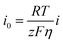Open Access Article
Open Access Article200 MPa cold isostatic pressing creates surface-microcracks in a Zn foil for scalable and long-life zinc anodes†
Di
Zhang‡
,
Hongfei
Lu‡
 ,
Nawei
Lyu‡
,
Xin
Jiang
,
Zili
Zhang
and
Yang
Jin
,
Nawei
Lyu‡
,
Xin
Jiang
,
Zili
Zhang
and
Yang
Jin
 *
*
Research Center of Grid Energy Storage and Battery Application, School of Electrical Engineering, Zhengzhou University, Zhengzhou 450001, Henan, China. E-mail: yangjin@zzu.edu.cn
First published on 7th January 2023
Abstract
The Zn anode suffers from severe dendrite growth and side reactions, which restrict its development in the realm of large-scale energy storage. Herein, in this study, we propose a method to create surface-microcracks in a Zn foil by 200 MPa cold isostatic pressing. The proposed pressing method can avoid the surface tip effect of Zn, and creates a subtly surface-microcracked zinc structure, providing more zinc ion transport channels, thereby effectively alleviating the dendrite growth and side reactions during the repeated Zn plating and stripping. Benefiting from these advantages, the 200 MPa Zn‖Zn symmetric cell can achieve a long cycle life (1525 h) of 1 mA h cm−2 at 2 mA cm−2. The 200 MPa Zn‖VO2 full cell can still maintain a capacity of 110 mA h g−1 after 1000 cycles at 0.1 A g−1. In addition, assembled pouch cells also show excellent cycling stability. The proposed cold isostatic pressing method is compatible with large-scale production applications and provides an effective strategy for realizing high-performance zinc anodes for zinc-ion batteries.
1. Introduction
Aqueous zinc-ion batteries are considered promising candidates for renewable energy storage due to their safety, low toxicity, and high theoretical capacity (820 mA h g−1).1–3 In recent years, although researchers have achieved an obvious development of high-performance zinc-ion battery cathodes and electrolytes, the issue of Zn metal itself has been underestimated.4,5 The inherent defects and uneven growth of zinc dendrites shorten the life of zinc-ion batteries.6,7 At the same time, due to the presence of tips and impurities on the surface of mass-produced Zn foils, these regions are conducive to the nucleation of Zn ions during Zn deposition,8,9 which leads to further deposition in subsequent cycles causing the dendrite growth.10 Therefore, how to solve the problem of zinc dendrite growth is an important challenge.11In order to solve this challenge of obtaining high-performance zinc anodes, the research procedure mainly involves the following ways to improve the performance of zinc anodes, (i) covering the zinc surface with a protective layer,12–14 (ii) adjusting the structure of the electrolyte,15–17 and (iii) changing the metal structure of the zinc anode.18–20 Recently, it has been reported that creating a copper–zinc alloy material on the zinc surface inhibits the electrochemical corrosion of aqueous zinc metal and improves the cycle performance.21 To guide the preferential deposition of zinc ions, the researcher prepared an AgZn3 coating on the zinc metal surface by the plasma sputtering method;22 others include Ag coating23 and Zn–Sn alloy coating.24,25 Coating a protective layer on the zinc surface can improve the cycle performance of zinc-ion batteries, but it has high requirements on the uniformity and thickness of the protective layer.26–28 In addition, a study has shown that electrolyte additive is a simple and effective way to improve the performance of zinc-ion batteries.29 For example, the performance of zinc-ion batteries was improved by adding a small amount of diethyl ether additive to the electrolyte of zinc-ion batteries;30 the structure of the electrolyte was adjusted by the addition of tetrabutylammonium sulfate, thereby inducing uniform zinc deposition.31 Adding electrolyte additives is a simple modification method, but these additives may destroy the original safety and economy of aqueous zinc batteries.32 Therefore, from the perspective of industrialization, the above methods are difficult for mass production.33,34
It is effective to change the original structure of zinc to create a structure on the zinc surface.35–37 For example, Zn–Cu alloys were prepared by combining the samples with the electrochemically assisted annealing thermal method.38 The structured Zn–Cu alloys can provide high electron and ion transport paths for the uniform deposition of Zn.39,40 Chen et al. reported a double porous Zn-3D@600 protective layer by coating a Zn@C zinc skeleton. This Zn-3D@600 anode exhibits high stability and low polarization voltage during the galvanization/stripping process. Li et al. reported a modification method for zinc anodes by utilizing a ball-milling clay precipitate (designated as BMC, consisting of the unetched MAX phase and the Ti3C2Tx phase) to construct a three-dimensional (3D) framework on the surface of a zinc foil (Zn@BMC).35 In addition, some researchers changed the structure of zinc metal by rolling from the problem of zinc metal itself,41 and adjusted the preferential deposition of zinc crystal plane.19,42 However, this method was developed by changing the zinc metal into a molten state at 500 °C, then cooling it to normal temperature, and repeatedly rolling it for 3 cycles to deform it. The structure is difficult to commercialize due to the complicated process and high cost.18 Since the tensile strength of the Zn metal is about 110 MPa,43 the surface microcrack structure cannot be achieved by simple rolling. Therefore, exploring simple and effective methods for improving the structure of zinc has high practical value for the rational design of zinc anodes.44,45
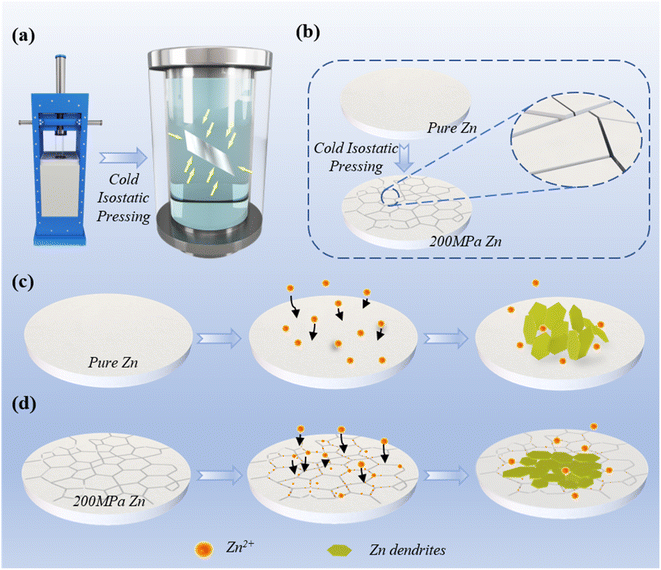
Herein, here, we propose a surface-microcracked Zn structure prepared by 200 MPa cold isostatic pressing. Cold isostatic pressing is a commonly used technology for the large-scale processing of metal structures (Fig. 1a). It is a simple physical treatment method and can be used to apply isobaric pressure to metals. It is proved that 200 MPa cold isostatic pressing can skillfully create zinc surface microcracks to achieve uniform deposition of Zn ions (Fig. 1b and S1†). These microcracks subtly create the Zn structure, which exposes more deposition of the sites. When the electrolyte is immersed inside the microcrack structure during deposition, it provides more zinc ion transport channels. These channels help regulate the uniform deposition of zinc ions, while effectively avoiding the “tip effect” during zinc production (Fig. 1d). However, due to the surface protrusions and impurities of pure zinc during the production process, the serious growth of dendrites affects the performance of the zinc anode (Fig. 1c). On the basis of these advantages, a long cycle life of over 1500 h for the 200 MPa Zn‖Zn symmetric cell and super cycling stability of up to 1000 cycles for the 200 MPa Zn‖VO2 full cell was achieved. This work provides an effective strategy for large-scale improvement in the performance of the zinc anode.
2. Results and discussion
2.1. Analysis of the interface characteristics and mechanism of a zinc anode
Compared with previous studies, the outstanding features of this treatment method are (i) large-scale mass production and simple process, (ii) clever design of the surface-microcrack structure to increase zinc ion transport channels and (iii) stable performance and more practicality. Fig. 2a shows the surface changes of the zinc foil (1 m long, 30 μm thick) before and after cold isostatic pressing at 200 MPa. Before the treatment, the surface of the zinc foil is rough with many impurities and protrusions, while the surface becomes relatively smooth and flat with metallic luster and dark yellow after the treatment. In Fig. 2b, SEM images show that 200 MPa cold isostatic pressing pressure-treated zinc foil subtly formed various microcracks on the surface (Fig. S2a†). From the SEM image of pure zinc (Fig. 2c), it can be found that the zinc surface has uneven protrusions. Due to the inevitable defects and impurities in the production process, the surface roughness of pure zinc affects the cycle performance (Fig. 2c and S2b†).Atomic force microscopy (AFM) images clearly showed various spikes on the surface of pure Zn, while the surface of 200 MPa Zn was relatively flat (Fig. 2c, d and S3†). This is related to the defects in zinc itself from the production process. Thermodynamically, the presence of dendrites favors the preferential deposition of Zn2+, resulting in a “tip effect”. This promotes the deposition of zinc ions and eventually leads to the growth of dendrites that puncture the membrane. Therefore, the presence of dendrites directly limits the battery life. In Fig. 2f, by selecting the zinc surface topography in the 0–4 μm region, the changes in the degree of concavity and convexity on the zinc surface can be seen more intuitively (Fig. S7†). Compared with pure Zn, 200 MPa Zn avoids the growth of dendrites along the dendrite direction, which can improve the cycle stability of zinc-ion batteries.
2.2. Comparative analysis of different pressure treatments of cold isostatic pressing
In order to find out the influence of the pressure in the cold isostatic pressing process on the performance of the zinc anode, the zinc foil was subjected to isobaric treatment at 50 MPa, 100 MPa, 200 MPa, and 300 MPa. The pressure holding time (120 s) was the same. XRD data show that the (002) crystal face of 200 MPa zinc foil had an increasing trend, which is beneficial to the uniform and dense deposition of zinc ions (Fig. S4†). Raman data are shown in Fig. S5,† it was found that there were oxide peaks on the zinc surface. However, the 200 MPa Zn oxide peak intensity was relatively small, indicating that there were relatively few impurity defects on the 200 MPa Zn surface. This is because the 200 MPa pressure could remove the oxide layer from the zinc surface.To visualize the mechanism of Zn surface dendrites, a schematic of Zn dendrite growth is presented here. As shown in Fig. 3a, due to the lack of any treatment on the surface of pure Zn, the impurities on the surface and the dendrite phenomenon lead to the disordered and uneven growth of dendrites. The cold isostatic pressing process flattens the defects and impurities on the surface of Zn, and the created microcrack structures will induce zinc ions in the surface (Fig. 3b). The surface is uniformly deposited, thereby increasing its cycling stability. After cycling, they were disassembled to observe the growth morphology of dendrites on the zinc surface (Fig. 3c–f, S6 and S12†). The results show that the effect of different pressure treatments is far more uniform than that of pure Zn. The most obvious is 200 MPa Zn, in which the zinc deposition is dense and uniform (Fig. 3e and S13†).
Through the in situ optical observations on pure Zn and 200 MPa Zn anodes, it can be intuitively found that several large dendrites grow disorderly on the pure Zn anode (Fig. 3g). The dendrites appeared in 1 hour, and the dendrites grew obviously in 2 hours. After 3 hours, the dendrites agglomerated and grew into larger particles. There are no large dendrites on the surface of the 200 MPa Zn anode, but flocculent growth was observed (Fig. 3h). Zinc ions are deposited densely and uniformly on the zinc surface. This is attributed to the microcracks inside zinc, which provide more transport channels for the zinc ions, inducing a uniform deposition of the zinc ions.
2.3. Analysis of the electrochemical performance of symmetrical batteries
In order to accurately evaluate the kinetic performance of the deposited Zn ions, the rate performance of the symmetric cells was tested at 2 to 10 mA cm−2 (Fig. 4a and S10†). 200 Mpa Zn has a smaller overpotential than pure Zn. In order to further intuitively describe the deposition process of Zn, the exchange current density of zinc-ion battery deposition is calculated by fitting the rate capability and the following formula:22,46Where i0 is the exchange current density; I is the current density; η is total overpotential; R is the gas constant; T is the thermodynamic temperature; F is the Faraday constant; z is the number of electrons involved in the electrode reaction.
The fitting result of the calculated data is shown in Fig. 4b (Table S1†). The exchange current density of the 200 mPa zinc-ion battery is 2.004 mA cm−2, which is lower than that of pure Zn (3.0013 mA cm−2). This shows that 200 mPa Zn has a small overpotential and good zinc ion deposition kinetics.46
The excellent long cycle life of the symmetric cell directly reflects the effectiveness of the cold isostatic pressing 200 mPa Zn treatment (Fig. 4c and d). In Fig. 4c, the Zn‖Zn symmetric cells are assembled from pure, 50 mPa, 100 mPa, 200 mPa, and 300 mPa Zn foil under the conditions of 2 mA cm−2 and 1 mA h cm−2. At the same time, the cell cycle performance and roll pressure were compared with those reported previously. The results show that 100 mPa, 200 mPa, and 300 mPa showed good electrochemical performance, of which, 200 MPa had the most obvious effect. The cycle life of the 200 MPa Zn‖Zn symmetrical cell (2 mA cm−2,1 mA h cm−2) reaches 1525 h, approximately 7 times longer than that of pure Zn (Fig. S15†). The EDS images of the dead 200 MPa and pure Zn cells showed that 200 MPa Zn is uniformly deposited on the surface (Fig. S6†). In addition, the cycle performance of the symmetric cell at high current densities of 5 mA cm−2 and 1 mA h cm−2 was tested (Fig. 4d and S8†). It was found that the cycle life of 200 MPa Zn can reach more than 1000 h, far exceeding that of pure Zn (155 h).
Since the corrosion behavior and side reactions in Zn-ion batteries are closely related, the linear polarization curves of the batteries assembled with pure Zn and 200 MPa Zn were investigated, as shown in Fig. 4e. The results show that the 200 MPa Zn anode has a higher positive corrosion potential, indicating an improved chemical stability and corrosion inhibition rate of 200 MPa Zn relative to pure Zn. Then, chronoamperometry (CA) characterization was performed on the pure Zn and 200 MPa Zn assembled symmetric cells to further verify the zinc ion deposition behavior (Fig. 4f). The change in the current density of the CA curves can directly reflect the change in the zinc surface morphology. The difference between the initial current and the final current values indicates the degree of increase of the active area, which can reflect the growth of zinc ions. The final current of pure Zn is 40.64 mA cm−2 and 23.53 mA cm−2 for 200 Mpa Zn. The difference between the two is 17.11 mA cm−2, which indicates that pure Zn has faster ion and electron transfer rates and it is easier to grow dendrites on the surface crystal. The EIS curve reflects the hindrance of ion and electron migration in the cell.47,48 The EIS curves of symmetric cells were also tested (as shown in Fig. 4g), verifying that the Rct values of 200 MPa Zn were much lower than those of pure Zn. 200 MPa Zn has a significantly reduced impedance relative to pure Zn, reflecting excellent electrochemical performance.
2.4. Analysis of the electrochemical performance of full batteries
To verify the effectiveness of 200 MPa Zn in the electrochemical performance of the full cell, here, the Zn‖VO2 full cell was assembled with VO2 as the cathode. The cathode was produced by the rolling sheet method. The loading of VO2 active material was about 5.4 mg cm−2. First, the rate performance of 200 MPa Zn‖VO2 and pure Zn‖VO2 full cells was tested. As shown in Fig. 5a, 200 MPa Zn exhibited excellent performance when the current density was from 100 to 1000 mA g−1. The results show that the rate performance of the 200 MPa Zn full cell is significantly better than that of pure Zn, showing excellent capacity retention. Then, at a scan rate of 0.1 mV s−1, the cyclic voltammetry (CV) test results of the full cell also confirmed that 200 MPa Zn could promote the electrode reaction (Fig. 5b).As shown in Fig. 5c, the active material quality of the full cell is controlled at ≈ 5.4 mg cm−2. After 1000 cycles, the specific capacity of the 200 MPa Zn‖VO2 full battery remains above 110 mA h g−1, while the pure Zn‖VO2 full cell is shorted at 400 cycles, and the capacity decays rapidly. Furthermore, when the cycle number increases from 100 to 1000 cycles, the 200 MPa Zn‖VO2 full cell exhibited higher capacity retention and smaller polarization compared with pure Zn cells (Fig. 5d). From the EIS measurements, it can be found that the 200 MPa Zn‖VO2 full cell has lower impedance and excellent electrochemical performance compared to pure Zn (Fig. 5e).
2.5. Analysis of the electrochemical performance of pouch batteries
In order to verify the validity and practicability of the 200 MPa cold isostatic pressings, further explanation is provided here through pouch batteries. 200 MPa Zn and pure Zn foils were assembled as 6 × 6 cm Zn‖Zn pouch symmetric cells, and the 200 MPa Zn pouch symmetric cell achieved a long cycle life (over 650 h) at 36 mA h (Fig. 6a and S9†). As the zinc foil area of the pouch battery is larger, it is more prone to short-circuit death than the button battery. This is because zinc dendrites can grow anywhere, pierce the separator, and cause short circuits. The symmetric cell life of the pouch assembled with 200 MPa Zn can be as long as more than 650 h, while that of the pure Zn pouch cell is short-circuited in only 84 h. When the electrode was disassembled to observe the deposition on the zinc surface, it can be found that pure zinc nucleated locally and had large dendrites, while 200 MPa Zn nucleated uniformly, with a dense surface and good nucleation morphology.In Fig. 6b, the pouch full cells assembled with an active material of ≈ 5.9 mg cm−2. After 300 cycles, the 200 MPa Zn‖VO2 cell had a capacity of 100 mA h g−1 with 80% retention, while the pure zinc cell capacity decayed rapidly and died after 200 cycles. It can be seen that 200 MPa Zn showed an extremely high practical value.
The pouch symmetric cell was disassembled after 40 cycles at 36 mA and 36 mA h, and the surface morphology of the zinc anode was observed (Fig. 6c). There is no obvious dendrite growth on the surface of 200 MPa Zn, while the surface of pure Zn is decayed and has obvious dendrites. The 200 MPa Zn‖VO2 pouch full cell was 6 cm × 6 cm with an open circuit voltage of 1.314 V (Fig. 6d). It could successfully light LEDs (0.06 W/pc) to demonstrate their effectiveness (Fig. 6e). To evaluate its safety against external influences, piercing and cutting experiments were performed on the pouch cells here (Fig. S11†). The pouch cells can still work normally by piercing or cutting, thus proving the excellent safety and practicability of 200 MPa Zn pouch cells in practical applications.
3. Conclusions
Large-scale processing of zinc foil by 200 MPa cold isostatic pressing can ingeniously create a microcracked structure, which can provide more transfer channels for zinc ions. Due to the existence of surface microcracks, the obvious tip dendrite effect is avoided during the deposition process, enabling a long cycle life for the uniform deposition of zinc ions. The data of symmetric cells show that the 200 MPa Zn can achieve an excellent electrochemical performance of up to 1525 h, far exceeding that of pure Zn batteries. At the same time, the surface of the zinc anode after cycling is dense and uniform. More importantly, the capacity of the 200 MPa Zn‖VO2 cell remains above 110 mA h g−1 after 1000 cycles, while the pure Zn cell decays rapidly, with only 400 cycles of short circuit and almost zero capacity decay. After 300 cycles of the pouch full cell, the specific capacity of 200 MPa Zn is 102 mA h g−1 higher than that of pure Zn, showing excellent cycle performance and a low decay rate. Therefore, the 200 MPa cold isostatic pressing strategy provides a new way for fundamental improvement and large-scale production of zinc anodes for zinc-ion batteries.Author contributions
Di Zhang: methodology, investigation, data curation, formal analysis, writing – original draft. Hongfei Lu: methodology, investigation, data curation, formal analysis, writing – original draft. Nawei Lyu: investigation, validation. Xin Jiang: formal analysis, writing review & editing. Zili Zhang: methodology, investigation, data curation. Yang Jin: conceptualization, writing – review & editing, supervision, Funding acquisition.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work is supported by the National Natural Science Foundation of China (Grant Nos. 52177223) and the Postgraduate Education Reform and Quality Improvement Project of Henan Province (YJS2021JD02).References
- Z. Yang, Q. Zhang, C. Xie, Y. Li, W. Li, T. Wu, Y. Tang and H. Wang, Energy Storage Mater., 2022, 47, 319–326 CrossRef
.
- X. Zhang, J. P. Hu, N. Fu, W. B. Zhou, B. Liu, Q. Deng and X. W. J. I. Wu, InfoMat, 2022, e12306 CAS
.
- M. Erdiwansyah, H. Husin, M. Z. Nasaruddin and A. Muhibbuddin, Prot. Control Mod. Power Syst., 2021, 6, 37–54 CrossRef
.
- C. Yuan, L. Yin, P. Du, Y. Yu, K. Zhang, X. Ren, X. Zhan and S. Gao, Chem. Eng. J., 2022, 442 Search PubMed
.
- C. Li, X. Xie, H. Liu, P. Wang, C. Deng, B. Lu, J. Zhou and S. Liang, Nat. Sci. Rev., 2022, 9, nwab177 CrossRef CAS PubMed
.
- J. Yang, B. Yin, Y. Sun, H. Pan, W. Sun, B. Jia, S. Zhang and T. Ma, Nano-Micro Lett., 2022, 14, 1–47 CrossRef PubMed
.
- Y. Bai, H. Zhang, M. Usman Tahir and B. Xiang, J. Colloid Interface Sci., 2022, 608, 22–29 CrossRef CAS PubMed
.
- B.-F. Cui, X.-P. Han and W.-B. Hu, Small Struct., 2021, 2, 2000128 CrossRef CAS
.
- H. Jia, Z. Wang, B. Tawiah, Y. Wang, C.-Y. Chan, B. Fei and F. J. N. E. Pan, Nano Energy, 2020, 70, 104523 CrossRef CAS
.
- G. Qian, G. Zan, J. Li, S. J. Lee, Y. Wang, Y. Zhu, S. Gul, D. J. Vine, S. Lewis and W. Yun, Adv. Energy Mater., 2022, 12, 2200255 CrossRef CAS
.
- Y. Guo, Prot. Control Mod. Power Syst., 2022, 7, 602–618 Search PubMed
.
- J. Ming, J. Guo, C. Xia, W. Wang and H. N. Alshareef, Mater. Sci. Eng. R Rep., 2019, 135, 58–84 CrossRef
.
- T. C. Li, Y. V. Lim, X. Xie, X. L. Li, G. Li, D. Fang, Y. Li, Y. S. Ang, L. K. Ang and H. Y. Yang, Small, 2021, 17, e2101728 CrossRef PubMed
.
- M. Idrees, S. Batool, J. Cao, M. S. Javed, S. Xiong, C. Liu and Z. Chen, Nano Energy, 2022, 100, 107505 CrossRef CAS
.
- Y. Wang, Z. Wang, F. Yang, S. Liu, S. Zhang, J. Mao and Z. Guo, Small, 2022, 2107033 Search PubMed.
- J. Xu, W. Lv, W. Yang, Y. Jin, Q. Jin, B. Sun, Z. Zhang, T. Wang, L. Zheng and X. J. A. n. Shi, ACS Nano, 2022, 16(7), 11392–11404 CrossRef CAS PubMed
.
- Y. Quan, W. Zhou, T. Wu, M. Chen, X. Han, Q. Tian, J. Xu and J. Chen, Chem. Eng. J., 2022, 446 Search PubMed
.
- N. Guo, W. Huo, X. Dong, Z. Sun, Y. Lu, X. Wu, L. Dai, L. Wang, H. Lin and H. Liu, Small Methods, 2022, 2200597 CrossRef CAS PubMed
.
- M. Zhou, S. Guo, J. Li, X. Luo, Z. Liu, T. Zhang, X. Cao, M. Long, B. Lu, A. Pan, G. Fang, J. Zhou and S. Liang, Adv. Mater., 2021, 33, e2100187 CrossRef PubMed
.
- X. Zhu, H. Zhang, Z. Wang, C. Zhang, L. Qin, D. Chen, S. Sun, C. Liu and J. Chen, Mater. Today Energy, 2022, 23, 100897 CrossRef CAS
.
- C. Li, X. Shi, S. Liang, X. Ma, M. Han, X. Wu and J. Zhou, Chem. Eng. J., 2020, 379 Search PubMed
.
- H. Lu, Q. Jin, X. Jiang, Z. M. Dang, D. Zhang and Y. Jin, Small, 2022, 18, e2200131 CrossRef PubMed
.
- Q. Lu, C. Liu, Y. Du, X. Wang, L. Ding, A. Omar and D. Mikhailova, ACS Appl. Mater. Interfaces, 2021, 13, 16869–16875 CrossRef CAS PubMed
.
- Z. Liu, J. Ren, F. Wang, X. Liu, Q. Zhang, J. Liu, P. Kaghazchi, D. Ma, Z. Chi and L. Wang, ACS Appl. Mater. Interfaces, 2021, 13, 27085–27095 CrossRef CAS PubMed
.
- Z. Miao, M. Du, H. Li, F. Zhang, H. Jiang, Y. Sang, Q. Li, H. Liu and S. Wang, EcoMat, 2021, 3 Search PubMed
.
- Y. Liu, T. Guo, Q. Liu, F. Xiong, M. Huang, Y. An, J. Wang, Q. An, C. Liu and L. Mai, Mater. Today Energy, 2022, 28, 101056 CrossRef CAS
.
- Z. Yi, G. Chen, F. Hou, L. Wang and J. J. A. E. M. Liang, Adv. Energy Mater., 2021, 11, 2003065 CrossRef CAS
.
- Z. Cao, P. Zhuang, X. Zhang, M. Ye, J. Shen and P. M. Ajayan, Adv. Energy Mater., 2020, 10, 2001599 CrossRef CAS
.
- T. Zhang, Y. Tang, S. Guo, X. Cao, A. Pan, G. Fang, J. Zhou and S. Liang, Energy Environ. Sci., 2020, 13, 4625–4665 RSC
.
- W. Xu, K. Zhao, W. Huo, Y. Wang, G. Yao, X. Gu, H. Cheng, L. Mai, C. Hu and X. Wang, Nano Energy, 2019, 62, 275–281 CrossRef CAS
.
- A. Bayaguud, X. Luo, Y. Fu and C. Zhu, ACS Energy Lett., 2020, 5, 3012–3020 CrossRef CAS
.
- H. Dong, J. Li, J. Guo, F. Lai, F. Zhao, Y. Jiao, D. J. Brett, T. Liu, G. He and I. P. Parkin, Adv. Mater., 2021, 33, 2007548 CrossRef CAS PubMed
.
- Y. Gao, Q. Cao, J. Pu, X. Zhao, G. Fu, J. Chen, Y. Wang and C. Guan, Adv. Mater., 2022, 2207573 CrossRef PubMed
.
- J. Hao, B. Li, X. Li, X. Zeng, S. Zhang, F. Yang, S. Liu, D. Li, C. Wu and Z. Guo, Adv. Mater., 2020, 32, 2003021 CrossRef CAS PubMed
.
- Y. M. Li, W. H. Li, W. Y. Diao, F. Y. Tao, X. L. Wu, X. Y. Zhang and J. P. Zhang, ACS Appl. Mater. Interfaces, 2022, 14(20), 23558–23569 CrossRef CAS PubMed
.
- C. Li, X. Shi, S. Liang, X. Ma, M. Han, X. Wu and J. Zhou, Chem. Eng. J., 2020, 379, 122248 CrossRef CAS
.
- W. Li, Q. Zhang, Z. Yang, H. Ji, T. Wu, H. Wang, Z. Cai, C. Xie, Y. Li and H. Wang, Small, 2022, 2205667 CrossRef CAS PubMed
.
- B. Liu, S. Wang, Z. Wang, H. Lei, Z. Chen and W. Mai, Small, 2020, 16, e2001323 CrossRef PubMed
.
- K. Chen, H. Guo, W. Li and Y. Wang, ACS Appl. Mater. Interfaces, 2021, 13, 54990–54996 CrossRef CAS PubMed
.
- P. Cao, J. Tang, A. Wei, Q. Bai, Q. Meng, S. Fan, H. Ye, Y. Zhou, X. Zhou and J. Yang, ACS Appl. Mater. Interfaces, 2021, 13, 48855–48864 CrossRef CAS PubMed
.
- M. Zhou, S. Guo, J. Li, X. Luo, Z. Liu, T. Zhang, X. Cao, M. Long, B. Lu and A. Pan, Adv. Mater., 2021, 33, 2100187 CrossRef CAS PubMed
.
- Z. Zhang, B. Xi, X. Ma, W. Chen, J. Feng and S. Xiong, SusMat, 2022, 2, 114–141 CrossRef
.
- Y. Qin, P. Wen, D. Xia, H. Guo, M. Voshage, L. Jauer, Y. Zheng, J. H. Schleifenbaum and Y. Tian, Adv. Mater., 2020, 33, 101134 CAS
.
- W. Zhang, Y. Dai, R. Chen, Z. Xu, J. Li, W. Zong, H. Li, Z. Li, Z. Zhang and J. Zhu, Angew. Chem., Int. Ed., 2022, e202212695 Search PubMed
.
- J. Zheng, G. Zhu, X. Liu, H. Xie, Y. Lin, Y. Zeng, Y. Zhang, A. N. Gandi, Z. Qi and Z. Wang, ACS Energy Lett., 2022, 7, 4443–4450 CrossRef CAS
.
- X. Xie, S. Liang, J. Gao, S. Guo, J. Guo, C. Wang, G. Xu, X. Wu, G. Chen and J. Zhou, Energy Environ. Sci., 2020, 13, 503–510 RSC
.
- W. Zong, H. Guo, Y. Ouyang, L. Mo, C. Zhou, G. Chao, J. Hofkens, Y. Xu, W. Wang and Y. E. Miao, Adv. Funct. Mater., 2022, 32, 2110016 CrossRef CAS
.
- W. Zong, N. Chui, Z. Tian, Y. Li, C. Yang, D. Rao, W. Wang, J. Huang, J. Wang and F. Lai, Adv. Sci., 2021, 8, 2004142 CrossRef CAS PubMed
.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2na00682k |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2023 |