Nanoscale pulverization effect in double-layered MOF-derived hierarchical G/Co@C composites for boosting electromagnetic wave dissipation†
Guanjie
Zeng
ac,
Xiaozhong
Huang
 bc,
Hailong
Hu
bc,
Hailong
Hu
 d,
Jianling
Yue
bc,
Yu
Liu
d,
Jianling
Yue
bc,
Yu
Liu
 bc,
Benhui
Fan
e,
Jia
Huang
*d and
Xiu-Zhi
Tang
bc,
Benhui
Fan
e,
Jia
Huang
*d and
Xiu-Zhi
Tang
 *d
*d
aSchool of Physics and Electronics, Central South University, Changsha, Hunan 410083, China
bState Key Laboratory of Powder Metallurgy, Central South University, Changsha, Hunan 410083, China
cHunan Key Laboratory of Advanced Fibers and Composites, Central South University, Changsha, Hunan 410083, China
dResearch Institute of Aerospace Technology, Central South University, Changsha, Hunan 410083, China. E-mail: huangjia2019@csu.edu.cn; xztang@csu.edu.cn; Fax: +731-88837927; Fax: +731-88837927; Tel: +731-88837927 Tel: +731-88837927
eCerema, Equipe Recherche ENDSUM, 23 avenue Amiral Chauvin, 49136, Les Ponts de Cé, France
First published on 22nd November 2022
Abstract
Metal–organic frameworks (MOFs) have drawn a lot of interest as prospective starting points for highly effective electromagnetic wave (EMW) absorbers. However, the inevitable shrinkage and probable densification that occur during pyrolysis significantly reduce the microwave-loss capacity. A dual-layer MOF, ZIF-8@ZIF-67, is created and effectively decorated on graphene sheets as a solution to this problem. The shrinkage and densification were then suppressed by the subsequent pulverization effect between the two MOFs. Due to suitable compositions and specialized microstructures, G/Co@C exhibits excellent impedance matching and dissipates EMW by combining magnetic and dielectric loss. The maximum reflection loss of G/Co@C-7/paraffin is −55.0 dB at 5.8 GHz with just 7% filler. Therefore, the preparation of high-efficiency MOF-derived microwave absorbers by the pulverization effect is demonstrated to be an efficient strategy.
Introduction
Electromagnetic wave (EMW) absorbing materials can convert EMW energy into heat, and accordingly, they are frequently employed to decrease electromagnetic radiation pollution.1,2 According to the mechanism of electromagnetic wave attenuation, typical absorbing materials can be categorized into three categories: conductive, dielectric, and magnetic.3–5 Nevertheless, it can be tough for a single-component material to satisfy all requirements for practical applications due to the mismatched impedance of conductive materials, the low attenuation capability of electromagnetic waves of dielectric materials, and the commonly high density of magnetic materials.6 Accordingly, the four main indicators for the next-generation absorbing material, including thin thickness, broad absorption bandwidth, lightweight, and strong absorption ability, were proposed by many researchers.7,8 To this end, there is an urgent need and a big challenge to develop high-performance and lightweight EMW-absorbing materials.An attractive lightweight absorber usually possesses a hierarchical porous structure and elaborately designed heterogeneous interfaces via structure optimization and ingredient regulation.9 Thus, developing EMW absorbers with multiple absorbing mechanisms has become an effective and widely recognized strategy.10–12 Because of this, metal–organic frameworks (MOFs), a form of porous material with flexible compositions, large specific surface areas, and designable structural features, have exhibited great advantages in fabricating high-performance EMW absorbers.13
Specifically, MOF materials with distinct structures and versatile chemical functionality can be synthesized by merging varied organic ligands and metal atoms via a facile wet chemical process. Unfortunately, raw MOFs are neither magnetic nor electrically conductive, and they must be carbonized or combined with other materials to absorb electromagnetic waves.14–16 Qiao et al. reported non-magnetic bimetallic MOF-derived porous composites which exhibited a minimum RL of −67.8 dB (2.16 mm, 13.0 GHz) and a maximum effective absorption bandwidth of 5.9 GHz.14 Wang et al. prepared a Ni–Co bimetallic hierarchical CNT/CoO/Ni2O3 composite by incorporating NiCo-MOF into a CNT matrix. Due to the integrity of CNT-based conductive network structures, as-prepared samples possess a maximum absorption of −49.6 dB at 9.47 GHz with a thickness of 2.5 mm while the EAB is 3.2 GHz.15 Jin et al. prepared a thickness-controllable MOF-derived Ni@N-doped carbon nanoflake, which achieved a wide effective absorption bandwidth of 6.21 GHz, ranging from 11.79 to 18.00 GHz with a thickness of 2.3 mm.16
By altering the thermal-treatment conditions, the magnetic metal ions in the MOF can convert from organometallic complexes to metal/C or metal oxide/C hybrids, thereby enhancing the electrical conductivity and magnetic permeability while generating a range of heterointerfaces. Moreover, the in situ-formed carbon can effectively inhibit the aggregation or etching of magnetic metal/metal oxide nanoparticles, which is beneficial for the stability of magnetic loss. In addition, the incorporation of MOF-derived particles into additional substances can not only adjust the dielectric loss of the composite but also connect these particles to form an integrated high-performance EMW absorber.17–19 Among those optimal substrates for carrying an MOF-derived carbide, reduced graphene oxide (RGO) is particularly suitable due to its exceptional electrical conductivity, large specific surface area, high aspect ratio, and excellent mechanical properties.20,21 On the one hand, many functional groups on the surface of graphene oxide can coordinate metal ions and provide abundant nucleation sites for forming MOFs, boosting the interaction between RGO and MOF-derived materials in the final composite products. On the other hand, incorporating RGO can expand the composite's overall conductive network while avoiding the aggregation of MOF-derived particles. Notably, coupling MOF-derived particles and RGO will generate multiple heterointerfaces, increasing the wave-absorption capacities of RGO/MOF-derived composites.22,23 Nevertheless, the carbonization of MOFs would cause a severe collapse of the porous structure, leading to a drastically reduced specific surface area and a significant reduction in interfacial polarization.
To address this problem, the methods adopted by most researchers primarily focus on creating a hollow structure to suppress densification and excessive shrinking.24–26 Kang et al. implanted MOF-derived core–shell Co/NPC@ZnO particles onto rGO nanosheets to construct 3D porous composites. The as-prepared composites were subsequently mixed with paraffin at 30 wt% and displayed an optimal RL of −45.4 dB and an EAB of 5.4 GHz, when the sample is 2 mm in thickness.24 Zhao et al. constructed a hollow granatohedron structure by combining N-doped carbon/CoNi polyhedra and rGO. When the filler loading is maintained at 30% and the thickness is 2.5 mm, the minimum RL and EAB reach up to −58.2 dB (10.62 GHz) and 4.03 GHz (8.80–12.83 GHz), respectively.25 Qiu et al. prepared a porous hollow Ni/C microsphere by in situ pyrolysis of a Ni-MOF. Benefiting from the hollow structure and the synergistic effect between carbon and nickel nanoparticles, the composite exhibits a substantially enhanced EMW absorption performance with a maximum RL of −57.25 dB at 16.1 GHz when the thickness is 1.8 mm, and the corresponding EAB is 5.1 GHz.26 On the other hand, in situ pulverized MOF loading on the support during pyrolysis is expected to improve absorptive performances because of the abundant interfaces formed. By generating a double-layer MOF structure, nanoscale pulverization of MOF particles during thermal breakdown might prevent the decrease in specific surface area induced by the intensification of MOF materials during pyrolysis. Despite this, there are a few published accounts of bilayer MOF-derived composite absorbers with densification-inhibiting properties.
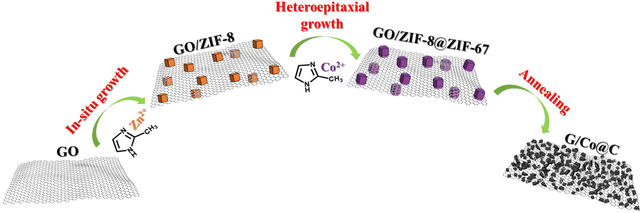
In this study, bilayer MOFs were decorated onto graphene sheets, and high temperature-induced thermal stress variations were subsequently utilized to pulverize the bilayer particle structure. The final porous composites consist of nanoscale cobalt/carbon nanoparticles assembled on RGO sheets. To be specific, we develop a bilayer MOF structure by first decorating ZIF-8 on GO and then in situ synthesizing ZIF-67 on the ZIF-8 surface. When the pyrolysis temperature exceeds 900 °C, ZIF-8@ZIF-67 carbonized and GO sheets were reduced simultaneously. During this process, ZIF-8 functions as the inner support to prevent the structural collapse of the outer ZIF-67 successfully, and the Zn vapor that stems from the disintegration of ZIF-8 promotes further nanocrystallization of ZIF-67-converted Co@C, resulting in a nanoscale pulverization effect that is bound by RGO sheets. Due to careful compositional adjustment and ingenious structural design, the final G/Co@C has achieved excellent impedance matching and high-performance microwave absorption.
2. Experimental section
2.1. Preparation of GO/ZIF-8 templates
Graphene oxide (GO) was manufactured according to our earlier work.27 First, 90 mg of GO was sonicated for one hour in 20 mL of deionized water. The GO suspension was then mixed with 326 mg of Zn(NO3)2·6H2O and 10 mg of cetyltrimethylammonium bromide (CTAB). After 50 minutes of agitation, 80 mL of DI water containing 5.65 g of 2-MeIm was added to the above mixtures with continuous stirring. After 0.5 h, the resulting precipitates were washed with C2H5OH four times. Finally, the freeze-drying method was employed to obtain the GO/ZIF-8 powder.2.2 Synthesis of GO/ZIF-8@ZIF-67 precursors
100 mg of GO/ZIF-8 powders was first dispersed in 23 mL of methanol and then reacted with the mixture of 190 mg of CoCl2·6H2O and 3 mg of CTAB by ultrasonication. Subsequently, a 3 mL methanol solution containing 895 mg of 2-MeIn was dropwise added before sealing in an autoclave. After that, the hydrothermal reaction was conducted at 90 °C for 12 h. Then, the GO/ZIF-8@ZIF-67 powder was obtained by centrifugation, washing, and freeze-drying. As a comparison, 100 mg of GO was substituted for GO/ZIF-8, and the above operation was repeated to obtain a sample of GO/ZIF-67.2.3 Fabrication of G/Co@C composites
G/Co@C composites were prepared by a pyrolysis process. Under continuous N2 flow, the precursor GO/ZIF-8@ZIF-67 was heated up to 900 °C for 6 h. The final product G/Co@C composites were collected at room temperature.The same operation was carried out for GO/ZIF-67, and the product obtained was named S-G/Co@C.
2.4 Characterization
Basic characterization methods, such as scanning electron microscopy (SEM, TESCAN MIRA3 LMU), transmission electron microscopy (TEM, EOL JEM-2100F), X-ray diffraction (XRD, X'Pert PRO MPD), X-ray photoelectron spectroscopy (XPS, ESCALAB 250Xi) and Raman spectroscopy (LabRam HR Evolution), have been carried out to reveal the morphology, elemental compositions, and crystal structure of the samples, respectively. The coaxial method was employed to measure the electromagnetic parameters of specimens in 2–18 GHz using a vector network analyzer (PNA-N5244A). Standard coaxial rings (ϕout = 7.00 mm, ϕin = 3.04 mm) were prepared by mixing 5, 7, and 10 wt% G/Co@C and 7% S-G/Co@C with wax uniformly and named G/Co@C-5, G/Co@C-7, G/Co@C-10 and S-G/Co@C respectively.3. Results and discussion
3.1 Structural characterization
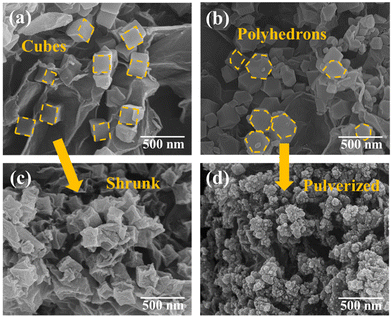
The fabrication process of G/Co@C is shown in Fig. 1. When Zn(NO3)2·6H2O was blended with the GO aqueous dispersion, the positive Zn2+ ions were electrostatically absorbed onto negative GO sheets. After adding the 2-MeIm solution, ZIF-8 nano-cubes formed on both sides of GO sheets via the in situ coordination reaction between 2-MeIm and Zn2+. Afterward, the as-obtained GO/ZIF-8 functioned as crystal seeds for the following epitaxial growth of ZIF-67 in the presence of Co2+ and 2-MeIm aqueous solution. Through a solvothermal reaction at 90 °C for 12 h, GO/ZIF-8@ZIF-67, composed of polyhedral ZIF-8@ZIF-67 crystals with a core–shell feature uniformly anchored on both sides of the GO sheet, was obtained. Subsequently, the G/Co@C composites were prepared after carbonization treatment of GO/ZIF-8@ZIF-67 under an inert atmosphere at 900 °C for 6 hours. During the carbonizing process, Zn would be restored from ZIF-8, then vaporized and escaped. Particle-like Co@C eventually formed on RGO substrates.Fig. 2 shows the morphology of various products. From the SEM image of GO/ZIF-67 shown in Fig. 2a, the ZIF-67 nanocrystals with an average size of 200 nm are embedded within the wrinkled GO sheets, implying the effective interaction between the oxygen-containing groups from the GO and the metal (Co) ions during the formation of ZIF-67. GO/ZIF-8 displayed a similar morphology to GO/ZIF-67 (Fig. S1†). During the epitaxial growth of ZIF-67 on ZIF-8, the nano-cubic ZIF-8 is converted to the polyhedral ZIF-8@ZIF-67 nanocrystal (Fig. 2b). After pyrolysis at 900 °C under N2, the morphology of GO/ZIF-67 was almost maintained in its pyrolyzed product S-G/Co@C, but the surface of the Co/C cube became concave as a result of severe shrinkage (Fig. 2c). In contrast, many nanoscale particles were observed in the GO/ZIF-8@ZIF-67 pyrolysis product G/Co@C (Fig. 2d). The nanoscale pulverization of ZIF-8@ZIF-67 nanocrystals was attributed to the decomposition of MOFs and the loss of Zn ions in ZIF-8 during high-temperature pyrolysis. The BET test was subsequently used to determine the precise surface areas of the objects. As shown in Fig. S2,† the characteristic type-IV nitrogen adsorption–desorption isotherm was seen in two samples suggesting the co-presence of micropores and mesopores. The Brunauer–Emmett–Teller (BET) surface area of G/Co@C was calculated to be 333.526 m2 g−1, larger than 228.052 m2 g−1 of S-G/Co@C, demonstrating that the pulverization effect can increase the specific surface area of the composite.
Furthermore, the microstructure of G/Co@C was investigated by TEM and HRTEM. In agreement with the SEM findings, many particles with a size of 10 to 80 nm are well attached to the surface RGO nanosheets (Fig. 3a). The corresponding elemental mapping analysis indicated the coexistence and the homogeneous dispersion of Co, N, and C elements within the hybrids (Fig. 3b–d). The wrinkled graphene sheets imply a well-exfoliated effect, while the uniformly dispersed nanoparticle demonstrated an RGO nanosheet ideal substrate. The details of the particles shown in the HRTEM image indicate the typical lattice spacing of 0.205 nm ascribed to Co (1 1 1) and the highly crystalline Co encapsulated by amorphous crystalline carbon (Fig. 3e). Zn ions in ZIF-8 were transformed into Zn vapor and released during the high-temperature pyrolysis process, and the N element doped in G/Co@C came from the organic ligand of ZIF-8@ZIF-67. Notably, the nitrogen-doping process and pulverization effect can bring about numerous interfaces and defects, thereby triggering more polarization of electromagnetic waves.16,21
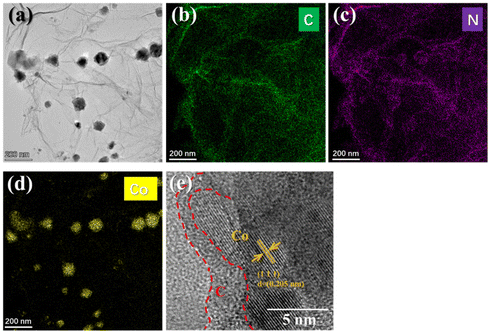 | ||
| Fig. 3 TEM image of G/Co@C (a) and the corresponding C (b), N (c), and Co (d) elemental mapping. (e) High-resolution TEM images of Co@C particle on graphene. | ||
The crystal structure and the reduction state of the samples were identified by XRD and Raman spectroscopy. As shown in Fig. 4a, before pyrolysis, the XRD patterns for the precursors GO/ZIF-8 and GO/ZIF-8@ZIF-67 showed a similar ZIF-8-type phase. After pyrolysis, two diffraction peaks, 44.2 and 51.5°, emerged in the XRD curve of G/Co@C, corresponding to two lattice planes of cobalt, which proved the presence of the Co nanoparticle.28Fig. 4b shows the Raman spectra of G/Co@C and its precursor. Two typical peaks, ∼1346 and ∼1583 cm−1, corresponding to the D and G bands of carbon-based materials, appeared in the spectra. The ID/IG of GO/ZIF-8@ZIF-67 (1.02) is smaller than that of G/Co@C (1.05), indicating that more defects were generated in G/Co@C during the annealing process.29
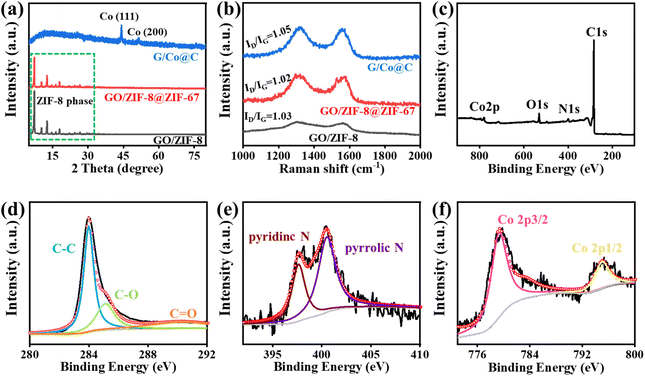 | ||
| Fig. 4 Basic characterization of GO/ZIF-8, GO/ZIF-8@ZIF-67, and G/Co@C: (a) XRD and (b) Raman spectrum; (c–f) XPS spectra of G/Co@C. | ||
Fig. 4c shows the XPS curve to further analyze the chemical states and elemental composition of G/Co@C. The broad scan spectrum of G/Co@C possesses four peaks corresponding to four elements, C, N, O, and Co, respectively. It further proved the disappearance of the Zn element in G/Co@C. In the C 1s spectrum, the broad peak can be fitted to C−C (∼284.8 eV), C–O (∼286.0 eV), and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (∼288.0 eV).30 The characteristic peak intensity of oxygen-containing bands was weak because of the reduction of GO. The N 1s spectrum was split into two peaks located around 398.7 eV and 400.0 eV, which can be assigned to pyridinic N and pyrrolic N respectively, which further proves N-doping in the composites.31 The Co 2p spectrum was fitted by two peaks, Co 2p3/2 (782.8 eV) and Co 2p1/2 (796.8 eV).32 These results indicate that G/Co@C consists of partially doped carbon and magnetic metal, an advantage for enhancing conductive loss and magnetic loss. Furthermore, the deficiencies and the rich interface of the hierarchical structure in G/Co@C can contribute to dielectric losses.
O (∼288.0 eV).30 The characteristic peak intensity of oxygen-containing bands was weak because of the reduction of GO. The N 1s spectrum was split into two peaks located around 398.7 eV and 400.0 eV, which can be assigned to pyridinic N and pyrrolic N respectively, which further proves N-doping in the composites.31 The Co 2p spectrum was fitted by two peaks, Co 2p3/2 (782.8 eV) and Co 2p1/2 (796.8 eV).32 These results indicate that G/Co@C consists of partially doped carbon and magnetic metal, an advantage for enhancing conductive loss and magnetic loss. Furthermore, the deficiencies and the rich interface of the hierarchical structure in G/Co@C can contribute to dielectric losses.
3.2 EMW absorption performance
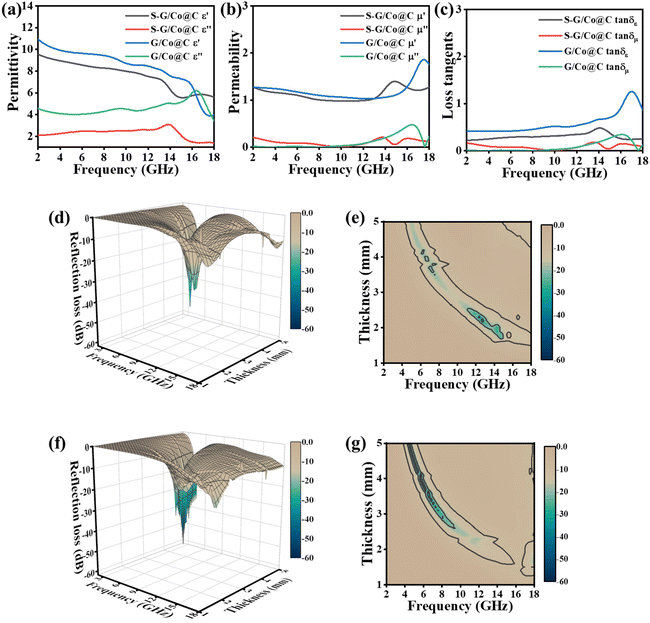
It is known that the complex permittivity (εr = ε′ − jε′′), the relative complex permeability (μr = μ′ − jμ′′), and the excellent impedance matching are essential parameters that determine the absorbing performance of a material. Their real parts represent the ability of the material to store electrical (ε′) and magnetic energies (μ′). In contrast, the imaginary parts correspond to the dissipation capability of electromagnetic wave energy within the medium. We measured the frequency-dependent electromagnetic parameters of S-G/Co@C and G/Co@C with 7 wt% filler loading to investigate the associated microwave absorption mechanism. As shown in Fig. 5a, the ε′ values for two absorbers decrease with increasing frequency, which is ascribed to the dispersion effect of frequency. The ε′ values of G/Co@C are in the range of 10.95–3.95 over 2–18 GHz, larger than those of S-G/Co@C (in the range of 9.21–5.29). The ε′′ values for G/Co@C and S-G/Co@C are in the ranges of 6.31–3.33 and 3.08–1.19, respectively. Thus, the dielectric loss of G/Co@C is significantly improved in comparison with S-G/Co@C, which is mainly due to the improved surface area and the large number of well-defined interfaces between the graphene sheets and the Go@C nano-particles. Besides, the fluctuations of complex permittivity of samples can be explained by the existence of dipole or interfacial polarization.16,20The permeability is shown in Fig. 5b, and the μ′ curves suggest that G/Co@C owns larger μ′ values than the other, attributed to its stronger magnetism. The peak after 15.00 GHz in the μ′ curve of G/Co@C and the peak between 13.00 and 17.00 GHz in the μ′ curve of S-G/Co@C could be attributed to the natural resonance. Concerning the imaginary part μ′′, two samples display similar average values with the presence of several peaks in the measured frequency. The prominent resonance peaks after 10.00 GHz might be attributed to the minor size effect, surface effects and spin-wave excitation-induced exchange resonance between magnetic nanoparticles. Based on the basic electromagnetic theory, the tangent value of permittivity (tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δε) and permeability (tan
δε) and permeability (tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δμ) can estimate the dielectric loss capacity and the magnetic loss capacity, respectively. From Fig. 5c, the tan
δμ) can estimate the dielectric loss capacity and the magnetic loss capacity, respectively. From Fig. 5c, the tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δε and tan
δε and tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δμ for G/Co@C were significantly higher than those for S-G/Co@C, indicating that G/Co@C possesses better dielectric loss capacity and magnetic loss capacity. Moreover, the larger tan
δμ for G/Co@C were significantly higher than those for S-G/Co@C, indicating that G/Co@C possesses better dielectric loss capacity and magnetic loss capacity. Moreover, the larger tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δε than tan
δε than tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δμ over the whole frequency range for the two samples suggests the dielectric loss dominant dissipation mechanism.
δμ over the whole frequency range for the two samples suggests the dielectric loss dominant dissipation mechanism.
The estimated RL is typically employed to evaluate the performance of EMW absorbers based on the measured electromagnetic characteristics and transmission line theory. As depicted in Fig. 5d and f, the three-dimensional representation of RL values vs. frequency was plotted while the thickness of the samples varied between 1.0 and 5.0 mm. It is noted that the green areas of G/Co@C are more significant than those of S-G/Co@C, indicating that G/Co@C has a greater reflection loss. At 5.8 GHz, when the thickness of G/Co@C is 3.9 mm, a minimal reflection loss of −55.0 dB is achieved. In addition, the 2D contour plots (Fig. 5e and g) reveal that the EAB of the two samples (−10 dB) exceeds 13.5 GHz from 4 to 18 GHz. The results confirm that the hierarchical G/Co@C composites exhibit superior EMW absorption capability.
Furthermore, the electromagnetic parameters of G/Co@C with different filler contents were analyzed. Fig. S2† shows the frequency dependence of complex relative permittivity and permeability with filler loadings from 5 to 10 wt%.
From Fig. S2a and b,† it could be found that the values of ε′ and ε′′ for G/Co@C increased as the filler contents increased when the content of G/Co@C exceeded 7 wt%, and both ε′ and ε′′ values presented a decreasing tendency in high frequency. Due to the enhanced dc conductivity, the conductivity loss makes the main attenuation contribution and the polarization relaxation process would be hidden. Thus, the absence of the relaxation peak could be attributed to the decreased interfacial polarization in the system. In addition, the average values of tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δε for G/Co@C-7 and G/Co@C-10 were slightly higher than those of G/Co@C-5 (Fig. S2e†).
δε for G/Co@C-7 and G/Co@C-10 were slightly higher than those of G/Co@C-5 (Fig. S2e†).
Based on the Debye theory, the dielectric behaviors of composites could be explained based on the Cole–Cole model, and each semicircle represents one relaxation process.33 Fig. S2g† shows the Cole–Cole curves of composites with different loading levels of G/Co@C from 5 wt% to 10 wt%. Several semicircles on the Cole–Cole curves of G/Co@C-5 and G/Co@C-7 implied that the interfacial polarization relaxation processes mainly ascribed to the relaxation processes. These relaxations should be attributed to the defects and abundant heterogeneous interfaces in G/Co@C, and when the content of G/Co@C was 10 wt%, the Cole–Cole plots present fewer semicircles and extend to a straight line. This means that the conductivity loss contributes to the imaginary permittivity when the mass fraction of G/Co@C is high enough. The polarization relaxation process would be hidden due to the enhanced electrical conductivity. The μ′ and μ′′ values of G/Co@C-5, G/Co@C-7, and G/Co@C-10 gradually increased as the amount of Co increased (Fig. S2c and d†). Simultaneously, G/Co@C-7 and G/Co@C-10 had an optimized magnetic loss capacity because of the higher tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δμ value (Fig. S2f†).
δμ value (Fig. S2f†).
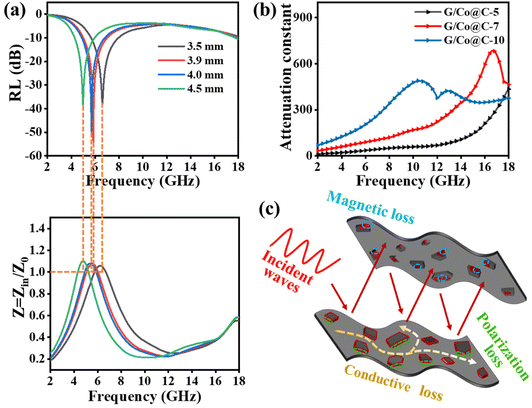
We investigated the filler-dependent microwave absorption properties of G/Co@C. Fig. 6 illustrates 3D representations of RL values and associated 2D contour plots for G/Co@C with varying filler amounts. Fig. 6a and b show that the maximum RL of G/Co@C-5 was −29.3 dB at 16.2 GHz with a sample thickness of 1.0 mm, and the EAB spanned largely between 12 and 18 GHz. As the G/Co@C content grew to 7 wt%, the maximum RL value reached −55.0 dB at 5.8 GHz with a sample thickness of 3.9 mm, while the largest EAB was obtained at 3.7 GHz, which corresponds to 8.3–12 GHz with a sample thickness of 2.5 mm (Fig. 5f). Fig. 5g indicates that upon varying the thickness of the sample from 1 to 5 mm, the EAB of G/Co@C-7 may cover the frequency range of 2 to 18 GHz. In addition, the greatest RL values were reduced to −16 dB (Fig. 6c) and the EAB coverage decreased as the G/Co@C content increased to 10 wt% (Fig. 6d).
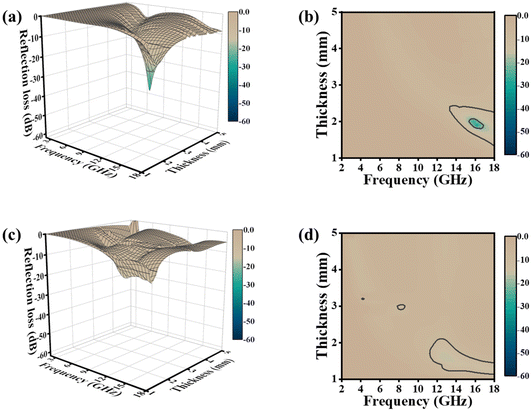 | ||
| Fig. 6 3D representations of RL values and the corresponding 2D contour plots for G/Co@C with filler loadings ranging from 5 wt% (a and b) to 10 wt% (c and d). | ||
Due to weak conductive loss and polarization ability, it is difficult to present good performance on the EM absorption properties at a low filler content. But when the filler loading is as high as 10.0 wt%, the electromagnetic wave absorption also performs poorly because the inappropriate permittivity destroys the necessary impedance matching and results in strong EM reflection at the sample surface. Thus, simply increasing the content of G/Co@C cannot improve the EMW absorbing performance. As an optimized result, the RL of G/Co@C-5 exhibits a satisfying electromagnetic absorption capacity.
Alternatively, good impedance matching is the basis of excellent EMW absorption of the materials because most electromagnetic waves can transmit into the absorber.34,35 Thus, in this part, we will further investigate the impedance matching of G/Co@C-7, according to the following equation:
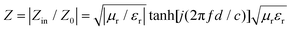 | (1) |
As shown in Fig. 7a, for the RL curves of G/Co@C-7 samples with different thicknesses (3.5, 3.9, 4.0, and 4.5 mm), the impedance in the absorption peak was very close to 1, which was one of the prerequisites for an excellent absorber. In addition, besides the impedance, the microwave attenuation ability of the absorber is viewed as another important parameter for characterizing the inherent ability to dissipate electromagnetic waves. The calculation of the attenuation constant (α) is as follows:36,37
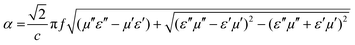 | (2) |
Benefitting from the reasonable composition and structural design, the loss coefficient of G/Co@C was high and increased with increasing frequency, as shown in Fig. 7b. Compared with G/Co@C-5 and G/Co@C-7, G/Co@C-10 possesses a higher loss coefficient and poorer EMW absorption capability, so it can be concluded that whether the EMW absorption performance of G/Co@C is exceptional or not is mainly dependent on the impedance matching. According to the above analysis and discussion, Fig. 7c shows the reasonable absorption mechanism of G/Co@C. Based on the composition and microstructure, G/Co@C exhibited good impedance matching; consequently, most EMW could enter the absorber. The EMW energy was consumed and converted into heat energy through dielectric loss and magnetic loss. Generally, dielectric loss includes conductive loss and polarization loss. In our case, the dielectric loss should be contributed by the conductive network of carbon particles, defects, the doping of the N element and the interfacial regions in G/Co@C. The magnetic loss in our case should come from the effect of Co nanoparticles. Furthermore, the RGO sheets loaded with Co@C nanoparticles would cause multiple reflections to the EMW, further enhancing the microwave attenuation efficiency.
Table 1 shows the ZIF-67-derived absorbers and their corresponding EM wave absorbing performance reported in the recent literature. Compared with most of the reported absorbents, our Co@C/RGO hybrids compete well with composites with much higher filler loadings.
| Sample | Filling ratio (%) | EAB (GHz) | Matching thickness (mm) | RLmin (dB) | Ref. |
|---|---|---|---|---|---|
| Co/C | 30 | 3.8 | 2.0 | −32.4 | 38 |
| H-Co/C | 10 | 4.6 | 2.9 | −50.7 | 28 |
| CoC-rGO | 10 | 5.2 | 2.1 | −44.77 | 39 |
| CN/C/Co-300 | 15 | 5.42 | 4.8 | −51.42 | 40 |
| HCF@NC/Co | 14 | 7.36 | 2.25 | −50.14 | 41 |
| CF@C/Co | 20 | 6.25 | 1.71 | −71.95 | 42 |
| HCNP | 35 | 4.16 | 2.0 | −41.08 | 43 |
| CPT-1-Co | 30 | 5.4 | 1.7 | −15.7 | 44 |
| HP-Co/C | 10 | 4.8 | 2.4 | −47.2 | 45 |
| G/Co@C | 7 | 3.7 | 3.9 | −55.0 | This work |
4. Conclusion
In this work, G/Co@C with excellent broadband microwave absorption properties has been obtained by implanting the Co@C adhesion on RGO. The hybrids of Co@C can effectively adjust the dielectric permittivity to achieve good impedance matching. Meanwhile, the magnetic Co particles can introduce magnetic loss that further enhances the dissipation capacity of the EMW. In addition, the dispersed hybrid particles can further improve the absorbing performance by complex interfacial scattering, which consumes the energy of EMW. Therefore, the composite based on G/Co@C-7 can reach a maximum RL of −55.0 dB at 5.8 GHz. The results of this study indicated that G/Co@C could be an ideal EMW absorber because of its lightweight, cost-effectiveness, and high efficiency.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This research was financially supported by the Natural Science Foundation of Hunan Province, China (No. 2020JJ4726).References
- Y. Li, X. Liu, X. Nie, W. Yang, Y. Wang, R. Yu and J. Shui, Microwave Absorbing Materials: Multifunctional Organic-Inorganic Hybrid Aerogel for Self-Cleaning, Heat-Insulating, and Highly Efficient Microwave Absorbing Material, Adv. Funct. Mater., 2019, 29(10), 1970059 CrossRef.
- X. Zhang, J. Zhu, P. Yin, A. Guo, A. Huang, L. Guo and G. Wang, Tunable High-Performance Microwave Absorption of Co1−xS Hollow Spheres Constructed by Nanosheets within Ultralow Filler Loading, Adv. Funct. Mater., 2018, 28(49), 1800761 CrossRef.
- A. Houbi, Z. A. Aldashevich, Y. Atassi, Z. B. Telmanovna, M. Saule and K. Kubanych, Microwave absorbing properties of ferrites and their composites: A review, J. Magn. Magn. Mater., 2021, 529, 167839 CrossRef CAS.
- S. Ren, H. Yu, L. Wang, Z. Huang, T. Lin, Y. Huang, J. Yang, Y. Hong and J. Liu, State of the Art and Prospects in Metal-Organic Framework-Derived Microwave Absorption Materials, Nano-Micro Lett., 2022, 14(1), 68 CrossRef CAS PubMed.
- D. Zhi, T. Li, J. Li, H. Ren and F. Meng, A review of three-dimensional graphene-based aerogels: Synthesis, structure and application for microwave absorption, Composites, Part B, 2021, 211, 108642 CrossRef CAS.
- X. Zeng, X. Cheng, R. Yu and G. D. Stucky, Electromagnetic microwave absorption theory and recent achievements in microwave absorbers, Carbon, 2020, 168, 606–623 CrossRef CAS.
- X. Qian, Y. Zhang, Z. Wu, R. Zhang, X. Li, M. Wang and R. Che, Multi-Path Electron Transfer in 1D Double-Shelled Sn@Mo2 C/C Tubes with Enhanced Dielectric Loss for Boosting Microwave Absorption Performance, Small, 2021, e2100283 CrossRef PubMed.
- Z. Zhang, H. Zhao, W. Gu, L. Yang and B. Zhang, A biomass-derived porous carbon for broadband and lightweight microwave absorption, Sci. Rep., 2019, 9(1), 18617 CrossRef CAS PubMed.
- H. Rong, T. Gao, Y. Zhang, X. Liu, X. Zhang and M. Yan, Carbonized fibers with multi-elemental doping and hollow architecture derived from natural cotton for tunable microwave absorption properties, J. Alloys Compd., 2021, 884, 161084 CrossRef CAS.
- S. Ayub, B. H. Guan, F. Ahmad, Y. A. Oluwatobi, Z. U. Nisa, M. F. Javed and A. Mosavi, Graphene and Iron Reinforced Polymer Composite Electromagnetic Shielding Applications: A Review, Polymers, 2021, 13(15), 2580 CrossRef CAS PubMed.
- C. Wang, L. Zong, Y. Pan, N. Li, Q. Liu, J. Wang and X. Jian, Preparation and characterization of branch-like heteroatoms-doped Ni@C nanofibers for high-performance microwave absorption with thin thickness, Composites, Part B, 2021, 223, 109114 CrossRef CAS.
- S. Zhang, B. Cheng, Z. Gao, D. Lan, Z. Zhao, F. Wei, Q. Zhu, X. Lu and G. Wu, Two-dimensional nanomaterials for high-efficiency electromagnetic wave absorption: An overview of recent advances and prospects, J. Alloys Compd., 2022, 893, 162343 CrossRef CAS.
- U. Ryu, S. Jee, P. C. Rao, J. Shin, C. Ko, M. Yoon, K. S. Park and K. M. Choi, Recent advances in process engineering and upcoming applications of metal-organic frameworks, Coord. Chem. Rev., 2021, 426, 213544 CrossRef CAS PubMed.
- J. Qiao, X. Zhang, C. Liu, L. Lyu, Y. Yang, Z. Wang, L. Wu, W. Liu, F. Wang and J. Liu, Non-Magnetic Bimetallic MOF-Derived Porous Carbon-Wrapped TiO2/ZrTiO4 Composites for Efficient Electromagnetic Wave Absorption, Nano-Micro Lett., 2021, 13(1), 75 CrossRef PubMed.
- J. Wang, J. Yang, J. Yang and H. Zhang, An Ni-Co bimetallic MOF-derived hierarchical CNT/CoO/Ni2O3 composite for electromagnetic wave absorption, J. Alloys Compd., 2021, 876, 160126 CrossRef CAS.
- L. Jin, P. Yi, L. Wan, J. Hou, P. Chen, J. Zu, B. Wei, Z. Yao and J. Zhou, Thickness-controllable synthesis of MOF-derived Ni@N-doped carbon hexagonal nanoflakes with dielectric-magnetic synergy toward wideband electromagnetic wave absorption, Chem. Eng. J., 2022, 427, 130940 CrossRef CAS.
- F. Chen, S. Zhang, B. Ma, Y. Xiong, H. Luo, Y. Cheng, X. Li, X. Wang and R. Gong, Bimetallic CoFe-MOF@Ti3C2Tx MXene derived composites for broadband microwave absorption, Chem. Eng. J., 2022, 431, 134007 CrossRef CAS.
- J. Yuan, Q. Liu, S. Li, Y. Lu, S. Jin, K. Li, H. Chen and H. Zhang, Metal-organic framework (MOF)-derived carbonaceous Co3O4/Co microframes anchored on RGO with enhanced electromagnetic wave absorption performances, Synth. Met., 2017, 228, 32–40 CrossRef CAS.
- Y. Zhao, W. Wang, J. Wang, J. Zhai, X. Lei, W. Zhao, J. Li, H. Yang, J. Tian and J. Yan, Constructing multiple heterogeneous interfaces in the composite of bimetallic MOF-derivatives and rGO for excellent microwave absorption performance, Carbon, 2021, 173, 1059–1072 CrossRef CAS.
- X. Zhang, S. Zhang, K. Zhang, F. Yan, C. Zhu, H. Yuan, X. Zhang and Y. Chen, Interface-induced enhanced electromagnetic wave absorption property of metal-organic frameworks wrapped by graphene sheets, J. Alloys Compd., 2019, 780, 718–726 CrossRef CAS.
- R. Shu, J. Zhang, C. Guo, Y. Wu, Z. Wan, J. Shi, Y. Liu and M. Zheng, Facile synthesis of nitrogen-doped reduced graphene oxide/nickel-zinc ferrite composites as high-performance microwave absorbers in the X-band, Chem. Eng. J., 2020, 384, 123266 CrossRef CAS.
- X. Huang, J. Wei, Y. Zhang, B. Qian, Q. Jia, J. Liu, X. Zhao and G. Shao, Ultralight Magnetic and Dielectric Aerogels Achieved by Metal-Organic Framework Initiated Gelation of Graphene Oxide for Enhanced Microwave Absorption, Nano-Micro Lett., 2022, 14(1), 107 CrossRef CAS PubMed.
- S. Song, A. Zhang, L. Chen, Q. Jia, C. Zhou, J. Liu and X. Wang, A novel multi-cavity structured MOF derivative/porous graphene hybrid for high performance microwave absorption, Carbon, 2021, 176, 279–289 CrossRef CAS.
- S. Kang, W. Zhang, Z. Hu, J. Yu, Y. Wang and J. Zhu, Porous core-shell zeolitic imidazolate framework-derived Co/NPC@ZnO-decorated reduced graphene oxide for lightweight and broadband electromagnetic wave absorber, J. Alloys Compd., 2020, 818, 152932 CrossRef CAS.
- Y. Zhao, W. Wang, J. Wang, J. Zhai, X. Lei, W. Zhao, J. Li, H. Yang, J. Tian and J. Yan, Constructing multiple heterogeneous interfaces in the composite of bimetallic MOF-derivatives and rGO for excellent microwave absorption performance, Carbon, 2021, 173, 1059–1072 CrossRef CAS.
- Y. Qiu, Y. Lin, H. Yang, L. Wang, M. Wang and B. Wen, Hollow Ni/C microspheres derived from Ni-metal organic framework for electromagnetic wave absorption, Chem. Eng. J., 2020, 383, 123207 CrossRef CAS.
- T. Guo, X. Chen, L. Su, C. Li, X. Huang and X.-Z. Tang, Stretched graphene nanosheets formed the “obstacle walls” in melamine sponge towards effective electromagnetic interference shielding applications, Mater. Des., 2019, 182, 108029 CrossRef CAS.
- L. Wang, X. Bai, B. Wen, Z. Du and Y. Lin, Honeycomb-like Co/C composites derived from hierarchically nanoporous ZIF-67 as a lightweight and highly efficient microwave absorber, Composites, Part B, 2019, 166, 464–471 CrossRef CAS.
- Y. Xiong, L. Xu, C. Yang, Q. Sun and X. Xu, Implanting FeCo/C nanocages with tunable electromagnetic parameters in anisotropic wood carbon aerogels for efficient microwave absorption, J. Mater. Chem. A, 2020, 8(36), 18863–18871 RSC.
- W. Feng, Y. Wang, J. Chen, L. Guo, J. Ouyang, D. Jia and Y. Zhou, Microwave absorbing property optimization of starlike ZnO/reduced graphene oxide doped by ZnO nanocrystal composites, Phys. Chem. Chem. Phys., 2017, 19(22), 14596–14605 RSC.
- J. Yan, Y. Huang, X. Han, X. Gao and P. Liu, Metal-organic framework (ZIF-67)-derived hollow CoS2/N-doped carbon nanotube composites for extraordinary electromagnetic wave absorption, Composites, Part B, 2019, 163, 67–76 CrossRef CAS.
- W. M. Zhang, Z. W. Yue, Q. M. Wang, X. X. Zeng, C. C. Fu, Q. Li, X. T. Li, L. D. Fang and L. Li, Carbon-encapsulated CoS2 nanoparticles anchored on N-doped carbon nanofibers derived from ZIF-8/ZIF-67 as anode for sodium-ion batteries, Chem. Eng. J., 2020, 380, 122548 CrossRef CAS.
- N. Yang, Z.-X. Luo, G.-R. Zhu, S.-C. Chen, X.-L. Wang, G. Wu and Y.-Z. Wang, Ultralight Three-Dimensional Hierarchical Cobalt Nanocrystals/N-Doped CNTs/Carbon Sponge Composites with a Hollow Skeleton toward Superior Microwave Absorption, ACS Appl. Mater. Interfaces, 2019, 11(39), 35987–35998 CrossRef CAS PubMed.
- L. Yan, C. Hong, B. Sun, G. Zhao, Y. Cheng, S. Dong, D. Zhang and X. Zhang, In Situ Growth of Core–Sheath Heterostructural SiC Nanowire Arrays on Carbon Fibers and Enhanced Electromagnetic Wave Absorption Performance, ACS Appl. Mater. Interfaces, 2017, 9(7), 6320–6331 CrossRef CAS PubMed.
- Y. Wang, C. Li, X. Han, D. Liu, H. Zhao, Z. Li, P. Xu and Y. Du, Ultrasmall Mo2C Nanoparticle-Decorated Carbon Polyhedrons for Enhanced Microwave Absorption, ACS Appl. Nano Mater., 2018, 1(9), 5366–5376 CrossRef CAS.
- Q. Li, Z. Zhang, L. Qi, Q. Liao, Z. Kang and Y. Zhang, Toward the Application of High-Frequency Electromagnetic Wave Absorption by Carbon Nanostructures, Adv. Sci., 2019, 6(8), 1801057 CrossRef PubMed.
- L. Shi, Y. Zhao, Y. Li, X. Han and T. Zhang, Octahedron Fe3O4 particles supported on 3D MWCNT/graphene foam: In-situ method and application as a comprehensive microwave absorption material, Appl. Surf. Sci., 2017, 416, 329–337 CrossRef CAS.
- R. Qiang, Y. Du, D. Chen, W. Ma, Y. Wang, P. Xu, J. Ma, H. Zhao and X. Han, Electromagnetic functionalized Co/C composites by in situ pyrolysis of metal-organic frameworks (ZIF-67), J. Alloys Compd., 2016, 681, 384–393 CrossRef CAS.
- H. Qiu, X. Zhu, P. Chen, S. Yang, X. Guo, J. Liu and X. Zhu, Magnetic Dodecahedral CoC-Decorated Reduced Graphene Oxide as Excellent Electromagnetic Wave Absorber, J. Electron. Mater., 2019, 49(2), 1204–1214 CrossRef.
- H. Jin, J. Wang, S. Yang, Q. Wu and B. Zhang, ZIF-67-derived micron-sized cobalt-doped porous carbon-based microwave absorbers with g-C3N4 as template, Ceram. Int., 2021, 47(8), 11506–11513 CrossRef CAS.
- Y. Guo, D. Wang, J. Wang, Y. Tian, H. Liu, C. Liu and C. Shen, Hierarchical HCF@NC/Co Derived from Hollow Loofah Fiber Anchored with Metal-Organic Frameworks for Highly Efficient Microwave Absorption, ACS Appl. Mater. Interfaces, 2022, 14(1), 2038–2050 CrossRef CAS PubMed.
- J. Tao, Z. Jiao, L. Xu, P. Yi, Z. Yao, F. Yang, C. Zhou, P. Chen, J. Zhou and Z. Li, Construction of MOF-Derived Co/C shell on carbon fiber surface to enhance multi-polarization effect towards efficient broadband electromagnetic wave absorption, Carbon, 2021, 184, 571–582 CrossRef CAS.
- B. Li, J. Xu, H. Xu, F. Yan, X. Zhang, C. Zhu, X. Zhang and Y. Chen, Grafting thin N-doped carbon nanotubes on hollow N-doped carbon nanoplates encapsulated with ultrasmall cobalt particles for microwave absorption, Chem. Eng. J., 2022, 435, 134846 CrossRef CAS.
- B.-Y. Zhu, P. Miao, J. Kong, X.-L. Zhang, G.-Y. Wang and K.-J. Chen, Co/C Composite Derived from a Newly Constructed Metal-Organic Framework for Effective Microwave Absorption, Cryst. Growth Des., 2019, 19(3), 1518–1524 CrossRef CAS.
- X. Sun, S. Zhu and L. Wang, MOF-derived hollow Co/C with order macropores prepared at low pyrolysis temperature as high-performance microwave absorber, Mater. Lett., 2022, 320, 132410 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2nr04851e |
| This journal is © The Royal Society of Chemistry 2023 |