Open Access Article
Open Access ArticleBoosted ability of ZIF-8 for early-stage adsorption and degradation of chemical warfare agent simulants†
Sojin
Oh
,
Sujeong
Lee
,
Gihyun
Lee
and
Moonhyun
Oh
 *
*
Department of Chemistry, Yonsei University, 50 Yonsei-ro, Seodaemun-gu, Seoul 03722, Republic of Korea. E-mail: moh@yonsei.ac.kr; Fax: +82-2-364-7050; Tel: +82-2-2123-5637
First published on 7th October 2023
Abstract
Efficient adsorption of hazardous substances from the environment is crucial owing to the considerable risks they pose to both humans and ecosystems. Consequently, the development of porous materials with strong adsorption capabilities for hazardous substances, such as chemical warfare agents (CWAs), is pivotal for safeguarding human lives. Specifically, the early-stage adsorption proficiency of the adsorbents plays a vital role in determining their effectiveness as ideal adsorbents. Herein, we report the efficient adsorption of CWA simulants using thermally treated ZIF-8 (T-ZIF-8). The T-ZIF-8 samples were prepared by subjecting ZIF-8 to a simple thermal treatment, which resulted in a more positive surface charge with extra open metal sites. Although the pore volume of T-ZIF-8 decreased after thermal treatment, the positive surface charge of T-ZIF-8 proved advantageous for the adsorption of the CWA simulants. As a result, the adsorption capacity of T-ZIF-8 for the CWA simulants improved compared to that of pure ZIF-8. Notably, T-ZIF-8 exhibited a remarkably enhanced adsorption ability in the early stage of exposure to the CWA simulants, possibly due to the effective polar interactions between T-ZIF-8 and the simulants via the electron-rich components within the CWA simulants. Moreover, the enhanced adsorption capacity of T-ZIF-8 led to the fast degradation of simulant compared to pure ZIF-8. T-ZIF-8 also demonstrated excellent stability over three adsorption cycles. These findings highlight that T-ZIF-8 is an outstanding material for the early-stage adsorption and degradation of CWA simulants, offering high effectiveness and stability.
1. Introduction
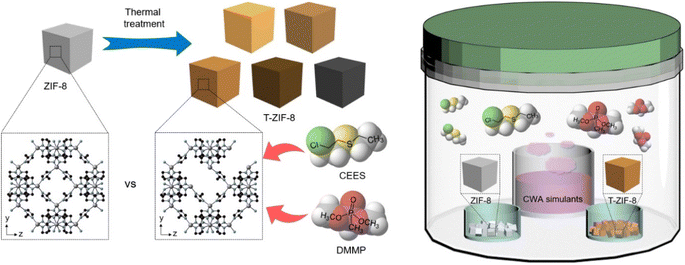
Metal–organic frameworks (MOFs) are an interesting class of porous materials that have garnered considerable attention owing to their unique properties and applications in various fields.1–28 These materials comprise metal ions or clusters linked by organic ligands, resulting in highly porous structures with large surface areas and tunable pore sizes. MOFs have a wide range of applications in gas storage, catalysis, sensing, adsorption, and separation.1–28 In particular, the use of MOFs for the adsorption and separation of toxic chemicals including chemical warfare agents (CWAs) is of utmost importance.15–28CWAs can trigger an immense long-term damage to humans and the environment.15–39 Isopropyl methylphosphonofluoridate known as sarin and bis(2-chloroethyl)sulfide known as sulfur mustard are well-known examples of highly toxic CWAs.21–28 For examples, sarin inhibits acetylcholinesterase and provokes muscle contraction and asphyxiation,21–26 and sulfur mustard damages exposed skin and tissue.24–28 Despite efforts to protect humans from the dangers of CWAs, their use in military activities or terrorist attacks remains prevalent. Therefore, developing efficient and effective methods for the removal and detoxification of CWAs from contaminated air and water is urgently required to protect humans from the adverse effects of CWAs.15–42 Adsorption is one such effective method, and porous materials, such as porous carbons, zeolites, and MOFs, have shown promising potential in this field.15–35 In particular, MOFs exhibit outstanding adsorption properties, making them promising candidates for CWA removal.15–28 Several studies have recently been conducted to explore the potential of MOFs for the adsorption and removal of CWAs.15–39 Developing porous adsorptive materials for effective adsorption of CWAs is crucial, and the use of MOFs in this context holds tremendous promise. In particular, an effective adsorption ability during the early stage of exposure to CWAs is critical in deeming an adsorbent ideal for use. The simulant compounds that have functionalities similar to those of CWAs but are less toxic and easier to handle are convenient for use in a laboratory. In general, 2-chloroethyl ethyl sulfide (CEES) and dimethyl methyl phosphonate (DMMP) were used as CWA simulants for the study.
Herein, we report the recent developments in the effective adsorption of CWA simulants using ZIF-8. The T-ZIF-8 samples (where T denotes the temperature), which have relatively high positive surface charges with extra open metal sites, were prepared by simple thermal treatment of ZIF-8. T-ZIF-8 displayed enhanced adsorption capacity for CWA simulants. Although the pore volume of T-ZIF-8 decreased after thermal treatment, the positive surface charge of T-ZIF-8 were beneficial for the adsorption of the CWA simulants. In particular, a dramatically enhanced adsorption ability of T-ZIF-8 was observed in the early stage of exposure to CWA simulants, possibly because of the effective polar interaction of T-ZIF-8 with the simulants via electron-rich moieties within the simulants. Moreover, T-ZIF-8 showed excellent stability during the three adsorption cycles. Not only the boosted ability for the rapid adsorption of simulant but also the enhanced ability for the degradation of simulant were demonstrated by T-ZIF-8. These findings suggest that T-ZIF-8 is an excellent material for the rapid adsorption and degradation of CWA simulants in the early stage of exposure, with high effectiveness and stability.
2. Experimental section
2.1. Materials and characterizations
All solvents and chemicals were purchased from commercial sources and used as received, unless stated otherwise. Deionized water was obtained from a Millipore Direct-Q®3. Scanning electron microscopy (SEM) images were acquired using a JEOL JSM-7001F field-emission scanning electron microscope (Yonsei Center for Research Facilities, Yonsei University). Energy-dispersive X-ray (EDX) spectra were obtained using a Hitachi SU 1510 SEM device equipped with a Horiba EMAX Energy E-250 EDX system. Powder X-ray diffraction (PXRD) patterns were obtained using a Rigaku Ultima IV instrument equipped with a graphite monochromated Cu Kα radiation source (40 kV, 40 mA). The adsorption–desorption isotherms of N2 (77 K) were measured using a BELSORP Max volumetric adsorption equipment system. All isotherms were measured after pretreatment under a dynamic vacuum at 25 °C for 3 h. X-ray photoelectron spectroscopy (XPS) was conducted using a Thermo Scientific K-Alpha KA1066 spectrometer with a monochromatic Al Ka X-ray source (hν = 1486.6 eV). The XPS spectra were calibrated with the measured C 1s peak position at 284.6 eV. Zeta-potential measurements were performed in an aqueous solution using a Malvern Nano-ZS Zetasizer. Water contact angle measurements of the prepared pellet samples were performed using a contact angle meter (Biolin Scientific ThetaLite 100) at 25 °C. Water was slowly dropped onto the pellet using a microsyringe, and the contact angle was measured. 1H NMR and 31P NMR spectra were recorded on a Bruker Avance III HD 300 spectrometer (1H NMR, 300 MHz) (31P NMR, 122 MHz), with chemical shifts reported relative to the residual deuterated solvent peaks. The infrared (IR) spectra of the solid samples and liquid CWA simulants were acquired using a Jasco FT/IR 4200 spectrometer and an attenuated total reflection module.2.2. Preparation of ZIF-8
Zn(NO3)2·6H2O (0.1 mmol, 29.7 mg), 2-methylimidazole (5.5 mmol, 451.6 mg), and hexadecyltrimethylammonium bromide (0.0014 mmol, 0.5 mg) were dissolved in 8 mL of deionized water. The resulting aqueous solutions were incubated at room temperature for 20 min. The resulting product was isolated and subsequently washed several times with deionized water and methanol via a centrifugation–redispersion cycle and dried under vacuum for 1 h.2.3. Preparation of T-ZIF-8 samples
Pure ZIF-8 was placed in a tube furnace, heated at 450, 500, 550, 570, or 600 °C for 5 h at a heating rate of 5 °C min−1 under N2 gas flow, and then cooled to room temperature. The resulting products are denoted as T-ZIF-8s, where T denotes the thermal treatment temperature.2.4. Adsorption of CWA simulants on ZIF samples
The adsorption of 2-chloroethyl ethyl sulfide (CEES) and dimethyl methylphosphonate (DMMP) was performed at room temperature using a jar-in-jar setup. To conduct the experiments, pure ZIF-8 and T-ZIF-8 powders (5.0 mg) were placed in a ceramic crucible pan, and a CWA simulant (2 mL) was taken in a 5 mL beaker. Subsequently, the ceramic crucible pan and beaker were placed in a large jar with the lid closed. The quantity of the CWAs simulants adsorbed onto ZIF-8 or T-ZIF-8s at different time intervals was measured using 1H NMR spectroscopy with CH2Br2 as an internal standard. The samples were digested in a mixed deuterated solvent of dimethyl sulfoxide-d6 and acetic acid-d4 to obtain 1H NMR spectra.2.5. Recycling of 550-ZIF-8 for CEES adsorption
Three cycles were conducted in the recycling experiment of 550-ZIF-8 for the adsorption of CEES. After 4 h, in the first adsorption step, 550-ZIF-8 was washed several times with methanol and dried under vacuum for 1 h. The adsorption procedure for the second and third cycles was performed under conditions identical to those of the first adsorption cycle.2.6. Adsorption kinetics of ZIF samples
The kinetics of the adsorption process provides essential information about reaction path and the rate of adsorbate.43–46 The pseudo-first order model is one of the representative models. The pseudo-first order model equation is as follows:| qt = qe(1 − e−kt) |
2.7. Degradation of DMMP on ZIF samples
For the degradation of DMMP on ZIF samples, DMMP solution was prepared by mixing DMMP (6 μL) and 3 mL of H2O and D2O mixture (H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D2O = 9
D2O = 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v). And then, DMMP solution was added to a 20 mL glass vial containing 100 mg of pure ZIF-8 or 550-ZIF-8. This mixture was continuously stirred at 60 °C. Every several hours, 300 μL of aliquot of the solvent was transferred into the NMR tube for 31P NMR measurements to determine the conversion of DMMP to MPA. The conversion was then calculated as the ratio of the integrated peak for MPA (the only hydrolysis product) to that of DMMP. After 72 h, the yields for the production of MPA were 67 and 26%, respectively, in the presence of 550-ZIF-8 and pure ZIF-8. Furthermore, the conversion of DMMP to MPA was confirmed by 1H NMR and 31P NMR (see ESI†). For comparison, the degradation of dimethyl 4-nitrophenylphosphate (DMNP) on 550-ZIF-8 was also conducted. For the degradation of DMNP on a ZIF sample, 2.1 mL of 0.45 M N-ethylmorpholine buffer solution was added to a 20 mL glass vial containing 50 mg of 550-ZIF-8 and then sonicated for 1 min to disperse homogeneously. After that, 4 μL of DMNP was added and the reaction was continuously stirred at room temperature. Every several hours, 200 μL of aliquot of the solvent was transferred into the NMR tube for 31P NMR measurements to determine the conversion of DMNP.
1 v/v). And then, DMMP solution was added to a 20 mL glass vial containing 100 mg of pure ZIF-8 or 550-ZIF-8. This mixture was continuously stirred at 60 °C. Every several hours, 300 μL of aliquot of the solvent was transferred into the NMR tube for 31P NMR measurements to determine the conversion of DMMP to MPA. The conversion was then calculated as the ratio of the integrated peak for MPA (the only hydrolysis product) to that of DMMP. After 72 h, the yields for the production of MPA were 67 and 26%, respectively, in the presence of 550-ZIF-8 and pure ZIF-8. Furthermore, the conversion of DMMP to MPA was confirmed by 1H NMR and 31P NMR (see ESI†). For comparison, the degradation of dimethyl 4-nitrophenylphosphate (DMNP) on 550-ZIF-8 was also conducted. For the degradation of DMNP on a ZIF sample, 2.1 mL of 0.45 M N-ethylmorpholine buffer solution was added to a 20 mL glass vial containing 50 mg of 550-ZIF-8 and then sonicated for 1 min to disperse homogeneously. After that, 4 μL of DMNP was added and the reaction was continuously stirred at room temperature. Every several hours, 200 μL of aliquot of the solvent was transferred into the NMR tube for 31P NMR measurements to determine the conversion of DMNP.
3. Results and discussion
3.1. Preparation of ZIF-8 and T-ZIF-8 samples
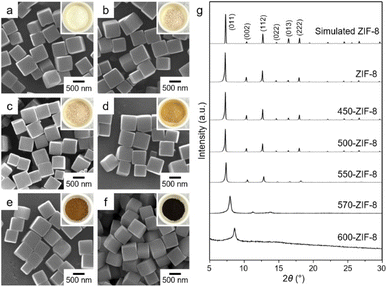
First, cubic ZIF-8 with exposed {100} planes was homogeneously synthesized via the reaction of Zn(NO3)2 and 2-methylimidazole in the presence of hexadecyltrimethylammonium bromide.47,48 The as-synthesized ZIF-8 was examined using scanning electron microscopy (SEM) to validate the production of consistent ZIF-8 cubes (Fig. 1a). The formation of crystalline ZIF-8 was verified using powder X-ray diffraction (PXRD). The PXRD pattern of the as-synthesized product displayed a representative pattern for ZIF-8 (Fig. 1g). In addition, the expected elements of ZIF-8, including zinc, carbon, and nitrogen, were detected in the energy-dispersive X-ray (EDX) spectrum (Fig. S1†). After confirming the formation of the ZIF-8 cubes, they were thermally treated in a conventional tube furnace at various temperatures (450, 500, 550, 570, and 600 °C) under N2 gas flow (Fig. 2). Hereafter, samples obtained after thermal treatment of ZIF-8 are designated with the treatment temperature, i.e., 550-ZIF-8 represents ZIF-8 obtained after thermal treatment at 550 °C. Typically, subjecting an MOF to thermal treatment at low temperatures causes the decomposition of some organic linkers, while maintaining the original structure. This process also leads to the creation of positively charged open metal sites.49–55 However, when the MOF is subjected to high temperature and exposed to an inert gas during thermal treatment, it undergoes complete decomposition and carbonization.56–61 The SEM images of all T-ZIF-8 samples displayed no morphological changes, despite the thermal treatment involved (Fig. 1). In addition, no critical structural changes were observed, as confirmed by the PXRD patterns of the T-ZIF-8 samples, except for 570-ZIF-8 and 600-ZIF-8 (Fig. 1g). Photographs of 450, 500, and 550-ZIF-8 displayed only minor color changes from white to beige (insets of Fig. 1). However, significant color changes to brown and black were observed for 570-ZIF-8 and 600-ZIF-8, indicating considerable decomposition and carbonization of ZIF-8 upon exposure to high temperatures.59–613.2. Porous properties and surface charges of T-ZIF-8 samples
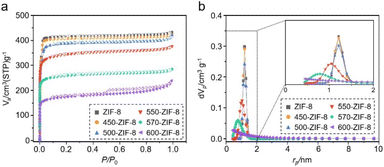
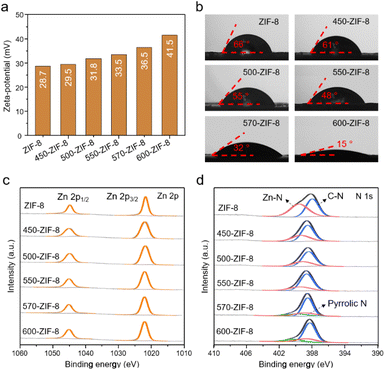
The change in the porosity of the T-ZIF-8 samples was examined by measuring the N2 sorption isotherms at 77 K (Fig. 3a). In general, when MOFs undergo thermal treatment, their pore volumes and surface areas tend to decrease owing to the decomposition of MOFs.49–51 This phenomenon was also observed in the T-ZIF-8 samples, as their N2 sorption abilities gradually decreased with increasing temperature during the thermal treatment (Fig. 3a and Table S1†). Specifically, dramatic decreases in N2 sorption were observed for 570-ZIF-8 and 600-ZIF-8. The Brunauer–Emmett–Teller (BET) surface areas of 570- ZIF-8 and 600-ZIF-8 were found to be 913.5 and 670.4 m2 g−1 respectively, and their total pore volumes were 0.44 and 0.37 cm3 g−1 respectively. These values are substantially lower than those of pure ZIF-8 at 1320.1 m2 g−1 (BET surface area) and 0.67 cm3 g−1 (total pore volume). However, no significant decrease in N2 sorption was observed for 450-, 500-, and 550-ZIF-8. In addition, the pore size distributions (Fig. 3b) of 450- and 500-ZIF-8 calculated using the non-local density functional theory (NLDFT) method remained unchanged compared to the original ZIF-8, which exhibited the characteristic pore dimensions of ZIF-8 at approximately 11.6 Å.22,62 Pore size of 10.4 Å was observed for 550-ZIF-8, which is slightly smaller than that of ZIF-8. However, the pore sizes of 570- and 600-ZIF-8 are significantly smaller than that of pure ZIF-8. In terms of pore volume, the thermal treatment of ZIF-8 is disadvantageous for the effective adsorption of the target molecule, as it results in a decrease in the adsorption capacity of the material.49,50,59The surface charge of the adsorbent plays a crucial role in determining the effectiveness of the CWA simulant adsorption. In particular, the positive charge of MOFs enhances the adsorption of CWA simulants via effective polar interactions with the electron-rich atoms present in the molecules.25,34,63,64 We examined the change in the surface charges of T-ZIF-8 samples caused by the thermal treatment. Zeta-potential measurements indicated a gradual shift towards more positive surface charges for the T-ZIF-8 samples with increasing temperature (Fig. 4a). This can be understood by considering that thermal treatment of the MOF causes the decomposition of some of organic part, leading to the creation of open metal sites carrying a positive charge.49–55 For example, pure ZIF-8 exhibited a surface charge at 28.7 mV; however, 550-ZIF, and 600-ZIF exhibited higher charges at 33.5 and 41.5 mV, respectively. Due to its large positive surface charge, T-ZIF-8 displayed the efficient adsorption of the negatively charged methyl orange (Fig. S2†). The binding energy of Zn 2p in the XPS spectra of the T-ZIF-8 samples shifted to a slightly higher position (Fig. 4c), while the binding energy of N 1s shifted to a slightly lower position (Fig. 4d) as the temperature increased, indicating an increase in Zn–N fractures.49–51 The water contact angles of the T-ZIF-8 samples were measured to determine their hydrophilicity (Fig. 4b). In general, the contact angles decreased with an increase in temperature. The water contact angles of the ZIF-8 and five T-ZIF-8 samples were found to be 66, 61, 55, 48, 32, and 15°, respectively, indicating increased hydrophilicity of the surface of the T-ZIF-8 samples.
3.3. Adsorption of CWA simulants on T-ZIF-8 samples
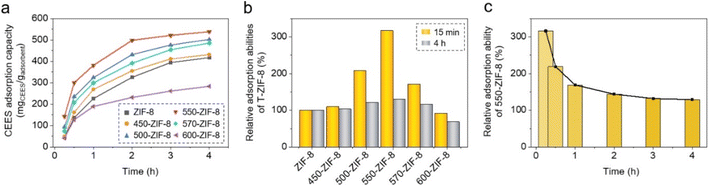
The adsorption of CEES onto pure ZIF-8 and T-ZIF-8 was investigated using a jar-in-jar setup at room temperature (Fig. 1).26 In this setup, small jars enclosing the ZIF-8 and T-ZIF-8 samples were placed in a large jar with CEES, and the ZIF samples were exposed to CEES vapors for various time intervals. After the exposure to CEES vapors, the ZIF samples were digested in a mixed deuterated solvent of dimethyl sulfoxide-d6 and acetic acid-d4, and the resulting solution was analyzed using 1H NMR spectroscopy. Peak integrations of CEES and a standard molecule (CH2Br2) were used to determine the amount of CEES per gram of ZIF (Fig. S3 and S4†). The CEES uptake was plotted against the exposure time of the ZIF samples (Fig. 5a). The amount of CEES adsorbed onto pure ZIF-8 was 417 mg of CEES per gram of ZIF-8 (417 mgCEES gadsorbent−1). On the other hand, the adsorption abilities of porous carbon and zeolite have been reported to be 74 and 109 mgCEES gadsorbent−1, respectively.32,33 Thus, ZIF-8 outperforms other porous adsorbents by a considerable margin. It is worth noting that, with the exception of 600-ZIF-8, all the T-ZIF-8 samples exhibited improved CEES adsorption capacities compared to pure ZIF-8. Out of a series of T-ZIF-8 samples, 550-ZIF-8 displayed a 29% increase at 538 mgCEES gadsorbent−1 in adsorption capacity compared with that of pure ZIF-8. However, excessive thermal treatment of ZIF-8 at high temperatures can lead to a decrease in its adsorption capacity by significantly reducing its porosity and surface area.49,50,61 Indeed, 570- and 600-ZIF-8 displayed lower adsorption abilities than 550-ZIF-8 (Fig. 5). A particularly exciting outcome of this experiment was the significant improvement in CEES adsorption observed in T-ZIF-8 during the early stage of exposure to CEES vapors. Except 600-ZIF-8, all the T-ZIF-8 samples demonstrated dramatically enhanced CEES adsorption capacities during the first 15 min of exposure to CEES vapors (Fig. 5b). Notably, 550-ZIF-8 exhibited an impressive 216% increase in the early-stage adsorption compared to that of pure ZIF-8 (Fig. 5b and c). This improvement can be attributed to the positive surface charge and the availability of open metal sites in the T-ZIF-8s. Specifically, the enhanced ability to adsorb CEES can be attributed to T-ZIF-8's effective polar interactions with the electron-rich sulfur atoms in CEES.25,34,63,64 The positive charge of the adsorbent enhances this interaction with the electron-rich sulfur atoms in CEES, thereby enhancing the adsorption capacity. These results highlighted the potential of T-ZIF-8 as a highly effective material for the rapid adsorption of CWAs. The ability to adsorb CWAs during the early stage of exposure, more than the maximum adsorption capacity during saturation, is crucial for the fast removal or detection of CWAs, given the critical nature of this concern.The successful adsorption of CEES onto the ZIF samples was confirmed using IR and EDX spectroscopy. Specifically, the IR spectra of the ZIF samples after exposure to and adsorption of CEES vapors displayed characteristic IR bands for CEES at 1262.2 and 1213.0 cm−1 (Fig. S5†).25,65 Additionally, the EDX spectra of the ZIF samples showed the presence of sulfur and chlorine originated from CEES (Fig. S6†). The SEM images and PXRD patterns of the ZIF-8 samples after CEES adsorption revealed no critical morphological or structural changes (Fig. S7 and S8†).
To further investigate the efficacy of T-ZIF-8 for CEES adsorption, the recyclability of the material was examined through three consecutive adsorption measurements (Fig. 6a). These results demonstrate that the CEES adsorption capacity of 550-ZIF-8 remained consistent during the three adsorption cycles. Furthermore, the SEM image and PXRD pattern (Fig. 6b and c, respectively) of 550-ZIF-8 after three adsorption cycles show no critical morphological or structural changes, which suggests that 550-ZIF-8 is a highly effective and stable material for CEES adsorption.
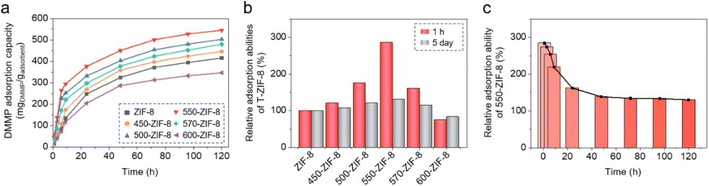
In addition, the DMMP adsorption abilities of the pure ZIF-8 and T-ZIF-8 samples were analyzed by measuring the DMMP uptake using the same jar-in-jar setup. The ZIF samples exposed to DMMP vapors for different time spans were digested in a mixed deuterated solvent, and the peak integrations of DMMP and the internal standard (CH2Br2) were used to determine the amounts of DMMP in the ZIF samples (Fig. S9 and S10†). The adsorption of DMMP on the ZIF samples was confirmed through the IR and EDX spectra (Fig. S11 and S12†). The IR spectra of the ZIF samples after exposure to and adsorption of DMMP vapors displayed the characteristic IR bands of DMMP (Fig. S11†). Additionally, the EDX spectra of the ZIF samples showed the presence of phosphorus from DMMP (Fig. S12†). Adsorption graphs showing the DMMP uptake are presented in Fig. 7a. The saturation of DMMP adsorption in ZIF-samples took 5 day, which was significantly longer than the time taken for the saturation of CEES adsorption, that is, 4 h. This is due to the lower vapor pressure of DMMP, 0.96 mmHg at 25 °C, compared to a vapor pressure of 3.4 mmHg for CEES, at the same temperature.66,67 The amount of DMMP adsorbed on pure ZIF-8 was found to be 416 mg per gram of ZIF-8 (416 mgDMMP gadsorbent−1). This capacity is comparable to those of other porous adsorbents. For example, the adsorption ability of other MOF (Zr-abtc) and porous carbon has been reported to be 351 and 460 mgDMMP gadsorbent−1, respectively.20,29 In addition, all the T-ZIF-8 samples exhibited improved DMMP adsorption capacities similar to that of CEES, compared to pure ZIF-8, except for 600-ZIF-8. Among them, 550-ZIF-8 displayed the highest increase (31%) in adsorption compared to that for pure ZIF-8. Importantly, a dramatic improvement in DMMP adsorption was found for T-ZIF-8 during the early stage of exposure to DMMP vapors. All the T-ZIF-8 samples exhibited superior DMMP adsorption capacities during the first 1 h of exposure to DMMP vapors, with the exception of 600-ZIF-8 (Fig. 7b). Especially, 550-ZIF-8 demonstrated an outstanding 185% increase in adsorption compared to pure ZIF-8 (Fig. 7b and c). SEM images and PXRD patterns showed no morphological or structural changes after DMMP adsorption, indicating that the ZIF samples retained their original properties (Fig. S13†). Furthermore, the adsorption kinetics of pure ZIF-8 and T-ZIF-8 samples were shown in Fig. S14 and Table S2.† The adsorption of CEES and DMMP on ZIF-8 and T-ZIF-8 samples was fitted to the pseudo-first order model, and kinetics of CEES adsorption was faster than DMMP adsorption. This indicated that DMMP adsorption process required a longer time to reach the equilibrium adsorption amount. The adsorption ability of 550-ZIF-8 in the presence of both CEES and DMMP was investigated via placing a 550-ZIF-8 sample in a large jar with both CEES and DMMP. After 1 h exposure to both CEES and DMMP vapors, 550-ZIF-8 sample holds 345 mgCEES gadsorbent−1 and 25 mgDMMP gadsorbent−1, respectively.
3.4. Catalytic degradation of DMMP using 550-ZIF-8
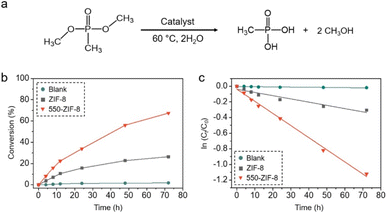
The catalytic degradation of DMMP using pure ZIF-8 and 550-ZIF-8 was investigated. It was anticipated that 550-ZIF-8 would exhibit an improved catalytic performance compared to ZIF-8 due to its enhanced adsorption capability during the early stages of exposure to DMMP. DMMP can be hydrolyzed by the catalyst to form methylphosphonic acid (MPA).21,40–42 The progression of the reaction was analyzed using 31P NMR spectroscopy. Peak integrations of DMMP and MPA were utilized to determine the conversion rate (Fig. 8, S15, and S16†). Initially, a background reaction without any catalyst was conducted, which resulted in only 2% conversion over 72 h. On the other hand, ZIF-8 exhibited a conversion rate of 26% over the same 72 h reaction time. However, the catalytic activity of 550-ZIF-8 for the hydrolysis of DMMP was significantly enhanced, achieving a conversion rate of 67% over 72 h (Fig. 8). This improvement was attributed to the significantly enhanced adsorption capacity of 550-ZIF-8, leading to higher conversion rates. The reaction mechanism for degradation of DMMP was well established in two steps; (i) the initial interaction of phosphate group of DMMP with the Lewis acid site of catalyst, (ii) subsequent nucleophilic attack by water.22,68,69 However, no critical catalytic degradation of DMMP in the presence of 550-ZIF-8 was observed at room temperature. In addition, the catalytic degradation of dimethyl 4-nitrophenylphosphate using 550-ZIF-8 was conducted in aqueous buffer solution at room temperature.26,70,71 550-ZIF-8 exhibited a conversion rate 84% over the 72 h reaction time (Fig. S17 and S18†).4. Conclusions
The efficient removal of hazardous substances, such as CWAs, from the environment is of paramount importance for mitigating the significant risks they pose to both humans and ecosystems. Adsorption has emerged as an effective method for addressing this challenge, and the development of porous materials with strong CWA adsorption capabilities is a crucial step toward safeguarding human lives. In this regard, the use of thermally treated ZIF-8 (T-ZIF-8) is a remarkable advancement in the efficient adsorption of CWA simulants. By subjecting ZIF-8 to a simple thermal treatment, T-ZIF-8 samples were prepared, resulting in several notable changes. The surface charge of T-ZIF-8 became more positive with open metal sites. These modifications positively influenced the adsorption capacity of CWA simulants by T-ZIF-8. Although the pore volume of T-ZIF-8 decreased after the thermal treatment, the positive surface charge were advantageous for adsorption. The most important finding was that T-ZIF-8 exhibited a significantly enhanced adsorption ability during the early stage of exposure to CWA simulants. This improvement can be attributed to the effective polar interactions between T-ZIF-8 and the simulants facilitated by the electron-rich components within the CWA simulants. T-ZIF-8 demonstrated excellent stability over three adsorption cycles, confirming its suitability for long-term applications. Furthermore, enhanced adsorption ability of T-ZIF-8 led to its improved activity for the degradation of simulant. Overall, the advances in the adsorption of CWA simulants using T-ZIF-8 present a promising prospect for the efficient removal of hazardous substances. T-ZIF-8 exhibits high adsorption effectiveness, particularly in the early stage of exposure, and maintains excellent stability over repeated adsorption cycles. These findings suggest that T-ZIF-8 is an outstanding material for early-stage removal of CWA simulants, offering both high efficacy and stability in the remediation of hazardous environments.Author contributions
Moonhyun Oh designed, coordinated the research, and drafted the manuscript. Sojin Oh, Sujeong Lee, and Gihyun Lee conducted experiments. Sojin Oh, Sujeong Lee, Gihyun Lee, and Moonhyun Oh conducted data analysis and revised the manuscript. The authors read and approved the final manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by the Challengeable Future Defense Technology Research and Development Program through the Agency for Defense Development (ADD) funded by the Defense Acquisition Program Administration (DAPA) in 2023 (No. 915019201) and by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (no. NRF-2022R1A2B5B03001450).References
- G. Cai, P. Yan, L. Zhang, H. C. Zhou and H. L. Jiang, Chem. Rev., 2021, 121, 12278–12326 CrossRef CAS PubMed.
- M. Ding, R. W. Flaig, H. L. Jiang and O. M. Yaghi, Chem. Soc. Rev., 2019, 48, 2783–2828 RSC.
- K. Nath, A. Ahmed, D. J. Siegel and A. J. Matzger, J. Am. Chem. Soc., 2022, 144, 20939–20946 CrossRef CAS.
- J. Liu, T. A. Goetjen, Q. Wang, J. G. Knapp, M. C. Wasson, Y. Yang, Z. H. Syed, M. Delferro, J. M. Notestein, O. K. Farha and J. T. Hupp, Chem. Soc. Rev., 2022, 51, 1045–1097 RSC.
- H. Yoon, S. Lee, S. Oh, H. Park, S. Choi and M. Oh, Small, 2019, 15, 1805232 CrossRef PubMed.
- H. Jun, S. Oh, G. Lee and M. Oh, Sci. Rep., 2022, 12, 14735 CrossRef CAS.
- J. J. Pang, Z. Q. Yao, K. Zhang, Q. W. Li, Z. X. Fu, R. Zheng, W. Li, J. Xu and X. H. Bu, Angew. Chem., Int. Ed., 2023, 62, e202217456 CrossRef CAS PubMed.
- T. Y. Luo, P. Das, D. L. White, C. Liu, A. Star and N. L. Rosi, J. Am. Chem. Soc., 2020, 142, 2897–2904 CrossRef CAS PubMed.
- W. Cho, H. J. Lee, G. Choi, S. Choi and M. Oh, J. Am. Chem. Soc., 2014, 136, 12201–12204 CrossRef CAS PubMed.
- C. S. Smoljan, Z. Li, H. Xie, C. J. Setter, K. B. Idrees, F. A. Son, F. Formalik, S. Shafaie, T. Islamoglu, L. K. Macreadie, R. Q. Snurr and O. K. Farha, J. Am. Chem. Soc., 2023, 145, 6434–6441 CrossRef CAS.
- M. I. Gonzalez, M. T. Kapelewski, E. D. Bloch, P. J. Milner, D. A. Reed, M. R. Hudson, J. A. Mason, G. Barin, C. M. Brown and J. R. Long, J. Am. Chem. Soc., 2018, 140, 3412–3422 CrossRef CAS PubMed.
- S. Zhou, O. Shekhah, A. Ramírez, P. Lyu, E. Abou-Hamad, J. Jia, J. Li, P. M. Bhatt, Z. Huang, H. Jiang, T. Jin, G. Maurin, J. Gascon and M. Eddaoudi, Nature, 2022, 606, 706–712 CrossRef CAS PubMed.
- Y. Gu, J. J. Zheng, K. i. Otake, M. Shivanna, S. Sakaki, H. Yoshino, M. Ohba, S. Kawaguchi, Y. Wang, F. Li and S. Kitagawa, Angew. Chem., Int. Ed., 2021, 60, 11688–11694 CrossRef CAS.
- H. S. Cho, H. Deng, K. Miyasaka, Z. Dong, M. Cho, A. V. Neimark, J. K. Kang, O. M. Yaghi and O. Terasaki, Nature, 2015, 527, 503–507 CrossRef PubMed.
- M. Woellner, S. Hausdorf, N. Klein, P. Mueller, M. W. Smith and S. Kaskel, Adv. Mater., 2018, 30, 1704679 CrossRef PubMed.
- T. Islamoglu, Z. Chen, M. C. Wasson, C. T. Buru, K. O. Kirlikovali, U. Afrin, M. R. Mian and O. K. Farha, Chem. Rev., 2020, 120, 8130–8160 CrossRef CAS PubMed.
- N. S Bobbitt, M. L. Mendonca, A. J. Howarth, T. Islamoglu, J. T. Hupp, O. K. Farha and R. Q. Snurr, Chem. Soc. Rev., 2017, 46, 3357–3385 RSC.
- N. Couzon, J. Dhainaut, C. Campagne, S. Royer, T. Loiseau and C. Volkringer, Coord. Chem. Rev., 2022, 467, 214598 CrossRef CAS.
- C. Montoro, F. Linares, E. Q. Procopio, I. Senkovska, S. Kaskel, S. Galli, N. Masciocchi, E. Barea and J. A. R Navarro, J. Am. Chem. Soc., 2011, 133, 11888–11891 CrossRef CAS PubMed.
- S. K. Chitale, Y. S. Ko, J. W. Choi, J. W. Yoon, D. Jo, S. K. Lee, K. H. Cho and U. H. Lee, J. Hazard. Mater. Lett., 2022, 3, 100066 CrossRef CAS.
- F. J. Ma, S. X. Liu, C. Y. Sun, D. D. Liang, G. J. Ren, F. Wei, Y. G. Chen and Z. M. Su, J. Am. Chem. Soc., 2011, 133, 4178–4181 CrossRef CAS PubMed.
- A. M. Ebrahim, A. M. Plonka, N. Rui, S. Hwang, W. O. Gordon, A. Balboa, S. D. Senanayake and A. I. Frenkel, ACS Appl. Mater. Interfaces, 2020, 12, 58326–58338 CrossRef CAS.
- K. Ma, T. Islamoglu, Z. Chen, P. Li, M. C. Wasson, Y. Chen, Y. Wang, G. W. Peterson, J. H. Xin and O. K. Farha, J. Am. Chem. Soc., 2019, 141, 15626–15633 CrossRef CAS PubMed.
- F. A. Son, M. C. Wasson, T. Islamoglu, Z. Chen, X. Gong, S. L. Hanna, J. Lyu, X. Wang, K. B. Idrees, J. J. Mahle, G. W. Peterson and O. K. Farha, Chem. Mater., 2020, 32, 4609–4617 CrossRef CAS.
- P. Asha, M. Sinha and S. Mandal, RSC Adv., 2017, 7, 6691–6696 RSC.
- T. Wu, F. Qiu, R. Xu, Q. Zhao, L. Guo, D. Chen, C. Li and X. Jiao, ACS Appl. Mater. Interfaces, 2023, 15, 1265–1275 CrossRef CAS PubMed.
- Y. R. Son, S. G. Ryu and H. S. Kim, Microporous Mesoporous Mater., 2020, 293, 109819 CrossRef CAS.
- C. Zhou, B. Yuan, S. Zhang, G. Yang, L. Lu, H. Li and C. A. Tao, ACS Appl. Mater. Interfaces, 2022, 14, 23383–23391 CrossRef CAS PubMed.
- K. Huynh, S. Holdren, J. Hu, L. Wang, M. R. Zachariah and B. W. Eichhorn, ACS Appl. Mater. Interfaces, 2017, 9, 40638–40644 CrossRef CAS PubMed.
- D. A. Giannakoudakis, M. Barczak, M. Florent and T. J. Bandosz, Chem. Eng. J., 2019, 362, 758–766 CrossRef CAS.
- E. J. Park, H. J. Kim, S. W. Han, J. H. Jeong, I. H. Kim, H. O. Seo and Y. D. Kim, Chem. Eng. J., 2017, 325, 433–441 CrossRef CAS.
- M. Florent, D. A. Giannakoudakis, R. Wallace and T. J. Bandosz, ACS Appl. Mater. Interfaces, 2017, 9, 26965–26973 CrossRef CAS PubMed.
- Y. R. Son, M. K. Kim, S. G. Ryu and H. S. Kim, ACS Appl. Mater. Interfaces, 2018, 10, 40651–40660 CrossRef CAS PubMed.
- M. Sadeghi, S. Yekta and D. Mirzaei, J. Alloys Compd., 2018, 748, 995–1005 CrossRef CAS.
- W. A. Khanday, Microporous Mesoporous Mater., 2017, 244, 15–20 CrossRef CAS.
- H. Jung, M. K. Kim, J. Lee, J. H. Kwon and J. Lee, Anal. Lett., 2021, 54, 468–480 CrossRef CAS.
- T. Islamoglu, A. Atilgan, S. Y. Moon, G. W. Peterson, J. B. DeCoste, M. Hall, J. T. Hupp and O. K. Farha, Chem. Mater., 2017, 29, 2672–2675 CrossRef CAS.
- Y. Li, Q. Gao, Y. Zhou, L. Zhang, Y. Zhong, Y. Ying, M. Zhang, Y. Liu and Y. Wang, J. Hazard. Mater., 2018, 358, 113–121 CrossRef CAS PubMed.
- M. K. Kim, S. H. Kim, M. Park, S. G. Ryu and H. Jung, RSC Adv., 2018, 8, 41633–41638 RSC.
- W. Guo, H. Lv, K. P. Sullivan, W. O. Gordon, A. Balboa, G. W. Wagner, D. G. Musaev, J. Bacsa and C. L. Hill, Angew. Chem., Int. Ed., 2016, 55, 7403–7407 CrossRef CAS PubMed.
- Y. L. Wu, Y. Q. Sun, X. X. Li and S. T. Zheng, Inorg. Chem. Commun., 2020, 113, 107815 CrossRef CAS.
- M. Sadeghi, H. Ghaedi, S. Yekta and E. Babanezhad, J. Environ. Chem. Eng., 2016, 4, 2990–3000 CrossRef CAS.
- Y. Sun, A. Spieß, C. Jansen, A. Nuhnen, S. Gökpinar, R. Wiedey, S.-J. Ernst and C. Janiak, J. Mater. Chem. A, 2020, 8, 13364–13375 RSC.
- H. Zhao, Q. Wang, Z. Xi, C. Liu and C. Miao, J. Solid State Chem., 2023, 324, 124135 CrossRef CAS.
- H. S. AlSalem, F. K. Algethami, S. T. Al-Goul and A. Shahat, ACS Omega, 2023, 8, 20125–20137 CrossRef CAS PubMed.
- A. A. G. Khamseh, S. A. Ghorbanian, Y. Amini and M. M. Shadman, Sci. Rep., 2023, 13, 8393 CrossRef CAS PubMed.
- M. L. Gao, L. Li, Z. X. Sun, J. R. Li and H. L. Jiang, Angew. Chem., Int. Ed., 2022, 61, e202211216 CrossRef CAS PubMed.
- X. Y. Liu, W. S. Lo, C. Wu, B. P. Williams, L. Luo, Y. Li, L. Y. Chou, Y. Lee and C. K. Tsung, Nano Lett., 2020, 20, 1774–1780 CrossRef CAS PubMed.
- J. D. Xiao, Q. N. Wang, Z. D. Feng, S. Tang, Y. Liu and C. Li, J. Catal., 2022, 406, 165–173 CrossRef CAS.
- S. Gadipelli, W. Travis, W. Zhou and Z. Guo, Energy Environ. Sci., 2014, 7, 2232–2238 RSC.
- Y. Yun, Y. Fang, W. Fu, W. Du, Y. Zhu, H. Sheng, D. Astruc and M. Zhu, Small, 2022, 18, 2107459 CrossRef CAS PubMed.
- J. Zhang, M. Zou, Q. Li, W. Dai, D. Wang, S. Zhang, B. Li, L. Yang, S. Luo and X. Luo, Appl. Surf. Sci., 2022, 572, 151408 CrossRef CAS.
- S. Chen, S. Mukherjee, B. E. G. Lucier, Y. Guo, Y. T. A. Wong, V. V. Terskikh, M. J. Zaworotko and Y. Huang, J. Am. Chem. Soc., 2019, 141, 14257–14271 CrossRef CAS PubMed.
- L. Xiang, H. Zhang, C. Huang, R. Fei, X. Hu and J. Li, Colloids Surf., A, 2023, 662, 131042 CrossRef CAS.
- P. Wu, Y. Shen, H. Li, W. Shi, L. Zhang, T. Pan, W. Pei, W. Zhang and F. Huo, Small Struct., 2021, 2, 2000119 CrossRef CAS.
- C. Wang, Y. Yao, J. Li and Y. Yamauchi, Acc. Mater. Res., 2022, 3, 426–438 CrossRef CAS.
- P. He, W. Ma, J. Xu, J. Wei, X. Liu, P. Zuo, Z. K. Cui and Q. Zhuang, Small, 2023, 19, 2204649 CrossRef CAS PubMed.
- H. Park, S. Oh, S. Lee, S. Choi and M. Oh, Appl. Catal., B, 2019, 246, 322–329 CrossRef CAS.
- S. Oh, S. Lee and M. Oh, ACS Appl. Mater. Interfaces, 2020, 12, 18625–18633 CrossRef CAS PubMed.
- S. Choi and M. Oh, Angew. Chem., Int. Ed., 2019, 58, 866–871 CrossRef CAS PubMed.
- S. Tanaka and Y. Tanaka, ACS Omega, 2019, 4, 19905–19912 CrossRef CAS PubMed.
- K. S. Park, Z. Ni, A. P. Côté, J. Y. Choi, R. Huang, F. J. Uribe-Romo, H. K. Chae, M. O'Keeffe and O. M. Yaghi, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 10186–10191 CrossRef CAS PubMed.
- J. A. Arcibar-Orozco, S. Panettieri and T. J. Bandosz, J. Mater. Chem. A, 2015, 3, 17080–17090 RSC.
- D. B. Mawhinney, J. A. Rossin, K. Gerhart and J. T. Yates Jr, Langmuir, 1999, 15, 4789–4795 CrossRef CAS.
- T. L. Thompson, D. A. Panayotov and J. T. Yates Jr, J. Phys. Chem. B, 2004, 108, 16825–16833 CrossRef CAS.
- A. B. Butrow, J. H. Buchanan and D. E. Tevault, J. Chem. Eng. Data, 2009, 54, 1876–1883 CrossRef CAS.
- M. Spiandore, A. Piram, A. Lacoste, D. Josse and P. Doumenq, Drug Test. Anal., 2014, 6, 67–73 CrossRef CAS PubMed.
- G. Wang, G. Sharp, A. M. Plonka, Q. Wang, A. I. Frenkel, W. Guo, C. Hill, C. Smith, J. Kollar, D. Troya and J. R. Morris, J. Phys. Chem. C, 2017, 121, 11261–11272 CrossRef CAS.
- Q. Hu, V. M. Jayasinghe-Arachchige, J. Zuchniarz and R. Prabhakar, Front. Chem., 2019, 7, 195 CrossRef CAS PubMed.
- S. Y. Moon, Y. Liu, J. T. Hupp and O. K. Farha, Angew. Chem., Int. Ed., 2015, 54, 6795–6799 CrossRef CAS PubMed.
- Z. Chen, T. Islamoglu and O. K. Farha, ACS Appl. Nano Mater., 2019, 2, 1005–1008 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3na00807j |
| This journal is © The Royal Society of Chemistry 2023 |