Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Time-domain Tollens reaction: synthesising silver nanoparticles with the formaldehyde clock†‡
Ronny
Kürsteiner
 a,
Maximilian
Ritter
a,
Maximilian
Ritter
 a,
Alla
Sologubenko
a,
Alla
Sologubenko
 b,
Laura
Stricker
b,
Laura
Stricker
 a and
Guido
Panzarasa
a and
Guido
Panzarasa
 *a
*a
aInstitute for Building Materials, Department of Civil, Environmental and Geomatic Engineering, ETH Zürich, Laura-Hezner-Weg 7, 8093 Zürich, Switzerland. E-mail: guidop@ethz.ch
bScientific Center for Light and Electron Microscopy (ScopeM), ETH Zürich, Otto-Stern-Weg 3, 8093 Zürich, Switzerland
First published on 16th March 2023
Abstract
The addition of silver(I) ions to the methylene glycol–sulphite (MGS) clock reaction results in the sudden formation of metallic silver nanoparticles. Stable suspensions are obtained in the presence of poly(vinylpyrrolidone). The time delay before the appearance of the particles, as well as their size, decreases with the initial methylene glycol concentration while their monodispersity increases.
In 1882, B. Tollens presented an extensive study on the reduction of silver(I) (hydr)oxide to metallic silver by aldehydes, reactions of great analytical as well as synthetic value.1,2 In the classical Tollens reaction, the reagent is not silver (hydr)oxide but its water-soluble complex with ammonia. Since then, the Tollens reaction has been used for depositing thin films and for nanoparticle synthesis, enabling a variety of applications ranging from electrical conductors to catalysis, from antimicrobial agents to plasmonics.3–7
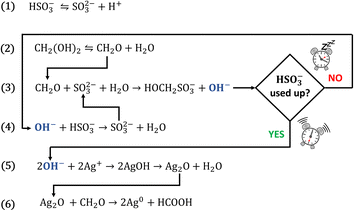
Our research concentrates on the materials science applications of clock reactions,8–15 and during our studies we realised that the methylene glycol–sulphite (MGS) reaction, also termed “formaldehyde clock”, could be suitable for the reduction of silver(I) ions to metallic silver, in close analogy with the classical Tollens reaction. The MGS reaction is a well-known pH clock featuring a sudden pH increase, from acidic (pHinitial ≈ 5.5) to alkaline (pHfinal ≈ 11), after a highly reproducible induction time which is a function of the initial reactant concentrations.16,17 The mechanism of the MGS clock is presented by eqn (1)–(4) in Scheme 1. Bisulphite HSO3− and sulphite SO32− species are in a dynamic equilibrium (eqn (1)), resulting in the formation of a pH-buffering system. Methylene glycol MG CH2(OH)2 spontaneously dehydrates into formaldehyde CH2O (eqn (2)), which quickly reacts with sulphite generating hydroxymethanesulphonate HOCH2SO3− and hydroxide ions OH− (eqn (3)). However, while bisulphite is present, the base is scavenged with the formation of sulphite (eqn (4)) which feeds back into the system. As a result, the pH remains fairly constant until the bisulphite buffer is depleted, after which it increases suddenly. The final amount of OH− generated equals that of the initial sulphite concentration, while for a fixed SO32−/HSO3− ratio the induction time is directly proportional to the initial concentration of formaldehyde (which remains always in excess).
Recently, the application of the MGS clock for the synthesis of hybrid (e.g. the metal–organic framework ZIF-8)18 and inorganic (e.g. iron (hydr)oxides)15 materials has been demonstrated. In the present work, we applied the same principle to obtain silver nanoparticles. When silver(I) ions are added to the MGS reaction, silver(I) hydroxide AgOH precipitates after the clock sets off, then spontaneously dehydrates to form the oxide Ag2O (eqn (5)). The latter is then reduced to metallic silver by the formaldehyde, following the route of the Tollens reaction (eqn (6)).
For our investigations we chose to use the following initial reactant concentrations: [SO32−]0 = 5 mM, [HSO3−]0 = 50 mM, and [MG]0 ranging from 100 to 500 mM. These conditions result in the generation of ca. 5 mM OH−, which is more than sufficient to react with 0.5 mM silver(I) ions (added as nitrate). Reactions were carried out at room temperature (23 ± 1 °C). Silver(I) is soluble in presence of sulphite in excess due to the formation of a complex, which is stable in the reaction conditions.19
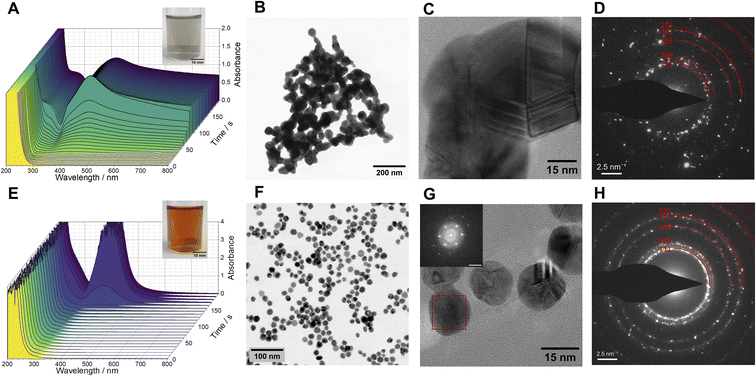
The initially transparent and colourless reaction mixture transforms into a greyish suspension. Later on, it forms black agglomerates, readily dispersible by sonication (inset of Fig. 1A, S1 and Movie S1‡). A qualitative test with acetic acid§ confirmed that the final product is metallic silver and not silver (hydr)oxide. UV-vis spectra acquired as a function of time (Fig. 1A) demonstrated a gradual increase of absorbance at the beginning, followed by prompt evolution of the curves to exhibit a distinct broad peak centred at 418 nm. This feature, along with the absorption tail at longer wavelengths, can be associated to the surface plasmon resonance (SPR) of aggregated silver nanoparticles. Scanning transmission electron microscopy (STEM) data (Fig. 1B) demonstrated that the product consists of aggregated spherical and elongated particles 40–60 nm in size. In addition, yet another silver-free component is detected in the material. Analyses of the selected area electron diffraction patterns (SAED, Fig. 1D) evidenced the presence of metallic silver in the product and suggests that the silver-free phase could be a sodium hydroxymethanesulphonate hydrate (see also Fig. S2‡). The energy dispersive X-ray spectroscopy (EDS) in the spectrum imaging (SI) mode of STEM (Fig. S3‡) indeed showed the presence of sulphur in the material, indicating that hydroxymethanesulphonate had likely adsorbed on the silver nanoparticle surfaces.
To prevent particle aggregation, we added 1%m/v poly(N-vinylpyrrolidone) (PVP, avg. Mw 10 kDa), a commonly used steric stabiliser for silver nanoparticles,20 to the MGS–Ag(I) system. In presence of PVP, the initially colourless reaction mixture evolved over time into a homogeneous amber-coloured and transparent suspension (inset of Fig. 1E and Movie S2‡). The induction time of the pH clock was not significantly affected by the presence of PVP (Fig. S4‡). As seen from the UV-vis spectrum as a function of time (Fig. 1E), an intense and relatively narrow peak, centred at 405 nm, developed rapidly after some latent period. This peak is characteristic for the surface plasmon resonance of well-dispersed, spherical, and small (<100 nm in diameter) silver nanoparticles.21 Indeed, the high-resolution TEM micrographs revealed a highly uniform spherical morphology of the particles, 15–20 nm in size (Fig. 1G), displaying the characteristic contrast of nano-sized twins in silver nanoparticles. The analyses of SAED patterns (Fig. 1H) and fast Fourier transforms (FFTs) of the HR-TEM images also confirmed that the product is metallic silver. We also tried reducing the concentration of PVP to 0.1 and 0.5%m/v, but this resulted in more polydisperse particles. Although the UV-vis spectra of the particle suspensions obtained with 0.5% and 1% PVP were virtually identical (Fig. S5‡), more detailed information was revealed by electron microscopy. The suspension made with 0.5% PVP exhibited significantly higher polydispersity (Fig. S6‡). Increasing the concentration of silver(I) ions (to 0.75 and 1 mM) in the reaction mixture led to particles with increased polydispersity, as pointed out by the UV-vis spectra (Fig. S7‡) and electron micrographs (Fig. S8‡).
PVP is known to form complexes with Ag(I) ions and to be able to reduce them to metallic silver thanks to its end-groups.22 To better understand its role in the MGS–Ag(I) system, we performed additional control experiments. As a result, we established that in the presence of PVP, no silver (hydr)oxide forms when 5 mM NaOH is added to 0.5 mM Ag(I). Instead, an amber-coloured suspension containing much more polydisperse Ag-particles with sizes ranging from 2 to 50 nm forms (Fig. S9 and Movie S3‡). Repeating this experiment with 200 mM of MG, we obtain a dark brown suspension which, within 20 min, evolves into a dichroic suspension (amber-coloured in transmission, hazy brown/grey in reflection, Movie S4‡). It contains roughly spherical, metallic silver particles up to ∼100 nm in size with a broad size distribution (Fig. S10‡). These control experiments showed the superiority of the MGS clock-based approach in terms of particle homogeneity and monodispersity.
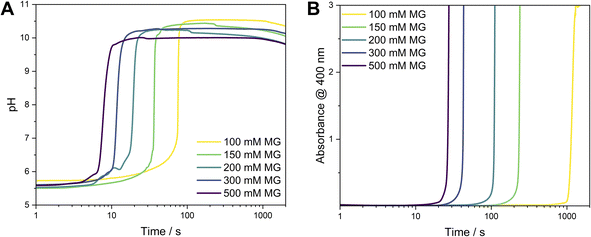
Moreover, we investigated the effect of the initial MG concentration on the behaviour of the MGS–Ag(I)–PVP system, simultaneously measuring the time-evolution of the pH value (indicative of the MGS clock reaction) and the evolution of absorbance (indicative of the formation of silver particles). Varying the [MG]0 in the MGS clock allows to tune the induction time of the pH increase. For the conditions used here, this amounted to about 8 s ([MG]0 = 500 mM) to 50 s ([MG]0 = 100 mM) time periods. Although both, pH (Fig. 2A) and absorbance (measured at 400 nm, Fig. 2B), show a sharp increase after an induction time, there is a significant time lag between them. A closer inspection of the absorbance profiles reveals a direct correlation between the two in the form of a small increase in absorbance (Fig. S11‡), which occurs at the moment of the pH increase. We attribute this behaviour to the formation of AgOH (Scheme 1, eqn (5)). The evolution of absorbance over time can be as fast as 27 s ([MG]0 = 500 mM) or can take up to 1200 s ([MG]0 = 100 mM) and is clearly auto-accelerating, a behaviour sometimes observed during the synthesis of silver nanoparticles with the Tollens reaction.23,24
In addition to varying the induction time for the formation of silver nanoparticles, we found that varying [MG]0 also leads to different particle sizes. The UV-vis spectra of samples obtained for [MG]0 ≥ 150 mM (Fig. S12‡) showed a narrow SPR peak at ∼400 nm. For [MG]0 = 100 mM a significant broadening is observed, indicative of larger particles. Image analyses (Fig. 3) of the STEM micrographs (Fig. 3 and S18–S22‡) showed that the size distribution remains virtually constant for [MG]0 ≥ 200 mM, while for [MG]0 ≤ 150 mM significant broadening and a shift to larger particle sizes was observed.
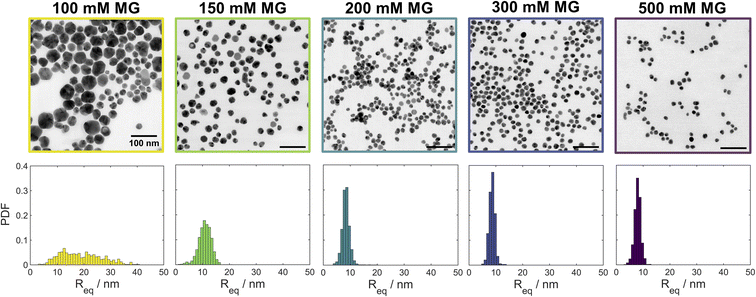 | ||
| Fig. 3 Bright field STEM micrographs and particle size distributions showing the effect of the initial methylene glycol concentration [MG]0 (indicated on top of each image) in the MGS–Ag(I)–PVP system on the morphology and size distribution of the resulting silver nanoparticles. The scale bar is 100 nm in all images. For the corresponding high-angle annular dark field (HAADF) and low-angle annular dark field (LAADF) micrographs see Fig. S13–S17.‡ | ||
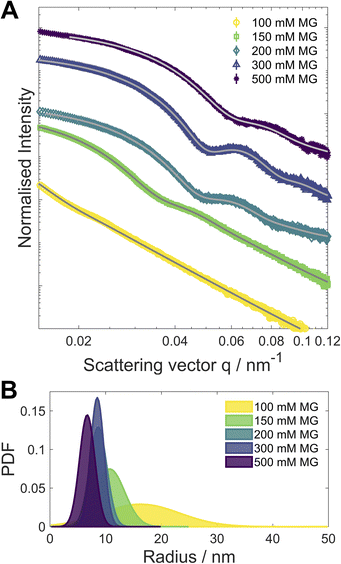
In order to probe a larger number of particles for size analysis than is available via analysis of the STEM images, small angle X-ray scattering (SAXS) was performed on both “clocked” and control systems. The size distribution was simulated using Xenocs XSACT (Fig. 4 and S23‡) and the pair distance distribution function (PDDF, Fig. S24‡) was analysed using the ATSAS (Version 3.0.5) software package.25 The average particle size decreases with [MG]0, most likely due to a faster initial nucleation. The particle size distribution becomes narrower for the higher [MG]0, except in the case of [MG]0 = 500 mM, for which the slight broadening is detected and is attributed to the presence of larger particles. It is noteworthy to mention that the particle size distribution analyses from STEM micrographs and from the SAXS data are in good agreement.
Conclusions
We demonstrated that the addition of silver(I) ions to the methylene glycol–sulphite (MGS) clock reaction results in the spontaneous formation of silver nanoparticles after an induction time. Together with the particle morphology, this induction time can be tuned by varying the initial MG concentration and by the addition of PVP. The base produced by the MGS clock results in the formation of silver (hydr)oxide, which in turn is reduced to metallic silver by the formaldehyde in excess, similar to what happens in the classical Tollens reaction. These results further show the usefulness of chemical clocks for the design of time-programmable autonomous self-assembly systems.Author contributions
Conceptualization: G. P.; formal analysis: L. S., R. K., M. R., A. S.; funding acquisition: G. P.; investigation: G. P., R. K., M. R., A. S.; methodology: G. P., R. K.; project administration: G. P.; supervision: G. P.; validation: G. P., R. K.; visualization: G. P., R. K.; writing – original draft: G. P., R. K.; writing – review and editing: G. P., R. K.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the ETH Career Seed Grant SEED-12 20-1. The authors are grateful to Prof. Dr Ingo Burgert (ETH Zurich) for his kind support. The authors gratefully acknowledge Dr Thomas Weber from the D-MATL X-Ray Service Platform at ETH Zurich for his support & assistance with SAXS characterisation in this work. The authors also thank the Scientific Center for Optical and Electron Microscopy (ScopeM) of ETH Zurich for providing the electron microscopy facilities.Notes and references
- B. Tollens, Ber. Dtsch. Chem. Ges., 1882, 15, 1635–1639 CrossRef.
- B. Tollens, Ber. Dtsch. Chem. Ges., 1882, 15, 1828–1830 CrossRef.
- Q. Huang, K. Zhang, Y. Yang, J. Ren, R. Sun, F. Huang and X. Wang, Chem. Eng. J., 2019, 370, 1048–1056 CrossRef CAS.
- M. Li, L. Shi, T. Xie, C. Jing, G. Xiu and Y.-T. Long, ACS Sens., 2017, 2, 263–267 CrossRef CAS PubMed.
- P. Prokopovich, M. Köbrick, E. Brousseau and P. Stefano, J. Biomed. Mater. Res., Part B, 2015, 103, 273–281 CrossRef PubMed.
- M. Zienkiewicz-Strzałka, S. Pasieczna-Patkowska, M. Kozak and S. Pikus, Appl. Surf. Sci., 2013, 266, 337–343 CrossRef.
- G. Upert, F. Bouillère and H. Wennemers, Angew. Chem., Int. Ed. Engl., 2012, 51, 4231–4234 CrossRef CAS PubMed.
- G. Panzarasa, A. Osypova, A. Sicher, A. Bruinink and E. R. Dufresne, Soft Matter, 2018, 14, 6415–6418 RSC.
- G. Panzarasa, T. Sai, A. L. Torzynski, K. Smith-Mannschott and E. R. Dufresne, Mol. Syst. Des. Eng., 2020, 5, 445–448 RSC.
- G. Panzarasa, A. L. Torzynski, T. Sai, K. Smith-Mannschott and E. R. Dufresne, Soft Matter, 2020, 16, 591–594 RSC.
- C. C. M. Sproncken, B. Gumi-Audenis, G. Panzarasa and I. K. Voets, ChemSystemsChem, 2020, 2, e2000005 CrossRef CAS.
- S. Riedel, T. Schweizer, K. Smith-Mannschott, E. R. Dufresne and G. Panzarasa, Soft Matter, 2021, 17, 1189–1193 RSC.
- S. Riedel and G. Panzarasa, Mol. Syst. Des. Eng., 2021, 6, 883–887 RSC.
- R. Kürsteiner, M. Ritter, Y. Ding and G. Panzarasa, Materials, 2022, 15, 1166 CrossRef PubMed.
- R. Kürsteiner, Y. Ding, M. Ritter and G. Panzarasa, Nanomaterials, 2022, 12, 3743 CrossRef PubMed.
- C. Wagner, Ber. Dtsch. Chem. Ges., 1929, 62, 2873–2877 CrossRef.
- R. L. Barrett, J. Chem. Educ., 1955, 32, 78 CrossRef.
- N. Német, G. Holló, G. Schuszter, D. Horváth, Á. Tóth, F. Rossi and I. Lagzi, Chem. Commun., 2022, 58, 5777–5780 RSC.
- T. H. James, J. Am. Chem. Soc., 1940, 62, 3411–3415 CrossRef CAS.
- K. M. Koczkur, S. Mourdikoudis, L. Polavarapu and S. E. Skrabalak, Dalton Trans., 2015, 44, 17883–17905 RSC.
- G. Panzarasa, J. Chem. Educ., 2014, 91, 696–700 CrossRef CAS.
- Y. Xiong, I. Washio, J. Chen, H. Cai, Z.-Y. Li and Y. Xia, Langmuir, 2006, 22, 8563–8570 CrossRef CAS PubMed.
- R. Dondi, W. Su, G. A. Griffith, G. Clark and G. A. Burley, Small, 2012, 8, 770–776 CrossRef CAS PubMed.
- Y. Yin, Z.-Y. Li, Z. Zhong, B. Gates, Y. Xia and S. Venkateswaran, J. Mater. Chem., 2002, 12, 522–527 RSC.
- K. Manalastas-Cantos, P. V. Konarev, N. R. Hajizadeh, A. G. Kikhney, M. V. Petoukhov, D. S. Molodenskiy, A. Panjkovich, H. D. T. Mertens, A. Gruzinov, C. Borges, C. M. Jeffries, D. I. Svergun and D. Franke, J. Appl. Crystallogr., 2021, 54, 343–355 CrossRef CAS PubMed.
Footnotes |
| † This work is dedicated to the 140th anniversary (1882–2022) of the Tollens reaction. |
| ‡ Electronic supplementary information (ESI) available: Complete experimental details, additional images and movies. See DOI: https://doi.org/10.1039/d3na00121k |
| § Silver (hydr)oxide is soluble in acetic acid, while metallic silver is not. |
| This journal is © The Royal Society of Chemistry 2023 |