Pd(II)-catalyzed selective β-C–H functionalization of azobenzene carboxamides†
Rayavarapu
Padmavathi
and
Srinivasarao Arulananda
Babu
 *
*
Department of Chemical Sciences, Indian Institute of Science Education and Research (IISER) Mohali, Knowledge City, Sector 81, SAS Nagar, Mohali, Manauli P.O., Punjab 140306, India. E-mail: sababu@iisermohali.ac.in
First published on 18th January 2023
Abstract
We report a Pd(II)-catalyzed bidentate directing group 8-aminoquinoline-aided, site-selective β-C–H functionalization protocol for assembling modified azobenzene carboxamides. Considering the importance of azobenzenes in chemical sciences, this paper reports a new route for enriching the library of modified azobenzene motifs.
The transition metal-catalyzed C–H activation and functionalization, especially, the Pd(II)-catalyzed C–H functionalization of organic substrates has transpired as one of the cornerstone strategies.1,2 The site-selective C–H activation and functionalization of organic substrates have been accomplished by using appropriate directing groups (DGs).1,2
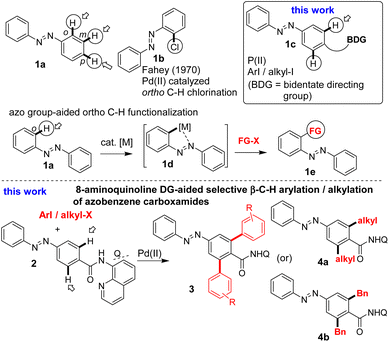
Azobenzenes are a phenomenal class of photochromic molecules and have found a wide range of applications in chemical sciences.3 Azobenzene-based molecules have been found to exhibit a wide range of biological activities, used as drug molecules and as tools to study the pharmacology of various bioactive compounds (Fig. 1).3,4 There have been constant efforts to synthesize novel and functionalized azobenzenes. Notably, the inherent –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– (azo) group-aided C–H functionalization strategy has been extended to functionalize the ortho position of azobenzene motif 1a (Scheme 1).5 Thus, there is immense scope for investigating the C–H bonds of other positions of azobenzenes via different C–H functionalization strategies.
N– (azo) group-aided C–H functionalization strategy has been extended to functionalize the ortho position of azobenzene motif 1a (Scheme 1).5 Thus, there is immense scope for investigating the C–H bonds of other positions of azobenzenes via different C–H functionalization strategies.
The Pd(II)-catalyzed, site-selective C–H functionalization of aromatic carboxamides assisted by the bidentate directing groups (e.g., 8-aminoquinoline (8-AQ) and picolinamide (PA)) has emerged as a benchmark strategy.2,6 Taking an impetus from this strategy, we herein report the Pd(II)-catalyzed 8-aminoquinoline DG-assisted, selective β-C–H arylation and alkylation of azobenzene motif 1c and synthesis of modified azobenzene carboxamides (Scheme 1).
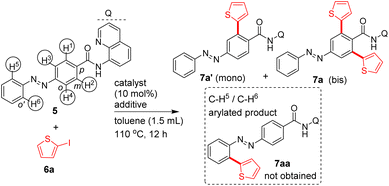
To commence with the Pd(II)-catalyzed aminoquinoline-assisted, site-selective β-C–H arylation and alkylation of azobenzene carboxamides, we prepared the azobenzene carboxamide 5 from its carboxylic acid and 8-AQ DG. Table 1 shows the optimization of reaction conditions for the β-C–H arylation of substrate 5 with 2-iodothiophene (6a) involving the Pd- or Ni-based catalysts and an iodide ion scavenger (e.g., AgOAc, Ag2CO3, K2CO3, etc.).2,6
| Entry | Substrate (mmol) | 6a (mmol) | Catalyst (mol%) | Additive (mmol) | Yield (%) | Yield (%) |
|---|---|---|---|---|---|---|
| a 36 h. b Toluene (2–3 mL). c p-Anisyl iodide (6b). d 4-Acetyliodobenzene (6c), toluene (1 mL), 160 °C, sealed tube, 40 h. | ||||||
| 1 | 5 (0.15) | 0.3 | Pd(OAc)2 | AgOAc (0.30) |
7a′![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) — — |
7a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35 35 |
| 2 | 5 (0.15) | 0.6 | Pd(OAc)2 | AgOAc (0.38) |
7a′![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) — — |
7a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
| 3 | 5 (0.15) | 0.6 | Nil | AgOAc (0.38) |
7a′![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) — — |
7a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) — — |
| 4 | 5 (0.15) | 0.6 | Pd(OAc)2 (5 mol%) | AgOAc (0.38) |
7a′![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) — — |
7a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 40 |
| 5 | 5 (0.15) | 0.6 | Pd(OAc)2 | AgOAc (0.30) |
7a′![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) — — |
7a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 38 38 |
| 6 | 5 (0.15) | 0.6 | Pd(OAc)2 | Ag2CO3 (0.30) |
7a′![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) — — |
7a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35 35 |
| 7 | 5 (0.15) | 0.6 | Pd(OAc)2 | NaHCO3 (0.30) |
7a′![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) — — |
7a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) — — |
| 8 | 5 (0.15) | 0.6 | Pd(OAc)2 | K2CO3 (0.30) |
7a′![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) — — |
7a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 43 43 |
| 9 | 5 (0.15) | 0.45 | Pd(OAc)2 | AgOAc (0.30) |
7a′![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) — — |
7a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 67 67 |
| 10a | 5 (0.2) | 0.8 | Ni(OTf)2 | Na2CO3 (0.4) |
7a′![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 47 47 |
7a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) — — |
| 11b | 10c (0.2) | 0.8 | Pd(OAc)2 | AgOAc (0.44) |
11e′![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) — — |
11e![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 32 32 |
| 12b | 10d (0.25) | 1 | Pd(OAc)2 | AgOAc (0.55) |
11f′![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) — — |
11f![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 51 51 |
| 13 | 10e (0.2) | 0.6c | Pd(OAc)2 | AgOAc (0.4) |
11g′![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) — — |
11g![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 41 41 |
| 14a,b | 10f (0.15) | 0.6c | Pd(OAc)2 | AgOAc (0.33) |
11h′![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) — — |
11h![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) — — |
| 15a | 10f (0.15) | 0.45d | Ni(OTf)2 | Na2CO3 (0.45) |
11i′![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) — — |
11i![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) — — |
| 16a,b | 10g (0.15) | 0.6c | Pd(OAc)2 | AgOAc (0.33) |
11j′![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) — — |
11j![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) — — |
|
||||||
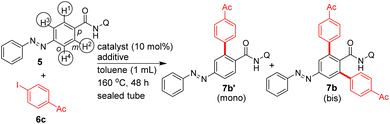
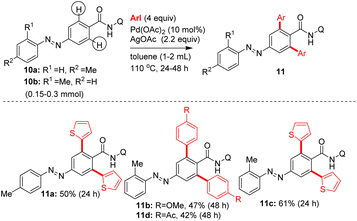
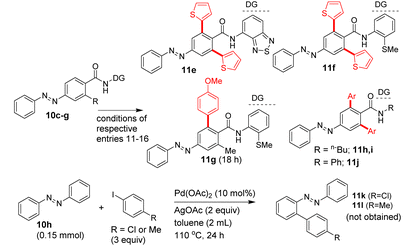
Heating a mixture of carboxamide 5, 2-iodothiophene (3 equiv.), Pd(OAc)2 (10 mol%) and AgOAc additive yielded the bis β-C–H arylated azobenzene carboxamide 7a in 67% yield (entry 9, Table 1). The column chromatography purification of the crude reaction mixtures (entries 1–9, Table 1) gave the bis β-C–H arylated product 7a. We did not obtain the mono β-C–H arylated product 7a′ or any other by-product such as the azo functionality-aided ortho C–H arylated product 7aa. We also tested the efficiency of other DGs. The C–H arylation of substrate 10c possessing the 4-amino-2,1,3-benzothiadiazole DG yielded the product 11e in 32% yield (Table 1). The C–H arylation of substrates 10d and 10e possessing the 2-(methylthio)aniline DG yielded the products 11f and 11g in 41–51% yields. However, the arylation of carboxamides 10f and 10g containing the corresponding simple amide moieties did not yield corresponding products 11h–j (Table 1). Treatment of azobenzene 10h with aryl iodides under the experimental conditions did not provide the other expected azo group-directed by-products 11k or 11l (Table 1). We then continued with optimization reactions to obtain the mono β-C–H arylated azobenzene product (e.g., 7a′) in good yield. Treatment of the carboxamide 5 with 6a or 6c in the presence of the Ni(OTf)2 catalyst and Na2CO3 additive in toluene gave the corresponding mono β-C–H arylated azobenzene carboxamides 7a′ or 7b′ in 47 and 50% yields (entry 10, Table 1 and entry 6, Table 2).
| Entry | 5 (mmol) | 6c (mmol) | Catalyst (mol%) | Additive (mmol) |
7b’![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) yield (%) yield (%) |
7b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) yield (%) yield (%) |
|---|---|---|---|---|---|---|
| a p-Xylene. b 36 h (in RB flask). c 5 (0.2 mmol), ArI (0.6 mmol), Na2CO3 (0.6 mmol), 36 h. d 5 (0.15 mmol), ArI (0.45 mmol), Na2CO3 (0.45 mmol), 36 h. e 5 (0.2 mmol), ArI (0.8 mmol), Na2CO3 (0.4 mmol), 36 h. f 5 (0.2 mmol), ArI (0.8 mmol), Na2CO3 (0.4 mmol), 36 h. | ||||||
| 1 | 0.15 | 0.45 | Ni(OTf)2 | KHCO3 (0.45) | 30 | — |
| 2 | 0.15 | 0.45 | Ni(OTf)2 | NaHCO3 (0.45) | 26 | — |
| 3 | 0.15 | 0.45 | Ni(OTf)2 | K2CO3 (0.45) | 39 | — |
| 4 | 0.15 | 0.45 | Ni(OTf)2 | Cs2CO3 (0.45) | — | — |
| 5 | 0.15 | 0.45 | Ni(OTf)2 | KOAc (0.45) | 11 | — |
| 6 | 0.15 | 0.45 | Ni(OTf)2 | Na2CO3 (0.9) | 50 | — |
| 7a | 0.15 | 0.45 | Ni(OTf)2 | Na2CO3 (0.45) | 40 | 17 |
| 8 | 0.15 | 0.45 | Ni(acac)2 | Na2CO3 (0.6) | 7 | — |
| 9 | 0.15 | 0.45 | NiCl2 | Na2CO3 (0.6) | — | — |
| 10 | 0.15 | 0.45 | Ni(OAc)2·4H2O | Na2CO3 (0.6) | 18 | — |
| 11b | 0.125 | 0.5 | Pd(OAc)2 | AgOAc (0.28) | — | 40 |
| 12 | 0.15 | 0.45 | Ni(OTf)2 | AgOAc (0.3) | — | — |
|
||||||
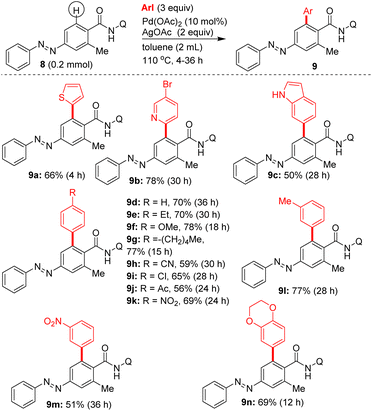
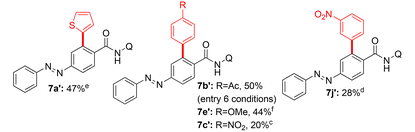
Subsequently, additional examples of mono β-C–H arylated azobenzene carboxamides 7e′ (44%), 7c′ (20%) and 7j′ (28%) were obtained in 20–50% yields (Table 2). Towards exploring the generality, the azobenzene carboxamides 5/8/10a,b were subjected to the Pd(II)-catalyzed 8-AQ DG-aided C–H arylation with various aryl iodides. These reactions yielded the corresponding bis and mono β-C–H arylated azobenzene carboxamides 7a–i,k,l (Scheme 2), 9a–n (Scheme 3) and 11a–d (Scheme 4) in 25–78% yields. The arylation of 5 with 6a was performed on a gram scale, which afforded 7a in 55% (1.04 g).
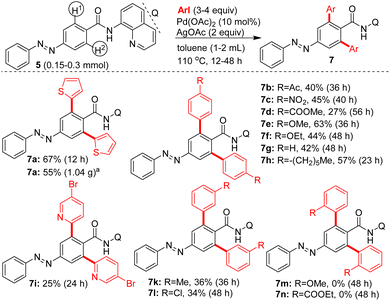 | ||
| Scheme 2 Pd(II)-catalyzed, 8-AQ DG-aided β-C–H arylation of 5. aGram scale reaction using 5 (1.29 g, 3.66 mmol), 6a (3 equiv.), Pd(OAc)2 (10 mol%), AgOAc (2 equiv.), toluene (3 mL), 110 °C, 12 h. | ||
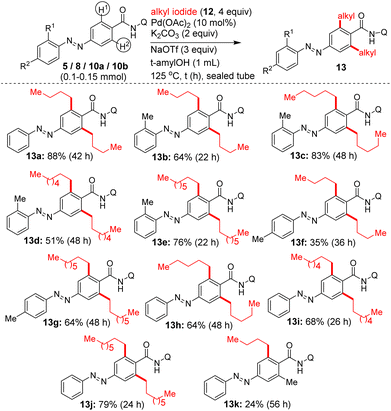
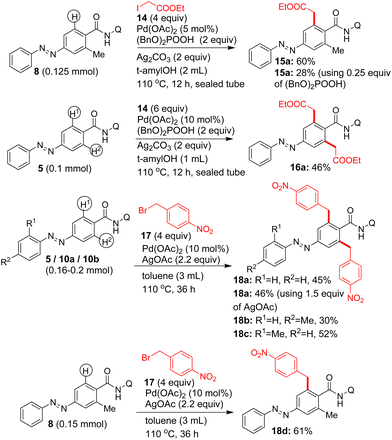
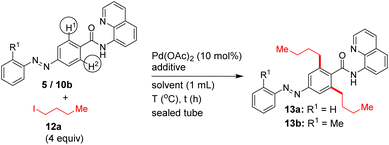
We then attempted the Pd(II)-catalyzed β-C–H alkylation of azobenzene carboxamides. Table 3 shows the optimization of reaction conditions for the C–H alkylation of 5/10b with 1-iodobutane (12a). Markedly, the Pd(II)-catalyzed, 8-AQ DG-aided β-C–H alkylation of 5 with 1-iodobutane in the presence of K2CO3 and NaOTf as additives in t-amylOH at 125 °C for 22–42 h successfully afforded the corresponding bis β-C–H alkylated azobenzene carboxamides 13a and 13b in 88 and 64% yields (entries 4 and 5, Table 3). Next, we performed the Pd(II)-catalyzed, 8-AQ DG-aided β-C–H alkylation of carboxamides 5/8/10a,b with various alkyl iodides, ethyl 2-iodoacetate and p-nitrobenzyl bromide. These reactions afforded the corresponding bis β-C–H alkylated azobenzene carboxamides 13a–j, 16a, 18a–c and mono β-C–H alkylated azobenzene carboxamide 13k, 15a and 18d in 24–88% yields (Schemes 5 and 6).
| Entry | Substrate (mmol) | Additive (mmol) | Solvent (mL) | Conditions | Yield (%) |
|---|---|---|---|---|---|
| a Catalyst = Ni(OTf)2. | |||||
| 1 | 5 (0.1) | Ag2CO3 (0.20), (BnO)2POOH (0.2) | t-AmylOH | 110 °C, 40 h |
13a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
| 2 | 5 (0.1) | KHCO3 (0.20), o-toluic acid (0.2) | 1,2-DCE | 110 °C, 40 h |
13a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
| 3 | 5 (0.1) | Ag2CO3 (0.40), CuBr2 (0.04) | H2O | 120 °C, 48 h |
13a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
| 4 | 5 (0.1) | K2CO3 (0.20), NaOTf (0.3) | t-AmylOH | 125 °C, 42 h |
13a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 88 88 |
| 5 | 10b (0.15) | K2CO3 (0.30), NaOTf (0.45) | t-AmylOH | 125 °C, 22 h |
13b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 64 64 |
| 6a | 10b (0.15) | Na2CO3 (0.30), PPh3 (20 mol%) | Toluene | 150 °C, 50 h |
13b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
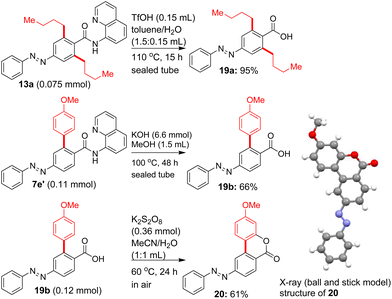
To reveal the synthetic utility, we attempted the removal of the 8-aminoquinoline DG after performing the Pd(II)-catalyzed β-C–H alkylation/arylation of azobenzene carboxamides. While we faced some issues in establishing the conditions for removing the 8-aminoquinoline DG, out of various attempts carried out, we succeeded in removing the 8-aminoquinoline DG from the bis β-C–H alkylated azobenzene carboxamide 13a by treating it with TfOH in toluene/H2O at 110 °C. This reaction yielded the azobenzene carboxylic acid 19a in 95% yield (Scheme 7). We also succeeded in removing the 8-aminoquinoline DG from the mono β-C–H arylated azobenzene carboxamides 7e′ by treating it with KOH in MeOH at 100 °C and this reaction yielded the azobenzene carboxylic acid 19b in 66% yield.
Finally, we also attempted the conversion of carboxylic acid 19b into the azobenzene lactone derivative 20via the δ-C–H lactonization of the azobenzene carboxylic acid 19b. Accordingly, treatment of 19b with K2S2O8 in MeCN/H2O at 60 °C for 24 h yielded the azobenzene-based lactone derivative 20 in 61% yield (Scheme 7). It may be noted that the synthesized azobenzene-based lactone derivative 20 is structurally similar to the lactone motifs of naturally occurring urolithin derivatives.7
The structure of the azobenzene-based lactone derivative 20 was unequivocally assigned based on the X-ray analysis. The X-ray structure of 20 also validates the following; (a) the Pd(II)-catalyzed C–H alkylation and arylation of azobenzene carboxamides selectively occurred at the β-C–H bonds next to the 8-AQ DG-linked carboxamide moiety, (b) based on the observed products from the arylation/alkylation of azobenzene carboxamides and the control experiment using 10h, the N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N functionality is intact and also the azo group-directed arylation did not occur under the experimental conditions used for the arylation of β-C–H bonds of azobenzene carboxamides containing a directing group (e.g., 8-AQ). Accordingly, the azo group-assisted ortho C–H functionalization or any other by-products were not obtained in characterizable amounts.
N functionality is intact and also the azo group-directed arylation did not occur under the experimental conditions used for the arylation of β-C–H bonds of azobenzene carboxamides containing a directing group (e.g., 8-AQ). Accordingly, the azo group-assisted ortho C–H functionalization or any other by-products were not obtained in characterizable amounts.
Conclusions
In summary, in this communication paper, we have shown the preliminary results comprising of the Pd(II)-catalyzed bidentate directing group 8-aminoquinoline-aided, site-selective β-C–H functionalization of azobenzene carboxamides. We have exemplified the usefulness of the Pd(II)-catalyzed 8-aminoquinoline DG-assisted C–H functionalization route for the synthesis of a wide range of novel and modified azobenzene carboxamides by negating the N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N (azo) group assistance.5 Considering the importance of azobenzenes in chemical sciences, this method comprising of the Pd(II)-catalyzed 8-aminoquinoline-assisted site-selective β-C–H functionalization of azobenzene carboxamides would serve as a useful method for enriching the libraries of azobenzene derivatives. Further studies to elaborate on the DG-assisted functionalization of azobenzenes and investigation of the photophysical properties of the synthesized compounds are in progress in our laboratory.
N (azo) group assistance.5 Considering the importance of azobenzenes in chemical sciences, this method comprising of the Pd(II)-catalyzed 8-aminoquinoline-assisted site-selective β-C–H functionalization of azobenzene carboxamides would serve as a useful method for enriching the libraries of azobenzene derivatives. Further studies to elaborate on the DG-assisted functionalization of azobenzenes and investigation of the photophysical properties of the synthesized compounds are in progress in our laboratory.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank IISER-M for funding, analytical facilities (NMR, HRMS and X-ray). Ms. Sonam Suwasia of our lab is thanked for helping in performing a few reactions during revision of this article.References
-
(a) F. Kakiuchi and S. Murai, Acc. Chem. Res., 2002, 35, 826 CrossRef CAS PubMed
; (b) S. Rej, A. Das and N. Chatani, Coord. Chem. Rev., 2021, 431, 213683 CrossRef CAS
; (c) J. Yamaguchi, A. D. Yamaguchi and K. Itami, Angew. Chem., Int. Ed., 2012, 51, 8960 CrossRef CAS PubMed
; (d) P. A. Arockiam, C. Bruneau and P. H. Dixneuf, Chem. Rev., 2012, 112, 5879 CrossRef CAS PubMed
; (e) T. Besset, T. Poisson and X. Pannecoucke, Chem. – Eur. J., 2014, 20, 16830 CrossRef CAS PubMed
; (f) A. Banerjee, S. Sarkar and B. K. Patel, Org. Biomol. Chem., 2017, 15, 505 RSC
; (g) M. Maraswami and T.-P. Loh, Synthesis, 2019, 51, 1049 CrossRef CAS
; (h) R. Mandal, B. Garai and B. Sundararaju, ACS Catal., 2022, 12, 3452 CrossRef CAS
; (i) M. Miura, T. Satoh and K. Hirano, Bull. Chem. Soc. Jpn., 2014, 87, 751 CrossRef CAS
; (j) R. Jazzar, J. Hitce, A. Renaudat, J. Sofack-Kreutzer and O. Baudoin, Chem. – Eur. J., 2010, 16, 2654 CrossRef CAS PubMed
; (k) J. He, M. Wasa, K. S. L. Chan, Q. Shao and J.-Y. Yu, Chem. Rev., 2017, 117, 8754 CrossRef CAS PubMed
; (l) L. Ackermann, R. Vicente and A. R. Kapdi, Angew. Chem., Int. Ed., 2009, 48, 9792 CrossRef CAS PubMed
; (m) T. Yoshino and S. Matsunaga, Adv. Synth. Catal., 2017, 359, 1245 CrossRef CAS
; (n) J. J. Topczewski and M. S. Sanford, Chem. Sci., 2015, 6, 70 RSC
; (o) S.-F. Ni, G. Huang, Y. Chen, J. S. Wright, M. Li and L. Dang, Coord. Chem. Rev., 2022, 455, 214255 CrossRef CAS
; (p) J. I. Higham and J. A. Bull, Org. Biomol. Chem., 2020, 18, 7291 RSC
; (q) S. K. Sinha, S. Guin, S. Maiti, J. P. Biswas, S. Porey and D. Maiti, Chem. Rev., 2022, 122, 5682 CrossRef CAS PubMed
; (r) R. Manoharan and M. Jeganmohan, Asian J. Org. Chem., 2019, 8, 1949 CrossRef CAS
; (s) R. Das, G. S. Kumar and M. Kapur, Eur. J. Org. Chem., 2017, 5439 CrossRef CAS
; (t) S. V. Kumar, S. Banerjee and T. Punniyamurthy, Org. Chem. Front., 2020, 7, 1527 RSC
; (u) T. Yanagi, K. Nogi and H. Yorimitsu, Tetrahedron Lett., 2018, 59, 2951 CrossRef CAS
; (v) M. M. Mingo, N. Rodríguez, R. G. Arrayás and J. C. Carretero, Chem. Commun., 2022, 58, 2034 RSC
.
-
(a) S. Rej, Y. Ano and N. Chatani, Chem. Rev., 2020, 120, 1788 CrossRef CAS PubMed
; (b) O. Daugulis, J. Roane and L. D. Tran, Acc. Chem. Res., 2015, 48, 1053 CrossRef CAS PubMed
; (c) X. Yang, G. Shan, L. Wang and Y. Rao, Tetrahedron Lett., 2016, 57, 819 CrossRef CAS
; (d) C. Sambiagio, D. Schönbauer, R. Blieck, T. Dao-Huy, G. Pototschnig, P. Schaaf, T. Wiesinger, M. F. Zia, J. Wencel-Delord, T. Besset, B. U. W. Maes and M. Schnürch, Chem. Soc. Rev., 2018, 47, 6603 RSC
; (e) R. K. Rit, M. R. Yadav, K. Ghosh and A. K. Sahoo, Tetrahedron, 2015, 71, 4450 CrossRef CAS
; (f) B. Liu, A. M. Romine, C. Z. Rubel, K. M. Engle and B.-F. Shi, Chem. Rev., 2021, 121, 14957 CrossRef CAS PubMed
; (g) G. He, B. Wang, W. A. Nack and G. Chen, Acc. Chem. Res., 2016, 49, 635 CrossRef CAS PubMed
; (h) S. A. Babu, Y. Aggarwal, P. Patel and R. Tomar, Chem. Commun., 2022, 58, 2612 RSC
.
-
(a) E. Merino, Org. Biomol. Chem., 2011, 40, 3835 CAS
; (b) W.-C. Geng, H. Sun and D.-S. Guo, J. Inclusion Phenom. Macrocyclic Chem., 2018, 92, 1 CrossRef CAS
; (c) M. Ghedini, I. Aiello, A. Crispini, A. Golemme, M. L. Deda and D. Pucci, Coord. Chem. Rev., 2006, 250, 1373 CrossRef CAS
; (d) C. Y. Chang, C. Fedele, A. Priimagi, A. Shishido and C. J. Barrett, Adv. Opt. Mater., 2019, 7, 1900091 CrossRef
; (e) X. Pang, J.-a. Lv, C. Zhu, L. Qin and Y. Yu, Adv. Opt. Mater., 2019, 31, 1904224 CrossRef CAS PubMed
; (f) H. A. Wegner, Angew. Chem., Int. Ed., 2012, 51, 4787 CrossRef CAS PubMed
; (g) A. Chevalier, P.-y. Renard and A. Romieu, Chem. – Asian J., 2017, 12, 2008 CrossRef CAS PubMed
; (h) A. S. Amrutha, K. R. S. Kumar and N. Tamaoki, ChemPhotoChem, 2019, 3, 337 CrossRef CAS
; (i) H. M. D. Bandara and S. C. Burdette, Chem. Soc. Rev., 2012, 41, 1809 RSC
; (j) S. Crespi, N. A. Simeth and B. König, Nat. Rev., 2019, 3, 133 CAS
; (k) M. Zhu and H. Zhou, Org. Biomol. Chem., 2018, 16, 8434 RSC
; (l) J. Vapaavuori, C. G. Bazuin and A. Prrimagi, J. Mater. Chem. C, 2018, 6, 2168 RSC
; (m) W. Wagner-Wysiecka, N. Łukasik, J. F. Biernat and E. Luboch, J. Inclusion Phenom. Macrocyclic Chem., 2018, 90, 189 CrossRef PubMed
; (n) M. D. Martino, L. Sessa, M. D. Matteo, B. Panunzi, S. Piotto and S. Concilio, Molecules, 2022, 27, 5643 CrossRef PubMed
.
-
(a) M. W. H. Hoorens, M. E. Ourailidou, T. Rodat, P. E. van der Wouden, P. Kobauri, M. Kriegs, C. Peifer, B. L. Feringa, F. J. Dekker and W. Szymanski, Eur. J. Med. Chem., 2019, 179, 133 CrossRef CAS PubMed
; (b) J. A. Frank, M. Moroni, R. Moshourab, M. Sumser, G. R. Lewin and D. Trauner, Nat. Commun., 2015, 6, 7118 CrossRef PubMed
; (c) B. Blanco, K. A. Palasis, A. Adwal, D. F. Callen and A. D. Abell, Bioorg. Med. Chem., 2017, 25, 5050 CrossRef CAS PubMed
; (d) S. A. Reis, B. Ghosh, J. A. Hendricks, D. M. Szantai-Kis, L. Törk, K. N. Ross, J. Lamb, W. Read-Button, B. Zheng, H. Wang, C. Salthouse, S. J. Haggarty and R. Mazitschek, Nat. Chem. Biol., 2016, 12, 317 CrossRef CAS PubMed
; (e) R. S. Stoll, M. V. Peters, A. Kühn, S. Heiles, R. Goddard, M. Buhl, C. M. Thiele and S. Hecht, J. Am. Chem. Soc., 2009, 131, 357 CrossRef CAS PubMed
.
-
(a) D. R. Fahey, J. Organomet. Chem., 1971, 27, 283 CrossRef CAS
; (b) D. R. Fahey, J. Chem. Soc. D, 1970, 417a RSC
; (c) T. H. L. Nguyen, N. Gigant and D. Joseph, ACS Catal., 2018, 8, 1546 CrossRef CAS
; (d) N. K. Mishra, J. Park, H. Oh, S. H. Han and I. S. Kim, Tetrahedron, 2018, 74, 6769 CrossRef CAS
; (e) G. Li, X. Ma, C. Jia, Q. Han, Y. Wang, J. Wang, L. Yu and S. Yang, Chem. Commun., 2017, 53, 1264 Search PubMed
.
-
(a) D. Shabashov and O. Daugulis, J. Am. Chem. Soc., 2010, 132, 3965 CrossRef CAS PubMed
; (b) N. Hoshiya, K. Takenaka, S. Shuto and J. Uenishi, Org. Lett., 2016, 18, 48 CrossRef CAS PubMed
; (c) K. S. Kanyiva, Y. Kuninobu and M. Kanai, Org. Lett., 2014, 16, 1968 CrossRef CAS PubMed
; (d) G. He, S.-Y. Zhang, W. A. Nack, Q. Li and G. Chen, Angew. Chem., Int. Ed., 2013, 52, 11124 CrossRef CAS PubMed
; (e) R. Parella and S. A. Babu, J. Org. Chem., 2015, 80, 12379 CrossRef CAS PubMed
; (f) R. Parella and S. A. Babu, J. Org. Chem., 2017, 82, 7123 CrossRef CAS PubMed
; (g) B. Gopalakrishnan, S. Mohan, R. Parella and S. A. Babu, J. Org. Chem., 2016, 81, 8988 CrossRef CAS PubMed
; (h) R. Padmavathi, R. Sankar, B. Gopalakrishnan, R. Parella and S. A. Babu, Eur. J. Org. Chem., 2015, 3727 CrossRef CAS
; (i) R. Tomar, D. Bhattacharya and S. A. Babu, Asian J. Org. Chem., 2022, 11, e202100736 CAS
; (j) R. Kaur, S. Banga and S. A. Babu, Org. Biomol. Chem., 2022, 20, 4391 RSC
; (k) R. Tomar, S. Suwasia, A. R. Choudhury, S. Venkataramani and S. A. Babu, Chem. Commun., 2022, 58, 12967 RSC
; (l) A. Seki and Y. Takahashi, Tetrahedron Lett., 2021, 74, 153130 CrossRef CAS
; (m) C. Wang, L. Zhang and J. You, Org. Lett., 2017, 19, 1690 CrossRef CAS PubMed
; (n) C. Reddy, N. Bisht, R. Parella and S. A. Babu, J. Org. Chem., 2016, 81, 12143 CrossRef CAS PubMed
.
- B. Cerdá, J. C. Espín, S. Parra, P. Martínez and F. A. Tomás-Barberán, Eur. J. Nutr., 2004, 43, 205 CrossRef PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2226308. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2ob02261c |
| This journal is © The Royal Society of Chemistry 2023 |