the nanometer scale.24–26 Membrane emulsification is a method for droplet formation in which a dispersed phase is extruded into a continuous phase through the pores of an emulsification membrane.27 Since the droplet size obtained by membrane emulsification depends on the pore size of the emulsification membrane, monodisperse emulsion droplets can be prepared by membrane emulsification using a membrane with uniform-sized pores. Anodic porous alumina is a nanohole array material obtained by the anodization of Al in an acidic or basic electrolyte. Ordered nanohole arrays composed of uniform-sized cylindrical pores can be obtained by anodization under appropriate anodizing conditions.28,29 In addition, the use of a depression pattern at the Al surface by texturing using a mold can produce anodic porous alumina with an ideally arranged array of cylindrical pores because each depression serves as a starting point for pore generation.30,31 The pore size uniformity of anodic porous alumina increases with the regularity of pore arrangement, so the pore size uniformity of ideally ordered anodic porous alumina is significantly high.32 Owing to its high pore size uniformity, ideally ordered anodic porous alumina is a suitable material for emulsification membranes to form droplets with uniform sizes. In our previous works, we showed that membrane emulsification using ideally ordered anodic porous alumina can generate monodisperse emulsion droplets with desired sizes depending on the pore size of anodic porous alumina.24,25,32
In this study, we investigated the preparation of nanoparticle assemblies with a controlled number of nanoparticles by membrane emulsification using ideally ordered anodic porous alumina. Here, we first studied membrane emulsification using an aqueous suspension of silica particles as a dispersed phase as a model system for the fabrication of nanoparticle assemblies with a controlled number of nanoparticles. The method reported in this paper is promising as an efficient technique for producing particle assemblies because the droplets that serve as templates for assembly formation are formed continuously through a dense array of pores in an anodic porous alumina membrane. In addition, the number of constituent particles can also be controlled by changing the droplet size or the concentration of particles in the dispersed phase. The present process is superior to conventional methods because it combines both control of the number of constituent particles and efficient production of assemblies. We also attempted to control the number of nanoparticles at the assemblies by changing the droplet size and concentration in the dispersed phase. Next, we investigated the fabrication of Au nanoparticle assemblies with a controlled number of nanoparticles by using an aqueous suspension of Au nanoparticles as a dispersed phase for membrane emulsification. The application of the obtained Au nanoparticle assemblies to the substrate for the measurement of surface-enhanced Raman scattering was also investigated. The nanoparticle assemblies with a controlled number of nanoparticles obtained by the present process will be applied to various functional devices, such as sensors, and catalysts, in addition to substrates for the measurement of surface-enhanced Raman scattering.
Experimental
Fig. 1 shows a schematic of the process of preparing nanoparticle assemblies by membrane emulsification using ideally ordered anodic porous alumina. Al sheets (99.99% purity) annealed at 450 °C for 1 h were used to prepare ideally ordered anodic porous alumina. Al sheets were electrochemically polished in a mixture of 80 vol% ethanol and 20 vol% perchloric acid at 0 °C for 4 min under a constant current of 0.1 A cm−2. An ordered depression pattern with a period of 500 nm was formed at the surface of the Al sheet by texturing using a Ni mold with an ideally hexagonal pattern of protrusions with a period of 500 nm. The Al sheet with the depression pattern was anodized in 0.1 M phosphoric acid at 0 °C under a constant voltage of 200 V for 90 min using a multirange DC power supply (PSW-720H800Y1H, Texio Technology Co., Japan). After anodization, the residual Al was dissolved selectively using a saturated iodine methanol solution, and the bottom of the alumina layer, which is the barrier layer, was removed by Ar ion milling. The pore size of the resulting alumina through-hole membrane was adjusted by etching with 10 wt% phosphoric acid at 30 °C. Prior to membrane emulsification, the surface of the alumina membrane was hydrophobically treated with octadecyltrichlorosilane.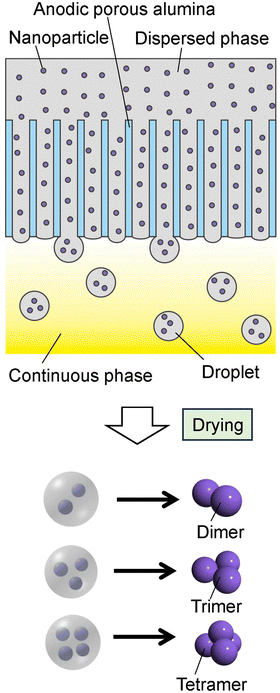 | ||
| Fig. 1 Schematic of the preparation process for the nanoparticle assemblies by membrane emulsification using anodic porous alumina. | ||
For the fabrication of silica nanoparticle assemblies, a commercially available suspension of silica nanoparticles with a diameter of 80 nm (Cataloid SI-80P, JGC Catalysts and Chemical Ltd, Japan; 40 wt% SiO2, 0.5 wt% NaO2, 59.5% H2O) was used as a dispersed phase for membrane emulsification. A kerosene-containing surfactant (2 wt% CR-310 and 1 wt% span 80) was used as a continuous phase. Emulsion droplets were formed by extruding the dispersed phase through the pores of the alumina membrane into the continuous phase by pressurization with N2 gas. The resulting emulsion was heated at 70 °C for 1 h to solubilize the aqueous phase in the droplets into the continuous phase, thereby agglomerating the silica nanoparticles in the droplets. In this study, we attempted to control the number of silica nanoparticles in the assemblies by controlling the concentration of silica nanoparticles in the dispersed phase and the pore size of the alumina membrane.
Au nanoparticle assemblies were prepared using a commercially available aqueous solution as a dispersed phase, in which Au nanoparticles had an average diameter of 50 nm (Immunochromato gold colloids WRGH1, Wineredchemical Co., Japan; 1 wt% Au). As with the silica nanoparticle assemblies, the Au nanoparticle assemblies were prepared by forming emulsion droplets in the kerosene-containing surfactant and heating the resulting emulsion.
The nanoparticle assemblies formed in kerosene solution by heat treatment were trapped on a filter (Anodisc with 0.1 μm pores, Whatman International Ltd), washed with hexane, and observed by scanning electron microscopy (SEM; JSM-7500F, JEOL). The intensity of the photoelectric field of the Au nanoparticle assemblies was simulated using the finite-difference time domain (FDTD) method. A Raman microscope (NRS-2000, JASCO, Japan) equipped with a He–Ne laser (λ = 633 nm) was used for the Raman measurement of pyridine with Au nanoparticle assemblies as substrates. The laser spot size and laser power were 5 μm and 0.9 mW.
Results and discussion
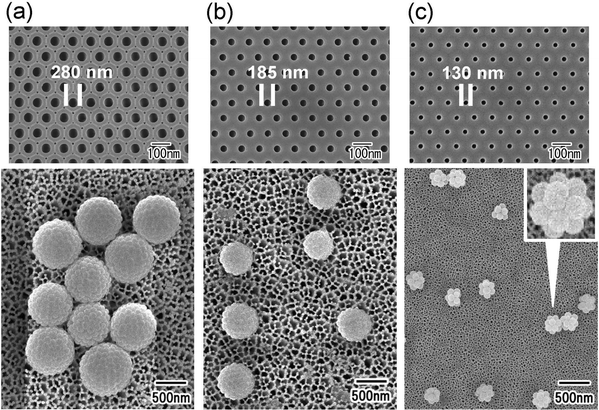
Fig. 2(a) shows an SEM image of the silica particles used to form the nanoparticle assemblies. The silica nanoparticles dispersed in the aqueous solution used as the dispersed phase were filtered and observed. From the SEM image, it was confirmed that each particle did not form aggregates and was dispersed as an isolated particle in the dispersed phase. Fig. 2(b) and (c) show the low- and high-magnification SEM images of silica nanoparticle assemblies fabricated by membrane emulsification using ideally ordered anodic porous alumina with a pore size of 280 nm. For membrane emulsification, 40 wt% silica nanoparticle solution was used as a dispersed phase. The thickness of the anodic porous alumina membrane used for the emulsification was 20 μm. In the emulsification using this membrane, the formation of emulsion droplets was carried out at 150 kPa. This pressure was the critical pressure at which droplets started to form from the 280 mn diameter pored of the alumina membrane. The SEM images show the formation of uniform-sized spherical silica nanoparticle assemblies. As observed from the SEM image, the nanoparticles were regularly arranged on the surface of the resulting assemblies. This is believed to be due to the formation of a close-packed structure of silica nanoparticles as water solubilizes from the droplet formed by membrane emulsification into the continuous phase, and the droplet size decreases. The assembled structures of silica nanoparticles could be retained even after trapping on the filter and washing with hexane. From the SEM images, the average diameter of the resulting silica nanoparticle assemblies was measured to be 1.3 μm. Assuming that the silica nanoparticles are packed without gaps inside the assembly, the number of nanoparticles in the aggregate was estimated to be about 4000. The droplet size formed by the membrane emulsification is larger than the pore size of the emulsification membrane.33 Optical microscopy confirmed that the size of the water droplets formed from pores with a diameter of 280 nm was approximately 1.8 μm. Therefore, the result that the size of the nanoparticle assemblies obtained by drying the droplets is 1.3 μm is considered reasonable. In our previous study, we found that in the membrane emulsification using anodic porous alumina, the droplet size obtained does not change even when the pressure is increased by about 100 kPa above the critical pressure at which the dispersed phase begins to be extruded from the pores.33 This indicates that the droplet size obtained in the membrane emulsification depends on the pore size of the emulsification membrane, not on the pressure. In this study, the formation of emulsion droplets by membrane emulsification was carried out at critical pressure. If the extrusion pressure was not excessively high, the number of nanoparticles in the resulting assemblies did not change with varying pressure.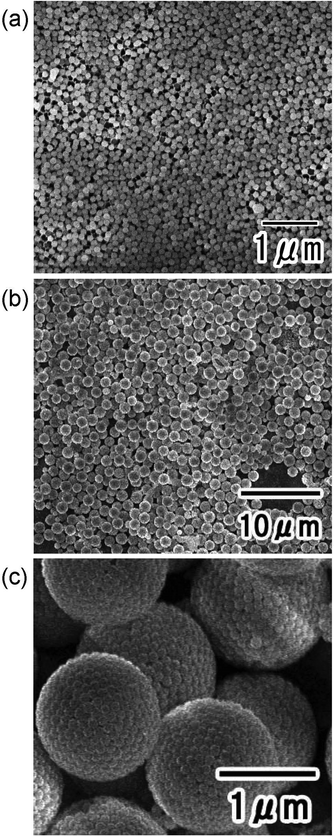 | ||
| Fig. 2 (a) SEM image of silica nanoparticles used to form assemblies. SEM images of silica nanoparticle assemblies: (b) low and (c) high magnifications. | ||
Fig. 3 shows the results of the preparation of nanoparticle assemblies using silica nanoparticle dispersions with silica concentrations of (a) 1.3, (b) 4, and (c) 20 wt%. The concentration of the silica nanoparticle suspension was adjusted by dilution with distilled water. In this experiment, ideally ordered anodic porous alumina with a pore size of 280 nm was used as an emulsification membrane. The SEM images show that spherical nanoparticle assemblies are formed regardless of the silica nanoparticle concentration of the dispersed phase. The size of the obtained nanoparticle assemblies was observed to decrease as the silica nanoparticle concentration of the dispersed phase decreased. The average diameters of the nanoparticle assemblies obtained at silica nanoparticle concentrations of 1.3, 4, and 20 wt% in a dispersed phase were 450, 760, and 1050 nm, respectively. The number of nanoparticles was estimated from the diameters of the nanoparticle assemblies to be 30, 630, and 1670. In our previous studies, it has been confirmed that the size of emulsion droplets obtained by membrane emulsification using anodic porous alumina depends on the pore size of the anodic porous alumina.24–26 Therefore, the size of an emulsion droplet containing silica nanoparticles is expected to be the same even when the concentration of silica nanoparticles in the dispersed phase was different because the pore size of anodic porous alumina used as an emulsification membrane was the same. This indicates that the number of silica nanoparticles in the droplets formed by membrane emulsification can be controlled by adjusting the concentration of nanoparticles in the dispersed phase, thus making it possible to control the number of nanoparticles constituting the resulting nanoparticle assembly.
 | ||
| Fig. 3 SEM images of silica nanoparticle assemblies fabricated using dispersed phases with silica nanoparticle concentrations of (a) 1.3, (b) 4, and (c) 20 wt%. | ||
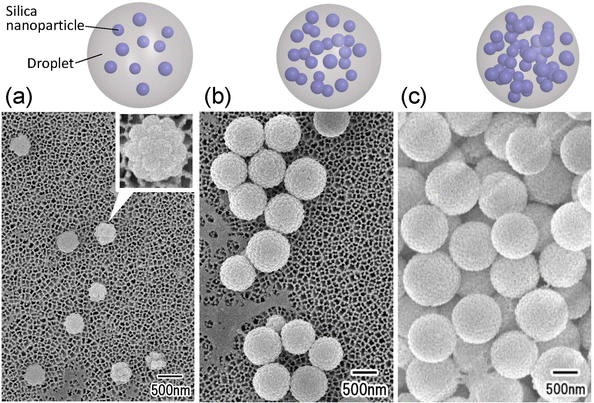
Fig. 4 shows the results of the formation of silica nanoparticle assemblies by membrane emulsification using ideally ordered anodic porous alumina with pore sizes of (a) 280, (b) 185, and (c) 130 nm. For membrane emulsification, a silica nanoparticle suspension with a silica concentration of 5 wt% was used. The SEM image in Fig. 5 shows that spherical nanoparticle assemblies are obtained even when the pore size of anodic porous alumina is varied. The diameter of the resulting nanoparticle assemblies decreases as the pore size of the anodic porous alumina decreases. The diameters of the nanoparticle assemblies were 800, 520, and 250 nm, and the number of nanoparticles constituting the assemblies was estimated to be 740, 200, and 15, respectively. In membrane emulsification, the droplet size obtained depends on the pore size of the emulsification membrane. This means that the number of nanoparticles in an assembly can be controlled by changing the size of emulsion droplets.
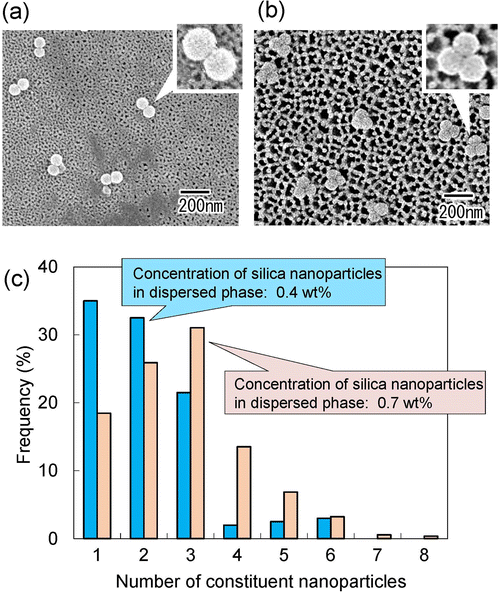
As shown in Fig. 3 and 4, it was found that the number of nanoparticles in an assembly can be controlled by changing the concentration of silica nanoparticles in the dispersed phase or the droplet size. On the basis of these results, we attempted to fabricate dimers and trimers of silica nanoparticles by adjusting the concentration of silica nanoparticles in the dispersed phase and the pore size of the anodic porous alumina. Fig. 5(a) and (b) show the SEM images of silica nanoparticle assemblies obtained by membrane emulsification using ideally ordered anodic porous alumina with a pore size of 130 nm and silica nanoparticle concentrations of (a) 0.4 and (b) 0.7 wt% in the dispersed phase. The resulting silica nanoparticle assemblies were trapped on the filters; (a) Anodisc with 0.02 μm pores (Whatman Ltd) and (b) Anodisc with 0.1 μm pores (Whatman Ltd). The SEM images show that dimers and trimers of silica nanoparticles are formed, although not completely. Fig. 5(c) shows a histogram of the number of nanoparticles in the nanoparticle assemblies obtained at each silica concentration. When the silica nanoparticle concentration was 0.4 wt%, the frequency of dimer formation was higher than that of trimer formation. When the solution with a silica concentration of 0.7 wt% was used for membrane emulsification, the frequency of trimer formation was higher than that of formation dimer. These results indicate that the frequencies of dimer and trimer formations can be controlled by changing the concentration of silica nanoparticles in the dispersed phase and the pore size of anodic porous alumina.
One application of nanoparticle assemblies with a controlled number of nanoparticles is the control of the photoelectric field on the surface of the nanoparticles. Fig. 6 shows the images of the photoelectric field enhancement obtained by simulation using the FDTD method when plasmon excitation is applied to (a) a dimer and (b) a trimer of Au nanoparticles. From Fig. 6, it was observed that very enhanced photoelectric fields are formed between the Au nanoparticles. The intensity of the photoelectric field was estimated to be enhanced 5-fold in the dimer and 40-fold in the trimer. This result suggests that controlling the number of Au nanoparticles in an Au nanoparticle assembly can improve its photoelectric properties.
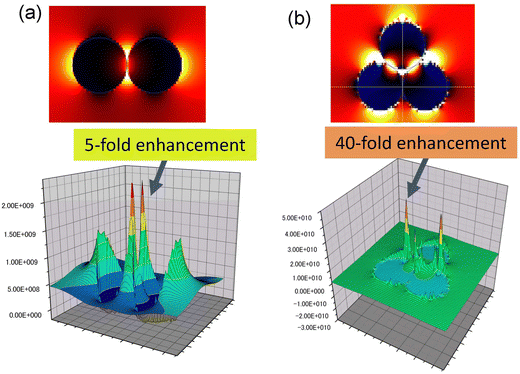 | ||
| Fig. 6 Images of the photoelectric field enhancement obtained by simulation using the FDTD method when plasmon excitation is applied to (a) dimer and (b) trimer of Au nanoparticles. | ||
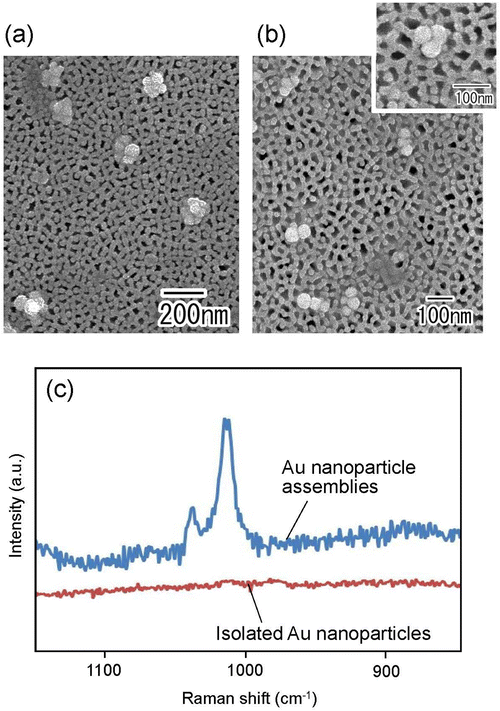
Fig. 7(a) and (b) show the SEM images of Au nanoparticle assemblies fabricated by membrane emulsification using Au nanoparticle dispersion solution as a dispersed phase. For membrane emulsification, (a) 1 wt% and (b) 0.2 wt% Au nanoparticle dispersion solutions diluted with distilled water were used. Ideally ordered anodic porous alumina with a pore size of 130 nm was used as an emulsification membrane. The SEM images show the formation of Au nanoparticle assemblies in both samples. In addition, the number of Au nanoparticles constituting an assembly was observed to be smaller when the concentration of Au nanoparticles in the dispersed phase was lower. The frequency of trimer formation was observed to increase when the concentration of Au nanoparticles in the dispersed phase was 0.2 wt%. Fig. 7(c) shows the Raman spectra of pyridine measured using isolated Au nanoparticles and Au nanoparticle assemblies as substrates. The Au nanoparticle assemblies prepared under the conditions shown in Fig. 7(b) were used in this experiment. Raman spectra were measured after the dropped pyridine and dried up on filter substrates with trapped isolated Au nanoparticles or Au nanoparticle assemblies. From the measured Raman spectra, no peaks attributable to pyridine were observed for isolated Au nanoparticles. However, the distinct peaks originating from pyridine were observed when Au nanoparticle assemblies were used as substrates. This is due to the effect of the light-enhanced photoelectric field of the Au nanoparticle assemblies, which enhanced Raman scattering.
Conclusions
Silica nanoparticle assemblies were formed by membrane emulsification using ideally ordered anodic porous alumina and the subsequent drying of obtained droplets in the continuous phase. It was shown that it is possible to control the number of silica nanoparticles in the resulting assemblies by changing the concentration of silica nanoparticles in the disperses phase and the pore size of ideally ordered anodic porous alumina. This process was also effective in fabricating Au nanoparticle assemblies with a controlled number of Au nanoparticles by using an aqueous solution containing suspended Au nanoparticles as the dispersed phase. The Raman spectra of pyridine were measured using the obtained Au nanoparticle assemblies, and it was observed that the Raman scattering of pyridine was enhanced owing to the effect of the enhanced photoelectric field associated with the formation of the Au nanoparticle assemblies. This process is expected to make it possible to produce nanoparticle assemblies with a controlled number of nanoparticles of various materials, including metals, semiconductors, and polymers, by using a solution containing a suspension of various nanoparticles as the dispersed phase in membrane emulsification. The nanoparticle assemblies with a controlled number of nanoparticles obtained by this process can be applied as key materials for fabricating various functional nanodevices.Author contributions
T. Y.: conceptualization, methodology, investigation, writing – original draft;. K. Y.: investigation; T. K.: simulation using FDTD method, H. M.: writing – review & editing. All authors read and approved the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The present work was supported by Izumi science and technology foundation.References
- Y. Xia, Y. Yin, Y. Lu and J. McLellan, Adv. Funct. Mater., 2003, 13, 907 CrossRef CAS.
- J. H. Moon, G.-R. Yi, S.-M. Yang, D. J. Pine and S. B. Park, Adv. Mater., 2004, 16, 605 CrossRef CAS.
- Y. Masuda, T. Itoh and K. Koumoto, Adv. Mater., 2005, 17, 841 CrossRef CAS.
- H. Li, H. Wang, A. Chen, B. Meng and X. Li, J. Mater. Chem., 2005, 15, 2551 RSC.
- S.-H. Hong, J. H. Moon, J.-M. Lim, S.-H. Kim and S.-M. Yang, Langmuir, 2005, 21, 10416 CrossRef CAS PubMed.
- Z. Nie, W. Li, M. Seo, S. Xu and E. Kumacheva, J. Am. Chem. Soc., 2006, 128, 9408 CrossRef CAS PubMed.
- S.-H. Kim, J.-M. Lim, W. C. Jeong, D.-G. Choi and S.-M. Yang, Adv. Mater., 2008, 20, 3211 CrossRef CAS.
- J. Yan, M. Bloom, S. C. Bae, E. Luijten and S. Granick, Nature, 2012, 491, 578 CrossRef CAS PubMed.
- J. He, X. Huang, Y.-C. Li, Y. Liu, T. Babu, M. A. Aronova, S. Wang, Z. Lu, X. Chen and Z. Nie, J. Am. Chem. Soc., 2013, 135, 7974 CrossRef CAS PubMed.
- J. Wang and J. Zhu, Eur. Polym. J., 2013, 49, 3420 CrossRef CAS.
- Y. Zhao, L. Shang, Y. Cheng and Z. Gu, Acc. Chem. Res., 2014, 47, 3632 CrossRef CAS PubMed.
- N. Vogel, M. Retsch, C.-A. Fustin, A. del Campo and U. Jonas, Chem. Rev., 2015, 115, 6265 CrossRef CAS PubMed.
- Y. Shelke, M. Sabapathy and E. Mani, Langmuir, 2017, 33, 6760 CrossRef CAS PubMed.
- H. Ding, C. Zhu, L. Tian, C. Liu, G. Fu, L. Shang and Z. Gu, ACS Appl. Mater. Interfaces, 2017, 9, 11933 CrossRef CAS PubMed.
- R. Sardar, T. B. Heap and J. S. Shumaker-Parry, J. Am. Chem. Soc., 2007, 129, 5356 CrossRef CAS PubMed.
- M. Yang, G. Chen, Y. Zhao, G. Silber, Y. Wang, S. Xing, Y. Han and H. Chen, Phys. Chem. Chem. Phys., 2010, 12, 11850 RSC.
- A. J. Pasquale, B. M. Reinhard and L. D. Negro, ACS Nano, 2011, 5, 6578 CrossRef CAS PubMed.
- K. L. Wustholz, A.-I. Henry, J. M. McMahon, R. G. Freeman, N. Valley, M. E. Piotti, M. J. Natan, G. C. Schatz and R. P. V. Duyne, J. Am. Chem. Soc., 2010, 132, 10903 CrossRef CAS PubMed.
- T. Uchida, T. Yoshikawa, M. Tamura, T. Iida and K. Imura, J. Phys. Chem. Lett., 2016, 7, 3652 CrossRef CAS PubMed.
- O. D. Velev, K. Furusawa and K. Nagayama, Langmuir, 1996, 12, 2374 CrossRef CAS.
- J. P. Novak, C. Nickerson, S. Franzen and D. L. Feldheim, Anal. Chem., 2001, 73, 5758 CrossRef CAS PubMed.
- Y. Yin, Y. Lu, B. Gates and Y. Xia, J. Am. Chem. Soc., 2001, 123, 8718 CrossRef CAS PubMed.
- Z. Yu, L. Chen and S. Chen, J. Mater. Chem., 2010, 20, 6182 RSC.
- T. Yanagishita, R. Fujimura, K. Nishio and H. Masuda, Langmuir, 2010, 26, 1516 CrossRef CAS PubMed.
- T. Yanagishita, Y. Maejima, K. Nishio and H. Masuda, RSC Adv., 2014, 4, 1538 RSC.
- T. Yanagishita, R. Otomo, K. Nishio and H. Masuda, RSC Adv., 2023, 13, 16549 RSC.
- T. Nakajima, M. Shimizu and M. Kukizaki, Key Eng. Mater., 1991, 61, 513 Search PubMed.
- H. Masuda and K. Fukuda, Science, 1995, 268, 1466 CrossRef CAS PubMed.
- M. Iwai, T. Kikuchi and R. O. Suzuki, Sci. Rep., 2021, 11, 7240 CrossRef CAS PubMed.
- H. Masuda, H. Yamada, M. Satoh, H. Asoh, M. Nakao and T. Tamamura, Appl. Phys. Lett., 1997, 71, 2770 CrossRef CAS.
- T. Yanagishita, A. Kato and H. Masuda, Jpn. J. Appl. Phys., 2017, 56, 035202 CrossRef.
- T. Yanagishita, Y. Maejima and H. Masuda, Mater. Res. Express, 2022, 9, 086404 CrossRef.
- T. Yanagishita, R. Asami and H. Masuda, Mater. Res. Express, 2021, 8, 025003 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2023 |