Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Unimodal polyethylenes of high linearity and narrow dispersity by using ortho-4,4′-dichlorobenzhydryl-modified bis(imino)pyridyl-iron catalysts†
Tian
Liu
ab,
Yanping
Ma
 a,
Gregory A.
Solan
*ac,
Yang
Sun
a and
Wen-Hua
Sun
a,
Gregory A.
Solan
*ac,
Yang
Sun
a and
Wen-Hua
Sun
 *abd
*abd
aKey Laboratory of Engineering Plastics and Beijing National Laboratory for Molecular Sciences, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, China. E-mail: whsun@iccas.ac.cn; Fax: +86-10-62618239; Tel: +86-10-62557955
bCAS Research/Education Center for Excellence in Molecular Sciences, University of Chinese Academy of Sciences, Beijing 100049, China
cDepartment of Chemistry, University of Leicester, University Road, Leicester LE1 7RH, UK. E-mail: gas8@leicester.ac.uk; Tel: +44-116-2522096
dState Key Laboratory for Oxo Synthesis and Selective Oxidation, Lanzhou Institute of Chemical Physics Chinese Academy of Sciences, Lanzhou 730000, China
First published on 24th February 2023
Abstract
Six different examples of 4,4′-dichlorobenzhydryl-substituted 2,6-bis(arylimino)pyridyl-iron(II) chloride complex, [2-{{2,6-((p-ClPh)2CH)2-4-MeC6H2}N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CMe}-6-(ArN
CMe}-6-(ArN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CMe)C5H3N]FeCl2 (Ar = 2,6-Me2C6H3Fe1, 2,6-Et2C6H3Fe2, 2,6-iPr2C6H3Fe3, 2,4,6-Me3C6H2Fe4, 2,6-Et2-4-MeC6H2Fe5, 2,6-((p-ClPh)2CH)2-4-MeC6H2Fe6), have been synthesized in good yield and characterized by various spectroscopic and analytical techniques. The molecular structures of Fe2 and Fe5 emphasize the uneven steric protection of the ferrous center imposed by the unsymmetrical N,N,N′-chelate. When treated with either MAO or MMAO (modified-MAO) as activators, Fe1–Fe5 exhibited very high productivities at elevated temperature with peak performance of 21.59 × 106 g PE mol−1(Fe) h−1 for Fe5/MMAO at 50 °C and 15.65 × 106 g PE mol−1(Fe) h−1 for Fe1/MAO at 60 °C. By contrast, the most sterically hindered Fe6 was either inactive (using MAO) or displayed very low activity (using MMAO). As a further feature, this class of iron catalyst was capable of displaying long lifetimes with catalytic activities up to 10.77 × 106 g PE mol−1(Fe) h−1 observed after 1 h. In all cases, strictly linear and unimodal polyethylene was formed with narrow dispersity, while the polymer molecular weight was strongly influenced by the aluminoxane co-catalyst (Mw using MAO > MMAO) and also by the steric properties of the second N-aryl group (up to 32.9 kg mol−1 for Fe3/MAO).
CMe)C5H3N]FeCl2 (Ar = 2,6-Me2C6H3Fe1, 2,6-Et2C6H3Fe2, 2,6-iPr2C6H3Fe3, 2,4,6-Me3C6H2Fe4, 2,6-Et2-4-MeC6H2Fe5, 2,6-((p-ClPh)2CH)2-4-MeC6H2Fe6), have been synthesized in good yield and characterized by various spectroscopic and analytical techniques. The molecular structures of Fe2 and Fe5 emphasize the uneven steric protection of the ferrous center imposed by the unsymmetrical N,N,N′-chelate. When treated with either MAO or MMAO (modified-MAO) as activators, Fe1–Fe5 exhibited very high productivities at elevated temperature with peak performance of 21.59 × 106 g PE mol−1(Fe) h−1 for Fe5/MMAO at 50 °C and 15.65 × 106 g PE mol−1(Fe) h−1 for Fe1/MAO at 60 °C. By contrast, the most sterically hindered Fe6 was either inactive (using MAO) or displayed very low activity (using MMAO). As a further feature, this class of iron catalyst was capable of displaying long lifetimes with catalytic activities up to 10.77 × 106 g PE mol−1(Fe) h−1 observed after 1 h. In all cases, strictly linear and unimodal polyethylene was formed with narrow dispersity, while the polymer molecular weight was strongly influenced by the aluminoxane co-catalyst (Mw using MAO > MMAO) and also by the steric properties of the second N-aryl group (up to 32.9 kg mol−1 for Fe3/MAO).
Introduction
Over recent years, rapid developments have been seen in the design of iron (and cobalt) complexes as catalysts for olefin polymerization. These have been largely inspired by the pioneering disclosures in the late 1990s that bis(imino)pyridine-chelated examples can display high catalytic activity (A, Chart 1).1–11 In a manner similar to the industrialization of Ziegler–Natta catalysts, researchers have been committed to improving both the activity and thermal stability of iron catalysts as well as the performance characteristics of the resulting polyolefinic materials.12–24 From an industrial standpoint, iron is one of the most abundant elements on the earth which offers, on account of its cost effectiveness, considerable opportunities to be integrated into a commercial process. However, the sensitivity of A-derived catalysts towards deactivation at higher operating temperatures has somewhat hindered their further industrial development.26 In particular, pathways involving alkylation reactions or deprotonation chemistry involving the imino-C methyl groups in the N,N,N-ligand frame have been proposed as likely deactivation routes.25 As a consequence, research endeavors in this area have largely focused on the improvement of the thermal stability of bis(imino)pyridyl-iron catalysts while maintaining or enhancing the overall effectiveness of the polymerization process.27–37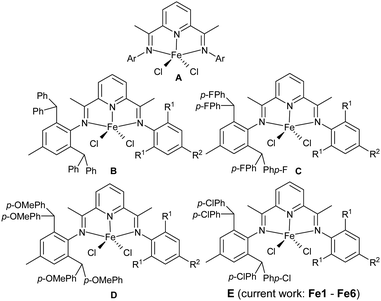 | ||
| Chart 1 Parent bis(imino)pyridyl-iron precatalyst A and its ortho-CH(p-RC6H4)2-substituted derivatives, B (R = H), C (R = F), D (R = OMe), and the target of the current work E (R = Cl). | ||
In the main, two strategies have been employed with a view to improve thermal stability and activity of N,N,N-iron catalysts, namely modification of the substituents on the N-aryl groups in A or changes to the ligand framework itself.38–51 Among these, most effort has been dedicated to the introduction of large sterically hindered substituents to the N-aryl group in A that not only retain the high catalytic activity, but also improve the thermal stability of the catalyst.49,50 Based on research conducted by our group and others, we have found that the introduction of ortho-benzhydryl (CHPh2) substituents to a single N-aryl group in an unsymmetrical version of A (B, Chart 1) can be beneficial to the thermal stability of the iron catalyst.52 Furthermore, when the benzhydryl group in B was affixed with electron-withdrawing para-fluoride substituents (C, Chart 1), improved catalytic activity was observed whereas the molecular weight of the polymer reduced.53 Conversely, the introduction of an electron-donating para-methoxy group to the benzhydryl substituent (D, Chart 1) significantly increased the molecular weight of the polyethylene whereas the catalytic activity lowered.54 Evidently, the electronic properties of the substituents on the benzhydryl groups have a crucial influence on the catalytic performance of the bis(imino)pyridine-iron complexes and the properties of the resulting polyethylene.
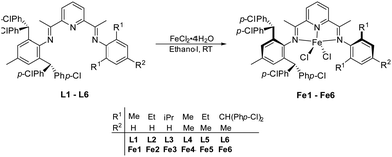
To further explore the influence of electronic effects on the performance of benzhydryl-substituted bis(imino)pyridine-iron catalysts, we target in this work a series of iron precatalysts incorporating N-2,6-bis(4,4′-dichlorobenzhydryl)-4-methylphenyl groups (E, Chart 1). More specifically, we disclose six examples of E (Fe1–Fe6) in which the second N-aryl group is systematically modified in terms of its steric and electronic properties. All iron precatalysts are subject to a comprehensive polymerization evaluation that explores how run temperature, co-catalyst, run time and ethylene pressure can impact on performance and polymer properties. In addition, full characterization for the new complexes is described.
Results and discussion
Synthesis and characterization
The ferrous chloride complexes, [2-{{2,6-((p-ClPh)2CH)2-4-MeC6H2}N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CMe}-6-(ArN
CMe}-6-(ArN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CMe)C5H3N]FeCl2 (Ar = 2,6-Me2C6H3Fe1, 2,6-Et2C6H3Fe2, 2,6-iPr2C6H3Fe3, 2,4,6-Me3C6H2Fe4, 2,6-Et2-4-MeC6H2Fe5) and [2,6-{{2,6-((p-ClPh)2CH)2-4-MeC6H2}N
CMe)C5H3N]FeCl2 (Ar = 2,6-Me2C6H3Fe1, 2,6-Et2C6H3Fe2, 2,6-iPr2C6H3Fe3, 2,4,6-Me3C6H2Fe4, 2,6-Et2-4-MeC6H2Fe5) and [2,6-{{2,6-((p-ClPh)2CH)2-4-MeC6H2}N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CMe}2C5H3N]FeCl2 (Fe6), were prepared in good yield by interaction of the corresponding bis(imino)pyridine, L1–L6, with iron(II) chloride hexahydrate in ethanol at ambient temperature (Scheme 1). The compounds, L1–L6, were prepared in two-steps from 2,6-diacetylpyridine using a previously described procedure.22 All iron complexes were characterized by FT-IR spectroscopy, ESI mass spectrometry and elemental analysis. In addition, the molecular structures of Fe2 and Fe5 were determined using single-crystal X-ray diffraction.
CMe}2C5H3N]FeCl2 (Fe6), were prepared in good yield by interaction of the corresponding bis(imino)pyridine, L1–L6, with iron(II) chloride hexahydrate in ethanol at ambient temperature (Scheme 1). The compounds, L1–L6, were prepared in two-steps from 2,6-diacetylpyridine using a previously described procedure.22 All iron complexes were characterized by FT-IR spectroscopy, ESI mass spectrometry and elemental analysis. In addition, the molecular structures of Fe2 and Fe5 were determined using single-crystal X-ray diffraction.
Crystals of Fe2 and Fe5 suitable for the X-ray determinations were grown as described in the Experimental section (see later). Views of Fe2 and Fe5 are depicted in Fig. 1 and 2, respectively, while selected bond lengths and angles are listed in Table 1.
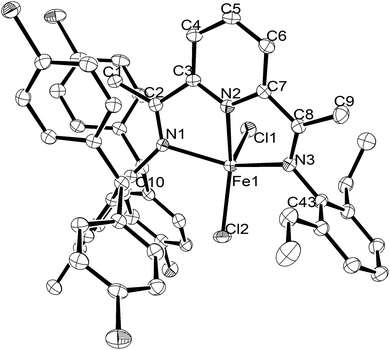 | ||
| Fig. 1 ORTEP representation of Fe2 with the thermal ellipsoids at the 30% probability level. All hydrogen atoms have been omitted for clarity. | ||
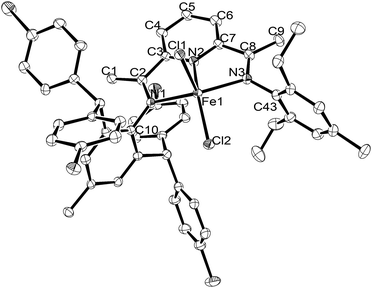 | ||
| Fig. 2 ORTEP representation of Fe5 with the thermal ellipsoids at the 30% probability level. All hydrogen atoms have been omitted for clarity. | ||
| Fe2 | Fe5 | |
|---|---|---|
| Bond lengths (Å) | ||
| Fe(1)–N(1) | 2.206(4) | 2.234(4) |
| Fe(1)–N(2) | 2.068(4) | 2.062(4) |
| Fe(1)–N(3) | 2.207(4) | 2.200(4) |
| Fe(1)–Cl(1) | 2.3375(13) | 2.3199(13) |
| Fe(1)–Cl(2) | 2.2384(14) | 2.2513(12) |
| Bond Angles (deg) | ||
| N(1)–Fe(1)–N(2) | 72.85(14) | 73.19(14) |
| N(1)–Fe(1)–N(3) | 139.59(14) | 139.62(14) |
| N(2)–Fe(1)–N(3) | 73.99(15) | 73.87(15) |
| N(1)–Fe(1)–Cl(2) | 100.12(11) | 99.45(10) |
| N(2)–Fe(1)–Cl(2) | 153.34(11) | 150.22(12) |
| N(3)–Fe(1)–Cl(2) | 99.18(11) | 97.61(11) |
| N(1)–Fe(1)–Cl(1) | 95.11(10) | 99.07(10) |
| N(2)–Fe(1)–Cl(1) | 89.61(10) | 93.11(12) |
| N(3)–Fe(1)–Cl(1) | 107.13(11) | 105.41(11) |
| Cl(2)–Fe(1)–Cl(1) | 116.87(6) | 116.66(5) |
The coordination geometry of both complexes can be described as distorted square pyramidal with N1, N2, N3 and Cl2 defining the square base and Cl1 the apical position (N(2)–Fe(1)–Cl(1): 89.61° (Fe2), 93.11° (Fe5)).51,52 More specifically the degree of distortion can be quantified in terms of the tau value (with tau = 0 perfectly square pyramidal and tau = 1 perfectly trigonal bipyramid),64 with Fe2 being 0.23 and Fe5 0.17. The iron atom itself sits at a distance of 0.531 Å above the basal plane for Fe2 and 0.571 Å for Fe5. For both structures, the planes of the inequivalent N-aryl groups are inclined almost perpendicularly to the neighboring imine vectors with dihedral angles of 84.02° and 87.98° for Fe2, and 77.50° and 87.15° for Fe5. In terms of the Fe–N bond lengths, it is apparent that there are some differences with the central Fe–Npyridine bond length [2.068(4) (Fe2), 2.062(4) (Fe5) Å] markedly shorter than the exterior Fe–Nimine ones [2.200(4)–2.234(4) Å]. This observation is widespread in this class of complex and highlights more effective coordination of the pyridine nitrogen with the iron center.57 Scrutiny of the Fe–Nimine distances reveals little variation in Fe2, whereas in Fe5, some minor difference is apparent with that involving the bulkier 2,6-bis(4,4′-dichlorobenzhydryl)-4-methylphenyl-substituted Nimine slightly longer [Fe(1)–N(1) 2.234(4) Å] than its 2,6-diethyl-4-methylphenyl-Nimine comparator [Fe(1)–N(3) 2.200(4) Å], suggesting steric factors exert some influence. With regard to the planarity of the bis(imino)pyridine, some minimal deviation between the neighboring imine vectors and the pyridine ring is also evident as is evidenced by the torsion angles for N(1)–C(2)–C(3)–N(2) [−3.38° (Fe2), 1.71° (Fe5)] and N(3)–C(8)–C(7)–N(2) [5.07° (Fe2), −2.86° (Fe5)]. There are no intermolecular contacts of note.
In the mass spectra of Fe1–Fe6, fragmentation peaks corresponding to the loss of one chloride are seen in each case, while their FT-IR spectra reveal stretching vibrations for the v(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N)imine groups in the range of 1607–1614 cm−1. When compared to their free ligands, L1–L6, these stretching vibrations are generally lower in wavenumber by ca. 30 cm−1, which provides further evidence of successful coordination of both Nimine donors of the ligand to the metal center.58
N)imine groups in the range of 1607–1614 cm−1. When compared to their free ligands, L1–L6, these stretching vibrations are generally lower in wavenumber by ca. 30 cm−1, which provides further evidence of successful coordination of both Nimine donors of the ligand to the metal center.58
Ethylene polymerization studies
To permit Fe1–Fe6 to be evaluated as precatalysts for ethylene polymerization, methylaluminoxane (MAO) and modified methylaluminoxane (MMAO) were deployed as co-catalysts. For each co-catalyst, Fe1 was utilized as the test precatalyst to allow an optimal set of polymerization conditions to be identified in terms of run temperature, molar ratio of Al![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe, run time and ethylene pressure. Typically, the runs were performed in toluene with ethylene pressure initially set at 10 atm. The resulting polyethylenes were then analyzed by gel permeation chromatography (GPC) and differential scanning calorimetry (DSC) to shed light on the polymer properties and in turn catalyst behavior.
Fe, run time and ethylene pressure. Typically, the runs were performed in toluene with ethylene pressure initially set at 10 atm. The resulting polyethylenes were then analyzed by gel permeation chromatography (GPC) and differential scanning calorimetry (DSC) to shed light on the polymer properties and in turn catalyst behavior.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe ratio kept at 2000
Fe ratio kept at 2000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and the run time at 30 min (entries 1–5, Table 2). Inspection of the data reveals the catalytic activity to initially increase with temperature reaching a peak value of 10.46 × 106 g PE mol−1(Fe) h−1 at 60 °C (entry 3, Table 2), and then decrease to 8.62 × 106 g PE mol−1(Fe) h−1 at 80 °C; this loss in performance at temperatures in excess of 60 °C can be attributed to the onset of catalyst deactivation and the lower solubility of ethylene in the solvent at these temperatures.59 Nonetheless, the observed polymerization activity at 80 °C is greater than those of a host of cobalt or iron analogues,51,54 which would point towards appreciable thermal stability. In terms of the molecular weight of the polyethylene, this was observed to decrease at higher run temperature, in accordance with an increased rate of chain termination (Fig. 3).
1 and the run time at 30 min (entries 1–5, Table 2). Inspection of the data reveals the catalytic activity to initially increase with temperature reaching a peak value of 10.46 × 106 g PE mol−1(Fe) h−1 at 60 °C (entry 3, Table 2), and then decrease to 8.62 × 106 g PE mol−1(Fe) h−1 at 80 °C; this loss in performance at temperatures in excess of 60 °C can be attributed to the onset of catalyst deactivation and the lower solubility of ethylene in the solvent at these temperatures.59 Nonetheless, the observed polymerization activity at 80 °C is greater than those of a host of cobalt or iron analogues,51,54 which would point towards appreciable thermal stability. In terms of the molecular weight of the polyethylene, this was observed to decrease at higher run temperature, in accordance with an increased rate of chain termination (Fig. 3).
| Entry | Precat. | T (°C) | Al![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe Fe |
t (min) | Activityb | M w | M w/Mnc | T m (°C) |
|---|---|---|---|---|---|---|---|---|
| a Conditions: 2.0 μmol of iron precatalyst, 10 atm ethylene, 100 mL toluene. b Activity: 106 g PE per mol (Fe) per h. c M w in kg per mol. Mw and Mw/Mn measured by GPC. d Measured by DSC. e 5 atm. f 1 atm. | ||||||||
| 1 | Fe1 | 40 | 2000 | 30 | 2.43 | 77.0 | 3.9 | 130.7 |
| 2 | Fe1 | 50 | 2000 | 30 | 3.37 | 67.3 | 3.5 | 129.2 |
| 3 | Fe1 | 60 | 2000 | 30 | 10.46 | 50.0 | 2.0 | 132.7 |
| 4 | Fe1 | 70 | 2000 | 30 | 10.01 | 23.3 | 2.8 | 130.7 |
| 5 | Fe1 | 80 | 2000 | 30 | 8.62 | 16.1 | 2.8 | 130.5 |
| 6 | Fe1 | 60 | 1500 | 30 | 8.44 | 71.9 | 3.9 | 131.0 |
| 7 | Fe1 | 60 | 2500 | 30 | 15.65 | 26.7 | 4.1 | 129.2 |
| 8 | Fe1 | 60 | 3000 | 30 | 13.87 | 15.7 | 3.0 | 132.3 |
| 9 | Fe1 | 60 | 3500 | 30 | 13.32 | 10.0 | 2.8 | 129.6 |
| 10 | Fe1 | 60 | 2500 | 5 | 39.24 | 5.4 | 1.6 | 128.0 |
| 11 | Fe1 | 60 | 2500 | 15 | 25.06 | 12.5 | 3.1 | 134.8 |
| 12 | Fe1 | 60 | 2500 | 45 | 11.97 | 41.1 | 6.2 | 135.1 |
| 13 | Fe1 | 60 | 2500 | 60 | 9.43 | 53.7 | 6.4 | 131.4 |
| 14e | Fe1 | 60 | 2500 | 30 | 13.87 | 20.8 | 5.9 | 131.8 |
| 15f | Fe1 | 60 | 2500 | 30 | 0.38 | 0.9 | 1.3 | 123.5 |
| 16 | Fe2 | 60 | 2500 | 30 | 11.64 | 21.0 | 3.8 | 131.1 |
| 17 | Fe3 | 60 | 2500 | 30 | 6.48 | 32.9 | 2.8 | 135.1 |
| 18 | Fe4 | 60 | 2500 | 30 | 14.82 | 10.5 | 1.8 | 130.8 |
| 19 | Fe5 | 60 | 2500 | 30 | 11.92 | 16.4 | 2.8 | 130.1 |
| 20 | Fe6 | 60 | 2500 | 30 | — | — | — | — |
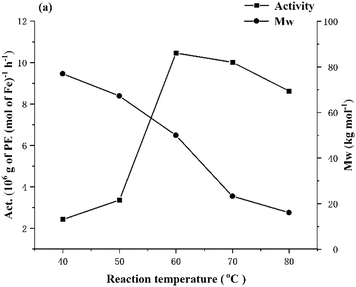 | ||
| Fig. 3 For Fe1/MAO: plots of catalytic activity and molecular weight of the polymer versus reaction temperature (entries 1–5, Table 2). | ||
Next, the molar ratio of Al![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe using Fe1/MAO was varied between 1500
Fe using Fe1/MAO was varied between 1500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 3500
1 and 3500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with the run temperature fixed at 60 °C (entries 3, 6–9, Table 2). With the ratio at 2500
1 with the run temperature fixed at 60 °C (entries 3, 6–9, Table 2). With the ratio at 2500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the highest activity of 15.65 × 106 g PE mol−1(Fe) h−1 was achieved (entry 7, Table 2). On the other hand, the highest molecular weight polymer (Mw = 71.9 kg mol−1) was obtained with the Al
1, the highest activity of 15.65 × 106 g PE mol−1(Fe) h−1 was achieved (entry 7, Table 2). On the other hand, the highest molecular weight polymer (Mw = 71.9 kg mol−1) was obtained with the Al![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe ratio at 1500
Fe ratio at 1500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, while further increasing the Al
1, while further increasing the Al![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe ratio to 3500
Fe ratio to 3500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the polymer molecular weight decreased to 10.0 kg mol−1 (Fig. 4). This drop in molecular weight would signify that higher ratios foster quicker chain transfer from the iron active center to the aluminum species and concomitant chain termination.60 As a further notable feature was the relatively narrow dispersity (Mw/Mn range: 2.0–4.0) displayed by these polyethylenes that highlights the good control and single-site-like nature of the active species (Fig. S2, ESI†). With regard to the polyethylene, these were of high linearity (Tm range: 129.2–132.7 °C) as is common using iron ethylene polymerization catalysts.54
1, the polymer molecular weight decreased to 10.0 kg mol−1 (Fig. 4). This drop in molecular weight would signify that higher ratios foster quicker chain transfer from the iron active center to the aluminum species and concomitant chain termination.60 As a further notable feature was the relatively narrow dispersity (Mw/Mn range: 2.0–4.0) displayed by these polyethylenes that highlights the good control and single-site-like nature of the active species (Fig. S2, ESI†). With regard to the polyethylene, these were of high linearity (Tm range: 129.2–132.7 °C) as is common using iron ethylene polymerization catalysts.54
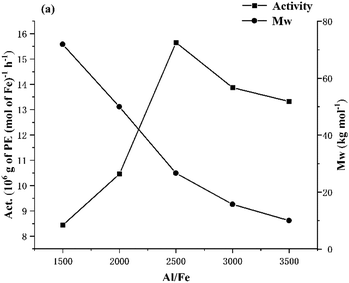 | ||
Fig. 4 For Fe1/MAO: plots of catalytic activity and molecular weight of the polymer versus Al![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe molar ratio (entries 3 and 6–9, Table 2). Fe molar ratio (entries 3 and 6–9, Table 2). | ||
With a view to exploring the lifetime of the active species formed using Fe1/MAO, the polymerization runs were performed at set run times between 5 and 60 min with the temperature kept at 60 °C and the Al![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe ratio fixed at 2500
Fe ratio fixed at 2500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (entries 7, 10–14, Table 2). The maximum activity of 39.24 × 106 g PE mol−1(Fe) h−1 was observed after 5 min, indicating that the active species was rapidly generated following addition of MAO and then progressively deactivated as the catalytic run time elapsed (Fig. 5). Although uncertain, it is plausible that an irreversible structural change of the catalyst occurs at high temperature leading to the observed deactivation of the catalyst. Alternatively, poisoning of the catalyst by trace impurities in the reaction system could account for this loss in activity. With respect to the ethylene pressure, a drop-in catalytic activity was noted as the pressure was lowered (entries 7, 14 and 15, Table 2), with a value of 15.65 × 106 g PE mol−1(Fe) h−1 obtained at PC2H4 = 10 atm that reduced to 13.87 × 106 g PE mol−1(Fe) h−1 at 5 atm and then more dramatically decreased to 0.38 × 106 g PE mol−1(Fe) h−1 at 1 atm. This dependency of the polymerization activity on the pressure can be attributed to the relative rates of insertion and ethylene coordination which can be enhanced at higher ethylene pressures.61
1 (entries 7, 10–14, Table 2). The maximum activity of 39.24 × 106 g PE mol−1(Fe) h−1 was observed after 5 min, indicating that the active species was rapidly generated following addition of MAO and then progressively deactivated as the catalytic run time elapsed (Fig. 5). Although uncertain, it is plausible that an irreversible structural change of the catalyst occurs at high temperature leading to the observed deactivation of the catalyst. Alternatively, poisoning of the catalyst by trace impurities in the reaction system could account for this loss in activity. With respect to the ethylene pressure, a drop-in catalytic activity was noted as the pressure was lowered (entries 7, 14 and 15, Table 2), with a value of 15.65 × 106 g PE mol−1(Fe) h−1 obtained at PC2H4 = 10 atm that reduced to 13.87 × 106 g PE mol−1(Fe) h−1 at 5 atm and then more dramatically decreased to 0.38 × 106 g PE mol−1(Fe) h−1 at 1 atm. This dependency of the polymerization activity on the pressure can be attributed to the relative rates of insertion and ethylene coordination which can be enhanced at higher ethylene pressures.61
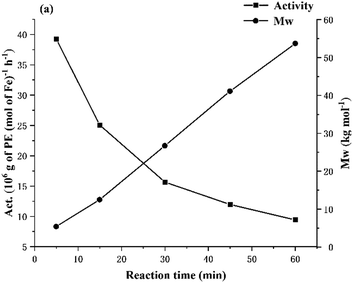 | ||
| Fig. 5 For Fe1/MAO: plots of catalytic activity and molecular weight of the polymer versus reaction time (entries 7 and 10–13, Table 2). | ||
With the optimum polymerization conditions identified for Fe1/MAO (viz. 60 °C, Al![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe ratio of 2500
Fe ratio of 2500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 10 atm ethylene and 30 min), the remaining iron complexes (Fe2–Fe6) were investigated as precatalysts for ethylene polymerization (entries 16–20, Table 2). In general, all iron/MAO systems displayed very good catalytic performance (range in activity: 6.48–15.65 × 106 g PE mol−1(Fe) h−1), with the exception of Fe6 which proved inactive. This latter observation can be attributed to the excessive steric hindrance provided by the four bulky 4,4′-dichlorobenzhydryl ortho-substituents in Fe6 which impedes ethylene coordination. With regard to the relative activities of Fe1–Fe5, their levels decreased in the order: Fe1 (2,6-dimethyl) > Fe4 (2,4,6-trimethyl) > Fe5 (2,6-diethyl-4-methyl) > Fe2 (2,6-diethyl) > Fe3 (2,6-diisopropyl) (Fig. 6). This trend emphasizes the role played by the steric properties of these ortho-substituents on the amenability to ethylene monomer coordination and insertion.62 In particular, it is apparent that the increase in steric properties from 2,6-diethyl to 2,6-diisopropyl leads to a steady decline in activity. By contrast, the variation in molecular weight with respect to iron precatalyst follows the order, Fe3 > Fe1 > Fe2 > Fe5 > Fe4, which indicates that greater hindrance leads to higher molecular weight polymer (up to 32.9 kg mol−1 for Fe3). As can be seen in Fig. S4 (ESI†), the resulting polyethylenes additionally showed reasonably narrow dispersity for all Fe/MAO combinations (Mw/Mn range: 1.8–3.8) in line with the good control displayed by the catalyst.
1, 10 atm ethylene and 30 min), the remaining iron complexes (Fe2–Fe6) were investigated as precatalysts for ethylene polymerization (entries 16–20, Table 2). In general, all iron/MAO systems displayed very good catalytic performance (range in activity: 6.48–15.65 × 106 g PE mol−1(Fe) h−1), with the exception of Fe6 which proved inactive. This latter observation can be attributed to the excessive steric hindrance provided by the four bulky 4,4′-dichlorobenzhydryl ortho-substituents in Fe6 which impedes ethylene coordination. With regard to the relative activities of Fe1–Fe5, their levels decreased in the order: Fe1 (2,6-dimethyl) > Fe4 (2,4,6-trimethyl) > Fe5 (2,6-diethyl-4-methyl) > Fe2 (2,6-diethyl) > Fe3 (2,6-diisopropyl) (Fig. 6). This trend emphasizes the role played by the steric properties of these ortho-substituents on the amenability to ethylene monomer coordination and insertion.62 In particular, it is apparent that the increase in steric properties from 2,6-diethyl to 2,6-diisopropyl leads to a steady decline in activity. By contrast, the variation in molecular weight with respect to iron precatalyst follows the order, Fe3 > Fe1 > Fe2 > Fe5 > Fe4, which indicates that greater hindrance leads to higher molecular weight polymer (up to 32.9 kg mol−1 for Fe3). As can be seen in Fig. S4 (ESI†), the resulting polyethylenes additionally showed reasonably narrow dispersity for all Fe/MAO combinations (Mw/Mn range: 1.8–3.8) in line with the good control displayed by the catalyst.
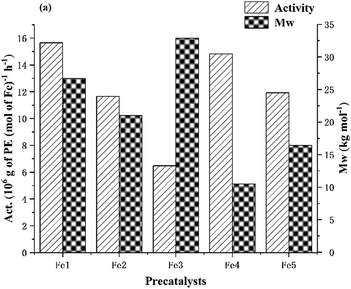 | ||
| Fig. 6 For Fe1–Fe5 using MAO as co-catalyst: a bar chart showing catalytic activity and molecular weight of the polymer versus the type of iron precatalyst (entries 7 and 16–20, Table 2). | ||
| Entry | Precat. | T (°C) | Al![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe Fe |
t (min) | Activityb | M w | M w/Mnc | T m (°C) |
|---|---|---|---|---|---|---|---|---|
| a Conditions: 2.0 μmol of iron precatalyst, 10 atm ethylene, 100 mL toluene. b Activity: 106 PE per mol (Fe) per h. c M w in kg per mol. Mw and Mw/Mn measured by GPC. d Measured by DSC. e 5 atm. f 1 atm. | ||||||||
| 1 | Fe1 | 40 | 2500 | 30 | 8.70 | 18.7 | 3.5 | 126.4 |
| 2 | Fe1 | 50 | 2500 | 30 | 17.31 | 15.4 | 2.9 | 128.3 |
| 3 | Fe1 | 60 | 2500 | 30 | 15.11 | 7.6 | 1.9 | 128.6 |
| 4 | Fe1 | 70 | 2500 | 30 | 13.44 | 4.7 | 1.6 | 125.6 |
| 5 | Fe1 | 80 | 2500 | 30 | 5.84 | 2.6 | 1.6 | 122.6 |
| 6 | Fe1 | 50 | 2000 | 30 | 13.80 | 16.0 | 1.4 | 127.4 |
| 7 | Fe1 | 50 | 3000 | 30 | 17.61 | 6.5 | 2.0 | 128.8 |
| 8 | Fe1 | 50 | 3500 | 30 | 18.12 | 4.7 | 1.3 | 131.2 |
| 9 | Fe1 | 50 | 4000 | 30 | 15.69 | 4.0 | 1.7 | 124.7 |
| 10 | Fe1 | 50 | 3500 | 5 | 57.12 | 3.0 | 1.5 | 123.9 |
| 11 | Fe1 | 50 | 3500 | 15 | 28.02 | 4.6 | 1.8 | 125.8 |
| 12 | Fe1 | 50 | 3500 | 45 | 13.09 | 7.1 | 2.7 | 127.2 |
| 13 | Fe1 | 50 | 3500 | 60 | 10.77 | 9.1 | 3.4 | 128.3 |
| 14e | Fe1 | 50 | 3500 | 30 | 13.80 | 3.5 | 2.3 | 127.1 |
| 15f | Fe1 | 50 | 3500 | 30 | 0.12 | 0.9 | 1.6 | 122.6 |
| 16 | Fe2 | 50 | 3500 | 30 | 20.12 | 12.7 | 4.2 | 129.8 |
| 17 | Fe3 | 50 | 3500 | 30 | 5.51 | 13.7 | 2.0 | 124.5 |
| 18 | Fe4 | 50 | 3500 | 30 | 16.66 | 4.4 | 1.5 | 125.7 |
| 19 | Fe5 | 50 | 3500 | 30 | 21.59 | 3.4 | 1.4 | 128.9 |
| 20 | Fe6 | 50 | 3500 | 30 | 0.29 | 4.6 | 2.1 | 127.3 |
With the Al![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe ratio fixed at 2500
Fe ratio fixed at 2500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the polymerization runs using Fe1/MMAO were performed at temperatures between 40 and 80 °C (entries 1–5, Table 3). The highest activity of 17.31 × 106 g PE mol−1(Fe) h−1 was achieved at 50 °C, while further increasing the temperature to 80 °C saw the activity decrease to 5.84 × 106 g PE mol−1(Fe) h−1 in line with deactivation of the catalyst occurring (Fig. 7). Similarly, the molecular weight of the polymer dropped from 18.7 to 2.6 kg mol−1 as a result of more effective chain termination occurring with increased temperature. By comparison with Fe1/MAO over a comparable temperature range, the polyethylene displayed much lower molecular weight and in turn the melting temperatures of the polymers were lower (Tm range: 122.6–128.6 °C).
1, the polymerization runs using Fe1/MMAO were performed at temperatures between 40 and 80 °C (entries 1–5, Table 3). The highest activity of 17.31 × 106 g PE mol−1(Fe) h−1 was achieved at 50 °C, while further increasing the temperature to 80 °C saw the activity decrease to 5.84 × 106 g PE mol−1(Fe) h−1 in line with deactivation of the catalyst occurring (Fig. 7). Similarly, the molecular weight of the polymer dropped from 18.7 to 2.6 kg mol−1 as a result of more effective chain termination occurring with increased temperature. By comparison with Fe1/MAO over a comparable temperature range, the polyethylene displayed much lower molecular weight and in turn the melting temperatures of the polymers were lower (Tm range: 122.6–128.6 °C).
The influence of Al![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe molar ratio on the performance of Fe1/MMAO was then explored with the ratio varied between 2000
Fe molar ratio on the performance of Fe1/MMAO was then explored with the ratio varied between 2000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 4000
1 and 4000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and the run temperature maintained at 50 °C (entries 2, 6–9, Table 3). With the ratio at 3500
1 and the run temperature maintained at 50 °C (entries 2, 6–9, Table 3). With the ratio at 3500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the uppermost activity of 18.12 × 106 g PE mol−1(Fe) h−1 was obtained which then dropped to 15.69 × 106 g PE mol−1(Fe) h−1 at 4000
1, the uppermost activity of 18.12 × 106 g PE mol−1(Fe) h−1 was obtained which then dropped to 15.69 × 106 g PE mol−1(Fe) h−1 at 4000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 as chain transfer to aluminum became more prominent.63 Unimodal and narrowly disperse polyethylene (Mw/Mn range: 1.3–2.9) was obtained in agreement with the existence of single-site active species at a run temperature of 50 °C.
1 as chain transfer to aluminum became more prominent.63 Unimodal and narrowly disperse polyethylene (Mw/Mn range: 1.3–2.9) was obtained in agreement with the existence of single-site active species at a run temperature of 50 °C.
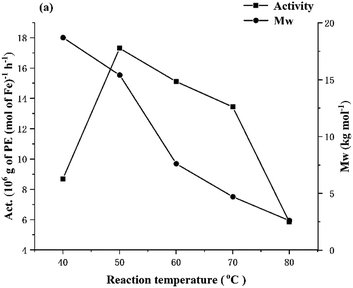 | ||
| Fig. 7 For Fe1/MMAO: plots of catalytic activity and molecular weight of the polymer versus reaction temperature. | ||
The time/activity profile of Fe1/MMAO was then investigated with the polymerization runs performed between 5 and 60 min (entries 8, 10–13, Table 3). As with Fe1/MAO, a short period of 5 min was sufficient to produce the active species leading to the highest activity of 57.12 × 106 g PE mol−1(Fe) h−1. As the run time was extended beyond 5 min, the active species gradually deactivated resulting in lower activities with the level reaching 10.77 × 106 g PE mol−1(Fe) h−1 after 1 h. Furthermore, and mirroring the MAO study, reducing the ethylene pressure greatly lowered the catalytic activity from 18.12 × 106 g PE mol−1(Fe) h−1 at PC2H4 = 10 atm to 0.12 × 106 g PE mol−1(Fe) h−1 at 1 atm.
On the basis of the optimized parameters established for Fe1/MMAO namely, run temperature = 50 °C, Al![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe molar ratio = 3500
Fe molar ratio = 3500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, PC2H4 = 10 atm and run time = 30 min, the remaining iron precatalysts Fe2–Fe6 were screened and their catalytic performance compared with Fe1/MAO. With the exception of Fe6, all the iron complexes exhibited high activities (range: 5.51–21.59 × 106 g PE mol−1(Fe) h−1), with levels in general exceeding that seen using MAO as co-catalyst (range: 6.48–15.65 × 106 g PE mol−1(Fe) h−1). In terms of the relative performance, these fell in the order: Fe5 (2,6-diethyl-4-methyl) > Fe2 (2,6-diethyl) > Fe1 (2,6-dimethyl) > Fe4 (2,4,6-trimethyl) > Fe3 (2,6-diisopropyl) ≫ Fe6 (2,6-bis(4,4′-dichlorobenzhydryl)-4-methyl) (Fig. 8). Once again, the activity exhibited by Fe6 was found at the bottom end of the range suggesting that the excessive steric hindrance around the active center, inhibits the coordination and insertion of ethylene monomer.62 On the other hand, the range in molecular weights of the polyethylenes produced was less than with MAO [3.4–13.7 kg mol−1vs. 10.5–32.9 kg mol−1 (MAO)], which was reflected by the lower melting temperature range [124.5–129.8 °C vs. 129.2–135.1 °C (MAO)]. Nonetheless, all polyethylenes possessed narrow dispersity and unimodal distributions.
1, PC2H4 = 10 atm and run time = 30 min, the remaining iron precatalysts Fe2–Fe6 were screened and their catalytic performance compared with Fe1/MAO. With the exception of Fe6, all the iron complexes exhibited high activities (range: 5.51–21.59 × 106 g PE mol−1(Fe) h−1), with levels in general exceeding that seen using MAO as co-catalyst (range: 6.48–15.65 × 106 g PE mol−1(Fe) h−1). In terms of the relative performance, these fell in the order: Fe5 (2,6-diethyl-4-methyl) > Fe2 (2,6-diethyl) > Fe1 (2,6-dimethyl) > Fe4 (2,4,6-trimethyl) > Fe3 (2,6-diisopropyl) ≫ Fe6 (2,6-bis(4,4′-dichlorobenzhydryl)-4-methyl) (Fig. 8). Once again, the activity exhibited by Fe6 was found at the bottom end of the range suggesting that the excessive steric hindrance around the active center, inhibits the coordination and insertion of ethylene monomer.62 On the other hand, the range in molecular weights of the polyethylenes produced was less than with MAO [3.4–13.7 kg mol−1vs. 10.5–32.9 kg mol−1 (MAO)], which was reflected by the lower melting temperature range [124.5–129.8 °C vs. 129.2–135.1 °C (MAO)]. Nonetheless, all polyethylenes possessed narrow dispersity and unimodal distributions.
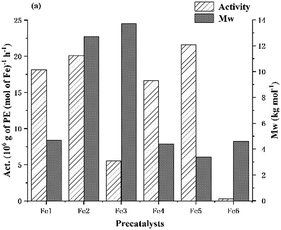 | ||
| Fig. 8 For Fe1–Fe6 with MMAO as co-catalyst: a bar chart showing catalytic activity and molecular weight of the polymer versus iron precatalyst (entries 8 and 16–20, Table 3). | ||
As is evident from the values of the melting temperatures of the polyethylenes shown in Tables 2 and 3, these materials all display a linear backbone. To lend further support for this linearity and to cast some light on their end-group composition, samples produced using Fe1/MAO at 60 °C (Mw = 26.7 kg mol−1; entry 7, Table 2) and Fe1/MMAO at 50 °C (Mw = 4.7 kg mol−1; entry 8, Table 3) were selected and characterized by 13C NMR spectroscopy. To engender suitable solubility, the spectra were recorded at 100 °C in 1,1,2,2-tetrachloroethane-d2. As is characteristic for both samples, the spectra show high intensity singlets at around δ 30.00 ppm (see Fig. 9 and Fig. S9, ESI†) which can be assigned to the –(CH2)n– repeat unit in support of the high linearity of the polyethylene. Furthermore, no peaks for saturated or unsaturated chain ends could be seen in the spectra which is likely due to high molecular weight of these samples.
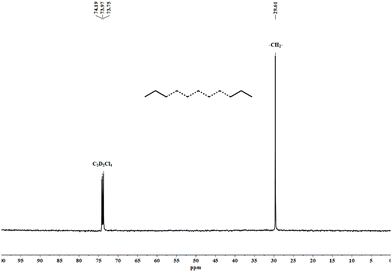 | ||
| Fig. 9 13C NMR spectrum of the polyethylene obtained using Fe1/MAO at 60 °C (entry 7, Table 2); recorded in 1,1,2,2-tetrachloroethane-d2 at 100 °C. | ||
Comparison of the current iron precatalysts with previously reported examples
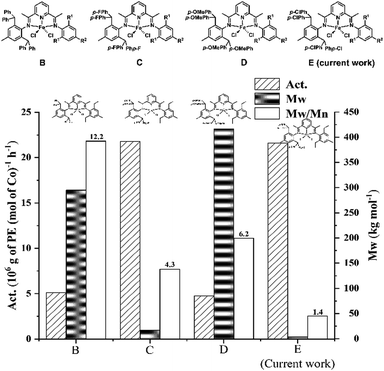
With the intent to understand how the introduction of the para-chlorides to the benzhydryl phenyl groups in E affect the polymerization performance (viz. catalytic activity, polymer molecular weight and dispersity), a comparison with the previously reported unsymmetrical iron precatalysts, B, C and D, was conducted (Fig. 10).52–54 In particular, the most active system, Fe5/MMAO, was selected as a representative of the E class and performance data for the most structurally related examples of B, C and D compared; all polymerization runs were undertaken with MMAO under their optimized reaction conditions at 10 atm of C2H4.In terms of the catalytic activity, it is evident that the 4,4′-dichlorobenzhydryl group in E has a positive impact on the level of activity with a value of 21.59 × 106 g PE mol−1(Fe) h−1 seen over 30 min, which is higher than that for B (benzhydryl), D (4,4′-dimethoxybenzhydryl), and close to that seen for C (4,4′-difluorobenzhydryl) which was performed over 15 min. Moreover, the polyethylene generated using E exhibits the lowest molecular weight (Mw: 3.4 kg mol−1) and narrowest dispersity (Mw/Mn: 1.4) and indeed has characteristics of a polyethylene wax. Overall, it is evident that E displays a closer similarity to it fluoride counterpart C than to B and D. The origin of these findings is uncertain but may relate to the electropositivity of the active iron center caused by the presence of the electron withdrawing para-halide. Nevertheless, it is clear that variations to the benzhydryl periphery, although remote from the metal center can not only enhance catalytic activity but also affect the molecular weight of the polymer.
Conclusions
To summarize, a series of unsymmetrical and symmetrical 2-[1-(2,6-bis(di(4-chlorophenyl)methyl)-4-methylphenylimino)ethyl]-6-[1-(arylimino)ethyl]pyridyl-iron(II) chloride complexes, Fe1–Fe6, were successfully synthesized by employing a straightforward and high yielding approach. Characterization of the complexes was achieved by using a range of techniques including single crystal X-ray diffraction for Fe2 and Fe5. On activation with MAO and MMAO, Fe1–Fe5 exhibited very good activities for ethylene polymerization with levels up to 21.59 × 106 g PE mol−1(Fe) h−1 for Fe5/MMAO at 50 °C and 15.65 × 106 g PE mol−1(Fe) h−1 for Fe1/MAO at 60 °C, producing strictly linear and narrowly disperse unimodal polyethylenes. Notably, the molecular weight of the polymer was influenced by not only the N,N,N'-ligand structure but also the aluminoxane employed with the MMAO runs displaying a predilection for forming lower molecular weight materials. In addition, by comparison with related benzhydryl-containing catalytic systems (B–D, Chart 1), we found that the introduction of the electron withdrawing Cl group is beneficial to the catalytic activity, while the catalysts with electron donating group (e.g. OCH3) can generate higher molecular weight polyethylene. Overall, we feel these findings provide an important insight into the design of new catalysts based on electronic variations.Experimental
General procedures
All manipulations making use of air- and moisture-sensitive compounds were carried out using standard Schlenk techniques or were performed in an inert atmosphere glovebox. Toluene, used for the polymerization studies, was dried over sodium and distilled under nitrogen prior to use. Methylaluminoxane (MAO, 1.30 M solution in toluene) and modified methylaluminoxane (MMAO, 1.93 M in n-heptane) were purchased from Anhiu Botai Electronic Materials Co. Other reagents were purchased from Aldrich, Acros or local suppliers (Beijing, China). High purity ethylene was purchased from Beijing Yansan Petrochemical Co. and used as received. The FT-IR spectra were recorded on a PerkinElmer System 2000 FT-IR spectrometer and elemental analysis were undertaken with a Flash EA 1112 microanalyzer. The molecular weights (Mw) and the dispersities (Mw/Mn) of the polyethylenes were carried out using an Agilent PL-GPC 220 GPC instrument (Beijing, China) at 150 °C with trichlorobenzene as the solvent. The melting points (Tm) of the polyethylenes were measured on a PerkinElmer TA-Q2000 differential scanning calorimeter (DSC) under a nitrogen atmosphere. In a typical procedure, a sample of about 5.0 mg was heated up to 160 °C at a rate of 20 °C min−1, maintained for 5 min at 160 °C to remove the thermal history and then cooled to −20 °C at a rate of 20 °C min−1. The 13C NMR spectra of the polyethylenes were recorded on a Bruker AVANCE III 500 MHz instrument at 100 °C; a weighed amount of polyethylene (20–40 mg) was dissolved in 1,1,2,2-tetrachloroethane-d2 (2 mL) with TMS as an internal standard. The bis(imino)pyridines, 2-{{2,6-((p-ClPh)2CH)2-4-MeC6H2}N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CMe}-6-(ArN
CMe}-6-(ArN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CMe)C5H3N (Ar = 2,6-Me2C6H3 (L1), 2,6-Et2C6H3 (L2), 2,6-iPr2C6H3 (L3), 2,4,6-Me3C6H2 (L4), 2,6-Et2-4-MeC6H2 (L5)) and 2,6-{{2,6-((p-ClPh)2CH)2-4-MeC6H2}N
CMe)C5H3N (Ar = 2,6-Me2C6H3 (L1), 2,6-Et2C6H3 (L2), 2,6-iPr2C6H3 (L3), 2,4,6-Me3C6H2 (L4), 2,6-Et2-4-MeC6H2 (L5)) and 2,6-{{2,6-((p-ClPh)2CH)2-4-MeC6H2}N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CMe}2C5H3N (L6) were prepared using a previously reported route.22
CMe}2C5H3N (L6) were prepared using a previously reported route.22
Synthesis of Syntheses of [2-{{2,6-((p-ClPh)2CH)2-4-MeC6H2}N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/h3_char_e001.gif) CMe}-6-(ArN
CMe}-6-(ArN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/h3_char_e001.gif) CMe)C5H3N]FeCl2 (Fe1–Fe6)
CMe)C5H3N]FeCl2 (Fe1–Fe6)
(a) Ar = 2,6-Me2C6H3 (Fe1). Under a nitrogen atmosphere, FeCl2·4H2O (0.043 g, 0.22 mmol) and L1 (0.20 g, 0.24 mmol) and were added together into a Schlenk tube and then freshly distilled ethanol (10 mL) introduced. The tube was sealed and the reaction mixture stirred for 12 h at room temperature to form a precipitate. Diethyl ether (10 mL) was added to complete the precipitation which was then filtered and washed with diethyl ether (3 × 10 mL). Following a period of drying under reduced pressure, Fe1 was isolated as a blue powder (0.17 g, 82%). FT-IR (cm−1): 2916 (w), 1614 (νC=N, m), 1582 (m), 1489 (s), 1468 (m), 1405 (m), 1370 (m), 1317 (w), 1265 (m), 1211 (m), 1182 (w), 1090 (s), 1014 (s), 828 (s), 804 (s), 769 (m), 731 (m), 682 (m). HRMS (ESI) m/z: [M − Cl]+, Calcd for C50H41Cl5FeN3 914.1090, Found 914.1086. Anal. Calc. for C50H41Cl6FeN3 (952.44): C, 63.05; H, 4.34; N, 4.41%, Found: C, 63.03; H, 4.34; N, 4.12%.
(b) Ar = 2,6-Et2C6H3 (Fe2). By adopting a similar procedure to that outlined for Fe1 but with L2 as the bis(imino)pyridine, Fe2 was isolated as a blue powder (0.077 g, 75%). FT-IR (cm−1): 2966 (w), 1973 (w), 1614 (νC=N, m), 1582 (m), 1489 (s), 1454 (m), 1405 (m), 1371 (m), 1319 (w), 1267 (m), 1210 (m), 1180 (w), 1090 (s), 1014 (s), 870 (m), 831 (s), 804 (s), 767 (s), 732 (m), 683 (m). HRMS (ESI) m/z: [M − Cl]+, Calcd for C52H45Cl5FeN3 942.1403, Found 942.1402. Anal. Calc. for C52H45Cl6FeN3 (980.50): C, 63.70; H, 4.63; N, 4.29%, Found: C, 63.66; H, 4.58; N, 3.97%.
(c) Ar = 2,6-iPr2C6H3 (Fe3). By adopting a similar procedure to that outlined for Fe1 but with L3 as the bis(imino)pyridine, Fe3 was isolated as a blue powder (0.058 g, 56%). FT-IR (cm−1): 2963 (w), 1973 (w), 1612 (νC=N, m), 1581 (m), 1489 (s), 1462 (m), 1406 (m), 1372 (m), 1318 (w), 1268 (m), 1211 (m), 1182 (m), 1090 (s), 1054 (s), 1014 (s), 939 (w), 870 (m), 833 (s), 804 (s), 765 (m), 733 (m), 683 (m). HRMS (ESI) m/z: [M − Cl]+, Calcd for C54H49Cl5FeN3 970.1716, Found 970.1716. Anal. Calc. for C54H49Cl6FeN3 (1008.55): C, 64.31; H, 4.90; N, 4.17%, Found: C, 64.64; H, 4.97; N, 3.75%.
(d) Ar = 2,4,6-Me3C6H2 (Fe4). By adopting a similar procedure to that outlined for Fe1 but with L4 as the bis(imino)pyridine, Fe4 was isolated as a blue powder (0.091 g, 87%). FT-IR (cm−1): 2912 (w), 1975 (w), 1607 (νC=N, m), 1577 (m), 1489 (s), 1407 (m), 1373 (m), 1312 (w), 1271 (m), 1218 (m), 1184 (w), 1150 (w), 1090 (s), 1013 (s), 850 (m), 832 (s), 810 (s), 733 (m), 684 (m). HRMS (ESI) m/z: [M − Cl]+, Calcd for C51H43Cl5FeN3 928.1246, Found 928.1246. Anal. Calc. for C51H43Cl6FeN3 (966.47): C, 63.38; H, 4.48; N, 4.35%, Found: C, 63.20; H, 4.48; N, 4.12%.
(e) Ar = 2,6-Et2-4-MeC6H2 (Fe5). By adopting a similar procedure to that outlined for Fe1 but with L5 as the bis(imino)pyridine, Fe5 was isolated as a blue powder (0.076 g, 92%). FT-IR (cm−1): 2967 (w), 1973 (w), 1612 (νC=N, m), 1580 (m), 1489 (s), 1460 (m), 1406 (m), 1373 (m), 1321 (w), 1268 (m), 1214 (m), 1181 (m), 1090 (s), 1015 (s), 832 (s), 810 (s), 733 (m), 683 (m). HRMS (ESI) m/z: [M − Cl]+, Calcd for C53H47Cl5FeN3 956.1559, Found 956.1562. Anal. Calc. for C53H47Cl6FeN3 (994.53): C, 64.01; H, 4.76; N, 4.23%, Found: C, 64.03; H, 5.02; N, 3.89%.
(f) Ar = 2,6-((p-ClPh)2CH)2-4-MeC6H2 (Fe6). By adopting a similar procedure to that outlined for Fe1 but with L6 as the bis(imino)pyridine, Fe6 was isolated as a blue powder (0.020 g, 25%). FT-IR (cm−1): 1972 (w), 1607 (νC=N, m), 1575 (m), 1489 (s), 1459 (m), 1405 (m), 1371 (m), 1269 (m), 1214 (m), 1091 (s), 1015 (s), 831 (s), 801 (s), 731 (m), 684 (m). Anal. Calc. for C75H55Cl10FeN3 (1408.63): C, 63.95; H, 3.94; N, 2.98%, Found: C, 63.81; H, 3.95; N, 2.82%.
X-ray crystallographic studies
Single crystals of Fe2 and Fe5 suitable for the X-ray determinations were obtained by layering diethyl ether onto a dichloromethane solution of the corresponding compound under a nitrogen atmosphere at room temperature. Data collection for Fe2 and Fe5 was carried out using mirror-monochromatic Cu-Kα radiation (λ = 1.54184 Å) at 170 K, cell parameters were obtained using global refinement of the positions of all collected reflections. Intensities were corrected for Lorentz and polarization effects and empirical absorption. The structures were solved by direct methods and refined with full-matrix least-squares on F2. All hydrogen atoms were placed in the calculated positions. Structural solution and refinement were performed by using the Olex2 1.2 package and SHELXTL.55 The SQUEEZE option of the crystallographic program PLATON was applied to remove free solvents from the structure of Fe5.56 Details of the crystal data and processing parameters are summarized in Table S1 (ESI†).Ethylene polymerization procedures
Author contributions
Tian Liu: investigation, formal analysis, validation, writing – original draft. Yanping Ma: formal analysis, data curation, software. Gregory A. Solan: investigation, writing – review & editing. Yang Sun: investigation, validation. Wen-Hua Sun: conceptualization, funding acquisition, methodology, project administration, resources, supervision, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 21871275). G. A. S. thanks the Chinese Academy of Sciences for a President's International Fellowship for Visiting Scientists.References
- B. L. Small, M. Brookhart and A. M. A. Bennett, J. Am. Chem. Soc., 1998, 120, 4049–4050 CrossRef CAS.
- G. J. P. Britovsek, V. C. Gibson, B. S. Kimberley, P. J. Maddox, S. J. McTavish, G. A. Solan, A. J. P. White and D. J. Williams, Chem. Commun., 1998, 849–850 RSC.
- G. J. P. Britovsek, M. Bruce, V. C. Gibson, B. S. Kimberley, P. J. Maddox, S. Mastroianni, S. J. McTavish, C. Redshaw, G. A. Solan, S. Strömberg, A. J. P. White and D. J. Williams, J. Am. Chem. Soc., 1999, 121, 8728–8740 CrossRef CAS.
- G. J. P. Britovsek, V. C. Gibson and D. F. Wass, Angew. Chem., Int. Ed., 1999, 38, 428–447 CrossRef CAS PubMed.
- G. J. P. Britovsek, V. C. Gibson, B. S. Kimberley, S. Mastroianni, C. Redshaw, G. A. Solan, A. J. P. White and D. J. Williams, J. Chem. Soc., Dalton Trans., 2001, 1639–1644 RSC.
- G. J. P. Britovsek, V. C. Gibson, S. K. Spitzmesser, K. P. Tellmann, A. J. P. White and D. J. Williams, J. Chem. Soc., Dalton Trans., 2002, 1159–1171 RSC.
- (a) V. C. Gibson, C. Redshaw and G. A. Solan, Chem. Rev., 2007, 107, 1745–1776 CrossRef CAS PubMed; (b) V. C. Gibson and G. A. Solan, Top. Organomet. Chem., 2009, 2, 107–158 CrossRef PubMed; (c) V. C. Gibson and G. A. Solan, Catalysis without Precious Metals, ed. R. M. Bullock, Wiley-VCH, Weinheim, Germany, 2010, pp. 111–141 Search PubMed.
- Z. Wang, Q. Liu, G. A. Solan and W.-H. Sun, Coord. Chem. Rev., 2017, 350, 68–83 CrossRef CAS.
- S. Wang, D. Liu, R. Huang, Y. Zhang and B. Mao, J. Mol. Catal. A: Chem., 2006, 245, 122–131 CrossRef CAS.
- A. S. Abu-Surrah, K. Lappalainen, U. Piironen, P. Lehmus, T. Repo and M. Leskela, J. Organomet. Chem., 2002, 648, 55–61 CrossRef CAS.
- R. Gao, Y. Li, F. Wang, W.-H. Sun and M. Bochmann, Eur. J. Inorg. Chem., 2009, 4149–4156 CrossRef CAS.
- K. Wang, K. Wedeking, W. Zuo, D. Zhang and W.-H. Sun, J. Organomet. Chem., 2008, 693, 1073–1080 CrossRef CAS.
- I. S. Paulino and U. Schuchardt, J. Mol. Catal. A: Chem., 2004, 211, 55–58 CrossRef CAS.
- M. J. Humphries, K. P. Tellmann, V. C. Gibson, A. J. P. White and D. J. Williams, Organometallics, 2005, 24, 2039–2050 CrossRef CAS.
- W.-H. Sun, S. Jie, S. Zhang, W. Zhang, Y. Song and H. Ma, Organometallics, 2006, 25, 666–677 CrossRef CAS.
- C. C. H. Atienza, C. Milsmann, E. Lobkovsky and P. J. Chirik, Angew. Chem., Int. Ed., 2011, 50, 8143–8147 CrossRef PubMed.
- N. Kleigrewe, W. Steffen, T. Blömker, G. Kehr, R. Fröhlich, B. Wibbeling, G. Erker, J.-C. Wasilke, G. Wu and G. C. Bazan, J. Am. Chem. Soc., 2005, 127, 13955 CrossRef CAS PubMed.
- L. Xiao, R. Gao, M. Zhang, Y. Li, X. Cao and W.-H. Sun, Organometallics, 2009, 28, 2225–2233 CrossRef CAS.
- S. Du, W. Zhang, E. Yue, F. Huang, T. Liang and W.-H. Sun, Eur. J. Inorg. Chem., 2016, 1748–1755 CrossRef CAS.
- C. Bianchini, G. Giambastiani, I. R. Guerrero, A. Meli, E. Passaglia and T. Gragnoli, Organometallics, 2004, 23, 6087–6089 CrossRef CAS.
- W. Zhang, W. Chai, W.-H. Sun, X. Hu, C. Redshaw and X. Hao, Organometallics, 2012, 31, 5039–5048 CrossRef CAS.
- T. Liu, M. Liu, Y. Ma, G. A. Solan, T. Liang and W.-H. Sun, Eur. J. Inorg. Chem., 2022, e20220396 Search PubMed.
- C. Görl and H. G. Alt, J. Mol. Catal. A: Chem., 2007, 273, 118–132 CrossRef.
- S.-F. Yuan, L. Wang, Y. Yan, T. Liu, Z. Flisak, Y. Ma and W.-H. Sun, RSC Adv., 2022, 12, 15741–15750 RSC.
- Q. Knijnenburg, S. Gambarotta and P. H. M. Budzelaar, Dalton Trans., 2006, 5442–5448 RSC.
- L. Zhang, W. Zhang, P. Serp, W.-H. Sun and J. Durand, ChemCatChem, 2014, 6, 1310–1316 CAS.
- M. W. Bouwkamp, E. Lobkovsky and P. J. Chirik, J. Am. Chem. Soc., 2005, 127, 9660 CrossRef CAS PubMed.
- S. Wang, B. Li, T. Liang, C. Redshaw, Y. Li and W.-H. Sun, Dalton Trans., 2013, 42, 9188–9197 RSC.
- R. Raucoules, T. de Bruin, P. Raybaud and C. Adamo, Organometallics, 2009, 28, 5358–5367 CrossRef CAS.
- Q. Mahmood, J. Guo, W. Zhang, Y. Ma, T. Liang and W.-H. Sun, Organometallics, 2018, 37, 957–970 CrossRef CAS.
- A. S. Ionkin, W. J. Marshall, D. J. Adelman, B. B. Fones, B. M. Fish and M. F. Schiffhauer, Organometallics, 2006, 25, 2978–2992 CrossRef CAS.
- W. Zhang, S. Wang, S. Du, C.-Y. Guo, X. Hao and W.-H. Sun, Macromol. Chem. Phys., 2014, 215, 1797–1809 CrossRef CAS.
- S. McTavish, G. J. P. Britovsek, T. M. Smit, V. C. Gibson, A. J. P. White and D. J. Williams, J. Mol. Catal. A: Chem., 2007, 261, 293–300 CrossRef CAS.
- L. Guo, H. Gao, L. Zhang, F. Zhu and Q. Wu, Organometallics, 2010, 29, 2118–2125 CrossRef CAS.
- B. L. Small, R. Rios, E. R. Fernandez, D. L. Gerlach, J. A. Halfen and M. J. Carney, Organometallics, 2010, 29, 6723–6731 CrossRef CAS.
- Q. Chen, W. Zhang, G. A. Solan, R. Zhang, L. Guo, X. Hao and W.-H. Sun, Organometallics, 2018, 37, 4002–4014 CrossRef CAS.
- A. Boudier, P.-A. Breuil, L. Magna, H. Olivier-Bourbigou and P. Braunstein, Chem. Commun., 2014, 50, 1398–1407 RSC.
- C. Huang, S. Du, G. A. Solan, Y. Sun and W.-H. Sun, Dalton Trans., 2017, 46, 6948–6957 RSC.
- F. Huang, Z. Sun, S. Du, E. Yue, J. Ba, X. Hu, T. Liang, G. B. Galland and W.-H. Sun, Dalton Trans., 2015, 44, 14281–14292 RSC.
- Z. Sun, F. Huang, M. Qu, E. Yue, I. V. Oleynik, I. I. Oleynik, Y. Zeng, T. Liang, K. Li, W. Zhang and W.-H. Sun, RSC Adv., 2015, 5, 77913–77921 RSC.
- F. Huang, W. Zhang, E. Yue, T. Liang, X. Hu and W.-H. Sun, Dalton Trans., 2016, 45, 657–666 RSC.
- Z. Zuo, Q. Zhang, M. Han, M. Liu, Y. Sun, Y. Ma and W.-H. Sun, Catalysts, 2022, 12, 1119 CrossRef CAS.
- F. He, W. Zhao, X.-P. Cao, T. Liang, C. Redshaw and W.-H. Sun, J. Organomet. Chem., 2012, 713, 209–216 CrossRef CAS.
- J. Ramos, V. Cruz, A. Muñoz-Escalona and J. Martínez-Salazar, Polymer, 2002, 43, 3635–3645 CrossRef CAS.
- N. E. Mitchell, W. C. Anderson Jr. and B. K. Long, J. Polym. Sci. Part A: Polym. Chem., 2017, 55, 3990–3995 CrossRef CAS.
- C. Huang, Y. Huang, Y. Ma, G. A. Solan, Y. Sun, X. Hu and W.-H. Sun, Dalton Trans., 2018, 47, 13487–13497 RSC.
- H. Suo, I. I. Oleynik, C. Bariashir, I. V. Oleynik, Z. Wang, G. A. Solan, Y. Ma, T. Liang and W.-H. Sun, Polymer, 2018, 149, 45–54 CrossRef CAS.
- Z. Wang, G. A. Solan, Q. Mahmood, Q. Liu, Y. Ma, X. Hao and W.-H. Sun, Organometallics, 2018, 37, 380–389 CrossRef CAS.
- L. Guo, M. Zada, W. Zhang, A. Vignesh, D. Zhu, Y. Ma, T. Liang and W.-H. Sun, Dalton Trans., 2019, 48, 5604–5613 RSC.
- W. Yang, Z. Ma, J. Yi and W.-H. Sun, Catalysts, 2017, 7, 120 CrossRef.
- M. Han, I. I. Oleynik, Y. Ma, I. V. Oleynik, G. A. Solan, X. Hao and W.-H. Sun, Eur. J. Inorg. Chem., 2022, e202200224 CAS.
- J. Yu, H. Liu, W. Zhang, X. Hao and W.-H. Sun, Chem. Commun., 2011, 47, 3257–3259 RSC.
- W.-H. Sun, W. Zhao, J. Yu, W. Zhang, X. Hao and C. Redshaw, Macromol. Chem. Phys., 2012, 231, 1266–1273 CrossRef.
- T. Liu, Y. Ma, G. A. Solan, T. Liang and W.-H. Sun, Appl. Organomet. Chem., 2021, 35, e6259 CAS.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Adv., 2015, A71, 3–8 CrossRef PubMed.
- G. M. Sheldrick, Acta Crystallogr., Sect. C: Struct. Chem., 2015, C71, 3–8 Search PubMed.
- Q. Zhang, R. Zhang, M. Han, W. Yang, T. Liang and W.-H. Sun, Dalton Trans., 2020, 49, 7384–7396 RSC.
- Q. Zhang, N. Wu, J. Xiang, G. A. Solan, H. Suo, Y. Ma, T. Liang and W.-H. Sun, Dalton Trans., 2020, 49, 9425–9437 RSC.
- M. Zada, A. Vignesh, H. Suo, Y. Ma, H. Liu and W.-H. Sun, Mol. Catal., 2020, 492, 110981 CrossRef CAS.
- M. Han, Q. Zhang, I. I. Oleynik, H. Suo, G. A. Solan, I. V. Oleynik, Y. Ma, T. Liang and W.-H. Sun, Dalton Trans., 2020, 49, 4774–4784 RSC.
- J. Guo, W. Zhang, I. I. Oleynik, G. A. Solan, I. V. Oleynik, T. Liang and W.-H. Sun, Dalton Trans., 2020, 49, 136–146 RSC.
- L. Guo, W. Zhang, F. Cao, Y. Jiang, R. Zhang, Y. Ma, G. A. Solan, Y. Sun and W.-H. Sun, Polym. Chem., 2021, 12, 4214–4225 RSC.
- M. Liu, S. Jiang, Y. Ma, G. A. Solan, Y. Sun and W.-H. Sun, Organometallics, 2022, 41, 3237–3248 CrossRef CAS.
- A. W. Addison, T. N. Rao, J. Reedijk, J. Von Rijn and G. C. Verschoor, J. Chem. Soc., Dalton Trans., 1984, 1349 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2231616 (Fe2) and 2231617 (Fe5). For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2nj06212g |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2023 |