An unnatural amino acid modified human insulin derivative for visual monitoring of insulin aggregation†
Shantanu
Sen
 a,
Rafat
Ali
a,
Rafat
Ali
 a,
Harminder
Singh
a,
Harminder
Singh
 a,
Akanksha
Onkar
b,
Pratibha
Bhadauriya
b,
Subramaniam
Ganesh
b and
Sandeep
Verma
a,
Akanksha
Onkar
b,
Pratibha
Bhadauriya
b,
Subramaniam
Ganesh
b and
Sandeep
Verma
 *a
*a
aDepartment of Chemistry, Indian Institution of Technology Kanpur, Kanpur-208016, UP, India. E-mail: sverma@iitk.ac.in
bDepartment of Biological Sciences and Bioengineering, Indian Institution of Technology Kanpur, Kanpur-208016, UP, India
First published on 28th August 2023
Abstract
Insulin often forms toxic fibrils during production and transportation, which are deposited as amyloids at repeated injection sites in diabetic patients. Distinguishing early fibrils from non-fibrillated insulin is difficult. Herein, we introduce a chemically modified human insulin derivative with a distinct visual colour transition upon aggregation, facilitating insulin quality assessment.
Introduction
Fibrillation of commercially available insulin formulations not only has detrimental effects on the health of diabetic patients but also causes a huge commercial burden.1 Insulin misfolding and its subsequent fibril formation is a multistep process. Under unfavorable conditions, partially unfolded insulin monomers interact with each other to form early immature shorter insulin oligomers that eventually develop into mature larger fibrils.2 A high propensity of insulin fibrils to culminate into amyloidogenic aggregates at repeated injection sites prevents normal insulin absorption into the bloodstream and also results in subcutaneous insulin resistance.3 Subcutaneous amyloid deposition of cytotoxic insulin fibrils also leads to the formation of ‘insulin balls’ or ‘injection-mediated amyloidosis’.4–6 In addition to unwanted pathophysiological consequences, insulin fibrillation consequently has a huge negative impact on its storage, distribution, supply chain, and shelf-life as well as the cost.7,8 The possibility of insulin aggregation upon storage is another concern, as it is difficult to predict the viability of the product due to a lack of ready detection methods. Thus, a real-time quality assessment will be helpful for its safe and optimal usage.Finding traces of toxic and invisible minuscule fibrils in insulin doses is possible through HPLC, DLS, FTIR, CD spectroscopy, or size exclusion chromatography.9–13 Fluorophore probes such as thioflavin T (ThT), thioflavin S (ThS), Congo red, etc. can also be widely used to mark the presence of such minuscule fibrils.14–17 However, these fluorophores often experience aggregation-caused quenching (ACQ) phenomena at high concentrations or in the aggregated state. The ACQ effect mainly occurs due to the strong intermolecular π–π stacking or the formation of excimers, significantly compromising dye sensitivity and limiting the scope of practical applications.18 These drawbacks could be overcome by fluorescence molecules that work via manifesting aggregation-induced emission (AIE).19,20 This unique phenomenon, first observed by Tang et al., is in direct contrast to ACQ.21,22 The molecules displaying AIE are generally nonemissive or emit weak fluorescence in dilute solutions; however, they emit strong fluorescence in concentrated solutions or in the aggregated state, mainly due to the restriction of intermolecular rotations and π–π interactions. Several AIE based fluorophores have been reported as added excipients to detect amyloids of different proteins, including insulin.20,23–28 These include tetraphenylethene (TPE), bis(triphenylphosphonium)tetraphenylethene (TPE-TPP), 1,2-bis[4-(3-sulfonatopropoxyl)phenyl]-1,2-diphenylethene salt (BSPOTPE), 4-aminobiphenyl, 4,4′,4′′,4′′′-(anthracene-9,10-diylbis(ethene-2,1,1-triyl))tetrakis(N,N,N trimethylbenzenaminium) iodide (BDVAI), etc.24,25,27,29,30
AIE luminogens (AIEgens) can also reveal changes in protein dynamics, thus making them a potential detection tool to differentiate between fresh and fibrillated proteins.31,32 Site-specific conjugation of AIE probes with amyloidogenic proteins amyloid-β protein (Aβ) and α-synuclein was recently carried out for high-throughput screening of small-molecule inhibitors of protein fibrillation.33 AIEgens have also been explored to sense protein unfolding, demonstrating fluorescence turn-on in the presence of natively folded human serum albumin.34 Although there are studies signifying the protein amyloid-induced emission phenomenon, they work outside the possibility of visible detection of amyloidogenic transformation of a polypeptide or a protein.
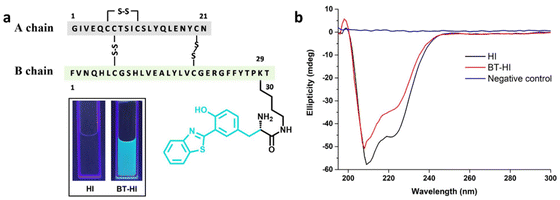
AIEgens exhibiting excited-state intramolecular proton transfer (ESIPT) phenomena are emerging as an important class of fluorescent probes with diverse applications, including bioimaging, metal ion detection, and latent fingerprinting, among others.35–37 ESIPT involves the rapid relaxation of energy in photoexcited molecules with intramolecular hydrogen bonds through very rapid keto–enol photo-tautomerization. This intriguing process imparts significant photophysical properties to the fluorescent molecules, such as a large Stokes shift, excellent photostability, high quantum yield, and low self-quenching.38–40 Herein, we introduce a novel approach for detecting insulin amyloids using the 3-(2-benzothiazolyl)tyrosine-conjugated human insulin derivative BT-HI (Fig. 1a). BT-HI exhibits a remarkable cyan-to-green colour change upon the initiation of insulin fibrillation, mainly due to the AIE-ESIPT phenomenon.
Results and discussion
In this work, 3-(2-benzothiazolyl)tyrosine37 is conjugated to the ε-amino group of the Lys-29 residue of the human insulin B chain to obtain a novel chemically modified human insulin derivative (BT-HI) through standard methods. The synthesis of BT-HI involved the selective coupling of tert-butyloxycarbonyl (Boc)-protected 3-(2-benzothiazolyl)tyrosine with Lys B29 of insulin in a sodium carbonate/sodium bicarbonate buffer of pH 10. The progress of the reaction was monitored using analytical reverse-phase HPLC. After completion, Boc-BT-HI was purified through preparative reverse-phase HPLC, followed by Boc deprotection using trifluoroacetic acid, resulting in the final product BT-HI. The structural integrity of BT-HI was affirmed by CD spectroscopy, where it shows a similar pattern to that of native human insulin with two minima at 208 nm and 222 nm (Fig. 1b), signifying a maintained or conserved native alpha-helical conformation even after the chemical modification. The purity of the synthesized insulin derivative was affirmed by analytical HPLC and SDS-PAGE (Fig. S12 and S7†).Benzothiazole groups have been explored in many instances to make AIE-probes sensitive to amyloid fibrils for both in vitro and in vivo imaging.31,32,41–47 One of such established ‘gold-standard’ tools for detecting amyloid fibrils is thioflavin T (ThT), a benzothiazole salt containing molecular-rotor dye.48,49 The ThT molecule contains a benzothiazole ring and a benzylamine moiety that rotate freely in solution through the carbon–carbon bond. Unrestrained rotation of these two rings leads to the transition of the ThT molecule into a non-fluorescent twisted internal charge transfer (TICT) state, which exhibits quenching of the excited state.48,50 Upon binding to β-sheet-rich amyloid fibrils, restricted molecular rotation of the ThT scaffold results in significant fluorescence enhancement. However, the ability of ThT is severely compromised in detecting small oligomers during the early aggregation phase due to its poor binding affinity and lower molar extinction coefficient.14,31,51
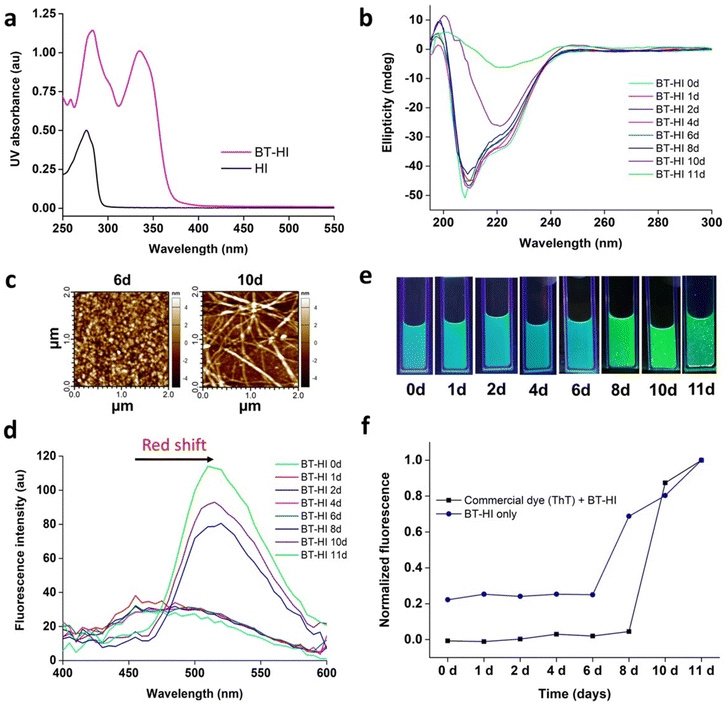
The photophysical properties of BT-HI were studied using UV-Vis, CD, and fluorescence spectroscopy. The added benzothiazole moiety causes spectral alterations in the UV-Vis spectra of BT-HI, which can be observed as an additional absorption peak at around 340 nm as compared to HI (Fig. 2a). To initiate the fibrillation, the BT-HI sample was incubated at an elevated temperature (65 °C) under acidic conditions (pH 1.6). The fibrillation kinetics of the incubated samples were studied by CD spectroscopy. The CD spectrum of the native insulin conformation is characterized by its major alpha-helical conformation with two minima in the spectrum at around 208 nm and 222 nm wavelengths, whereas denatured insulin molecules having extended β-sheets are self-assembled into a macromolecular architecture to form a cross-β insulin amyloid structure, represented by a single peak minimum in the CD spectrum at around 218 nm.12 The change in such an insulin secondary structure during fibrillation can be easily correlated from the pronounced difference in its CD spectra.
When subjected to incubation conditions (65 °C, pH 1.6), the three-dimensional structure of BT-HI gradually misfolded. The misfolding process can be evaluated from the reduction in alpha helicity in the CD spectra of the incubated BT-HI samples. In the early stages of fibrillation, only a small number of misfolded insulin molecules contribute to the formation of β-sheet structures which is reflected by a slight decrease in the overall helicity of BT-HI on the eighth day (Fig. 2b). However, as fibrillation progresses, the β-sheet population becomes increasingly dominant. By the tenth day of incubation, a complete structural distortion of the modified insulin is observed, as the alpha helix undergoes a noticeable transformation into a cross-β sheet structure. This transformation is confirmed by the disappearance of the characteristic alpha helix peaks at 208 nm and 222 nm in the CD spectra, accompanied by the appearance of a peak at 218 nm, which signifies the prevalence of β-sheet populations (Fig. 2b). From the time-based CD spectra of BT-HI, the conversion of the intact insulin structure into its unstructured form can be tracked easily. This transition of the alpha helix structure of BT-HI into fibrillated structures can also be confirmed by atomic force microscopy (AFM). The AFM micrograph of the tenth day incubated sample shows the prevalence of fibrillar morphologies as compared to the non-fibrillated insulin on the sixth day of incubation (Fig. 2c).
The 3-(2-benzothiazolyl)tyrosine upon aggregation is known to show AIE + ESIPT phenomena, which can be easily studied with the help of fluorescence spectroscopy.37 This AIE property of 3-(2-benzothiazolyl)tyrosine can be exploited to study the initiation of fibrillation in the newly synthesized BT-HI. In the fluorescence emission spectra of BT-HI, we observed that upon fibrillation initiation on the eight-day incubated samples, we observed a bathochromic shift of approximately 50 nm with enhancement in the peak intensity of their fluorescence emission spectra (Fig. 2d). The spectral alteration of BT-HI is observed with a red shift upon aggregation, a characteristic attributed to the conjugated 3-(2-benzothiazolyl)tyrosine moiety. The fluorescence emission intensity was further enhanced upon the complete transition of the alpha helix structure into the insoluble beta-sheet population due to the phenomenon of AIE.
Unrestrained rotation in the soluble form of protein (alpha helix) is expected to be restricted due to the formation of an insoluble population (beta sheet). Such changes in the protein conformation of BT-HI can be envisaged for altering the fluorescence properties of the conjugated 3-(2-benzothiazolyl) tyrosine moiety, which results in emission enhancement accompanied by a red shift in the fluorescence emission spectra (Fig. 2d). However, no such spectroscopic changes in the emission spectra were observed for the native insulin solution under similar conditions (Fig. S3†). This observation emphasizes the pivotal role of the 3-(2-benzothiazolyl)tyrosine group and its altered spatial arrangement between the non-fibrillated and fibrillated states of BT-HI in influencing the photophysical properties of the modified insulin. The changes in the protein conformations during BT-HI aggregation are directly linked to the shift observed in its fluorescence emission spectra. The change in the fluorescence properties of BT-HI upon initiation of fibrillation can also be visualized with the naked eye by irradiating the protein samples with UV light of wavelength 254 nm. We observed a prominent colour alteration of the BT-HI solution due to insulin fibrillation. The cyan colour of fresh BT-HI solution turned greenish as soon as fibrillation commenced (Fig. 2e).
To assess the sensitivity in detecting the initial fibrillation, the fluorescence emission spectra of the newly synthesized BT-HI was compared with the well-established amyloid detection dye thioflavin T (Fig. 2f). The incubated BT-HI samples were taken at different time intervals and mixed with the ThT dye and the fluorescence emission intensity at 488 nm of dye mixed samples was plotted. The ThT fluorescence intensity of the BT-HI samples exhibited the classical sigmoid trend of aggregation kinetics detection, suggesting that fibrillation began after 8 days of incubation. However, the CD spectra (Fig. 2b) revealed a decrease in the helicity of BT-HI after only 6 days of incubation, and the fluorescence emission spectra (Fig. 2d) showed a significant red shift at the same time point, indicating the formation of the initial fibrils. This highlights that ThT fluorescence is not highly sensitive for detecting the initial fibrils. On the other hand, when we plotted the auto-fluorescence emission intensity at 510 nm (λex 254 nm) of the BT-HI samples at different intervals, we observed a steep rise in the intensity of the spectrum after 6 days, signifying the initiation of fibrillation. This suggests that BT-HI autofluorescence is superior to ThT fluorescence in detecting the rare abundance of early insulin fibrillation. Furthermore, the enhanced fluorescence emission at 510 nm on the eighth day, along with a significant change in the colour simultaneously, allowed us to trace the presence of early cytotoxic insulin oligomers in BT-HI. Hence, BT-HI demonstrated higher sensitivity and accuracy compared to the available insulin aggregation detection probe.
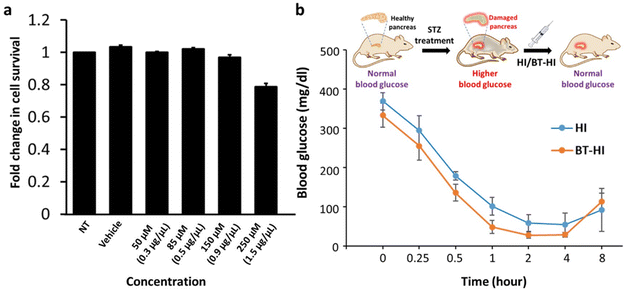
To determine whether the conjugation of 3-(2-benzothiazolyl)tyrosine influences the biological properties of insulin, we examined the biocompatibility and the bioactivity of BT-HI under in vitro and in vivo conditions. To test the biocompatibility of the newly synthesized insulin derivative BT-HI, we performed the MTT cytotoxicity assay (Fig. 3a), where we treated the HEK293T cells with varying concentrations of BT-HI. We observed that the compound displayed no cytotoxicity on the growth of HEK293T cells, even at higher concentrations of 150 micromoles.
Next, we estimated the bio-efficacy of the insulin derivative in the streptozotocin (STZ) induced diabetic mouse model. STZ destroys insulin-producing beta cells of the pancreas, depriving the mouse of its natural source of insulin and causing a spike in its blood glucose levels.52 For our experiments, we subjected the STZ-treated mice to overnight fasting and then subcutaneously injected either the native insulin (HI) or the modified insulin (BT-HI) at a specific dosage of 1 μg per 100 μl per 30 g of body weight. We closely monitored the blood glucose levels of the mice after injection, and the readings were recorded using a glucometer. The absolute blood glucose levels (mg dl−1) were then plotted against time for both experimental groups (Fig. 3b).
We observed that treatment with the modified insulin (BT-HI) reduced blood glucose levels in STZ-induced diabetic mice without causing any obvious phenotypic abnormalities (e.g., loss of motor functions) or mortality, and the blood glucose was comparable to the treatment with wild-type human insulin (HI). The blood glucose levels reached the original values on the next day of the experiment, with no mortality in either of the experimental groups. Though the blood glucose drop was below normal levels for both the HI and BT-HI treated animals which were hypoglycemic between the 2–4 h period at the used dosage, the similarity in the curves between the two treatment regimens indicates that BT-HI has a physiological activity equivalent to the wild-type insulin and a dose standardization to avoid such hypoglycemic phenotypes is required for future work. These results demonstrate that the newly synthesized insulin derivative is non-toxic to cells and equally effective as native human insulin in reducing blood glucose in diabetic mice. Nevertheless, additional research is imperative to thoroughly assess its efficacy and safety before considering its potential use for human consumption.
Conclusions
In conclusion, we have developed a novel fluorescent human insulin analog by blending the phenomena of AIE and ESIPT for visual self-reporting of insulin quality with the naked eye. This insulin analog can be used to monitor the early fibrillation onset or the rare abundance of oligomeric species through the change in its autonomous optical properties during fibrillation initiation. Insulin quality deteriorates due to protein aggregation, altering the way light interacts with the insulin-conjugated 3-(2-benzothiazolyl)tyrosine moiety. This novel approach promises superior sensitivity in determining insulin quality visibly, mainly beyond the scope of existing amyloid-detecting probes. This study gives consumers the independence to self-supervise insulin potency before its administration. Thus, on-demand disposal of insulin dosage aborts unnecessary insulin dumping and the resulting commercial loss.Ethical statement
All animal experiments were approved by the Animal Ethics Committee of Indian Institute of Technology Kanpur (protocol no: IITK/IAEC/2018/1048), and the experiments were conducted according to the guidelines proposed by the Committee for the Purpose of Control and Supervision of Experiments on Animals, Government of India.Conflicts of interest
An Indian Patent application has been filed on part of this work with SS, RA, HS and SV as the inventors (Patent Application No. 202311030590, filed on 28/04/2023).Acknowledgements
SV thanks the JC Bose Fellowship (SERB) and CSIR for financial support; SS and AO thank MHRD for a pre-doctoral fellowship; RA thanks DST for the INSPIRE Faculty Fellowship (DST/INSPIRE/04/2019/002454); HS thanks IIT Kanpur for financial support through the Institute Postdoctoral Fellowship. SG thanks the Science and Engineering Research Board (SERB) for the grant-in-aid in the form of the JC Bose National Fellowship (JCB/2022/000007).References
- S. Sen, R. Ali, A. Onkar, S. Ganesh and S. Verma, ChemBioChem, 2022, 23, e202100678 CrossRef CAS PubMed
.
- A. Das, M. Shah and I. Saraogi, ACS Bio Med Chem Au, 2022, 2, 205–221 CrossRef CAS PubMed
.
- M. Nakamura, Y. Misumi, T. Nomura, W. Oka, A. Isoguchi, K. Kanenawa, T. Masuda, T. Yamashita, Y. Inoue, Y. Ando and M. Ueda, Diabetes, 2019, 68, 609–616 CrossRef CAS
.
- F. E. Dische, C. Wernstedt, G. T. Westermark, P. Westermark, M. B. Pepys, J. A. Rennie, S. G. Gilbey and P. J. Watkins, Diabetologia, 1988, 31, 158–161 CrossRef CAS PubMed
.
- Y. Gupta, G. Singla and R. Singla, Indian J. Endocrinol. Metab., 2015, 19, 174–177 CrossRef PubMed
.
- B. N. Ratha, M. Kim, B. Sahoo, K. Garai, D. Lee and A. Bhunia, Biochim. Biophys. Acta, Biomembr., 2018, 1860, 1917–1926 CrossRef CAS PubMed
.
- R. C. Weiss, D. van Amerongen, G. Bazalo, M. Aagren and J. R. Bouchard, Manag. Care, 2011, 20, 42–47 Search PubMed
.
- L. Heinemann, K. Braune, A. Carter, A. Zayani and L. A. Krämer, J. Diabetes Sci. Technol., 2021, 15, 147–159 CrossRef CAS PubMed
.
- J. A. Karas, N. A. Patil, J. Tailhades, M.-A. Sani, D. B. Scanlon, B. E. Forbes, J. Gardiner, F. Separovic, J. D. Wade and M. A. Hossain, Angew. Chem., Int. Ed., 2016, 55, 14743–14747 CrossRef CAS PubMed
.
- H. B. Bohidar, Colloid Polym. Sci., 1989, 267, 159–166 CrossRef CAS
.
- S. Delbeck and H. M. Heise, J. Diabetes Sci. Technol., 2020, 15, 865–873 CrossRef PubMed
.
- S. Sen, P. Singh, N. K. Mishra, S. Ganesh, S. Sivakumar and S. Verma, Bioorg. Chem., 2021, 111, 104899 CrossRef CAS PubMed
.
- B. M. Teska, A. Kumar, J. F. Carpenter and M. F. Wempe, J. Pharm. Sci., 2015, 104, 1555–1560 CrossRef CAS PubMed
.
- L. P. Jameson, N. W. Smith and S. V. Dzyuba, ACS Chem. Neurosci., 2012, 3, 807–819 CrossRef CAS
.
- V. Kumar, N. Sami, T. Kashav, A. Islam, F. Ahmad and M. I. Hassan, Eur. J. Med. Chem., 2016, 124, 1105–1120 CrossRef CAS PubMed
.
- R. Dec, V. Babenko and W. Dzwolak, RSC Adv., 2016, 6, 97331–97337 RSC
.
- M. Ziaunys and V. Smirnovas, J. Phys. Chem. B, 2019, 123, 8727–8732 CrossRef CAS PubMed
.
-
B. Valeur, Molecular Fluorescence: Principles and Applications, Bernard, 2001, vol. 8 Search PubMed
.
- Y. Hong, J. W. Y. Lam and B. Z. Tang, Chem. Soc. Rev., 2011, 40, 5361–5388 RSC
.
- D. Ding, K. Li, B. Liu and B. Z. Tang, Acc. Chem. Res., 2013, 46, 2441–2453 CrossRef CAS PubMed
.
- J. Luo, Z. Xie, J. W. Y. Lam, L. Cheng, H. Chen, C. Qiu, H. S. Kwok, X. Zhan, Y. Liu, D. Zhu and B. Z. Tang, Chem. Commun., 2001, 1740–1741 RSC
.
- X. Ma, R. Sun, J. Cheng, J. Liu, F. Gou, H. Xiang and X. Zhou, J. Chem. Educ., 2016, 93, 345–350 CrossRef CAS
.
- Y. Tang, D. Zhang, Y. Zhang, Y. Liu, L. Cai, E. Plaster and J. Zheng, J. Mater. Chem. B, 2021, 10, 2280–2295 RSC
.
- Y. Jia, S. Guo, Q. Han, J. Zhu, X. Zhang, N. Na and J. Ouyang, J. Mater. Chem. B, 2021, 9, 5128–5135 RSC
.
- Q. Huang, J. Xie, Y. Liu, A. Zhou and J. Li, Bioconjugate Chem., 2017, 28, 944–956 CrossRef CAS
.
- M. Cingolani, L. Mummolo, F. Lugli, M. Zaffagnini and D. Genovese, New J. Chem., 2021, 45, 14259–14268 RSC
.
- Y. Hong, L. Meng, S. Chen, C. W. T. Leung, L. T. Da, M. Faisal, D. A. Silva, J. Liu, J. W. Y. Lam, X. Huang and B. Z. Tang, J. Am. Chem. Soc., 2012, 134, 1680–1689 CrossRef CAS PubMed
.
- D. Su, W. Diao, J. Li, L. Pan, X. Zhang, X. Wu and W. Mao, ACS Chem. Neurosci., 2022, 13, 540–551 CrossRef CAS PubMed
.
- N. Pradhan, D. Jana, B. K. Ghorai and N. R. Jana, ACS Appl. Mater. Interfaces, 2015, 7, 25813–25820 CrossRef CAS PubMed
.
- M. Kumar, Y. Hong, D. C. Thorn, H. Ecroyd and J. A. Carver, Anal. Chem., 2017, 89, 9322–9329 CrossRef CAS PubMed
.
- L. M. Needham, J. Weber, J. A. Varela, J. W. B. Fyfe, D. T. Do, C. K. Xu, L. Tutton, R. Cliffe, B. Keenlyside, D. Klenerman, C. M. Dobson, C. A. Hunter, K. H. Müller, K. O'Holleran, S. E. Bohndiek, T. N. Snaddon and S. F. Lee, Chem. Sci., 2020, 11, 4578–4583 RSC
.
- A. Kaur, L. D. Adair, S. R. Ball, E. J. New and M. Sunde, Angew. Chem., Int. Ed., 2022, 61, e202112832 CrossRef CAS PubMed
.
- L. Jia, W. Wang, Y. Yan, R. Hu, J. Sang, W. Zhao, Y. Wang, W. Wei, W. Cui, G. Yang, F. Lu, J. Zheng and F. Liu, ACS Appl. Mater. Interfaces, 2020, 12, 31182–31194 CrossRef CAS PubMed
.
- Y. Hong, C. Feng, Y. Yu, J. Liu, J. W. Y. Lam, K. Q. Luo and B. Z. Tang, Anal. Chem., 2010, 82, 7035–7043 CrossRef CAS PubMed
.
- P. Zhou and K. Han, Aggregate, 2022, 3, e160 CrossRef CAS
.
- T. Xiao, C. Bao, L. Zhang, K. Diao, D. Ren, C. Wei, Z.-Y. Li and X.-Q. Sun, J. Mater. Chem. A, 2022, 10, 8528–8534 RSC
.
- H. Singh and S. Verma, Chem. Commun., 2021, 57, 5290–5293 RSC
.
- A. C. Sedgwick, L. Wu, H.-H. Han, S. D. Bull, X.-P. He, T. D. James, J. L. Sessler, B. Z. Tang, H. Tian and J. Yoon, Chem. Soc. Rev., 2018, 47, 8842–8880 RSC
.
- Y. Li, D. Dahal, C. S. Abeywickrama and Y. Pang, ACS Omega, 2021, 6, 6547–6553 CrossRef CAS PubMed
.
- C. S. Abeywickrama, K. A. Bertman, L. J. Mcdonald, N. Alexander, D. Dahal, H. J. Baumann, C. R. Salmon, C. Wesdemiotis, M. Konopka, C. A. Tessier and Y. Pang, J. Mater. Chem. B, 2019, 7, 7502–7514 RSC
.
- N. Gour, V. Kshtriya, S. Gupta, B. Koshti, R. Singh, D. Patel and K. B. Joshi, ACS Appl. Bio Mater., 2019, 2, 4442–4455 CrossRef CAS
.
- F. Gorka, S. Daly, C. M. Pearson, E. Bulovaite, Y. P. Zhang, A. Handa, S. G. N. Grant, T. N. Snaddon, L. M. Needham and S. F. Lee, J. Phys. Chem. B, 2021, 125, 13710–13717 CrossRef CAS PubMed
.
- G. E. Lancioni, N. N. Singh, M. F. O'reilly, J. Sigafoos, F. Buonocunto, V. Sacco, J. Navarro, L. M. Addante and I. D'agostino, Percept. Mot. Skills, 2010, 111, 485–495 CrossRef PubMed
.
- V. L. Villemagne, Ageing Res. Rev., 2016, 30, 95–106 CrossRef CAS PubMed
.
- H. Watanabe, M. Ono, T. Ariyoshi, R. Katayanagi and H. Saji, ACS Chem. Neurosci., 2017, 8, 1656–1662 CrossRef CAS
.
- M. Ono, S. Hayashi, H. Kimura, H. Kawashima, M. Nakayama and H. Saji, Bioorg. Med. Chem., 2009, 17, 7002–7007 CrossRef CAS PubMed
.
- C. Gan, L. Zhou, Z. Zhao and H. Wang, Med. Chem. Res., 2013, 22, 4069–4074 CrossRef CAS
.
- M. Biancalana and S. Koide, Biochim. Biophys. Acta, Proteins Proteomics, 2010, 1804, 1405–1412 CrossRef CAS PubMed
.
- K. Gade Malmos, L. M. Blancas-Mejia, B. Weber, J. Buchner, M. Ramirez-Alvarado, H. Naiki and D. Otzen, Amyloid, 2017, 24, 1–16 CrossRef CAS PubMed
.
- V. I. Stsiapura, A. A. Maskevich, V. A. Kuzmitsky, V. N. Uversky, I. M. Kuznetsova and K. K. Turoverov, J. Phys. Chem. B, 2008, 112, 15893–15902 CrossRef CAS PubMed
.
- A. L. Cloe, J. P. R. O. Orgel, J. R. Sachleben, R. Tycko and S. C. Meredith, Biochemistry, 2011, 50, 2026–2039 CrossRef CAS PubMed
.
- A. A. Like and A. A. Rossini, Science, 1976, 193, 415–417 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and spectral data. See DOI: https://doi.org/10.1039/d3ob01038d |
| This journal is © The Royal Society of Chemistry 2023 |