Proteomic mapping of intercellular synaptic environments via flavin-dependent photoredox catalysis†
Tyler J.
Bechtel
a,
Jayde M.
Bertoch
a,
Aleksandra K.
Olow
 b,
Margaret
Duich
a,
Cory H.
White
a,
Tamara
Reyes-Robles
*a,
Olugbeminiyi O.
Fadeyi‡
*a and
Rob C.
Oslund‡
b,
Margaret
Duich
a,
Cory H.
White
a,
Tamara
Reyes-Robles
*a,
Olugbeminiyi O.
Fadeyi‡
*a and
Rob C.
Oslund‡
 *a
*a
aExploratory Science Center, Merck & Co., Inc., Cambridge, MA 02139, USA. E-mail: tamara.reyes.robles@merck.com; niyi@induprolabs.com; rob@induprolabs.com
bGenetics and Pharmacogenomics, Merck & Co., Inc., South San Francisco, CA 94080, USA
First published on 2nd December 2022
Abstract
Receptor–ligand interactions play essential signaling roles within intercellular contact regions. This is particularly important within the context of the immune synapse where protein communication at the surface of physically interacting T cells and antigen-presenting cells regulate downstream immune signaling responses. To identify protein microenvironments within immunological synapses, we combined a flavin-dependent photocatalytic labeling strategy with quantitative mass spectrometry-based proteomics. Using α-PD-L1 or α-PD-1 single-domain antibody (VHH)-based photocatalyst targeting modalities, we profiled protein microenvironments within the intercellular region of an immune synapse-forming co-culture system. In addition to enrichment of both PD-L1 and PD-1 with either targeting modality, we also observed enrichment of both known immune synapse residing receptor–ligand pairs and surface proteins, as well as previously unknown synapse residing proteins.
Introduction
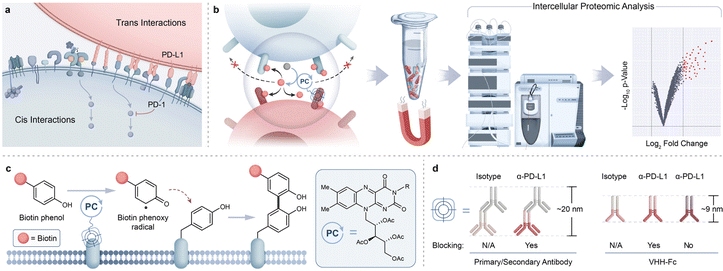
Many important protein-mediated biological processes occur at the cell surface through cis and trans protein interactions to drive intracellular signal transduction and communication between neighboring cells (Fig. 1a).1 For example, physical cellular association between T cells and antigen-presenting cells (APCs) involve key protein-mediated interactions that include T cell receptors (TCRs) and peptide-MHC complexes (pMHCs), adhesion molecules, and costimulatory/checkpoint proteins.2,3 How these proteins arrange and engage within these intercellular environments ultimately drive T cell activity with physiological and pathophysiological consequences. For example, transcellular interactions between immune checkpoint ligand PD-L1, overexpressed on tumor cells, and its receptor PD-1, on T cells, results in T cell inactivation that hinders T cell-mediated killing of tumors.4 Elucidation of this interaction has led to the development of PD-1/PD-L1 immunotherapies, highlighting the importance of understanding immune synapse protein environments. To this end, technologies that unbiasedly assess proteins within these intercellular microenvironments can play an important role in providing a more comprehensive picture of synaptic protein landscapes (Fig. 1b). Ideally, an approach that leverages the use of therapeutic modalities that effectively target these intercellular regions would open opportunities for profiling synaptic protein microenvironments in a high-resolution manner.Proximity labeling strategies such as BioID,5 APEX,6 and TurboID7 have been developed that rely on the use of an engineered ligase or peroxidase enzyme fused to a protein of interest to generate reactive biotin-containing species to tag neighboring protein environments. These methods have been extensively used to proteomically profile subcellular compartments,8–10 as well as cell–cell interfaces.11,12 While these approaches have been transformative in identifying protein networks within subcellular regions, the enzyme dependency of these technologies may perturb the overall physical properties of the targeting therapeutic modality as well as limit the ability for temporal control of the labeling reaction. Also, the requirement for cell engineering hinders utility within cell types that are not amenable to recombinant expression. Other emerging proximity labeling methods include the use of a two-step photooxidation process wherein enzymes or fluorescent dyes are activated to first generate singlet oxygen species for promiscuous protein oxidation followed by a second step condensation- or disulfide-based trapping of oxidized protein residues.13–16 Ultimately, despite the many successful efforts to profile a wide range of cellular regions using proteomic-based proximity labeling, implementation of a direct labeling technology within intercellular environments remains a challenge.
Photocatalytic-based methods offer an attractive alternative to the above approaches through their relatively much smaller size compared to enzymes, their exquisite spatiotemporal control, and their ability to induce direct activation of a biotin probe for targeted surface protein labeling.17–19 Indeed, such efforts have been deployed for covalent labeling and mass spectrometry (MS)-based identification of cis-interacting protein environments on mammalian cell surfaces.20–23 Photocatalytic-based approaches are also expanding into more complex cellular environments as demonstrated through our recently disclosed photocatalytic cell tagging (PhoTag) technology.24 This method exploits visible light-activation of flavin co-factors to generate phenoxy radicals21 (Fig. 1c) for selective tagging of physically associated cellular interfaces to facilitate downstream transcriptome analysis of cell–cell interactions. Given the importance of identifying proteins that reside within synaptic microenvironments, we envisioned that this flavin photocatalytic system would be well suited for extending beyond current photocatalyst-based profiling activities in monoculture systems and enable the proteomic analysis of intercellular protein interactions. Here we describe the development of a robust MS-based intercellular proteomics platform that leverages the use of VHH-based photocatalyst conjugates to capture and identify synaptic protein microenvironments (Fig. 1b).
Results and discussion
To identify an optimal targeting modality for profiling the immune synapse, we began our studies by first investigating the use of a primary/secondary antibody or a blocking/non-blocking VHH-Fc photocatalyst conjugate system to label PD-L1 microenvironments on the surface of B cells in monoculture (Fig. 1d).24 Accordingly, we site-selectively functionalized a blocking α-PD-L1 VHH-Fc that disrupts the PD-1/PD-L1 interaction with riboflavin tetraacetate (RFT) (ESI Fig. 1a†) for PD-L1-targeted proximity labeling. JY B cells overexpressing PD-L1 (hereafter referred to as JY PD-L1 cells) were incubated with α-PD-L1 VHH-Fc-RFT followed by the addition of biotin phenol (ESI Fig. 1b†) and irradiated with visible blue light (450 nm). Western blot analysis showed light- and time-dependent biotinylation compared to an isotype control antibody and confocal imaging analysis confirmed that these labeling events were selective for the cell surface (Fig. 2a and ESI Fig. 2†). Next, biotinylated proteins were enriched on streptavidin magnetic beads and identified by tandem mass tag (TMT)-based quantitative LC-MS/MS proteomic analysis (Fig. 2b and ESI Table 1†). Targeted labeling with α-PD-L1 VHH-Fc-RFT resulted in PD-L1 being the most highly significantly enriched protein with enrichment of other immune relevant surface proteins such as HLA class I and II molecules, CD40, and ICAM protein family members, highlighting the ability of this technology to identify proteins on the surface of B cells in a targeted and proximity-based fashion (Fig. 2b). In an analogous experiment, a primary/secondary antibody system, comprised of a commercially available blocking α-PD-L1 primary antibody and a goat anti-mouse secondary antibody non-site selectively labeled with RFT, yielded similar results by western blot and confocal microscopy (ESI Fig. 3†). Likewise, proteomic analysis of the two-antibody system showed strong enrichment for PD-L1 as well as several other proteins similarly identified by VHH-Fc (ESI Fig. 4a, 5 and Table 2†).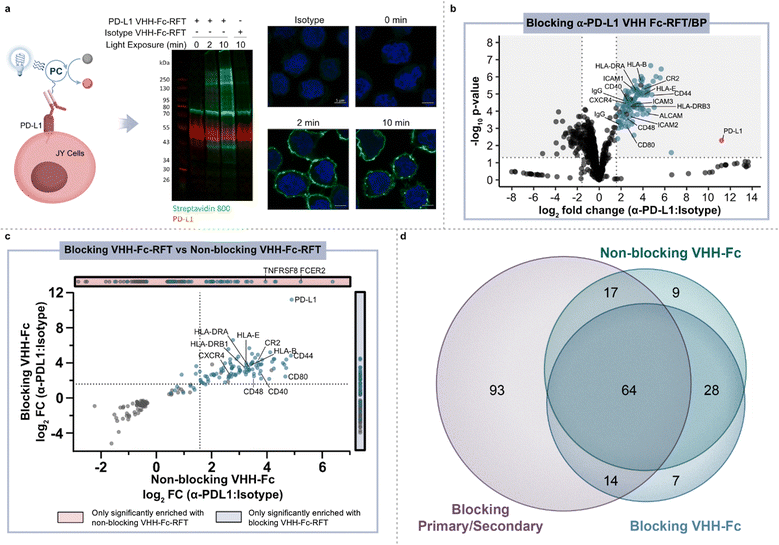 | ||
Fig. 2 Photocatalytic proximity labeling of PD-L1 microenvironments on the surface of B cells. (a) Schematic depicting α-PD-L1 VHH-Fc-RFT targeted labeling on the surface of JY PD-L1 cells and western blot and immunofluorescent microscopy analysis of labeling events. Biotinylation levels (green) increase as a function of blue-light irradiation time in PD-L1 targeted samples, but not isotype controls. Nuclei are labeled with Hoechst stain (blue) and scale bars indicate 5 μm. (b) Volcano plot of statistical significance vs. fold-enrichment for α-PD-L1 VHH-Fc targeted vs. isotype-targeted biotinylation on JY PD-L1 cells following 2 minutes of blue-light irradiation. Significantly enriched cell-surface proteins (p-value <0.05 and log2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) fold change > 1.58) are indicated as blue dots and PD-L1 is indicated as a red dot (n = 3 experiments). (c) Quadrant plot comparing log2 fold change > 1.58) are indicated as blue dots and PD-L1 is indicated as a red dot (n = 3 experiments). (c) Quadrant plot comparing log2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) fold change (FC) enrichment of proteins in blocking VHH-Fc and non-blocking VHH-Fc PD-L1 targeted datasets. Only proteins that are significantly enriched (p-value <0.05) in both datasets are displayed. Cell-surface-localizing proteins, determined by Uniprot25 and the Surfaceome,26 are indicated with a blue dot. (d) Venn diagram depicting the overlap of significantly enriched proteins (p value <0.05 and log2 fold change (FC) enrichment of proteins in blocking VHH-Fc and non-blocking VHH-Fc PD-L1 targeted datasets. Only proteins that are significantly enriched (p-value <0.05) in both datasets are displayed. Cell-surface-localizing proteins, determined by Uniprot25 and the Surfaceome,26 are indicated with a blue dot. (d) Venn diagram depicting the overlap of significantly enriched proteins (p value <0.05 and log2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) FC > 1.58) identified with α-PD-L1 targeting modalities that include a blocking primary/secondary antibody-RFT, blocking VHH-Fc-RFT, and non-blocking VHH-Fc-RFT. FC > 1.58) identified with α-PD-L1 targeting modalities that include a blocking primary/secondary antibody-RFT, blocking VHH-Fc-RFT, and non-blocking VHH-Fc-RFT. | ||
Encouraged by the targeted labeling results of our PD-L1 blocking modalities, we next explored the use of a non-blocking α-PD-L1 VHH-Fc-RFT that does not disrupt PD-1/PD-L1 interactions and serves as a spectator within the PD-L1 microenvironment. As expected, PD-L1 was highly enriched along with other surface proteins that included a well-known PD-L1 cis interactor, CD80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 27 (ESI Fig. 4b and Table 3†). Enrichment of this cis interactor resulted from the use of the non-blocking VHH that, in addition to lacking PD-1/PD-L1 blocking capacity, does not block PD-L1/CD80 interactions.24 Differences in protein enrichment between blocking and non-blocking α-PD-L1 VHH-Fc targeting modalities were analyzed using a quadrant plot that compares the fold change enrichment for statistically significantly enriched proteins identified in blocking and/or non-blocking proximity labeling experiments (Fig. 2c). While many proteins, such as PD-L1, CD44, ALCAM, CR2, CXCR4, ICAM1, and HLA molecules were highly enriched by both VHH modalities, CD80 enrichment was much more pronounced with the non-blocking VHH-Fc compared to the blocking VHH-Fc which blocks both PD-1/PD-L1 and PD-L1/CD80 interactions.
27 (ESI Fig. 4b and Table 3†). Enrichment of this cis interactor resulted from the use of the non-blocking VHH that, in addition to lacking PD-1/PD-L1 blocking capacity, does not block PD-L1/CD80 interactions.24 Differences in protein enrichment between blocking and non-blocking α-PD-L1 VHH-Fc targeting modalities were analyzed using a quadrant plot that compares the fold change enrichment for statistically significantly enriched proteins identified in blocking and/or non-blocking proximity labeling experiments (Fig. 2c). While many proteins, such as PD-L1, CD44, ALCAM, CR2, CXCR4, ICAM1, and HLA molecules were highly enriched by both VHH modalities, CD80 enrichment was much more pronounced with the non-blocking VHH-Fc compared to the blocking VHH-Fc which blocks both PD-1/PD-L1 and PD-L1/CD80 interactions.
Venn diagram analysis of the enriched protein hits across all three PD-L1 targeting modalities (primary/secondary antibody, blocking VHH, non-blocking VHH) on JY PD-L1 monoculture cells revealed shared overlap of 64 proteins (Fig. 2d and ESI Table 4†), many of which have been implicated in PD-L1 biology. For instance, ADAM10 and ADAM17 have been reported to directly cleave the PD-L1 surface domain leading to increased checkpoint inhibitor resistance.28 EPHA3 has been recently shown to physically interact with PD-L1 in a checkpoint inhibitor (atezolizumab) dependent fashion.29 Other proteins such as CR2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 and HLA molecules31,32 have been observed to co-express with PD-L1. Interestingly, inhibition of CXCR4
30 and HLA molecules31,32 have been observed to co-express with PD-L1. Interestingly, inhibition of CXCR4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 33 and SEMA4D34 as well as a CD40
33 and SEMA4D34 as well as a CD40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35,36 agonist (all proteins enriched from PD-L1 targeting) are being investigated for evidence of preclinical enhancement in combination with PD-1/PD-L1 checkpoint blockade in patients. To the best of our knowledge, the combined identification of CXCR4, SEMA4D and CD40 has not been previously shown to be proximal to PD-L1 outside of our photocatalyst labeling technologies (ESI Fig. 6†), highlighting the use of therapeutic-based modalities as an attractive approach for profiling relevant cell surface environments for discovery of novel drug targets. Finally, in further comparison of the RFT-based VHH and primary/secondary antibody systems, we observed a higher overlap of shared proteins between the VHH targeting modalities as well as a much smaller number of total enriched proteins using the VHHs compared to the primary/secondary antibody system (Fig. 2d). Collectively, these results showcase the high-resolution nature of this technology where targeting modality size (primary/secondary antibody vs. VHH) and/or binding mode (blocking vs. non-blocking) ultimately impact the microenvironment that is captured on the cell surface.
35,36 agonist (all proteins enriched from PD-L1 targeting) are being investigated for evidence of preclinical enhancement in combination with PD-1/PD-L1 checkpoint blockade in patients. To the best of our knowledge, the combined identification of CXCR4, SEMA4D and CD40 has not been previously shown to be proximal to PD-L1 outside of our photocatalyst labeling technologies (ESI Fig. 6†), highlighting the use of therapeutic-based modalities as an attractive approach for profiling relevant cell surface environments for discovery of novel drug targets. Finally, in further comparison of the RFT-based VHH and primary/secondary antibody systems, we observed a higher overlap of shared proteins between the VHH targeting modalities as well as a much smaller number of total enriched proteins using the VHHs compared to the primary/secondary antibody system (Fig. 2d). Collectively, these results showcase the high-resolution nature of this technology where targeting modality size (primary/secondary antibody vs. VHH) and/or binding mode (blocking vs. non-blocking) ultimately impact the microenvironment that is captured on the cell surface.
Having showcased this protein labeling technology in a monoculture system, we next explored the feasibility of applying our labeling technology within immune synapse environments. For this experiment, we used a co-culture system comprised of engineered Jurkat T cells (CD8+, PD-1+) that also express a TCR with specificity for HLA-A02![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 37 (hereafter referred to as Jurkat JS86-PD-1 cells) and JY PD-L1 cells (HLA-A02 positive). Co-incubation of these two cell types results in T cell activation as measured by IL-2 production (ESI Fig. 7†). For immunosynaptic PD-L1-targeted proximity labeling, JY PD-L1 cells were pre-labeled with the non-blocking α-PD-L1 VHH-Fc-RFT and co-incubated with Jurkat JS86-PD-1 cells for 30 minutes to allow for cell interaction, after which biotin phenol was added and cells were irradiated with blue light. Under these conditions, flow cytometry analysis confirmed cis and trans labeling of both cell types and confocal microscopy revealed highly selective labeling events within APC-T cell contact sites (ESI Fig. 8 and 9†). To confirm that we are not only tagging PD-L1, but also achieving transcellular tagging of the cognate receptor, PD-1, labeled cells were lysed and biotinylated proteins were enriched using streptavidin magnetic beads followed by elution and downstream LC-MS based proteomic analysis (Fig. 3a). As anticipated, we observed strong enrichment of both PD-L1 and PD-1, (Fig. 3b, middle panel and ESI Table 5†) confirming the ability of our method to achieve protein labeling on both sides of the immune synapse.
37 (hereafter referred to as Jurkat JS86-PD-1 cells) and JY PD-L1 cells (HLA-A02 positive). Co-incubation of these two cell types results in T cell activation as measured by IL-2 production (ESI Fig. 7†). For immunosynaptic PD-L1-targeted proximity labeling, JY PD-L1 cells were pre-labeled with the non-blocking α-PD-L1 VHH-Fc-RFT and co-incubated with Jurkat JS86-PD-1 cells for 30 minutes to allow for cell interaction, after which biotin phenol was added and cells were irradiated with blue light. Under these conditions, flow cytometry analysis confirmed cis and trans labeling of both cell types and confocal microscopy revealed highly selective labeling events within APC-T cell contact sites (ESI Fig. 8 and 9†). To confirm that we are not only tagging PD-L1, but also achieving transcellular tagging of the cognate receptor, PD-1, labeled cells were lysed and biotinylated proteins were enriched using streptavidin magnetic beads followed by elution and downstream LC-MS based proteomic analysis (Fig. 3a). As anticipated, we observed strong enrichment of both PD-L1 and PD-1, (Fig. 3b, middle panel and ESI Table 5†) confirming the ability of our method to achieve protein labeling on both sides of the immune synapse.
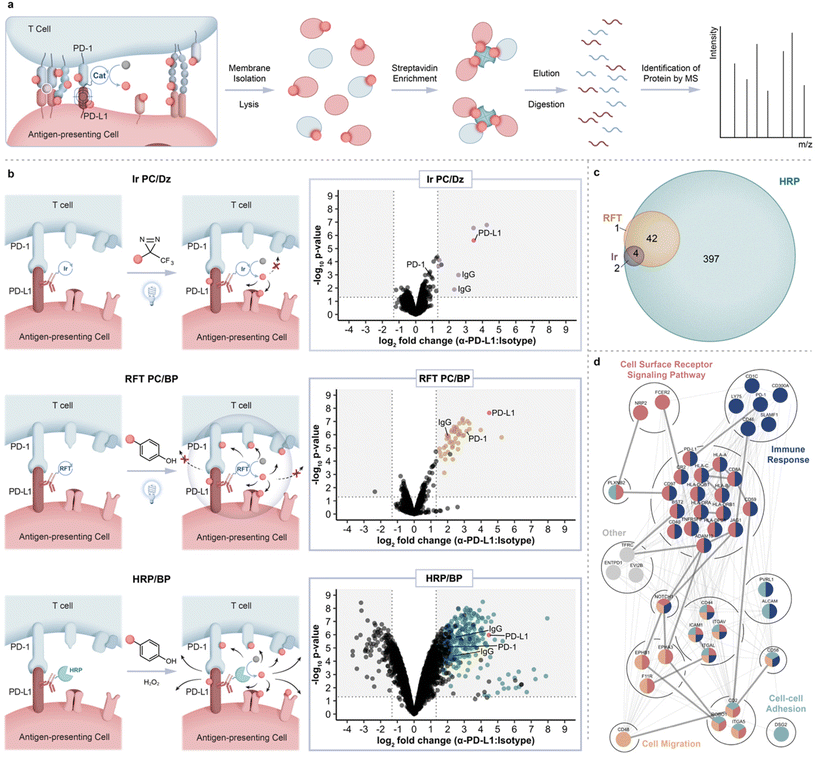 | ||
Fig. 3 Intercellular proteomic platform for identification of PD-L1 microenvironments within the immunological synapse of Jurkat JS86-PD-1 T cells and JY PD-L1 B cells. (a) Schematic depicting intercellular proximity labeling within the immunological synapse and downstream proteomic workflow. (b) Comparison of PD-L1 targeted catalytic labeling technologies including an iridium photocatalyst (top panel, purple), RFT (middle panel, yellow), and horseradish peroxidase (bottom panel, blue), within the immune synapse. Data are represented as volcano plots of statistical significance vs. fold-enrichment for non-blocking VHH-Fc PD-L1 targeted vs. isotype-targeted biotinylation. Significantly enriched cell-surface proteins (p-value <0.05 and log2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) FC > 1.32) are indicated as purple, yellow, or blue dots and PD-L1 is indicated as a red dot (n = 3 experiments). (c) Venn diagram depicting overlap between Ir, RFT, and HRP intercellular proximity labeling approaches. Significantly enriched proteins from each method were included (p-value <0.05 and log2 FC > 1.32) are indicated as purple, yellow, or blue dots and PD-L1 is indicated as a red dot (n = 3 experiments). (c) Venn diagram depicting overlap between Ir, RFT, and HRP intercellular proximity labeling approaches. Significantly enriched proteins from each method were included (p-value <0.05 and log2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) FC > 1.32). (d) STRING38 protein interaction network of significantly enriched PD-L1 proximal proteins within the immune synapse (p-value <0.05 and log2 FC > 1.32). (d) STRING38 protein interaction network of significantly enriched PD-L1 proximal proteins within the immune synapse (p-value <0.05 and log2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) FC > 1.32). Proteins are further grouped by biological process GO39 terms. Coloration indicates membership within broad gene ontology terms and multiple colors indicate nodes within multiple terms. Thick edges represent interaction evidence from experimental evidence while thin edges indicate evidence from other sources. FC > 1.32). Proteins are further grouped by biological process GO39 terms. Coloration indicates membership within broad gene ontology terms and multiple colors indicate nodes within multiple terms. Thick edges represent interaction evidence from experimental evidence while thin edges indicate evidence from other sources. | ||
To compare the ability of other methods to target and enrich PD-L1 and PD-1 within the synapse, we performed a similar labeling experiment using either a peroxidase enzyme that also generates a phenoxy radical for protein labeling or an iridium photocatalyst (Ir PC)-based micromap method that we recently disclosed20 for carbene-mediated proximal protein labeling. Accordingly, we conjugated non-blocking α-PD-L1 VHH-Fc to HRP or the iridium photocatalyst for labeling within the JY-Jurkat co-culture system. While proximity labeling via HRP in the presence of biotin phenol resulted in enrichment of PD-L1 and other proteins in similar fashion to RFT-mediated labeling, the overall number of significantly enriched proteins was nearly 10-fold higher with HRP (Fig. 3b, bottom panel, Fig. 3c and ESI Table 6†). Furthermore, PD-1 protein enrichment was much less pronounced with HRP-mediated labeling compared to the RFT system (Fig. 3b, middle and bottom panel). These results are consistent with previous observations of promiscuous labeling that occurs inside and outside of the synaptic region using HRP24 and as further confirmed by confocal imaging within this JY-Jurkat co-culture system (ESI Fig. 9†). On the other end of the spectrum, targeted labeling with iridium (ESI Fig. 1c†) in the presence of diazirine biotin (ESI Fig. 1d†) led to significant enrichment of PD-L1 in similar fashion to RFT but did not strongly enrich other proteins including PD-1 from the T cell surface (Fig. 3b, top and middle panel, Fig. 3c and ESI Table 7†). This observation is supported by the shorter labeling radius of the carbene intermediate that displays a much shorter half-life20 than the phenoxy radical from RFT or HRP labeling.17,18 These combined results demonstrate the enhanced ability of RFT-mediated synaptic labeling to co-enrich PD-L1 and its cognate receptor, PD-1, from synaptic environments compared to HRP and iridium-based micromap methods.
In addition to PD-L1 and PD-1 enrichment in the synapse with the RFT method, we detected other immune synapse ligand–receptor partners that include ICAM-1/LFA-1α, CD2/CD2 ligands (CD48, CD58 and CD59), JAG1/NOTCH1, CD8A/HLA class I molecules as well as other proteins with known roles within the immune synapse (CD40, CD46, CR2, and HLA class II molecules) (ESI Fig. 10 and Tables 5, 8†). We also identified several proteins with no known association within the immune synapse including adhesion molecule DSG2, receptor tyrosine kinase EPHA3, semaphorin cell surface receptor PLXNB2, and integrin ITGAV suggesting a potential role for these proteins within T cell–APC interactions (ESI Tables 5 and 8†). Analysis of all significantly enriched synaptic proteins using the search tool for retrieval of interacting genes (STRING)38 revealed mostly predicted (195) versus experimentally annotated (30) protein–protein interactions (Fig. 3d), further showcasing the utility of our approach to expand on what is known about these protein interactions, particularly within the context of immune synapse environments. Finally, reverse proximity labeling through use of a non-blocking α-PD-1 VHH Fc-RFT within the JY-Jurkat cell system strongly enriched for both PD-1 and PD-L1 (ESI Fig. 10, 11 and Table 9†). Of the total enriched protein hits from α-PD-1 targeted labeling, 13 proteins overlapped with the PD-L1 targeted list (Fig. 4a) that varied in protein expression levels on either Jurkat JS86-PD-1 or JY PD-L1 cells (ESI Fig. 12 and Table 10†). In contrast, enriched proteins that were unique to PD-1 or PD-L1 targeted labeling showed a closer correlation to protein levels detected on Jurkat JS86-PD-1 or JY PD-L1 cells, respectively (ESI Fig. 12 and Table 10†). As expected, the combined enrichment of proteins from α-PD-1 and/or α-PD-L1 showed GO39 term enrichment for immune-related biological processes that includes regulation of the immune response, positive regulation of cytokine production, and heterotypic cell–cell adhesion (Fig. 4b and ESI Table 11†). In addition, several of the enriched proteins (CD1c, CD40 and LY75) are currently undergoing clinical assessment in combination with PD-1/PD-L1 blockade, while others (CD2, ENTPD1, CD46 and EPHA3) are being evaluated as emerging cancer-based immunotherapies (ESI Table 8†). Collectively, these observations underscore the robust nature of the flavin-based photocatalytic system for capturing functionally relevant protein microenvironments from targeted labeling of either side of the synapse.
We next explored gene co-expression of the combined enriched protein hits from Fig. 4a with PD-L1 in tumor compared to healthy tissue using data from the cancer genome atlas (TCGA) and genotype-tissue expression (GTEX) (Fig. 4c). Specifically, this was evaluated using a separation score adapted from Dannenfelser et al.40 (see Methods section). Briefly, the separation score evaluates the tumor-specific targeting potential for two antigens using modalities that allow for Boolean gate antigen binding approaches, e.g. CAR-T, ADC, bispecifics. Specifically, in our case we are evaluating pairs of antigen 1 “AND” antigen 2, antigen 1 “AND NOT” antigen 2, or antigen 2 “AND NOT” antigen 1, where highest scoring pairs represent a good segregation of expression of tumor from normal samples when analyzed in unified expression space. Using this analysis, we observed that POSTN (pancreas), DSG2 (esophagus and lung), PECAM1 (lung), and APOA1 (testis) displayed increased segregation with PD-L1 in tumor types relative to healthy tissue (Fig. 4c). Furthermore, when we monitored gene expression across all enriched synaptic protein hits, pancreatic cancer stood out with strong gene expression correlation broadly observed in tumor versus healthy tissue compared to other tumor types such as breast cancer (ESI Fig. 13†). This result highlights potential novel therapeutic targeting approaches for pancreatic cancer, a tumor type with clinically unmet needs. In addition to co-expression analysis in tumor versus normal tissue, we explored the gene expression profiles of the enriched proteins list across various cell types using a single-cell RNA sequencing (scRNAseq) database to understand gene expression beyond the T and B cell co-culture system used in this study (Fig. 4d and ESI Table 12†). While expression of PDCD1 and other genes such as CD2, CD8a and ITGAL are specific to lymphoid and T cell lineages, many of the hits such as CD44, CD46, CD47, CD59, BST2 are broadly expressed across different immune cell types.
Conclusions
In this study we report the development of a flavin-based photoredox labeling platform for proteomic analysis of protein microenvironments within cellular synaptic regions. While most intercellular labeling technologies rely on the use of enzyme-based methods with a primary focus on imaging analysis of physical cell interactions,41–45 only two enzyme-based approaches (BioID and HRP) have reported proteomic profiling within cell–cell contact environments.11,12 Whereas those two methods utilized same-cell synapse forming monoculture systems, our approach enriched protein microenvironments from co-culture systems consisting of synapses formed between two different cell types. Furthermore, in contrast to the enzyme-based methods, a key feature of our technology includes the use of visible light for photocatalyst activation. As a result, light can be considered as a discretionary reagent that can be added or removed to instantaneously start or stop the protein labeling reaction and offers the unique ability for temporal control of protein labeling at any given phase of a biological event. Recently, Müller et al.14 reported the use of an MHC peptide antigen modified on the N-terminus with a fluorescent dye oxygen photosensitizer for proximal oxidation, followed by biotinylation capture of oxidized protein species in a co-culture system. However, sensitivity of other surface biomolecules such as lipids to singlet oxygen-mediated oxidation,46 absence of orthogonal selective synaptic labeling evidence (i.e. confocal imaging analysis), and highly variable binding properties displayed by N-terminally modified MHC class I peptides,47 limits the synaptic labeling selectivity and generalizability of this method. Through our method, we achieve selective and direct synaptic protein labeling on very short timescales (2 minutes) through use of non-blocking VHH modalities that act as spectators on the PD-1 on PD-L1 protein. Moreover, the ability to label from both sides of the synapse with our technology allows for one to adopt this labeling strategy to profile any intercellular junction in a targeting agnostic manner.While this technology holds promise for unbiased assessment of cis and trans protein interaction environments, disclosure of the initial version of this technology comes with the following considerations. Our technology requires the use of a non-blocking targeting modality consisting of a VHH-Fc fusion that is site selectively labeled with photocatalyst. As demonstrated with the blocking versus non-blocking VHH-Fc, protein enrichment within the protein microenvironment is sensitive to the nature of the targeting modality. Thus, care must be taken in the selection of targeting modalities that do not interfere with the biology under investigation. Moreover, the small size of the photocatalyst (<1 kDa) opens the possibility for direct attachment to other targeting modalities including proteins expressed on the cell surface. Finally, use of the iridium photocatalyst/diazirine biotin probe pair within the immune synapse led to minimal enrichment of surface proteins compared to the RFT system highlighting that while the iridium system is more suitable for target deconvolution or surveying highly localized protein microenvironments, the RFT photocatalyst/biotin phenol probe pair has optimal properties for broad capture of intercellular environments.
Using this flavin-based photocatalytic labeling system, we were able to selectively label, enrich, and identify transcellular protein interactions. This included our targeted interaction of PD-L1/PD-1, other known synaptic receptor–ligand pairs and surface proteins, as well as previously unidentified synaptic surface proteins. Ultimately, we envision that the synapse protein microenvironment information generated by this technology within immune synapse and other critical cell–cell contact regions can be combined with genomic and transcriptomic datasets/technologies for a more comprehensive understanding of cell–cell interaction biology to drive novel therapeutic development strategies.
Data availability
All ESI figures, experimental details, and synthesis information are included in ESI.†Author contributions
O. O. F. and R. C. O. conceived of the work. T. J. B., J. M. B., A. K. O., M. D., C. H. W., T. R. R., O. O. F., and R. C. O. designed and executed experiments. T. J. B., O. O. F., and R. C. O. wrote the manuscript with input from all authors.Conflicts of interest
T. J. B., J. M. B., A. K. O., M. D., C. H. W., T. R. R., O. O. F., and R. C. O. were employed by Merck & Co., Inc. during the experimental planning, execution and/or preparation of this manuscript. Merck & Co., Inc. has filed two patents (WO2020247725A1 and 63/285,520) related to this technology.Acknowledgements
We are very grateful to Nick Haining, Scott Lesley, Erik Hett, and Daria Hazuda (Merck & Co., Inc.) for helpful discussions and Eddie Bowman, Omar Nourzaie, and Sabine Le Saux (Merck & Co., Inc.) for providing protein and cell line reagents.References
- M. S. Almen, K. J. Nordstrom, R. Fredriksson and H. B. Schioth, BMC Biol., 2009, 7, 50 CrossRef PubMed.
- M. L. Dustin, Cancer Immunol. Res., 2014, 2, 1023–1033 CrossRef CAS PubMed.
- M. L. Dustin, A. K. Chakraborty and A. S. Shaw, Cold Spring Harbor Perspect. Biol., 2010, 2, a002311 CAS.
- H. O. Alsaab, S. Sau, R. Alzhrani, K. Tatiparti, K. Bhise, S. K. Kashaw and A. K. Iyer, Front. Pharmacol., 2017, 8, 561 CrossRef PubMed.
- K. J. Roux, D. I. Kim, M. Raida and B. Burke, J. Cell Biol., 2012, 196, 801–810 CrossRef CAS PubMed.
- H. W. Rhee, P. Zou, N. D. Udeshi, J. D. Martell, V. K. Mootha, S. A. Carr and A. Y. Ting, Science, 2013, 339, 1328–1331 CrossRef CAS PubMed.
- T. C. Branon, J. A. Bosch, A. D. Sanchez, N. D. Udeshi, T. Svinkina, S. A. Carr, J. L. Feldman, N. Perrimon and A. Y. Ting, Nat. Biotechnol., 2018, 36, 880–887 CrossRef CAS PubMed.
- A. C. Gingras, K. T. Abe and B. Raught, Curr. Opin. Chem. Biol., 2019, 48, 44–54 CrossRef CAS PubMed.
- C. D. Go, J. D. R. Knight, A. Rajasekharan, B. Rathod, G. G. Hesketh, K. T. Abe, J. Y. Youn, P. Samavarchi-Tehrani, H. Zhang, L. Y. Zhu, E. Popiel, J. P. Lambert, E. Coyaud, S. W. T. Cheung, D. Rajendran, C. J. Wong, H. Antonicka, L. Pelletier, A. F. Palazzo, E. A. Shoubridge, B. Raught and A. C. Gingras, Nature, 2021, 595, 120–124 CrossRef CAS PubMed.
- W. Qin, K. F. Cho, P. E. Cavanagh and A. Y. Ting, Nat. Methods, 2021, 18, 133–143 CrossRef CAS PubMed.
- O. Shafraz, B. Xie, S. Yamada and S. Sivasankar, Proc. Natl. Acad. Sci. U. S. A., 2020, 117, 31157–31165 CrossRef CAS PubMed.
- K. H. Loh, P. S. Stawski, A. S. Draycott, N. D. Udeshi, E. K. Lehrman, D. K. Wilton, T. Svinkina, T. J. Deerinck, M. H. Ellisman, B. Stevens, S. A. Carr and A. Y. Ting, Cell, 2016, 166, 1295–1307 CrossRef CAS PubMed.
- T. Tamura, M. Takato, K. Shiono and I. Hamachi, Chem. Lett., 2020, 49, 145–148 CrossRef CAS.
- M. Müller, F. Gräbnitz, N. Barandun, Y. Shen, F. Wendt, S. N. Steiner, Y. Severin, S. U. Vetterli, M. Mondal, J. R. Prudent, R. Hofmann, M. van Oostrum, R. C. Sarott, A. I. Nesvizhskii, E. M. Carreira, J. W. Bode, B. Snijder, J. A. Robinson, M. J. Loessner, A. Oxenius and B. Wollscheid, Nat. Commun., 2021, 12, 7036 CrossRef PubMed.
- T. L. To, K. F. Medzihradszky, A. L. Burlingame, W. F. DeGrado, H. Jo and X. K. Shu, Bioorg. Med. Chem. Lett., 2016, 26, 3359–3363 CrossRef CAS PubMed.
- K. Nakane, S. Sato, T. Niwa, M. Tsushima, S. Tomoshige, H. Taguchi, M. Ishikawa and H. Nakamura, J. Am. Chem. Soc., 2021, 143, 7726–7731 CrossRef CAS PubMed.
- T. J. Bechtel, T. Reyes-Robles, O. O. Fadeyi and R. C. Oslund, Nat. Chem. Biol., 2021, 17, 641–652 CrossRef CAS PubMed.
- K. A. Ryu, C. M. Kaszuba, N. B. Bissonnette, R. C. Oslund and O. O. Fadeyi, Nat. Rev. Chem., 2021, 5, 322–337 CrossRef CAS.
- C. P. Seath, A. D. Trowbridge, T. W. Muir and D. W. C. MacMillan, Chem. Soc. Rev., 2021, 50, 2911–2926 RSC.
- J. B. Geri, J. V. Oakley, T. Reyes-Robles, T. Wang, S. J. McCarver, C. H. White, F. P. Rodriguez-Rivera, D. L. Parker Jr., E. C. Hett, O. O. Fadeyi, R. C. Oslund and D. W. C. MacMillan, Science, 2020, 367, 1091–1097 CrossRef CAS PubMed.
- T. Hope, T. Reyes-Robles, K. A. Ryu, N. Removski, J. Maisonneuve, R. Oslund, O. Fadeyi and M. Frenette, ChemRxiv, 2021, DOI:10.26434/chemrxiv-2022-w8xz2.
- Z. Liu, X. Xie, Z. Huang, F. Lin, S. Liu, Z. Chen, S. Qin, X. Fan and P. R. Chen, Chem, 2022, 8, 2179–2191 CAS.
- N. E. S. Tay, K. A. Ryu, J. L. Weber, A. K. Olow, D. C. Cabanero, D. R. Reichman, R. C. Oslund, O. O. Fadeyi and T. Rovis, Nat. Chem., 2022 DOI:10.1038/s41557-022-01057-1.
- R. C. Oslund, T. Reyes-Robles, C. H. White, J. H. Tomlinson, K. A. Crotty, E. P. Bowman, D. Chang, V. M. Peterson, L. Li, S. Frutos, M. Vila-Perelló, D. Vlerick, K. Cromie, D. H. Perlman, S. Ingale, S. D. O. Hara, L. R. Roberts, G. Piizzi, E. C. Hett, D. J. Hazuda and O. O. Fadeyi, Nat. Chem. Biol., 2022, 18, 850–858 CrossRef CAS PubMed.
- The UniProt Consortium, Nucleic Acids Res., 2021, 49, D480–D489 CrossRef PubMed.
- D. Bausch-Fluck, U. Goldmann, S. Müller, M. van Oostrum, M. Müller, O. T. Schubert and B. Wollscheid, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, E10988–E10997 CrossRef CAS PubMed.
- Y. Zhao, C. K. Lee, C. H. Lin, R. B. Gassen, X. Xu, Z. Huang, C. Xiao, C. Bonorino, L. F. Lu, J. D. Bui and E. Hui, Immunity, 2019, 51, 1059–1073 CrossRef CAS PubMed.
- J. J. Orme, K. A. Jazieh, T. H. Xie, S. Harrington, X. Liu, M. Ball, B. Madden, M. C. Charlesworth, T. U. Azam, F. Lucien, B. Wootla, Y. L. Li, J. C. Villasboas, A. S. Mansfield, R. S. Dronca and H. D. Dong, OncoImmunology, 2020, 9, 1744980 CrossRef PubMed.
- E. Verschueren, B. Husain, K. Yuen, Y. Sun, S. Paduchuri, Y. Senbabaoglu, I. Lehoux, T. A. Arena, B. Wilson, S. Lianoglou, C. Bakalarski, Y. Franke, P. Chan, A. W. Wong, L. C. Gonzalez, S. Mariathasan, S. J. Turley, J. R. Lill and N. Martinez-Martin, Cell, 2020, 182, 329–344 CrossRef CAS PubMed.
- J. L. Platt, I. Silva, S. J. Balin, A. R. Lefferts, E. Farkash, T. M. Ross, M. C. Carroll and M. Cascalho, JCI Insight, 2017, 2, e90201 CrossRef PubMed.
- S. Aust, S. Felix, K. Auer, A. Bachmayr-Heyda, L. Kenner, S. Dekan, S. M. Meier, C. Gerner, C. Grimm and D. Pils, Sci. Rep., 2017, 7, 42929 CrossRef PubMed.
- M. G. M. Roemer, R. A. Redd, F. Z. Cader, C. J. Pak, S. Abdelrahman, J. Ouyang, S. Sasse, A. Younes, M. Fanale, A. Santoro, P. L. Zinzani, J. Timmerman, G. P. Collins, R. Ramchandren, J. B. Cohen, J. P. De Boer, J. Kuruvilla, K. J. Savage, M. Trneny, S. Ansell, K. Kato, B. Farsaci, A. Sumbul, P. Armand, D. S. Neuberg, G. S. Pinkus, A. H. Ligon, S. J. Rodig and M. A. Shipp, J. Clin. Oncol., 2018, 36, 942–950 CrossRef CAS PubMed.
- F. Grande, M. A. Occhiuzzi, B. Rizzuti, G. Ioele, M. De Luca, P. Tucci, V. Svicher, S. Aquaro and A. Garofalo, Molecules, 2019, 24, 550 CrossRef PubMed.
- L. Tamagnone and G. Franzolin, Cancer Res., 2019, 79, 5146–5148 CrossRef CAS PubMed.
- F. Barlesi, M. Lolkema, K. S. Rohrberg, C. Hierro, A. Marabelle, A. A. Razak, L. Teixeira, V. Boni, W. H. Miller, C. Aggarwal, M. Stern, O. Cirovic, O. Cirovic, B. Romagnoli, R. Christen, R. Dodia, K. Smart, B. Reis, N. Staedler, C. Watson and N. Steeghs, J. Immunother. Cancer, 2020, 8, A178–A178 Search PubMed.
- H. S. Ma, B. Poudel, E. Roussos, T. J. W. Sidhom, T. M. Robinson, B. Christmas, B. Scott, K. Cruz, S. Woolman, V. Z. Wall, T. Armstrong and E. M. Jaffee, Cancer Immunol. Res., 2019, 7, 428–442 CrossRef CAS PubMed.
- J. Borst, E. Devries, H. Spits, J. E. Devries, A. W. Boylston and E. A. Matthews, J. Immunol., 1987, 139, 1952–1959 CAS.
- D. Szklarczyk, A. L. Gable, D. Lyon, A. Junge, S. Wyder, J. Huerta-Cepas, M. Simonovic, N. T. Doncheva, J. H. Morris, P. Bork, L. J. Jensen and C. V. Mering, Nucleic Acids Res., 2019, 47, D607–D613 CrossRef CAS PubMed.
- Gene Ontology Consortium, Nucleic Acids Res., 2021, 49, D325–D334 CrossRef PubMed.
- R. Dannenfelser, G. M. Allen, B. VanderSluis, A. K. Koegel, S. Levinson, S. R. Stark, V. Yao, A. Tadych, O. G. Troyanskaya and W. A. Lim, Cell Syst., 2020, 11, 215–228 CrossRef CAS PubMed.
- K. F. Cho, T. C. Branon, S. Rajeev, T. Svinkina, N. D. Udeshi, T. Thoudam, C. Kwak, H. W. Rhee, I. K. Lee, S. A. Carr and A. Y. Ting, Proc. Natl. Acad. Sci. U. S. A., 2020, 117, 12143–12154 CrossRef CAS PubMed.
- Q. Liu, J. Zheng, W. P. Sun, Y. B. Huo, L. Y. Zhang, P. L. Hao, H. P. Wang and M. Zhuang, Nat. Methods, 2018, 15, 715–722 CrossRef CAS PubMed.
- J. D. Martell, M. Yamagata, T. J. Deerinck, S. Phan, C. G. Kwa, M. H. Ellisman, J. R. Sanes and A. Y. Ting, Nat. Biotechnol., 2016, 34, 774–780 CrossRef CAS PubMed.
- D. S. Liu, K. H. Loh, S. S. Lam, K. A. White and A. Y. Ting, PLoS One, 2013, 8, e52823 CrossRef CAS PubMed.
- M. A. Carpenter, Y. Wang, C. A. Telmer, B. F. Schmidt, Z. Yang and M. P. Bruchez, ACS Chem. Biol., 2020, 15, 2433–2443 CrossRef CAS PubMed.
- P. Di Mascio, G. R. Martinez, S. Miyamoto, G. E. Ronsein, M. H. G. Medeiros and J. Cadet, Chem. Rev., 2019, 119, 2043–2086 CrossRef CAS PubMed.
- M. A. Garstka, A. Fish, P. H. N. Celie, R. P. Joosten, G. M. C. Janssen, I. Berlin, R. Hoppes, M. Stadnik, L. Janssen, H. Ovaa, P. A. van Veelen, A. Perrakis and J. Neefjes, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 1505–1510 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ob02103j |
| ‡ Current address: InduPro Therapeutics, Cambridge, MA, 02139, USA. |
| This journal is © The Royal Society of Chemistry 2023 |