MoS2 nanoflower-decorated lignin nanoparticles for superior lubricant properties†
Lucie
Lindenbeck
 a,
Björn B.
Beele
a,
Björn B.
Beele
 a,
Mohammad
Morsali
a,
Mohammad
Morsali
 bd,
Serhiy
Budnyk
c,
Marcella
Frauscher
c,
Jianhong
Chen
b,
Mika H.
Sipponen
bd,
Serhiy
Budnyk
c,
Marcella
Frauscher
c,
Jianhong
Chen
b,
Mika H.
Sipponen
 bd,
Adam
Slabon
a and
Bruno V. M.
Rodrigues
bd,
Adam
Slabon
a and
Bruno V. M.
Rodrigues
 *a
*a
aChair of Inorganic Chemistry, University of Wuppertal, Wuppertal, Germany. E-mail: manzolli@uni-wuppertal.de
bDepartment of Materials and Environmental Chemistry, Stockholm University, Stockholm, Sweden
cAC2T Research GmbH, Wiener Neustadt, Austria
dWallenberg Wood Science Center, Department of Materials and Environmental Chemistry, Stockholm University, SE-10691 Stockholm, Sweden
First published on 24th March 2023
Abstract
Lignin has been, for a long time, treated as a low-value waste product. To change this scenario, high-value applications have been recently pursued, e.g., the preparation of hybrid materials with inorganic components. Although hybrid inorganic-based materials can benefit from the reactive lignin phenolic groups at the interface, often responsible for optimizing specific properties, this is still an underexplored field. Here, we present a novel and green material based on the combination of hydroxymethylated lignin nanoparticles (HLNPs) with molybdenum disulfide (MoS2) nanoflowers grown via a hydrothermal route. By bringing together the lubricant performance of MoS2 and the structural stability of biomass-based nanoparticles, a MoS2-HLNPs hybrid is presented as a bio-derived additive for superior tribological performances. While FT-IR analysis confirmed the structural stability of lignin after the hydrothermal growth of MoS2, TEM and SEM micrographs revealed a homogeneous distribution of MoS2 nanoflowers (average size of 400 nm) on the HLNPs (average size of 100 nm). Regarding the tribological tests, considering a pure oil as reference, only HLNPs as bio-derived additives led to a reduction in the wear volume of 18%. However, the hybrid of MoS2-HLNPs led to a considerably higher reduction (71%), pointing out its superior performance. These results open a new window of opportunity for a versatile and yet underexplored field that can pave the way for a new class of biobased lubricants.
Introduction
Tribological contacts account for approximately 23% (119 EJ) of the world's total energy consumption – 103 EJ dedicated only to overcome friction.1 While containing several functional additives, engine lubricants are essential components of internal combustion engines. Zinc dialkyldithiophosphate (ZDDP) is a well-known example of antiwear additive, which forms protective and stable chemisorbed and chemically reacting films on metal surfaces, via in situ decomposition. However, despite the reduced use of organometallic additives in recent years, ZDDP continues to be employed due to its great performance.As one would expect, there are many drawbacks related to the use of Zn-based additives, as with any other organometallic substance. While studies have been revealing the occurrence of metal fume fever by the inhalation of zinc-containing fumes (particle size <1 μm),2 dermal contact can cause irritation and oral intake of high Zn doses can even lead to death. Ultimately, the complex chemistry behind ZDDP degradation products may be responsible for the formation of potentially hazardous and toxic nanoparticles, which contain inorganic components embedded in carbonaceous matrices.3 Zinc is therefore present in drained engine oil in the form of oxides or mixed phosphate/sulfate glass.3 With increasing restrictions towards pollutant elements, such as phosphorus and sulfur, the replacement of ZDDP and other organometallics as antiwear additives becomes crucial.
Within the context of the development and deployment of new tribological solutions, society has been facing a rising interest in renewable and environmentally friendly materials. Therefore, much research has been centred on the production of green and/or biobased materials, where lignocellulosic biomass has found a prominent space.4–6
Together with cellulose and hemicelluloses, lignin is one of the main constituents of biomass, being the second most abundant natural biomacromolecule on Earth. Lignin consists of phenyl propane aromatic units, namely monolignols, along with other aromatic and non-aromatic units.7 Because of this heterogeneous, recalcitrant and complex structure, direct applications of lignin are often difficult, a reason why 95% – more than necessary – of lignin dissolved in chemical pulping processes is currently burned to supply energy and recover the chemicals necessary for the pulping industry.4,5,8
Traditionally, lignin has been considered as a low-value waste product. This scenario has been changing, however, since high-value applications using this complex plant polyphenol have been pursued, e.g., in the production of carbon fibres, syngas, phenolic compounds, oxidized products and multifunctional nanoparticles.4–6,9–11 Due to several unique properties of lignin, such as its surface activity, redox activity and amphiphilicity, this biomacromolecule is an excellent candidate for materials containing inorganic interfaces.12–14 In addition, hybrid inorganic materials can benefit from the reactive lignin phenolic groups at the interface, which is often responsible for optimizing specific properties while providing new functionalities for a wide range of applications.13
Recently, much attention has been given to nanoparticles and hydrocolloids as crucial components in many technical and life-science applications.15–17 In this context, the gradual replacement of fossil-derived nanoparticles by new biobased alternatives, while keeping the multifunctionality of the final products, is strictly necessary. With the advent of lignin hydrocolloids as biobased building blocks, new possibilities have been aroused for high-added value applications. Recently, Morsali et al.17 reported on the preparation of internally cross-linked nanoparticles from hydroxymethylated lignin (HL) and their subsequent hydrothermal curing. In such a process, Morsali et al.17 not only revealed a scalable approach, where the phenolic groups of lignin are preserved, but also provided a straightforward approach to produce hydroxymethylated lignin nanoparticles (HLNPs) with improved reactivity and stability.
In pursuit of inorganic components for decorating lignin nanoparticles, our interest arose around molybdenum disulfide (MoS2), which is a well-known 2D-layered nanostructured material comprising a network of Mo and S atoms linked via covalent and metallic bonds (Fig. 1B). The resulting MoS2 layers, bonded to each other via van der Waals interactions, can act as load-bearing and interlayer slips due to the mutual existence of low inter-layer binding and high intra-layer bonding forces.18 Thus, MoS2-based nanomaterials have been extensively used in the research of friction reduction and wear resistance.18–20 However, at the same time, MoS2 presents low friction coefficient as a friction-reduction material, and this inorganic compound is more charged within the layers compared to other common 2D-nanomaterials. Hence, MoS2 tends to agglomerate under electromagnetic action in a conducive environment, which makes its dispersion difficult while weakening its lubricating effect.21 Therefore, finding new strategies for solving or reducing the agglomeration is vital.
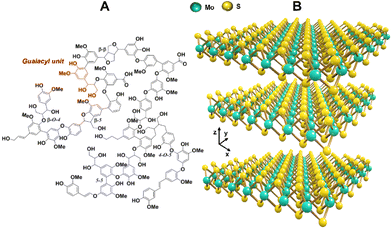 | ||
| Fig. 1 (A) Chemical structure of the insoluble fraction of softwood Kraft lignin, proposed by Crestini et al.7 and (B) 3D representation of the MoS2 crystal structure showing stacked atomic layers. | ||
Combining inorganic and organic materials is often used as an approach to generate hybrids with enhanced chemical and/or physical properties.13,22–25 These hybrids find a wide range of applications in a varied number of sectors, including energy production, medicine and wastewater treatment. For example, lignin-functionalized ZrO2 and SiO2 have been used for removing C.I. Basic Yellow 2 and C.I. Basic Blue 3 dyes from aqueous solutions.26 In a more recent application, a lignin-supported heterogeneous BiOBr photocatalyst has been applied for the direct generation of H2O2 from seawater under visible light illumination.14
In another vertex of science, several investigations have described the use of lignin and lignin-based materials as additives for lubricants.27–30 While investigating the properties of lignin in choline-amino acid ionic liquids, Zhu et al.31 reported improved anti-wear properties and friction stability of steel. Furthermore, the lubricating properties were further enhanced by introducing heteroatoms into the lignin structure, such as nitrogen and phosphorus. Anticorrosive properties on aluminium and iron were also reported by using lignin as an additive to lubricants based on ethylene glycol and poly(ethylene glycol).32 While there are plenty of examples to explore on the use of lignin as an additive to other lubricant systems, the generation of inorganic–organic interfaces via a combination of lignin and inorganic nanoparticles is still underexplored in this field.
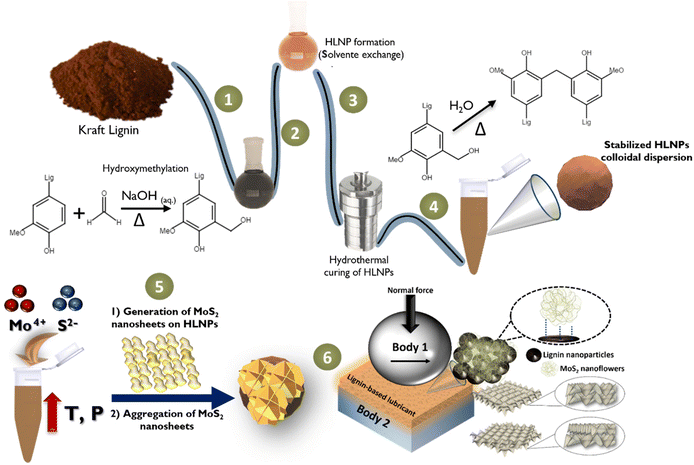
In this investigation we aimed to combine the optimum features from both worlds, namely natural and synthetic chemistry: a versatile and functional class of highly stable colloidal hydroxymethylated lignin nanoparticles (HLNPs) prepared from Kraft lignin (Fig. 1A), containing a high biobased content (97%), and the superior friction-reduction performance of MoS2 (Fig. 1B) nanoparticles. The use of lignin nanoparticles (LNPs) instead of bulk lignin overcomes the limitations of lignin stemming from its heterogeneous structure and wide molecular weight distribution, which are two important factors affecting the efficiency of lignin as a lubricating additive.33 Additionally, introducing lignin in the form of nanoparticles can improve its dispersibility, while the spherical geometry can result in rolling bearing effects.33 In addition, the hydroxymethylation of lignin serves the purpose of increasing its hydroxyl content while adding hydroxymethyl moieties adjacent to the phenolic structure of lignin as cross-linking sites, helping to increase the hydroxyl content of lignin after hydrothermal cross-linking. Therefore, we prepared composite powders of MoS2-HLNPs via a simple and green hydrothermal synthesis route, where the final product could combine the structural stability of biomass-based nanoparticles with the lubricant performance of the inorganic phase.
Materials and methods
Materials
Softwood Kraft lignin (SKL) (BioPiva 100 pine Kraft lignin), sodium hydroxide (VWR), formalin solution (formaldehyde 37%) (Sigma-Aldrich Co.), hydrochloric acid 35% (VWR) and acetone (Honeywell), hexaammonium heptamolybdate tetrahydrate (Sigma-Aldrich Co.) and thiourea (Sigma-Aldrich Co.) were all used as received.Synthesis of hydroxymethylated lignin nanoparticles (HLNPs)
Stabilized HLNPs in this work were prepared from the same lignin batch and according to the previously reported procedure.17 Briefly, in a round-bottom flask, 5 g of Kraft lignin, 11.25 mL of aqueous sodium hydroxide solution (1 M, 11.25 mmol), and 8.75 mL of deionized water were mixed using a stirrer. In a rubber-capped flask, the temperature was increased to 60 °C and 5 mL of formalin solution (37% formaldehyde, 66 mmol) was added to the lignin solution and allowed to react for 3 h. Then, the flask was cooled down in cold water, and the product was adjusted to pH 3 by the drop-by-drop addition of 0.1 M hydrochloric acid. The precipitate was centrifuged and washed with water 3 times to remove excess acid until the pH of the product becomes 3.2. Next, the product was freeze-dried and collected with a 90% reaction yield to be used for preparation of colloidal nanoparticles. To prepare a colloidal dispersion, 0.5 g of hydroxymethylated lignin was dissolved in 40 g of a solution mixture of acetone and water (mass ratio 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) for 3 h at room temperature. The solution was vacuum filtered using a glass-fibre filter (0.45 μm pore size) to eliminate any insoluble fraction. Eventually, 120 g of deionized water was added to the stirring solution to form colloidal particles (HL60NP). The remaining acetone was removed using a rotary evaporator under reduced pressure at 40 °C. To internally cross-link the particles, 20 mL of colloidal dispersion was hydrothermally cured in a clean 40 mL Teflon-sealed reactor at 150 °C overnight (HL60NP-150).
1) for 3 h at room temperature. The solution was vacuum filtered using a glass-fibre filter (0.45 μm pore size) to eliminate any insoluble fraction. Eventually, 120 g of deionized water was added to the stirring solution to form colloidal particles (HL60NP). The remaining acetone was removed using a rotary evaporator under reduced pressure at 40 °C. To internally cross-link the particles, 20 mL of colloidal dispersion was hydrothermally cured in a clean 40 mL Teflon-sealed reactor at 150 °C overnight (HL60NP-150).
Synthesis of MoS2 nanoflower-decorated lignin nanoparticles (MoS2-HLNPs)
MoS 2 -HLNPs were prepared following a hydrothermal route. In a typical preparation, 247.25 mg of hexaammonium heptamolybdate tetrahydrate (0.2 mmol, (NH4)6 Mo7O24·4H2O, i.e., 1.4 mmol of Mo) and 456.75 mg of thiourea (6 mmol) were dissolved in ultrapure water (40 mL) under vigorous stirring to form a homogeneous solution. Then, aliquots of 8.5 mL were transferred into 20 mL Teflon-lined stainless-steel autoclaves containing 7.5 mL of HLNP (HL60NP-150) solution and maintained at 180 °C for 24 h. After this period, the reaction system was cooled down to room temperature. The final product was washed thoroughly with water and ethanol to remove any possible ions, and the as-prepared final solutions were then directly dehydrated via a freeze-drying process. MoS2 nanoparticles were prepared in a similar procedure, except using HLNPs as templates.Material characterization and tribological evaluation
Fourier-transform infrared spectroscopy (FT-IR) analysis was carried out using a Varian 610-IR FT-IR spectrometer. The samples were analysed in the ATR mode at room temperature, in the range of 400–4000 cm−1, with 16 scans at 4 cm−1 resolution and 1 cm−1 interval. SEM micrographs were recorded on a JEOL JSM-7000F, with an accelerating voltage of 15 kV. The samples were previously coated with a thin layer of gold to improve the image acquisition. The TEM micrographs were obtained using electron diffraction and high-resolution transmission electron microscopy on a JEOL 2100F microscope, operated at 200 kV.Tribological tests were performed using pure reference oil and reference oil plus (mineral oil, 0.1 wt% succinimide) bio-derived additives: HL60NP, HL60NP-150 and MoS2-HLNPs. The tribometer data were collected with an NTR3 Nano Tribometer (Anton Paar) in linear oscillation mode using a 2 mm diameter 100Cr6 ball on a 100Cr6 base body. The measurements were carried out over 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles for an overall duration of 83 min. 50 datapoints were collected tip-to-tip (25 datapoints per stroke) per cycle. The input parameters were applied as follows: 0.8 N normal force, 0.85 GPa Hertzian mean contact pressure, 1.27 Gpa maximum contact pressure, 1 mm stroke, 2 Hz frequency, 25 °C and 6.28 × 10−3 m s−1 sliding speed. Surface topography analyses were performed with a Leica DCM 3D confocal microscope (Ernst Leitz GmbH, Wetzlar, Germany) for determining the removed wear volume after the experiments. Viscosity measurements were performed at 40 °C and 100 °C, according to ASTM D704234 using an SVM 3000 Stabinger viscometer (Anton Paar GmbH, Graz, Austria).
000 cycles for an overall duration of 83 min. 50 datapoints were collected tip-to-tip (25 datapoints per stroke) per cycle. The input parameters were applied as follows: 0.8 N normal force, 0.85 GPa Hertzian mean contact pressure, 1.27 Gpa maximum contact pressure, 1 mm stroke, 2 Hz frequency, 25 °C and 6.28 × 10−3 m s−1 sliding speed. Surface topography analyses were performed with a Leica DCM 3D confocal microscope (Ernst Leitz GmbH, Wetzlar, Germany) for determining the removed wear volume after the experiments. Viscosity measurements were performed at 40 °C and 100 °C, according to ASTM D704234 using an SVM 3000 Stabinger viscometer (Anton Paar GmbH, Graz, Austria).
XPS characterisation of the contact surface
XPS measurements were performed on a Theta Probe (Thermo Fisher Scientific). The X-ray source was a monochromatic Al K(alpha) source, operated at 1386.6 eV. The spectrometer was calibrated to the 368.21 eV binding energy (BE) of the Ag 3d5/2 line for metallic silver and the linearity was corrected to the BE of metallic 932.62 eV for the Cu 2p3/2 line and 83.96 eV for Au 4f7/2. Sputtering was performed with an EX05 ion gun with Ar+ at 3 keV, 1 μA, 4 mm2. The lateral resolution of the X-ray spot was set to 400 μm for all measurements. The survey spectra were recorded with a pass energy of 200 eV BE and the detail spectra with a pass energy of 50 eV. The base pressure in the analytical chamber was <5 × 10−8 Pa. The binding states of elements detected were analysed with reference to the NIST XPS database (NIST, 2012).Results and discussion
As previously approached, within the context of the development of new tribological solutions, strategies to avoid or decrease MoS2 agglomeration in different media become crucial. As MoS2-based nanomaterials present low friction coefficients as friction-reduction materials, combining these inorganic nanoparticles with organic materials evolves as an interesting and yet underexplored strategy to generate hybrids with enhanced chemical and physical properties. For this, our investigation aimed to merge the best features from a functional class of highly stable colloidal lignin nanoparticles with the superior friction-reduction performance of MoS2 nanoparticles.Fig. 2 summarizes the established route for the preparation of the hybrid MoS2-HLNPs. Firstly, Kraft lignin is hydroxymethylated (step 1) via electrophilic aromatic substitution using formaldehyde. Both the characterization of the softwood Kraft lignin35 used in this work and the reaction17,36 are described in previous works. Next, the hydroxymethylated lignin is used to generate the hydrocolloids, which are later hydrothermally cured to cross-link the colloidal particles (steps 2–4). HLNPs before (HL60NP) and after (HL60NP-150) curing were considered in this work for tribological evaluation. Then, an MoS2-HLNP nanocomposite is prepared via a hydrothermal route using HLNPs (HL60NP-150, after curing) in solution in the presence of hexaammonium heptamolybdate and thiourea (step 5), and further used as an additive to oil to generate the biobased lubricants (step 6).
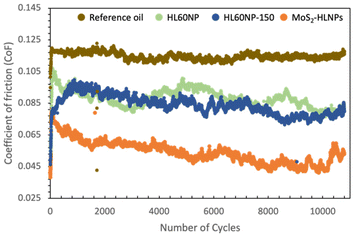
Fig. 3 shows the coefficient of friction (μ) versus the number of cycles for the reference oil (mineral oil, 0.1 wt% succinimide) and reference oil plus HL60NP, HL60NP-150 and MoS2-HLNPs as additives. One can see a clear reduction in the CoF values during all 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles (83 min) when HLNPs and hybrid MoS2-HLNPs were used as additives. On average, the reference oil presented a CoF of 0.11, which reduced by about 54% (a CoF of 0.05 – on average over 10
000 cycles (83 min) when HLNPs and hybrid MoS2-HLNPs were used as additives. On average, the reference oil presented a CoF of 0.11, which reduced by about 54% (a CoF of 0.05 – on average over 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles) when MoS2-HLNPs was used as an additive. When MoS2 was not present in the additive formulation, i.e., in the HLNPs before (HL60NP) and after (HL60NP-150) curing, a lower decrease in the CoF was still observed (around 26%), as expected.
000 cycles) when MoS2-HLNPs was used as an additive. When MoS2 was not present in the additive formulation, i.e., in the HLNPs before (HL60NP) and after (HL60NP-150) curing, a lower decrease in the CoF was still observed (around 26%), as expected.
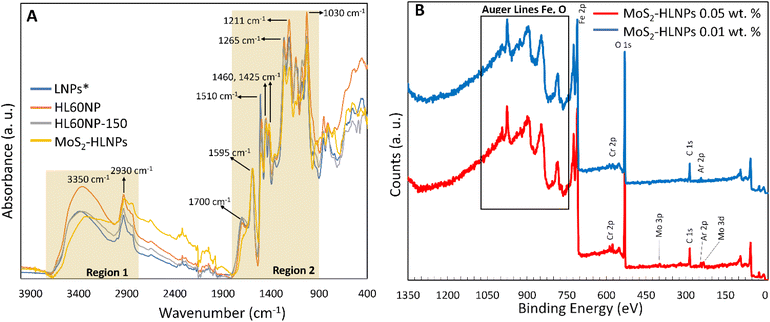
Fig. 4 shows the comparison of the tribological traces on the flat steel surface after the tribological tests. The performance of HL60NP as an additive is relatively poor, with the resulting wear volume in the same order of magnitude as for the pure reference oil without additives. Nevertheless, the presence of HL60NP-150 reduced the wear volume by 18%, with the most significant wear-reducing effect observed in the presence of MoS2-HLNPs – a 71% reduction confirmed by confocal microscopy measurements. XPS characterisation of the obtained wear tracks (Fig. 6B and Table S2†) confirms the presence of MoS2-related states (molybdenum oxide forms, sulphides, and sulphates) at 0.05 wt%MoS2-HLNPs, characteristic of lignin and hydrocarbon bonds in the hydrocarbon matrix. A unique combination of sulphate in the outer surface of the tribofilm in combination with small amounts of molybdenum disulphide in the lignin matrix demonstrates significant improvement of anti-wear properties by reduction of 71% at 0.05% MoS2-containing additives.
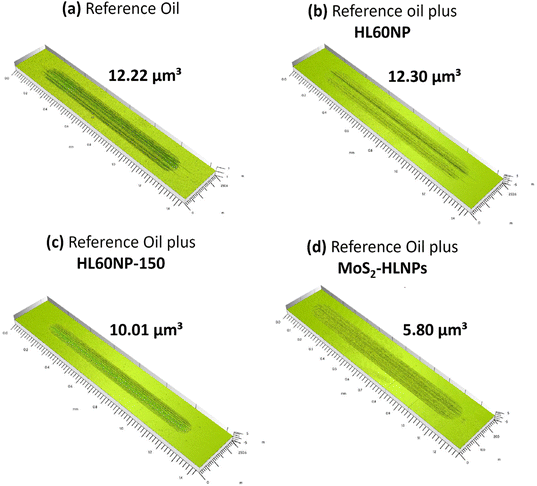 | ||
| Fig. 4 3D surface profiles of the wear tracks on a disc using (a) reference oil and reference oil plus (b) HL60NP, (c) HL60NP-150 and (d) MoS2-HLNPs (HL60NP-150) as additives at 0.05 wt%. | ||
Kinematic viscosity is an important property in tribology and plays a critical role in determining the lubrication regime of a fluid, which is a key factor in controlling friction and wear between two surfaces in relative motion.37 It is a crucial parameter that determines the thickness of the lubricant film.37 In the present investigation, although the addition of HL60NP, HL60NP-150 and MoS2-HLNPs at low concentrations (0.05 wt%) promoted a significant reduction in the CoF values and wear volume, they did not promote any significant changes in the kinematic viscosity of the reference oil (mineral base oil + 0.1 wt% succinimide). The values remained in the range of 33.43–33.56 mm2 s−1 at 40 °C and 5.58–5.61 mm2 s−1 at 100 °C for all samples.
Fig. 6A shows the FT-IR spectra of lignin nanoparticles (LNPs, no hydroxymethylation), hydroxymethylated LNPs before (HL60NP) and after (HL60NP-150) curing, and MoS2-HLNPs. The spectra of LNPs and HLNPs (before and after curing) are very similar. Characteristic lignin signals can be observed in regions 1 and 2 (Fig. 6A).17 In region 1, the broad absorption band at 3350 cm−1 is associated with the hydroxyl groups (–OH) present in the lignin structure. The peak at 1700 cm−1 in region 2 is attributed to the carbonyl groups (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) and the peak at 1595 cm−1 to the aromatic skeletal vibration. However, one can note an increase in the hydroxyl content (band at 3350 cm−1) in the HL60NP spectrum compared to LNPs, due to hydroxymethylation. Additionally, because of hydroxymethylation, changes at 1125, 1290, 1452, and 3350 cm−1 bands can be observed. As expected, hydrothermal curing resulted in a decrease in the hydroxyl content due to the aromatic ring cross-linking reactions via condensation of the hydroxymethyl groups (HL60NP-150 spectrum). A more detailed band assignment can be found in Table S1.† The spectrum of MoS2-HLNPS also shows the same peaks, confirming the structural stability of the HLNPs after the hydrothermal synthesis.
O) and the peak at 1595 cm−1 to the aromatic skeletal vibration. However, one can note an increase in the hydroxyl content (band at 3350 cm−1) in the HL60NP spectrum compared to LNPs, due to hydroxymethylation. Additionally, because of hydroxymethylation, changes at 1125, 1290, 1452, and 3350 cm−1 bands can be observed. As expected, hydrothermal curing resulted in a decrease in the hydroxyl content due to the aromatic ring cross-linking reactions via condensation of the hydroxymethyl groups (HL60NP-150 spectrum). A more detailed band assignment can be found in Table S1.† The spectrum of MoS2-HLNPS also shows the same peaks, confirming the structural stability of the HLNPs after the hydrothermal synthesis.
Fig. 5A and B depict the morphology of MoS2 nanoflowers; a nanosheet-assembled woven flowerlike structure can be seen from the SEM micrographs, with an average size in the range of 1.1–1.3 μm. These flowerlike architectures are composed of accumulated MoS2 nanosheets perpendicular to the spherical particle surface. Even though the MoS2 nanoflowers suffered from severe aggregation, which can be proved by SEM microscopy, it is possible to confirm that the average size of MoS2 nanoflowers in this work is larger than those in the other works prepared via hydrothermal38 or solvothermal methods.39 However, the average size of MoS2 nanoflowers drastically decreased once combined with lignin nanoparticles, with an average size in the range of 350–480 nm, as seen from the TEM micrographs (Fig. 5C and D). The use of HLNPs as the substrate for MoS2 nucleation and growth can not only decrease the average size but also relieve the aggregation. It should be noted that, within the scope of the current manuscript, we did not perform a detailed optimization regarding the growth mechanism of MoS2 with respect to temperature, pressure and concentrations of the precursors.
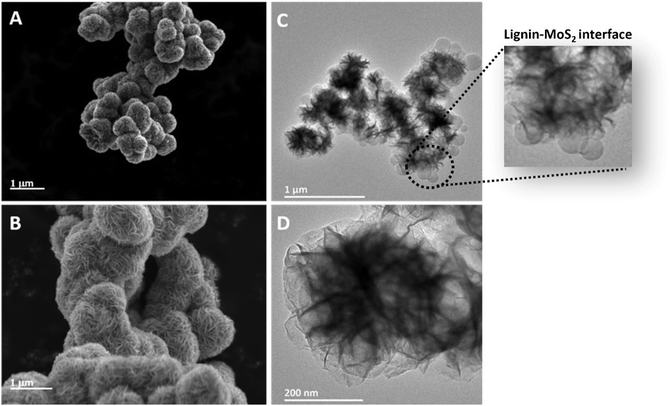 | ||
| Fig. 5 SEM micrographs of (A and B) MoS2 nanoflowers and TEM micrographs (C and D) of MoS2 nanoflower-decorated LNPs. | ||
The TEM micrographs confirm the formation of nanoflower-structured MoS2 on spherical lignin nanoparticles, as depicted in Fig. 5C. The highly magnified TEM image (Fig. 5D) further assures the presence of nanoflowers formed by nanosheets in MoS2-decorated lignin nanoparticles.
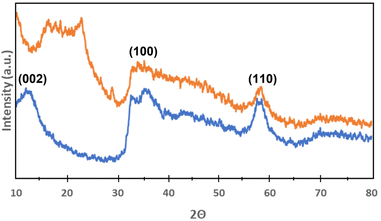
X-ray powder diffraction analysis of MoS2 nanoflowers and hybrid MoS2-HLNPs was performed to better understand their morphology. The XRD patterns of MoS2 nanoflowers and hybrid MoS2-HLNPs are shown in Fig. 7. The XRD patterns of MoS2 nanoflowers show diffraction peaks (2θ) at 12°, 35°, 40° and 59°, which are generally consistent with the reference XRD patterns of hexagonal MoS2 (JCPSD 01-075-1539).40 These peaks are assigned to the (002), (100) (103) and (110) lattice planes. The (002) peak is caused by stacking layers. This gives an indication of the layer structure of MoS2.41 For hybrid MoS2-HLNPs, the peaks at 35° and 59° can also be seen, which also belong to the lattice planes (100) and (110). However, the peaks belonging to the lattice planes (002) and (103) are missing. Instead, a peak (2θ) at 22° can be seen, which is attributed to lignin.42
Conclusions
The critical advancement of this work is a straightforward and green methodology to generate a powerful inorganic–organic interface based on lignin, a low-value waste product, and MoS2 nanoparticles. By hydrothermally growing MoS2 nanoflowers using hydroxymethylated lignin nanoparticles (HLNPs) as the substrate, we reached a hybrid material that combined the structural stability of the organic phase with the lubricant performance of the inorganic component. While the use of LNPs as bio-derived additives led to a reduction of 18% in the wear volume, hybrid MoS2-HLNPs was responsible for a reduction of 71%. These results open a window of opportunities for a versatile and yet underexplored field that can pave the way for a new class of circular biobased lubricants.Author contributions
M. M. synthesized the LNPs and provided their characterization. L. L. and B. V. M. R. synthesized the biobased materials combining MoS2 and LNPs. B. B. B. and J. C. characterized the materials structurally and morphologically. S. B. and M. F. characterized the tribological performance of all materials. B. V. M. R. and A. S. designed the experiments and supervised the synthesis and analysis. B. V. M. R. and L. L. wrote the final draft. M. H. S., M. M., M. F. and S. B. provided further specific discussion for the manuscript. B. V. M. R. and A. S. reviewed the final manuscript. All authors have given approval to the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank the Swedish Foundation for Strategic Environmental Research (Mistra: project Mistra SafeChem, project number 2018/11) for its financial support. We acknowledge additional financial support from the COMET InTribology project (FFG No. 872176, project coordinator: AC2T research GmbH). The work was carried out in collaboration with the “Excellence Centre of Tribology” AC2T research GmbH. The authors acknowledge funding from Vinnova (“C1Bio” project, grant number 2019-03174) and the Knut and Alice Wallenberg Foundation (KAW) through the Wallenberg Wood Science Center (grant number KAW 2021.0313). The authors would also like to thank the University of Wuppertal for additional financial support.References
- K. Holmberg and A. Erdemir, Friction, 2017, 5, 263–284 CrossRef CAS.
- L. M. Plum, L. Rink and H. Haase, Int. J. Environ. Res. Public Health, 2010, 7, 1342–1365 CrossRef CAS PubMed.
- T. Thersleff, I. Z. Jenei, S. Budnyk, N. Dörr and A. Slabon, ACS Appl Nano Mater, 2021, 4, 220–228 CrossRef CAS.
- M. Bilal, M. Asgher, H. M. N. Iqbal, H. Hu and X. Zhang, Int. J. Biol. Macromol., 2017, 98, 447–458 CrossRef CAS PubMed.
- A. M. Borrero-López, C. Valencia and J. M. Franco, Polymers, 2022, 14, 881, DOI:10.3390/polym14050881.
- S. Sugiarto, R. R. Pong, Y. C. Tan, Y. Leow, T. Sathasivam, Q. Zhu, X. J. Loh and D. Kai, Mater Today Chem, 2022, 26, 101022, DOI:10.1016/j.mtchem.2022.101022.
- C. Crestini, H. Lange, M. Sette and D. S. Argyropoulos, Green Chem., 2017,(17), 4104–4121 RSC.
- V. K. Ponnusamy, D. D. Nguyen, J. Dharmaraja, S. Shobana, J. R. Banu, R. G. Saratale, S. W. Chang and G. Kumar, Bioresour. Technol., 2019, 271, 462–472 CrossRef CAS PubMed.
- A. Moreno, J. Delgado-Lijarcio, J. C. Ronda, V. Cádiz, M. Galià, M. H. Sipponen and G. Lligadas, Small, 2022, 2205672 Search PubMed.
- A. Moreno, J. Liu, R. Gueret, S. E. Hadi, L. Bergström, A. Slabon and M. H. Sipponen, Angew. Chem., Int. Ed., 2022, 60, 20897–20905 CrossRef PubMed.
- M. Österberg, M. H. Sipponen, B. D. Mattos and O. J. Rojas, Green Chem., 2020, 22 Search PubMed.
- T. M. Budnyak, J. Onwumere, I. v. Pylypchuk, A. Jaworski, J. Chen, A. Rokicińska, M. E. Lindström, P. Kuśtrowski, O. Sevastyanova and A. Slabon, Chemosphere, 2021, 279, 130538 CrossRef CAS PubMed.
- T. M. Budnyak, A. Slabon and M. H. Sipponen, ChemSusChem, 2020, 13, 4344–4355 CrossRef CAS PubMed.
- A. Gopakumar, P. Ren, J. Chen, B. V. M. Rodrigues, H. Y. Vincent Ching, A. Jaworski, S. van Doorslaer, A. Rokicińska, P. Kuśtrowski, G. Barcaro, S. Monti, A. Slabon and S. Das, J. Am. Chem. Soc., 2022, 144, 2603–2613 CrossRef CAS PubMed.
- B. Zhu, Y. Xu and H. Xu, Nano Futures, 2022, 6, 032004, DOI:10.1088/2399-1984/ac8400.
- Y. Wang, X. Ji, Q. Wang, Z. Tian, S. Liu, G. Yang and H. Liu, Int. J. Biol. Macromol., 2022, 222, 2498–2511 CrossRef CAS PubMed.
- M. Morsali, A. Moreno, A. Loukovitou, I. Pylypchuk and M. H. Sipponen, Biomacromolecules, 2022, 23, 4597–4606 CrossRef CAS PubMed.
- G. Tang, J. Zhang, C. Liu, D. Zhang, Y. Wang, H. Tang and C. Li, Ceram. Int., 2014, 40, 11575–11580 CrossRef CAS.
- J. Yin, H. Yan, M. Cai, S. Song, X. Fan and M. Zhu, J. Ind. Eng. Chem., 2023, 117, 450–460 CrossRef CAS.
- P. Tonge, A. Roy, P. Patel, C. J. Beall and P. Stoyanov, Sustainable Mater. Technol., 2023, 35, e00552 CrossRef CAS.
- S. Zhou, H. Liu, S. Wang, L. Gan, J. Huang, G. Zhao and Y. Liu, Mater. Res. Express, 2019, 6, 115075 CrossRef.
- W. al Zoubi, M. P. Kamil, S. Fatimah, N. Nisa and Y. G. Ko, Prog. Mater. Sci., 2020, 112, 100663, DOI:10.1016/j.pmatsci.2020.100663.
- P. J. Hagrman, D. Hagrman and J. Zubieta, Angew. Chem., Int. Ed., 1999, 38, 2638–2684 CrossRef PubMed.
- S. Atri and R. Tomar, ChemistrySelect, 2021, 6, 9351–9362 CrossRef CAS.
- J. Piątek, B. V. M. Rodrigues and A. Slabon, DOI:10.1515/zkri-2022-0054.
- M. Wawrzkiewicz, B. Podkościelna, T. Jesionowski and Ł. Klapiszewski, Ind. Crops Prod., 2022, 180, 114785 CrossRef CAS.
- M. G. A. da Cruz, T. M. Budnyak, B. V. M. Rodrigues, S. Budnyk and A. Slabon, Green Chem. Lett. Rev., 2021, 14, 356–379 Search PubMed.
- M. A. Jedrzejczyk, S. van den Bosch, J. van Aelst, K. van Aelst, P. D. Kouris, M. Moalin, G. R. M. Haenen, M. D. Boot, E. J. M. Hensen, B. Lagrain, B. F. Sels and K. V. Bernaerts, ACS Sustainable Chem. Eng., 2021, 9, 12548–12559 CrossRef CAS.
- J. Zhang, D. Cao, S. Wang, X. Feng, J. Zhu, X. Lu and L. Mu, Biomass Bioenergy, 2022, 161, 106470, DOI:10.1016/j.biombioe.2022.106470.
- S. Shylesh, A. A. Gokhale, C. R. Ho and A. T. Bell, Acc. Chem. Res., 2017, 50, 2589–2597 CrossRef CAS PubMed.
- L. Mu, Y. Shi, X. Guo, T. Ji, L. Chen, R. Yuan, L. Brisbin, H. Wang and J. Zhu, RSC Adv., 2015, 5, 66067–66072 RSC.
- L. Mu, Y. Shi, H. Wang and J. Zhu, ACS Sustainable Chem. Eng., 2016, 4, 1840–1849 CrossRef CAS.
- W. Liu, X. Qiao, S. Liu and P. Chen, Nanomaterials, 2022, 12, 3780, DOI:10.3390/nano12213780.
- ASTM, American National Standard Institute.
- M. H. Sipponen, M. Farooq, J. Koivisto, A. Pellis, J. Seitsonen and M. Österberg, Nat. Commun., 2015, 9, 2300, DOI:10.1038/s41467-018-04715-6.
- A. Eraghi Kazzaz, Z. Hosseinpour Feizi and P. Fatehi, Green Chem., 2019, 21, 5714–5752 RSC.
- A. Wolak, G. Zajac and T. Słowik, Sensors, 2021, 21, 2530, DOI:10.3390/s21072530.
- J. Singh, Rishikesh, S. Kumar and R. K. Soni, J. Alloys Compd., 2020, 849, 15602, DOI:10.1016/j.jallcom.2020.156502.
- C. Mutalik, D. I. Krisnawati, S. B. Patil, M. Khafid, D. S. Atmojo, P. Santoso, S. C. Lu, D. Y. Wang and T. R. Kuo, ACS Sustainable Chem. Eng., 2021, 9, 7904–7912 CrossRef CAS.
- D. H. Youn, J. W. Jang, J. Y. Kim, J. S. Jang, S. H. Choi and J. S. Lee, Sci. Rep., 2014, 4, 5492, DOI:10.1038/srep05492.
- J. Y. Lin, C. Y. Chan and S. W. Chou, Chem. Commun., 2013, 49, 1440–1442 RSC.
- R. A. C. Gomide, A. C. S. de Oliveira, D. A. C. Rodrigues, C. R. de Oliveira, O. B. G. de Assis, M. V. Dias and S. V. Borges, J. Polym. Environ., 2020, 28, 1326–1334 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3nr00458a |
| This journal is © The Royal Society of Chemistry 2023 |