High-efficiency electrosynthesis of ammonia with selective reduction of nitrite over an Ag nanoparticle-decorated TiO2 nanoribbon array†
Xiaoya
Fan
ab,
Xun
He
 b,
Xianchang
Ji
b,
Longcheng
Zhang
b,
Xianchang
Ji
b,
Longcheng
Zhang
 b,
Jun
Li
b,
Long
Hu
b,
Xiuhong
Li
b,
Shengjun
Sun
c,
Dongdong
Zheng
b,
Yongsong
Luo
b,
Yan
Wang
b,
Jun
Li
b,
Long
Hu
b,
Xiuhong
Li
b,
Shengjun
Sun
c,
Dongdong
Zheng
b,
Yongsong
Luo
b,
Yan
Wang
 b,
Lisi
Xie
d,
Qian
Liu
d,
Binwu
Ying
b,
Lisi
Xie
d,
Qian
Liu
d,
Binwu
Ying
 *a and
Xuping
Sun
*a and
Xuping
Sun
 *bc
*bc
aDepartment of Laboratory Medicine, West China Hospital, Sichuan University, Chengdu 610041, Sichuan, China. E-mail: yingbinwu@scu.edu.cn
bInstitute of Fundamental and Frontier Sciences, University of Electronic Science and Technology of China, Chengdu 610054, Sichuan, China. E-mail: xpsun@uestc.edu.cn; xpsun@sdnu.edu.cn
cCollege of Chemistry, Chemical Engineering and Materials Science, Shandong Normal University, Jinan 250014, Shandong, China
dInstitute for Advanced Study, Chengdu University, Chengdu 610068, Sichuan, China
First published on 11th January 2023
Abstract
Electrochemical nitrite (NO2−) reduction can yield value-added ammonia (NH3) while removing NO2− as an environmental pollutant in wastewater; however, it involves a six-electron transfer process and requires highly efficient and selective electrocatalysts. In this study, we report high-efficiency electrosynthesis of NH3via NO2− reduction enabled by an Ag nanoparticle-decorated TiO2 nanoribbon array on a titanium plate (Ag@TiO2/TP). When tested in 0.1 M NaOH containing 0.1 M NO2−, such Ag@TiO2/TP shows a large NH3 yield of 514.3 μmol h−1 cm−2 and a high faradaic efficiency of 96.4% at −0.5 V vs. a reversible hydrogen electrode. Significantly, it also demonstrates excellent durability for 12 h electrolysis.
Ammonia (NH3) is widely applied to manufacture nitrogen fertilizers, explosives, chemical products, etc., and it is also considered as an attractive hydrogen carrier and zero-carbon fuel.1–3 Although the Haber–Bosch method realizes industrial NH3 synthesis from hydrogen and nitrogen under high temperature and high pressure, this process is highly energy-intensive and emits a mass of greenhouse gases.4 Electrochemical nitrogen reduction is thus deemed as a potential alternative to the Haber–Bosch process for ambient NH3 synthesis, although the competitive hydrogen evolution reaction and unsatisfactory adsorption and cleavage effects of N2 severely hinder the selectivity and activity of the electrochemical nitrogen reduction reaction.5–14
NH3 synthesis via electrochemical nitrite (NO2−) reduction, in contrast, needs lower energy to cleave the N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond with faster reaction kinetics and achieves higher reaction substrate concentrations, leading to a larger NH3 yield and higher faradaic efficiency (FE).1,15,16 In addition, excess NO2− accumulated in groundwater could destroy the ecological balance and harm human health.17 Electrochemical conversion of waste NO2− can produce value-added NH3 under ambient conditions and simultaneously remove NO2−, which provides a solution for restoring the imbalance in the global nitrogen cycle. However, the electrochemical NO2− reduction reaction (NO2−RR) involves a complex six-electron pathway with various possible by-products (N2H4, N2, and H2), thus requiring highly active catalysts for selective NO2−-to-NH3 conversion.18–27
O bond with faster reaction kinetics and achieves higher reaction substrate concentrations, leading to a larger NH3 yield and higher faradaic efficiency (FE).1,15,16 In addition, excess NO2− accumulated in groundwater could destroy the ecological balance and harm human health.17 Electrochemical conversion of waste NO2− can produce value-added NH3 under ambient conditions and simultaneously remove NO2−, which provides a solution for restoring the imbalance in the global nitrogen cycle. However, the electrochemical NO2− reduction reaction (NO2−RR) involves a complex six-electron pathway with various possible by-products (N2H4, N2, and H2), thus requiring highly active catalysts for selective NO2−-to-NH3 conversion.18–27
Noble metal (Au,28 Pd,28,29 Ru,30 Ir,31 Pt32)-based catalysts are active for the NO2−RR, but their scarcity hinders large-scale applications. Compared with the above noble metals, Ag is relatively low in price and high in abundance, and it also performs efficiently in NO2− reduction electrocatalysis.33 As an Earth-abundant transition metal oxide with high chemical and structural stability, TiO2 is widely used as a support to load noble metal nanoparticles for catalysis applications.34–39 Our recent studies also suggest that it is active for the NO2−RR and its activity can be enhanced by introducing oxygen vacancies40 and P doping.41 We believe that TiO2 could be an ideal support for Ag nanoparticles for an enhanced NO2−-to-NH3 conversion performance with much less usage of noble metals, which, however, has not been reported to date.
In this study, we constructed an Ag nanoparticle-decorated TiO2 nanoribbon array on a titanium plate (Ag@TiO2/TP) as a highly selective NO2−RR catalyst for NH3 synthesis. When tested in NO2−-containing solution, Ag@TiO2/TP is capable of delivering a large NH3 yield of 514.3 μmol h−1 cm−2 with a high FE of 96.4% at −0.5 V vs. a reversible hydrogen electrode (RHE). Furthermore, Ag@TiO2/TP exhibits robust stability for long-term electrolysis.
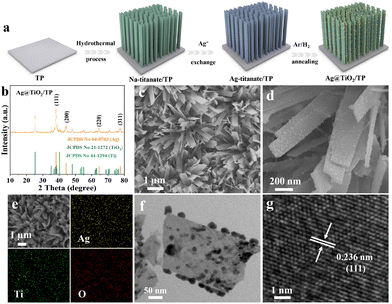
As shown in Fig. 1a, Ag@TiO2/TP was synthesized through a hydrothermal method in an alkaline solution, Ag+ exchange, and an annealing process under an Ar/H2 atmosphere (see the ESI† for details). Fig. 1b depicts the X-ray diffraction (XRD) pattern of Ag@TiO2/TP. The diffraction peaks at 38.15°, 44.30°, 64.43°, and 77.50° correspond to the (111), (200), (220), and (311) lattice planes of Ag, respectively (JCPDS No. 04-0783),33 while the other diffraction peaks can be assigned to metallic Ti (JCPDS No. 44-1294) and TiO2 (JCPDS No. 21-1272), and these are in accordance with those for TiO2/TP (Fig. S1†). As depicted in Fig. S2 and S3,† the scanning electron microscopy (SEM) images show that the TiO2 nanoribbon array was grown on TP. With regard to Ag@TiO2/TP, plenty of nanoparticles are decorated on the surface of the TiO2 nanoribbon (Fig. 1c and d). Additionally, the SEM image and corresponding energy-dispersive X-ray (EDX) elemental mapping images of Ag@TiO2/TP confirm the existence of Ag, Ti, and O elements with a homogeneous distribution (Fig. 1e). Furthermore, the result of the EDX spectrum confirms that the Ag content in Ag@TiO2/TP is approximately 13.63% (Fig. S4†). The transmission electron microscopy (TEM) image also provides evidence of the formation of a large number of nanoparticles without agglomeration on the nanoribbon, as shown in Fig. 1f. A high-resolution TEM (HRTEM) image taken from one such nanoparticle displays a lattice spacing of 0.236 nm indexed to the (111) plane of Ag (Fig. 1g). All these observations confirm the successful fabrication of an Ag nanoparticle-decorated TiO2 nanoribbon array.
The X-ray photoelectron spectroscopy (XPS) survey spectrum (Fig. 2a) also shows the presence of Ag, O, and Ti elements. The Ag 3d region spectrum (Fig. 2b) is divided into two peaks at 368.28 and 374.28 eV, which are ascribed to Ag 3d5/2 and Ag 3d3/2, respectively.42,43 In the Ti 2p spectrum, two fitting peaks at 459.38 and 465.08 eV are assigned to Ti 2p3/2 and Ti 2p1/2, respectively (Fig. 2c).44,45 In addition, two fitting peaks in the O 1s spectrum are attributed to metal–oxygen bonds (M–O, 530.78 eV) and adsorbed surface hydroxyl groups (M–OH, 533.18 eV) (Fig. 2d).42,45
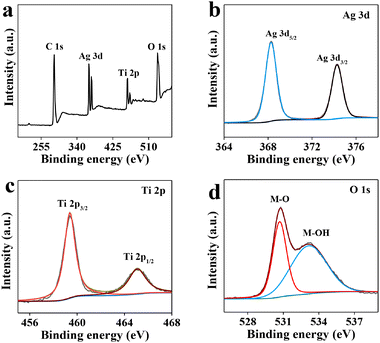 | ||
| Fig. 2 (a) XPS survey spectrum, and high resolution XPS spectra in the (b) Ag 3d, (c) Ti 2p, and (d) O 1s regions of Ag@TiO2. | ||
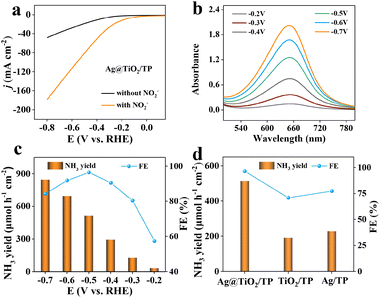
The electrochemical experiments of Ag@TiO2/TP, Ag/TP, and TiO2/TP toward the NO2−RR were implemented in Ar-saturated NO2−-free and NO2−-containing 0.1 M NaOH electrolytes. UV–vis spectra and related calibration curves are depicted in Fig. S5 and S6.† Linear scanning voltammetry (LSV) of Ag@TiO2/TP was firstly conducted. Obviously, a markedly enhanced current density (j) emerges upon the addition of NO2− (Fig. 3a), verifying that Ag@TiO2/TP enables efficient NO2− reduction. In comparison, Ag/TP and TiO2/TP display lower j with NO2−-containing electrolytes (Fig. S7†), confirming that the electrocatalytic NO2−RR activity of Ag@TiO2/TP is superior to those of Ag/TP and TiO2/TP. Chronoamperometry (CA) measurements at given potentials (from −0.2 V to −0.7 V) were then executed to study the NH3-generation ability of Ag@TiO2/TP (Fig. S8†), where the peak intensity of the relevant UV–vis spectra strengthens with an increase in the given potential (Fig. 3b), manifesting that a more negative potential results in more NH3. Furthermore, we evaluated NH3 FEs and yields of Ag@TiO2/TP in test windows (Fig. 3c). Noticeably, as the cathode potential negatively shifts, the NH3 yields of Ag@TiO2/TP progressively increase, and eventually the largest value of 846.3 μmol h−1 cm−2 (14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 387.1 μg h−1 cm−2) at −0.7 V is obtained. Furthermore, the maximum FE of NH3 production is 96.4% at −0.5 V with an NH3 yield of 514.3 μmol h−1 cm−2 (8743.1 μg h−1 cm−2), confirming an excellent NO2−RR electrocatalyst. The NH3 yields and FEs of Ag@TiO2/TP exceed those of most reported NO2−RR electrocatalysts (Table S1†). As shown in Fig. 3d, Ag@TiO2/TP exhibits a much better performance than Ag/TP (77.38%, 228.5 μmol h−1 cm−2) and TiO2/TP (70.8%, 190.9 μmol h−1 cm−2).
387.1 μg h−1 cm−2) at −0.7 V is obtained. Furthermore, the maximum FE of NH3 production is 96.4% at −0.5 V with an NH3 yield of 514.3 μmol h−1 cm−2 (8743.1 μg h−1 cm−2), confirming an excellent NO2−RR electrocatalyst. The NH3 yields and FEs of Ag@TiO2/TP exceed those of most reported NO2−RR electrocatalysts (Table S1†). As shown in Fig. 3d, Ag@TiO2/TP exhibits a much better performance than Ag/TP (77.38%, 228.5 μmol h−1 cm−2) and TiO2/TP (70.8%, 190.9 μmol h−1 cm−2).
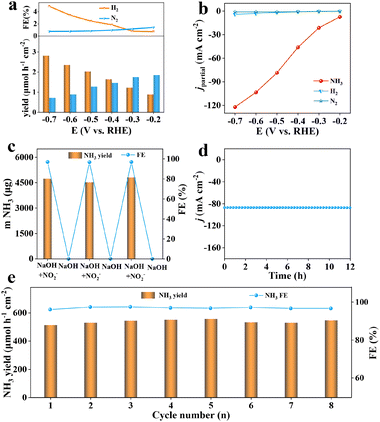
The NO2− reduction process of Ag@TiO2/TP was further assessed by quantifying various by-products (N2H4, H2, and N2). As exhibited in Fig. S9,† no N2H4 signals were monitored as was proved by identical UV–vis absorption spectral peaks at different potentials. Meanwhile, traces of H2 and N2 were detected (Fig. 4a) with the maximal H2 and N2 yields being 2.82 μmol h−1 cm−2 and 1.85 μmol h−1 cm−2, with FEs of 4.9% and 1.42%, respectively, much lower than that of NH3 at every potential, verifying the superb selectivity of such Ag@TiO2/TP electrocatalysts for NH3 synthesis. Furthermore, the partial current densities (jpartial) of Ag@TiO2/TP for NH3 reach −122.1 mA cm−2 at −0.7 V, clearly higher than that of H2 (−4.1 mA cm−2) and N2 (−1.04 mA cm−2) (Fig. 4b), again proving great NO2−RR selectivity towards NH3 electrosynthesis. Control experiments were then performed to determine whether the synthesized NH3 just comes from the NO2−RR on Ag@TiO2/TP. It is clearly seen that the amounts of NH3 generated after 1 h of electrolysis in a blank solution (0.29 μg) and open circuit potential (OCP, 0.66 μg) are extremely small (Fig. S10†), which excludes possible interference factors from the electrolytic solution and device.
Six alternative-cycle measurements were then carried out in NO2−-free/NO2−-containing electrolytes at −0.5 V, and NH3 only is generated in NO2−-containing electrolytes (Fig. 4c), demonstrating that NH3 just originates from NO2−via the NO2−RR on Ag@TiO2/TP. Additionally, stability is an extremely important parameter of the NO2−RR process for NH3 synthesis. We thus implemented a 12 h electrolysis test, as displayed in Fig. 4d, and the Ag@TiO2/TP electrode maintained an initial j of nearly 100% with almost no fluctuation, confirming the excellent tolerance of our catalyst. Furthermore, we carried out 8 consecutive measurements on Ag@TiO2/TP at −0.5 V, and the volatility of NH3 yields and FEs was negligible, again proving the durability of Ag@TiO2/TP (Fig. 4e and S11†), which is also in good accordance with the LSV curve (Fig. S12†), XRD pattern (Fig. S13†), and SEM images (Fig. S14†) of Ag@TiO2/TP after long-term electrolysis. These results suggest that Ag@TiO2/TP has excellent stability for the electrocatalytic reduction of NO2− to NH3.
In summary, a Ag nanoparticle-decorated TiO2 nanoribbon array is proved to be an efficient and stable NO2−RR catalyst for NO2−-to-NH3 conversion in an alkaline electrolyte, producing a remarkable NH3 yield of 8743.1 μg h−1 cm−2 with a large FE of 96.4%. This study not only offers a highly selective electrocatalyst for ambient NH3 synthesis via NO2− reduction, but also opens up a new avenue to construct a nanostructured Ag/TiO2 hybrid array for applications.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 22072015).References
- J. Liang, Q. Liu, A. A. Alshehri and X. Sun, Recent advances in nanostructured heterogeneous catalysts for N-cycle electrocatalysis, Nano Res. Energy, 2022, 1, e9120010 CrossRef.
- J. Liang, W. Hu, B. Song, T. Mou, L. Zhang, Y. Luo, Q. Liu, A. A. Alshehri, M. S. Hamdy, L. Yang and X. Sun, Efficient nitric oxide electroreduction toward ambient ammonia synthesis catalyzed by a CoP nanoarray, Inorg. Chem. Front., 2022, 9, 1366–1372 RSC.
- D. Qi, F. Lv, T. Wei, M. Jin, G. Meng, S. Zhang, Q. Liu, W. Liu, D. Ma, M. S. Hamdy, J. Luo and X. Liu, High-efficiency electrocatalytic NO reduction to NH3 by nanoporous VN, Nano Res. Energy, 2022, 1, e9120022 CrossRef.
- I. Dybkjaer, in Ammonia, catalysis and manufacture, ed. A. Nielsen, Springer, Heidelberg, 1995, pp. 199–327 Search PubMed.
- Y. Ji, L. Li, W. Cheng, Y. Xiao, C. Li and X. Liu, A CeP nanoparticle-reduced graphene oxide hybrid: an efficient electrocatalyst for the NH3 synthesis under ambient conditions, Inorg. Chem. Front., 2021, 8, 2103–2106 RSC.
- L. Li, C. Tang, H. Jin, K. Davey and S.-Z. Qiao, Main-group elements boost electrochemical nitrogen fixation, Chem, 2021, 7, 3232–3255 CAS.
- N. Cao, Z. Chen, K. Zang, J. Xu, J. Zhong, J. Luo, X. Xu and G. Zheng, Doping strain induced Bi-Ti3+ pairs for efficient N2 activation and electrocatalytic fixation, Nat. Commun., 2019, 10, 2877 CrossRef.
- C. Guo, J. Ran, A. Vasileff and S. Qiao, Rational design of electrocatalysts and photo(electro)catalysts for nitrogen reduction to ammonia (NH3) under ambient conditions, Energy Environ. Sci., 2018, 11, 45–56 RSC.
- D. Chanda, R. Xing, T. Xu, Q. Liu, Y. Luo, S. Liu, R. A. Tufa, T. H. Dolla, T. Montini and X. Sun, Electrochemical nitrogen reduction: recent progress and prospects, Chem. Commun., 2021, 57, 7335–7349 RSC.
- Y. Luo, Q. Li, Y. Tian, Y. Liu and K. Chu, Amorphization engineered VSe2−x nanosheets with abundant Se-vacancies for enhanced N2 electroreduction, J. Mater. Chem. A, 2022, 10, 1742–1749 RSC.
- Y. Li, Y. Liu, J. Wang, Y. Guo and K. Chu, Plasma-engineered NiO nanosheets with enriched oxygen vacancies for enhanced electrocatalytic nitrogen fixation, Inorg. Chem. Front., 2020, 7, 455–463 RSC.
- S. Zhang, C. Zhao, Y. Liu, W. Li, J. Wang, G. Wang, Y. Zhang, H. Zhang and H. Zhao, Cu doping in CeO2 to form multiple oxygen vacancies for dramatically enhanced ambient N2 reduction performance, Chem. Commun., 2019, 55, 2952–2955 RSC.
- C. Liu, S. Li, Z. Li, L. Zhang, H. Chen, D. Zhao, S. Sun, Y. Luo, A. A. Alshehri, M. S. Hamdy, Q. Liu and X. Sun, Ambient N2-to-NH3 fixation over CeO2 nanoparticles decorated three-dimensional carbon skeleton, Sustainable Energy Fuels, 2022, 6, 3344–3348 RSC.
- Q. Li, P. Shen, Y. Tian, X. Li and K. Chu, Metal-free BN quantum dots/graphitic C3N4 heterostructure for nitrogen reduction reaction, J. Colloid Interface Sci., 2022, 606, 204–212 CrossRef CAS.
- X. He, X. Li, X. Fan, J. Li, D. Zhao, L. Zhang, S. Sun, Y. Luo, D. Zheng, L. Xie, A. M. Asiri, Q. Liu and X. Sun, Ambient electroreduction of nitrite to ammonia over Ni nanoparticle supported on molasses-derived carbon sheets, ACS Appl. Nano Mater., 2022, 5, 14246–14250 CrossRef CAS.
- S. Li, J. Liang, P. Wei, Q. Liu, L. Xie, Y. Luo and X. Sun, ITO@TiO2 nanoarray: an efficient and robust NO2− reduction reaction electrocatalyst toward NH3 production under ambient conditions, eScience, 2022, 2, 382–388 CrossRef.
- X. Zhu, X. Zeng, X. Chen, W. Wu and Y. Wang, Inhibitory effect of nitrate/nitrite on the microbial reductive dissolution of arsenic and iron from soils into pore water, Ecotoxicology, 2019, 28, 528–538 CrossRef CAS.
- Q. Liu, Q. Liu, X. Lisi, L. Yue, T. Li, Y. Luo, N. Li, B. Tang, L. Yu and X. Sun, 3D FeOOH nanotube array: an efficient catalyst for ammonia electrosynthesis by nitrite reduction, Chem. Commun., 2022, 58, 5160–5163 RSC.
- C. Wang, W. Zhou, Z. Sun, Y. Wang, B. Zhang and Y. Yu, Integrated selective nitrite reduction to ammonia with tetrahydroisoquinoline semi-dehydrogenation over a vacancy-rich Ni bifunctional electrode, J. Mater. Chem. A, 2021, 9, 239–243 RSC.
- L. Hu, D. Zhao, C. Liu, Y. Liang, D. Zheng, S. Sun, Q. Li, Q. Liu, Y. Luo, Y. Liao, L. Xie and X. Sun, Amorphous CoB nanoarray as a high-efficiency electrocatalyst for nitrite reduction to ammonia, Inorg. Chem. Front., 2022, 9, 6075–6079 RSC.
- X. Zhang, Y. Wang, Y. Wang, Y. Guo, X. Xie, Y. Yu and B. Zhang, Recent advances in electrocatalytic nitrite reduction, Chem. Commun., 2022, 58, 2777–2787 RSC.
- L. Ouyang, L. Yue, Q. Liu, Q. Liu, Z. Li, S. Sun, Y. Luo, A. A. Alshehri, M. S. Hamdy, Q. Kong and X. Sun, Cu nanoparticles decorated juncus-derived carbon for efficient electrocatalytic nitrite-to-ammonia conversion, J. Colloid Interface Sci., 2022, 624, 394–399 CrossRef CAS.
- J. Wang, T. Feng, J. Chen, V. Ramalingam, Z. Li, D. M. Kabtamu, J. He and X. Fang, Electrocatalytic nitrate/nitrite reduction to ammonia synthesis using metal nanocatalysts and bio-inspired metalloenzymes, Nano Energy, 2021, 86, 106088 CrossRef CAS.
- L. Mattarozzi, S. Cattarina, N. Comissoa, P. Guerriero, M. Musiani, L. Vázquez-Gómez and E. Verlatoa, Electrochemical reduction of nitrate and nitrite in alkaline media at CuNi alloy electrodes, Electrochim. Acta, 2013, 89, 488–496 CrossRef CAS.
- R. Zhang, S. Zhang, Y. Guo, C. Li, J. Liu, Z. Huang, Y. Zhao, Y. Li and C. Zhi, A Zn–nitrite battery as an energy-output electrocatalytic system for high-efficiency ammonia synthesis using carbon-doped cobalt oxide nanotubes, Energy Environ. Sci., 2022, 15, 3024–3032 RSC.
- S. E. Braley, J. Xie, Y. Losovyj and J. M. Smith, Graphite conjugation of a macrocyclic cobalt complex enhances nitrite electroreduction to ammonia, J. Am. Chem. Soc., 2021, 143, 7203–7208 CrossRef CAS PubMed.
- Z. Chen, A. Jaworski, J. Chen, T. M. Budnyak, I. Szewczyk, A. Rokicińska, R. Dronskowski, N. Hedin, P. Kuśtrowski and A. Slabon, Graphitic nitrogen in carbon catalysts is important for the reduction of nitrite as revealed by naturally abundant 15N NMR spectroscopy, Dalton Trans., 2021, 50, 6857–6866 RSC.
- H. Li, S. Guo, K. Shin, M. S. Wong and G. Henkelman, Design of a Pd–Au nitrite reduction catalyst by identifying and optimizing active ensembles, ACS Catal., 2019, 9, 7957–7966 CrossRef CAS.
- H. Shin, S. Jung, S. Bae, W. Lee and H. Kim, Nitrite reduction mechanism on a Pd surface, Environ. Sci. Technol., 2014, 48, 12768–12774 CrossRef CAS PubMed.
- X. Huo, D. J. Van Hoomissen, J. Liu, S. Vyas and T. J. Strathmann, Hydrogenation of aqueous nitrate and nitrite with ruthenium catalysts, Appl. Catal., B, 2017, 211, 188–198 CrossRef CAS.
- H. Li, C. Yan, H. Guo, K. Shin, S. M. Humphrey, C. J. Werth and G. Henkelman, CuxIr1−x nanoalloy catalysts achieve near 100% selectivity for aqueous nitrite reduction to NH3, ACS Catal., 2020, 10, 7915–7921 CrossRef CAS.
- M. C. Figueiredo, V. Climent and J. M. Feliu, Nitrite reduction on bismuth modified Pt (111) surfaces in different electrolytic media, Electrocatalysis, 2011, 2, 255–262 CrossRef CAS.
- Q. Liu, G. Wen, D. Zhao, L. Xie, S. Sun, L. Zhang, Y. Luo, A. A. Alshehri, M. S. Hamdy, Q. Kong and X. Sun, Nitrite reduction over Ag nanoarray electrocatalyst for ammonia synthesis, J. Colloid Interface Sci., 2022, 623, 513–519 CrossRef CAS PubMed.
- S. Chang and X. Xu, Au nanocrystals decorated TiO2 nanotubes for photocatalytic nitrogen fixation into ammonia, Inorg. Chem. Front., 2020, 7, 620–624 RSC.
- W. Shi, A.-H. Park, S. Xu, P. J. Yoo and Y.-U. Kwon, Continuous and conformal thin TiO2-coating on carbon support makes Pd nanoparticles highly efficient and durable electrocatalyst, Appl. Catal., B, 2021, 284, 119715 CrossRef CAS.
- A. Shoaib, M. Ji, H. Qian, J. Liu, M. Xu and J. Zhang, Noble metal nanoclusters and their in situ calcination to nanocrystals: precise control of their size and interface with TiO2 nanosheets and their versatile catalysis applications, Nano Res., 2016, 9, 1763–1774 CrossRef CAS.
- S. Zhang, W. Wang, Y. Gao, S. Deng, L. Ding, H. Zhuo, Z. Bao, W. Ji, C. Qiu and J. Wang, Pd-Co alloy supported on TiO2 with oxygen vacancies for efficient N2 and O2 electrocatalytic reduction, Appl. Surf. Sci., 2021, 567, 150680 CrossRef CAS.
- L.-N. Chen, S.-H. Wang, P.-Y. Zhang, Z.-X. Chen, L. Xiao, H.-J. Yang, T. Sheng, W.-F. Lin, N. Tian, S.-G. Sun and Z.-Y. Zhou, Ru nanoparticles supported on partially reduced TiO2 as highly efficient catalyst for hydrogen evolution, Nano Energy, 2021, 88, 106211 CrossRef CAS.
- Z.-W. Wei, H.-J. Wang, C. Zhang, K. Xu, X.-L. Lu and T.-B. Lu, Reversed charge transfer and enhanced hydrogen spillover in platinum nanoclusters anchored on titanium oxide with rich oxygen vacancies boost hydrogen evolution reaction, Angew. Chem., Int. Ed., 2021, 60, 16622–16627 CrossRef CAS PubMed.
- D. Zhao, J. Liang, J. Li, L. Zhang, K. Dong, L. Yue, Y. Luo, Y. Ren, Q. Liu, M. S. Hamdy, Q. Kong, Q. Liu and X. Sun, A TiO2−x nanobelt array with oxygen vacancies: an efficient electrocatalyst toward nitrite conversion to ammonia, Chem. Commun., 2022, 58, 3669–3672 RSC.
- L. Ouyang, X. He, S. Sun, Y. Luo, D. Zheng, J. Chen, Y. Li, Y. Lin, Q. Liu, A. M. Asirif and X. Sun, Enhanced electrocatalytic nitrite reduction to ammonia over P-doped TiO2 nanobelt array, J. Mater. Chem. A, 2022, 10, 23494–23498 RSC.
- Q. Qin, Y. Li, W. Bu, L. Meng, X. Chuai, Z. Zhou and C. Hu, Self-template-derived ZnCo2O4 porous microspheres decorated by Ag nanoparticles and their selective detection of formaldehyde, Inorg. Chem. Front., 2021, 8, 811–820 RSC.
- Y. Fang, S. Zhang, Z.-P. Wu, D. Luan and X. W. Lou, A highly stable lithium metal anode enabled by Ag nanoparticle-embedded nitrogen-doped carbon macroporous fibers, Sci. Adv., 2021, 7, eabg3626 CrossRef CAS PubMed.
- Y. Guo, R. Zhang, S. Zhang, Y. Zhao, Q. Yang, Z. Huang, B. Dong and C. Zhi, Pd doping-weakened intermediate adsorption to promote electrocatalytic nitrate reduction on TiO2 nanoarrays for ammonia production and energy supply with zinc-nitrate batteries, Energy Environ. Sci., 2021, 14, 3938–3944 RSC.
- X. Fan, C. Ma, D. Zhao, Z. Deng, L. Zhang, Y. Wang, Y. Luo, D. Zheng, T. Li, J. Zhang, S. Sun, Q. Liu and X. Sun, Unveiling selective nitrate reduction to ammonia with Co3O4 nanosheets/TiO2 nanobelt heterostructure catalyst, J. Colloid Interface Sci., 2023, 630, 714–720 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section and supplementary figures. See DOI: https://doi.org/10.1039/d2qi02409h |
| This journal is © the Partner Organisations 2023 |