Dual-infinite coordination polymer-engineered nanomedicines for dual-ion interference-mediated oxidative stress-dependent tumor suppression†
Junlie
Yao
ab,
Jie
Xing
a,
Fang
Zheng
a,
Zihou
Li
a,
Shunxiang
Li
a,
Xiawei
Xu
a,
Devrim
Unay
d,
Young Min
Song
e,
Fang
Yang
 *ac and
Aiguo
Wu
*ac and
Aiguo
Wu
 *ac
*ac
aCixi Institute of Biomedical Engineering, International Cooperation Base of Biomedical Materials Technology and Application, Chinese Academy of Sciences (CAS) Key Laboratory of Magnetic Materials and Devices, Zhejiang Engineering Research Center for Biomedical Materials, Ningbo Institute of Materials Technology and Engineering, CAS, Ningbo 315201, China. E-mail: yangf@nimte.ac.cn; aiguo@nimte.ac.cn
bUniversity of Chinese Academy of Sciences, No. 1 Yanqihu East Road, Huairou District, Beijing, 101408, China
cAdvanced Energy Science and Technology Guangdong Laboratory, Huizhou 516000, China
dElectrical and Electronic Engineering Izmir Democracy University, Karabaglar 35140, Turkey
eSchool of Electrical Engineering and Computer Science, Gwangju Institute of Science and Technology, Gwangju 61005, Republic of Korea
First published on 10th March 2023
Abstract
Recently, nanomedicine design has shifted from simple nanocarriers to nanodrugs with intrinsic antineoplastic activities for therapeutic performance optimization. In this regard, degradable nanomedicines containing functional inorganic ions have blazed a highly efficient and relatively safe ion interference paradigm for cancer theranostics. Herein, given the potential superiorities of infinite coordination polymers (ICPs) in degradation peculiarity and functional integration, a state-of-the-art dual-ICP-engineered nanomedicine is elaborately fabricated via integrating ferrocene (Fc) ICPs and calcium–tannic acid (Ca–TA) ICPs. Thereinto, Fc ICPs, and Ca–TA ICPs respectively serve as suppliers of ferrous iron ions (Fe2+) and calcium ions (Ca2+). After the acid-responsive degradation of ICPs, released TA from Ca–TA ICPs facilitated the conversion of released ferric iron (Fe3+) from Fc ICPs into highly active Fe2+. Owing to the dual-path oxidative stress and neighboring effect mediated by Fe2+ and Ca2+, such a dual-ICP-engineered nanomedicine effectively induces dual-ion interference against triple-negative breast cancer (TNBC). Therefore, this work provides a novel antineoplastic attempt to establish ICP-engineered nanomedicines and implement ion interference-mediated synergistic therapy.
New conceptsAs a promising oncotherapy paradigm with low invasiveness and high controllability, the exploitation of nanoprodrugs is still in its infancy. This work proposes the concept of two-or-more-infinite coordination polymer (ICP)-engineered nanomedicines and confirms their feasibility as a nanoprodrug. Concretely, acid-responsive degradable Fc@Ca–TA nanosystems have been fabricated in a novel way without following cumbersome synthetic procedures using integrating ferrocene (Fc) derivative-ferric iron (Fe3+) ICPs and tannic acid (TA)–calcium (Ca2+) ICPs. Thereinto, Ca–TA ICPs, which have never been reported in the nanotechnology and medicine fields, realize the improvement of Ca2+ overload nanoplatforms, since previously reported nanoplatforms were mainly CaCO3, CaO2, or CaP-based nanomaterials. Furthermore, dual-ion interference (a cutting-edge antitumor strategy) based on Fc@Ca–TA nanomedicines, namely ferrous iron ion (Fe2+) based reactive oxygen species (ROS) generation and Ca2+ based overload effect, can significantly realize synergistically induced oxidative stress against triple-negative breast cancer (TNBC). In addition, the neighboring effect based on ion interference has been innovatively proposed and initially verified. As the first report about two-or-more-ICP-engineered nanomedicines for multi-ion interference, this work holds great potential for the establishment of nanoprodrugs and the advancement of synergistic tumor therapy. |
Introduction
Recent decades have witnessed the blooming of nanomedicines and realize a paradigm shift in oncotherapy and secure their places as “magic bullets” driven by the breakthrough of nanomaterials.1 Significantly distinguished from the first-generation nanomedicines dedicated to the morphological and structural regulation for efficient drug payload, the latest-generation nanomedicines have transferred their functionality from nanocarriers to nanodrugs featuring intrinsic antineoplastic activities.2–4 Impressively, this trend has brought about numerous superiorities, such as optimized therapeutic efficiency (integrating antitumor effects and nano-level pharmacokinetics), simplified synthesis procedure (omitting drug encapsulation) as well as biosecurity management (no longer administering the toxicity and immunogenicity profiles of nanocarriers and drugs separately).5 Nevertheless, the adverse effects of such nanomedicines remain a stumbling block, since they generally offer high natural toxicity to achieve potent antitumor efficacy. Nanoprodrugs, a promising concept to solve the aforementioned dilemma, are extremely low-toxic drug precursors meeting the requirements to become active therapeutic agents in the target region through chemical conversion.6 In this sense, the exploitation of nanoprodrugs based on revolutionized nanomaterial combination and design is expected to blaze a new path for the next-generation nanomedicines, yet a fairly slow, challenging, and demanding process.As an essential property of nanomedicines in biological applications, biodegradability is tremendously arousing great interest and offering significant inspiration recently.7 Depending on the chemical composition, the degradation of nanomedicines can generate a mass of organic molecules and inorganic ions, which used to be regarded as waste components and smoothly discharged from the body.8 Nowadays, ion interference has been a central antitumor topic overturning the traditional thinking mode, which is implicated in the aberrant distribution of ions in cancer cells and the resulting dysfunction of biological processes (e.g., irreversible physical damage, cytotoxic ingredient generation, and apoptosis induction).9,10 For instance, calcium (Ca2+) overload is eligible to induce severe oxidative stress, which involves reactive oxygen species (ROS) generation, mitochondrial morphology change, and mitochondrial respiration disturbance.11 Meanwhile, ferrous iron (Fe2+) can catalyze the hydrogen peroxide (H2O2) over-expressed in tumor mitochondria through a localized Fenton reaction, thus yielding highly active hydroxyl radical (˙OH) to trigger mitochondrial dysfunction.12 Conceivably, the variety and quantity of therapeutic ions for ion interference can be predesigned in the fabrication of nanoparticles (NPs) and generated as required during degradation. In parallel, with the introduction of the tumor microenvironment (TME) responsive concept, therapeutic ions that are initially confined in degradable nanosystems and eventually released in the TME can be relatively low off-target toxic, hence exhibiting sufficient potential as a promising prodrug.
Deemed as a type of cutting-edge degradable nanosystems, infinite coordination polymers (ICPs) are amorphous compounds harnessing coordination bonds to connect ligands and metal ions directly.13 For instance, burgeoning metal–phenolic ICPs are formed by the coordination between phenolic ligands and metal ions, which therefore inherit the biological activity of polyphenols and the imaging/therapeutic properties of metal ions.14 As the coordination bonds within ICPs are extremely sensitive to acid, ICPs realize biosafety and biodegradability, i.e., not disintegrating until reaching the acidic tumor tissue.15 Currently, ICPs have been a welcome addition to the nanoprodrugs available for oncotherapy, owing to their high agent payload, perfect therapeutic performance, minimal side effects, extended functionality, and so forth.16 Nevertheless, the investigations in this direction are still in their infancy due to the need for multi-disciplinary integration (e.g., material, chemical, pharmaceutical, biological, and medical sciences). In particular, there is a lack of two-or-more-ICP-engineered nanomedicines providing remarkable super-additive therapeutic effects for synergistic antineoplastic strategies.
Despite the progress in various nanomedicines, triple-negative breast cancer (TNBC) still remains a huge threat to society due to the resistance mechanism, which urgently waits for more in-depth explorations of innovative therapeutic options.17 Herein, with the prospects of highlighting the underlying superiorities of ICP-engineered nanomedicines, two types of ICPs based on ferrocene (Fc) derivative/ferric iron (Fe3+) and tannic acid (TA)/Ca2+ respectively, were integrated into a novel nanosystem (Fc@Ca–TA NPs) as a nanoprodrug model against TNBC. In this nanosystem, the interior Fc ICPs served as a Fe2+ supplier to generate ˙OH and destroy oxidation equilibrium, thereby existing as an effective form of mitochondrial destruction.18 Withal, owing to the pH-dependent degradation characteristic of Fc@Ca–TA NPs, unconfined Fe2+/Fe3+ from Fc ICPs imparted TME-responsive magnetic resonance (MR) imaging for the visualization of the tumor area.19 Furthermore, during the degradation process, the exterior Ca–TA ICPs were eligible to release both TA and Ca2+. Thereinto, the TA was able to convert Fe3+ released from Fc ICPs into high catalytic-active Fe2+, thus replenishing the Fe2+ supply for Fc ICPs to induce more efficient oxidative stress.20 In addition, the up-regulation of Ca2+ was especially beneficial to disturb mitochondrial function, which collaborated with Fe2+ interference to overturn oxidation homeostasis.21 When two functional ions induce cell apoptosis, liberated ions from dead or dying cells can further infect surrounding cells, thus amplifying their therapeutic effects through the neighboring effect.22 By taking full advantage of the aforementioned synergistic mechanisms, tumor proliferation can be availably restrained by the cell death regulation of Fc@Ca–TA NPs. In summary, this work enlightens the design and exploitation of ICP-engineered nanomedicines and proposes a cutting-edge orientation for future ion interference-mediated synergistic therapy.
Results and discussion
Preparation and characterization of Fc@Ca–TA NPs
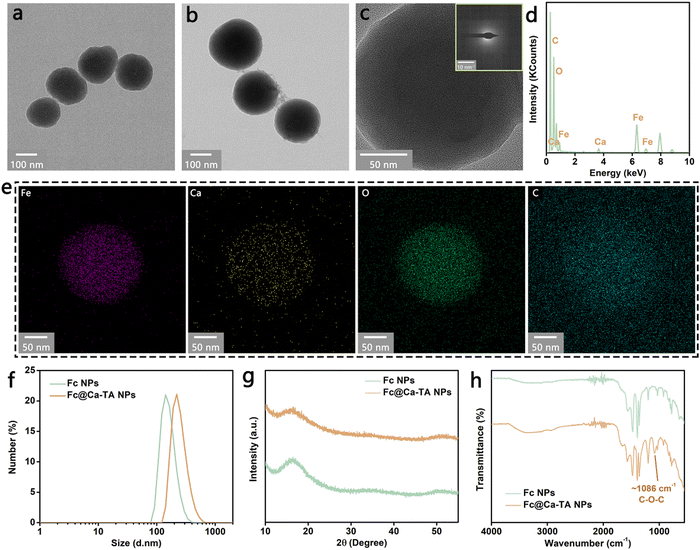
In brief, the preparation technique of Fc@Ca–TA NPs was divided into two steps: the synthesis of Fc NPs and the introduction of Ca–TA (Fig. S1, ESI†). First, Fc ICPs were prepared by referencing a previous method,23 which involved the light-triggered Fe2+ release and 1,1′-ferrocenedicarboxylic acid deprotonation, the oxidation of free Fe2+ into Fe3+, and the coordination of Fe3+ and deprotonated 1,1′-ferrocenedicarboxylic acid. Importantly, LED light with power as low as 0.02 W (a daily table lamp) was adapted as an exogenous energy source for photodegradation instead of previously reported natural light and xenon light to make the preparation more flexible in time and cast off expensive equipment to some extent. After the cladding of DSPE-PEG on the surface of Fc ICPs, spherical ICPs NPs were synthesized, as shown in Fig. 1a and Fig. S2 (ESI†). In addition to improving the hydrophilicity, DSPE-PEG effectively protected the spherical frame of Fc ICPs from coordination competition in the subsequent Ca–TA coordination process (Fig. S3, ESI†). Second, Ca–TA ICPs, to our knowledge, firstly proposed in the nanotechnology and medicine fields, were manufactured on the surface of Fc NPs through the surface adsorption of free TA/small TA complexes to DSPE-PEG and subsequent coordination of Ca2+ and digalloyl groups in polyphenol TA (Fig. S4, ESI†).24 This strategy of growing ICPs on liposomes holds promise for further utilization in a variety of engineered cells and bacteria as well as membrane mimetic biomaterials. Due to the hydrogen-bond interaction between TA and PVP, highly biocompatible PVP is well-suited for further encapsulation to obtain dual-ICP-engineered Fc@Ca–TA NPs, as shown in Fig. 1b and Fig. S5, S6 (ESI†).25,26Since the principal components were amorphous ICPs, the obtained Fc@Ca–TA NPs were featured with an obvious amorphous state, without distinct lattice fringe in the transmission electron microscopy (TEM) analysis and diffraction characteristic in the selected area electron diffraction (SAED) analysis (Fig. 1c). Withal, the energy-dispersive X-ray spectroscopy (EDS) and elemental mapping analyses verified the efficient introduction of Ca2+ into Fc@Ca–TA NPs (Fig. 1d and e), compared with Fc NPs in the previous step (Fig. S7, ESI†). Besides, the dynamic light scattering (DLS) analysis illustrated that the average sizes of Fc NPs and Fc@Ca–TA NPs (containing hydration layers) were, respectively, 186.3 nm and 249.7 nm (Fig. 1f). Simultaneously, the polydispersity index of Fc@Ca–TA NPs was 0.12, which embodied a suitable size distribution for biological applications. With regard to the crystal structures, typical widening peaks rather than diffraction peaks in the powder X-ray diffraction (XRD) analysis further proved the amorphous structure of Fc NPs and Fc@Ca–TA NPs (Fig. 1g). Besides, the X-ray photoelectron spectroscopy (XPS) analysis further illustrated the existence of Ca and Fe composition in the Fc@Ca–TA NPs (Fig. S8, S9, and S10, ESI†). Inductively coupled plasma optical emission spectrometry (ICP–OES) also indicated that the content ratio of Fe to Ca in the Fc@Ca–TA NPs was 72.7%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 27.3% (Fig. S11, ESI†). In parallel, there were no significant absorption peaks in the ultraviolet-visible (UV-vis) spectra of Fc NPs and Fc@Ca–TA NPs (Fig. S12, ESI†). Furthermore, Fourier transform infrared (FT-IR) and zeta potential analyses were engaged for the superficial property detections. As shown in Fig. 1h, the bands appeared at 1086 cm−1 referred to the C–O–C of TA, which could adequately certify the effective integration of Fc NPs and Ca–TA ICPs by combining with the previously confirmed presence of Ca2+. Additionally, both water-dispersible Fc NPs and Fc@Ca–TA NPs were negatively charged with electricity (Fig. S13, ESI†). In a word, the aforementioned results demonstrated the successful prescription and preparation of dual-ICP-engineered Fc@Ca–TA NPs.
27.3% (Fig. S11, ESI†). In parallel, there were no significant absorption peaks in the ultraviolet-visible (UV-vis) spectra of Fc NPs and Fc@Ca–TA NPs (Fig. S12, ESI†). Furthermore, Fourier transform infrared (FT-IR) and zeta potential analyses were engaged for the superficial property detections. As shown in Fig. 1h, the bands appeared at 1086 cm−1 referred to the C–O–C of TA, which could adequately certify the effective integration of Fc NPs and Ca–TA ICPs by combining with the previously confirmed presence of Ca2+. Additionally, both water-dispersible Fc NPs and Fc@Ca–TA NPs were negatively charged with electricity (Fig. S13, ESI†). In a word, the aforementioned results demonstrated the successful prescription and preparation of dual-ICP-engineered Fc@Ca–TA NPs.
Theranostic performance of Fc@Ca–TA NPs
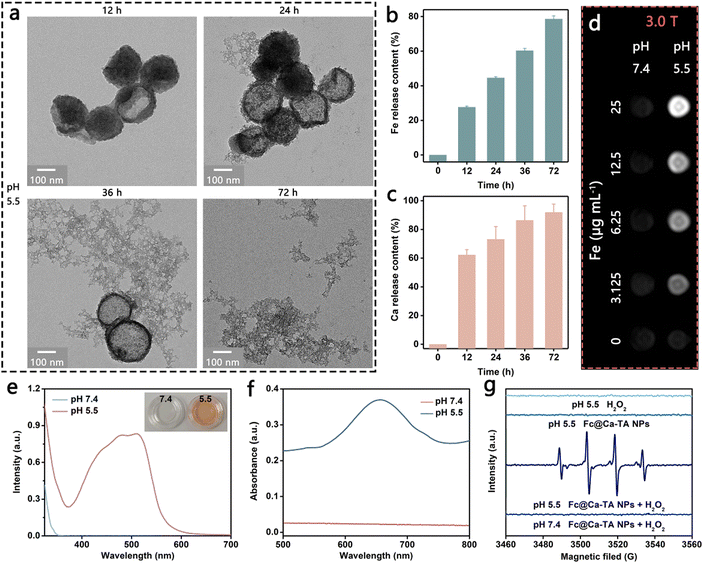
Above all, the degradation properties of Fc@Ca–TA NPs were comprehensively evaluated to explore the development potential as a degradable nanoprodrug. Intuitively, the structure of Fc@Ca–TA NPs decomposed over time in a slightly acidic environment at pH 5.5 (Fig. 2a and Fig. S14, ESI†). As a supplementary, proton-triggered Fe and Ca release after Fc@Ca–TA NPs degradation were investigated, and their release amounts at 24 h were 44.6% and 73.2%, respectively, thus revealing the pH-responsive dual-ion provision of Fc@Ca–TA NPs (Fig. 2b and c). Meanwhile, Ca–TA ICPs on the outside were preferentially in contact with the proton compared to the Fc ICPs on the inside of the DSPE-PEG layer, thus showing a higher ion release efficiency. In addition, MR imaging performance based on the Fe release was traced to determine the feasibility of providing tumor lesion information in real time. Obviously, T1-weighted MR contrast enhancement was positively correlated with Fe concentration at pH 5.5, accompanied by a longitudinal relaxivity (r1) value up to 7.502 mM−1 s−1, while no significant change in the signal intensity arose at pH 7.4 (Fig. 2d and Fig. S15, S16, ESI†). From another aspect, the catalytic properties of Fc@Ca–TA NPs as a Fenton agent were methodically investigated. In the 1,10-phenanthroline colorimetric assay for Fe2+ detection, the generated orange ferrous complexes at pH 5.5, with the characteristic absorption peak at 510 nm, strongly supported the existence of free Fe2+, which may be ascribed to the direct supply pathway of 1,1′-ferrocenedicarboxylic acid as well as the indirect supply pathway of Fe3+ and TA (Fig. 2e). Analogously, in the TMB assay for ˙OH detection, the characteristic absorption peak of oxidized TMB at 652 nm signifying the ˙OH generation only appeared at pH 5.5, due to the structure integrality of Fc@Ca–TA NPs at pH 7.4 and the resulting absence of free Fe2+ (Fig. 2f and Fig. S17, ESI†). In good accordance with the TMB assay, there was a strong ˙OH signal at pH 5.5 by using ESR as a more intuitive measurement. In a nutshell, Fc@Ca–TA NPs had the potential to serve as a degradable nanoprodrug, offering a dramatic opportunity to integrate dual-ion interference and pH-responsive MR imaging.Synergistic effect of calcium and iron
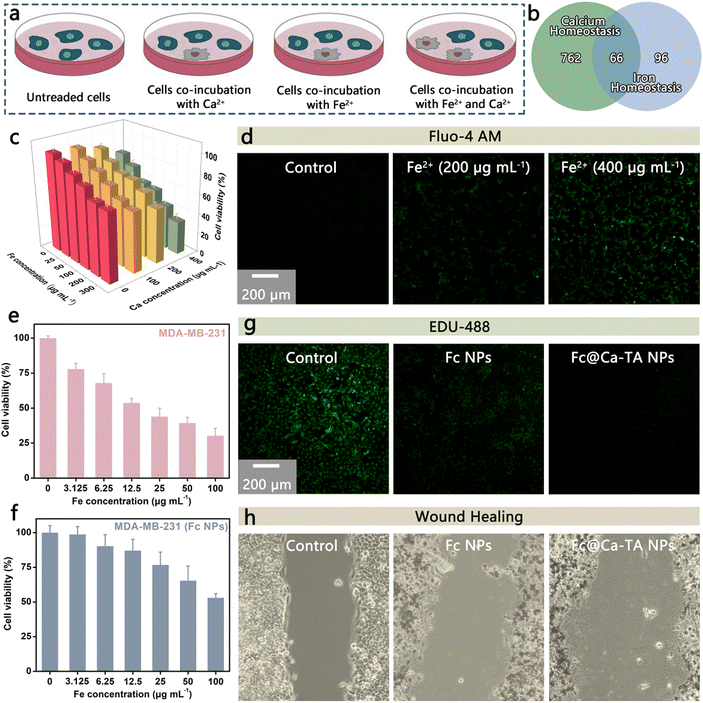
Ca2+ overload and intracellular free radicals are promising to mediate a synergistic antineoplastic effect due to their mutual causation.11 In this regard, Fe2+ holds great promise in ˙OH up-regulation, thus showing an inextricable link to calcium homeostasis. Based on this, we infer that Ca2+ and Fe2+ could induce a synergistic antitumor effect and conduct related investigations (Fig. 3a). For starters, there is a total of 66 overlapping genes among calcium and iron homeostasis-related genes from MSigDB online database (https://www.gsea-msigdb.org/gsea/login.jsp), showing a significant intersection between calcium and iron homeostasis (Fig. 3b). In order to explore the combination therapeutic potential of Ca2+ and Fe2+, the death situation of TNBC breast cancer cells (MDA-MB-231) incubated with dual ions was explored (Fig. 3c). Compared with single ion interference, combination interference achieved a more efficient inhibition effect at a lower dosage, availably demonstrating the effectiveness and safety of combination strategy. Additionally, Fe2+ ions were capable even to up-regulate the intracellular level of Ca2+ ions indirectly, which demonstrated the close association between calcium and iron homeostasis (Fig. 3d). Furthermore, the MDA-MB-231 line was selected to compare the antitumor effects of Fc NPs (Fe2+ supply) with Fc@Ca–TA NPs (Ca2+ and Fe2+ dual-supply). As expected, the survival rate of MDA-MB-231 cells treated with Fc@Ca–TA NPs was lower than those treated with Fc NPs (Fig. 3e and f). In addition to more efficiently inhibiting cell proliferation, Fc@Ca–TA NPs had a more obvious influence on cell metastasis than Fc NPs, embodying that calcium overload induced by direct Ca2+ up-regulation could effectively cooperate with iron interference (Fig. 3g and h). Therefore, the aberrant distribution of Ca2+ and Fe2+ ions mediated by Fc@Ca–TA NPs is a promising way to induce a synergistic intervention effect efficiently interfering with the functional homeostasis of cancer cells.Biosafety of Fc@Ca–TA NPs
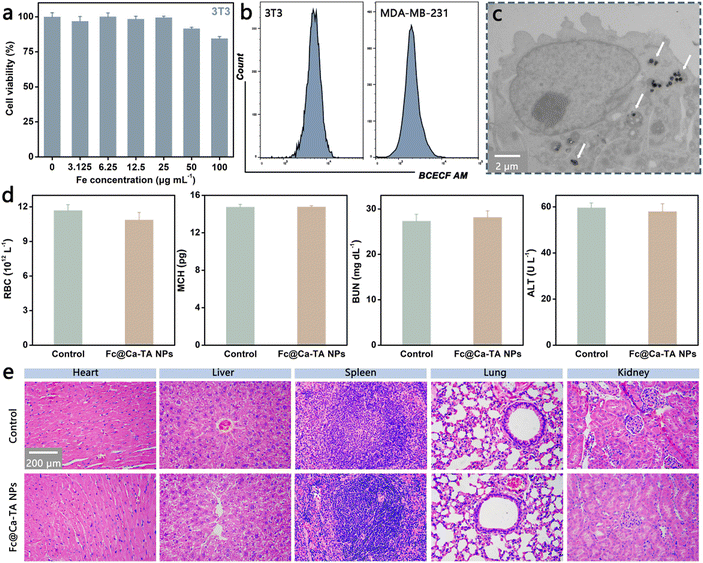
Systematic experiments at the cellular and animal levels were further executed to evaluate the biological potential of Fc@Ca–TA NPs. Initially, the normal fibroblast cell line (3T3), MDA-MB-231 line, and estrogen receptor (ER)-positive breast cancer cell line (MCF-7) were selected for the biocompatibility and cytotoxicity of Fc@Ca–TA NPs by the CCK-8 assay. The survival rate of 3T3 cells incubated with diverse concentrations of Fc@Ca–TA NPs degraded slightly. It still remained at 84.5% even though the Fe concentration was high (100 μg mL−1), demonstrating the safety of Fc@Ca–TA NPs in normal cells (Fig. 4a). Nevertheless, both MDA-MB-231 and MCF-7 cells incubated with Fc@Ca–TA NPs exhibited significant concentration-dependent mortality (Fig. 3e and Fig. S18, ESI†). Specifically, as the Fe concentration of Fc@Ca–TA reached 100 μg mL−1, the survival rate of MDA-MB-231 cells declined to 30.2%. To explain this phenomenon, the intracellular pH difference between 3T3 and MDA-MB-231 cells was explored via the fluorescence probe BCECF AM. There was a higher fluorescence intensity (i.e., higher pH value) in 3T3 cells, which was adverse to the degradation of Fc@Ca–TA and subsequent ion interference. Meanwhile, normal cells exhibited much lower uptake than tumor cells, which was another reason for their high tolerance (Fig. S19, ESI†). In parallel, significantly reduced cell colonies in the crystal violet staining assay also indicated the satisfactory inhibitory effect of Fc@Ca–TA NPs on MDA-MB-231 cell proliferation (Fig. S20, ESI†). As for the cellular uptake behavior of Fc@Ca–TA NPs, the bio-TEM image confirmed the efficient ingestion of MDA-MB-231 cells to Fc@Ca–TA NPs (Fig. 4c). Furthermore, the blood biochemistry and hematology assays, as an indispensable step before clinical application, were conducted to further notarize the suitability of Fc@Ca–TA NPs. By comparison, various indexes of BALB/c mice receiving intravenous injections of Fc@Ca–TA NPs were maintained within a normal range (Fig. 4d and Fig. S21, ESI†). In addition, no pathological damage appeared in the major organs of the experimental mice receiving intravenous injection of Fc@Ca–TA NPs (Fig. 4e). In short, the above-mentioned results convincingly illustrated that Fc@Ca–TA NPs at the right concentration were distinguished with engaging biosafety as a promising antitumor nanoprodrug.Antitumor mechanisms of Fc@Ca–TA NPs
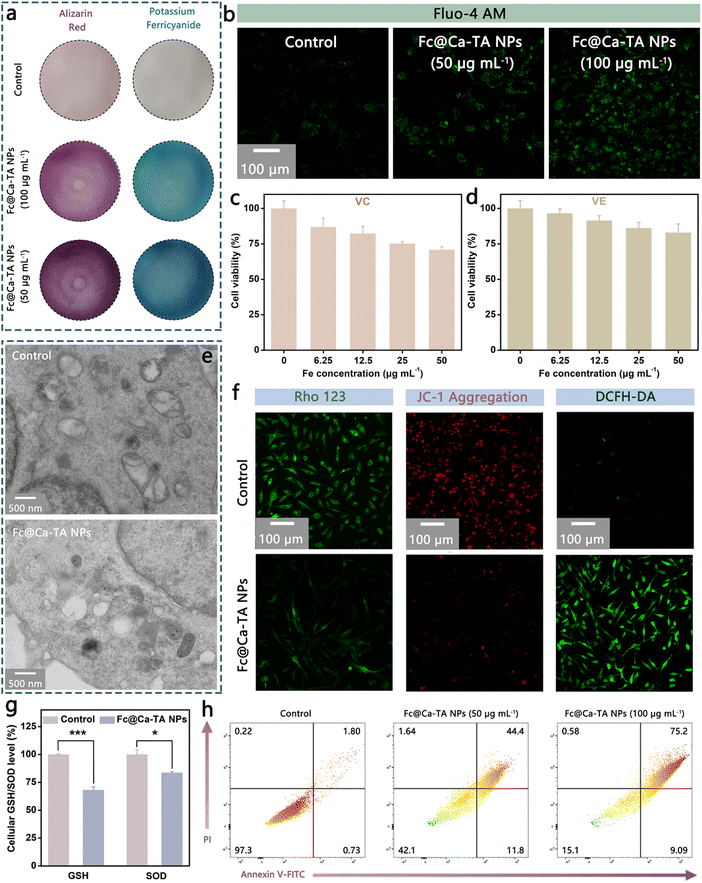
Inspired by the theranostic properties of Fc@Ca–TA NPs, their specific antitumor mechanisms were further explored using the MDA-MB-231 cell line. According to the pH-responsive degradation characteristic of Fc@Ca–TA NPs, the generation of Fe2+ and Ca2+ inside MDA-MB-231 cells was respectively monitored by potassium ferricyanide staining and alizarin red staining. As shown in Fig. 5a and Fig. S22 (ESI†), conspicuously amplified blue and red areas implied the formation of metal complexes after co-incubation with Fc@Ca–TA NPs, which provided potent evidence of the produced Fe2+ and Ca2+. Besides, Fluo-4 AM (a Ca2+ fluorescent probe) was applied to complementarily illustrate the occurrence of Ca2+ overload. Analogously, significantly elevated green fluorescence appeared after co-incubation with Fc@Ca–TA NPs (Fig. 5b). Thereafter, the synergistically induced oxidative stress based on Fe2+ and Ca2+ was systematically investigated. First of all, vitamin C (VC) and vitamin E (VE) are regarded as natural antioxidants to alleviate oxidative stress, contributing to improving the survival of MDA-MB-231 cells treated by Fc@Ca–TA NPs, which suggests that the oxidation balance disruption is an important cause of cell death (Fig. 5c and d). Meanwhile, there was an obvious volume reduction of mitochondria and extensive vacuolization of the endoplasmic reticulum in the treated MDA-MB-231 cells, implying effective oxidative stress and organelle damage (Fig. 5e).27 Ulteriorly, intracellular ROS generation, and mitochondrial membrane potential were directly measured using DCFH-DA, JC-1, and Rho 123 fluorescent probes, certifying the noticeable ROS up-regulation as well as mitochondrial dysfunction induced by Fc@Ca–TA NPs (Fig. 5f). Accompanied by the mitochondrial dysfunction, the intracellular ATP level was significantly decreased (Fig. S23, ESI†). Moreover, intracellular GSH and SOD (two types of natural intracellular antioxidants) levels were down-regulated visibly by Fc@Ca–TA NPs, indirectly displaying the destruction of Fc@Ca–TA NPs to the oxidation defense system of MDA-MB-231 cells (Fig. 5g).28,29 To further explore the cell death mode, the antitumor efficiency of Fc@Ca–TA NPs was investigated through the annexin V-FITC/PI assay. Unlike normal MDA-MB-231 cells, those incubated with Fc@Ca–TA NPs were seriously impaired due to apoptosis induction. To be specific, the apoptosis rate of MDA-MB-231 cells increased to 84.3% when the Fe concentration was 100 μg mL−1 (Fig. 5h). Furthermore, common apoptosis factors, namely, Caspase-3, Caspase-8, and Caspase-9 were effectively up-regulated (Fig. S24, ESI†).30 Based on the above analyses, Fc@Ca–TA NPs may induce an apoptosis cellular regulatory pathway and override the resistance mechanism of MDA-MB-231 cells to achieve cell death. Therefore, these results at the cellular level demonstrated that Fc@Ca–TA NPs effectively mediated dual-ion interference-based mitochondrial oxidative stress, thus relentlessly striking and eliminating MDA-MB-231 cells.Neighboring effect of Fc@Ca–TA NPs
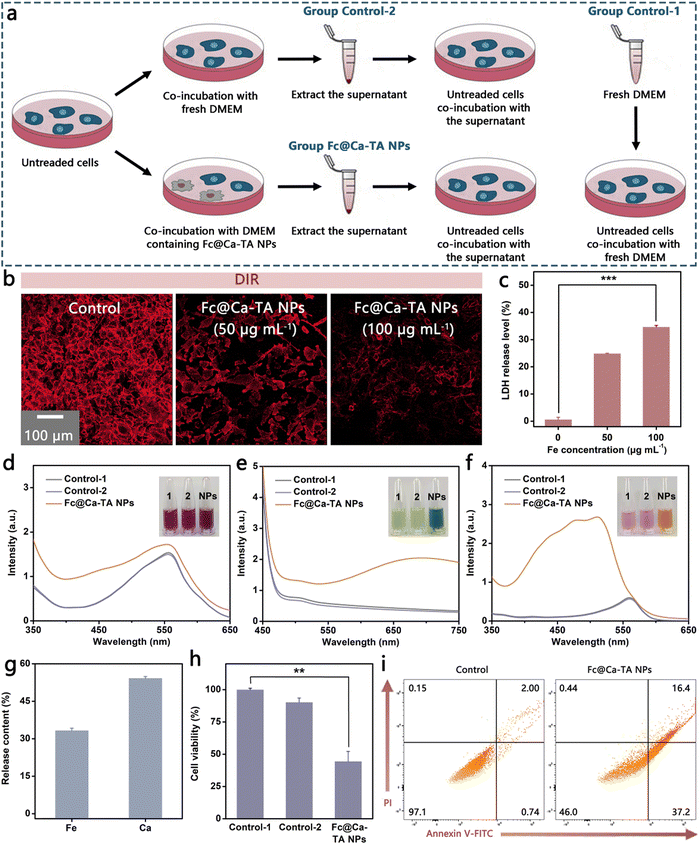
The neighboring effect means that tumor cells may have the potential to be converted into an in situ drug depot, once as-ingested drugs are superfluous and liberated from dead or dying cells after inducing cell apoptosis.22 Based on this, we suppose that liberated ions could execute an effective neighboring effect after the tumor cell death for intercellular transport and continued apoptosis induction and conduct relevant investigations (Fig. 6a). As expected, there was significant membrane damage in the MDA-MB-231 cells after co-incubation with Fc@Ca–TA NPs, testified by markedly diminished fluorescence in DiR membrane staining (Fig. 6b). In addition, the release of cytoplasmic lactate dehydrogenase (LDH) was also valid as an argument for plasma membrane rupture (Fig. 6c). Besides, the cellular morphology of MDA-MB-231 cells after co-incubation with Fc@Ca–TA NPs was appraised by SEM (Fig. S25, ESI†). There was a marked rupture in MDA-MB 231 cells, thereby embodying the possibility of a large outflow of ions. Whereafter, fresh DMEM (Group Control-1), DMEM after 24 h co-incubation with MDA-MB-231 cells (Group Control-2), and DMEM after 24 h co-incubation with MDA-MB-231 cells and Fc@Ca–TA NPs (Group Fc@Ca–TA NPs) were collected for subsequent apoptosis induction. After staining with alizarin red, potassium ferricyanide, and 1,10-phenanthroline, respectively, the dark red color of DMEM in Group Fc@Ca–TA NPs implied the presence of large amounts of released Ca2+, while the green and orange color of DMEM in Group Fc@Ca–TA NPs corresponded to the presence of Fe2+ (Fig. 6d, e and f). Meanwhile, the release amounts of co-existing Ca2+ and Fe2+ severally reached 54.2% and 33.3% (Fig. 6g). Moreover, DMEM in Group Fc@Ca–TA NPs was relatively acidic due to the leakage of intracellular acid substance after the plasma membrane damage (Fig. S26, ESI†). After incubation with the above three types of DMEM, only the MDA-MB-231 cells in Group Fc@Ca–TA NPs died obviously on account of dual-ion interference-mediated apoptosis induction effects (Fig. 6h and i). Consequently, Fc@Ca–TA possessed the potential to amplify its antitumor effect through the neighboring effect based on the dual ions.Tumor inhibition of Fc@Ca–TA NPs
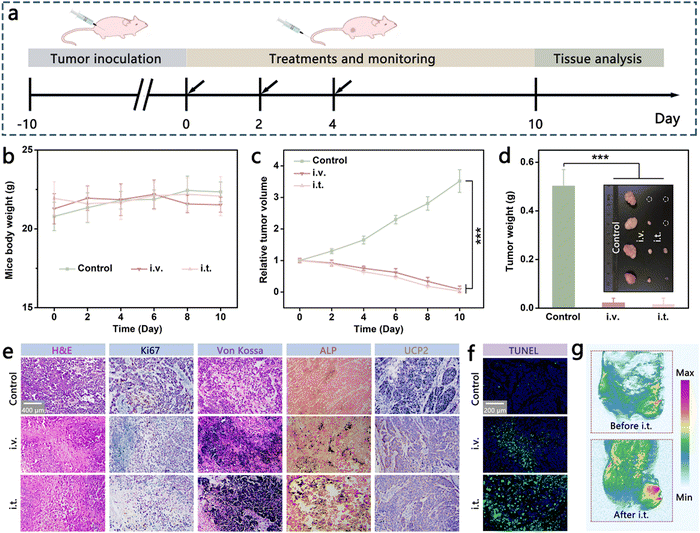
To investigate the tumor inhibition performance of Fc@Ca–TA NPs under clinically relevant conditions, the MDA-MB-231 tumor-bearing mice model was established and validated to monitor the treatment response (Fig. 7a). During the therapeutic process, the body weight of mice fluctuated mildly within the normal range, displaying that the adopted therapeutic schemes were safe with little adverse effects (Fig. 7b). With regard to therapeutic efficacy, conspicuous tumor elimination occurred after the injection of Fc@Ca–TA NPs either intravenously or intratumorally (Fig. 7c and d). In addition, pathological histology analysis was imparted to further explore the pathological change in tumor tissue mediated by Fc@Ca–TA NPs (Fig. 7e and f). Specifically, H&E staining, TUNEL staining, and Ki67 staining affirmed the excellent tumor inhibition performance of Fc@Ca–TA NPs, demonstrated by obvious nucleus disappearance and membrane fragmentation. Meanwhile, there were noticeable calcified nodule and mitochondrial dysfunction characteristics, separately in the Von Kossa staining, alkaline phosphatase (ALP) staining, and uncoupling protein 2 (UCP 2, a typical mitochondrial dysfunction indicator) staining assays.31,32 Moreover, there was a clearly enhanced T1 signal intensity within the tumor region at the post-intravenous or intratumoral injection of Fc@Ca–TA NPs, thereby demonstrating the pH-triggered imaging and therapeutic supervision abilities of Fc@Ca–TA NPs (Fig. 5f and Fig. S27, ESI†). As a consequence, as-prepared Fc@Ca–TA NPs ingeniously integrated therapeutic and imaging modalities, offering a reliable theranostic paradigm for MDA-MB-231 tumor Inhibition.Conclusions
In summary, ICP-engineered Fc@Ca–TA NPs were fabricated via dexterously integrating Fc ICPs and Ca–TA ICPs, which served as “magic bullets” for highly efficient dual-ion-induced and MR imaging-guided mitochondrial oxidative stress against TNBC. Herein, the biosafety of Fc@Ca–TA NPs was demonstrated in detail, and their prominent therapeutic effects and mechanisms were comprehensively illustrated. On the one hand, this work is promising to enlighten the design and construction of nanomedicines in the near future, e.g., functionalized ICPs, Ca2+ overload nanoplatforms, and MR imaging nanosystems. On the other, this work may provide a novel channel for applying multi-ion interference for oxidative stress-mediated tumor eradication.Author contributions
Junlie Yao: methodology, investigation, formal analysis, data curation, writing – original draft. Jie Xing: investigation, conceptualization, writing – review, and editing. Fang Zheng: data curation, formal analysis. Zihou Li: formal analysis, validation. Sunxiang Li: methodology, validation. Xiawei Xu: methodology, validation. Devrim Unay: image analysis, visual images. Young Min Song: data curation, image analysis. Fang Yang: conceptualization, funding acquisition, supervision, writing – review, and editing. Aiguo Wu: funding acquisition, resources, project administration, supervision, writing – review, and editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are grateful for the financial support from the National Natural Science Foundation of China (32025021, 31971292, 32111540257), Youth Innovation Promotion Association, Chinese Academy of Sciences (2022301), and Ningbo 3315 Innovative Talent Project (2018-05-G).Notes and references
- J. Shi, P. W. Kantoff, R. Wooster and O. C. Farokhzad, Nat. Rev. Cancer, 2017, 17, 20–37 CrossRef CAS PubMed.
- F. Tang, L. Li and D. Chen, Adv. Mater., 2012, 24, 1504–1534 CrossRef CAS PubMed.
- Y. Wang, J. Tang, Y. Yang, H. Song, J. Fu, Z. Gu and C. Yu, Angew. Chem., Int. Ed., 2020, 59, 2695–2699 CrossRef CAS PubMed.
- S. Zhang, Z. Wang, Z. Kong, Y. Wang, X. Zhang, B. Sun, H. Zhang, Q. Kan, Z. He, C. Luo and J. Sun, Theranostics, 2021, 11, 6019–6032 CrossRef CAS PubMed.
- S. Onoue, S. Yamada and H. K. Chan, Int. J. Nanomed., 2014, 9, 1025–1037 CrossRef CAS PubMed.
- J. Rautio, H. Kumpulainen, T. Heimbach, R. Oliyai, D. Oh, T. Jarvinen and J. Savolainen, Nat. Rev. Drug Discovery, 2008, 7, 255–270 CrossRef CAS PubMed.
- J. Nicolas, S. Mura, D. Brambilla, N. Mackiewicz and P. Couvreur, Chem. Soc. Rev., 2013, 42, 1147–1235 RSC.
- M. Huo, L. Wang, Y. Chen and J. Shi, Nat. Commun., 2017, 8, 357 CrossRef PubMed.
- S. V. Rana, J. Trace Elem. Med. Biol., 2008, 22, 262–284 CrossRef CAS PubMed.
- F. Lang and E. K. Hoffmann, Compr. Physiol., 2012, 2, 2037–2061 Search PubMed.
- M. Zhang, R. X. Song, Y. Y. Liu, Z. G. Yi, X. F. Meng, J. W. Zhang, Z. M. Tang, Z. W. Yao, Y. Liu, X. G. Liu and W. B. Bu, Chem, 2019, 5, 2171–2182 CAS.
- C. Zhang, W. B. Bu, D. L. Ni, S. J. Zhang, Q. Li, Z. W. Yao, J. W. Zhang, H. L. Yao, Z. Wang and J. L. Shi, Angew. Chem., Int. Ed., 2016, 55, 2101–2106 CrossRef CAS PubMed.
- A. M. Spokoyny, D. Kim, A. Sumrein and C. A. Mirkin, Chem. Soc. Rev., 2009, 38, 1218–1227 RSC.
- W. Sang, Z. Zhang, G. H. Wang, L. S. Xie, J. Li, W. X. Li, H. Tian and Y. L. Dai, Adv. Funct. Mater., 2022, 32, 2113168 CrossRef CAS.
- S. Xu, J. Liu, D. Li, L. M. Wang, J. Guo, C. C. Wang and C. Y. Chen, Biomaterials, 2014, 35, 1676–1685 CrossRef CAS PubMed.
- S. Zhang, S. Zhang, S. Y. Luo and D. C. Wu, Coord. Chem. Rev., 2021, 445, 214059 CrossRef CAS.
- K. Li, C. Lin, M. Li, K. Xu, Y. He, Y. Mao, L. Lu, W. Geng, X. Li, Z. Luo and K. Cai, ACS Nano, 2022, 16, 2381–2398 CrossRef CAS PubMed.
- Y. Qin, F. Tong, W. Zhang, Y. Zhou, S. Q. He, R. Xie, T. Lei, Y. S. Wang, S. J. Peng, Z. F. Li, J. Leong, H. L. Gao and L. G. Lu, Adv. Funct. Mater., 2021, 31, 2104645 CrossRef CAS.
- B. Yu, B. S. Choi, W. G. Li and D. H. Kim, Nat. Commun., 2020, 11, 1–9 CrossRef PubMed.
- Y. Xu, Y. Guo, C. Zhang, M. Zhan, L. Jia, S. Song, C. Jiang, M. Shen and X. Shi, ACS Nano, 2022, 16, 984–996 CrossRef CAS PubMed.
- X. L. Ding, M. D. Liu, Q. Cheng, W. H. Guo, M. T. Niu, Q. X. Huang, X. Zeng and X. Z. Zhang, Biomaterials, 2022, 281, 121369 CrossRef CAS PubMed.
- D. Zhao, W. Tao, S. Li, Y. Chen, Y. Sun, Z. He, B. Sun and J. Sun, Sci. Adv., 2021, 7, 0880 Search PubMed.
- L. Zhang, X. Gao, L. Yang, P. Yu and L. Mao, ACS Appl. Mater. Interfaces, 2013, 5, 8120–8124 CrossRef CAS PubMed.
- H. Ejima, J. J. Richardson, K. Liang, J. P. Best, M. P. van Koeverden, G. K. Such, J. Cui and F. Caruso, Science, 2013, 341, 154–157 CrossRef CAS PubMed.
- V. Kozlovskaya, O. Zavgorodnya, Y. Chen, K. Ellis, H. M. Tse, W. Cui, J. A. Thompson and E. Kharlampieva, Adv. Funct. Mater., 2012, 22, 3389–3398 CrossRef CAS PubMed.
- J. Chen, S. Ratnayaka, A. Alford, V. Kozlovskaya, F. Liu, B. Xue, K. Hoyt and E. Kharlampieva, ACS Nano, 2017, 11, 3135–3146 CrossRef CAS PubMed.
- P. X. Wang, S. L. Liu, M. X. Hu, H. W. Zhang, D. M. Duan, J. Y. He, J. J. Hong, R. T. Lv, H. S. Choi, X. Y. Yan and M. M. Liang, Adv. Funct. Mater., 2020, 30, 2000647 CrossRef CAS.
- Y. Xiong, C. Xiao, Z. Li and X. Yang, Chem. Soc. Rev., 2021, 50, 6013–6041 RSC.
- E. Kim, Y. Jeen, H. Cho, B. Keum, Y. Kim, H. Lee, H. Chun, C. Kim and H. Ryu, J. Clin. Oncol., 2011, 29, 463 Search PubMed.
- L. Galluzzi, A. Lopez-Soto, S. Kumar and G. Kroemer, Immunity, 2016, 44, 221–231 CrossRef CAS PubMed.
- Y. Jiang, W. Meng, L. Wu, K. Shao, L. Wang, M. Ding, J. Shi and X. Kong, Adv. Healthcare Mater., 2021, 10, 2100789 CrossRef CAS PubMed.
- W. Bao, M. Liu, J. Meng, S. Liu, S. Wang, R. Jia, Y. Wang, G. Ma, W. Wei and Z. Tian, Nat. Commun., 2021, 12, 6399 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3mh00001j |
| This journal is © The Royal Society of Chemistry 2023 |