Open Access Article
Open Access ArticleAtomically precise AuxAg25−x nanoclusters with a modulated interstitial Au–Ag microenvironment for enhanced visible-light-driven photocatalytic hydrogen evolution†
Ye
Liu
a,
Zhi
Li
b,
Xiao-He
Liu
b,
Nicola
Pinna
 *a and
Yu
Wang
*a and
Yu
Wang
 *a
*a
aDepartment of Chemistry, IRIS Adlershof & The Center for the Science of Materials Berlin, Humboldt-Universität zu Berlin, 12489, Berlin, Germany. E-mail: nicola.pinna@hu-berlin.de; wangyuxx@hu-berlin.de
bSchool of Geology and Environment, Xi'an University of Science and Technology, Xi'an 710049, Shaanxi, China
First published on 15th August 2023
Abstract
Herein, we report the study of atomically precise AuxAg25−x nanoclusters (NCs) toward photocatalytic hydrogen evolution. The incorporation of Au atoms into Ag25 NCs not only narrowed the HOMO–LUMO gaps but also created an interstitial Au–Ag microenvironment, which promoted the photogenerated charge carrier utilization and optimized the reaction dynamics.
New conceptsAs one of the emerging light absorbing materials, metal nanoclusters with distinctive electronic structures have stimulated intensive research in solar energy conversion. In the past decade, efforts have been made towards the synthesis and structure determination of nanoclusters, while studies on the relationship between their structures and physiochemical properties are rare. Studying nanoclusters from the perspective of structure–activity relationships is of great significance to push nanoclusters into practical applications. In this work, we synthesized Au25, Ag25 and alloy (AuAg)25 nanoclusters protected by 2,4-dimethylbenzenethiol, which were subsequently utilized as photosensitizers for photocatalytic hydrogen evolution. Physiochemical characterizations and DFT calculations allowed us to gain insight into the enhanced photocatalytic activities at the molecular level: a unique Au–Ag microenvironment was created by the simultaneous functioning of Au and Ag sites in the nanoclusters, which not only contributed to the separation of photogenerated charge carriers, but also balanced the hydrogen adsorption/desorption on the surface of the nanoclusters. Besides, alloying Au into Ag25 nanoclusters strengthened the light adsorption capabilities, which could also contribute to the photocatalytic activities. As a result, the reactivities of the alloy (AuAg)25 nanoclusters outperformed their single metal nanocluster counterparts (up to ∼5 times higher). |
Metal doping or alloying in heterogeneous catalysis provides opportunities to tailor the properties of catalysts, enhance their activity, selectivity and stability, and enable new catalytic reactions.1–3 In the nanoparticle regime, bimetallic nanocrystals exhibit superior performance over individual metal nanocrystals due to the synergistic effect. By varying the ratio of the two metals, it is possible to control the surface composition and electronic structure of the nanocrystals, thus allowing for fine-tuning of the catalytic activity, selectivity, and reaction kinetics.4–6 However, achieving precise atomic-level control over the composition of bimetallic nanocrystals is challenging due to synthetic limitations.
Metal nanoclusters (NCs), with atomic precision, represent a class of materials with tunable geometric structures at the atomic level.7,8 This unique characteristic makes them ideal models for studying the relationship between defined NC structures and their physicochemical properties.9–12 Furthermore, the broad light absorption within the UV-visible region and excellent stability in corrosive redox environments make metal NCs promising for solar energy conversion devices.13–15 For instance, using a glutathione-protected Au18 NC as a sensitizer, a solar cell achieved a power conversion efficiency of 3.79%. Incorporating Ag into Au18 increased the efficiency to 4.22%.16 Although the impact of hetero metal doping on the performance of pristine metal NCs has been extensively investigated, systematic studies on bimetallic NCs and their individual metal counterparts have been hindered by synthesis challenges.17–23
Due to the small size of metal NCs, they possess a high surface-to-volume ratio, with most of the metal atoms located on the surface and bound to ligands.24,25 Since ligand affinities to different metals vary, the coordination modes and NC structures may differ.10,26 As an example, the well-known Au25 NCs with a solved structure are protected by aliphatic 2-phenylethanethiol (PET), while the Ag25 NCs with a solved structure are protected with aromatic thiol. Doping Ag into Au25(PET)18 successfully yields (AuAg)25(PET)18.27 However, Ag25 NCs protected by PET remain unavailable.28 Recently, Wang et al. reported a universal synthesis approach for preparing Au25 NCs protected by various aromatic thiols, enabling a comparison between the properties of bimetallic NCs and their individual metal counterparts while maintaining the same ligand.29
In this study, Au25, Ag25, and (AuAg)25 NCs protected by 2,4-dimethylbenzenethiol are synthesized and utilized as photosensitizers in a TiO2-based system for photocatalytic H2 production under visible light. To our surprise, the catalytic performance doesn’t completely follow the order of the light absorption ability of the NCs. Although Au25 has the second narrowest highest occupied molecular orbital (HOMO)–lowest unoccupied molecular orbital (LUMO) gap among the five NCs, the hydrogen production rates of the bimetallic NC decorated TiO2 systems outperform their single metal NC counterparts. DFT calculations reveal that the presence of Ag–Au dual metal sites could facilitate the formation of electron acceptor centers. In this way, the photoinduced electrons prefer to remain in the NCs for reducing the adsorbed hydrogen ions. Moreover, the Au–Ag environments in the bimetallic (AuAg)25 NCs not only upshift the d-band centers (εd) but also balance the hydrogen adsorption/desorption dynamics, further optimizing the catalytic activities of the bimetallic NCs toward hydrogen production.
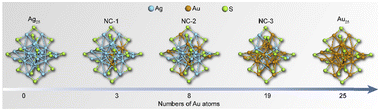
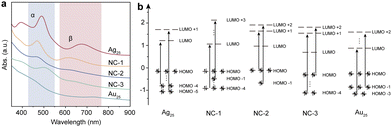
Scheme 1 shows the schematic illustration of the AuxAg25−x NCs. They were synthesized by the methods reported previously with some modifications (see ESI† for details).28–31 The (AuAg)25 NCs, namely NC-1, NC-2, and NC-3, consist of a series of bimetallic NCs, all sharing the common formula [AuxAg25−x(SR)18]−. Here, x varies within the following ranges: 1 ≤ x ≤ 3 for NC-1, 3 ≤ x ≤ 8 for NC-2, and 19 ≤ x ≤ 23 for NC-3 (Fig. S1, ESI†). Tandem mass study revealed that there is a site preference for the metals (Fig. S2 and S3, ESI†). The UV-Vis absorption spectra in Fig. 1a showed different absorption profiles, where significant blue shifts were observed for NC-1, NC-2 and NC-3 when compared to the pristine Ag25 NCs. To gain a deeper insight into the absorption behaviors of the NCs, density functional theory calculations were performed. As is shown in Fig. 1b, the absorption bands located at around 675 nm and 488 nm correspond to the LUMO+1 ← HOMO−5 transition (α) and LUMO ← HOMO−4 (β) transition for Ag25 NCs, which are mainly contributed by the intraband transitions. The Au25 NCs show similar transition behaviors compared to Ag25 NCs, while the excited electrons come from shallower HOMO states (LUMO ← HOMO−3 and LUMO+2 ← HOMO−1) for α and β transitions, respectively. However, the cases for the bimetallic NCs are different. The intraband transitions give rise to two absorption bands located at 612 nm and 468 nm, which could be assigned as LUMO+3 ← HOMO−1 transition (α) and LUMO ← HOMO−4 (β) transitions for NC-1 and LUMO+2 ← HOMO−1 transition (α) and LUMO+1 ← HOMO (β) for NC-2, respectively. As for NC-3, the α and β transitions arise from LUMO+1 ← HOMO−4 transitions and LUMO+2 ← HOMO transitions. Compared to Ag25 NCs, the HOMO–LUMO gaps decrease with the increase of the amount of Au atoms doped into the Ag25 NCs, with the values being 1.34 eV, 1.30 eV, 1.2 eV, 1.05 eV and 1.16 eV for Ag25, NC-1, NC-2, NC-3 and Au25, respectively (Table S1, ESI†).
The Kohn–Sham molecular orbitals (MO), energies, and atomic orbital (AO) contributions are shown in Fig. S4 (ESI†). Except for the main contribution from sp atomic orbitals of C and S atoms, the low-lying LUMO states and HOMO states of all the NCs were largely constructed from the d10 atomic orbitals of Ag and Au atoms, which constituted the d-bands. The LUMO state of Au25 was triply degenerated, while the others were doubly degenerated. It is also interesting to note that only the HOMO of NC-3 was doubly degenerated. It could also be concluded that the intraband α and β transitions originated from sp–sp transitions.
To investigate the photocatalytic activities of the NCs, we built a NCs/TiO2 composite system to carry out the photocatalytic measurements. Accordingly, the composite samples were named NC-3/TiO2, NC-2/TiO2, NC-1/TiO2, Au25/TiO2 and Ag25/TiO2. TiO2 is known to be inactive in the visible region due to its large band gap, limiting its photoresponse.32 To overcome this limitation, photosensitizers are often employed to extend the photoresponse of titania.33,34 One effective approach to further improve the catalytic performance is narrowing the HOMO–LUMO gap of the light absorber, which enhances the light absorption of the composite. Fig. 2a and Fig. S5 (ESI†) demonstrate that TiO2 decorated with metal NCs exhibits a favorable response to visible light. Among the four NC-based composites, Ag25/TiO2 displayed the lowest hydrogen evolution reaction (HER) rate at 2.45 μmol gcatal.−1 h−1, while NC-1/TiO2 showed an HER rate of 6.65 μmol gcatal.−1 h−1. The HER rates of NC-2 and NC-3 significantly outweighed Ag25/TiO2 and Au25/TiO2, which reached 12.00 μmol gcatal.−1 h−1 and 14.86 μmol gcatal.−1 h−1. This suggests that the doping of Au atoms into Ag25 significantly thus contributes to the catalytic activity of the system. It is worth noting that Au25 has a smaller HOMO–LUMO gap than NC-1 and NC-2. But surprisingly, the photocatalytic activities of either NC-1/TiO2 or NC-2/TiO2 outperform Au25/TiO2, indicating that the catalytic activities do not solely depend on the light absorption capabilities (Fig. 2a and Fig. S6, ESI†).
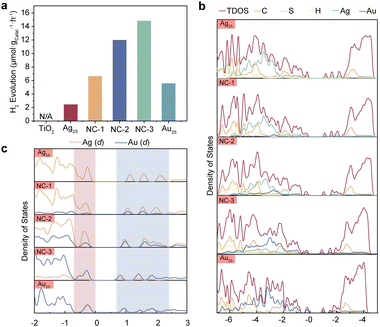 | ||
| Fig. 2 (a) Hydrogen evolution under visible light irradiation. (b) Density of states projected to each atom. (c) Density of states projected to the d states of Ag and Au atoms. | ||
Previous studies have revealed the formation of a staggered energy level between small band gap metal NCs and n-type semiconductors upon band alignment.35–37 The higher LUMO+X states compared to the conduction band (CB) of the semiconductor facilitate the flow of photoinduced electrons from the LUMO+X states to the CB (Fig. S7, ESI†). However, there are other competitive pathways for these electrons. In the case of photoexcited dye systems, electrons on the LUMO can return to their ground state or participate in reduction reactions.38 Since most dyes are catalytically inactive, hydrogen evolution only occurs when these electrons flow to the semiconductor. In contrast, metal NCs exhibit both photoactivity and catalytic activity.39–41 Therefore, enhancing the composite's performance under visible light is achievable if the photoinduced electrons remain at the metal NCs and reduce the hydrogen ions adsorbed onto them. This proposed mechanism is illustrated in Fig. S8 (ESI†). In order to gain these molecular-level insights, we performed a series of DFT calculations, including the electronic structures and the charge dynamics when hydrogen was absorbed on the NC surface. Firstly, we started with the electronic structures of the NCs by comparing the projected density of states of the NCs. As it is shown in Fig. 2b, the HOMO and LUMO states were greatly contributed by the metallic d states from Ag and Au. Different from the case of pristine Ag25 NCs, the PDOS of Au atoms in the Au25 NCs exhibited broad distribution rather than isolated peaks within a wide range of energies of HOMO states (Fig. 2c). This indicates that the d states in the Au25 NCs are more delocalized than that in the pristine Ag25 NCs. Similar phenomena were also observed in the bimetallic clusters, indicating that alloying has a great impact on the electronic structures. It can also be observed that both the bonding and antibonding d states of Au have been strongly overlapped with the Ag counterparts in the bimetallic NCs. This overlapping PDOS of the d orbitals of the Ag and Au atoms suggests the formation of diffuse superatomic d-type orbitals, which could facilitate the transfer of hot electrons in space.42,43
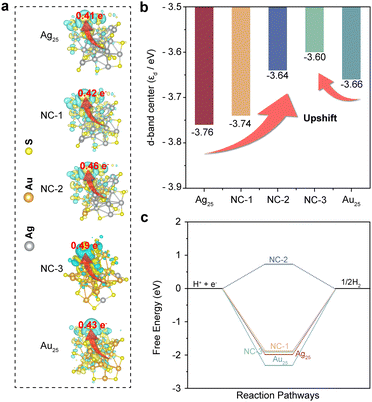
Besides, the Bader charge and charge density difference analysis provided direct evidence for this electron transfer behavior. The net charges on each atom with a negative sign (depletion) indicating the loss of charge (positively charged) and a positive sign (accumulation) indicating the excess of charge (negatively charged). The calculated Bader charges of H* for hydrogen-adsorbing metal sites on each NC are shown in Fig. 3a, where the values were calculated to be 0.41 e−, 0.42 e−, 0.46 e−, 0.49 e− and 0.43 e− for Ag25, NC-1, NC-2, NC-3 and Au25, respectively. Therefore, electrons are more likely to be transferred to the adsorbed proton to catalyze the hydrogen evolution in bimetallic NCs, especially NC-3. Moreover, after the absorption of a hydrogen atom, all the bimetallic NCs displayed increased charge density area around both the Ag and Au site, which was clearly shown in the isosurfaces of charge density difference in Fig. 3a. Hence, the Au–Ag sites would be electron acceptor centers for catalyzing the hydrogen evolution, which is in accordance with the results obtained from Bader charge analysis.24 Moreover, the d-band features (εd) of the clusters were also investigated. The εd of Au and Ag atoms in the bimetallic NCs showed upward shifts compared to the pristine Ag25 and Au25 NCs (Fig. 3b). The upward shift of εd would result in a decrease in the electron filling of the antibonding states, which could contribute to the adsorption of the reaction intermediate on the catalyst surface. In contrast, the downward shift of εd would facilitate the desorption of the reaction intermediate on the catalyst surface.44–46 Based on the results obtained above, the impact of the dual metal sites on charge dynamics in the bimetallic NCs can be proposed. The introduction of Au sites into Ag25 NCs leads to the delocalization and hybridization of metallic d orbitals, forming diffuse superatomic d-type orbitals. As verified by Bader charge and charge density difference analysis, the diffuse superatomic d-type orbitals can facilitate the transfer of photoinduced electrons to the adsorbed proton for photocatalytic reactions, which hinders the electrons from bulk recombination or migration to the CB of TiO2. However, with the increase of the amount of Au sites in NC-3 the Ag25 NCs, the positive impact on the charge dynamics for HER reactions would decrease, as confirmed by the downward shifted εd and Bader charge values of Au25 compared to NC-2 and NC-3. Therefore, the unique microenvironment created by the Au–Ag dual metal sites also plays a vital role in optimizing the photocatalytic activities.
Moreover, further calculations from the perspective of reaction dynamics were performed to investigate the reaction pathways on the surfaces of the NCs. H+ + e−, adsorbed hydrogen (H*) and 1/2H2 were considered as the initial, intermediate and final states of the reaction, respectively. The calculated free energy diagrams of the HER process on the Ag atoms of each NC were presented in Fig. 3c. Ag25, NC-1, NC-3 and Au25 NCs showed large upslopes with negative values of −1.95 eV, −1.94 eV, −1.86 eV and −2.31 eV, respectively, while NC-2 showed the smallest upslope with a positive value of 0.73 eV. However, the hydrogen adsorption/desorption was balanced by the Au–Ag sites because of orbital modulation, giving rise to the smallest upslope of 0.73 eV. Therefore, hydrogen desorption could be considered as the rate-determining step in the HER process for Ag25, NC-1, NC-3 and Au25 NCs. Compared with Au25, who had the largest upslope with a negative value of −2.31 eV, the other three clusters showed more positive free energy changes, especially in the case of NC-2. The interaction energy made them more energetically favored for hydrogen adsorption compared to Au25 NCs. It is reported that the reaction rate of the HER depends on the upslope process in the HER free energy diagram. With a much smaller positive upslope value, the interaction between hydrogen and the NC-2 surface would reach a more balanced hydrogen adsorption/desorption process involved in the HER process.47–49 Combining the results obtained from εd and reaction free energy analysis, it is proved that the unique microenvironment created by the dual Au–Ag sites not only optimizes the charge dynamics, but also the adsorption/desorption of intermediates. As a result, the HER sites on the NCs were readily modulated, making the HER rates of the bimetallic NCs much faster than the pristine Ag25 and Au25 counterparts.
Conclusions
In summary, we have studied the sensitizing capabilities and reaction dynamics of bimetallic AuxAg25−x NCs toward visible-light-driven photocatalytic hydrogen evolution. The incorporation of Au atoms into Ag25 NCs narrowed the HOMO–LUMO gaps and modified the electronic configurations of the NCs. The unique microenvironment created by the dual Au–Ag sites in the bimetallic NCs helped to form electron-acceptor sites and upshifted the d-band centers, which hinders the electrons from bulk recombination and promotes the H2 evolution process. On the other hand, the simultaneous role of the dual metal sites offered moderate hydrogen adsorption/desorption dynamics, which further optimized the overall photocatalytic process. As a result, the photocatalytic activities of the NCs followed an order of NC-3 > NC-2 > NC-1 > Au25 > Ag25. These results provide molecular insights into the origin of the photocatalytic activities of NC-based materials.Author contributions
Y. W. and N. P.: conceptualization. Y. L. synthesized all the NCs, and performed all the physiochemical characterizations and DFT calculations. X. H. L. and Z. L. conducted the photocatalysis experiments. Data plotting and analysis were performed by Y. L. The manuscript was drafted by Y. L. and Y. W. and then revised by all the co-authors.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank the German Research Foundation (DFG, Project 456075917) for the financial support of this work. Dr C. Zhao is acknowledged for the MS study. Y. L. acknowledges financial support from China Scholarship Council (CSC, No. 201908420279). X. H. L. and Z. L. acknowledge the financial support by the National Natural Science Foundation of China (52206277) and the China Postdoctoral Science Foundation (2022MD723821).Notes and references
- J. Ran, T. Y. Ma, G. Gao, X.-W. Du and S. Z. Qiao, Energy Environ. Sci., 2015, 8, 3708–3717 RSC.
- W. Wu, Q. Zhang, X. Wang, C. Han, X. Shao, Y. Wang, J. Liu, Z. Li, X. Lu and M. Wu, ACS Catal., 2017, 7, 7267–7273 CrossRef CAS.
- H. Fakhouri, E. Salmon, X. Wei, S. Joly, C. Moulin, I. Russier-Antoine, P.-F. Brevet, X. Kang, M. Zhu and R. Antoine, J. Phys. Chem. C, 2022, 126, 21094–21100 CrossRef CAS.
- H. Nishiyama, T. Yamada, M. Nakabayashi, Y. Maehara, M. Yamaguchi, Y. Kuromiya, Y. Nagatsuma, H. Tokudome, S. Akiyama, T. Watanabe, R. Narushima, S. Okunaka, N. Shibata, T. Takata, T. Hisatomi and K. Domen, Nature, 2021, 598, 304–307 CrossRef CAS PubMed.
- P. Babu and B. Naik, Inorg. Chem., 2020, 59, 10824–10834 CrossRef CAS PubMed.
- J. Zhao, J. Liu, Z. Li, K. Wang, R. Shi, P. Wang, Q. Wang, G. I. N. Waterhouse, X. Wen and T. Zhang, Nat. Commun., 2023, 14, 1909 CrossRef CAS PubMed.
- P. Chakraborty, A. Nag, A. Chakraborty and T. Pradeep, Acc. Chem. Res., 2019, 52, 2–11 CrossRef CAS PubMed.
- S. Hossain, D. Hirayama, A. Ikeda, M. Ishimi, S. Funaki, A. Samanta, T. Kawawaki and Y. Negishi, Aggregate, 2023, 4, e255 CrossRef CAS.
- Z. Qin, J. Zhang, C. Wan, S. Liu, H. Abroshan, R. Jin and G. Li, Nat. Commun., 2020, 11, 6019 CrossRef CAS PubMed.
- R. Jin, C. Zeng, M. Zhou and Y. Chen, Chem. Rev., 2016, 116, 10346–10413 CrossRef CAS PubMed.
- H. Qian, D. E. Jiang, G. Li, C. Gayathri, A. Das, R. R. Gil and R. Jin, J. Am. Chem. Soc., 2012, 134, 16159–16162 CrossRef CAS PubMed.
- Y. Liu, X. Chai, X. Cai, M. Chen, R. Jin, W. Ding and Y. Zhu, Angew. Chem., Int. Ed., 2018, 57, 9775–9779 CrossRef CAS PubMed.
- M. A. Abbas, P. V. Kamat and J. H. Bang, ACS Energy Lett., 2018, 3, 840–854 CrossRef CAS.
- M. S. Bootharaju, C. W. Lee, G. Deng, H. Kim, K. Lee, S. Lee, H. Chang, S. Lee, Y.-E. Sung, J. S. Yoo, N. Zheng and T. Hyeon, Adv. Mater., 2023, 35, 2207765 CrossRef CAS PubMed.
- F.-X. Xiao, S.-F. Hung, J. Miao, H.-Y. Wang, H. Yang and B. Liu, Small, 2015, 11, 554–567 CrossRef CAS PubMed.
- M. H. Naveen, R. Khan, M. A. Abbas, E. Cho, G. J. Lee, H. Kim, E. Sim and J. H. Bang, Chem. Sci., 2020, 11, 6248–6255 RSC.
- O. J. H. Chai, Z. Liu, T. Chen and J. Xie, Nanoscale, 2019, 11, 20437–20448 RSC.
- Q.-L. Mo, B.-J. Liu and F.-X. Xiao, J. Phys. Chem. C, 2021, 125, 22421–22428 CrossRef CAS.
- Z. Gan, N. Xia and Z. Wu, Acc. Chem. Res., 2018, 51, 2774–2783 CrossRef CAS PubMed.
- S. Wang, Q. Li, X. Kang and M. Zhu, Acc. Chem. Res., 2018, 51, 2784–2792 CrossRef CAS PubMed.
- A. Ghosh, O. F. Mohammed and O. M. Bakr, Acc. Chem. Res., 2018, 51, 3094–3103 CrossRef CAS PubMed.
- S. Hossain, Y. Niihori, L. V. Nair, B. Kumar, W. Kurashige and Y. Negishi, Acc. Chem. Res., 2018, 51, 3114–3124 CrossRef CAS PubMed.
- S. Sharma, K. K. Chakrahari, J.-Y. Saillard and C. W. Liu, Acc. Chem. Res., 2018, 51, 2475–2483 CrossRef CAS PubMed.
- G. Hu, Z. Wu and D.-E. Jiang, J. Mater. Chem. A, 2018, 6, 7532–7537 RSC.
- Y. Wang, X.-K. Wan, L. Ren, H. Su, G. Li, S. Malola, S. Lin, Z. Tang, H. Häkkinen, B. K. Teo, Q.-M. Wang and N. Zheng, J. Am. Chem. Soc., 2016, 138, 3278–3281 CrossRef CAS PubMed.
- Y. Du, H. Sheng, D. Astruc and M. Zhu, Chem. Rev., 2020, 120, 526–622 CrossRef CAS PubMed.
- D. R. Kauffman, D. Alfonso, C. Matranga, H. Qian and R. Jin, J. Phys. Chem. C, 2013, 117, 7914–7923 CrossRef CAS.
- C. P. Joshi, M. S. Bootharaju, M. J. Alhilaly and O. M. Bakr, J. Am. Chem. Soc., 2015, 137, 11578–11581 CrossRef CAS PubMed.
- Z. Lei, J. J. Li, Z. A. Nan, Z. G. Jiang and Q. M. Wang, Angew. Chem., Int. Ed., 2021, 60, 14415–14419 CrossRef CAS PubMed.
- Y. Negishi, T. Iwai and M. Ide, Chem. Commun., 2010, 46, 4713–4715 RSC.
- M. S. Bootharaju, C. P. Joshi, M. R. Parida, O. F. Mohammed and O. M. Bakr, Angew. Chem., Int. Ed., 2016, 55, 922–926 CrossRef CAS PubMed.
- A. Fujishima and K. Honda, Nature, 1972, 238, 37–38 CrossRef CAS PubMed.
- M. Lv, D. Zheng, M. Ye, J. Xiao, W. Guo, Y. Lai, L. Sun, C. Lin and J. Zuo, Energy Environ. Sci., 2013, 6, 1615–1622 RSC.
- C. Yu, G. Li, S. Kumar, H. Kawasaki and R. Jin, J. Phys. Chem. Lett., 2013, 4, 2847–2852 CrossRef CAS.
- Y. Liu, D. Long, A. Springer, R. Wang, N. Koch, M. Schwalbe, N. Pinna and Y. Wang, Sol. RRL, 2023, 7, 2201057 CrossRef CAS.
- Y. Wang, X. H. Liu, Q. Wang, M. Quick, S. A. Kovalenko, Q. Y. Chen, N. Koch and N. Pinna, Angew. Chem., Int. Ed., 2020, 59, 7748–7754 CrossRef CAS PubMed.
- X. H. Liu, Y. He, Z. Li, A. Cheng, Z. Song, Z. Yu, S. Chai, C. Cheng and C. He, J. Colloid Interface Sci., 2023, 651, 368–375 CrossRef CAS PubMed.
- S. Maity, S. Kolay, S. Ghosh, S. Chakraborty, D. Bain and A. Patra, J. Phys. Chem. Lett., 2022, 13, 5581–5588 CrossRef CAS PubMed.
- Y. Wang, X.-H. Liu, R. Wang, B. Cula, Z.-N. Chen, Q. Chen, N. Koch and N. Pinna, J. Am. Chem. Soc., 2021, 143, 9595–9600 CrossRef CAS PubMed.
- Y. Liu, E. Wierzbicka, A. Springer, N. Pinna and Y. Wang, J. Phys. Chem. C, 2022, 126, 1778–1784 CrossRef CAS.
- Y. Wang, X.-H. Liu, S. A. Kovalenko, Q.-Y. Chen and N. Pinna, Chem. – Eur. J., 2019, 25, 4814–4820 CrossRef CAS PubMed.
- X. Yu, Y. Sun, W. W. Xu, J. Fan, J. Gao, X. Jiang, Y. Su and J. Zhao, Nanoscale Horiz., 2022, 7, 1192–1200 RSC.
- Y.-Y. Liu, Z. Wei, S. Meng, R. Wang, X. Jiang, R. Huang, S.-S. Li and L.-W. Wang, Phys. Rev. B, 2021, 104, 115310 CrossRef CAS.
- Y. Sun, X. Li, T. Zhang, K. Xu, Y. Yang, G. Chen, C. Li and Y. Xie, Angew. Chem., Int. Ed., 2021, 60, 21575–21582 CrossRef CAS PubMed.
- W. Wang, W. Geng, L. Zhang, Z. Zhao, Z. Zhang, T. Ma, C. Cheng, X. Liu, Y. Zhang and S. Li, Small, 2023, 19, e2206808 CrossRef PubMed.
- J. K. Norskov, F. Abild-Pedersen, F. Studt and T. Bligaard, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 937–943 CrossRef CAS PubMed.
- X. Chen, Y. Gu, G. Tao, Y. Pei, G. Wang and N. Cui, J. Mater. Chem. A, 2015, 3, 18898–18905 RSC.
- J. K. Nørskov, T. Bligaard, A. Logadottir, J. R. Kitchin, J. G. Chen, S. Pandelov and U. Stimming, J. Electrochem. Soc., 2005, 152, J23 CrossRef.
- F. Li, G. F. Han, H. J. Noh, J. P. Jeon, I. Ahmad, S. Chen, C. Yang, Y. Bu, Z. Fu, Y. Lu and J. B. Baek, Nat. Commun., 2019, 10, 4060 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3nh00235g |
| This journal is © The Royal Society of Chemistry 2023 |