Open Access Article
Open Access ArticleAsymmetric Strecker reaction at the solid/solid interface†
Yuki
Yoshimura
,
Yudai
Tanaka
,
Ryota
Kobayashi
,
Kohei
Niikura
and
Tsuneomi
Kawasaki
 *
*
Department of Applied Chemistry, Tokyo University of Science, Kagurazaka, Shinjuku-ku, Tokyo 162-8601, Japan. E-mail: tkawa@rs.tus.ac.jp
First published on 17th November 2022
Abstract
Related to absolute asymmetric synthesis, a stereospecific reaction at the solid/solid interface arising from crystal chirality of the achiral or racemic substrates has not yet been reported. Here, we demonstrate the asymmetric Strecker-type solid/solid reaction between the chiral crystal of a racemic cyanohydrin (kryptoracemate) and the achiral crystal of an ammonium salt to afford highly enantioenriched α-aminonitrile in combination with amplification of chirality. rac-Cyanohydrin provides its chiral surface as a reactive site and the reaction proceeds with dissociation of cyanohydrin; thus, an asymmetric Strecker-type reaction takes place at the interface of the substrate crystals. Strecker synthesis coupled with cyanohydrin synthesis offers a credible abiotic synthesis mechanism of α-amino acids and α-hydroxy acids. For the first time, stereochemical relationship has been found between the two chiral intermediates, aminonitrile and cyanohydrin, which are in equilibrium in the synthesis mechanism.
Introduction
Overwhelming enantioenrichment of chiral bioorganic compounds as exemplified by L-amino acids and D-sugars is a fundamental prerequisite of living organisms; the origin and amplification of chirality are therefore key issues regarding the origin of life. Louis Pasteur first reported the separation of the enantiomorphs of sodium ammonium tartrate.1 Following on from the association between the optical activity and molecular chirality, plausible theories for the origin of chirality have been proposed as a mechanism of prebiotic evolution of biological homochirality.2–14 Among the theories, chiral crystallization of achiral compounds, including racemates, is one of the topics associated with absolute asymmetric synthesis.15 Stereospecific reactions utilizing chiral crystals as both substrate and source of chirality have been demonstrated.2,16–19 Regarding chiral crystals of racemic compounds, [2 + 2] cycloaddition and enantiomer-specific racemization under photoirradiation have been shown to proceed stereospecifically.20,21Enantiotopic crystal faces can also provide a reactive surface, affording enantioenriched compounds.22–25 In addition, a chiral crystal acts as a heterogeneous chiral trigger for the asymmetric autocatalysis (the Soai reaction).4,26,27 However, to our knowledge, a stereospecific solid/solid reaction at the interface resulting solely from the crystal chirality remains unsolved.28,29
In the current research, we report on an asymmetric Strecker-type solid/solid reaction between the chiral crystal of racemic cyanohydrin and achiral crystal of amine as its ammonium salt to afford, in co-operation with the amplification of enantiomeric excess (ee),30–33 highly enantioenriched aminonitrile with the corresponding absolute configuration. The source of asymmetry is only the chiral arrangement of the racemic substrate under solvent-free conditions.34 This result therefore expands the concept of stereoselective reaction35 towards the solid/solid interface.
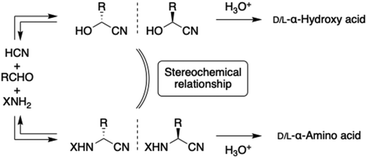
On the other hand, Strecker amino acid synthesis,36 including cyanohydrin synthesis, has been considered as a mechanism for prebiotic synthesis of α-amino acids and α-hydroxy acids, both of which are biologically important classes of compounds.37–40 They have also been identified in meteorites.41 The chiral intermediates in the mechanism are α-aminonitrile and α-cyanohydrin, synthesized by cyanide addition reactions to achiral imine and aldehyde, respectively. Because aminonitrile and cyanohydrin are in equilibrium through the achiral intermediates, a stereochemical relationship between cyanohydrin and aminonitrile has not been considered. The asymmetric Strecker-type reaction described here, in which cyanohydrin in racemic form is utilized as a chiral substrate, would have implications for the origin and induction of enantioenriched α-amino acids (Fig. 1).
Results and discussion
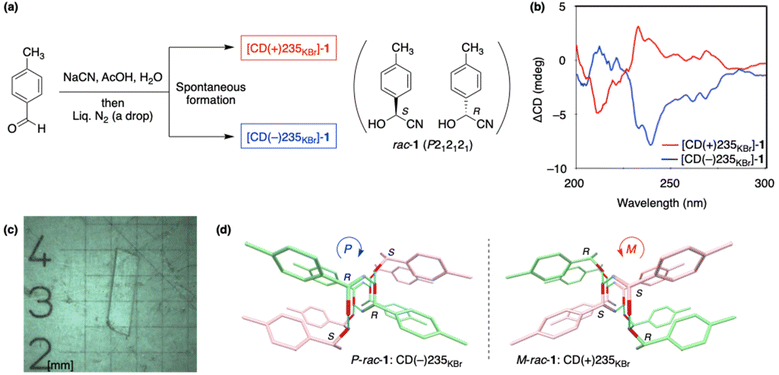
We previously reported on a spontaneous absolute asymmetric Strecker-type reaction based on the conglomerate formation of α-aminonitriles.33,42 In this case, the ee of aminonitriles can be significantly enhanced from ca. 0.05% ee to near enantiopure state only by the thermally operated cycles of partial dissolution and recrystallization in its suspension. Because D- and L-α-amino acids can act as a chiral trigger for the amplification of their own D- and L-intermediate aminonitriles, respectively, the process, including hydrolysis, represents the self-replication of chiral α-amino acids.43 In addition, the enantiotopic single-crystal face of achiral imine25 and isotopically chiral amine arising from 2H/1H substitution44 are responsible for the enantioselective synthesis of aminonitriles. Therefore, further research on the asymmetric Strecker-type reaction using a chiral crystal of racemic cyanohydrin would expand the focus on the origin and induction of enantioenriched α-amino acids (Fig. 1).We found that 4-methylmandelonitrile (1) forms the chiral crystal of racemic compounds, i.e., kryptoracemate.45rac-Cyanohydrin 1 can be synthesized by the reaction between p-tolualdehyde and sodium cyanide in the presence of acetic acid in water (Fig. 2a). The addition of a drop of liquid nitrogen to the resulting mixture induced the crystallization of rac-1 to afford, under stirred conditions,5 powder-like crystals with solid-state chirality (Fig. 2b and ESI, Table S1†). The precipitate 1 had either a plus or minus Cotton effect at approximately 235 nm in the solid-state circular dichroism (CD) spectra. The sequence of cyanide addition and crystallization was repeatedly conducted, six times, to afford the powder-like crystal [CD(+)235KBr]-1 three times and its enantiomorphic [CD(−)235KBr]-1 three times (ESI, Table S1†). Thus, the enantioenriched chiral powder-like crystal 1 formed spontaneously, without the use of any chiral materials.
X-ray single-crystal structure analysis shows the formation of racemic compound 1 (Fig. 2c), which belongs to the chiral space group (P212121) (ESI†). Therefore, equal numbers of (S)- and (R)-1 included in the unit cell and the enantiomers were connected to each other through intermolecular hydrogen bonds between the cyano (CN) and hydroxy (OH) groups (Fig. 2d). Racemic cyanohydrin 1 is chirally arranged having right-handed P-helicity in one crystal and left-handed M-helicity in its enantiomorph. Absolute handedness could be determined as [CD(+)235KBr]-1 with M-helicity and [CD(−)235KBr]-1 with P-helicity (ESI, Fig. S1†). It should be noted that any detectable enrichment between (S)- and (R)-1 in the crystal was not observed (Fig. 2c) by the analysis using high performance liquid chromatography (HPLC) on a chiral stationary phase.
Next, we examined the Strecker-type reaction46 between 1 and achiral amine to achieve the formation of α-aminonitrile. We selected benzhydrylamine (2) as substrate because the enantiomeric imbalance of the resulting aminonitrile 3 can be amplified to near enantiopure state.43 Because dissolution or melt of rac-1 causes the disappearance of the chirality, it seemed ideal to perform the reaction without using solvents. When an equimolar amount of 2 (mp. 11–13 °C) was added to the solid of rac-1, the Strecker-type reaction readily proceeded to afford 3 as a solid product, quantitatively. However, the reaction progressed via a glassy, seemingly homogeneous state, in which amine 2 acted as solvent. Because enantioenrichment of the resulting product 3 was below the level of detection, solid product 3 was submitted to the thermally operated amplification cycle. Although enantioenriched 3 was obtained with an ee above the detection level, stereochemical relationships between P/M-crystal chirality of rac-1 and the resulting D/L-molecular handedness of 3 were not observed.
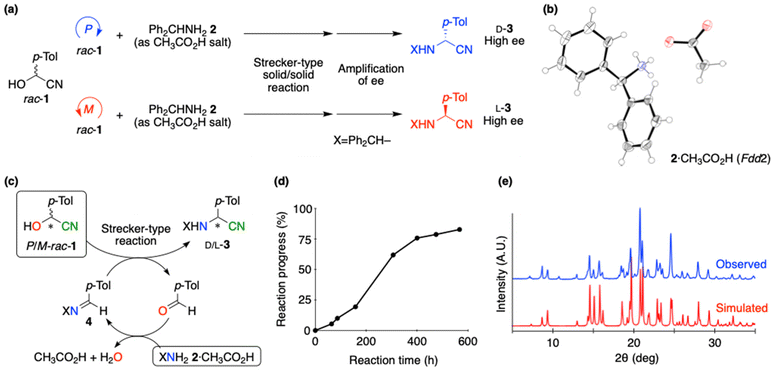
Therefore, the solid/solid reaction was examined using an ammonium salt of benzhydrylamine (2) (Fig. 3). After examination of several salts (ESI, Tables S2 and S3†), the acetate (2·CH3CO2H), which belongs to achiral space group Fdd2 (ESI†), was selected as a suitable reactant (Fig. 3a and b). Ammonium acetate 2·CH3CO2H and an equimolar amount of rac-1 were mixed, using an agate mortar and pestle, to give a finely powdered reaction mixture. The powder X-ray diffraction pattern of the latter seems to be the sum of each of the substrates, in comparison with the simulated patterns obtained from the single-crystal X-ray data of rac-1 and 2·CH3CO2H (ESI, Fig. S2†). The mixture was stored in a sealed screw-cap vial, without stirring, and the reaction progress over time was monitored by 1H NMR analysis of a sample of the mixture. The conversion was measured from the ratio of the integrated α-protons of both substrate 1 and product 3. The powder form of the solid mixture was maintained throughout the reaction. After 20–25 days, >80% of 1 was converted to 3.
It is conceivable that the dissociation of a part of 1 into aldehyde and HCN, following formation of imine 4 and HCN addition, occurred continuously at the solid/solid interface to afford 3 (Fig. 3c). No unexpected compounds arising from side reactions were detected. The released water and/or acetic acid might promote further reaction, as observed in the sigmoidal reaction profile (Fig. 3d). The X-ray powder diffraction pattern of a solid product is essentially the same as the simulated pattern of conglomerate 3 (space group: P21) (Fig. 3e).33 The ee of 3 was amplified to ensure that the stereoselectivity originated from the crystal chirality of rac-1 (Fig. 3a). After the removal of volatiles under vacuum, methanol was added to the mixture, affording a suspension of 3. Unreacted 1 and 2·CH3CO2H completely converted to form 3 upon the addition of methanol. After stirring overnight, 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU), which promotes the solution-phase racemization of 3, was added to this suspension. The latter was submitted to the thermally operated asymmetric amplification.43 In this cycle, nearly equimolar amounts of D- and L-3 dissolve during heating to afford a reduced amount of suspended 3 with amplified ee. Then, during cooling, the crystal recovers with no decrease in ee under a solution-phase racemization.
The stereochemical results of the asymmetric Strecker-type solid/solid reaction and following asymmetric amplification are summarized (Table 1, ESI, Table S4†). When P-enriched rac-1 ([CD(−)235KBr]-1) was reacted with 2·CH3CO2H under solid/solid conditions at room temperature, after the amplification of ee by the thermal operation, D-aminonitrile 3 with >99% ee was synthesized and collected in 49% yield by filtration (Table 1, entry 1). In contrast, the reaction of M-enriched rac-1 with 2·CH3CO2H gave the oppositely configured L-3 with high enantioenrichment thanks to the amplification of ee (entry 2). These stereochemical outcomes are reproducible, as is evident in entries 3 and 4. When the solid/solid reaction was performed at higher temperatures (35 and 40 °C), it required a shorter reaction time (4 and 2 days, respectively), affording the product 3. After the asymmetric amplification, the same stereochemical relationships could be reproducibly observed, i.e., D- and L-3 were synthesized by the reaction of P- and M-enriched rac-1, respectively (entries 5–8). When 20 mol% of the corresponding solid imine 4 was added to the initial reaction mixture, the Strecker-type solid/solid reaction proceeded to afford aminonitrile 3 with the same stereochemical relationship (entries 9 and 10). Therefore, the crystal chirality of rac-1 is responsible for the enantioselective synthesis of aminonitrile 3 at the solid/solid interface. It should be noted that stochastic stereochemical outcomes were obtained when a suspension of racemic conglomerate 3, synthesized without using any chiral materials, was subjected to thermal operation.42
| Entrya | rac-1b | Solid/solid reaction | Aminonitrile 3 | ||
|---|---|---|---|---|---|
| Temp/°C | Time/days | eec/% (config.) | Yield/% | ||
a Unless otherwise noted, the molar ratio used was rac-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2·CH3CO2H = 1 2·CH3CO2H = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (mmol). The powder state of the reaction mixture is maintained throughout the reactions.
b Absolute handedness of the arrangement of the racemate is indicated and the sign of the Cotton effect at 235 nm in solid-state CD spectra with KBr matrix is indicated in parentheses.
c The ee value was determined by HPLC on a chiral stationary phase. Values were obtained after 7–10 cycles of asymmetric amplification.
d The yield indicates the amount of solid product 3 collected by filtration after the amplification procedure. The amount of 3 in the filtrate is not included.
e Imine 4, prepared from 2 and p-tolualdehyde, was added to the solid/solid reaction. The molar ratio used was 4 1 (mmol). The powder state of the reaction mixture is maintained throughout the reactions.
b Absolute handedness of the arrangement of the racemate is indicated and the sign of the Cotton effect at 235 nm in solid-state CD spectra with KBr matrix is indicated in parentheses.
c The ee value was determined by HPLC on a chiral stationary phase. Values were obtained after 7–10 cycles of asymmetric amplification.
d The yield indicates the amount of solid product 3 collected by filtration after the amplification procedure. The amount of 3 in the filtrate is not included.
e Imine 4, prepared from 2 and p-tolualdehyde, was added to the solid/solid reaction. The molar ratio used was 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) rac-1 rac-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2·CH3CO2H = 0.2 2·CH3CO2H = 0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (mmol). The remaining 4 after the solid/solid reaction reacted with HCN to afford 3 at the amplification stage. 1 (mmol). The remaining 4 after the solid/solid reaction reacted with HCN to afford 3 at the amplification stage.
|
|||||
| 1 | P (−) | rt | 20 | >99 (D) | 49 |
| 2 | M (+) | rt | 20 | 92 (L) | 53 |
| 3 | P (−) | rt | 20 | 99 (D) | 55 |
| 4 | M (+) | rt | 20 | 91 (L) | 65 |
| 5 | P (−) | 35 | 4 | 97 (D) | 44 |
| 6 | P (−) | 40d | 2 | 99 (D) | 60 |
| 7 | M (+) | 35d | 4 | 97 (L) | 52 |
| 8 | M (+) | 40d | 2 | 92 (L) | 58 |
| 9e | P (−) | 35 | 4 | >99 (D) | 54 |
| 10e | M (+) | 35 | 4 | >99 (L) | 58 |
The source of chirality of the present reaction is only the helical molecular arrangement of racemic cyanohydrin 1, whose chirality would be lost upon dissolution or melt; therefore, the asymmetry induced at the solid/solid interface of cyanohydrin 1 and ammonium salt 2·CH3CO2H. Degradation of cyanohydrin to aldehyde and HCN is indispensable for the progress of the present transformation. Hence, different from the lattice-controlled stereospecific reactions, here chiral solid substrate 1 provides its surface as a chiral reactive site, at which formation of imine 4 followed by cyanide addition to this 4 should occur in an enantioselective manner.25
Possible interactions inducing the enantioenrichment may include the following: (i) enantioface-selective adsorption of imine 4; (ii) asymmetric addition of chirally modified HCN adsorbed on the chiral surface of 1; and (iii) chiral recognition of the produced 3 by the crystal 1. The enantioselectivity of the reaction at the solid/solid interface is below the detection level but it is sufficient for amplification. Therefore, highly enantioenriched 3 with the corresponding absolute configuration to that of rac-1 was obtained reproducibly.
Conclusions
In summary, we have demonstrated the stereospecific Strecker-type reaction between the chiral P/M-crystal of racemic 4-methylmandelonitrile (1) and the achiral crystal of benzhydryl ammonium acetate (2·CH3CO2H) at the solid/solid interface. The stereoselectivity was induced solely by the crystal chirality of racemic cyanohydrin 1 to afford highly enantioenriched aminonitrile 3 in combination with amplification of ee. Chiral crystallization of racemic cyanohydrin acted as an origin of chirality for the synthesis of highly enantioenriched amino acids, which are hydrolyzed products of aminonitriles. The described Strecker-type reaction, under solvent-free conditions, expands the potential to address enantioenriched α-amino acids from the perspectives of synthetic, systems and prebiotic chemistry.Author contributions
T. K conceptualized and planned the research. Y. Y., Y. T. and R. K. performed the experiments. Y. Y. and K. N. analyzed the data. T. K. and Y. Y. interpreted the results and wrote the manuscript. T. K. supervised the work.Conflicts of interest
There are no conflicts to declare.Acknowledgements
T. K. acknowledges support from JSPS KAKENHI (Grant No JP16K05692 & JP19K22190). The authors thank Dr. Naoya Takamatsu and Professor Yuji Tokunaga for the preliminary researches.References
- L. Pasteur, Ann. Chim. Phys., 1848, 24, 442 Search PubMed
.
- I. Weissbuch and M. Lahav, Chem. Rev., 2011, 111, 3236 CrossRef CAS
.
- K.-H. Ernst, Phys. Status Solidi B, 2012, 249, 2057 CrossRef CAS
.
- K. Soai, T. Shibata, H. Morioka and K. Choji, Nature, 1995, 378, 767 CrossRef CAS
.
- D. K. Kondepudi, R. J. Kaufman and N. Singh, Science, 1990, 250, 975 CrossRef CAS PubMed
.
- R. Naaman, Y. Paltiel and D. H. Waldeck, Nat. Rev. Chem., 2019, 3, 250 CrossRef CAS
.
- J. M. Ribó, J. Crusats, F. Sagués, J. Claret and R. Rubires, Science, 2001, 292, 2063 CrossRef PubMed
.
- C. Viedma, Phys. Rev. Lett., 2005, 94, 065504 CrossRef PubMed
.
- W. L. Noorduin, A. A. C. Bode, M. van der Meijden, H. Meekes, A. F. van Etteger, W. J. P. van Enckevort, P. C. M. Christianen, B. Kaptein, R. M. Kellogg, T. Rasing and E. Vlieg, Nat. Chem., 2009, 1, 729 CrossRef CAS
.
- M. Sakamoto, N. Uemura, R. Saito, H. Shimobayashi, Y. Yoshida, T. Mino and T. Omatsu, Angew. Chem., Int. Ed., 2021, 60, 12819 CrossRef CAS
.
- Y. Inoue, Chem. Rev., 1992, 92, 741 CrossRef CAS
.
- B. L. Feringa and R. A. van Delden, Angew. Chem., Int. Ed., 1999, 38, 3418 CrossRef
.
- T. Kawasaki, Y. Matsumura, T. Tsutsumi, K. Suzuki, M. Ito and K. Soai, Science, 2009, 324, 492 CrossRef CAS
.
- J. D. Horvath, A. Koritnik, P. Kamakoti, D. S. Sholl and A. J. Gellman, J. Am. Chem. Soc., 2004, 126, 14988 CrossRef CAS PubMed
.
- K. Penzien and G. M. J. Schmidt, Angew. Chem., Int. Ed. Engl., 1969, 8, 608 CrossRef CAS
.
- M. Sakamoto, M. Kato, Y. Aida, K. Fujita, T. Mino and T. Fujita, J. Am. Chem. Soc., 2008, 130, 1132 CrossRef CAS
.
- A. Johansson and M. Håkansson, Chem. – Eur. J., 2005, 11, 5238 CrossRef CAS
.
- H. Koshima, K. Ding, Y. Chisaka and T. Matsuura, J. Am. Chem. Soc., 1996, 118, 12059 CrossRef CAS
.
- M. Hasegawa, C. M. Chung, N. Muro and Y. Maekawa, J. Am. Chem. Soc., 1990, 112, 5676 CrossRef CAS
.
- J. van Mil, L. Addadi, M. Lahav and L. Leiserowitz, J. Chem. Soc., Chem. Commun., 1982, 584 RSC
.
- Y. T. Osano, A. Uchida and Y. Ohashi, Nature, 1991, 352, 510 CrossRef CAS
.
- P. C. Chenchaiah, H. L. Holland and M. F. Richardson, J. Chem. Soc., Chem. Commun., 1982, 436 RSC
.
- A. Kuhn and P. Fischer, Angew. Chem., Int. Ed., 2009, 48, 6857 CrossRef CAS
.
- T. Kawasaki, S. Kamimura, A. Amihara, K. Suzuki and K. Soai, Angew. Chem., Int. Ed., 2011, 50, 6796 CrossRef CAS
.
- S. Miyagawa, K. Yoshimura, Y. Yamazaki, N. Takamatsu, T. Kuraishi, S. Aiba, Y. Tokunaga and T. Kawasaki, Angew. Chem., Int. Ed., 2017, 56, 1055 CrossRef CAS PubMed
.
- K. Soai, T. Kawasaki and A. Matsumoto, Acc. Chem. Res., 2014, 47, 3643 CrossRef CAS PubMed
.
- T. Kawasaki, K. Jo, H. Igarashi, I. Sato, M. Nagano, H. Koshima and K. Soai, Angew. Chem., Int. Ed., 2005, 44, 2774 CrossRef CAS PubMed
.
- B. Rodríguez, T. Rantanen and C. Bolm, Angew. Chem., Int. Ed., 2006, 45, 6924 CrossRef
.
- G. Rothenberg, A. P. Downie, C. L. Raston and J. L. Scott, J. Am. Chem. Soc., 2001, 123, 8701 CrossRef CAS
.
- Y. Kaji, N. Uemura, Y. Kasashima, H. Ishikawa, Y. Yoshida, T. Mino and M. Sakamoto, Chem. – Eur. J., 2016, 22, 16429 CrossRef CAS PubMed
.
- R. R. E. Steendam, M. M. Verkade, T. J. B. van Benthem, H. Meekes, W. J. P. van Enckevort, J. Raap, F. P. J. T. Rutjes and E. Vlieg, Nat. Commun., 2014, 5, 5543 CrossRef CAS PubMed
.
- Q. Zhang, L. Jia, J.-R. Wang and X. Mei, CrystEngComm, 2016, 18, 8834 RSC
.
- T. Kawasaki, N. Takamatsu, S. Aiba and Y. Tokunaga, Chem. Commun., 2015, 51, 14377 RSC
.
- F. Toda and K. Tanaka, Chem. Rev., 2000, 100, 1025 CrossRef
.
-
E. L. Eliel and S. W. Wilen, Stereochemistry of organic compounds, Wiley, New York, 1994 Search PubMed
.
- A. Strecker, Ann. Chem. Pharm., 1850, 75, 27 CrossRef
.
- S. L. Miller, Science, 1953, 117, 528 CrossRef CAS
.
- E. T. Peltzer, J. L. Bada, G. Schlesinger and S. L. Miller, Adv. Space Res., 1984, 4, 69 CrossRef CAS PubMed
.
- J. Bocková, N. C. Jones, U. J. Meierhenrich, S. V. Hoffmann and C. Meinert, Commun. Chem., 2021, 4, 86 CrossRef
.
- K. Harada, Nature, 1963, 200, 1201 CrossRef CAS PubMed
.
- E. T. Peltzer and J. L. Bada, Nature, 1978, 272, 443 CrossRef CAS
.
- S. Miyagawa, S. Aiba, H. Kawamoto, Y. Tokunaga and T. Kawasaki, Org. Biomol. Chem., 2019, 17, 1238 RSC
.
- S. Aiba, N. Takamatsu, T. Sasai, Y. Tokunaga and T. Kawasaki, Chem. Commun., 2016, 52, 10834 RSC
.
- T. Kawasaki, H. Kubo, S. Nishiyama, T. Saijo, R. Yokoi and Y. Tokunaga, J. Am. Chem. Soc., 2021, 143, 19525 CrossRef CAS
.
- S. Clevers and G. Coquerel, CrystEngComm, 2020, 22, 7407 RSC
.
- K. Harada, T. Okawara and K. Matsumoto, Bull. Chem. Soc. Jpn., 1973, 46, 1865 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2117047, 2204872 and 2117487. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2ob01802k |
| This journal is © The Royal Society of Chemistry 2023 |