Open Access Article
Open Access ArticleGrowth factor-encapsulated triphasic scaffolds of electrospun polylactic acid–polycaprolactone (PLA–PCL) nanofibrous mats combined with a directionally freeze-dried chitosan hydrogel for periodontal tissue regeneration
Weihan
Hua
a,
Jie
Xiang
a,
Yeke
Wu
b,
Wei
Yang
 *c and
Lixing
Zhao
*c and
Lixing
Zhao
 *a
*a
aState Key Laboratory of Oral Diseases & National Clinical Research Center for Oral Diseases & Department of Orthodontics, West China Hospital of Stomatology, Sichuan University, China. E-mail: zhaolixing@scu.edu.cn
bDepartment of Stomatology, Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, China
cCollege of Polymer Science and Engineering, Sichuan University, State Key Laboratory of Polymer Materials Engineering, Chengdu 610065, Sichuan, China. E-mail: weiyang@scu.edu.cn
First published on 8th September 2023
Abstract
Cementum, periodontal ligament, alveolar bone, and gingiva make up the three-dimensional, multicellular structure known as periodontal tissue, which is crucial for maintaining the healthy alignment and function of teeth. Simulating the highly layered and orderly structure of periodontal tissue is the key to achieving its regeneration. In this study, through electrospinning, directional freeze-drying, and cross-linking, we successfully fabricated poly lactic acid (PLA)–poly ε-caprolactone (PCL) electrospun nanofibrous mats with the freeze-dried chitosan of the directionally arranged microporous channel structure. Moreover, we used nanoparticles to load three different growth factors of the target layer. We constructed triphasic scaffolds that simulated the physiological periodontal tissue. These scaffolds with superior mechanical properties were tested in vitro with periodontal ligament stem cells (PDLSCs), and the results supported that the triphasic scaffolds with growth factors had superior biocompatibility and reduced cytotoxicity. Furthermore, we performed in vivo experiments, and the triphasic scaffolds loaded with growth factors were able to promote the repair of periodontal defects by promoting the formation of alveolar bone-like tissue, periodontal ligament-like tissue, and cementum-like tissue. The successful construction of the triphasic scaffold provides a novel approach for remodeling the physiologic organization and function of the periodontal tissue with multiphasic scaffolds.
Introduction
During the process of dental and maxillofacial development, the microenvironment of dental follicles promotes stem cells to differentiate into cementoblasts, fibroblasts, and osteoblasts, thereby forming the cementum, periodontal ligament, and alveolar bone. However, this microenvironment no longer exists after the completion of periodontal tissue, resulting in great difficulties in self-repair.1–4 Moreover, the chronic infectious disease periodontitis might occur with bacterial infection or trauma, which leads to the destruction of periodontal tissues and even tooth loss, and seriously affects the oral health-related quality of life.5–7 At present, the purpose of inflammation control is mainly achieved by removing plaque and local irritants,8,9 while for the structural-functional reconstruction of defects, there has been no efficient therapeutic strategy that can only prevent further tissue destruction yet.10With the development of biomaterials, tissue engineering technology has been continuously shown to be a promising approach for periodontal tissue regeneration. At present, most scaffolds for periodontal tissue engineering are biphasic constructs, which focus mainly on the ectopic or in situ regeneration of periodontal ligaments and the restoration of alveolar bone height. The creation of new periodontal ligament-like tissue and alveolar bone-like tissue is visible in the defect location after ectopic or in situ implantation of these scaffolds in vivo.11–16 In our previous study, we synthesized a 3D multilayered electrospun scaffold to simulate the microenvironment of periodontium.17 It was observed that aligned electrospun nanofibers could induce cells in an oriented alignment through contact guidance and cell–fiber interactions, which further promoted cytoskeleton rearrangement to guide oriented tissue regeneration. Notably, the periodontal complex has a stratified sandwich-like structure, with periodontal ligament located in the middle layer, containing directionally arranged collagen fiber bundles inserted into the mineralized cementum and alveolar bone at both ends. Thus, the three components are connected as a whole.11–16 Periodontal tissue engineering can only fully simulate the physical structure and function of the physiological periodontal tissue by achieving cementum regeneration and forming an anchoring connection structure-the Sharpey fiber, at the interface between cementum, periodontal ligament, and alveolar bone.18–21
According to recent research, the incorporation of poly lactic acid (PLA) into poly ε-caprolactone (PCL) can compensate for the shortcomings of the two and make the performance of the material better.22,23Electrospun PLA–PCL nanofibrous scaffolds would have a shorter degradation time, higher mechanical properties (e.g., stiffness and modulus), and better bioactivity than pure PCL scaffolds. Besides, researchers have found that PLA–PCL (wt/wt, 70/30) showed that the separation of the phase would not be a major concern.24,25 In this study, we have successfully developed PLA–PCL (wt/wt, 70/30) nanofibrous mats using an electrospinning technique and chitosan with an orderly aligned microporous channel structure by directional freeze-drying technology.
Besides, bone morphogenetic protein 2 (BMP2), recombinant human cementum protein 1 (rhCEMP1), and connective tissue growth factor (CTGF) are encapsulated into the target layer of the scaffold by chitosan-stabilized bovine serum albumin (BSA) nanoparticles. After that, the shape, structure, surface hydrophilicity, drug loading, and release of the nanoparticle were examined. And the in vitro micromorphology, mechanical properties, biocompatibility, and cytotoxicity of the triphasic scaffolds were also determined. Additionally, tissue regeneration was evaluated after the nanofibrous composite was implanted into rat periodontal defects. Collectively, our studies looked at the potential of a biomimetic multiphasic scaffold in the field of periodontal tissue regeneration, potentially providing light on complex tissue regeneration with clinical viability.
Materials and methods
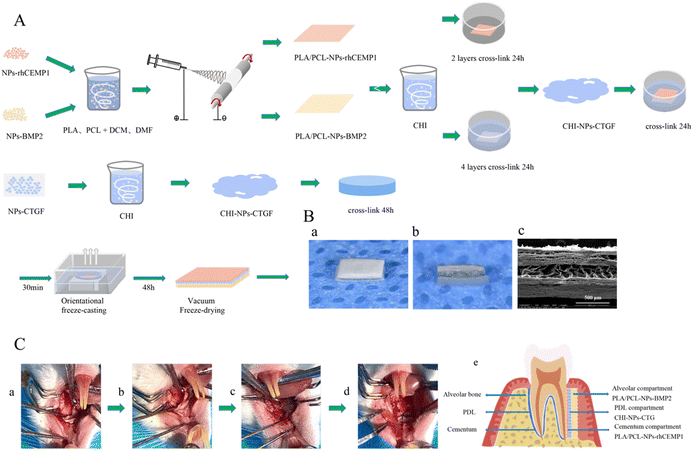
Scaffold preparation
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 20 minutes white precipitated aggregates were collected and stored at −20 °C. The supernatant was collected for further research on encapsulation effectiveness and in vitro release of GFs. Finally, the precipitate was freeze-dried for 48 h in a vacuum freeze dryer (Beijing Boyikang Experimental Instrument Co., Ltd, Beijing, China) to obtain NPs loaded with BMP2, rhCEMP1, and CTGF (NPs-BMP2, NPs-rhCEMP1, and NPs-CTGF).
000 rpm for 20 minutes white precipitated aggregates were collected and stored at −20 °C. The supernatant was collected for further research on encapsulation effectiveness and in vitro release of GFs. Finally, the precipitate was freeze-dried for 48 h in a vacuum freeze dryer (Beijing Boyikang Experimental Instrument Co., Ltd, Beijing, China) to obtain NPs loaded with BMP2, rhCEMP1, and CTGF (NPs-BMP2, NPs-rhCEMP1, and NPs-CTGF).
Scaffold characterization
Besides, 13 μg PLA–PCL-NPs-BMP2 (containing 1 ng BMP2), 65 μg PLA–PCL-NPs-rhCEMP1 (containing 5 ng rhCEMP1), and 110 μg CHI-NPs-CTGF (containing 10 ng CTGF) were shaken and incubated with 0.5 mL phosphate buffer saline (PBS) at 37 °C. On the certain time-points of 6 h, 12 h, 1 d, 3 d, 5 d, 7 d, 9 d, 12 d, 15 d, 18 d, 21 d, 25 d, and 30 d, 0.5 mL of the PBS solution was removed and BMP2, rhCEMP1, and CTGF concentrations were measured using a BMP2 enzyme-linked immunosorbent assay (ELISA) kit (LinkBio, Wuhan, China), rhCEMP1 ELISA kit (Huamibi, Wuhan, China), and CTGF ELISA kit (LinkBio, Wuhan, China). The withdrawn solution was replaced with the same volume of brand-new PBS. Software called CurveExpert was used to establish the standard curve (Huami Bio, Wuhan, China). Three parallel samples were set for each group of materials.
In vitro evaluations
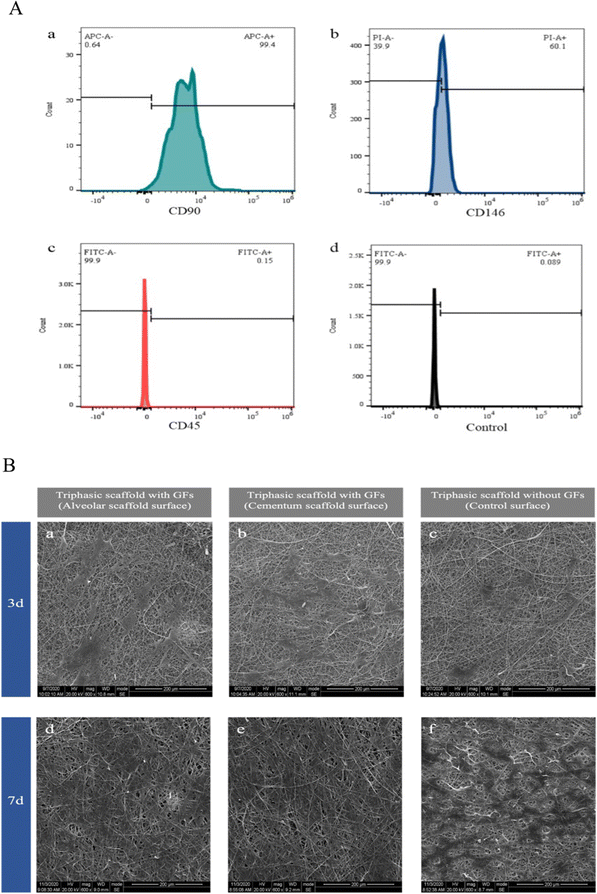
The isolated hPDLSCs were observed under a U-LH100L-3 inverted phase contrast microscope (Olympus, Tokyo, Japan). For identification of the mesenchymal stem cells phenotype, hPDLSCs (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ×
× ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 105 cells) were incubated with allophycocyanin (APC)-, propidium iodide (PI)- or fluorescein isothiocyanate (FITC)-conjugated monoclonal antibodies for human CD90, CD146, and CD45 (BD Biosciences, Franklin Lakes, NJ, USA), or isotype-matched control IgGs. Then, a Beckman Cytomic FC500 flow cytometer (Beckman Coulter, Brea, CA, USA) was used to analyze the flow cytometry. Three parallel samples were set for each group of materials at each time point.
105 cells) were incubated with allophycocyanin (APC)-, propidium iodide (PI)- or fluorescein isothiocyanate (FITC)-conjugated monoclonal antibodies for human CD90, CD146, and CD45 (BD Biosciences, Franklin Lakes, NJ, USA), or isotype-matched control IgGs. Then, a Beckman Cytomic FC500 flow cytometer (Beckman Coulter, Brea, CA, USA) was used to analyze the flow cytometry. Three parallel samples were set for each group of materials at each time point.
| Gene | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| RUNX2 | CCGCCTCAGTGATTTAGGGC | GGGTCTGTAATCTGACTCTGTCC |
| ALP | AACATCAGGGACATTGACGTG | GTATCTCGGTTTGAAGCTCTTCC |
| CAP | TCCAGACATTTGCCTTGCTT | TTACAGCAATAGAAAAACAGCATGA |
| CEMP1 | GGGCACATCAAGCACTGACAG | CCCTTAGGAAGTGGCTGTCCAG |
| Periostin | CTCATAGTCGTATCAGGGGTCG | ACACAGTCGTTTTCTGTCCAC |
| Scleraxis | AGAAAGTTGAGCAAGGACC | CTGTCTGTACGTCCGTCT |
| COL1A1 | GTGCGATGACGTGATCTGTGA | CGGTGGTTTCTTGGTCGGT |
| COL3A1 | TTGAAGGAGGATGTTCCCATCT | ACAGACACATATTTGGCATGGTT |
| GAPDH | GGAGCGAGATCCCTCCAAAAT | GGCTGTTGTCATACTTCTCATGG |
In vivo studies
| Triphasic | GFs | Directional lyophilization | |
|---|---|---|---|
| Triphasic scaffold (with GFs) | + | + | + |
| Triphasic scaffold (without GFs) | + | − | + |
| Monophasic scaffold (CHI-NPs-CTGF) | − | + | + |
| Monophasic scaffold (PLA/PCL) | − | + | − |
Statistical analysis
The data were analyzed using the Statistical Package for Social Science (version 25.0; SPSS, Chicago, IL, USA) for Windows. Data were expressed as mean ± SD, and each experiment was repeated at least three times. The one-way analysis of variance (ANOVA) with Tukey's post hoc test was used to statistically compare the groups. Statistics were considered significant when *P < 0.05.Results
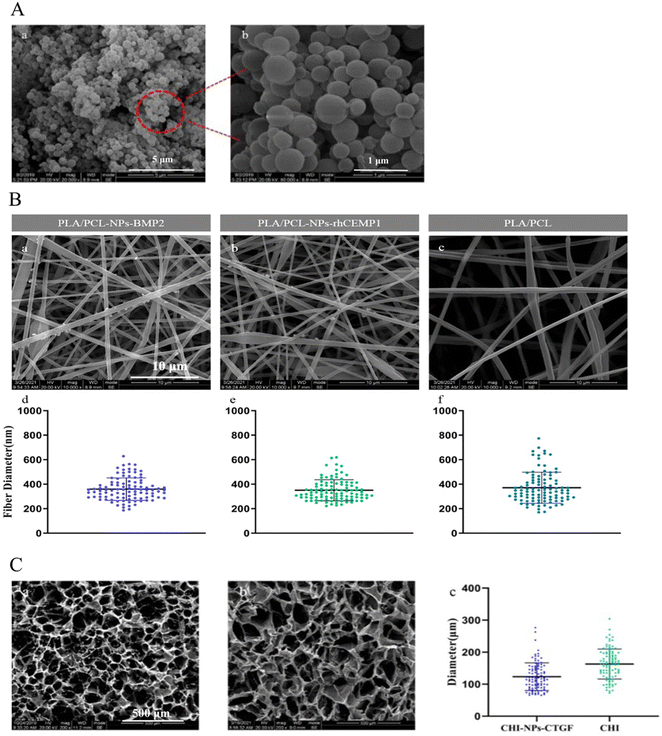
Micromorphology of NPs, electrospun nanofiber mats, and CHI hydrogels
The micromorphology of the CHI-stabilized BSA NPs was observed by SEM and is shown in Fig. 2(A). The results measured by DLS showed that the three NPs were similar in diameter: 434.72 nm (NPs-BMP2), 441.70 nm (NPs-rhCEMP1), and 425.64 nm (NPs-CTGF), with an average diameter of 434.02 ± 8.05 nm.In contrast to the pure PLA–PCL nanofibers, which showed a smooth appearance, both the PLA–PCL-NPs-BMP2 and the PLA–PCL-NPs-rhCEMP1 nanofibers presented a slightly rougher surface with small nodules bulging outward, demonstrating the successful incorporation of NPs-BMP2 and NPs-rhCEMP1 within the nanofiber matrix during the electrospinning process (Fig. 2(B)). The average diameters of each nanofiber were approximately 359.85 ± 91.60 nm (PLA–PCL-NPs-BMP2), 350.41 ± 8 6.00 nm (PLA–PCL-NPs-rhCEMP1), and 370.96 ± 127.02 nm (PLA–PCL), without statistically significant differences among them (P > 0.05), indicating that the loading of NPs-BMP2 and NPs-rhCEMP1 had no remarkable influence on the diameter of the electrospun nanofibers. For pore size distributions, the average pore size of CHI-NPs-CTGF was 123.48 ± 43.17 μm, whereas the pore size of pure CHI (162.90 ± 46.78 μm) was much larger (P < 0.05) (Fig. 2(C)). Importantly, all these diameters and pore sizes are within the nanometer range and close to the natural ECM fibers, which represents favorable biomimicry.28
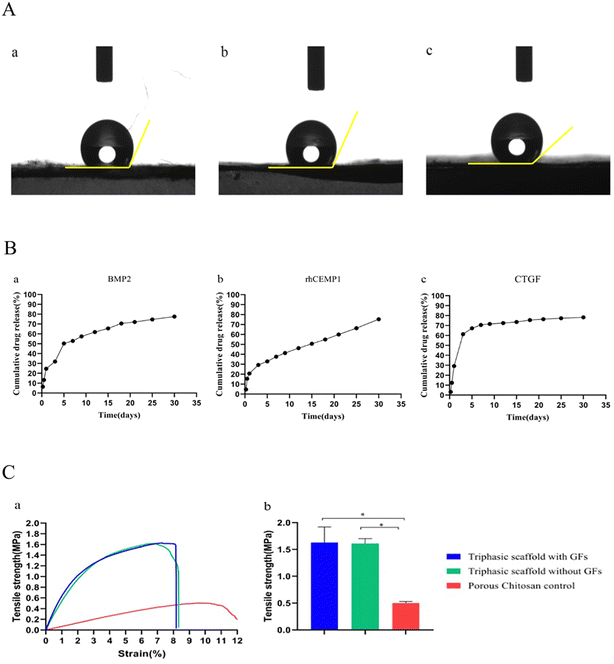
Characterizations of scaffolds
In vitro bioactivity of scaffolds
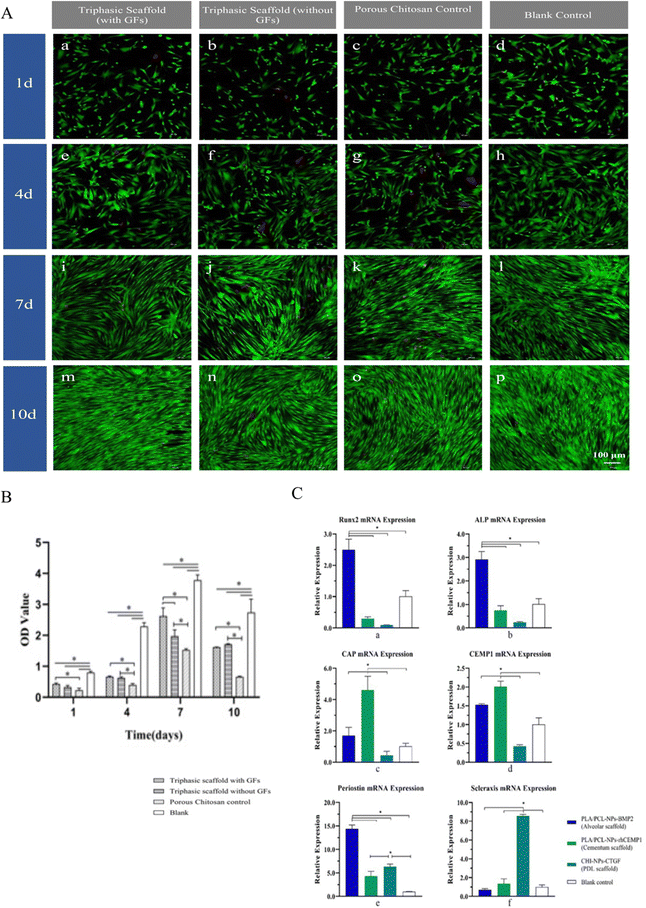
Then the seventh day was when all groups showed the maximum levels of cell proliferation, according to the CCK-8 results, and the blank control had significantly higher levels of proliferation activity at each time point than the other three groups (P < 0.05). However, the triphasic scaffolds with GFs showed higher cell proliferation activity compared to the porous CHI control (P < 0.05 on the 1st, 4th, 7th, and 10th days) and the triphasic scaffolds without GFs (P < 0.05 on the 7th day), indicating that the loading of GFs promoted hPDLSCs proliferation and alleviated the moderate cytotoxicity of the scaffolds on cells (Fig. 5(B)).
Scaffolds guided repair in rat periodontal defects
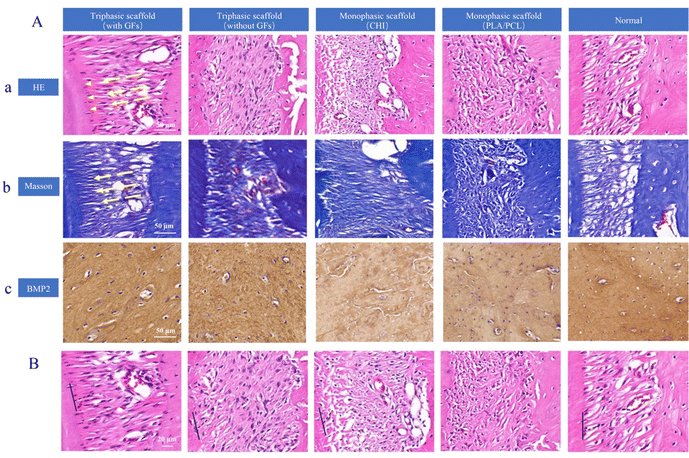
To further support periodontal regenerations, Masson trichrome staining was then performed (Fig. 7(A)-b). Blue dye was used to color the newly produced collagen strands, and it was noticeable that denser and larger amounts of collagen as well as mineralized bone tissue were presented in the triphasic scaffold with the GFs group. This result was in line with the H&E staining result that the triphasic scaffold with GFs had the best restoration effectiveness of all the groups. Furthermore, we conducted immunohistochemical staining to assess BMP2 expression (Fig. 7(A)-c). The staining in the triphasic scaffold with the GFs group indicated that this group has the best osteoinductive capacity and periodontal repair efficacy. Besides, BMP2 staining was significantly positive in both triphasic scaffold groups and the monophasic scaffold groups showed marginally positive staining.
Discussion
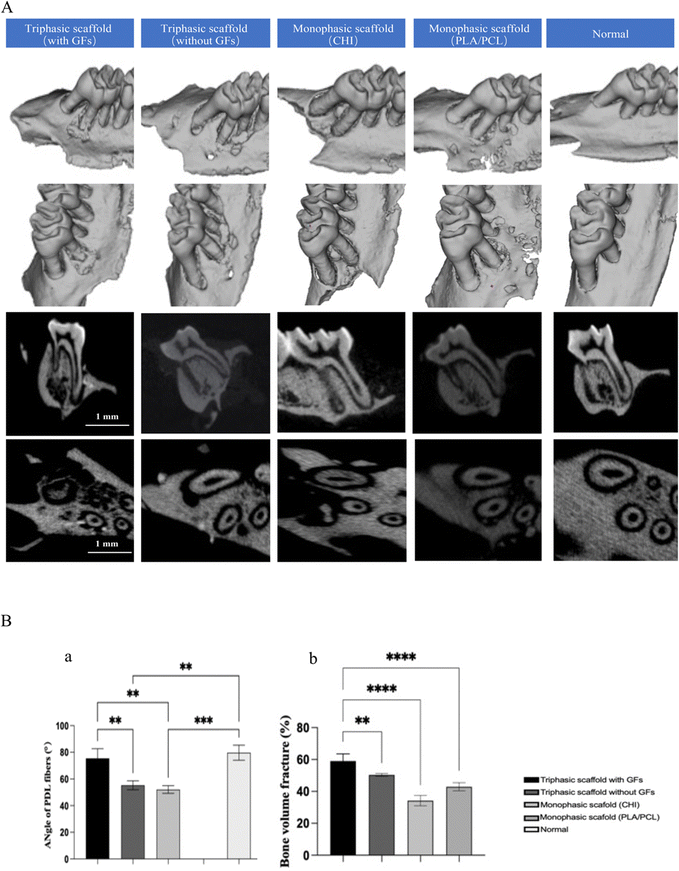
The periodontium's hierarchical architecture necessitates a highly coordinated regeneration process in which each component (cementum, PDL, and alveolar bone) reacts in accordance with distinct, occasionally overlapping spatiotemporal sequences.31–33 Since predictable periodontal regeneration hardly occurs during conventional therapy, the utilization of multiphasic constructs with either cells or biochemical cues, recapitulating the native anatomical topology, especially the submicron interfacial PDL geometry, has recently been advocated as the most promising strategy.34 The current study suggests that putative alveolar bone, cementum-like structures, and a PDL-like tissue interface can be produced both in vitro and in vivo using PLA–PCL- and CHI-based triphasic scaffolds with three distinct microstructures and spatiotemporal delivery of BMP2, rhCEMP1, and CTGF in NPs. Each compartment's thickness complied with the periodontium's anatomical requirements.14,19,35–39The diameters of electrospun nanofibers were in the range of 200–600 nm, while the pore sizes of CHI were in the range of 60–200 μm, which met the criteria of the natural ECM fibers to maintain sufficient mechanical strength and provide a favorable microenvironment for cell attachment, proliferation, and migration.40 Also, the electrospun nanofibers on both sides of the triphasic scaffold were tightly stacked, simulating the lamellar structure of cementum and alveolar bone, according to the results of our SEM scanning.35,41 The CHI hydrogel in the middle had a directionally arranged microporous channel structure, resulting in high porosity and interconnectivity, as well as low stiffness, which may be considered an important design criterion for tissue integration.42 The structure provided the possibility to guide the directional regeneration of the PDL.
It is significant that the scaffold with a specified mechanical strength promotes selective cell repopulation and wound stability, prevents undesirable tissue invasion, and serves as space maintenance to promote the development of functional tissues that are dimensionally stable over time.34,43,44 The PCL adopted is extensively used in the regeneration of mineralized tissues owing to superior biocompatibility, water degradability, toughness, and mechanical strength.45 However, the low surface energy of PCL impedes cell adhesion and tissue integration, and the slow degradation rate was also unfavorable to further ingrowth and maturation of regenerated tissues.46,47 In contrast, PLA has higher brittleness but good biocompatibility.22,23 As a naturally degradable polysaccharide in ECM, CHI possesses excellent hydrophily, fluidity, adhesiveness, and antibacterial properties and is widely applied in tissue regeneration, wound healing, and other fields.48 Moreover, CHI has a morphological structure analogous to the extracellular matrix (ECM), and it acts as a biological glue to bond materials such as electrospun nanofibers.49 With the current study's freeze-dried CHI hydrogel, a specific porosity structure allowed for huge transportation to help with biological delivery, cell infiltration, and tissue regeneration.50–52
BMP2 is a member of the transforming growth factor-β superfamily, which promotes the differentiation of various stem cells such as dental follicle cells and hPDLSCs into osteoblasts, and this can accelerate the formation of alveolar bone tissue.53–55 CEMP1 isolated from cementum, promotes the differentiation of hPDLSCs into cementoblasts while inhibiting its osteogenic differentiation.56–58 CTGF plays an important role in maintaining and repairing periodontal ligament integrity by promoting fibrogenic differentiation and upregulating the expression of collagen type I and III and periostin.19,59,60 Due to the short half-life and easy inactivation of bioactive components in vitro, local delivery and sustained release of them cannot be achieved if they are directly combined with a scaffold by immersion, covalent binding, or other methods.61–64 Therefore, it is crucial to develop more advanced methods to distribute these bioactive compounds in a controlled and sustained manner while also minimizing their negative effects. Using NPs stabilized by BSA and CHI, we loaded BMP2, rhCEMP, and CTGF into the alveolar bone, cement, and PDL compartments, respectively, based on the procedures outlined in earlier investigations.26 The EE of NPs was rather high, and their sustained release could be detected until the 30th day, which was consistent with the previous study.26 Interestingly, we found that CTGF, which promotes ligament differentiation, was released in a more robust manner than BMP2 and rhCEMP1 during the first three days, which is speculated to be better for the repair and regeneration of periodontal tissue.
On the 7th day, the number and fusion area of hPDLSCs on the alveolar and cementum surfaces of triphasic scaffolds with GFs were significantly larger than the other groups, proving the capacity of cell adhesion and alignment guidance for both the alveolar bone and cementum compartments. Indicated time points showed that the triphasic scaffold group with GFs had higher cell proliferation activity than the other groups, demonstrating that the GFs loading increased hPDLSCs proliferation and reduced the mild cytotoxicity of the scaffolds on cells in a time-dependent manner. We hypothesized that this pattern might be related to the cumulative concentrations of BMP2, rhCEMP1, and CTGF in a certain period, which promoted cell proliferation and differentiation.65–67 The studies of Cattaneo,68 Jhala,69 and Mcbeath70 all showed that the cells with flattened morphology tended to differentiate into osteoblasts and cementoblasts rather than fibroblasts. Results showed that both PLA–PCL-NPs-BMP2 and PLA–PCL-NPs-rhCEMP1 tended to induce the osteogenic differentiation of hPDLSCs. Unexpectedly, we discovered that the expression of Periostin in the CHI-NPs-CTGF was lower than that in the PLA–PCL-NPs-BMP2. The reason might be that Periostin expression was not specifically confined to the PDL, it is also highly expressed in the bone tissue and could be effectively promoted by BMP2 treatment.66,71,72
Here, we have successfully generated a rat model of surgically induced periodontal lesions in the maxillary first molar and assessed the in vivo effectiveness of the scaffolds. Collagen bundles were oriented between the newly created alveolar bone-like tissue and root in the triphasic scaffold with the GFs group. In addition, intricate integrations between collagen bundles and tissue that resembled the cementum and the PDL–cementum interface were created at the same time. The fibrous connective tissue in the other groups, however, was disordered and lacked trim configurations, and no collagen bundles had developed. The anchoring of the newly generated fiber bundles into a cementum-like mineralized layer on the root surface was further confirmed by Masson and IHC staining. Our research has shown that triphasic scaffolds with biomimetic geometries can effectively support the regrowth, arranging, and structuring of physiological periodontal tissues.
Despite all these encouraging outcomes for this scaffold in vitro and in vivo, there are still several constraints. The recent research primarily showed that both ends of fibrous PDL bundles were anchored into the cementum and alveolar bone, which act as connectors between mineralized tissues, although the underlying mechanisms were still not fully understood. However, the in vivo models were still limited to larger animals. This hampered the direct translation into preclinical and clinical treatment. Consequently, further research will need to be done in the future to confirm the translational use of this scaffold.
Conclusions
In this study, we successfully fabricated a biomimetic GF-loaded triphasic scaffold with regional-specific geometry, which highly simulated the native architecture of alveolar bone, PDL, and cementum. In vitro, the scaffolds were validated to possess favorable mechanical properties, excellent biocompatibility, and low cytotoxicity. Each compartment of the construct with indicated GFs could promote the expression of osteogenesis-related, periodontal ligament-related, and cementogenesis-related genes in PDLSCs. In vivo, the biomimetic scaffolds loaded with GFs were also demonstrated to repair the periodontal defects in rats by promoting the formation of the physiological periodontium. Overall, despite some drawbacks, our work provides a novel approach for recapitulating the physiologic organization and function of the periodontal tissue with multiphasic scaffolds.Author contributions
Weihan Hua: conceptualization, methodology, data curation, formal analysis, writing – original draft, and validation. Jie Xiang: methodology, validation, formal analysis, and software. Yeke Wu: methodology, data curation, funding acquisition, and validation. Wei Yang: conceptualization, project administration, and writing – review & editing. Lixing Zhao: conceptualization, funding acquisition, project administration, and writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by grants from the National Nature Science Foundation of China (no. 81973684, 31271052, 31670992) and the Nature Science Foundation of Sichuan (no. 2574).References
- W. Guo, L. Chen, K. Gong, B. Ding, Y. Duan and Y. Jin, Tissue Eng., Part A, 2012, 18, 459–470 CrossRef CAS PubMed.
- S. Yao, F. Pan, V. Prpic and G. E. Wise, J. Dent. Res., 2008, 87, 767–771 CrossRef CAS PubMed.
- P. M. Bartold, S. Shi and S. Gronthos, Periodontol 2000, 2006, 40, 164–172 CrossRef PubMed.
- S. Sowmya, K. P. Chennazhi, H. Arzate, P. Jayachandran, S. V. Nair and R. Jayakumar, Tissue Eng., Part C, 2015, 21, 1044–1058 CrossRef CAS PubMed.
- C. A. Ramseier, A. Anerud, M. Dulac, M. Lulic, M. P. Cullinan, G. J. Seymour, M. J. Faddy, W. Bürgin, M. Schätzle and N. P. Lang, J. Clin. Periodontol., 2017, 44, 1182–1191 CrossRef PubMed.
- J. Pyo, M. Lee, M. Ock and J. Lee, Int. J. Environ. Res. Public Health, 2020, 17, 4895 CrossRef PubMed.
- D. R. Meusel, J. C. Ramacciato, R. H. Motta, R. B. Brito Júnior and F. M. Flório, J. Oral Sci., 2015, 57, 87–94 CrossRef CAS PubMed.
- A. Caygur, M. R. Albaba, A. Berberoglu and H. G. Yilmaz, J. Int. Med. Res., 2017, 45, 1168–1174 CrossRef CAS PubMed.
- D. E. Deas, A. J. Moritz, R. S. Sagun, Jr., S. F. Gruwell and C. A. Powell, Periodontol 2000, 2016, 71, 128–139 CrossRef PubMed.
- I. Darby, Aust. Dent. J., 2011, 56(Suppl 1), 107–118 CrossRef PubMed.
- C. Vaquette, S. Saifzadeh, A. Farag, D. W. Hutmacher and S. Ivanovski, J. Dent. Res., 2019, 98, 673–681 CrossRef CAS PubMed.
- P. F. Costa, C. Vaquette, Q. Zhang, R. L. Reis, S. Ivanovski and D. W. Hutmacher, J. Clin. Periodontol., 2014, 41, 283–294 CrossRef CAS PubMed.
- C. Vaquette, W. Fan, Y. Xiao, S. Hamlet, D. W. Hutmacher and S. Ivanovski, Biomaterials, 2012, 33, 5560–5573 CrossRef CAS PubMed.
- C. H. Park, H. F. Rios, Q. Jin, M. E. Bland, C. L. Flanagan, S. J. Hollister and W. V. Giannobile, Biomaterials, 2010, 31, 5945–5952 CrossRef CAS PubMed.
- C. H. Park, H. F. Rios, Q. Jin, J. V. Sugai, M. Padial-Molina, A. D. Taut, C. L. Flanagan, S. J. Hollister and W. V. Giannobile, Biomaterials, 2012, 33, 137–145 CrossRef CAS PubMed.
- E. C. Carlo Reis, A. P. Borges, M. V. Araújo, V. C. Mendes, L. Guan and J. E. Davies, Biomaterials, 2011, 32, 9244–9253 CrossRef CAS PubMed.
- W. Jiang, L. Li, D. Zhang, S. Huang, Z. Jing, Y. Wu, Z. Zhao, L. Zhao and S. Zhou, Acta Biomater., 2015, 25, 240–252 CrossRef CAS PubMed.
- C. H. Park, J. H. Oh, H. M. Jung, Y. Choi, S. U. Rahman, S. Kim, T. I. Kim, H. I. Shin, Y. S. Lee, F. H. Yu, J. H. Baek, H. M. Ryoo and K. M. Woo, Acta Biomater., 2017, 61, 134–143 CrossRef CAS PubMed.
- C. H. Lee, J. Hajibandeh, T. Suzuki, A. Fan, P. Shang and J. J. Mao, Tissue Eng., Part A, 2014, 20, 1342–1351 CrossRef CAS PubMed.
- S. Sowmya, U. Mony, P. Jayachandran, S. Reshma, R. A. Kumar, H. Arzate, S. V. Nair and R. Jayakumar, Adv. Healthcare Mater., 2017, 6, 10 Search PubMed.
- M. Wu, J. Wang, Y. Zhang, H. Liu and F. Dong, Med. Sci. Monit., 2018, 24, 1112–1123 CrossRef CAS PubMed.
- F. Diomede, A. Gugliandolo, P. Cardelli, I. Merciaro, V. Ettorre, T. Traini, R. Bedini, D. Scionti, A. Bramanti, A. Nanci, S. Caputi, A. Fontana, E. Mazzon and O. Trubiani, Stem Cell Res. Ther., 2018, 9, 104 CrossRef CAS PubMed.
- T. Xu, Q. Yao, J. M. Miszuk, H. J. Sanyour, Z. Hong, H. Sun and H. Fong, Colloids Surf., B, 2018, 171, 31–39 CrossRef CAS PubMed.
- M. Sun and S. Downes, J. Mater. Sci.: Mater. Med., 2009, 20, 1181–1192 CrossRef CAS PubMed.
- M. Sun, P. J. Kingham, A. J. Reid, S. J. Armstrong, G. Terenghi and S. Downes, J. Biomed. Mater. Res., Part A, 2010, 93, 1470–1481 CAS.
- L. Li, G. Zhou, Y. Wang, G. Yang, S. Ding and S. Zhou, Biomaterials, 2015, 37, 218–229 CrossRef CAS PubMed.
- P. Divakar, K. Yin and U. G. K. Wegst, J. Mech. Behav. Biomed. Mater., 2019, 90, 350–364 CrossRef CAS PubMed.
- G. Huang, F. Li, X. Zhao, Y. Ma, Y. Li, M. Lin, G. Jin, T. J. Lu, G. M. Genin and F. Xu, Chem. Rev., 2017, 117, 12764–12850 CrossRef CAS PubMed.
- Z. M. Goudarzi, T. Behzad, L. Ghasemi-Mobarakeh and M. Kharaziha, Polymer, 2021, 213, 123313 CrossRef.
- M. Zhang, Y. Ma, R. Li, J. Zeng, Z. Li, Y. Tang and D. Sun, J. Biomater. Sci., Polym. Ed., 2017, 28, 2205–2219 CrossRef CAS PubMed.
- Y. Yang, F. M. Rossi and E. E. Putnins, Biomaterials, 2010, 31, 8574–8582 CrossRef CAS PubMed.
- R. Raju, M. Oshima, M. Inoue, T. Morita, Y. Huijiao, A. Waskitho, O. Baba, M. Inoue and Y. Matsuka, Sci. Rep., 2020, 10, 1656 CrossRef CAS PubMed.
- S. Ivanovski, Aust. Dent. J., 2009, 54(Suppl 1), S118–S128 CrossRef PubMed.
- C. Vaquette, S. P. Pilipchuk, P. M. Bartold, D. W. Hutmacher, W. V. Giannobile and S. Ivanovski, Adv. Healthcare Mater., 2018, 7, e1800457 CrossRef PubMed.
- S. P. Ho, M. P. Kurylo, K. Grandfield, J. Hurng, R. P. Herber, M. I. Ryder, V. Altoe, S. Aloni, J. Q. Feng, S. Webb, G. W. Marshall, D. Curtis, J. C. Andrews and P. Pianetta, Bone, 2013, 57, 455–467 CrossRef CAS PubMed.
- A. Nanci and D. D. Bosshardt, Periodontol 2000, 2006, 40, 11–28 CrossRef PubMed.
- M. G. Kim and C. H. Park, Molecules, 2020, 25, 4802 CrossRef CAS PubMed.
- G. Rasperini, S. P. Pilipchuk, C. L. Flanagan, C. H. Park, G. Pagni, S. J. Hollister and W. V. Giannobile, J. Dent. Res., 2015, 94, 153s–157s CrossRef CAS PubMed.
- S. P. Pilipchuk, A. Monje, Y. Jiao, J. Hao, L. Kruger, C. L. Flanagan, S. J. Hollister and W. V. Giannobile, Adv. Healthcare Mater., 2016, 5, 676–687 CrossRef CAS PubMed.
- W. Zhang, Z. He, Y. Han, Q. Jiang, C. Zhan, K. Zhang, Z. Li and R. Zhang, Composites, Part A, 2020, 137, 106009 CrossRef CAS PubMed.
- T. van den Bos and W. Beertsen, J. Periodontal Res., 1999, 34, 1–6 CrossRef CAS PubMed.
- C. H. Park, K. H. Kim, H. F. Rios, Y. M. Lee, W. V. Giannobile and Y. J. Seol, J. Dent. Res., 2014, 93, 1304–1312 CrossRef CAS PubMed.
- J. Gottlow, T. Karring and S. Nyman, J. Periodontol., 1990, 61, 680–685 CrossRef CAS PubMed.
- J. Gottlow, S. Nyman, J. Lindhe, T. Karring and J. Wennström, J. Clin. Periodontol., 1986, 13, 604–616 CrossRef CAS PubMed.
- T. K. Teh, S. L. Toh and J. C. Goh, Tissue Eng., Part A, 2013, 19, 1360–1372 CrossRef CAS PubMed.
- R. Dwivedi, S. Kumar, R. Pandey, A. Mahajan, D. Nandana, D. S. Katti and D. Mehrotra, J. Oral Biol. Craniofacial Res., 2020, 10, 381–388 CrossRef PubMed.
- W. Wang, G. Caetano, W. S. Ambler, J. J. Blaker, M. A. Frade, P. Mandal, C. Diver and P. Bártolo, Materials, 2016, 9, 992 CrossRef PubMed.
- A. Francesko and T. Tzanov, Adv. Biochem. Eng./Biotechnol., 2011, 125, 1–27 CAS.
- I. Y. Kim, S. J. Seo, H. S. Moon, M. K. Yoo, I. Y. Park, B. C. Kim and C. S. Cho, Biotechnol. Adv., 2008, 26, 1–21 CrossRef CAS PubMed.
- Y. Liao, H. Li, R. Shu, H. Chen, L. Zhao, Z. Song and W. Zhou, Front. Cell. Infect. Microbiol., 2020, 10, 180 CrossRef CAS PubMed.
- E. M. Varoni, S. Vijayakumar, E. Canciani, A. Cochis, L. De Nardo, G. Lodi, L. Rimondini and M. Cerruti, J. Dent. Res., 2018, 97, 303–311 CrossRef CAS PubMed.
- D. G. Miranda, S. M. Malmonge, D. M. Campos, N. G. Attik, B. Grosgogeat and K. Gritsch, J. Biomed. Mater. Res., Part B, 2016, 104, 1691–1702 CrossRef CAS PubMed.
- M. Ou, Y. Zhao, F. Zhang and X. Huang, Connect. Tissue Res., 2015, 56, 204–211 CrossRef CAS PubMed.
- S. Y. Hyun, J. H. Lee, K. J. Kang and Y. J. Jang, Mol. Cells, 2017, 40, 550–557 CrossRef CAS PubMed.
- J. Tan, M. Zhang, Z. Hai, C. Wu, J. Lin, W. Kuang, H. Tang, Y. Huang, X. Chen and G. Liang, ACS Nano, 2019, 13, 5616–5622 CrossRef CAS PubMed.
- M. Komaki, K. Iwasaki, H. Arzate, A. S. Narayanan, Y. Izumi and I. Morita, J. Cell. Physiol., 2012, 227, 649–657 CrossRef CAS PubMed.
- H. Arzate, M. Zeichner-David and G. Mercado-Celis, Periodontol 2000, 2015, 67, 211–233 CrossRef PubMed.
- L. Hoz, E. Romo, M. Zeichner-David, M. Sanz, J. Nuñez, L. Gaitán, G. Mercado and H. Arzate, Cell Biol. Int., 2012, 36, 129–136 CrossRef CAS PubMed.
- A. Yuda, H. Maeda, S. Fujii, S. Monnouchi, N. Yamamoto, N. Wada, K. Koori, A. Tomokiyo, S. Hamano, D. Hasegawa and A. Akamine, J. Cell. Physiol., 2015, 230, 150–159 CrossRef CAS PubMed.
- J. W. Choi, C. Arai, M. Ishikawa, S. Shimoda and Y. Nakamura, J. Periodontal Res., 2011, 46, 513–521 CrossRef CAS PubMed.
- C. Uggen, J. Dines, M. McGarry, D. Grande, T. Lee and O. Limpisvasti, Arthroscopy, 2010, 26, 1456–1462 CrossRef PubMed.
- T. Tokunaga, J. Ide, H. Arimura, T. Nakamura, Y. Uehara, H. Sakamoto and H. Mizuta, Arthroscopy, 2015, 31, 1482–1491 CrossRef PubMed.
- M. S. Lee, T. Ahmad, J. Lee, H. K. Awada, Y. Wang, K. Kim, H. Shin and H. S. Yang, Biomaterials, 2017, 124, 65–77 CrossRef CAS PubMed.
- Y. C. Jiang, X. F. Wang, Y. Y. Xu, Y. H. Qiao, X. Guo, D. F. Wang, Q. Li and L. S. Turng, Biomacromolecules, 2018, 19, 3747–3753 CrossRef CAS PubMed.
- G. N. King and F. J. Hughes, J. Clin. Periodontol., 2001, 28, 465–475 CrossRef CAS PubMed.
- A. Skodje, S. B. Idris, Y. Sun, S. Bartaula, K. Mustafa, A. Finne-Wistrand, U. M. Wikesjö and K. N. Leknes, J. Biomed. Mater. Res., Part A, 2015, 103, 1991–1998 CrossRef CAS PubMed.
- N. H. Heng, J. Zahlten, V. Cordes, M. M. Ong, B. T. Goh, P. D. N’Guessan and N. Pischon, J. Periodontol., 2015, 86, 569–577 CrossRef PubMed.
- V. Cattaneo, C. Rota, M. Silvestri, C. Piacentini, A. Forlino, A. Gallanti, G. Rasperini and G. Cetta, J. Periodontal Res., 2003, 38, 568–574 CrossRef CAS PubMed.
- D. Jhala, H. Rather and R. Vasita, Biomater. Sci., 2016, 4, 1584–1595 RSC.
- R. McBeath, D. M. Pirone, C. M. Nelson, K. Bhadriraju and C. S. Chen, Dev. Cell, 2004, 6, 483–495 CrossRef CAS PubMed.
- J. Du and M. Li, Adv. Exp. Med. Biol., 2019, 1132, 63–72 CrossRef CAS PubMed.
- K. Inai, R. A. Norris, S. Hoffman, R. R. Markwald and Y. Sugi, Dev. Biol., 2008, 315, 383–396 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2023 |