Design of LCST-type phase separation of poly(4-hydroxystyrene)†
Natsuki
Inaba
a,
Kota
Hashimoto
a,
Miwa
Kubota
a,
Keitaro
Matsuoka
 ab and
Kazuki
Sada
ab and
Kazuki
Sada
 *ab
*ab
aGraduate School of Chemical Sciences and Engineering, Hokkaido University, Sapporo 060-0810, Japan. E-mail: sadatcm@sci.hokudai.ac.jp
bDepartment of Chemistry, Faculty of Science, Hokkaido University, Sapporo 060-0810, Japan
First published on 30th September 2022
Abstract
Commercially available poly(4-hydroxystyrene) was introduced as a new LCST (lower critical solution temperature)-type thermo-responsive polymer at ambient temperature. The LCST-type phase separation was designed by control of the solvation and desolvation of the polymer chain in the mixtures of hydrogen-bonding solvents for solvation and non-polar solvents as a non-solvent for desolvation. As examples, the mixture of THF/1,2-dichloroethane (DCE) at a 3/7 ratio provided the LCST-type phase separation with the cloud point at 45 °C, monitored by the transmittance change. At the cloud point, the THF molecules were not able to solvate the polymer chain due to the thermal cleavage of the hydrogen bonds between the polymer and the THF molecules, which induced aggregation of the polymer chains. In conclusion, LCST-type thermo-responsive polymers can be easily realized by designing the hydrogen bonds between the polymer and the solvent molecules through controlling the solvation and desolvation of the polymer chain. This should provide another important molecular design for LCST-type thermo-responsive polymers in organic media.
Design, System, ApplicationWater-soluble amphiphilic polymers with a small alkyl group have been well accepted as LCST thermo-responsive polymers in water. However, designing LCST thermo-responsive homopolymers in organic media other than water is still difficult due to the lack of the grand design widely applicable for many organic homopolymers. In this report, we focus on designed LCST-type phase separation of poly(4-hydroxystyrene) in the mixtures of hydrogen-bonding solvents for solvation to the polymer chain and non-polar solvents as a non-solvent for desolvation. The cleavage of the hydrogen bond between the OH group of the polymer chain and the hydrogen-bonding groups of solvent molecules easily induced the LCST-type phase separation, which was realized in a wide range of the organic solvent mixtures. Within our knowledge, this is still a rare example for the designed LCST-type phase separation by using a commodity homopolymer. Combining our previous reports, this approach should provide a new class of LCST-type thermo-responsive polymers including many common organic homopolymers with a hydrogen bonding group such as poly(vinyl alcohol) (PVA) and poly(2-hydroxyethyl methacrylate) (polyHEMA). It will spark great interest for polymer scientists and materials engineers for smart materials such as polymer gels in water as well as in organic solvents. |
Introduction
Thermo-responsive polymers have been attracting considerable interest in polymer science due to the drastic coil–globule change of the polymer chain by a slight temperature change.1–5 Especially, the molecular designs of LCST-type thermo-responsive polymers in an aqueous solution have been widely studied such as poly(N-isopropylacrylamide) and poly(ethylene glycol). The coil–globule transition of the polymer chain in water and the desolvation of water molecules from the solvated polymer play a key role in designing the LCST-type phase separation. Water-soluble amphiphilic flexible polymers with a small alkyl group have been used as the typical design of the LCST-type thermo-responsive polymers.1–3,6–12 Many related amphiphilic homopolymers and copolymers have been developed toward stimuli-responsive polymers to various stimuli13–23 and biomedical applications.24–27Compared to these extensive studies of LCST-type thermo-responsive polymers in aqueous media in the past three decades, the studies in organic media have been limited due to the difficulties in designing the coil–globule transition of the polymer chain associated with solvation-desolvation of the solvent molecules. As classical examples, commodity organic polymers, such as polystyrene and polyethylene, have been documented for the LCST-type phase separation at an extremely high temperature due to the aggregation of the polymer chain by the vigorous motions of the solvent molecules.8,28–33 Although other LCST-type thermo-responsive polymers in organic solvents34–45 and ionic liquids46–58 have also been found, these findings were made case-by-case through trial-and-error or by chance, and the strategy for designing LCST-type polymers has not yet been clarified in media other than water. Therefore, it is impossible to predict what kinds of polymers will exhibit the LCST-type phase separation behavior in a given organic medium, and what kinds of solvents will be useful for the LCST-type phase separation of a given organic homopolymer.
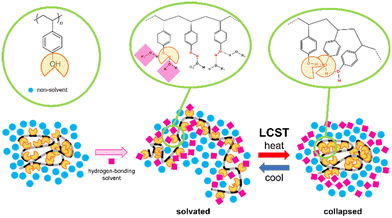
Recently, we have demonstrated LCST-type thermo-responsive polymers based on the supramolecular designs through the solvation and desolvation of the polymer chain, rather than the coil–globule transition. The addition of pseudo-solvating molecules, called effectors, to the polymer suspension in a non-solvent59–64 induced the LCST-type phase separation under ambient conditions in non-aqueous media. At room temperature, the polymer chain was solubilized by the complex formation with the effector, similar to solvation, and at elevated temperature, the polymer chain collapsed by the dissociation of the effector from the polymer complex, similar to desolvation. The two kinds of supramolecular interactions, hydrogen bonding61–64 and charge transfer complexation,59,60 were used to realize a desirable LCST-type phase separation. However, these designs were not accepted as the general strategy for the LCST-type phase separation because they were constructed by controlling the supramolecular interactions between specially-designed polymers and specially-designed effectors. In this report, our aim is to demonstrate the applicability of this molecular design and to investigate what kinds of solvent mixtures with hydrogen-bonding solvent and non-solvent will be efficient for the LCST-phase separations by using poly(4-hydroxystyrene) (PHS) as one of the commercially available commodity polymers. PHS has been well investigated as a dielectric layer in organic electronics and the plastic base of photolithography as a chemically-amplified resist.65–68 The polymer blends with hydrogen-bonding polymers have been studied as the control regarding the properties in the bulk phase.69–73 The solution property was investigated to determine the solubility parameters of PHS.74–76 However, to date, few attempts have been carried out for the investigation of thermal change of solubility in the organic solution. Therefore, for designing the LCST-type phase separation, we selected PHS as a candidate due to the hydroxyl group in the phenol moiety as the solvation group through hydrogen bonding and the polystyrene group as a flexible and hydrophobic polymer chain compatible with non-polar solvents. To control of solvation and desolvation of PHS, the mixtures of a hydrogen-bonding solvent as an effector and a non-polar solvent as the non-solvent were used as schematically shown in Fig. 1.
Results and discussion
According to a previous report,76 we first confirmed the solubility of PHS (average Mw: ∼25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 purchased from Sigma-Aldrich) in organic solvents. Most of the polar solvents such as DMF, alcohols, ketones, and esters, were good solvents of PHS (ca. 25 mg mL−1) at low temperature (0 °C), room temperature (ca. 25 °C) and elevated temperature (ca. 100 °C) due to the formation of hydrogen bonds with the phenol group of the polymer chain. Non-polar solvents, such as hexane, toluene, and DCE, were practically immiscible at these temperatures. We then investigated the thermal behaviors in the mixtures of these solvents at various mixing ratios. As a typical example, the polymer solution (ca. 25 mg mL−1) in the mixtures of THF and DCE resulted in a thermal response around the ratio of 3.0
000 purchased from Sigma-Aldrich) in organic solvents. Most of the polar solvents such as DMF, alcohols, ketones, and esters, were good solvents of PHS (ca. 25 mg mL−1) at low temperature (0 °C), room temperature (ca. 25 °C) and elevated temperature (ca. 100 °C) due to the formation of hydrogen bonds with the phenol group of the polymer chain. Non-polar solvents, such as hexane, toluene, and DCE, were practically immiscible at these temperatures. We then investigated the thermal behaviors in the mixtures of these solvents at various mixing ratios. As a typical example, the polymer solution (ca. 25 mg mL−1) in the mixtures of THF and DCE resulted in a thermal response around the ratio of 3.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
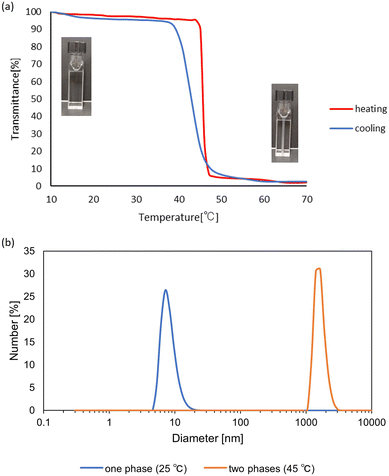
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.0 (molar ratio) for THF and DCE. The transmittance measurement at 800 nm at various temperatures demonstrated the LCST-type phase separation around 45 °C as the cloud point. As expected, at the lower temperature, the mixture was a clear solution, and the solution became turbid at the elevated temperature to collapse the polymer chain, as shown in Fig. 2a. The thermal dissociation of THF from the solvated PHS should occur. The desolvation of THF played a key role in the coil–globule change and the growth of the polymer particles for the LCST-type phase separation. This was also confirmed by the slight growth of the polymer particles of PHS by elevating the solution temperature from 25 °C to 40 °C in the dynamic light scattering (DLS) measurement, as shown in Fig. 2b. This is the first observation of the LCST-type phase separation of PHS at ambient temperature.
7.0 (molar ratio) for THF and DCE. The transmittance measurement at 800 nm at various temperatures demonstrated the LCST-type phase separation around 45 °C as the cloud point. As expected, at the lower temperature, the mixture was a clear solution, and the solution became turbid at the elevated temperature to collapse the polymer chain, as shown in Fig. 2a. The thermal dissociation of THF from the solvated PHS should occur. The desolvation of THF played a key role in the coil–globule change and the growth of the polymer particles for the LCST-type phase separation. This was also confirmed by the slight growth of the polymer particles of PHS by elevating the solution temperature from 25 °C to 40 °C in the dynamic light scattering (DLS) measurement, as shown in Fig. 2b. This is the first observation of the LCST-type phase separation of PHS at ambient temperature.
Further control of the cloud point was investigated by changing the mixing ratios. The polymer was practically insoluble in the mixed solvent at ratios of THF and DCE lower than 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.5 and soluble in the mixed solvent at ratios higher than 3.8
7.5 and soluble in the mixed solvent at ratios higher than 3.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.2 between 0 °C and 100 °C. At the solvent ratios from 2.6
6.2 between 0 °C and 100 °C. At the solvent ratios from 2.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.4 to 3.7
7.4 to 3.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
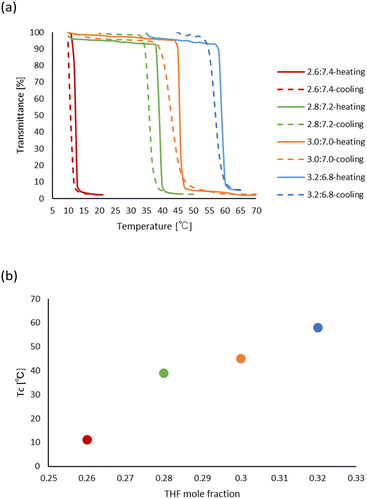
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.3, PHS showed the LCST-type phase separations, and the cloud point increased as the THF ratio raised, as shown in Fig. 3a. Additionally, as shown in Fig. 3b, the linear plot of the cloud point vs. the mol fraction of THF in the mixtures demonstrated the easy control of the phase separation temperature. As a higher temperature was required for the desolvation with the cleavage of the hydrogen bonds between the polymer and THF by increasing the amount of THF, the thermal cleavage of the hydrogen bonds between THF and PHS would induce the LCST-type phase separations.
6.3, PHS showed the LCST-type phase separations, and the cloud point increased as the THF ratio raised, as shown in Fig. 3a. Additionally, as shown in Fig. 3b, the linear plot of the cloud point vs. the mol fraction of THF in the mixtures demonstrated the easy control of the phase separation temperature. As a higher temperature was required for the desolvation with the cleavage of the hydrogen bonds between the polymer and THF by increasing the amount of THF, the thermal cleavage of the hydrogen bonds between THF and PHS would induce the LCST-type phase separations.
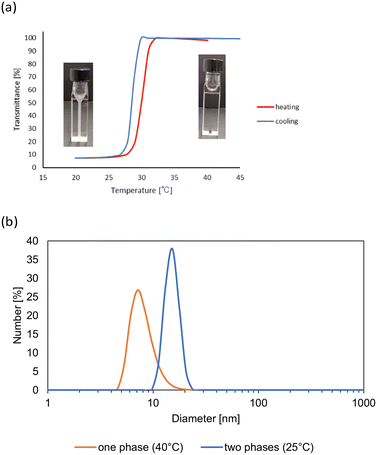
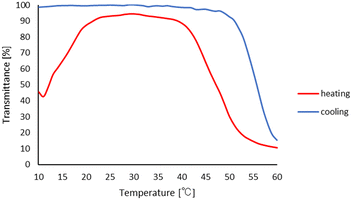
Subsequently, we investigated the effects of the hydrogen-bonding solvents for the thermo-response of PHS in the mixtures with DCE as the non-solvent. As summarized in Table 1, the common hydrogen-bonding solvents, such as amides, carboxylic acids, ketones, ethers, alcohols, nitriles, and esters, showed the thermo-responsive behaviors. Most of the polar solvents we investigated, such as DMF, methanol, 2-butanone, and ethyl acetate, provided the LCST-type phase separations. The versatility of the hydrogen-bonding solvents from 1,4-dioxane (ε = 2) to DMF (ε = 52) indicated the wide applicability of the coil–globule transition and LCST-type thermo-response of PHS in the media with a wide range of polarity. On the other hand, when acetic acid or nitriles was used as hydrogen-bonding solvents with DCE as a non-solvent, UCST (upper critical solution temperature)-type phase separation was observed. In these mixtures, PHS became soluble at the elevated temperature, which was confirmed by the transmittance change of the solution. As shown in Fig. 4a, the reversible transmittance change occurred during the change in the temperature, and the DLS measurement in Fig. 4b indicated the change in the polymer state between 25 °C and 40 °C. These observations might be derived from low solvating ability via the hydrogen bonds of acetic acid or nitriles. In both cases, an elevated temperature should be required for the solvation of the polymer chain to provide the UCST-type thermal response, and no further LCST-type thermal response was observed at the higher temperature.
| Hydrogen-bonding solvent | Dielectric constant (ε) | Mol. ratio hydrogen-bonding solvent![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DCE DCE |
Phase separation (cloud point) |
|---|---|---|---|
| a The conditions: PHS (ca. 25 mg) in the mixed organic solvents (1.0 mL) from ca. 20 °C to ca. 100 °C. b Cloud point was determined by a transmittance change (90% of transmission) at 800 nm. For detailed measurement conditions and results, see the ESI.† | |||
| DMF | 52 | 1.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8.4 8.4 |
LCST (34 °C) |
| DMSO | 46 | 2.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8.0 8.0 |
LCST |
| Dimethylacetoamide | 38 | 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9.0 9.0 |
LCST |
| Acetonitrile | 36 | 6.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.0 4.0 |
UCST |
| Methanol | 33 | 3.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.3 6.3 |
LCST (43 °C) |
| Propionitrile | 29 | 6.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.0 4.0 |
UCST |
| Ethanol | 25 | 2.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.1 7.1 |
LCST (68 °C) |
| Benzonitrile | 25 | 9.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 1.0 |
UCST(29 °C) |
| Tetramethylurea | 24 | 0.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9.2 9.2 |
LCST |
| 1-Propanol | 21 | 2.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.6 7.6 |
LCST (75 °C) |
| 2-Butanone | 19 | 4.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.7 5.7 |
LCST (37 °C) |
| 2-Methyl-1-propanol | 18 | 2.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8.0 8.0 |
LCST |
| 1-Butanol | 18 | 2.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8.0 8.0 |
LCST (75 °C) |
| 2-Butanol | 17 | 2.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8.0 8.0 |
LCST |
| Pyridine | 13 | 2.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8.0 8.0 |
LCST (43 °C) |
| 1-Hexanol | 13 | 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8.5 8.5 |
LCST (60 °C) |
| 2-Methyl-2-propanol | 12 | 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.5 7.5 |
LCST |
| 1-Octanol | 11 | 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8.5 8.5 |
LCST |
| THF | 7 | 3.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.0 7.0 |
LCST (45 °C) |
| 1,2-Dimethoxyethane | 7 | 4.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.5 5.5 |
LCST |
| Acetic acid | 6 | 8.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.0 2.0 |
UCST (26 °C) |
| Ethyl acetate | 6 | 4.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.3 5.3 |
LCST (24 °C) |
| 1,4-Dioxane | 2 | 4.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.0 6.0 |
LCST (53 °C) |
To gain insights into the role of the non-solvents, we investigated the thermal behaviors in the mixtures of toluene or hexane instead of DCE with THF as the hydrogen-bonding solvent, as summarized in Table S1 in ESI.† Although the compatibility to PHS decreased, the tendency of the thermo-response was not significantly changed except for the mixed solvents of hexane and THF. The amount of the non-solvent decreased with the decreasing polarity and compatibility of the media. For the solvation, the higher amount of the hydrogen-bonding solvents should be required. As shown in Fig. 5, the UCST-LCST double phase separation was observed in the mixture at 9.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 for THF and hexane. The small increase in the mol fraction of hexane allowed the disappearance of the thermal phase separations to be totally insoluble. The increased incompatibility in the hexane mixtures induced the generation of the UCST-type phase separation at the lower temperature than the LCST-type phase separation due to the formation of the collapsed state by the aggregation of the polymer chain.
1.0 for THF and hexane. The small increase in the mol fraction of hexane allowed the disappearance of the thermal phase separations to be totally insoluble. The increased incompatibility in the hexane mixtures induced the generation of the UCST-type phase separation at the lower temperature than the LCST-type phase separation due to the formation of the collapsed state by the aggregation of the polymer chain.
Conclusions
In this report, we demonstrated the LCST-type phase separation of PHS in various organic media at ambient temperature as an example of commodity organic polymers with a hydrogen-bonding group. Many mixtures of a hydrogen-bonding solvent and a non-polar solvent provided the LCST-type phase separation. The thermal cleavage of the hydrogen bonds between the solvent molecule and the polymer chain induced the collapse of the polymer chain by heating. Therefore, the LCST-type phase separation can be easily realized by controlling the hydrogen bonds between the polymer chain and the solvent molecules in the mixtures of a hydrogen-bonding solvent and a non-polar solvent. Since many commercially available organic polymers have a hydrogen bonding group in the polymer chain such as poly(vinyl alcohol) (PVA), poly(2-hydroxyethyl methacrylate) (polyHEMA), poly(acrylic acid) (PAA) and poly (N-isopropylacrylamide) (PNIPAM), they could serve as LCST-type thermo-responsive polymers in suitable organic solvent mixtures other than water. This strategy should be a new powerful method for designing the LCST-type phase separation for various polymers. Further exploration of the LCST-type phase separations of the common organic polymers with the hydrogen-bonding group is under current investigation.Author contributions
Natsuki Inaba: investigation, methodology, visualisation. Kota Hashimoto: investigation, methodology. Miwa Kubota: investigation, methodology. Keitaro Matsuoka: investigation, supervision, writing – review & editing. Kazuki Sada: conceptualization, funding acquisition, project administration, supervision, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge Prof. K. Konishi in HU for DLS measurement. This work was supported in part by JSPS KAKENHI Grant Number JP18H04638, JP20H04796 in Hybrid Catalysis, and JP21H01980 for K. S.Notes and references
- V. Aseyev, H. Tenhu and F. M. Winnik, Adv. Polym. Sci., 2011, 242, 29 CrossRef CAS.
- D. Roy, W. L. A. Brooks and B. S. Sumerlin, Chem. Soc. Rev., 2013, 42, 7214 RSC.
- C. Zhao, Z. Ma and X. X. Zhu, Prog. Polym. Sci., 2019, 90, 269 CrossRef CAS.
- Q. Zhang and R. Hoogenboom, Prog. Polym. Sci., 2015, 48, 122 CrossRef CAS.
- J. Seuring and S. Agarwal, ACS Macro Lett., 2013, 2, 597 CrossRef CAS PubMed.
- A. Halperin, M. Kröger and F. M. Winnik, Angew. Chem., Int. Ed., 2015, 54, 15342 CrossRef CAS.
- H. G. Schild, Prog. Polym. Sci., 1992, 17, 163 CrossRef CAS.
- S. Saeki, N. Kuwahara, M. Nakata and M. Kaneko, Polymer, 1976, 17, 685 CrossRef CAS.
- C. Weber, R. Hoogenboom and U. S. Schubert, Prog. Polym. Sci., 2012, 37, 686 CrossRef CAS.
- F. A. Plamper, A. Schmalz, M. Ballauff and A. H. E. Müller, J. Am. Chem. Soc., 2007, 129, 14538 CrossRef CAS PubMed.
- J. F. Lutz, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 3459 CrossRef CAS.
- J. F. Lutz, Ö. Akdemir and A. Hoth, J. Am. Chem. Soc., 2006, 128, 13046 CrossRef CAS PubMed.
- M. Irie and D. Kungwatchakun, Proc. Jpn. Acad., Ser. B, 1992, 68, 127 CrossRef CAS.
- M. Irie, Adv. Polym. Sci., 1993, 110, 49 CrossRef CAS.
- D. Roy, J. N. Cambre and B. S. Sumerlin, Prog. Polym. Sci., 2010, 35, 278 CrossRef CAS.
- J. M. Korde and B. Kandasubramanian, Ind. Eng. Chem. Res., 2019, 58, 9709 CrossRef CAS.
- M. A. C. Stuart, W. T. S. Huck, J. Genzer, M. Müller, C. Ober, M. Stamm, G. B. Sukhorukov, I. Szleifer, V. V. Tsukruk, M. Urban, F. Winnik, S. Zauscher, I. Luzinov and S. Minko, Nat. Mater., 2010, 9, 101 CrossRef PubMed.
- F. Liu and M. W. Urban, Prog. Polym. Sci., 2010, 35, 3 CrossRef CAS.
- S. Wang, Q. Liu, L. Li and M. W. Urban, Macromol. Rapid Commun., 2021, 42, 1 Search PubMed.
- M. Wei, Y. Gao, X. Li and M. J. Serpe, Polym. Chem., 2017, 8, 127 RSC.
- Y. F. Gao, M. L. Wei, X. Li, W. W. Xu, A. Ahiabu, J. Perdiz, Z. N. Liu and M. J. Serpe, Macromol. Res., 2017, 25, 513 CrossRef CAS.
- F. D. Jochum and P. Theato, Chem. Soc. Rev., 2013, 42, 7468 RSC.
- P. Schattling, F. D. Jochum and P. Theato, Polym. Chem., 2014, 5, 25 RSC.
- E. S. Gil and S. M. Hudson, Prog. Polym. Sci., 2004, 29, 1173 CrossRef CAS.
- D. Schmaljohann, Adv. Drug Delivery Rev., 2006, 58, 1655 CrossRef CAS PubMed.
- J. F. Mano, Adv. Eng. Mater., 2008, 10, 515 CrossRef CAS.
- S. Ganta, H. Devalapally, A. Shahiwala and M. Amiji, J. Controlled Release, 2008, 126, 187 CrossRef CAS.
- S. Saeki, N. Kuwahara, S. Konno and M. Kaneko, Macromolecules, 1973, 6, 246 CrossRef CAS.
- S. Saeki, N. Kuwahara and S. Konno, Macromolecules, 1973, 6, 589 CrossRef CAS.
- A. Imre and W. A. vanHook, J. Polym. Sci., Part B: Polym. Phys., 1996, 34, 751 CrossRef CAS.
- B. Folie and M. Radosz, Ind. Eng. Chem. Res., 1995, 34, 1501 CrossRef CAS.
- N. Kuwahara, S. Saeki, T. Chiba and M. Kaneko, Polymer, 1974, 15, 777 CrossRef CAS.
- M. Schnell, S. Stryuk and B. A. Wolf, Ind. Eng. Chem. Res., 2004, 43, 2852 CrossRef CAS.
- Z. Liu, Y. Guo and K. Inomata, Polym. J., 2011, 43, 676 CrossRef CAS.
- Y. Guo, Z. Liu and K. Inomata, Fluid Phase Equilib., 2012, 315, 29 CrossRef CAS.
- K. Grygiel, W. Zhang, C. Detrembleur and J. Yuan, RSC Adv., 2016, 6, 57117 RSC.
- S. Matsumura, A. R. Hlil, C. Lepiller, J. Gaudet, D. Guay, Z. Shi, S. Holdcroft and A. S. Hay, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 7207 CrossRef.
- K. Wang, Q. Liu, G. Liu and Y. Zeng, Polymer, 2020, 203, 122746 CrossRef CAS.
- S. Li, L. Feng, H. Lu and S. Feng, New J. Chem., 2017, 41, 1997 RSC.
- S. Jana, M. Anas, T. Maji, S. Banerjee and T. K. Mandal, Polym. Chem., 2019, 10, 526 RSC.
- E. Cazares-Cortes, B. C. Baker, K. Nishimori, M. Ouchi and F. Tournilhac, Macromolecules, 2019, 52, 5995 CrossRef CAS.
- J. Lee, B. Lee, J. Park, J. Oh, T. Kim, M. Seo and S. Y. Kim, Polymer, 2018, 153, 430 CrossRef CAS.
- K. Pagonis and G. Bokias, Polymer, 2004, 45, 2149 CrossRef CAS.
- N. Metz and P. Theato, Eur. Polym. J., 2007, 43, 1202 CrossRef CAS.
- E. Sato, Y. Masuda, J. Kadota, T. Nishiyama and H. Horibe, Eur. Polym. J., 2015, 69, 605 CrossRef CAS.
- T. Ueki and M. Watanabe, Bull. Chem. Soc. Jpn., 2012, 85, 33 CrossRef CAS.
- T. Ueki, Polym. J., 2014, 46, 646 CrossRef CAS.
- T. Ueki, A. A. Arai, K. Kodama, S. Kaino, N. Takada, T. Morita, K. Nishikawa and M. Watanabe, Pure Appl. Chem., 2009, 81, 1829 CrossRef CAS.
- Y. Kohno, S. Saita, Y. Men, J. Yuan and H. Ohno, Polym. Chem., 2015, 6, 2163 RSC.
- Y. Kobayashi, Y. Kitazawa, K. Hashimoto, T. Ueki, H. Kokubo and M. Watanabe, Langmuir, 2017, 33, 14105 CrossRef CAS PubMed.
- W. Li and P. Wu, Polym. Chem., 2014, 5, 5578 RSC.
- H. Rodríguez and R. D. Rogers, Fluid Phase Equilib., 2010, 294, 7 CrossRef.
- Y. Biswas, P. Banerjee and T. K. Mandal, Macromolecules, 2019, 52, 945 CrossRef.
- D. Yokota, A. Kanazawa and S. Aoshima, Macromolecules, 2019, 52, 6241 CrossRef CAS.
- A. Kharel, C. Hall, P. Černoch, P. Stepanek and T. P. Lodge, Macromolecules, 2020, 53, 885 CrossRef CAS.
- H. N. Lee and T. P. Lodge, J. Phys. Chem. Lett., 2010, 1, 1962 CrossRef CAS.
- H. N. Lee and T. P. Lodge, J. Phys. Chem. B, 2011, 115, 1971 CrossRef CAS PubMed.
- W. Li and P. Wu, Polym. Chem., 2014, 5, 761 RSC.
- S. Amemori, K. Kokado and K. Sada, Angew. Chem., Int. Ed., 2013, 52, 4174 CrossRef CAS PubMed.
- D. H. Gharib, S. Amemori, M. Naya, K. Kokado and K. Sada, RSC Adv., 2015, 5, 89319 RSC.
- S. Amemori, K. Iseda, S. Anan, T. Ono, K. Kokado and K. Sada, Polym. Chem., 2017, 8, 3921 RSC.
- M. Naya, K. Kokado and K. Sada, ACS Appl. Polym. Mater., 2020, 2, 4415 CrossRef CAS.
- S. Amemori, K. Kokado and K. Sada, J. Am. Chem. Soc., 2012, 134, 8344 CrossRef CAS.
- M. Naya, K. Kokado, K. B. Landenberger, S. Kanaoka, S. Aoshima and K. Sada, Macromol. Chem. Phys., 2020, 221, 1900455 CrossRef CAS.
- M. H. Yoon, H. Yan, A. Facchetti and T. J. Marks, J. Am. Chem. Soc., 2005, 127, 10388 CrossRef CAS PubMed.
- L. Wang, M. H. Yoon, G. Lu, Y. Yang, A. Facchetti and T. J. Marks, Nat. Mater., 2006, 5, 893 CrossRef CAS.
- A. Takahashi and H. Yamamoto, Polym. J., 1980, 12, 79 CrossRef CAS.
- S. H. Ko, H. Pan, C. P. Grigoropoulos, C. K. Luscombe, J. M. J. Fréchet and D. Poulikakos, Nanotechnology, 2007, 18, 345202 CrossRef.
- M. T. Garay, M. Rodriguez, J. L. Vilas and L. M. León, J. Macromol. Sci., Part B: Phys., 2004, 43, 437 CrossRef.
- H. L. Chen, S. F. Wang and T. L. Lin, Macromolecules, 1998, 31, 8924 CrossRef CAS.
- D. Li and J. Brisson, Polymer (Guildf), 1998, 39, 793 CrossRef CAS.
- P. Xing, L. Dong, Y. An, Z. Feng, M. Avella and E. Martuscelli, Macromolecules, 1997, 30, 2726 CrossRef CAS.
- A. Compostizo, S. M. Cancho, R. G. Rubio and A. C. Colin, J. Phys. Chem., 1995, 99, 10261 CrossRef CAS.
- G. Luengo, G. Rojo, R. G. Rubio, M. G. Prolongo and R. M. Masegosa, Macromolecules, 1991, 24, 1315 CrossRef CAS.
- S. Arichi, N. Sakamoto, M. Yoshida and S. Himuro, Polymer, 1986, 27, 1761 CrossRef CAS.
- S. Arichi and S. Himuro, Polymer, 1989, 30, 686 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2me00165a |
| This journal is © The Royal Society of Chemistry 2023 |