 Open Access Article
Open Access ArticleOpportunities and challenges in aqueous nitrate and nitrite reduction beyond electrocatalysis
Guanling
Yang
ac,
Pengfei
Zhou
ac,
Jinsheng
Liang
ac,
Hao
Li
 *b and
Fei
Wang
*b and
Fei
Wang
 *ac
*ac
aKey Laboratory of Special Functional Materials for Ecological Environment and Information (Hebei University of Technology), Ministry of Education, Tianjin 300130, China. E-mail: wangfei@hebut.edu.cn
bAdvanced Institute for Materials Research (WPI-AIMR), Tohoku University, Sendai 980-8577, Japan. E-mail: li.hao.b8@tohoku.ac.jp
cInstitute of Power Source and Ecomaterials Science, Hebei University of Technology, Tianjin 300130, China
First published on 3rd April 2023
Abstract
Nitrate (NO3−) and nitrite (NO2−) ions are common health-threatening contaminants in water. To reduce nitrate and nitrite, catalytic thermal reduction using molecular hydrogen as the reducing agent is a strategy with some clear advantages. However, this method is relatively less explored, with some vital open questions still to be addressed. In this paper, we review the current stages of thermal nitrate and nitrite reduction on the aspects of catalyst synthesis, mechanistic insights, reaction activity, product selectivity, design guidelines for promising catalysts, and the efficiency comparison between thermal and electrocatalytic reduction. The main opportunities and challenges of this thermal reduction method in drinking water and wastewater treatment are discussed. Moreover, in addition to discussing this reaction's typical N2 formation selectivity, we also discuss the possibility of forming ammonia by tuning the reaction selectivity via rational catalyst design. This review shows that in addition to the currently popular electrocatalytic nitrate reduction, scientific efforts should also be devoted towards the thermal reduction of nitrate and nitrite. We hope that this review will arouse more interest from the catalyst community to explore the mechanistic insights and design guidelines for thermal nitrate and nitrite reduction.
1. Introduction
Surface and groundwater pollution has attracted broad attention in recent years, while nitrate (NO3−) is one of the most concerning pollutants.1 As a health-threatening pollutant, NO3− accounts for ∼70% of the groundwater samples and is ∼13 times more frequent than other contaminants.2 Generally, the level of NO3− in nature is relatively low, but human activities (e.g., septic systems, animal husbandry, and farming) have dramatically increased the NO3− concentration.3–7 When ingested, NO3− can easily be reduced in vivo to nitrite (NO2−), which can cause methemoglobinemia (the so-called “blue-baby syndrome”).8 NO2− can react with amines and amides in the gastrointestinal tract to form carcinogenic N-nitroso compounds.3,5,7,9 In addition, long-term exposure to low-concentration NO3−/NO2− may cause various cancers,7 which is one of the reasons why NO3− and NO2− have become serious contaminants in water treatment. The maximum contaminant levels (MCLs) of NO3− and NO2− have been set at 10 and 1.0 mg N L−1, respectively (calculated from the nitrogen weight, denoted as mg N L−1), by the United States Environmental Protection Agency (US EPA).10 The European Drinking Water Directive has set concentration limits for NO3− and NO2− at 11.3 and 0.15 mg N L−1, respectively.11 Besides, the World Health Organization guideline values of NO3− and NO2− are 11.3 and 0.91 mg N L−1, respectively.12Some wastewater treatment methods have been applied for NO3− and NO2− removal, such as electrodialysis,13 reverse osmosis,14 ion exchange,15 and biological treatment.16 However, the main drawbacks and challenges of these methods have been reported, as summarized in Table 1. When NO3−/NO2− is treated with adsorbents or reverse osmosis membranes, it leads to the accumulation of contaminants on the adsorbent surface and in the permeate wastewater, for which secondary treatment with high-concentration NO3−/NO2− is required. Biological nitrification can convert NO3−/NO2− to harmless N2, but it takes a long operation time.16 In terms of biological treatment, microorganisms can contaminate pure water to some extent. Moreover, the microbial reaction is temperature-dependent, and the reactivity is significantly lower in winter when the temperature is low.17 Among various treatment technologies, electrocatalytic processes show great potential for NO3−/NO2− removal but have some significant disadvantages, including the addition of electrolytes and chloride ions, and the need to maintain a high pH, which greatly limits their widespread use. In addition, the generally high overpotentials will lead to various side reactions and corrosive gases during electrolysis, which may corrode the electrodes and limit the energy utilization efficiency.18,19
| Method | Ion exchange | Reverse osmosis | Biological denitrification | Electrocatalytic reduction | Thermal catalytic reduction |
|---|---|---|---|---|---|
| Fate of nitrate | Adsorbed | Concentrated | N2 | N2 and NH3 | N2 and NH3 |
| Waste | Waste brine | Waste brine | Bacteria sludge | Waste brine | None |
| Chemical additives | Sodium chloride | Sulfuric acid alkali | Ethanol and phosphoric acid | Electrolytes | H2 |
| Percentage of efficiency | 85–98% | 75–80% | 98% | 98% | 100% |
| Flexibility in variable | Medium | Medium | Low | Medium | High |
| Energy use | Low | High | Medium | High | Low |
| Space | Limited | Limited | High | Limited | Limited |
| Movable | Yes | Yes | No | Good | Yes |
| Manageability | Good | Good | Poor | Good | Good |
| Type of operation | Periodic regeneration | Continuous | Continuous | Continuous | Continuous |
| Sensitivity to deactivation | Medium | High | High | High | High |
| Automatic control | Simple | Simple | Complex | Simple | Simple |
| Start-up time | Immediate | Immediate | Up to 1 month | Immediate | Immediate |
| Monitoring required | Little | Little | Intensive | Little | Little |
| Selectivity of the process | Low | Low | High | High | High |
| Odors | No | No | Yes | Yes | No |
| Noise | Some | High | No | No | No |
Thermal catalytic reduction of nitrate and nitrite by hydrogenation is an alternative beyond electrocatalysis. This technique was first systemically studied in 1989 as a promising method for nitrite removal.20 The main reason why thermal catalysis stands out among many chemical treatments is its high hydrogen utilization rate and the high selectivity for harmless N2. The H2 used in thermal catalytic reduction can be obtained by the electrolysis of water using renewable energy sources (e.g., wind and solar). The reduction of nitrate by noble metal catalysts in the liquid-phase usually proceeds in two steps: (1) reduction of nitrate to nitrite and (2) reduction of nitrite to N2 or NH3/NH4+. Nitrite reduction was found to be a structure–sensitive reaction during the reaction catalyzed by Pt–Cu bimetallic catalysts, while nitrate reduction to nitrite was found to be a structure-insensitive process.21–23 Meanwhile, related studies have shown that the selectivity of N2 during the reaction on the catalyst is determined by the reduction of NO2−, and the toxicity of nitrate in water is mainly from NO2−.24 For this reason, an in-depth understanding of the reaction process of nitrite reduction and the corresponding catalyst design is essential. This is one of the main reasons why a comprehensive review of thermal catalytic nitrate and nitrite reduction is urgently needed.
Based on the statistical analysis of previous publications (Fig. 1), most of the research focused on the electrocatalytic reduction of nitrate, while there were fewer studies involving nitrite reduction or thermal reduction, of which there were very few studies on the thermal reduction of nitrite. Unlike the research hotspot, electrocatalytic nitrate reduction,25 the thermal reduction of nitrate and nitrite is much less studied, and thus its importance is generally dismissed. In this review, we provide comprehensive discussions on thermal nitrate and nitrite reduction on the aspects of catalyst synthesis, mechanistic insights, reaction activity, product selectivity, design guidelines for promising catalysts, and the efficiency comparison between thermal and electrocatalytic reduction.
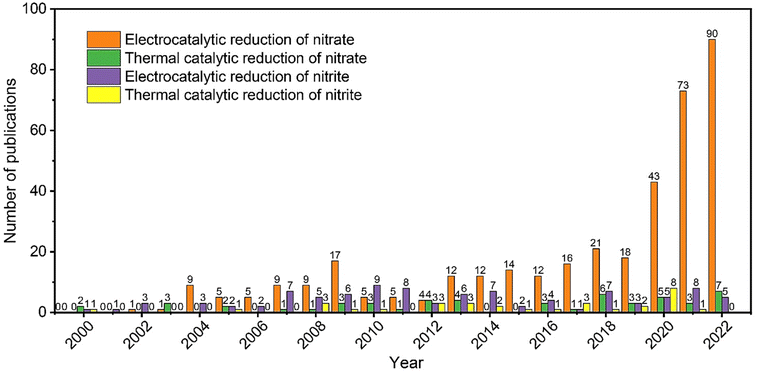 | ||
| Fig. 1 Statistical summary of the number of publications reporting electrocatalytic and thermal reduction of nitrate and nitrite. Source: Web of Science. | ||
2. Discussion
2.1. Reaction process
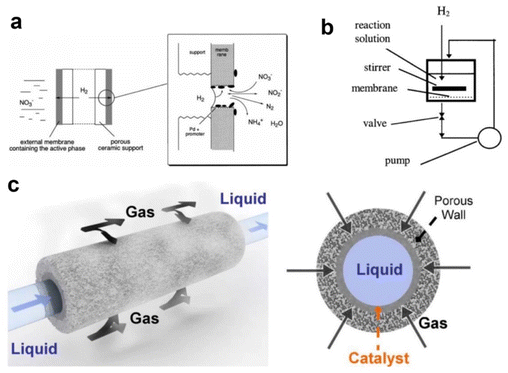 | ||
| Fig. 2 (a) Schematic illustration of a catalytic membrane reactor for nitrate and nitrite reduction. (b) The flow-through configuration of a catalytic membrane reactor.49 Copyright 2013, American Chemical Society. (c) The gas–liquid–solid contact in a porous ceramic reactor.50 Copyright 2011, Elsevier. | ||
2.2. Reaction mechanism
In terms of the reaction mechanism, herein, we first discuss the catalytic reduction of nitrate to nitrite. Thermal reduction of nitrate to nitrite requires a metal that can activate H2 (e.g., Pd or Pt) and a promoter metal (e.g., In, Sn, or Cu) to help reduce nitrate to nitrite. The promoter metal itself usually cannot dissociate H2, and it uses the spilt hydrogen from Pd (or Pt) to facilitate nitrate reduction (Fig. 3a).51–53 Previous studies provided experimental observations that in the presence of some special elements as the promoters (e.g., In, Cu, or Sn), nitrate reduction to nitrite is facile and not rate-limiting, in contrast to the more sluggish subsequent reduction of nitrite to N2 or NH3/NH4+.54 However, many promoters do not continue the reduction of nitrite. Although some studies have discussed the unique role of In as a promoter for nitrate reduction,55 the reason why these promoters can only reduce nitrate to nitrite is not clear and remains an open question.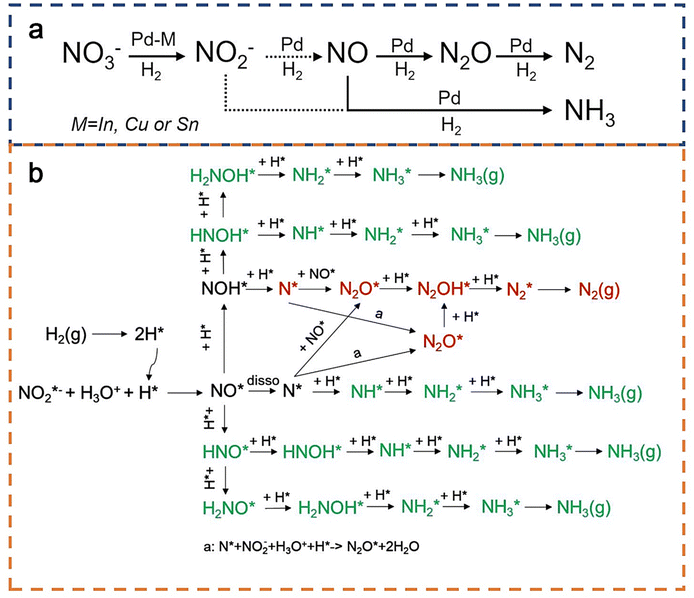 | ||
| Fig. 3 (a) A general pathway for thermal nitrate reduction catalyzed by Pd-based catalysts with special element M as the promoter (M = In, Cu, or Sn).29 Copyright 2017, American Chemical Society. (b) Reaction network of nitrite reduction using H2 as the reducing agent. Red and green pathways represent the reaction selectivity towards N2 and NH3, respectively.36 Copyright 2019, American Chemical Society. Note that after the formation of NH3, NH4+ formation (not shown) is considered a facile process in the liquid-phase. | ||
In nitrate and nitrite reduction, the rate-determining step usually exists in nitrite reduction.56 Therefore, we focus more on the mechanism of thermal catalytic reduction of nitrite to N2/NH3, which was derived from previous combined theoretical and experimental studies (Fig. 3b). Firstly, H2 (after H–H activation) is used as a reducing agent to reduce nitrite to NO*, which is a proven highly spontaneous redox reaction. Subsequently, NO* can dissociate to form N* or form NOH*/HNO* by hydrogenation.57 Due to the multiple available hydrogenation pathways, there are at least five pathways for NH3/NH4+ formation (Fig. 3b, green pathways). In contrast, N2O* formation from N* is a proven key step in N2 formation.57 Shin et al. found that N2O* can be formed via the reaction of N*, H*, nitrite, and water, in good agreement with experimental observations.15 Subsequently, N2O* is rapidly consumed to form N2*.58,59 In conclusion, it can be seen from Fig. 3b that there are at least five possible pathways for the formation of NH3/NH4+ and only two pathways for N2. However, N2 formation is usually thermodynamically more favorable.36 Kinetically speaking, H*-rich conditions may provide selectivity for NH3/NH4+, while nitrite-rich conditions favor N2 formation, which is consistent with the conclusions from previous studies.15,60 Detailed theoretical and experimental analyses regarding reaction selectivity will be discussed later in this review.
3. Activity
3.1. Thermal nitrite reduction by monometallic catalysts: activity
Due to the more favorable thermodynamics of the reaction pathways (Fig. 3, red pathways), the main product of thermal nitrate/nitrite reduction is usually N2.24,56,61,62 Previous studies have shown that many metal catalysts contribute to the catalytic initiation of nitrate (e.g., Cu, Sn, or In).45,63–69 However, the subsequent reduction of nitrite and the production of subsequent intermediates depend mainly on the precious metal Pd. Seminal studies by Hörold et al.70 and Soares et al.71 tested the performance of Pt-group monometallics, including Pt, Ru, Ir, and Rh, but found low nitrite reduction activities. Besides, many properties of the catalysts (e.g., particle size, electronic structure, and the type of catalyst carrier) have been found to affect the nitrite reduction activity.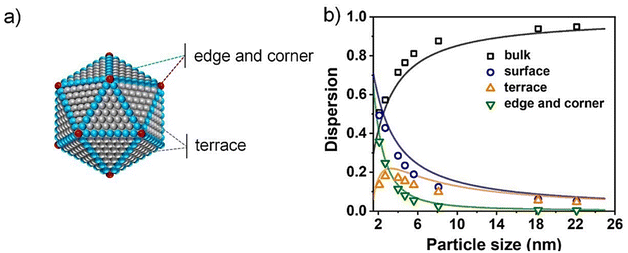 | ||
| Fig. 4 (a) Schematic diagram of a structurally stable icosahedral Pd NP. (b) The ratio of bulk, surface, terrace, edge, and corner atoms on icosahedral Pd NPs with different sizes.72 Copyright 2020, the Royal Society of Chemistry. | ||
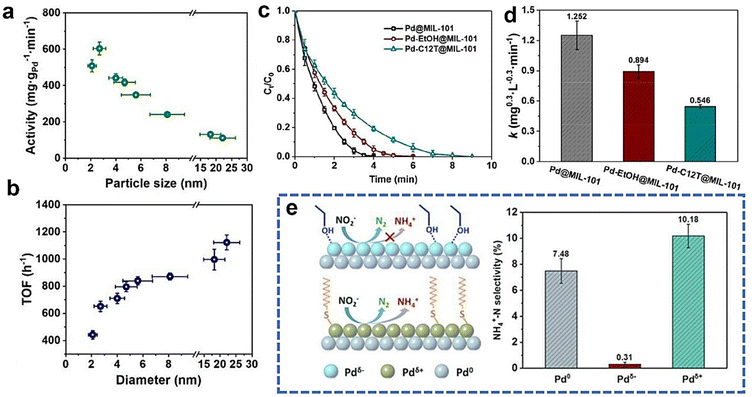 | ||
| Fig. 5 (a and b) Nitrite reduction mass activity and TOF of Pd/C NPs with various sizes.72 (c and d) Nitrite reduction performances of Pd catalysts with various treatment methods. Copyright 2020, the Royal Society of Chemistry. (e) Proposed relationships between the electronic structure of Pd catalysts and the experimentally measured nitrite reduction selectivity.38 Copyright 2018, the Royal Society of Chemistry. | ||
3.2. Catalytic nitrate reduction by bimetallic catalysts: activity
During the past decades, great progress has been made in researching bimetallic alloy catalysts. It can be seen from Table 2 that many previous studies focused on developing alloy catalysts for thermal nitrate and nitrite reduction. The main reason for studying bimetallics is that when two metals are combined, the activity and selectivity are tuned by synergistic effects.83 These effects can intrinsically be classified into electronic (or ligand), strain, ensemble, and spin effects.84,85 Seraj et al. showed that PdAu alloy NPs have higher catalytic reduction activities compared to pure Pd NPs.29 Similar conclusions were obtained by Qian et al.85 A series of bimetallic core@shell (Au@Pd) NPs were designed using a combination of experimental and computational approaches by Li et al.;36 their catalyst design resulted in a high density of reactive Pd-sites on the surface of metal NPs, enhancing nitrite reduction activity.| Materials | T (K) | Activity | S N2 (%) | S NH3 (%) | Ref. |
|---|---|---|---|---|---|
| a Nitrite reduction. b Nitrate reduction. | |||||
| PdAu | Room temp. | — | ∼100 | — |
36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a a |
| Pd/TiO2 | Room temp. | — | 90.9 | 9.1 |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a a |
| Pd–PVA/Al2O3 | 294 | 1480 μmol−1 L−1 g−1 min−1 | — | — |
86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a a |
| Pd/Al2O3 | 294 | 1720 μmol−1 L−1 g−1 min−1 | — | — |
86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a a |
| Pd/γ-Al2O3 | 293 | 1.7 ± 0.4 mmol g−1 min−1 | — | — |
86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a a |
| Pd/C | 298 | 603.6 mg g−1Pd min−1 | 93.2 | 6.8 |
72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a a |
| Pd@MIL-101 | 298 | 1.252 mg0.3 L−0.3 min−1 | 18 | 82 |
38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a a |
| Pd-ethanol@MIL-101 | 298 | 0.894 mg0.3 L−0.3 min−1 | 92.52 | 7.48 |
38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a a |
| Pd-C12T@MIL-101 | 298 | 0.546 mg0.3 L−0.3 min−1 | 99.62 | 0.38 |
38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a a |
| Rh/Al2O3 | Room temp. | ∼3–24 L g−1surface metal min−1 | ∼5–32 | ∼68–95 |
87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a a |
| AuNPs | 295 | Not active | — | — |
29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a a |
| Pd53Au47 NPs | 295 | 5.12 L g−1metal min−1 | — | <2 |
29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a a |
| Pd NPs | 295 | 1.99 L g−1metal min−1 | — | <2 |
29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a a |
| Pd50Cu50/PVP | Room temp. | 2.9 L h−1 mmol−1metal | 96.8 | 3.2 |
60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a a |
| Pd70Cu30/PVP | Room temp. | 4.2 L h−1 mmol−1metal | 97 | 3 |
60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a a |
| Pd/PVP | Room temp. | 0.3 L h−1 mmol−1metal | — | — |
60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a a |
| 80sc% Pd-on-AuNPs | N/A | 576 L g−1Pd min−1 | 99.6 | 0.4 |
85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a a |
| Au@Pdmonolayer NPs | Room temp. | 246 L g−1surface Pd min−1 | 97.5 | 2.5 |
36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a a |
| Pd95Ag5NP–SiO2 | Room temp. | 4.61 L g−1Pd min−1 |
34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a a |
||
| PdNP–SiO2 | Room temp. | 1.35 L g−1Pd min−1 |
34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a a |
||
| Pd/Al2O3 | ∼296 | — | — | ∼100 |
87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a a |
| Pd/CNFs | 298 | — | 18 | 82 |
88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a a |
| CuxIr1−x | Room temp. | — | ∼100 |
89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a a |
|
| Pd–Cu/C | 333 | — | 3.6 | 96.4 |
90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a a |
| CNFs/Ni | Room temp. | — | ∼30 | ∼70 |
91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a a |
| 40 sc% In-on-Pd NPs | Room temp. | 7.57 ± 0.65 L g−1surface metal min−1 | — | — |
55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
| Cu/NZVI | Room temp. | — | 18 | — |
92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
| Cu–Pd/NZVI | Room temp. | 2.67 mM NO3− per gcat per h | 60 | — |
93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
| Cu–Pd/TiO2 | Room temp. | — | 50.2 | 47.7 |
94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
| Pd–Cu/TiO2 | 293 | 0.139 g−1cat min−1 | 86.2 |
95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
|
| Sn–Pd-NZSM-5 | Room temp. | 410 × 10−2 L min−1 g−1Pd | 81 |
96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
|
| Cu–Pd-NBeta | Room temp. | — | 92.68 |
97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
|
| Pd/Cu pillared clays | 298 | 68.9 mmol min−1 g−1Pd | — | 25 |
98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
| Pd/Sn pillared clays | 298 | 82.7 mmol min−1 g−1Pd | — | 16.5 |
98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
| Pd/In pillared clays | 298 | 57.4 mmol min−1 g−1Pd | — | 12.2 |
98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
| Pd/Al particles | Room temp. | — | 5.8 | 93.4 |
99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
| Ni/Al particles | Room temp. | — | — | 99.6 |
99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
| Cu/Al particles | Room temp. | — | — | 99.9 |
99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
| Sn–Pd/γ-Al2O3 | Room temp. | 0.42 mg L−1 g−1 min−1 | 70.1 | — |
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
| Cu–Pd/AC | Room temp. | 2.017 mmol h−1 g−1cat | 2.4 | — |
101![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
| Cu–Pd/TiO2 | Room temp. | 0.356 mmol h−1 g−1cat | 16.7 | — |
101![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
| Cu–Pd/ZrO2 | Room temp. | 0.376 mmol h−1 g−1cat | 8.9 | — |
101![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
| Cu–Pd/Al2O3 | Room temp. | 0.355 mmol h−1 g−1cat | 0 | — |
101![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
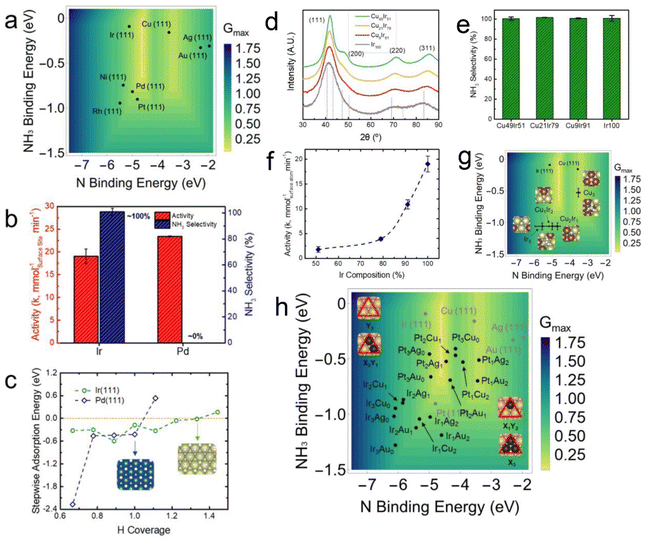
The composition and structure of bimetallic alloys can also affect the catalyst's activity. Some previous research analyzed the effects of the catalyst composition and structure on nitrite reduction. For example, Qian et al. studied the catalytic structure–performance relationships of Pd NPs.85 They used a two-step method to synthesize a catalyst with a special Pd-on-Au structure and found that the activity was related to the surface Pd coverage (Fig. 6e). Seraj et al.29 found that Pd–Au alloy NPs have higher catalytic activities and resistance to sulfur-poisoning compared to pure Pd NPs. Li et al.36 developed a combined experimental and theoretical strategy to find that the nitrite reduction performance of both monometallic and bimetallic catalysts can be evaluated by simple reaction descriptors, including the binding energies of N, N2, and NH3 at a surface site (Fig. 6a–c). They also found that bimetallic Pd-on-Au catalysts can optimize the surface activity of the active Pd sites and, meanwhile, maintain the maximum number of active Pd-sites on a surface. They found that a thin Pd layer [Pd1monolayer/Au (111)] deposited on Au NPs can lead to high nitrite reduction activity, with the experimental TOF superior to those of Pd and other Pd–Au alloys (Fig. 6f and g).
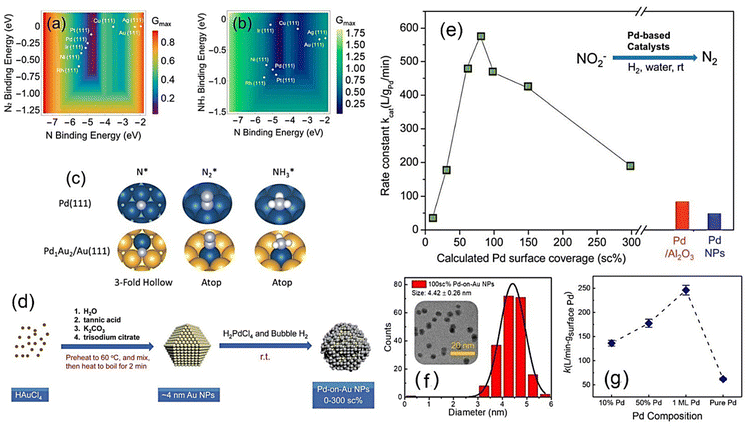 | ||
| Fig. 6 (a and b) Volcano-shaped predictive models for nitrite reduction through (a) N2 and (b) NH3/NH4+ formation pathways. (c) Typical optimized adsorption geometries on monometallic and bimetallic sites,36 where the blue, yellow, light blue, and which spheres represent Pd, Au, N, and H atoms, respectively. (d) Two-stage synthesis of Pd-on-Au NPs considered for thermal nitrite reduction. Copyright 2019, American Chemical Society. (e) Experimentally determined nitrite reaction rate constants of various Pd-on-Au NPs.85 Copyright 2014, Royal Society of Chemistry. (f) Particle size distributions of 100 sc% Pd-on-Au NPs. (g) Reaction kinetics of nitrite reduction on Pd-on-Au, Pd–Au alloys, and pure Pd NPs.36 Copyright 2019, American Chemical Society. | ||
4. Selectivity
4.1. Catalytic nitrite reduction by monometallic catalysts: selectivity
In drinking water treatment, N2 formation is desirable, while NH3/NH4+ is more desirable for some emerging applications (e.g., ammonia production from waste treatment). Notably, N2 or NH3/NH4+ production is mainly at the active sites of some stronger-binding elements (e.g., Pd, Pt, and Rh).18,20,36,38,102–105 Because of that, the selectivity of N2 and NH3/NH4+ production depends mainly on the reduction phase of nitrite on a strong-binding monometallic site.24,106 Therefore, it is important to study the catalytic mechanism and factors that affect these metal sites and analyze the structure–selectivity relationships in nitrate and nitrite reduction.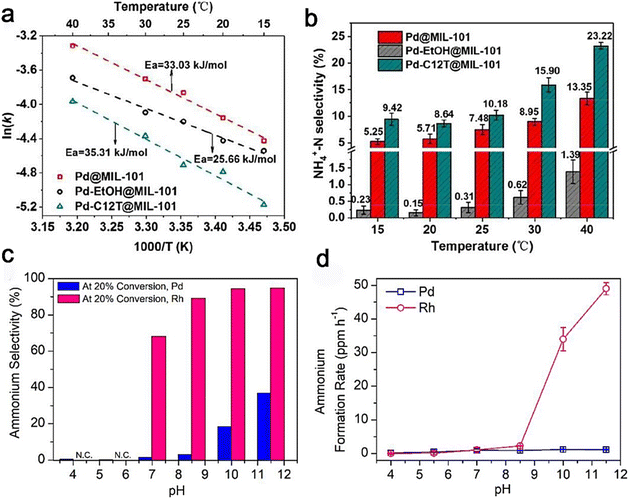 | ||
| Fig. 7 (a) Experimental estimation of nitrite reduction activation energies (Ea) on different Pd surfaces. (b) Effects of the reaction temperature on ammonia selectivity on various Pd surfaces.38 Copyright 2018, the Royal Society of Chemistry. (c and d) Effects of varying pH values on the selectivity of ammonia and the ammonia production rates on Pd and Rh.87 Copyright 2019, American Chemical Society. | ||
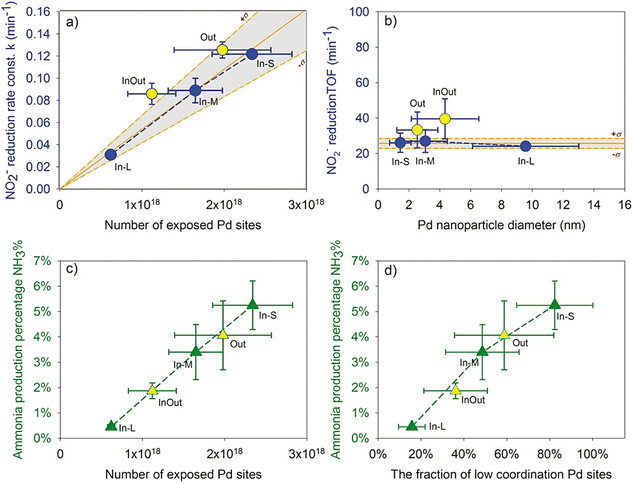 | ||
| Fig. 8 (a) First-order rate constant (k) versus the number of exposed Pd sites. (b) TOFs versus Pd NP diameters. (c) Ammonia production percentage (NH3%) versus the number of exposed Pd sites. (d) Ammonia production percentage (NH3%) versus the fraction of low-coordination Pd sites.35 Copyright 2021, American Chemical Society. | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H ratio increases, which in turn increases the chance of N–N pairing and thus favors N2 production.
H ratio increases, which in turn increases the chance of N–N pairing and thus favors N2 production.
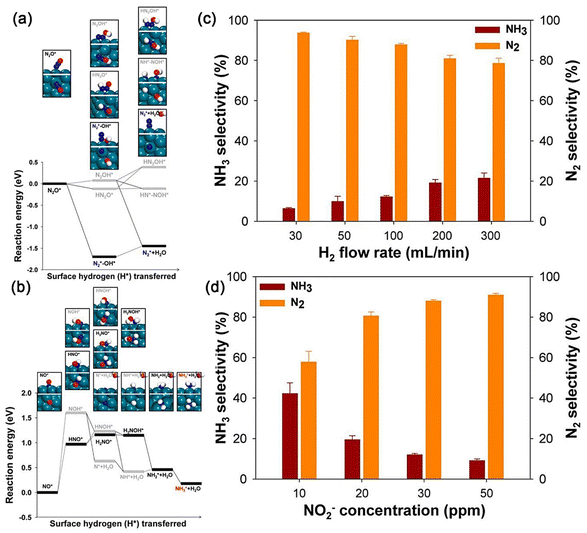 | ||
| Fig. 9 (a) Energy diagram of the hydrogenation pathway of NO* on a Pd(111) surface. (b) Energy diagram of the reduction of N2O* on Pd(111). Insets show the reaction intermediates considered for calculations. Green, blue, red, and white spheres represent Pd, N, O, and H atoms, respectively. (c and d) Experimentally measured N2 and NH3 selectivity of nitrite reduction at various (c) H2 flow rates and (d) NO2− concentrations.15 Copyright 2014, American Chemical Society. | ||
4.2. Catalyst design to improve activity and target-product selectivity
The rational design of a catalyst helps reduce the exhaustive experimental “trial-and-error” process in catalyst search. Based on the seminal Sabatier principle, a good heterogeneous catalyst should neither bond to the adsorbate too strongly nor too weakly.110 Therefore, almost all the derived heterogeneous catalytic models present a “volcano-shape” as a function of the key adsorbate binding energies (i.e., adsorption energies) of the reaction.111–123 A catalytic volcano model allows people to derive the optimal activity of catalysis. Therefore, catalyst design with the help of a volcano model can efficiently design promising catalysts with high activity and/or target-product selectivity.Model validation and active site identification. Before proceeding to design a new catalyst, the volcano activity plot should be determined beforehand. For thermal nitrite reduction, Li et al. derived the first volcano activity model for thermal nitrite reduction based on the linear scaling relations between the binding energies of N* and all other intermediate species.36 The accuracy of the model was then evaluated by the benchmarking analyses with the experimental results of Seraj et al.29 and Guy et al.60Fig. 10a and b shows the volcano activity model for nitrite reduction toward N2 formation as a function of N* and N2* binding energies, with the plotted binding energies calculated at various Pd–Au and Pd–Cu alloy (111) surfaces. It can be seen that only the 3-fold Pd3 triatomic site on Pd0.50Au0.50(111) and Pd0.75Cu0.25(111) can reach the volcano peak, suggesting that ∼50% and ∼75% Pd on PdAu and PdCu, respectively, can lead to optimized nitrite reduction activity compared to other compositions. These are in excellent agreement with previous experiments that the optimal catalysts for nitrite reduction were ∼53% and ∼80% Pd, respectively, in Pd–Au and Pd–Cu nano-catalysts.29,60 These benchmarking analyses clearly showed that this volcano model is precise and can be used for predicting promising nitrite reduction catalysts as a function of nitrogen bonding strengths, which can significantly reduce computation costs. Interestingly, we can see that on Pd-based alloys, there is a synergy between the ligand and strain effects in tuning the N-binding energy at alloy surfaces. As shown in Fig. 10c and d, the N* binding energy at the close-packed Pd3-site can reach the optimal region of the volcano due to the synergistic effects of the electronic change arising from the charge transfer between Pd and Au/Cu and the strain arising from the lattice difference between Pd and Au/Cu.
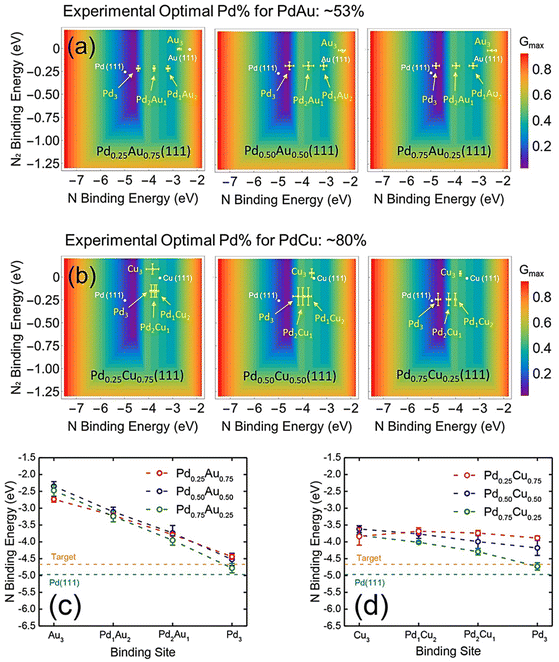 | ||
| Fig. 10 (a and b) Predictions of thermal nitrite reduction at triatomic ensembles on PdAu and PdCu alloy surfaces. (c and d) N binding energies at the triatomic ensembles of PdAu and PdCu (111).36 Copyright 2019, American Chemical Society. | ||
Optimizing and maximizing the most active sites: design of highly effective metal-on-metal catalysts. Volcano models can be used for the computational screening of promising catalysts. Those strongly bound metals are closer to the target binding energy than Au, Ag, and Cu. Also, X-on-Y (or core@shell-like) bimetallic structures can provide the maximum number of active sites on the catalytic surface, which can further increase the overall activity of a catalyst. For this purpose, Li et al.36 modeled X-on-Y structures with X being a strong-binding metal (e.g., Pd, Pt, Ir, Rh, and Ni) and Y being a typical weak-binding metal (e.g., Au, Ag, and Cu) (Fig. 11). Fig. 11a–c shows that at least six of the screened catalysts are able to achieve the target activity (i.e., theoretical optimal activity) indicated by the volcano model [Pd3ML/Cu(111), Pd2ML/Cu(111), Pd1ML/Ag(111), Pt3ML/Cu(111), Pd1ML/Au(111), and Ni1ML/Cu(111)]. Interestingly, most of these candidates are Pd-based catalysts, suggesting that Pd is a promising element for nitrite reduction. Furthermore, most of these optimized catalysts have relatively weak N2* binding but strong NH3* binding, suggesting facile N2 desorption and high selectivity for N2 formation. To evaluate the stability of these candidates, their surface segregation energies were calculated (Fig. 11d). With N* adsorption, only the Pd2ML/Cu(111) and Pd3ML/Cu(111) surfaces have negative surface segregation energies. Therefore, it is expected that the remaining four catalysts could be stable in nitrite reduction under mild reaction conditions (e.g., room temperature). Because Pd–Au can be synthesized by well-established methods, Pd-on-Au NPs were selected for experimental verification; the results showed that Pd-on-Au NPs had a relatively high selectivity (∼100%) for N2 formation compared to pure Pd NPs. In summary, the rational design of bimetallic catalysts based on the theoretical knowledge of alloying effects can help address complicated environmental problems.
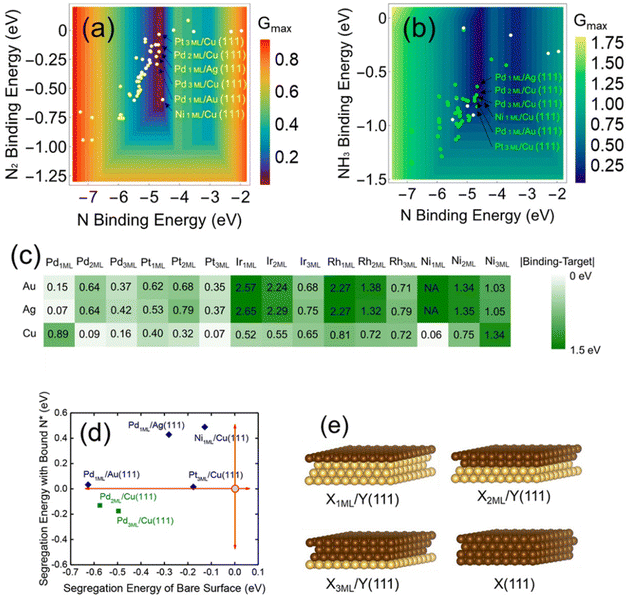 | ||
| Fig. 11 (a and b) Volcano activity models with the plotted nitrogen bindings at X-on-Y (X = Pd, Pt, Rh, Ir, and Ni; Y = Au, Ag, and Cu) catalysts through the (a) N2 and (b) NH3 formation pathways illustrated in Fig. 4. (c) Matrix showing the surfaces with function quantified by |N binding-target binding|. (d) Calculated segregation energies with and without adsorbed N* on the X-on-Y catalysts. (e) Schematic pictures of X-on-Y models considered for theoretical calculations. Brown and gold spheres represent the X and Y elements, respectively.36 Copyright 2019, American Chemical Society. | ||
 | ||
| Fig. 12 (a) Volcano activity plot for nitrite reduction through the NH3/NH4+ formation pathway. (b) Nitrite reduction activities and their ammonia selectivity on Pd and Ir NPs. (c) Stepwise adsorption energies of H* on Pd and Ir surfaces. (d) PXRD patterns of CuxIr(100−x) alloy NPs with different compositions. (e and f) Ammonia selectivity and the measured reaction rate constants of nitrite reduction on CuxIr(100−x) NPs. (g) Volcano activity model for nitrite reduction through the NH3/NH4+ formation pathways with the data points of Ir, Cu, and the triatomic ensembles sampled from Cu25Ir75 surfaces. (h) Volcano activity model for nitrite reduction through the NH3/NH4+ formation pathways with the triatomic ensembles at various alloy surfaces.89 Copyright 2020, American Chemical Society. | ||
5. Catalyst carrier versus activity and selectivity
To improve the dispersion of metals and to facilitate the separation and recovery of catalysts, Pd and other metals were often immobilized on support materials. Not only the properties of the metal particles but also the nature of the carrier and the strength of the metal–carrier interaction have significant impacts on the catalyst performance. Reaction systems based on the thermal reduction of nitrite involve liquid–solid–gas triads. For this reason, the ideal surface properties of the catalyst are essential to ensure appropriate contact of the three phases and to eliminate internal and external diffusion limitations, thus achieving high activity and selectivity.5.1. Oxide carriers
Oxide carriers (e.g., γ-Al2O3, SiO2, and TiO2) were widely used as catalyst carriers due to their desirable properties (e.g., high surface area and porosity) and chemical stability. The abundant hydroxyl groups on the oxide surface can interact with the metal precursors and thus influence the position of the metal on the carrier.133 This plays an important role in dispersing the active metal. According to previous studies,20,134 γ-Al2O3 showed the highest catalytic activities with the highest nitrite reduction rates. It was found that the enhanced catalytic activity may be related to the small catalyst particle size produced on the γ-Al2O3 support. The void structure of the support plays an important role in product selectivity. As studied by Krawczyk et al., SiO2 with the smallest pore size of 1–5 nm showed the lowest N2 selectivity (∼56%), while TiO2 with the largest pore size of 10–30 nm showed the highest N2 selectivity (∼88%).135 This is related to the formation of an internal OH− gradient in narrow pores:136 OH− formed in the narrow pore structure is difficult to neutralize because the diffusion of OH− from the pore into the reaction medium is limited, resulting in the increase of local pH within the pore. As discussed in section 4.1.2, a higher pH promotes the formation of NH3/NH4+. Therefore, small pore size supports tend to be more favorable for NH3/NH4+ formation.135,1375.2. Carbon carriers
Carbon material carriers for nitrite reduction mainly include activated carbon (AC), carbon nanotubes (CNTs), and CNFs, among which AC is the most widely used because of its low cost and high availability. The high specific surface area and porosity allow the active metal to be fully dispersed on the carrier surface, resulting in smaller-size metal particles. These properties make carbon materials competitive candidates as carriers for Pd-based catalysts.138 For AC carriers, due to the abundance of micropores, a rich void structure makes it easy to generate local pH gradients within the voids, favoring the production of NH3/NH4+. According to previous studies, the selectivity of AC-supported PdCu catalysts for NH3/NH4+ is often higher than 30%.139,140 In addition, carbon materials are conductive, accelerating the electron transfer from the accessible Pd in the support shell to the less accessible Pd in the pores, subsequently leading to an increase in H* concentration,43 which in turn is more favorable for NH3/NH4+ production.6. Thermal catalytic reduction versus electrocatalytic reduction
With the progress and development of detection technology and experimental technology, both thermal reduction and electrocatalytic reduction are promising strategies for removing nitrate and/or nitrite.27,141–151 A recent significant study compared the efficiency of these two methods for ammonia production via nitrate reduction (Fig. 13).146Fig. 13 (by Wang et al.146) illustrates that thermal nitrate reduction requires the availability of H2, while electrocatalysis involves the participation of electrons and applied potentials.11,21 Meanwhile, N2 and NH3/NH4+ are the main products for both methods. They analyzed the catalytic efficiency of the two methods in terms of the driving chemical potential, pH, nitrate concentration, and type of catalyst. Their experimental results, as summarized in Fig. 14, showed that increasing hydrogen pressure or electrochemical potential could promote nitrate conversion. It was also found that for alloy catalysts, the addition of Ru increased the adsorption strengths of nitrate and hydrogen as well as intermediate species, leading to higher catalytic activity. When the nitrate concentration was low, thermal and electrocatalytic nitrate reduction showed the same trend that a higher nitrate concentration can promote nitrite reduction. However, with a further increase in nitrate concentration (i.e., >0.5 M), the rate of electrocatalytic nitrate reduction will decrease. This indicates that nitrate may block the surface sites for hydrogen adsorption and inhibit the reaction. The higher activity and lower Ea of thermal nitrite reduction at a lower pH are shown in Fig. 14c and d. These suggest that the higher nitrite hydrogenation TOF at a lower pH is mainly due to the increased surface coverage of reaction intermediates such as *NO and *HNO.147 When pH = 1, the reaction had a lower Ea value, possibly due to the more favorable conversion of intermediates to ammonia at a low pH value. The effects of pH on electrocatalytic nitrate reduction are shown in Fig. 14c and d. Wang et al.146 suggested that pH affects the charge on the electrode surface, which in turn can tune the catalytic activity. The essential part of the work by Wang et al.146 is the feasibility comparison between the two reduction methods. An ideal catalytic system must be of low cost and must exhibit higher ammonia production efficiency as shown in Fig. 14f. For thermal nitrate reduction (reaction conditions: PtRu/C; pH = 1), the ammonia production rate was high, and the process cost was in line with the economic requirements. This shows that thermal nitrate reduction is expected to be a potential alternative to the Haber–Bosch method under milder conditions.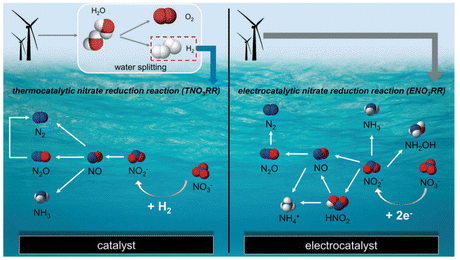 | ||
| Fig. 13 Simplified reaction mechanism of the thermal nitrate reduction reaction (TNO3RR) and the electrocatalytic nitrate reduction reaction (ENO3RR).146 Copyright 2018, the Royal Society of Chemistry. | ||
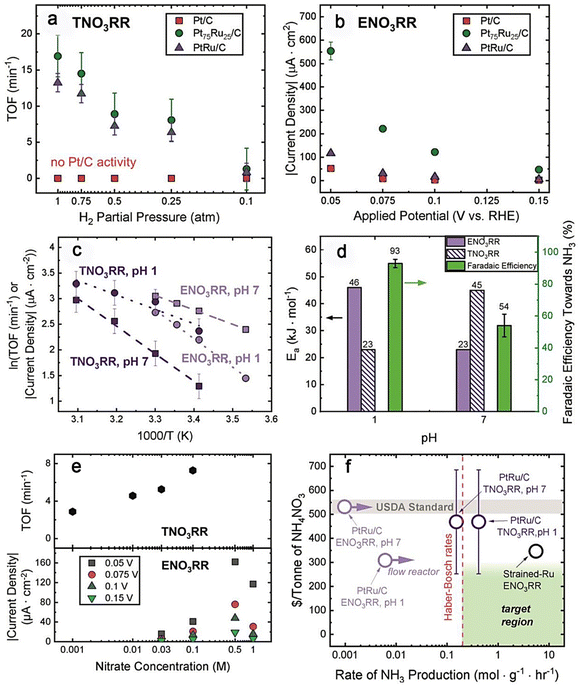 | ||
| Fig. 14 (a) TOFs of thermal catalytic nitrate reduction versus hydrogen partial pressures on different Pt-based catalysts. (b) Current densities of electrocatalytic nitrate reduction versus the applied potentials on different Pt-based catalysts. (c) Arrhenius plots of nitrate reduction on PtRu/C. (d) Comparison of apparent activation energy (Ea) versus faradaic efficiency (FE) towards NH3/NH4+ across different solution pH values and systems. (e) The activity of PtRu/C as a function of nitrate concentration at pH = 7. (f) Comparison of the economic value of two different methods.146 Copyright 2021, the Royal Society of Chemistry. | ||
Interestingly, there is a difference in the proposed mechanisms for thermal and electrocatalytic nitrate reduction. For electrocatalytic nitrate reduction, the reduction of nitrate to nitrite could be a rate-determining step on many catalysts,152 while for thermal nitrate reduction, this step is considered non-rate-limiting on some typical catalysts (e.g., In–Pd catalysts).54 Regarding why thermal nitrate reduction to nitrite on some In-based catalysts is not rate-limiting, one possibility is that the surface states (e.g., adsorbate coverage) of a catalyst under electrocatalytic conditions and thermal hydrogenation could be very different,153 leading to different energetics in the first step of nitrate reduction. However, to the best of our knowledge, almost no previous study has paid attention to this difference in the reaction mechanism between thermal and electrocatalytic nitrate reduction, and thus it is still an interesting open question in the nitrate conversion process. Note that one of the most obvious differences in designing thermal and electrocatalytic nitrate/nitrite reduction catalysts is that their surface states (i.e., adsorbate coverage) under reaction conditions could be very different. For example, in thermal nitrate/nitrite reduction, hydrogen will be activated and will precover on the surface; therefore, the adsorbate coverage will depend on the reaction temperature, nitrate/nitrite concentration, and H2 pressure. While in room-temperature electrocatalytic nitrate/nitrite reduction, due to the equilibrium between water and the adsorbates after potential-induced water activation (e.g., H*, HO*, and O*),153 the adsorbate coverage will depend on the applied potential, nitrate/nitrite concentration, and pH.154 These intrinsic differences in the surface states would lead to very different reaction environments that make the reaction energetics different. Therefore, when designing thermal and electrocatalytic nitrate/nitrite reduction catalysts, the surface phase diagrams should be analyzed before analyzing the reaction activity.
7. Challenges in the thermal catalytic reduction of nitrate and nitrite
Despite the great potential and opportunities of thermal nitrate and nitrite reduction in water treatment, some key obstacles may hamper its large-scale applications, which are summarized as follows.(1) Most of the catalysts reported to date are based on precious metals, which are associated with high costs in catalyst preparation. This also hampers the potential commercialization of this nitrate/nitrite removal method. Many of the lower-cost materials that can potentially be stable under reaction conditions, such as less-noble metals and some metal X-ides (TMX, where X = O, N, C, B, F, Cl, Br, etc.), are much less explored. This may be because the research population to study thermal nitrate/nitrite reduction is relatively small (Fig. 1).
(2) The harsh conditions in wastewater bodies impose stricter requirements on thermal nitrate and nitrite reduction catalysts, including high thermal and chemical stability. However, long-term stability evaluation of the related catalysts reported so far was generally dismissed. After long-term operating conditions for nitrate/nitrite reduction, active-site poisoning/blocking or adsorbate-induced surface segregation could occur, which may in turn lead to catalyst deactivation.
(3) There is no uniform standard so far to fairly evaluate the activity of a thermal nitrate and nitrite reduction catalyst. For example, how to confirm the nitrogen source of the formed N2 and NH3/NH4+? Perhaps, isotope labeling experiments, which have been proven essential for electrocatalytic ammonia synthesis, could also be necessary for detecting the products in thermal nitrate/nitrite reduction.
(4) It is difficult to find a catalyst with high activity and target-product selectivity, especially catalysts targeting ammonia formation. So far, the catalyst search strategy for thermal nitrate/nitrite reduction still mainly relies on a trial-and-error process from experiments. Better catalyst design strategies, such as theory-orientated catalyst design,89 should receive more attention.
8. Perspectives and conclusion
Although thermal nitrate and nitrite reduction is still not as “popular” as the electrochemical reduction of nitrate/nitrite, it is expected to be an active research area in the future given that it is important in wastewater treatment and its significant potential to form value-added ammonia upon rational catalyst design. The tremendous development of green hydrogen production and safe hydrogen transportation techniques endow the thermal reduction of nitrate and nitrite with a new future. Because this reaction is much less explored compared to electrocatalytic reduction, some fundamental and engineering questions need to be further explored, including:(1) Why some promoters (e.g., Cu, Sn, and In) can reduce nitrate to nitrite but cannot effectively reduce nitrite?
(2) Are there any effective and target-product selective catalysts beyond precious metals?
(3) Can we use other safer sources (e.g., hydrogen storage materials) to provide H* in water treatment instead of molecular hydrogen?
To address these key questions, in situ/operando methods and more comprehensive theoretical models need to be developed. For example, surface phase diagrams need to be developed to fully understand the state of the Cu, Sn, or In promoter on a Pd-based catalyst surface in a reaction environment, while in situ surface probing methods would be helpful in understanding the surface state of the catalyst. With solid answers to the above questions, the key challenges of nitrate and nitrite reduction can be addressed by developing a more rational design strategy for a catalyst, accompanied by new engineering methods for wastewater treatment.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Key R&D Program of China (No. 2021YFC1910605), the Iwatani Naoji Foundation, JSPS KAKENHI (No. JP23K13703), the National Natural Science Foundation of China (No. 51874115), the National Projects Funded by the Central Government to Guide Local Scientific and Technological Development of China (No. 216Z3803G), and the Excellent Young Scientist Foundation of Hebei province, China (No. E2018202241).References
- A. Liu, J. Ming and R. O. Ankumah, Nitrate contamination in private wells in rural Alabama, United States, Sci. Total Environ., 2005, 346, 112–120 CrossRef CAS PubMed.
- B. T. Nolan and J. D. Stoner, Nutrients in groundwaters of the conterminous United States, 1992–1995, Environ. Sci. Technol., 2000, 34, 1156–1165 CrossRef CAS.
- L. Fewtrell, Drinking-water nitrate, methemoglobinemia, and global burden of disease: a discussion, Environ. Health Perspect., 2004, 112, 1371–1374 CrossRef PubMed.
- S. Guo, C. D. Powell, D. Villagrán and M. S. Wong, Magnetic In-Pd catalysts for nitrate degradation, Environ. Sci.: Nano, 2020, 7, 2681–2690 RSC.
- A. R. Townsend, R. W. Howarth, F. A. Bazzaz, M. S. Booth, C. C. Cleveland, S. K. Collinge and A. H. Wolfe, Human health effects of a changing global nitrogen cycle, Front. Ecol. Environ., 2003, 1, 240–246 CrossRef.
- R. L. Seiler, Combined use of 15N and 18O of nitrate and 11B to evaluate nitrate contamination in groundwater, Appl. Geochem., 2005, 20, 1626–1636 CrossRef CAS.
- P. J. Weyer, J. R. Cerhan and B. C. Kross, Municipal drinking water nitrate level and cancer risk in older women: the Iowa Women's Health Study, Epidemiology, 2001, 327–338 CrossRef CAS PubMed.
- C. P. Ahada and S. Suthar, Groundwater nitrate contamination and associated human health risk assessment in southern districts of Punjab, India, Environ. Sci. Pollut. Res., 2018, 25, 25336–25347 CrossRef CAS PubMed.
- S. M. A. Adelana, Nitrate health effects, Water encyclopedia, 2005, 4, 30–42 Search PubMed.
- US.EPA, National Primary Drinking Water Regulations and Contaminant Candidate List, US.EPA, 2008 Search PubMed.
- EU, Council Directive 98/83/EC, Official Journal of the European Communities, Brussel, 1998.
- WHO, Guidelines for drinking-water quality, World Health Organization, 3rd edn, 2006 Search PubMed.
- L. Mattarozzi, S. Cattarin, N. Comisso, P. Guerriero, M. Musiani, L. Vázquez-Gómez and E. Verlato, Electrochemical reduction of nitrate and nitrite in alkaline media at CuNi alloy electrodes, Electrochim. Acta, 2013, 89, 488–496 CrossRef CAS.
- E. Filloux, J. Wang, M. Pidou, W. Gernjak and Z. Yuan, Biofouling and scaling control of reverse osmosis membrane using one-step cleaning-potential of acidified nitrite solution as an agent, J. Membr. Sci., 2015, 495, 276–283 CrossRef CAS.
- H. Shin, S. Jung, S. Bae, W. Lee and H. Kim, Nitrite reduction mechanism on a Pd surface, Environ. Sci. Technol., 2014, 48, 12768–12774 CrossRef CAS PubMed.
- J. Chung, R. Nerenberg and B. E. Rittmann, Evaluation for biological reduction of nitrate and perchlorate in brine water using the hydrogen-based membrane biofilm reactor, J. Environ. Eng., 2007, 133, 157–164 CrossRef CAS.
- G. Centi and S. Perathoner, Remediation of water contamination using catalytic technologies, Appl. Catal., B, 2003, 41, 15–29 CrossRef CAS.
- J. Martínez, A. Ortiz and I. Ortiz, State-of-the-art and perspectives of the catalytic and electrocatalytic reduction of aqueous nitrates, Appl. Catal., B, 2017, 207, 42–59 CrossRef.
- W. Teng, N. Bai, Y. Liu, J. Fan and W. X. Zhang, Selective nitrate reduction to dinitrogen by electrocatalysis on nanoscale iron encapsulated in mesoporous carbon, Environ. Sci. Technol., 2018, 52, 230–236 CrossRef CAS PubMed.
- K. D. Vorlop and T. Tacke, Erste schritte auf dem weg zur edelmetallkatalysierten nitrat-und nitrit-entfernung aus trinkwasser, Chem. Ing. Tech., 1989, 61, 836–837 CrossRef CAS.
- F. Epron, F. Gauthard, C. Pinéda and J. Barbier, Catalytic reduction of nitrate and nitrite on Pt-Cu/Al2O3 catalysts in aqueous solution: role of the interaction between copper and platinum in the reaction, J. Catal., 2001, 198, 309–318 CrossRef CAS.
- A. Pintar, J. Batista, J. Levec and T. Kajiuchi, Kinetics of the catalytic liquid-phase hydrogenation of aqueous nitrate solutions, Appl. Catal., B, 1996, 11, 81–98 CrossRef CAS.
- I. Balint, A. Miyazaki and K. I. Aika, NO reduction by CH4 over well-structured Pt nanocrystals supported on γ-Al2O3, Appl. Catal., B, 2002, 37, 217–229 CrossRef CAS.
- U. Prüsse and K. D. Vorlop, Supported bimetallic palladium catalysts for water-phase nitrate reduction, J. Mol. Catal. A: Chem., 2001, 173, 313–328 CrossRef.
- J. Wang, T. Feng, J. Chen, V. Ramalingam, Z. Li, D. M. Kabtamu and X. Fang, Electrocatalytic nitrate/nitrite reduction to ammonia synthesis using metal nanocatalysts and bio-inspired metalloenzymes, Nano Energy, 2021, 86, 106088 CrossRef CAS.
- N. M. Deraz, The comparative jurisprudence of catalysts preparation methods: I. Precipitation and impregnation methods, J. Ind. Environ. Chem., 2018, 2, 19–21 Search PubMed.
- J. Aluha, K. Bere, N. Abatzoglou and F. Gitzhofer, Synthesis of nano-catalysts by induction suspension plasma technology (SPS) for Fischer-Tropsch reaction, Plasma Chem. Plasma Process., 2016, 36, 1325–1348 CrossRef CAS.
- J. Taghavimoghaddam, G. P. Knowles and A. L. Chaffee, Preparation and characterization of mesoporous silica supported cobalt oxide as a catalyst for the oxidation of cyclohexanol, J. Mol. Catal. A: Chem., 2012, 358, 79–88 CrossRef CAS.
- S. Seraj, P. Kunal, H. Li, G. Henkelman, S. M. Humphrey and C. J. Werth, PdAu alloy nanoparticle catalysts: effective candidates for nitrite reduction in water, ACS Catal., 2017, 7, 3268–3276 CrossRef CAS.
- K. G. N. Quiton, M. C. Lu and Y. H. Huang, Synthesis and catalytic utilization of bimetallic systems for wastewater remediation: A review, Chemosphere, 2021, 262, 128371 CrossRef CAS PubMed.
- A. Devard, M. A. Ulla and F. A. Marchesini, Synthesis of Pd/Al2O3 coating onto a cordierite monolith and its application to nitrite reduction in water, Catal. Commun., 2013, 34, 26–29 CrossRef CAS.
- P. Xu, S. Agarwal and L. Lefferts, Mechanism of nitrite hydrogenation over Pd/γ-Al2O3 according a rigorous kinetic study, J. Catal., 2020, 383, 124–134 CrossRef CAS.
- A. F. Lee, P. J. Ellis, I. J. Fairlamb and K. Wilson, Surface catalysed Suzuki-Miyaura cross-coupling by Pd nanoparticles: an operando XAS study, Dalton Trans., 2010, 39, 10473–10482 RSC.
- J. P. Troutman, H. Li, A. M. Haddix, B. A. Kienzle, G. Henkelman, S. M. Humphrey and C. J. Werth, PdAg alloy nanocatalysts: toward economically viable nitrite reduction in drinking water, ACS Catal., 2020, 10, 7979–7989 CrossRef CAS.
- D. Shuai, J. K. Choe, J. R. Shapley and C. J. Werth, Enhanced activity and selectivity of carbon nanofiber supported Pd catalysts for nitrite reduction, Environ. Sci. Technol., 2021, 46, 2847–2855 CrossRef PubMed.
- H. Li, S. Guo, K. Shin, M. S. Wong and G. Henkelman, Design of a Pd-Au nitrite reduction catalyst by identifying and optimizing active ensembles, ACS Catal., 2019, 9, 7957–7966 CrossRef CAS.
- J. Lee, Y. G. Hur, M. S. Kim and K. Y. Lee, Catalytic reduction of nitrite in water over ceria-and ceria-zirconia-supported Pd catalysts, J. Mol. Catal. A: Chem., 2015, 399, 48–52 CrossRef CAS.
- Z. Zhang, W. Shi, W. Wang, Y. Xu, X. Bao, R. Zhang and F. Cui, Interfacial electronic effects of palladium nanocatalysts on the by-product ammonia selectivity during nitrite catalytic reduction, Environ. Sci.: Nano, 2018, 5, 338–349 RSC.
- A. Miyazaki, T. Asakawa, Y. Nakano and I. Balint, Nitrite reduction on morphologically controlled Pt nanoparticles, Chem. Commun., 2005, 29, 3730–3732 RSC.
- J. P. Troncéa, S. V. I. Parvulescu and P. Granger, Unexpected kinetic behavior of structured Pd/CeO2-ZrO2 toward undesired ammonia formation and consumption during nitrites reduction: role of the reactivity of oxygen from ceria, Catal. Today, 2022, 383, 330–338 CrossRef.
- S. D. Ebbesen, B. L. Mojet and L. Lefferts, In situ ATR-IR study of nitrite hydrogenation over Pd/Al2O3, J. Catal., 2021, 256, 15–23 CrossRef.
- J. K. Chinthaginjala, A. Villa, D. S. Su, B. L. Mojet and L. Lefferts, Nitrite reduction over Pd supported CNFs: Metal particle size effect on selectivity, Catal. Today, 2012, 183, 119–123 CrossRef CAS.
- J. K. Chinthaginjala and L. Lefferts, Support effect on selectivity of nitrite reduction in water, Appl. Catal., B, 2010, 101, 144–149 CrossRef CAS.
- B. P. Chaplin, J. R. Shapley and C. J. Werth, The selectivity and sustainability of a Pd-In/γ-Al2O3 catalyst in a packed-bed reactor: the effect of solution composition, Catal. Lett., 2009, 130, 56–62 CrossRef CAS.
- Y. X. Chen, Y. Zhang and H. Y. Liu, Reduction of nitrate from groundwater: powder catalysts and catalytic membrane, J. Environ. Sci., 2003, 15, 600–606 CAS.
- Y. X. Chen, Y. Zhang, H. Y. Liu, K. R. Sharma and G. H. Chen, Hydrogen-based tubular catalytic membrane for removing nitrate from groundwater, Environ. Technol., 2004, 25, 227–234 CrossRef CAS PubMed.
- K. Daub, G. Emig, M. J. Chollier, M. Callant and R. Dittmeyer, Studies on the use of catalytic membranes for reduction of nitrate in drinking water, Chem. Eng. Sci., 1999, 54, 1577–1582 CrossRef CAS.
- N. Wehbe, N. Guilhaume, K. Fiaty, S. Miachon and J. A. Dalmon, Hydrogenation of nitrates in water using mesoporous membranes operated in a flow-through catalytic contactor, Catal. Today, 2010, 156, 208–215 CrossRef CAS.
- H. C. Aran, J. K. Chinthaginjala, R. Groote, T. Roelofs, L. Lefferts, M. Wessling and R. G. Lammertink, Porous ceramic mesoreactors: a new approach for gas-liquid contacting in multiphase microreaction technology, Chem. Eng. J., 2011, 169(1–3), 239–246 CrossRef CAS.
- N. Diban, A. T. Aguayo, J. Bilbao, A. Urtiaga and I. Ortiz, Membrane reactors for in situ water removal: a review of applications, Ind. Eng. Chem. Res., 2013, 52, 10342–10354 CrossRef CAS.
- S. Hörold, K. D. Vorlop, T. Tacke and M. Sell, Development of catalysts for a selective nitrate and nitrite removal from drinking water, Catal. Today, 1993, 17, 21–30 CrossRef.
- B. P. Chaplin, M. Reinhard, W. F. Schneider, C. Schüth, J. R. Shapley, T. J. Strathmann and C. J. Werth, Critical review of Pd-based catalytic treatment of priority contaminants in water, Environ. Sci. Technol., 2012, 46, 3655–3670 CrossRef CAS PubMed.
- H. L. Tierney, A. E. Baber, J. R. Kitchin and E. C. H. Sykes, Hydrogen dissociation and spillover on individual isolated palladium atoms, Phys. Rev. Lett., 2009, 103, 246102 CrossRef PubMed.
- S. Guo, H. Li, K. N. Heck, X. Luan, W. Guo, G. Henkelman and M. S. Wong, Gold boosts nitrate reduction and deactivation resistance to indium-promoted palladium catalysts, Appl. Catal., B, 2022, 305, 121048 CrossRef CAS.
- S. Guo, K. Heck, S. Kasiraju, H. Qian, Z. Zhao, L. C. Grabow and M. S. Wong, Insights into nitrate reduction over indium-decorated palladium nanoparticle catalysts, ACS Catal., 2018, 8, 503–515 CrossRef CAS.
- U. Prüsse, M. Hähnlein, J. Daum and K. D. Vorlop, Improving the catalytic nitrate reduction, Catal. Today, 2000, 55, 79–90 CrossRef.
- J. Sá and J. A. Anderson, FTIR study of aqueous nitrate reduction over Pd/TiO2, Appl. Catal., B, 2008, 77, 409–417 CrossRef.
- R. Zhang, D. Shuai, K. A. Guy, J. R. Shapley, T. J. Strathmann and C. J. Werth, Elucidation of nitrate reduction mechanisms on a Pd-In bimetallic catalyst using isotope labeled nitrogen species, ChemCatChem, 2013, 5, 313–321 CrossRef CAS.
- S. Jung, S. Bae and W. Lee, Development of Pd-Cu/hematite catalyst for selective nitrate reduction, Environ. Sci. Technol., 2014, 48, 9651–9658 CrossRef CAS PubMed.
- K. A. Guy, H. Xu, J. C. Yang, C. J. Werth and J. R. Shapley, Catalytic nitrate and nitrite reduction with Pd-Cu/PVP colloids in water: Composition, structure, and reactivity correlations, J. Phys. Chem. C, 2009, 113, 8177–8185 CrossRef CAS.
- B. P. Chaplin, E. Roundy, K. A. Guy, J. R. Shapley and C. J. Werth, Effects of natural water ions and humic acid on catalytic nitrate reduction kinetics using an alumina supported Pd-Cu catalyst, Environ. Sci. Technol., 2006, 40, 3075–3081 CrossRef CAS PubMed.
- V. Urbain, R. Benoit and J. Manem, Membrane bioreactor: a new treatment tool, J. - Am. Water Works Assoc., 1996, 88, 75–86 CrossRef CAS.
- Y. B. Yin, S. Guo, K. N. Heck, C. A. Clark, C. L. Conrad and M. S. Wong, Treating water by degrading oxyanions using metallic nanostructures, ACS Sustainable Chem. Eng., 2018, 6, 11160–11175 CrossRef CAS.
- X. Zhu, X. C. Zeng, X. Chen, W. Wu and Y. Wang, Inhibitory effect of nitrate/nitrite on the microbial reductive dissolution of arsenic and iron from soils into pore water, Ecotoxicology, 2019, 28, 528–538 CrossRef CAS PubMed.
- B. P. Chaplin, J. R. Shapley and C. J. Werth, Regeneration of sulfur-fouled bimetallic Pd-based catalysts, Environ. Sci. Technol., 2007, 41, 5491–5497 CrossRef CAS PubMed.
- F. A. Marchesini, S. Irusta, C. Querini and E. Miró, Nitrate hydrogenation over Pt, In/Al2O3 and Pt, In/SiO2. Effect of aqueous media and catalyst surface properties upon the catalytic activity, Catal. Commun., 2008, 9, 1021–1026 CrossRef CAS.
- F. A. Marchesini, S. Irusta, C. Querini and E. Miró, Spectroscopic and catalytic characterization of Pd-In and Pt-In supported on Al2O3 and SiO2, active catalysts for nitrate hydrogenation, Appl. Catal., A, 2008, 348, 60–70 CrossRef CAS.
- A. Pintar, J. Batista and I. Muševič, Palladium-copper and palladium-tin catalysts in the liquid phase nitrate hydrogenation in a batch-recycle reactor, Appl. Catal., B, 2004, 52, 49–60 CrossRef CAS.
- Y. Xie, H. Cao, Y. Li, Y. Zhang and J. C. Crittenden, Highly selective PdCu/amorphous silica-alumina (ASA) catalysts for groundwater denitration, Environ. Sci. Technol., 2011, 45, 4066–4072 CrossRef CAS PubMed.
- S. Hörold, T. Tacke and K. D. Vorlop, Catalytical removal of nitrate and nitrite from drinking water: 1. Screening for hydrogenation catalysts and influence of reaction conditions on activity and selectivity, Environ. Technol., 1993, 14, 931–939 CrossRef.
- O. S. G. Soares, J. J. Órfão and M. F. R. Pereira, Activated carbon supported metal catalysts for nitrate and nitrite reduction in water, Catal. Lett., 2008, 126, 253–260 CrossRef CAS.
- Z. Zhang, J. Lu, B. Zhang, W. Shi, Y. Guo and F. Cui, Insight into the size effect of Pd nanoparticles on the catalytic reduction of nitrite in water over Pd/C catalysts, Environ. Sci.: Nano, 2020, 7, 2117–2129 RSC.
- J. K. Nørskov, T. Bligaard, B. Hvolbæk, F. Abild-Pedersen, I. Chorkendorff and C. H. Christensen, The nature of the active site in heterogeneous metal catalysis, Chem. Soc. Rev., 2008, 37, 2163–2171 RSC.
- D. Gao, H. Zhou, J. Wang, S. Miao, F. Yang, G. Wang and X. Bao, Size-dependent electrocatalytic reduction of CO2 over Pd nanoparticles, J. Am. Chem. Soc., 2015, 137, 4288–4291 CrossRef CAS PubMed.
- S. H. Joo, J. Y. Park, J. R. Renzas, D. R. Butcher, W. Huang and G. A. Somorjai, Size effect of ruthenium nanoparticles in catalytic carbon monoxide oxidation, Nano Lett., 2010, 10, 2709–2713 CrossRef CAS PubMed.
- H. U. I. Zhang, M. Jin, Y. Xiong, B. Lim and Y. Xia, Shape-controlled synthesis of Pd nanocrystals and their catalytic applications, Acc. Chem. Res., 2013, 46, 1783–1794 CrossRef CAS PubMed.
- X. F. Yang, A. Wang, B. Qiao, J. Li, J. Liu and T. Zhang, Single-atom catalysts: a new frontier in heterogeneous catalysis, Acc. Chem. Res., 2013, 46, 1740–1748 CrossRef CAS PubMed.
- J. Y. Park, Y. Zhang, S. H. Joo, Y. Jung and G. A. Somorjai, Size effect of RhPt bimetallic nanoparticles in catalytic activity of CO oxidation: Role of surface segregation, Catal. Today, 2012, 181, 133–137 CrossRef CAS.
- G. Chen, C. Xu, X. Huang, J. Ye, L. Gu, G. Li and N. Zheng, Interfacial electronic effects control the reaction selectivity of platinum catalysts, Nat. Mater., 2016, 15, 564–569 CrossRef CAS PubMed.
- K. Gong, F. Du, Z. Xia, M. Durstock and L. Dai, Nitrogen-doped carbon nanotube arrays with high electrocatalytic activity for oxygen reduction, science, 2009, 323, 760–764 CrossRef CAS PubMed.
- H. Zhang, T. Watanabe, M. Okumura, M. Haruta and N. Toshima, Catalytically highly active top gold atom on palladium nanocluster, Nat. Mater., 2012, 11, 49–52 CrossRef PubMed.
- L. Jin, B. Liu, S. S. Duay and J. He, Engineering surface ligands of noble metal nanocatalysts in tuning the product selectivity, Catalysts, 2017, 7, 44 CrossRef.
- H. Li, K. Shin and G. Henkelman, Effects of ensembles, ligand, and strain on adsorbate binding to alloy surfaces, J. Chem. Phys., 2018, 149(17), 174705 CrossRef PubMed.
- A. Cao, V. J. Bukas, V. Shadravan, Z. Wang, H. Li, J. Kibsgaard and J. K. Nørskov, A spin promotion effect in catalytic ammonia synthesis, Nat. Commun., 2022, 13, 1–7 Search PubMed.
- H. Qian, Z. Zhao, J. C. Velazquez, L. A. Pretzer, K. N. Heck and M. S. Wong, Supporting palladium metal on gold nanoparticles improves its catalysis for nitrite reduction, Nanoscale, 2014, 6, 358–364 RSC.
- Y. Zhao, N. K. Rao and L. Lefferts, Adsorbed species on Pd catalyst during nitrite hydrogenation approaching complete conversion, J. Catal., 2016, 337, 102–110 CrossRef CAS.
- C. A. Clark, C. P. Reddy, H. Xu, K. N. Heck, G. Luo, T. P. Senftle and M. S. Wong, Mechanistic insights into pH-controlled nitrite reduction to ammonia and hydrazine over rhodium, ACS Catal., 2019, 10, 494–509 CrossRef.
- Y. Yoshinaga, T. Akita, I. Mikami and T. Okuhara, Hydrogenation of nitrate in water to nitrogen over Pd-Cu supported on active carbon, J. Catal., 2002, 207, 37–45 CrossRef CAS.
- H. Li, C. Yan, H. Guo, K. Shin, S. M. Humphrey, C. J. Werth and G. Henkelman, Cux,Ir1−x Nanoalloy Catalysts Achieve Near 100% Selectivity for Aqueous Nitrite Reduction to NH3, ACS Catal., 2020, 10, 7915–7921 CrossRef CAS.
- I. Mikami, Y. Sakamoto, Y. Yoshinaga and T. Okuhara, Kinetic and adsorption studies on the hydrogenation of nitrate and nitrite in water using Pd-Cu on active carbon support, Appl. Catal., B, 2003, 44, 79–86 CrossRef CAS.
- R. Brunet Espinosa and L. Lefferts, Ni in CNFs: highly active for nitrite hydrogenation, ACS Catal., 2016, 6, 5432–5440 CrossRef CAS.
- S. Bae, S. Hamid, J. Jung, Y. Sihn and W. Lee, Effect of promoter and noble metals and suspension pH on catalytic nitrate reduction by bimetallic nanoscale Fe0 catalysts, Environ. Technol., 2016, 37, 1077–1087 CrossRef CAS PubMed.
- S. Hamid, S. Bae, W. Lee, M. T. Amin and A. A. Alazba, Catalytic nitrate removal in continuous bimetallic Cu-Pd/nanoscale zerovalent iron system, Ind. Eng. Chem. Res., 2015, 54, 6247–6257 CrossRef CAS.
- S. Bae, J. Jung and W. Lee, The effect of pH and zwitterionic buffers on catalytic nitrate reduction by TiO2-supported bimetallic catalyst, Chem. Eng. J., 2013, 232, 327–337 CrossRef CAS.
- Q. Wang, W. Wang, B. Yan, W. Shi, F. Cui and C. Wang, Well-dispersed Pd-Cu bimetals in TiO2 nanofiber matrix with enhanced activity and selectivity for nitrate catalytic reduction, Chem. Eng. J., 2017, 326, 182–191 CrossRef CAS.
- S. Hamid, M. A. Kumar and W. Lee, Highly reactive and selective Sn-Pd bimetallic catalyst supported by nanocrystalline ZSM-5 for aqueous nitrate reduction, Appl. Catal., B, 2016, 187, 37–46 CrossRef CAS.
- S. Hamid, M. A. Kumar, J. I. Han, H. Kim and W. Lee, Nitrate reduction on the surface of bimetallic catalysts supported by nano-crystalline beta-zeolite (NBeta), Green Chem., 2017, 19, 853–866 RSC.
- A. H. Pizarro, C. B. Molina, J. J. Rodriguez and F. Epron, Catalytic reduction of nitrate and nitrite with mono-and bimetallic catalysts supported on pillared clays, J. Environ. Chem. Eng., 2015, 3(4), 2777–2785 CrossRef CAS.
- W. Zhao, X. Zhu, Y. Wang, Z. Ai and D. Zhao, Catalytic reduction of aqueous nitrates by metal supported catalysts on Al particles, Chem. Eng. J., 2014, 254, 410–417 CrossRef CAS.
- S. Hao and H. Zhang, High catalytic performance of nitrate reduction by synergistic effect of zero-valent iron (Fe0) and bimetallic composite carrier catalyst, J. Cleaner Prod., 2017, 167, 192–200 CrossRef CAS.
- Y. Sakamoto, Y. Kamiya and T. Okuhara, Selective hydrogenation of nitrate to nitrite in water over Cu-Pd bimetallic clusters supported on active carbon, J. Mol. Catal. A: Chem., 2016, 250(1–2), 80–86 Search PubMed.
- R. Gavagnin, L. Biasetto, F. Pinna and G. Strukul, Nitrate removal in drinking waters: the effect of tin oxides in the catalytic hydrogenation of nitrate by Pd/SnO2 catalysts, Appl. Catal., B, 2002, 38, 91–99 CrossRef CAS.
- O. S. G. Soares, J. J. Orfao and M. F. R. Pereira, Pd-Cu and Pt-Cu catalysts supported on carbon nanotubes for nitrate reduction in water, Ind. Eng. Chem. Res., 2010, 49, 7183–7192 CrossRef CAS.
- P. Granger, S. Troncéa, J. P. Dacquin, M. Trentesaux and V. I. Parvulescu, Support-induced effect on the catalytic properties of Pd particles in water denitrification: Impact of surface and structural features of mesoporous ceria-zirconia support, Appl. Catal., B, 2018, 224, 648–659 CrossRef CAS.
- A. S. Sekhar, A. Zaki, S. Troncea, S. Casale, C. P. Vinod, J. P. Dacquin and P. Granger, Enhanced selectivity of 3-D ordered macroporous Pt/Al2O3 catalysts in nitrites removal from water, Appl. Catal., A, 2018, 564, 26–32 CrossRef CAS.
- C. A. Boasiako, Z. Zhou, X. Huo and T. Ye, Development of Pd-based Catalysts for Hydrogenation of Nitrite and Nitrate in Water: A Review, J. Hazard. Mater., 2002, 130661 Search PubMed.
- Y. Zhao, J. A. Baeza, N. K. Rao, L. Calvo, M. A. Gilarranz, Y. D. Li and L. Lefferts, Unsupported PVA-and PVP-stabilized Pd nanoparticles as catalyst for nitrite hydrogenation in aqueous phase, J. Catal., 2014, 318, 162–169 CrossRef CAS.
- T. Tacke and K. D. Vorlop, Kinetische charakterisierung von katalysatoren zur selektiven entfernung von nitrat und nitrit aus wasser, Chem. Ing. Tech., 1993, 65, 1500–1502 CrossRef CAS.
- S. K. Ghosh, S. Nath, S. Kundu, K. Esumi and T. Pal, Solvent and ligand effects on the localized surface plasmon resonance (LSPR) of gold colloids, J. Phys. Chem. B, 2004, 108, 13963–13971 CrossRef CAS.
- A. J. Medford, A. Vojvodic, J. S. Hummelshøj, J. Voss, F. Abild-Pedersen, F. Studt and J. K. Nørskov, From the Sabatier principle to a predictive theory of transition-metal heterogeneous catalysis, J. Catal., 2015, 328, 36–42 CrossRef CAS.
- H. Li, S. Kelly, D. Guevarra, Z. Wang, Y. Wang, J. A. Haber and J. K. Nørskov, Analysis of the limitations in the oxygen reduction activity of transition metal oxide surfaces, Nat. Catal., 2021, 4, 463–468 CrossRef CAS.
- M. Stamatakis, Kinetic modelling of heterogeneous catalytic systems, J. Phys.: Condens. Matter, 2014, 27, 013001 CrossRef PubMed.
- A. Cao, Z. Wang, H. Li and J. K. Nørskov, Relations between surface oxygen vacancies and activity of methanol formation from CO2 hydrogenation over In2O3 surfaces, ACS Catal., 2021, 11, 1780–1786 CrossRef CAS.
- H. Li, S. Xu, M. Wang, Z. Chen, F. Ji, K. Cheng and W. Yang, Computational design of (100) alloy surfaces for the hydrogen evolution reaction, J. Mater. Chem. A, 2020, 8, 17987–17997 RSC.
- G. W. Piburn, H. Li, P. Kunal, G. Henkelman and S. M. Humphrey, Rapid Synthesis of Rhodium-Palladium Alloy Nanocatalysts, ChemCatChem, 2018, 10, 329–333 CrossRef CAS.
- H. Li, A. Cao and J. K. Nørskov, Understanding Trends in Ethylene Epoxidation on Group IB Metals, ACS Catal., 2021, 11, 12052–12057 CrossRef CAS.
- L. Zhou, H. Li, Y. Lai, M. Richter, K. Kan, J. A. Haber and J. M. Gregoire, Stability and Activity of Cobalt Antimonate for Oxygen Reduction in Strong Acid, ACS Energy Lett., 2022, 7, 993–1000 CrossRef CAS.
- S. Pan, H. Li, D. Liu, R. Huang, X. Pan, D. Ren and X. Zhang, Efficient and stable noble-metal-free catalyst for acidic water oxidation, Nat. Commun., 2022, 13, 1–10 Search PubMed.
- W. Yang, X. Chen, Y. Feng, F. Wang, Z. Gao, Y. Liu and H. Li, Understanding trends in the mercury oxidation activity of single-atom catalysts, Environ. Sci.: Nano, 2022, 9, 2041–2050 RSC.
- H. Li, C. S. Abraham, M. Anand, A. Cao and J. K. Nørskov, Opportunities and Challenges in Electrolytic Propylene Epoxidation, J. Phys. Chem. Lett., 2022, 13, 2057–2063 CrossRef CAS PubMed.
- Y. Liu, C. Xu, W. Cen and H. Li, Design strategy of bifunctional catalysts for CO oxidation, Fuel, 2022, 320, 123909 CrossRef CAS.
- W. Yang, Y. Feng, X. Chen, C. Wu, F. Wang, Z. Gao and H. Li, Understanding Trends in the NO Oxidation Activity of Single-Atom Catalysts, J. Environ. Chem. Eng., 2022, 10, 108744 CrossRef CAS.
- W. Yang, B. Zhou, Z. Jia, C. Wu, L. Wei, Z. Gao and H. Li, Coordination Engineering of Single-Atom Iron Catalysts for Oxygen Evolution Reaction, ChemCatChem, 2022, 14, e202201016 CAS.
- J. W. Erisman, M. A. Sutton, J. Galloway, Z. Klimont and W. Winiwarter, How a century of ammonia synthesis changed the world, Nat. Geosci., 2008, 1, 636–639 CrossRef CAS.
- S. García, L. Zhang, G. W. Piburn, G. Henkelman and S. M. Humphrey, Microwave synthesis of classically immiscible rhodium-silver and rhodium-gold alloy nanoparticles: Highly active hydrogenation catalysts, ACS Nano, 2014, 8, 11512–11521 CrossRef PubMed.
- H. Li, W. Chai and G. Henkelman, Selectivity for ethanol partial oxidation: the unique chemistry of single-atom alloy catalysts on Au, Ag, and Cu (111), J. Mater. Chem. A, 2019, 7, 23868–23877 RSC.
- H. Guo, H. Li, K. Jarvis, H. Wan, P. Kunal, S. G. Dunning and S. M. Humphrey, Microwave-assisted synthesis of classically immiscible Ag-Ir alloy nanoparticle catalysts, ACS Catal., 2018, 8, 11386–11397 CrossRef CAS.
- H. Guo, Z. Fang, H. Li, D. Fernandez, G. Henkelman, S. M. Humphrey and G. Yu, Rational design of rhodium-iridium alloy nanoparticles as highly active catalysts for acidic oxygen evolution, ACS Nano, 2019, 13, 13225–13234 CrossRef CAS PubMed.
- G. Xiao, R. Lu, J. Liu, X. Liao, Z. Wang and Y. Zhao, Coordination environments tune the activity of oxygen catalysis on single atom catalysts: A computational study, Nano Res., 2022, 15, 3073–3081 CrossRef CAS.
- M. Mavrikakis, B. Hammer and J. K. Nørskov, Effect of strain on the reactivity of metal surfaces, Phys. Rev. Lett., 1998, 81, 2819 CrossRef.
- P. Liu and J. K. Nørskov, Ligand and ensemble effects in adsorption on alloy surfaces, Phys. Chem. Chem. Phys., 2001, 3, 3814–3818 RSC.
- E. M. Fernández, P. G. Moses, A. Toftelund, H. A. Hansen, J. I. Martínez, F. Abild-Pedersen and J. K. Nørskov, Scaling relationships for adsorption energies on transition metal oxide, sulfide, and nitride surfaces, Angew. Chem., Int. Ed., 2008, 47, 4683–4686 CrossRef PubMed.
- J. A. Schwarz, C. Contescu and A. Contescu, Methods for preparation of catalytic materials, Chem. Rev., 1995, 95, 477–510 CrossRef CAS.
- B. A. Mehrabadi, S. Eskandari, U. Khan, R. D. White and J. R. Regalbuto, A review of preparation methods for supported metal catalysts, Adv. Catal., 2017, 61, 1–35 CAS.
- N. Krawczyk, S. Karski and I. Witońska, The effect of support porosity on the selectivity of Pd-In/support catalysts in nitrate reduction, React. Kinet., Mech. Catal., 2011, 103, 311–323 CrossRef CAS.
- M. D'Arino, F. Pinna and G. Strukul, Nitrate and nitrite hydrogenation with Pd and Pt/SnO2 catalysts: the effect of the support porosity and the role of carbon dioxide in the control of selectivity, Appl. Catal., B, 2004, 53, 161–168 CrossRef.
- A. H. Pizarro, I. Torija and V. M. Monsalvo, Enhancement of Pd-based catalysts for the removal of nitrite and nitrate from water, J. Water Supply: Res. Technol.–AQUA, 2018, 67, 615–625 Search PubMed.
- F. Rodriguez-Reinoso, The role of carbon materials in heterogeneous catalysis, Carbon, 1998, 36, 159–175 CrossRef CAS.
- L. Calvo, M. A. Gilarranz, J. A. Casas, A. F. Mohedano and J. J. Rodriguez, Denitrification of water with activated carbon-supported metallic catalysts, Ind. Eng. Chem. Res., 2020, 49, 5603–5609 CrossRef.
- J. Trawczyński, P. Gheek, J. Okal, M. Zawadzki and M. I. Gomez, Reduction of nitrate on active carbon supported Pd-Cu catalysts, Appl. Catal., A, 2011, 409, 39–47 CrossRef.
- O. S. G. Soares, J. J. Órfão and M. F. R. Pereira, Bimetallic catalysts supported on activated carbon for the nitrate reduction in water: Optimization of catalysts composition, Appl. Catal., B, 2009, 91, 441–448 CrossRef CAS.
- Z. Wang, S. D. Young, B. R. Goldsmith and N. Singh, Increasing electrocatalytic nitrate reduction performance by tuning adsorption through PtRu alloying, in ECS Meeting Abstracts, IOP Publishing, 2020, vol. MA2020-02, p. 3267 Search PubMed.
- Z. Wang, D. Richards and N. Singh, Recent discoveries in the reaction mechanism of heterogeneous electrocatalytic nitrate reduction, Catal. Sci. Technol., 2021, 11, 705–725 RSC.
- P. H. van Langevelde, I. Katsounaros and M. T. Koper, Electrocatalytic nitrate reduction for sustainable ammonia production, Joule, 2021, 5, 290–294 CrossRef.
- N. Barrabés and J. Sá, Catalytic nitrate removal from water, past, present and future perspectives, Appl. Catal., B, 2011, 104, 1–5 CrossRef.
- Z. Wang, E. M. Ortiz, B. R. Goldsmith and N. Singh, Comparing electrocatalytic and thermocatalytic conversion of nitrate on platinum-ruthenium alloys, Catal. Sci. Technol., 2021, 11, 7098–7109 RSC.
- S. D. Ebbesen, B. L. Mojet and L. Lefferts, Effect of pH on the nitrite hydrogenation mechanism over Pd/Al2O3 and Pt/Al2O3: Details obtained with ATR-IR spectroscopy, J. Phys. Chem. C, 2011, 115, 1186–1194 CrossRef CAS.
- R. Yang, L. Mei, Y. Fan, Q. Zhang, H. G. Liao, J. Yang and Z. Zeng, Fabrication of liquid cell for in situ transmission electron microscopy of electrochemical processes, Nat. Protoc., 2023, 18, 555–578 CAS.
- Q. Zhang, J. Ma, L. Mei, J. Liu, Z. Li, J. Li and Z. Zeng, In situ TEM visualization of LiF nanosheet formation on the cathode-electrolyte interphase (CEI) in liquid-electrolyte lithium-ion batteries, Matter, 2022, 5, 1235–1250 CrossRef CAS.
- Y. Zhang, R. Yang, H. Li and Z. Zeng, Boosting electrocatalytic reduction of CO2 to HCOOH on Ni single atom anchored WTe2 monolayer, Small, 2022, 18, 2203759 CrossRef CAS PubMed.
- Y. Li, G. Tian, B. Chen and J. Liang, Self-templating construction of flower-like mesoporous magnesium silicate composites from sepiolite for high-efficiency adsorption of aflatoxin B1, Sep. Purif. Technol., 2022, 291, 120953 CrossRef CAS.
- Y. Wang, C. Wang, M. Li, Y. Yu and B. Zhang, Nitrate electroreduction: mechanism insight, in situ characterization, performance evaluation, and challenges, Chem. Soc. Rev., 2021, 50, 6720–6733 RSC.
- H. Liu, X. Jia, A. Cao, L. Wei, C. D′agostino and H. Li, The surface states of transition metal X-ides under electrocatalytic conditions, J. Chem. Phys., 2023, 158, 124705 CrossRef CAS PubMed.
- W. Yang, Z. Jia, B. Zhou, L. Wei, Z. Gao and H. Li, Surface states of dual-atom catalysts should be considered for analysis of electrocatalytic activity, Commun. Chem., 2023, 6, 6 CrossRef CAS PubMed.
| This journal is © the Partner Organisations 2023 |
