Open Access Article
Open Access ArticleRadioluminescence from polymer dots based on thermally activated delayed fluorescence†
Daiki
Asanuma‡
a,
Hieu Thi
Minh Nguyen‡
 b,
Zuoyue
Liu
a,
Sachiko
Tojo
a,
Hajime
Shigemitsu
b,
Zuoyue
Liu
a,
Sachiko
Tojo
a,
Hajime
Shigemitsu
 c,
Minoru
Yamaji
c,
Minoru
Yamaji
 e,
Kiyohiko
Kawai
e,
Kiyohiko
Kawai
 ad,
Tadashi
Mori
ad,
Tadashi
Mori
 c,
Toshiyuki
Kida
c,
Toshiyuki
Kida
 c,
Guillem
Pratx
c,
Guillem
Pratx
 *b,
Mamoru
Fujitsuka
*b,
Mamoru
Fujitsuka
 *af and
Yasuko
Osakada
*af and
Yasuko
Osakada
 *afg
*afg
aSANKEN, Osaka University, Mihogaoka 8-1, Ibaraki, Osaka 567-0047, Japan. E-mail: yosakada@sanken.osaka-u.ac.jp; fuji@sanken.osaka-u.ac.jp
bDepartment of Radiation Oncology and Medical Physics, Stanford University, 300 Pasteur Dr., Stanford, CA 94305, USA. E-mail: pratx@stanford.edu
cDepartment of Applied Chemistry, Graduate School of Engineering, Osaka University, 2-1 Yamadaoka, Suita, Osaka 565-0871, Japan
dDepartment of Life Science and Technology, Tokyo Institute of Technology, B-52, 4259 Nagatsuta, Midori-ku, Yokohama, Kanagawa 226-8501, Japan
eDivision of Molecular Science, Graduate School of Science and Engineering, Gunma University, Ota, Gunma 373-0057, Japan
fInnovative Catalysis Science Division, Institute for Open and Transdisciplinary Research Initiatives (ICS-OTRI), Osaka University, 2-1 Yamadaoka, Suita, Osaka 565-0871, Japan
gInstitute for Advanced Co-Creation Studies, Osaka University, Yamadagaoka 1-1, Suita, Osaka 565-0871, Japan
First published on 23rd May 2023
Abstract
We demonstrate that polymer dots doped with thermally activated delayed fluorescence (TADF) molecules clearly exhibit blue radio-luminescence upon hard X-ray and electron beam irradiation, which is a new design for nano-sized scintillators.
Thermally activated delayed fluorescence (TADF) molecules are an active area of research in applications of photoluminescent materials such as organic light-emitting diodes (OLEDs) and optoelectronic devices.1,2 Fluorescence is observable because of reverse intersystem crossing caused by thermal excitation from the lowest triplet excited state (T1) to the lowest singlet excited state (S1).3 Therefore, TADF molecules are characterized by the ability to achieve a long photoluminescence lifetime and high quantum yield without using heavy atoms.4 Therefore, in addition to OLED applications, applications such as bio-imaging are beginning to be considered, taking advantages of their brightness.5
Recently, molecules belong to TADF have been used for X-ray scintillation and imaging in the solid state.6,7 Their long lifetime of triplet excitons is an important factor for efficient X-ray scintillation and radiation detection in the solid state.8 However, these scintillation phenomena have only been reported in the solid state; further investigation on scintillation in nano-probes is necessary to develop imaging nano-probes.9 Previously, we investigated hard X-ray excited luminescence from nanomaterials such as polymer nanoparticles (P-dots) and nanoclusters as imaging probes in H2O.10–12 We also attributed the long lifetime of photoluminescence as an important requirement for efficient scintillation, but such luminescence has been limited to nanomaterials containing heavy atoms such as gold and iridium.10,11,13 We hypothesized that nano-probes containing TADF molecules would also work as scintillators for radiation (including hard X-ray and electron beams) in nanomaterials. In this study, we synthesized TADF molecule doped P-dots and characterized their emission properties, including hard X-ray and electron beam excited luminescence.
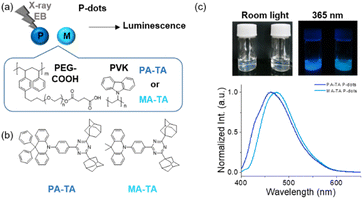
TADF molecule doped P-dots were synthesized by the precipitation method with slight modification (Fig. 1a).10,14,15 PA-TA and MA-TA, TADF molecules that exhibit blue photoluminescence, were selected as the molecules for doping (Fig. 1b).16 The synthesis method is as follows: TADF molecules, poly(n-vinylcarbazole) (PVK) and amphiphilic PEG polymers were dissolved in tetrahydrofuran (THF) and dispersed in water under sonication; Table S1† shows the concentrations of the constituents for the synthesis. Thereafter, THF was removed by evaporation to prepare a P-dot aqueous solution. PVK and PEG polymers are important for the composition of P-dots, with PVK and PEG polymer, with styrene, expected to act as a carrier for TADF molecules and interact with radiation, while PEG is expected to improve the dispersibility of P-dots in aqueous solution due to its amphiphilic nature.10 The average particle size of the synthesized P-dots was estimated by dynamic light scattering and transmission electron microscopy (TEM) (Fig. S1 and Table S2†). The hydrodynamic diameters of the P-dots were 73 and 63 nm for PA-TA and MA-TA P-dots, respectively. The core sizes of the P-dots determined by TEM were 26 and 25 nm, respectively. These sizes are somewhat smaller than other molecule-doped ones to date reported by us.17
The UV-vis absorption spectra of the P-dots (Fig. S2†) indicate absorption of the TADF molecules at 365 nm, and PVK-derived absorption at 300 and 345 nm in both of the P-dots. The photoluminescence spectra recorded by excitation at 355 nm (Fig. 1c) indicate emission with maxima at 456 and 473 nm, respectively, similar to those of PA-TA and MA-TA monomers, with slightly increased FWHM values in P-dots compared with monomers (Fig. S2 and Table S2†). We measured the photoluminescence absolute quantum yields of monomer TADF molecules in toluene and P-dots in water under Ar and aerobic conditions (Fig. S3, S4 and Table S3†). The quantum yields of P-dots subjected to oxygen quenching were not much lower than those of the respective monomers, indicating suppression of oxygen quenching by P-dot formation. The quantum yields of P-dots doped with a previously reported Ir complex [Ir(dfppy)3] dramatically decreased to 10% that the monomer. The TADF molecule doped P-dots did not exhibit a marked decrease in photoluminescence after P-dot formation and exhibited an improved photoluminescence quantum yield.
Next, we examined the photoluminescence dynamics. First, we measured the photoluminescence lifetime of the monomer TADF molecules in organic solvents, in the presence and absence of oxygen (Fig. S3, S4 and Table S4†). Under degassed conditions, the lifetimes of PA-TA and MA-TA were 6.0 and 5.2 μs, respectively; whereas in the presence of oxygen (i.e. air-saturated conditions), we observed substantial decreases in the lifetimes: as low as 0.011 and 0.012 μs, respectively. These changes indicate that oxygen quenched the emissive state of the TADF systems. Next, we measured the photoluminescence lifetimes of P-dots doped with TADF molecules. Under Ar-saturated conditions, the lifetimes of PA-TA and MA-TA P-dots were 23 and 19 μs, respectively; and in the presence of oxygen, 6.4 and 7.8 μs, respectively. Even in the presence of oxygen, we observed luminescence in the microsecond region, indicating inhibition of quenching by molecular oxygen due to difficulties with oxygen permeability inside the P-dots; thus, we observed efficient luminescence.10 This result agrees with the luminescence quantum yield results.
To further examine the relaxation process of the excited states in detail, we recorded transient absorption spectra. First, pulse radiolysis techniques were employed to observe the triplet excited state of the TADF monomers. This method refers to applying which nanosecond-pulsed electron beams to a sample and observing the time evolution of reactions induced by the radiation in the material. When a substrate is dissolved in an organic solvent, such as benzene or toluene, and irradiated with an electron beam pulse, an excited state of the substrate is generated. Here, we irradiated TADF molecules dissolved in toluene with electron beam pulses accelerated by the L-band LINAC (Fig. S5†). In the cases of PA-TA and MA-TA molecules, we observed transient absorption attributable to the triplet excited state at ca. 500 nm. We also recorded the transient absorption spectra of the excited states of the monomers by laser flash photolysis (Fig. S6†). After 355 nm picosecond laser irradiation, we observed emission at 450 nm as well as transient absorption attributable to the singlet excited and triplet excited states. We performed similar measurements on the P-dots (Fig. S6†). Upon 355 nm laser irradiation, we observed emission at 450 nm as well as transient absorption attributable to the singlet excited and triplet states at ca. 450 and 500 nm, respectively. These results indicate a minimal effect of P-dot formation on the excited states.
Next, we investigated the effect of oxygen on the triplet excited state in more detail. First, we compared the time traces of PA-TA and MA-TA for the transient absorption at 600 nm. Under degassed conditions, the lifetimes were 4.0 and 3.4 μs, respectively, whereas in the presence of oxygen, the lifetimes were 0.068 and 0.057 μs, respectively, indicating decreased lifetimes (Fig. S7†). These results are consistent with those obtained from photoluminescence experiments. We also measured the time traces of PA-TA and MA-TA P-dots. Under degassed conditions, the lifetimes were 84 and 37 μs, respectively; whereas in the presence of oxygen, we obtained lifetimes on the order of microseconds (i.e., 47 and 18 μs, respectively) (Fig. S8†). Rate constants (kq) for molecular oxygen quenching of the monomers were on the order of 109 L mol−1 s−1, close to the diffusion limit of toluene [kdiff(toluene) = 1.1 × 1010 L mol−1 s−1]; whereas the kq values for P-dots were on the order of 107 L mol−1 s−1 significantly smaller than the diffusion rate limit of H2O [kdiff(H2O) = 6.5 × 109 L mol−1 s−1].18 These results further demonstrate that doping TADF molecules into P-dots inhibited quenching by oxygen molecules, rendering them more luminescent.
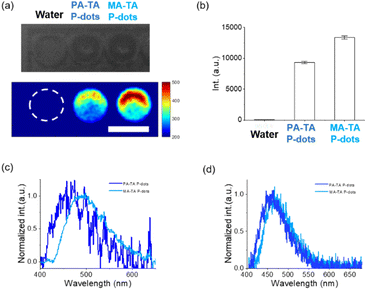
Next, we studied hard-X-ray-induced-luminescence (Fig. 2a–c). We placed an aqueous solution of P-dot sample in a 96-well plate, and then we directed a beam of hard X-rays (60 kVp) toward the side of the plate. We observed the luminescence of the sample from the top. Fig. 2a shows the results of luminescence imaging. We observed X-ray-induced luminescence with both P-dots. The luminescence intensities of PA-TA and MA-TA P-dots were 203× and 290× higher than that of the control water, respectively. We also recorded the emission spectra under hard X-ray excitation. Additionally, we embedded the P-dots into films (Fig. S9†) and recorded luminescence spectra. We placed the films in a hard X-ray irradiator and recorded spectra on a fiber-coupled spectrometer. These P-dots films exhibited blue emission with an emission maximum at ca. 470 nm. These results indicate that P-dots doped with TADF molecules emitted blue light when irradiated with hard X-rays. The mechanism of hard X-ray-induced luminescence is considered to be the excitation of the inner-shell electrons of the P-dots by hard X-ray irradiation; followed by ionization, de-excitation, and other processes. Then, the TADF molecule formed the lowest excited state, leading to luminescence.
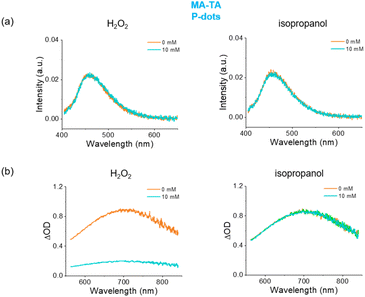
We then examined the luminescence by electron beam excitation (Fig. 2d). We irradiated the sample with electron beams accelerated from the L-band LINAC, and recorded the spectra. We also measured Ir(dfppy)3 P-dots for comparison.10 We observed emission maxima of PA-TA and MA-TA P-dots at 456 and 473 nm, respectively; and blue emission by electron beam excitation as in the case of hard X-ray irradiation. To investigate the mechanisms of electron beam induced luminescence, we added scavengers for hydroxyl radicals and hydrated electrons to the sample. Even at a high concentration (10 mM), there was no significant difference in the emission spectra under electron beam excitation (Fig. 3), whereas the transient absorption of hydrated electrons indicated clear quenching after adding H2O2 (Fig. 3b). These results suggest that the reaction mechanism for the luminescence of P-dots by electron beam excitation is independent of hydrated electrons and hydroxyl radicals, and that direct excitation of P-dots and energy transfer from the solvent are the major contributors to the luminescence.
In this study, we synthesized P-dots doped with TADF molecules and investigated their luminescence properties. The photoluminescence quantum yields of P-dots doped with TADF molecules were higher and less sensitive to molecular oxygen than those of monomers. Importantly, TADF molecule-doped P-dots emitted blue luminescence upon hard X-ray and electron beam excitation, demonstrating the potential of this material for developing of new nano-sized scintillators in imaging and therapeutic aspects.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the members of the Radiation Laboratory of SANKEN, Osaka University, for running the linear accelerator and Dr. Tatsuo Nakagawa at UNISOKU for the RIPT method on transient absorption measurements of P-dots. This work was partly supported by JSPS KAKENHI Grant Number JP17K19103 and the initiative for realizing diversity in the research environment project at Osaka University to YO. MY acknowledges a financial support from the Cooperative Research Program “Network Joint Research Center for Materials and Devices”. The authors gratefully acknowledge funding through Stanford Molecular Imaging Scholars (SMIS) Program (NIH T32 CA118681) to HTMN. We thank Michael Scott Long, PhD, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.Notes and references
- Y.-Z. Shi, H. Wu, K. Wang, J. Yu, X.-M. Ou and X.-H. Zhang, Chem. Sci., 2022, 13, 3625–3651 RSC.
- D. Barman, K. Narang, R. Gogoi, D. Barman and P. K. Iyer, J. Mater. Chem. C, 2022, 10, 8536–8583 RSC.
- H. Uoyama, K. Goushi, K. Shizu, H. Nomura and C. Adachi, Nature, 2012, 492, 234–238 CrossRef CAS PubMed.
- Z. Chen, Z. Wu, F. Ni, C. Zhong, W. Zeng, D. Wei, K. An, D. Ma and C. Yang, J. Mater. Chem. C, 2018, 6, 6543–6548 RSC.
- L. Xu, J. Wang, Q. Luo, G. Chen, F. Ni, Z. Zhu, Q. Zhao, G. Zhang and C. Yang, Mater. Chem. Front., 2020, 4, 2389–2397 RSC.
- W. Ma, Y. Su, Q. Zhang, C. Deng, L. Pasquali, W. Zhu, Y. Tian, P. Ran, Z. Chen, G. Yang, G. Liang, T. Liu, H. Zhu, P. Huang, H. Zhong, K. Wang, S. Peng, J. Xia, H. Liu, X. Liu and Y. M. Yang, Nat. Mater., 2022, 21, 210–216 CrossRef CAS PubMed.
- J.-X. Wang, L. Gutiérrez-Arzaluz, X. Wang, T. He, Y. Zhang, M. Eddaoudi, O. M. Bakr and O. F. Mohammed, Nat. Photonics, 2022, 16, 869–875 CrossRef CAS.
- A. Jana, S. Park, S. Cho, H. Kim and H. Im, Matter, 2022, 5, 20–22 CrossRef CAS.
- N. Gan, X. Zou, M. Dong, Y. Wang, X. Wang, A. Lv, Z. Song, Y. Zhang, W. Gong, Z. Zhao, Z. Wang, Z. Zhou, H. Ma, X. Liu, Q. Chen, H. Shi, H. Yang, L. Gu, Z. An and W. Huang, Nat. Commun., 2022, 13, 3995 CrossRef CAS PubMed.
- Y. Osakada, G. Pratx, L. Hanson, P. E. Solomon, L. Xing and B. Cui, Chem. Commun., 2013, 49, 4319–4321 RSC.
- Z. Liu, K. O. Jung, R. Takahata, M. Sakamoto, T. Teranishi, M. Fujitsuka, G. Pratx and Y. Osakada, RSC Adv., 2020, 10, 13824–13829 RSC.
- Y. Osakada, G. Pratx, C. Sun, M. Sakamoto, M. Ahmad, O. Volotskova, Q. Ong, T. Teranishi, Y. Harada, L. Xing and B. Cui, Chem. Commun., 2014, 50, 3549–3551 RSC.
- C. Wang, O. Volotskova, K. Lu, M. Ahmad, C. Sun, L. Xing and W. Lin, J. Am. Chem. Soc., 2014, 136, 6171–6174 CrossRef CAS PubMed.
- Y. Osakada, T. Fukaminato, Y. Ichinose, M. Fujitsuka, Y. Harada and T. Majima, Chem.–Asian J., 2017, 12, 2660–2665 CrossRef CAS PubMed.
- Y. Osakada, L. Hanson and B. Cui, Chem. Commun., 2012, 48, 3285–3287 RSC.
- Y. Wada, S. Kubo and H. Kaji, Adv. Mater., 2018, 30, 1705641 CrossRef PubMed.
- Z. Liu, Y. Okada, Y. Ichinose, D. Saitoh, N. Ieda, S. Yamasaki, K. Nishino, H. Nakagawa, M. Fujitsuka and Y. Osakada, ACS Appl. Nano Mater., 2023, 6, 1487–1495 CrossRef CAS.
- Handbook of photochemistry, ed. M. Montalti, A. Credi, L. Prodi and M. T. Gandolfi, 3rd edn, 2006 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3na00308f |
| ‡ Equally contributed to this work. |
| This journal is © The Royal Society of Chemistry 2023 |