Design, synthesis and immunological evaluation of monophosphoryl lipid A derivatives as adjuvants for a RBD-hFc based SARS-CoV-2 vaccine†
Shiwei
Su
a,
Liqing
Chen
ab,
Menglan
Yang
a,
Dan
Liang
c,
Bixia
Ke
c,
Zhongqiu
Liu
a,
Changwen
Ke
c,
Guochao
Liao
 *ad,
Liang
Liu
*bde and
Xiang
Luo
*a
*ad,
Liang
Liu
*bde and
Xiang
Luo
*a
aJoint Laboratory for Translational Cancer Research of Chinese Medicine of the Ministry of Education of the People's Republic of China, International Institute for Translational Chinese Medicine, Guangzhou University of Chinese Medicine, Guangzhou, China. E-mail: liao@gzucm.edu.cn; luoxiang@gzucm.edu.cn
bState Key Laboratory of Dampness Syndrome of Chinese Medicine, The Second Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou, China. E-mail: lliu@gzucm.edu.cn
cGuangdong Provincial Center for Disease Control and Prevention, Guangzhou, China
dGuangdong Hengda Biomedical Technology Co., Ltd., Guangzhou, China
eGuangzhou Laboratory, Guangzhou, China
First published on 14th October 2022
Abstract
Toll-like receptor 4 (TLR4) is a reliable target for the development of vaccine adjuvants. To identify novel TLR4 ligands with improved immunological properties for use as adjuvants for a RBD-hFc based SARS-CoV-2 vaccine, herein, natural E. coli monophosphoryl lipid A (MPLA) and nine of its derivatives were designed and synthesized. Immunological evaluation showed that compounds 1, 3, 5 and 7 exhibited comparative or better adjuvant activity than clinically used Al adjuvants, and are expected to be a promising platform for the development of new adjuvants used for a RBD-hFc based SARS-CoV-2 vaccine. Preliminary structure–activity relationship analysis of the MPLA derivatives showed that the replacement of the functional groups at the C-1, C-4′ or C-6′ position of E. coli MPLA has an effect on its biological activity. In addition, we found that the combination of MPLA and Al was feasible for immunotherapy and could further enhance immune responses, providing a new direction toward the immunological enhancement of RBD-hFc based SARS-CoV-2 vaccines.
Introduction
Adjuvants, which can regulate immune responses without antigenic molecules, are usually co-administered with vaccines to boost antigen-specific immune responses through modulating innate and adaptive immunity.1,2 Adjuvants can also reduce the dose of expensive antigens and assist in generating more rapid and durable immune responses.3 Although they play an important role in vaccine development, only six adjuvants have been approved by the Food and Drug Administration (FDA), including alum, MF-59, AS03, AS04, AS01B and CpG1018.Toll-like Receptor 4 (TLR4), a mammalian transmembrane receptor protein, widely exists in immune system cells including neutrophils, macrophages, splenocytes, lymphocytes, dendritic cells, etc.4 TLR4 was the first discovered human homolog of the Drosophila Toll protein and is responsible for the selective recognition of lipopolysaccharides (LPS),5 a main virulent factor covering the outer surface membrane of Gram-negative bacteria. LPS can induce TLR4 and myeloid differentiation factor 2 (MD-2) to form a homodimerization of ternary TLR4/MD-2/LPS complex. Then, this complex is internalized into the endosome and triggers the MyD88-dependent and TRIF-dependent pathways, leading to the production of various pro-inflammatory cytokines and chemokines, such as tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and interferon-β (IFN-β).6–9 The activation of TLR4 contributes to the enhancement of innate and adaptive immune responses, and these features make it a hot target for the development of vaccine adjuvants.
Lipid A, the main essential ingredient responsible for the immunostimulatory activity of LPS, is a potent adjuvant for vaccine development. However, extreme toxicity, including the induction of sepsis and septic shock, impeded its clinical use.10 To reduce the toxicity and improve the beneficial immunostimulatory activity, a plethora of lipid A derivatives have been investigated.11–14 Among them, the 1-O-dephosphorylated form of lipid A from Salmonella minnesota RC595, known as monophosphoryl lipid A (MPLA), has been proved to have a strong adjuvant activity with reduced toxicity when co-administered with various antigens.15 Adjuvant systems in which MPLA is combined with an aluminum salt (AS04) or QS-21 (AS01B) have been approved for vaccines against human papillomavirus (Cervarix) and herpes zoster virus (Shingrix), respectively.
Indeed, MPLA was initially semi-synthesized from lipid A that is purified from fed-batch fermentation.16 The properties and bioactivity are strongly influenced by the heterogeneous chemical composition containing several carbohydrate functionalities, limiting its clinical application.15 To address this deficiency, chemosynthetic methods were established. These could provide practical access to structurally defined derivatives, contributing to structure–activity relationship analysis and structural modification. To date, many modified MPLA structures have been designed, synthesized and evaluated,12,13,17 revealing the important characteristics that affect the bioactivity of MPLA, such as the number, length, saturation and branching of fatty chains. However, the impact of functional groups at the C-1, C-4′ or C-6′ position of MPLA has been rarely reported. Kusama et al. synthesized three Escherichia coli (E. coli) MPLA analogs bearing a phosphonooxyethyl group with the α- or β-configuration at the C-1 position, and tested their antitumor activity.18,19 Mochizuki and co-workers reported a series of MPLA derivatives bearing a carboxylic acid group at the reducing end,20 and the effect of substituents at the C-6′-position including hydroxy, methoxy and fluorine groups was examined.21 Corsaro and co-workers presented a semisynthetic method to prepare C-6′ functionalized MPLA derivatives, which showed promise as immunoadjuvant candidates.22 These studies reveal that substituents at the C-1, C-4′ or C-6′ position also have substantial effects on the activity of MPLA.
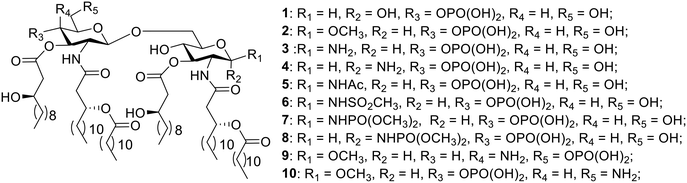
As part of our continuing study on the derivatization of MPLA,23,24 herein, the natural E. coli MPLA25 (1) and its nine derivatives were designed and synthesized to further investigate the influence of functional groups at the C-1, C-4′ and C-6′ positions (Fig. 1). A methoxy group (compound 2) was introduced to examine the influence of the conversion of a hydrogen donor to a hydrogen acceptor. An amino group, a bioisostere of the hydroxy group, was introduced to the C-1 position to explore whether it could increase the activity. As the configuration of the substituent affects the activity of carbohydrates, MPLA derivatives with β- and α-configurations at the C-1 position were both designed (compounds 3 and 4). As we know, lipid A, which has two phosphate groups at the C-1 and C-4′ positions, respectively, showed more activity and toxicity than MPLA. The objective of the introduction of different acyl groups including acetyl, mesyl and phosphoryl groups was to obtain higher activity and less toxic MPLA derivatives (compounds 5–8). Compounds 9 and 10 were synthesized to explore the influence of the site of phosphorylation. The potent adjuvant activities of these compounds were evaluated by mixing with a subunit vaccine candidate RBD-hFc protein against SARS-CoV-2. Through these studies, we anticipated identifying novel adjuvants with improved immunological properties useful for the development of RBD-hFc based SARS-CoV-2 vaccines.
Results and discussion
The synthesis of compounds 1–10
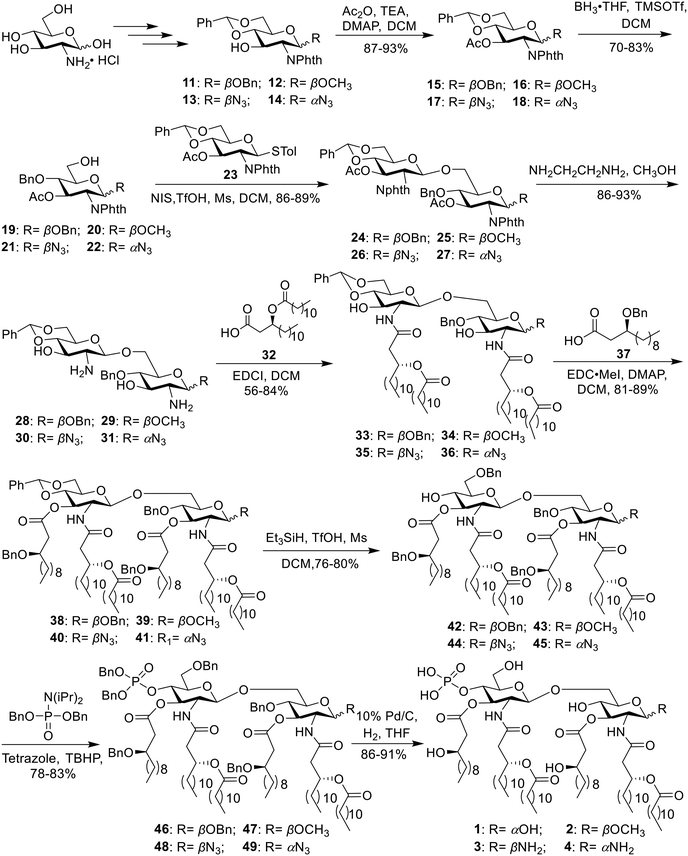
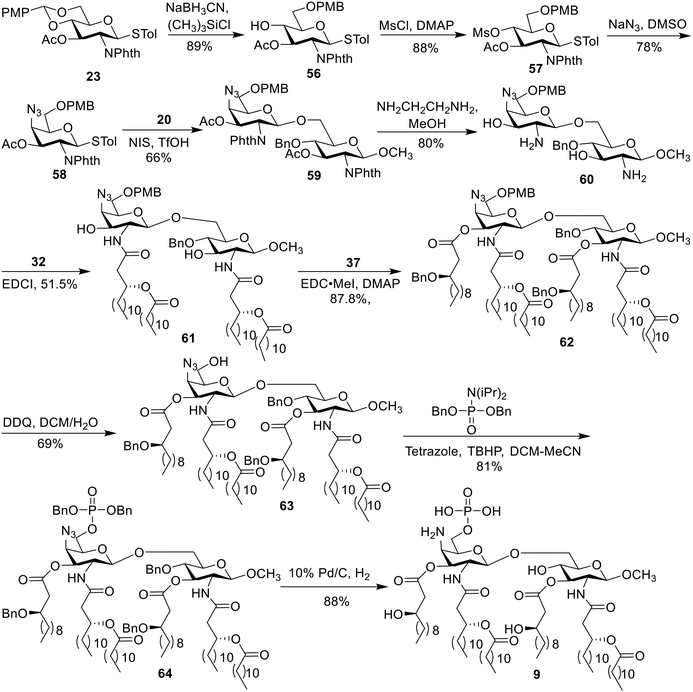
The chiral fatty acid 32, which was synthesized using published procedures from commercially available (R)-epichlorohydrin and 1-bromodecane,28 was simultaneously introduced to the free amino groups using 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDCI) as the promoter. Noteworthily, although two free hydroxyl groups were present in compounds 28–31, the reaction was selective for the amino groups, producing 33–36 in 78–84% yield. This is probably because the amino group is more active. Then, acylation of hydroxy groups was performed with benzyl-protected fatty acid 37 using 4-dimethylaminopyridine (DMAP) as a catalyst in the presence of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide methiodide (EDC·MeI) to give 38–41 in 81–89% yield. Next, the benzylidene ring in 38–41 was selectively opened from the 4′-O-position under Et3SiH and TfOH conditions, generating 42–45 with a free hydroxy group in 76–80% yield. Phosphorylation was subsequently accomplished using the phosphoramidite method in a two-step one-pot manner to produce intermediates 46–49 in 78–83% yield. Finally, all benzyl groups in 46–49 were removed along with the reduction of the azide group in 48 and 49 through hydrogenolysis catalyzed by Pd under a H2 atmosphere, affording the target products 1–4.
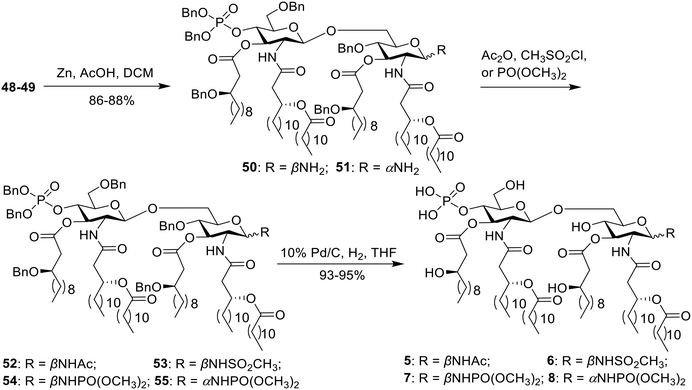
Compounds 5–8 were synthesized directly from the key intermediate 48 or 49 (Scheme 2). Initially, the azide group was reduced to a primary amine with zinc powder and acetic acid, delivering 50 and 51 in 86–88% yield. Then, acylation proceeded with acetic anhydride, methylsulfonyl chloride or trimethyl phosphite to give intermediates 52–55. Finally, hydrogenolysis was performed to remove all benzyl groups, yielding the desired compounds 5–8.
Immunological studies of compounds 1–10 adjuvanted RBD-hFc protein
With these target compounds in hand, we next explored their adjuvant activity by mixing with RBD-hFc fusion protein, a recombinant subunit vaccine that consists of a receptor-binding domain (RBD) in the spike protein of SARS-CoV-2 (residues 319–583) and human IgG1 Fc fragment linked by 6 amino acids (ADDDDK). These experiments were commenced using female BALB/c mice (6–7 weeks old). All animal studies were conducted under the Guiding Principles in the Care and Use of Animals (China) and were approved by the Laboratory Animal Ethics Committee of Guangzhou University of Chinese Medicine. Compounds 1–10 were prepared into liposomes using the sonication dispersion method with 1–10, cholesterol and 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC) in a molar ratio of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50
50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 65. For immunization, mice were intramuscularly injected with the mixture of RBD-hFc protein (5 μg) and the liposomes of compounds 1–10 (20 μg) or clinically used alum adjuvant (100 μg, positive control) on days 0 and 14. Each mouse was bled on days 14, 28 and 42 post-first immunization, and antisera were prepared with standard protocols. The titers of IgG and IgG isotype (IgG1, IgG2a, IgG2b, and IgG3) antibodies induced by the mixture of RBD-hFc protein and 1–10 (RBD-hFc/1–10) or alum (RBD-hFc/Al) were determined using enzyme-linked immunosorbent assay (ELISA).
65. For immunization, mice were intramuscularly injected with the mixture of RBD-hFc protein (5 μg) and the liposomes of compounds 1–10 (20 μg) or clinically used alum adjuvant (100 μg, positive control) on days 0 and 14. Each mouse was bled on days 14, 28 and 42 post-first immunization, and antisera were prepared with standard protocols. The titers of IgG and IgG isotype (IgG1, IgG2a, IgG2b, and IgG3) antibodies induced by the mixture of RBD-hFc protein and 1–10 (RBD-hFc/1–10) or alum (RBD-hFc/Al) were determined using enzyme-linked immunosorbent assay (ELISA).
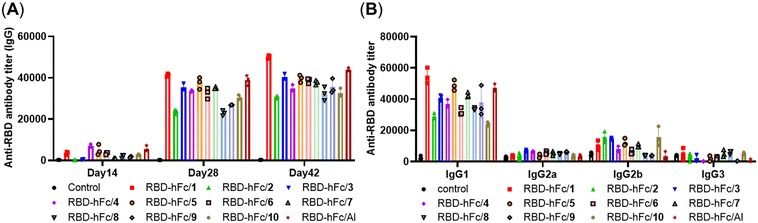
As shown in Fig. 2A, a degree of anti-RBD IgG antibodies in day 14 antisera of all groups was detected and the titers further increased after the booster immunization, revealing that all the mixtures of RBD-hFc with 1–10 or Al could trigger a robust immune response in mice. The IgG antibody titers in days 28 and 42 sera revealed that MPLA with different substituents had different effects on the adjuvant activity, although this difference did not reach statistical significance. Among them, compound 1 showed higher activity than Al, and the adjuvant activities of compounds 3, 5, 6 and 7 were comparable to that of Al. The analysis of IgG antibody titers in groups 1–3 suggested that the hydroxy group at the C-1 position of 1 substituted by an amino or methoxy group will slightly decrease the activity. The comparison of RBD-hFc/3 and RBD-hFc/4 revealed that the β-configuration of a substituent at the C-1 position may provoke a stronger immune response than the corresponding α-configuration. Compared to that by RBD-hFc/3, the level of IgG antibodies induced by RBD-hFc/5–7 did not obviously increase or decrease. These results indicated that the acylation of the amino group at the C-1 position may not have a significant impact on the activity. RBD-hFc/7 elicited a higher level of IgG antibodies than RBD-hFc/8, further demonstrating the benefit of the β-configuration of the substituent at the C-1 position. The comparison of RBD-hFc/2 and RBD-hFc/10 suggested that the hydroxy group at the C-6 position of 1 substituted by an amino group could achieve a similar result. Interestingly, we found that RBD-hFc/9 exhibited a better activity than RBD-hFc/10. This result suggested that phosphorylation of the C-6 position may improve the activity.
The analysis of IgG isotype antibodies presented mainly IgG1 and a low level of IgG2a, IgG2b, and IgG3 antibodies (Fig. 2B), revealing that all 1–10 and alum adjuvanted RBD-hFc vaccines elicited a predominantly humoral immune response, which is a feature of recombinant subunit protein vaccines. Compared to RBD-hFc/Al, MPLA derivative adjuvanted RBD-hFc showed higher IgG2b. This result demonstrated that a Th1-type response was triggered by the MPLA derivatives.
It has been reported that SARS-CoV-2 could bind to ACE2, facilitating human-to-human transmission.29 We next used fluorescence-activated cell sorting (FACS) assay to evaluate the ability of antisera to inhibit the RBD protein binding to ACE2. Human ACE2-transfected HEK293T cells (HEK293T/ACE2) were used in this study.30 The cells were incubated with RBD-His protein and diluted with day 28 antisera or PBS (positive control). For the negative control, neither antisera nor RBD-His was added.
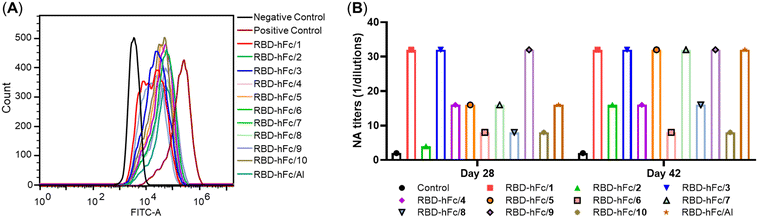
As shown in Fig. 3A, RBD-His protein exhibited a strong binding ability to HEK293T/ACE2 cells. Compared to it, the fluorescence peaks significantly shifted to the left in all RBD-hFc/1–10 and RBD-hFc/Al groups. These results revealed that all antisera induced by RBD-hFc/1–10 and RBD-hFc/Al could inhibit RBD-His binding to HEK293T/ACE2 cells. The median fluorescence intensity (MFI) of all groups follows the order: 9 ∼ 2 > 4 > 8 ∼ 6 > 5 ∼ Al > 10 > 3 > 7 ∼ 1. These results suggested that the natural E. coli MPLA and compound 7 exhibited the best activity.
The neutralization activities of days 28 and 42 antisera induced by all groups against live SARS-CoV-2 infection in Vero-E6 cells were also determined.30 As shown in Fig. 3B, the neutralizing antibody (NA) level reveals that the compounds 1, 3, 5, 7 and 9 adjuvanted groups elicit equal or stronger neutralizing titers than that adjuvanted with Al. The neutralizing titers of the 1–4 adjuvanted groups follow the order: 1 = 3 > 4 > 2. These results indicated that replacing the hydroxy group at the C-1 position of 1 by its bioisostere will decrease the activity. The comparison of 3 and 4 revealed that substituents with different configurations have obvious effects on the activity, and the β-configuration is a better choice, which is in accordance with the ELISA results. The comparison of the neutralizing titers induced by 3 and 5–8 suggested that the acylation of the amino group will decrease the activity. Unexpectedly, compound 9 with the phosphate group transferred into the C-6′ position and the original phosphate group at the C-4′ position replaced by an amino group exhibited comparable activity to 1. Compound 10 showed a similar NA titer to 2, further indicating that the hydroxy group at the C-6 position of 2 substituted by an amino group could achieve a similar result.
The efficacy of compound 1 combined with Al
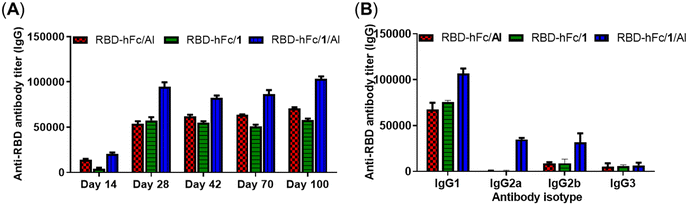
Encouraged by the above results, compound 1 was selected as a representative to explore whether MPLA derivatives could be combined with alum to further enhance the efficacy of RBD-hFc protein vaccines. Each group containing five mice was immunized with RBD-hFc/1, RBD-hFc/Al or RBD-hFc/1/Al with a dosage of 5 μg of RBD-hFc protein, 20 μg of 1 or/and 100 μg of Al using the same protocol as the above experiments. In order to test the effect in more detail, we extended the period of observation to 100 days in this study, and each mouse was bled on days 14, 28, 42, 70 and 100 post-first immunization.As shown in Fig. 4A, all antisera exhibited low IgG antibody titers on day 14. After booster immunization, the levels of IgG antibodies were effectively increased in all groups. The adjuvant activity of compound 1 and Al was still comparable. Compared to RBD-hFc/1 and RBD-hFc/Al, RBD-hFc/1/Al elicited much higher IgG antibody titers. After the booster immunization, the titers of IgG antibodies in day 28 antisera induced by RBD-hFc/1/Al is more than 1.6-fold that elicited by RBD-hFc/1 and RBD-hFc/Al (94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 537 vs. 53
537 vs. 53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 823 and 57
823 and 57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 386). It is noteworthy that the IgG titers still remained at a high level on days 42, 70 and 100. These results preliminary revealed that the combination of 1 and Al was feasible for immunotherapy, and a better immune response could be achieved than that with 1 or Al alone. Tests of the isotypes of the IgG antibody showed that the combination of 1 and Al could increase the production of IgG1, IgG2a and IgG2b (Fig. 4B). This result suggested that the combination of 1 and Al could boost both humoral and cellular responses. Compared to IgG1, IgG2a and IgG2b increased in a higher proportion. This result suggested that the combination of 1 and Al may efficiently promote the Th1-type immune response.
386). It is noteworthy that the IgG titers still remained at a high level on days 42, 70 and 100. These results preliminary revealed that the combination of 1 and Al was feasible for immunotherapy, and a better immune response could be achieved than that with 1 or Al alone. Tests of the isotypes of the IgG antibody showed that the combination of 1 and Al could increase the production of IgG1, IgG2a and IgG2b (Fig. 4B). This result suggested that the combination of 1 and Al could boost both humoral and cellular responses. Compared to IgG1, IgG2a and IgG2b increased in a higher proportion. This result suggested that the combination of 1 and Al may efficiently promote the Th1-type immune response.
Then, the interleukin 4 (IL-4) and gamma interferon (IFN-γ) levels in pooled antisera on day 28 were evaluated to investigate whether the combination of 1 and Al can provoke a higher production of cytokines. As shown in Fig. 5, all groups elicited high expressions of IL-4 and IFN-γ. Among them, RBD-hFc/1/Al exhibited the highest levels of IL-4 and IFN-γ. The level of IL-4 in the RBD-hFc/1/Al group was 1.9-fold higher than that of RBD-hFc/Al and RBD-hFc/1 alone (1884.5 vs. 987.2 and 819.6 pg mL−1). The level of IFN-γ in the RBD-hFc/1/Al group was more than 1.3-fold that of the RBD-hFc/Al and RBD-hFc/1 groups (43.4 vs. 32.5 and 17.4 pg mL−1). These results suggested that the combination of 1 and Al could increase the production of IL-4 and IFN-γ, enhancing the humoral and cellular responses.
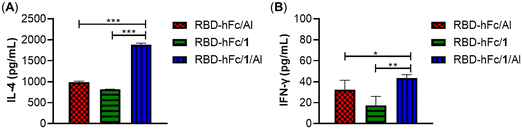 | ||
| Fig. 5 IL-4 (A) and IFN-γ (B) concentrations of day 28 antisera provoked by RBD-hFc/Al, RBD-hFc/1 and RBD-hFc/1/Al. Data are presented as mean ± SD; *p < 0.05, **p < 0.01, ***p < 0.001. | ||
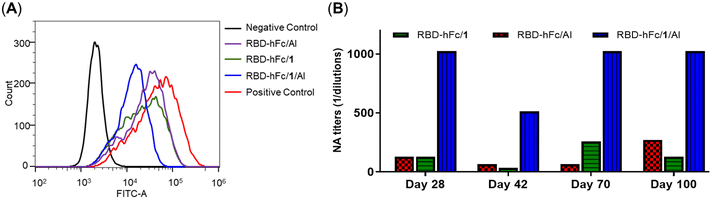
The ability of antisera to inhibit the RBD-His protein binding to HEK293T/ACE2 cells was also determined by FACS assay. As shown in Fig. 6A, compared to the positive control (RBD-His), all antisera exhibited the effective inhibition of RBD-His binding to HEK293T/ACE2 cells. The order of the inhibition is: RBD-hFc/1/Al > RBD-hFc/1 ∼ RBD-hFc/Al, suggesting the highest adjuvant activity of the combination of 1 and Al, which was consistent with the ELISA results.
The neutralizing antibody levels of RBD-hFc/Al, RBD-hFc/1 and RBD-hFc/1/Al are depicted in Fig. 6B. Compared to those in RBD-hFc/1 and RBD-hFc/Al, higher level neutralizing titers were also observed in the RBD-hFc/1/Al group. The titer in mice vaccinated with RBD-hFc/1/Al was 8-fold higher than that with RBD-hFc/1 or RBD-hFc/Al alone in day 28 sera. Similar to those by IgG antibodies, the neutralizing antibody titers induced by RBD-hFc/1/Al were also maintained at a high peak level of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1024 on day 100. These results further indicated that the combination of 1 and Al can more effectively improve the immunogenicity of the RBD-hFc protein.
1024 on day 100. These results further indicated that the combination of 1 and Al can more effectively improve the immunogenicity of the RBD-hFc protein.
Conclusion
TLR4 is a reliable target for the development of vaccine adjuvants and shows promising prospects. Though many MPLA analogs have been reported, the impact of functional groups at the C-1, C-4′ and C-6′ positions of MPLA has been rarely reported. To identify novel adjuvants with improved immunological properties, E. coli MPLA and its nine derivatives were designed using the bioisosterism strategy and synthesized through a facile and efficient method.Next, we explored the adjuvant activities of these compounds by mixing with the RBD-Fc fusion protein using the clinically used alum adjuvant as control. Immunological evaluation demonstrated that RBD-hFc could trigger a robust immune response in mice with the assistance of compounds 1–10. The adjuvant activities of 1–10 were almost comparable to that of Al, and compound 1 showed the best result. The introduction of substituents and the change of configuration may affect the binding of the MPLA derivative to the amino acid residues of TLR4 or MD-2. Moreover, the MPLA derivatives could trigger a Th1-type immune response, which may contribute to the clearance of the virus. FACS analysis revealed that all antisera could effectively inhibit RBD-His protein binding to HEK293T/ACE2 cells, and the effects of MPLA derivatives 1 and 7 were better than that of Al. The neutralizing antibody measurement reveals that the compounds 1, 3, 5, 7 and 9 adjuvanted groups elicit equal or stronger neutralizing titers than that adjuvanted with Al. Collectively, MPLA and its derivatives have the potential to be an alternative of the Al adjuvant used for the development of RBD-hFc based SARS-CoV-2 vaccines.
In addition, we found that the combination of 1 and Al was feasible for immunotherapy, which could achieve a better adjuvant activity than 1 or Al alone. Meanwhile, 1 combined with Al could efficiently promote the Th1-type immune response. Furthermore, RBD-hFc adjuvanted with 1 and Al could further effectively inhibit the binding of RBD-His to HEK293T/ACE2 cells, and elicited higher neutralizing antibody levels.
In conclusion, E. coli MPLA and its nine derivatives were designed and synthesized. The immunological evaluation revealed that MPLA derivatives are a promising platform for the development of novel adjuvants used for RBD-hFc based SARS-CoV-2 vaccines. Preliminary structure–activity relationship analysis demonstrated that the substituents at the C-1, C-4′ and C-6′ positions of E. coli MPLA have an effect on its biological activity, providing a reference for the structural modification of MPLA. The combination of MPLA and Al could be feasible for immunotherapy and further improve immune responses, providing a new direction toward the enhancement of RBD-hFc based SARS-CoV-2 vaccines.
Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
We gratefully acknowledge the financial support from the Emergency Key Program of Guangzhou Laboratory (Grant No. EKPG21-23), the Department of Science and Technology of Guangdong Province (Grant No. 2022B1111020005), the Key Laboratory of Guangdong Provincial Food and Drug Administration (2021ZDB03), the Department of Education of Guangdong Province, China (No. 2020KQNCX016), and the Basic and Applied Research Project of Guangzhou (202201011563).References
- A. Di Pasquale, S. Preiss, F. T. Da Silva and N. Garçon, Vaccines, 2015, 3, 320–343 CrossRef CAS.
- S. G. Reed, M. T. Orr and C. B. Fox, Nat. Med., 2013, 19, 1597–1608 CrossRef CAS.
- C. Pifferi, Nat. Rev. Chem., 2021, 5, 197–216 CrossRef CAS.
- C. Mohan and J. Zhu, Mediators Inflammation, 2010, 781235 Search PubMed.
- R. Medzhitov, P. Preston-Hurlburt and C. A. Janeway, Nature, 1997, 388, 394–397 CrossRef CAS PubMed.
- F. Cochet and F. Peri, Int. J. Mol. Sci., 2017, 18, 2318 CrossRef.
- T. L. Gioannini, A. Teghanemt, D. S. Zhang, N. P. Coussens, W. Dockstader, S. Ramaswamy and J. P. Weiss, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 4186–4191 CrossRef CAS.
- A. Brieger, L. Rink and H. Haase, J. Immunol., 2013, 191, 1808–1817 CrossRef CAS.
- V. Mata-Haro, C. Cekic, M. Martin, P. M. Chilton, C. R. Casella and T. C. Mitchell, Science, 2007, 316, 1628–1633 CrossRef CAS PubMed.
- A. Molinaro, O. Holst, F. Di Lorenzo, M. Callaghan, A. Nurisso, G. D'Errico, A. Zamyatina, F. Peri, R. Berisio, R. Jerala, J. Jiménez-Barbero, A. Silipo and S. Martín-Santamarí, Chem. – Eur. J., 2015, 21, 500–519 CrossRef CAS.
- D. H. Persing, R. N. Coler, M. J. Lacy, D. A. Johnson, J. R. Baldridge, R. M. Hershberg and S. G. Reed, Trends Microbiol., 2002, 10, 32–37 CrossRef.
- J. Gao and Z. Guo, Med. Res. Rev., 2018, 38, 556–601 CrossRef CAS.
- S. Federico, L. Pozzetti, A. Papa, G. Carullo, S. Gemma, S. Butini, G. Campiani and N. Relitti, J. Med. Chem., 2020, 63, 13466–13513 CrossRef CAS PubMed.
- D. A. Johnson, D. S. Keegan, C. G. Sowell, M. T. Livesay, C. L. Johnson, L. M. Taubner, A. Harris, K. R. Myers, J. D. Thompson, G. L. Gustafson, M. J. Rhodes, J. T. Ulrich, J. R. Ward, Y. M. Yorgensen, J. L. Cantrell and V. G. Brookshire, J. Med. Chem., 1999, 42, 4640–4649 CrossRef CAS PubMed.
- J. T. Ulrich and K. R. Myers, Pharm. Biotechnol., 1995, 6, 495–524 CAS.
- N. Qureshi, P. Mascagni, E. Ribi and K. Takayama, J. Biol. Chem., 1985, 260, 5271–5278 CrossRef CAS PubMed.
- A. Zamyatina and S. Strobl, Synthesis of bioactive lipid A and analogs, Elsevier Inc., 2020 Search PubMed.
- T. Kusama, T. Soga, E. Shioya, K. Nakayama, H. Nakajima, Y. Osada, Y. Ono, S. Kusumoto and T. Shiba, Chem. Pharm. Bull., 1990, 38, 3366–3372 CrossRef CAS PubMed.
- E. Kumazawa, A. Tohgo, T. Soga, T. Kusama and Y. Osada, Cancer Immunol. Immunother., 1992, 5461, 307–314 CrossRef.
- M. Shiozaki, S. Kurakata, T. Tatsuta, H. Maeda and M. Nishijima, Tetrahedron, 1997, 53, 16041–16060 CrossRef CAS.
- T. Mochizuki, Y. Iwano, M. Shiozaki, S. I. Kurakata, S. Kanai and M. Nishijima, Tetrahedron, 2000, 56, 7691–7703 CrossRef CAS.
- D. D'Alonzo, M. Cipolletti, G. Tarantino, M. Ziaco, G. Pieretti, A. Iadonisi, G. Palumbo, A. Alfano, M. Giuliano, M. De Rosa, C. Schiraldi, M. Cammarota, M. Parrilli, E. Bedini and M. M. Corsaro, Chem. – Eur. J., 2016, 22, 11053–11063 CrossRef.
- G. Liao, Z. Zhou, S. Suryawanshi, M. A. Mondal and Z. Guo, ACS Cent. Sci., 2016, 2, 210–218 CrossRef CAS PubMed.
- L. Gao, Q. Lian, L. Ma, S. Su, M. Yang, Y. Fang, Z. Liu, X. Luo and G. Liao, Chin. Chem. Lett., 2021, 32, 3011–3014 CrossRef CAS.
- Y. L. Tzeng, A. Datta, V. Kumar Kolli, R. W. Carlson and D. S. Stephens, J. Bacteriol., 2002, 184, 2379–2388 CrossRef CAS.
- G. Wasonga, Y. Tatara, I. Kakizaki and X. Huang, J. Carbohydr. Chem., 2013, 32, 392–409 CrossRef CAS PubMed.
- Q. Wang, J. Xue and Z. Guo, Chem. Commun., 2009, 5536–5537 RSC.
- L. Wang, S. Feng, S. Wang, H. Li, Z. Guo and G. Gu, J. Org. Chem., 2017, 82, 12085–12096 CrossRef CAS PubMed.
- D. Wrapp, N. Wang, K. S. Corbett, J. A. Goldsmith, C. L. Hsieh, O. Abiona, B. S. Graham and J. S. McLellan, Science, 2020, 367, 1260–1263 CrossRef CAS PubMed.
- X. Qi, B. Ke, Q. Feng, D. Yang, Q. Lian, Z. Li, L. Lu, C. Ke, Z. Liu and G. Liao, Chem. Commun., 2020, 56, 8683–8686 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2md00298a |
| This journal is © The Royal Society of Chemistry 2023 |