Open Access Article
Open Access ArticleDiruthenium(II,III) paddlewheel complexes: effects of bridging and axial ligands on anticancer properties
Iogann
Tolbatov
 a,
Elisabetta
Barresi
a,
Elisabetta
Barresi
 b,
Sabrina
Taliani
b,
Diego
La Mendola
b,
Tiziano
Marzo
b,
Sabrina
Taliani
b,
Diego
La Mendola
b,
Tiziano
Marzo
 b and
Alessandro
Marrone
b and
Alessandro
Marrone
 *c
*c
aInstitute of Chemical Research of Catalonia (ICIQ), The Barcelona Institute of Science and Technology, av. Paisos Catalans, 16, Tarragona, 43007, Spain
bDepartment of Pharmacy, University of Pisa, Via Bonanno Pisano, 6, Pisa, 56126, Italy
cDepartment of Pharmacy, University “G d'Annunzio” Chieti-Pescara, Via dei Vestini, 31, Chieti, 66100, Italy. E-mail: amarrone@unich.it
First published on 15th March 2023
Abstract
This article provides an overview of the application of diruthenium(II,III) paddlewheel complexes for anticancer purposes. The use of this coordinative construct is indeed attractive because it provides an excellent opportunity to combine the pharmacological properties of the dimetallic ruthenium center with those derived from the specific choice of ligands bearing a carboxylic function capable of coordination towards the Ru–Ru core. Indeed, the combination of carboxylate ligands with specific anticancer properties and the dimetallic center permits the production of new entities endowed with improved biological profiles. Additionally, these systems allow the simultaneous multiple deliveries of a drug to the target site. Nevertheless, in order to obtain the desired effects, it is mandatory to consider some relevant chemico-physical aspects such as the steric hindrance of the ligands or the possibility of their release under specific biological conditions that should be taken into account in the design of effective complexes. Accordingly, through various examples from the literature, the key features of this family of unconventional compounds are summarized here, also providing useful hints for the design of improved diruthenium(II,III) paddlewheel complexes.
1. Introduction
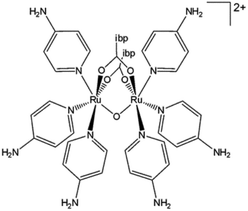
Nowadays metallodrugs are important competitors of the conventional purely organic agents due to several important inherent advantages, such as structural diversity, accessible redox states, a broad spectrum of coordination numbers and geometries, and the possibility to fine-tune both the thermodynamics and kinetics of ligand substitution.1,2 Since the discovery of the remarkable anticancer properties of cisplatin,3 huge efforts have been devoted to the identification of improved anticancer metallodrugs. In this context, beyond platinum, several other transition metals have been exploited for the synthesis of complexes characterized by a variety of geometries and oxidation states in an attempt to (1) design metal compounds with enhanced pharmacokinetic and pharmacodynamic profiles, and (2) overcome limitations of platinum-based chemotherapy such as heavy side effects and resistance.4 Several strategies have been developed and applied to the discovery of novel compounds endowed with improved pharmacological profiles,5 including the assembly of active ligands with metal scaffolds to obtain dual drugs. Such an approach is often applied to induce a multifaceted optimization of the drug delivery by effectively stabilizing nonconventional geometries of the compound, which can serve various purposes, such as peculiar ways of action, improved permeation of cell membranes, augmented solubility, or modified redox behaviour.6,7 On the other hand, the disassembling of the dual drug structure is also exploited to trigger two active mechanisms in the target tissue to achieve a potentiated pharmacological response.In this context, paddlewheel complexes composed of a Ru(II)Ru(III) bimetallic moiety and biologically active carboxylate ligands have been identified as novel dual anticancer drugs. The general structure of these active metal complexes is shown in Fig. 1. The four carboxylate ligands play a twofold role, by carrying and protecting the bimetallic moiety, thus favoring tissue targeting, and by exerting their own biological activity in parallel and/or synergizing with the activity of the bimetallic core. Therefore, the axial ligands on the Ru(II)Ru(III) unit may act as additional modulators of their target selectivity, thus enhancing the versatility of these metal drugs.
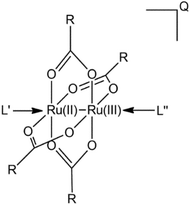 | ||
| Fig. 1 General structure of Ru(II)Ru(III) paddlewheel complexes. R-COO− = carboxylate ligand; L′, L′′ = chloro, aquo, hydroxo ligand, and total charge Q. | ||
Since the synthesis of the first diruthenium paddlewheel complex [Ru2Cl(O2CC3H7)4] in 1969,8 this dimetal scaffold has demonstrated overall oxidation states varying from IV to VI. The most stable form has been found to be Ru2(II,III), whereas both the complexes Ru2(II,II) and Ru2(III,III) were found to be much less stable, with the latter one especially unstable. On the one hand, the Ru2(II,III) scaffold was found to be well stabilized by four carboxylate bridging ligands (Fig. 1); in contrast, the axial chloro ligands are the ones that form the polymeric structure with dimetal units in the solid state. In solution, on the other hand, the axial chloro ligand is substituted by a water molecule; thus, Ru2 may exist in either a cationic (aquo) or neutral (hydroxo) form, depending on the pH of the milieu. The three unpaired electrons in these d5–d6 bimetallic moieties (quadruplet ground state) yield exceptional magnetic features as they display high magnetic moments of 3.8–4.4 B.M. per diruthenium unit. These complexes demonstrate reduction potentials of 0.30 to 0.50 V (versus normal hydrogen electrode), which means biological attainability.9
2. Experimental observations
A wide variety of paddlewheel complexes of the diruthenium(II,III) unit have been prepared since 1969, with the synthesis of the Ru2Cl(O2CC3H7)4 complex by Cotton et al.8 These complexes have been then recognized to be usable in the preparation of other paddlewheel complexes, thus increasing the structural variety in these compounds, ranging from steric effects to charge modulation, e.g. the preparation of the [Ru2(CO3)4]3− anion.10The electronic structure of the [Ru2(μ-O2CR)4]+ complexes has been theoretically characterized by Norman et al.,11,12 and later on confirmed by much experimental evidence.13,14 The calculations performed on the [Ru2(μ-O2CH)4]+ species have shown a σ2π4δ2π*2δ*1 configuration, in which two degenerate π* and one δ* orbitals were semioccupied, i.e. possessing spin S = 3/2. The close energy proximity of the π* and one δ* orbitals in these complexes has been mainly ascribed to the combinations involving the orbitals of μ-bridged carboxylates, whereas the axial ligands have a smaller impact on the electronic configuration.11
In the search for functionally improved metallodrugs, a myriad of diruthenium paddlewheel complexes has been developed with various axial and equatorial or bridging carboxylate ligands (Table 1), among which the most ubiquitous is the complex with four carboxylate ligands with the general formula [Ru2(O2CR)4X], where R may be an alkyl, aryl, or alkoxy group, or a metallocene moiety, whereas X is usually a Lewis base or a halide. Three groups of diruthenium paddlewheel carboxylate complexes are described in the following subsections.
| Axial ligand (L′′) | Axial ligand (L′′) | Charge (Q) | Anion | Target | Ref. |
|---|---|---|---|---|---|
| Cl | — | 0 | — | Leukemia P 388 | 15 |
| Cl | — | 0 | — | Leukemia P 388; Asp, Arg, Cys, His, Lys, Sec | 16–20 |
| H2O | H2O | +1 | PF6− | HeLa and multidrug resistant CoLo; glutathione, ascorbic acid, side chains | 9 and 19–22 |
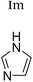
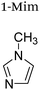
|
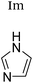
|
+1 | PF6− | HeLa and multidrug resistant CoLo | 9, 22 and 23 |
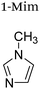
|
|
+1 | PF6− | HeLa and multidrug resistant CoLo | 9 and 22 |
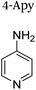
|
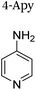
|
+1 | PF6− | No data | 24 |
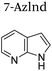
|
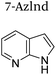
|
+1 | PF6− | No data | 23 |
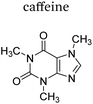
|
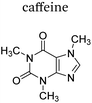
|
+1 | PF6− | No data | 23 |
2.1 Diruthenium(II,III) tetraacetates
Diruthenium tetraacetates [Ru2(CH3CO2)4X] are a new emerging scaffold for antitumor prodrugs. It is well demonstrated by several studies presented below that many therapeutically active agents bearing a carboxylate motif R-COO− can be utilized as bridging ligands in these paddlewheel complexes.15 By fine-tuning the complex interplay of steric and electronic features of its ligands, it is possible to formulate a prodrug for dual action, derived from the presence of the diruthenium core accompanied by the chance of simultaneous delivery of up to four units of therapeutically active ligand per molecule of complex. The role of the diruthenium center had been studied before in 1989 when it was shown that diruthenium tetraacetate is active against the P388 leukemia system, reaching T/C values of 125% (T/C value represents the median survival time/tumor weight of treated (= T) animals versus the median survival time/tumor weight of control (= C) animals × 100).25 Indeed, it was recently demonstrated that this compound, at a mean concentration between 120 and 950 micromol per dm3, killed 50% of P388 leukemia cell lines.22 Nevertheless, it was concluded that the low solubility of these complexes hampered their cytotoxic activity substantially. Additionally, it was shown that the HeLa cancer cells were 5 times less susceptible to drug-induced death than CoLo cells.Based on the body of studies available on diruthenium(II,III) tetraacetate complexes, we assumed these compounds as typical to analyse the effects of the axial ligands on the biological activity of these metal complexes.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and adenosine (1
1) and adenosine (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2).16 The metalation of adenine produced the polymeric structure in which the diruthenium tetraacetate monomeric units are bridged by adenine, linking their axial vacancies through adenine's N3 and N9. On the other hand, the metalation of adenosine occurred via N(7). Despite the finding that the metalation of these two molecules formed the DNA building blocks, the experiment showed that the complex [Ru2(CH3CO2)4Cl] exhibits a lower toxicity in comparison with monometallic ruthenium complexes. Nevertheless, the observed metalation of adenine and adenosine is a significant indication of the potential of the diruthenium(II,III) complexes as agents targeting DNA.16
2).16 The metalation of adenine produced the polymeric structure in which the diruthenium tetraacetate monomeric units are bridged by adenine, linking their axial vacancies through adenine's N3 and N9. On the other hand, the metalation of adenosine occurred via N(7). Despite the finding that the metalation of these two molecules formed the DNA building blocks, the experiment showed that the complex [Ru2(CH3CO2)4Cl] exhibits a lower toxicity in comparison with monometallic ruthenium complexes. Nevertheless, the observed metalation of adenine and adenosine is a significant indication of the potential of the diruthenium(II,III) complexes as agents targeting DNA.16
The versatility of Ru2(CH3CO2)4Cl in the synthesis of novel paddlewheel scaffolds with improved features has been shown by the preparation of mono-substituted diruthenium complexes.26,27 This synthetic methodology has been later adapted to prepare either mono-substituted complexes such as Ru2(APy)(CH3CO2)3Cl, with APy representing variably decorated aniline–pyridine ligands,28 or the so-called open paddlewheel diruthenium complexes in which one μ-bridged position is replaced by two chloro ligands.29
The investigation of thermodynamics of the axial substitution of water by chloride in the complex [Ru2(CH3COO)4(H2O)]+ and kinetic studies on its reactions with cysteine, glycine, histidine, and tryptophan corroborated the conservation of the [Ru2(RCOO)4]+ paddlewheel structure in water and in the presence of amino acids.18 The diaqua-Ru2 species formed from the dissolution of the [Ru2(CH3COO)4Cl] complex in water may be subjected to the substitution of its axial ligands on both sides. Computed reaction parameters ΔH0, ΔS0, and ΔV0 demonstrated that the consecutive axial ligand exchange of water by chloride is thermodynamically favourable. It was revealed that this diruthenium complex easily undergoes the axial replacement of the first water molecule by amino acids in the following order of reaction rate: cysteine ≫ tryptophan ∼ glycine. Nevertheless, the deconvolution of the UV-Vis absorption spectra demonstrated that a considerable quantity of the initial diaqua-Ru2 complexes is nonetheless preserved in the solutions.18
2.2 Diruthenium(II,III) complexed with NSAIDs
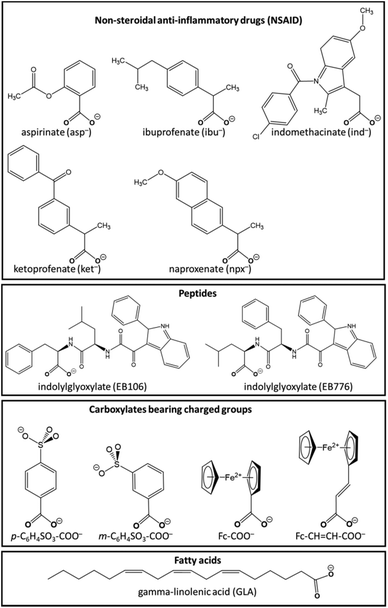
Non-steroidal anti-inflammatory drugs (NSAIDs) are anti-inflammatory, anti-fever, and pain-killing drugs and their main target is cyclooxygenase, the enzyme producing inflammation-inducing prostaglandin.32 Nevertheless, the side effects may be serious, even lethal.33 Recent studies discovered the anti-cancer activity of aspirin and other NSAIDs; in particular, the use of these agents diminished greatly the likelihood of gastrointestinal, prostate, breast, skin, and lung tumors.34,35 However, the anti-cancer efficacy of NSAIDs lasts only as long as the drugs are taken, and once they are stopped, the tumours recrudesce. The most probable source of their anti-cancer activity is their targeting of the cyclooxygenase (COX) enzyme, especially isoform COX-2 that is overexpressed in many types of cancers.36Numerous studies have profited from the availability of the carboxylic acid moiety COOH in NSAIDs in order to utilize this functional group as the bridging ligand in diruthenium paddlewheel complexes.37 These investigations have obtained metal complexes with promising pharmacological properties.9,38 Indeed, as demonstrated in a review,39 NSAIDs coordinated to metal ions have clear medicinal effects superior to those of uncomplexed NSAID molecules; moreover, their properties differ greatly from those of their parent drugs. It was shown that the stability of the diruthenium–NSAID complex in the biological milieu is the reason for this advantage.
It is noteworthy that the recently synthesized μ-oxo-diruthenium(III,III)-ibuprofen-(4-aminopyridine) chloride derivative of the Ru2 paddlewheel complex (Fig. 2) has shown antiproliferative effects (at 5–50 μmol L−1) and cytotoxicity (>20 μmol L−1) against cells of U87MG human glioblastoma, indicating that this novel scaffold as a promising choice for innovative treatment of gliomas.24
In the following sections, the anticancer activity of diruthenium–NSAID complexes bearing different axial ligands (2.2.1 and 2.2.2) or formulated in specialized drug delivery systems (2.2.3) has been examined.
Therefore, it was also found that the inhibition of C6 glioma cell proliferation by [Ru2(Ibp)4Cl] is remarkably more potent than by free ibuprofen.43 An in-depth study has been directed at the effects of [Ru2(Ibp)4Cl] upon C6 cell reactive species generation, mitochondrial membrane potential, cycle distribution, and mRNA as well as protein expression of E2F1, cyclin D1, c-myc, pRb, p21, p27, p53, Ku70, Ku80, Bax, Bcl2, COX1 and COX2.
The titled protein targets have been selected based on their pivotal role in cellular pathways potentially implicated in anticancer response. For example, the transcription factor E2F1 may bind to the retinoblastoma protein (pRb) to form a complex that blocks cell cycle progression; similarly, p53-p21-pRb signalling also induces a tumour suppressor response.44
The overexpression of cyclin D1 and c-myc has been detected in tumours, thus suggesting their oncogenic role and their targeting as a valuable anticancer strategy.45,46
Ku70 and Ku80 are DNA-binding proteins involved in the mechanisms of DNA repair, and, in particular, in repairing double-strand breaks that are markers of cellular aging.47 The Bax/Bcl2 heterodimers are onco-suppressors that activate the mitochondrial apoptotic pathway through their interaction with the mitochondrial voltage-dependent anion channel and the concomitant release of cytochrome c.48
Both isoforms COX1 and COX2 of the cyclooxygenase enzyme are implicated in the biosynthesis of prostaglandins, e.g. the most important mediators of inflammation. A body of evidence is available to show the upregulation of these enzymes in several cancer tissues, wherein a “prostaglandin storm” is typically induced.49
The increase of the cyclin-dependent kinase inhibitors p21 and p27 indicated the crucial alterations in mRNA and protein expression, which were also accompanied by the major decline in mitochondrial membrane potential and in anti-apoptotic Bcl2 expression, as well as a modest increase in apoptosis and in pro-apoptotic Bax expression. The drop in prostaglandin E2 production coupled with the augmented COX1 expression were attributed to the effects of ibuprofen. It could be inferred that the inhibition of C6 rat glioma proliferation by [Ru2Cl(Ibp)4] depends on changes in the mitochondrial membrane potential, expression of proteins p21, p27, and the Bax/Bcl2 ratio.43 In another study, it was shown that [Ru2(Ibp)4Cl] achieved a 41% inhibition of tumour area without significant toxic effects in a rat model, coupled with a decline in blood lymphocytes and an increase in blood monocytes and neutrophils. Moreover, the direct injection of the metallodrug resulted in 45% improved tumour inhibition without side effects.50
The role of axial ligands in the diruthenium(II,III)–ibuprofenato complexes was meticulously studied in another recent study, in which these metal scaffolds were used for inhibition of the U87MG and A172 human glioma cell proliferation.42 The axial ligands were added to the [Ru2(Ibp)4]+ scaffold, producing Ru2(Ibp)4(CF3SO3) and [Ru2(Ibp)4(EtOH)2]+, and their cytotoxic activity was compared with that of conventional Ru2(Ibp)4Cl. It was concluded that the chloride ligand plays a crucial role, since its presence confers the highest antiproliferative activity, i.e., effects on U87MG and A172 human glioma cell proliferation, apoptosis, mitosis, and cell migration in vitro. Nevertheless, all three studied complexes exhibited good cell permeation, caused apoptosis of a substantial number of cells, and decreased the fraction of mitotic cells. Moreover, evidence was obtained for the inhibition of cell migration by the diruthenium–ibuprofenato complexes in both human glioma cell lines, leading to the conclusion that these drugs have potent inhibition activity against cell mitosis and cell migration, thus providing a therapeutic response on two crucial chemotherapeutic objectives in high grade gliomas.42
Another study compared the anti-inflammatory activity of the diruthenium(II,III)–ibuprofen complex with the activity of the copper(II)–ibuprofenato complex.51 Both complexes, tested in vivo for their anti-inflammatory activity, were orally administered and resulted in the inhibition of advancement of the carrageenan-induced edema in rats, even though their efficiency was comparable to that of free ibuprofen. Nevertheless, the side effects, especially associated with the gastric damage, were less acute than in the case of ibuprofen. It was found out that the diruthenium–ibuprofenato scaffold produced a defensive influence in the case of light ulceration, whereas in the case of more severe ulceration, the efficacy of the copper–ibuprofenato complex was higher.51
In a recent study, the augmented efficacy of diruthenium(II,III) complexes with ibuprofenato or naproxenato bridging ligands encapsulated in intravenously injectable solid polymer–lipid nanoparticles (SPLNs) against prostate and breast cancer cells was studied.53 The Ru2(NSAID)-SPLNs were developed with a round shape and an average size of 120 nm. The nanoparticles were loaded 17–18% with metal complexes and demonstrated excellent colloidal stability in serum at the physiological temperature. The reported results describe the new Ru2(NSAID)-SPLN nanoformulations as exceptionally promising for medical use. Primarily, these complexes displayed adequate biodistribution and high accretion in tumors after being intravenously administered in an orthotopic breast tumor model. Moreover, the Ru2(NSAID)-SPLNs revealed appreciable cytotoxicity (IC50 = 60–100 μmol L−1), comparable with that of the unencapsulated Ru2(NSAID) complexes, as revealed by in vitro investigations in prostate DU145 and breast EMT6 and MDA-MB-231 cancer cells. Clearly, the nanoparticle stability and their appropriateness for intravenous administration coupled with the augmented anticancer properties are the reasons behind the active progress in this direction.53
2.3 Diruthenium(II,III) complexed with other bridging ligands
Various research groups have synthesized diruthenium paddlewheel complexes using diverse bridging ligands. For example, a propionate bridge was used in one of the earlier studies in this field, showing substantial activity against P388 leukemia cells.25In the following subsections, we examine the structure and anticancer activity of paddlewheel complexes of the diruthenium(II,III) scaffold bearing μ-bridged carboxylate ligands that present either steric or charge/electronic peculiarities.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH-COO−, Fc-COO− (Fig. 3), was examined for cytotoxicity against HeLa and multidrug resistant CoLo 320DM human cancer cell lines.22 All the tested complexes demonstrated weak antineoplastic action versus P388 leukemia cell lines. Nevertheless, the antineoplastic activity of the highly water-soluble m-C6H4SO3-COO− derivative was found to be the most robust, whereas other complexes lacked cytotoxicity due to the limited solubility in water. Another observed effect was that these metal complexes caused cell death of CoLo 320DM cancer cells 5 times more efficiently than of HeLa cells.
CH-COO−, Fc-COO− (Fig. 3), was examined for cytotoxicity against HeLa and multidrug resistant CoLo 320DM human cancer cell lines.22 All the tested complexes demonstrated weak antineoplastic action versus P388 leukemia cell lines. Nevertheless, the antineoplastic activity of the highly water-soluble m-C6H4SO3-COO− derivative was found to be the most robust, whereas other complexes lacked cytotoxicity due to the limited solubility in water. Another observed effect was that these metal complexes caused cell death of CoLo 320DM cancer cells 5 times more efficiently than of HeLa cells.
2.4 pH- and time-dependent release
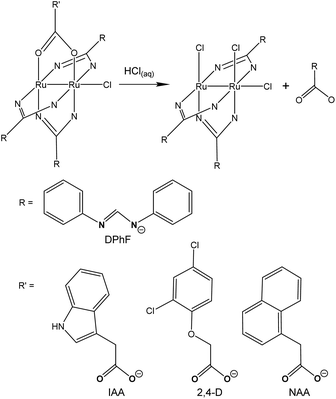
In the field of biomedical applications of prodrugs, the controlled detachment of the therapeutically active molecules is crucial; thus, the efficiency of the prodrugs is dependent on the occurrence of the conditions for the release.58 Usually, metallodrugs are developed in such a way that the conditions of the biological circumambience, including pH, determine that the release of the biologically active moiety takes place right in the target tissue. For instance, the augmented production of lactic acid and low rates of its diffusion are the reasons behind the extracellular acidosis displayed by solid tumours, i.e., pH values in the range 6.5–7.1 or even lower, whereas healthy tissues demonstrate a pH of ∼7.4.59–62 The lower pH of tumour tissue can be the circumambience condition to be exploited in the activation of anticancer prodrugs. In order to study the dependence of the release of bridging ligands in diruthenium(II,III) scaffolds, three metal complexes were synthesized, each comprising three bridging N,N′-diphenylformamidinates (DPhF) and one auxin-related hormone, either indole-3-acetate (IAA), 2,4-dichlorophenoxyacetate (2,4-D), or 1-naphthaleneacetate (NAA) (Fig. 4).63 The produced complexes [Ru2Cl(μ-DPhF)3(μ-IAA)], [Ru2Cl(μ-DPhF)3(μ-2,4-D)], and [Ru2Cl(μ-DPhF)3(μ-NAA)] were tested for their capability to detach the bound hormone at various pH values, via a very sensitive assay prepared on the basis of Arabidopsis thaliana plants; results demonstrated that the auxin ligands were released more rapidly at acidic and neutral pH than in slightly alkaline media. Indeed, as the auxins were found to be mostly in their anionic forms in solutions, their release from the diruthenium(II,III) scaffold necessitates a protonation step, thus being favored in acidic pH.633. Computational points of view
Density functional theory (DFT) provides for an accurate and robust computational paradigm, widely used in bioinorganic and organometallic chemistry,64–66 including in the computational design of metallodrugs as well as analysis of their modus operandi.67,68 Furthermore, DFT is known to yield accurate geometries and reaction profiles for transition-metal-containing scaffolds69,70 and the Ru-based anticancer compounds.71,72The investigation of diruthenium paddlewheel complexes by means of quantum chemistry approaches is expectedly quite interesting. Indeed, their stability is determined by both μ-bridges and axial ligands that cooperate to influence the electronic/electrostatic nature of the bimetallic moiety and modulate the strength of the metal–carboxylate bonds. As a matter of fact, the X-ray structure representing the HEWL protein metalated by diRh scaffolds suggested that paddlewheel dimetallic complexes may tend to break into two monometallic fragments upon binding to protein residues.17
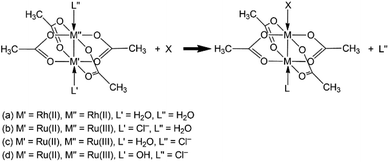
The impact of binding nucleophilic protein side chains at the axial positions of the complexes Rh2(μ-O2CCH3)4(H2O) and Ru2(μ-O2CCH3)4Cl has been analysed using DFT calculations.19,20
These computations revealed a weakening of the metal–metal bonds in both Rh- and Ru-based complexes upon the axial binding to Sec− and His. Interestingly, it was demonstrated that the coordination at the soft protein residue side chains caused a marginal weakening of the μ-coordination of acetates, which was the major effect was detected in [Ru2(μ-O2CCH3)4ClX] complexes. This means that diruthenium complexes are probably more prone to cleave into two monometallic fragments, thus facilitating the decomplexation of the bridging carboxylates.
The investigation of the thermodynamic rationale behind the axial ligand exchange reactions (Fig. 5) evidenced that the main targets of both diRh and diRu scaffolds in physiological conditions are Asp−, C-term−, His, and Sec−.
On the other hand, the DFT study of the kinetics of the reaction of both paddlewheel complexes with protein nucleophilic sites20 allowed determination of the most reactive protein side chains towards the paddlewheel scaffolds (Fig. 5). It was found that the acidic environment favours the reactions involving the exchange of axial water (reactions (a) and (b)) and brings them under thermodynamic control, selectively targeting Arg, Cys−, and Sec−. On the other hand, the presence of an axial hydroxide, following water coordination, makes chloride substitution possible (reaction (d)), but only at high pH that favors the hydroxo over the aquo form.20
Moreover, the calculated geometries of the transition states for the axial ligand substitution yielded the structural basis to elucidate how the hindrance of the paddlewheel complexes may also contribute to the chemoselectivity toward the analyzed protein residue side chains.20 From a structural point of view, the earliness or lateness of a transition state has a major effect on the degree of steric hindrance that can be possibly exerted by a bridging ligand. For instance, the early transition states feature the approaching ligand, i.e., a protein residue, farther from the bimetallic core, so the μ-ligands are located farther from the attacking nucleophile, and thus their effect is minimal (reactions (a) and (b)). Contrarily, the protein residue is located closer to the metal centers in an intermediate or late transition state, thus, it is more likely to sterically clash with the bulky bridging ligands (reactions (c) and (d)).
The highest structural control of the chemoselectivity of these paddlewheel complexes can be possibly combined when the operative conditions favor reaction (d) and yielding (1) higher selectivity of the complex [Ru2(μ-O2CR)4(OH)Cl]− toward the Lys, Cys−, and Sec− residues, and (2) an inherent propensity to form early TS structures in the reaction with protein residues, and due to the steric hindrance exerted by the bulky bridging ligands. Such an amalgamation of effects may eventuate in increased selectivity of these bimetallic agents toward typical anticancer targets, for example, thioredoxin reductase of cancer cells, although only at high pH.
Further computational exploration of the effects of the richly decorated bridging acetate-based ligands is required as the steric and electronic effects of these bridging moieties may substantially alter the pharmacodynamics of these bimetallic scaffolds.
4. A biological insight
The described structural and reactive features of diruthenium paddlewheel complexes evidenced how these chemical structures are characterised by a multifaceted response to stimuli exerted in the biological milieu. The cationic nature of diruthenium(II,III) tetracarboxylate complexes ensures high solubility in body fluids and, therefore, an efficient perfusion of vascularized tissues, such as neoplastic formations.Santos et al.73 have also shown that diruthenium NSAID complexes can form adducts with human serum proteins, mostly via non-covalent interactions, thus corroborating the good distribution of these complexes via blood circulation.
Therefore, we hypothesize that the organic pendants of the carboxylate ligands may enable good permeation of the biological membranes, which favours these complexes entering the target cells. An evaluation of either membrane permeation or, more generally, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values of diruthenium tetracarboxylate paddlewheel complexes could provide for a preliminary insight of the tissue-targeting features of these species, although, to the best of our knowledge, these data have not been reported yet.
P values of diruthenium tetracarboxylate paddlewheel complexes could provide for a preliminary insight of the tissue-targeting features of these species, although, to the best of our knowledge, these data have not been reported yet.
Different responsiveness to the various biological compartments may also be expected for these diruthenium complexes. Indeed, the composition of the biological medium in a specific compartment may affect the ligand coordination at the axial positions, modulating the overall stability of the paddlewheel complex. For example, chloride ions are characterized by a 15-fold higher concentration in the extracellular compartment than in the intracellular compartment, so that the axial chloro species of diruthenium paddlewheel complexes are expectedly more stable in the cytosol.
The possible articulation of the Ru(II)/Ru(III) states in the structure of bimetallic paddlewheel complexes is based on their redox responsiveness. Highly oxidative biological compartments can be found in peroxisomes or in conditions of upregulated production of radical species, especially in the mitochondrial compartments. In this case, the bimetallic scaffold is oxidized to the less stable Ru(III)Ru(III) species, and the release of carboxylate ligands is expected to be accelerated. Thus, we envision that the targeting of highly oxidative compartments may lead to a biological response that is mainly attributable to the carboxylate ligand activity.
On the other hand, highly reductive environments can be commonly encountered in the cancer tissues overproducing glutathione. For example, cisplatin-resistant cancer cells often develop overexpression of detoxifying mechanisms such as the overproduction of reduced GSH.74 In this context, the diruthenium Ru(II)Ru(III) tetracarboxylate complexes may be reduced to the corresponding Ru(II)Ru(II) forms, characterized by a low rate of decomplexation and increased hydrophobicity, thus prompting their reaction with protein targets.
Several cancerous tissues have shown responsiveness to diruthenium(II,III) tetracarboxylate scaffolds. Both the complexes with either acetate or carboxylate bearing charged groups have been found active on cell lines modelling either liquid or solid tumours (Table 1), probably based on the high water solubility of these complexes. On the other hand, the diruthenium paddlewheel complexes bearing NSAID μ-bridging ligands have been found majorly active against tumour cell lines of the nervous system, in particular, gliomas and glioblastomas. We envision that lipophilicity of the μ-bridged carboxylate ligands may represent the most important structural modulator of the biological activity. Indeed, even within the subclass of NSAID-based diruthenium complexes, the most lipophilic [Ru2(O2Ib)4]+ scaffold has been found to be overall the most active.75
Although the activity of these complexes have been so far studied mostly in vitro, we hypothesize that bearing μ-bridged ligands with an optimal balance between water solubility and lipophilicity may improve the perfusion in the target tissues, and, eventually, enhance the therapeutic response.
5. Conclusions
In this review, we described the potential application of diruthenium(II,III) paddlewheel complexes as valuable dual metallodrugs. The coordination of the dimetallic ruthenium center on protein targets can be exploited to release carboxylate active ligands, thus adding further advantageous pharmacological responses. Experimental and theoretical studies have evidenced that diruthenium(II,III) paddlewheel complexes can be structurally and functionally modulated to enhance the pharmacological profile. The choice of labile axial ligands can influence the target selectivity of these diruthenium complexes, thus favoring the binding of specific protein sites. On the other hand, the steric hindrance exerted by the equatorial carboxylic ligands softens the reactivity of these paddlewheel complexes with biological targets, especially providing for the kinetic stabilization of the bimetallic scaffold. Therefore, computational studies have evidenced that these two structural aspects, i.e. the lability of axial ligands and hindrance of the “paddle” ligands, do cooperate to draw the reactive and target profiles of these complexes in the biological milieu.Hence, we envision that diruthenium paddlewheel complexes will receive increasing interest in the field of bioinorganic chemistry, by representing valuable examples of metallodrugs in which the coordination–activity relationships play a prominent role.
Author contributions
IT and AM: conceptualization, writing – original draft, writing – review and editing and resources. EB, ST, DLM, TM: conceptualization, writing – review and editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work has been developed with the support of the Italian Ministry of Research (MUR).References
- I. Yousuf, M. Bashir, F. Arjmand and S. Tabassum, Advancement of metal compounds as therapeutic and diagnostic metallodrugs: Current frontiers and future perspectives, Coord. Chem. Rev., 2021, 445, 214104, DOI:10.1016/j.ccr.2021.214104.
- G. Ferraro and A. Merlino, Metallodrugs: Mechanisms of action, molecular targets and biological activity, Int. J. Mol. Sci., 2022, 23, 3504, DOI:10.3390/ijms23073504.
- B. Rosenberg, L. Vancamp, J. E. Trosko and V. H. Mansour, Platinum compounds: A new class of potent antitumour agents, Nature, 1969, 222(5191), 385–386, DOI:10.1038/222385a0.
- K. Barabas, R. Milner, D. Lurie and C. Adin, Cisplatin: A review of toxicities and therapeutic applications, Vet. Comp. Oncol., 2008, 6(1), 1–18, DOI:10.1111/j.1476-5829.2007.00142.x.
- D. Cirri, F. Bartoli, A. Pratesi, E. Baglini, E. Barresi and T. Marzo, Strategies for the improvement of metal-based chemotherapeutic treatments, Biomedicines, 2021, 9, 504, DOI:10.3390/biomedicines9050504.
- S. Spreckelmeyer, C. Orvig and A. Casini, Cellular transport mechanisms of cytotoxic metallodrugs: An overview beyond cisplatin, Molecules, 2014, 19, 15584–15610, DOI:10.3390/molecules191015584.
- I. Tolbatov, A. Marrone, C. Coletti and N. Re, Computational studies of Au(I) and Au(III) anticancer metallodrugs: A survey, Molecules, 2021, 26, 7600, DOI:10.3390/molecules26247600.
- M. J. Bennett, K. G. Caulton and F. A. Cotton, Structure of tetra-n-butyratodiruthenium chloride, a compound with a strong metal-metal bond, Inorg. Chem., 1969, 8, 1–6, DOI:10.1021/ic50071a001.
- M. A. Aquino, Recent developments in the synthesis and properties of diruthenium tetracarboxylates, Coord. Chem. Rev., 2004, 248, 1025–1045, DOI:10.1016/j.ccr.2004.06.016.
- F. A. Cotton, L. Labella and M. Shang, Further study of tetracarbonato diruthenium (II, III) compounds, Inorg. Chem., 1992, 31(12), 2385–2389, DOI:10.1021/ic00038a017.
- J. G. Norman Jr., G. E. Renzoni and D. A. Case, Electronic structure of Ru2(O2CR)4+ and Rh2(O2CR)4+ complexes, J. Am. Chem. Soc., 1979, 101(18), 5256–5267, DOI:10.1021/ja00512a025.
- J. G. Norman Jr. and H. J. Kolari, Strength and trans influence of the rhodium-rhodium bond in rhodium(II) carboxylate dimers, J. Am. Chem. Soc., 1978, 100(3), 791–799, DOI:10.1021/ja00471a022.
- D. S. Martin, R. A. Newman and L. M. Vlasnik, Crystal structure and polarized electronic spectra for diruthenium tetraacetate chloride, Inorg. Chem., 1980, 19(11), 3404–3407, DOI:10.1021/ic50213a038.
- R. J. Clark and L. T. Ferris, Resonance Raman, excitation profile and electronic structural studies of diruthenium tetracarboxylate complexes, Inorg. Chem., 1981, 20(9), 2759–2766, DOI:10.1021/ic50223a003.
- M. A. Aquino, Diruthenium and diosmium tetracarboxylates: Synthesis, physical properties and applications, Coord. Chem. Rev., 1998, 170, 141–202, DOI:10.1016/S0010-8545(97)00079-9.
- S. Gangopadhyay and P. K. Gangopadhyay, Adenine and adenosine derivatives of tetraacetatodiruthenium (II, III) cation, J. Inorg. Biochem., 1997, 66, 175–178, DOI:10.1016/S0162-0134(96)00199-7.
- L. Messori, T. Marzo, R. N. Sanches, D. de Oliveira Silva and A. Merlino, Unusual structural features in the lysozyme derivative of the tetrakis (acetato) chloridodiruthenium (II, III) complex, Angew. Chem., Int. Ed., 2014, 53, 6172–6175, DOI:10.1002/anie.201403337.
- R. L. Santos, R. van Eldik and D. de Oliveira Silva, Thermodynamics of axial substitution and kinetics of reactions with amino acids for the paddlewheel complex tetrakis (acetato) chloridodiruthenium (II, III), Inorg. Chem., 2012, 51, 6615–6625, DOI:10.1021/ic300168t.
- I. Tolbatov and A. Marrone, Reaction of dirhodium and diruthenium paddlewheel tetraacetate complexes with nucleophilic protein sites: A computational study, Inorg. Chim. Acta, 2022, 530, 120684, DOI:10.1016/j.ica.2021.120684.
- I. Tolbatov and A. Marrone, Kinetics of reactions of dirhodium and diruthenium paddlewheel tetraacetate complexes with nucleophilic protein sites: Computational Insights, Inorg. Chem., 2022, 61, 16421–16429, DOI:10.1021/acs.inorgchem.2c02516.
- R. L. Santos, R. van Eldik and D. de Oliveira Silva, Kinetic and mechanistic studies on reactions of diruthenium (II, III) with biologically relevant reducing agents, Dalton Trans., 2013, 42, 16796–16805, 10.1039/C3DT51763B.
- C. E. van Rensburg, E. Kreft, J. C. Swarts, S. R. Dalrymple, D. M. MacDonald, M. W. Cooke and M. A. Aquino, Cytotoxicity of a series of water-soluble mixed valent diruthenium tetracarboxylates, Anticancer Res., 2002, 22, 889–892 CAS.
- B. R. Bland, H. J. Gilfoy, G. Vamvounis, K. N. Robertson, T. S. Cameron and M. A. Aquino, Hydrogen bonding in diruthenium (II, III) tetraacetate complexes with biologically relevant axial ligands, Inorg. Chim. Acta, 2005, 358(13), 3927–3936, DOI:10.1016/j.ica.2005.06.023.
- S. R. Alves, R. L. Santos, B. Fornaciari, A. Colquhoun and D. de Oliveira Silva, A novel μ-oxo-diruthenium (III, III)-ibuprofen-(4-aminopyridine) chloride derived from the diruthenium (II, III)-ibuprofen paddlewheel metallodrug shows anticancer properties, J. Inorg. Biochem., 2021, 225, 111596, DOI:10.1016/j.jinorgbio.2021.111596.
- B. K. Keppler, M. Henn, U. M. Juhl, M. R. Berger, R. Niebl and F. E. Wagner, New ruthenium complexes for the treatment of cancer, in: Ruthenium and other non-platinum metal complexes in cancer chemotherapy, Springer, Berlin, Heidelberg, 1989, pp. 41–69. DOI:10.1007/978-3-642-74760-1_3.
- A. Terán, M. Cortijo, Á. Gutiérrez, A. E. Sánchez-Peláez, S. Herrero and R. Jiménez-Aparicio, Ultrasound-assisted synthesis of water-soluble monosubstituted diruthenium compounds, Ultrason. Sonochem., 2021, 80, 105828, DOI:10.1016/j.ultsonch.2021.105828.
- W. R. Osterloh, G. Galindo, M. J. Yates, E. Van Caemelbecke and K. M. Kadish, Synthesis, structural and physicochemical properties of water-soluble mixed-ligand diruthenium complexes containing anilinopyridinate bridging ligands, Inorg. Chem., 2019, 59(1), 584–594, DOI:10.1021/acs.inorgchem.9b02838.
- A. Terán, G. Ferraro, A. E. Sánchez-Peláez, S. Herrero and A. Merlino, Effect of equatorial ligand substitution on the reactivity with proteins of paddlewheel diruthenium complexes: Structural studies, Inorg. Chem., 2023, 62(2), 670–674, DOI:10.1021/acs.inorgchem.2c04103.
- G. Lozano, R. Jimenez-Aparicio, S. Herrero and E. Martinez-Salas, Fingerprinting the junctions of RNA structure by an open-paddlewheel diruthenium compound, RNA, 2016, 22(3), 330–338, DOI:10.1261/rna.054353.115.
- F. Lupi, T. Marzo, G. D'Adamio, S. Cretella, F. Cardona, L. Messori and A. Goti, Diruthenium diacetate catalysed aerobic oxidation of hydroxylamines and improved chemoselectivity by immobilisation to lysozyme, ChemCatChem, 2017, 9, 4225–4230, DOI:10.1002/cctc.201701083.
- H. T. Chifotides and K. R. Dunbar, Interactions of metal – metal-bonded antitumor active complexes with DNA fragments and DNA, Acc. Chem. Res., 2005, 38(2), 146–156, DOI:10.1021/ar0302078.
- F. Díaz-González and F. Sánchez-Madrid, NSAIDs: Learning new tricks from old drugs, Eur. J. Immunol., 2015, 45, 679–686, DOI:10.1002/eji.201445222.
- K. Brune and P. Patrignani, New insights into the use of currently available non-steroidal anti-inflammatory drugs, J. Pain Res., 2015, 8, 105, DOI:10.2147/JPR.S75160.
- S. M. Crusz and F. R. Balkwill, Inflammation and cancer: Advances and new agents, Nat. Rev. Clin. Oncol., 2015, 12, 584–596, DOI:10.1038/nrclinonc.2015.105.
- Z. Zhang, F. Chen and L. Shang, Advances in antitumor effects of NSAIDs, Cancer Manage. Res., 2018, 10, 4631, DOI:10.2147/CMAR.S175212.
- P. Rao and E. E. Knaus, Evolution of nonsteroidal anti-inflammatory drugs (NSAIDs): Cyclooxygenase (COX) inhibition and beyond, J. Pharm. Pharm. Sci., 2008, 11, 81s–110s, DOI:10.18433/J3T886.
- G. Golbaghi and A. Castonguay, Rationally designed ruthenium complexes for breast cancer therapy, Molecules, 2020, 25, 265, DOI:10.3390/molecules25020265.
- D. de Oliveira Silva, Perspectives for novel mixed diruthenium-organic drugs as metallopharmaceuticals in cancer therapy, Anti-Cancer Agents Med. Chem., 2010, 10, 312–323, DOI:10.2174/187152010791162333.
- C. N. Banti and S. K. Hadjikakou, Non–steroidal anti–inflammatory drugs (NSAIDs) in metal complexes and their effect at the cellular level, Eur. J. Inorg. Chem., 2016, 19, 3048–3071, DOI:10.1002/ejic.201501480.
- G. Ribeiro, M. Benadiba, A. Colquhoun and D. de Oliveira Silva, Diruthenium (II, III) complexes of ibuprofen, aspirin, naproxen and indomethacin non-steroidal anti-inflammatory drugs: Synthesis, characterization and their effects on tumor-cell proliferation, Polyhedron, 2008, 27, 1131–1137, DOI:10.1016/j.poly.2007.12.011.
- R. L. Santos, A. Bergamo, G. Sava and D. de Oliveira Silva, Synthesis and characterization of a diruthenium (II, III)–ketoprofen compound and study of the in vitro effects on CRC cells in comparison to the naproxen and ibuprofen derivatives, Polyhedron, 2012, 42, 175–181, DOI:10.1016/j.poly.2012.05.012.
- T. E. Freitas, R. N. Gomes, A. Colquhoun and D. de Oliveira Silva, Axially-modified paddlewheel diruthenium (II, III)-ibuprofenato metallodrugs and the influence of the structural modification on U87MG and A172 human glioma cell proliferation, apoptosis, mitosis and migration, J. Inorg. Biochem., 2016, 165, 181–191, DOI:10.1016/j.jinorgbio.2016.10.003.
- M. Benadiba, R. R. Dos Santos, D. de Oliveira Silva and A. Colquhoun, Inhibition of C6 rat glioma proliferation by [Ru2Cl(Ibp)4] depends on changes in p21, p27, Bax/Bcl2 ratio and mitochondrial membrane potential, J. Inorg. Biochem., 2010, 104, 928–935, DOI:10.1016/j.jinorgbio.2010.04.011.
- K. Engeland, Cell cycle regulation: p53-p21-RB signaling, Cell Death Differ., 2022, 29, 946–960, DOI:10.1038/s41418-022-00988-z.
- S. Chen and L. Li, Degradation strategy of cyclin D1 in cancer cells and the potential clinical application, Front. Oncol., 2022, 12, 949688, DOI:10.3389/fonc.2022.949688.
- S. K. Madden, A. D. de Araujo and M. Gerhardt, et al., Taking the Myc out of cancer: toward therapeutic strategies to directly inhibit c-Myc, Mol. Cancer, 2021, 20, 3, DOI:10.1186/s12943-020-01291-6.
- H. Li, H. Vogel, V. B. Holcomb, Y. Gu and P. Hasty, Deletion of Ku70, Ku80, or both causes early aging without substantially increased cancer, Mol. Cell. Biol., 2007, 27, 8205–8214, DOI:10.1128/MCB.00785-07.
- Z. N. Oltvai, C. L. Milliman and S. J. Korsmeyer, Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death, Cell, 1993, 74, 609–619, DOI:10.1016/0092-8674(93)90509-o.
- A. Pannunzio and M. Coluccia, Cyclooxygenase-1 (COX-1) and COX-1 Inhibitors in Cancer: A Review of Oncology and Medicinal Chemistry Literature, Pharmaceuticals, 2018, 11, 101, DOI:10.3390/ph11040101.
- M. Benadiba, I. de M. Costa, R. L. Santos, F. O. Serachi, D. de Oliveira Silva and A. Colquhoun, Growth inhibitory effects of the Diruthenium-Ibuprofen compound, [Ru2Cl(Ibp)4], in human glioma cells in vitro and in the rat C6 orthotopic glioma in vivo, J. Biol. Inorg. Chem., 2014, 19, 1025–1035, DOI:10.1007/s00775-014-1143-4.
- A. Andrade, S. F. Namora, R. G. Woisky, G. Wiezel, R. Najjar, J. A. Sertié and D. de Oliveira Silva, Synthesis and characterization of a diruthenium–ibuprofenato complex: Comparing its anti-inflammatory activity with that of a copper(II)–ibuprofenato complex, J. Inorg. Biochem., 2000, 81, 23–27, DOI:10.1016/S0162-0134(00)00106-9.
- S. R. Alves, A. Colquhoun, X. Y. Wu and D. de Oliveira Silva, Synthesis of terpolymer-lipid encapsulated diruthenium (II, III)-anti-inflammatory metallodrug nanoparticles to enhance activity against glioblastoma cancer cells, J. Inorg. Biochem., 2020, 205, 110984, DOI:10.1016/j.jinorgbio.2019.110984.
- S. R. Rico, A. Z. Abbasi, G. Ribeiro, T. Ahmed, X. Y. Wu and D. de Oliveira Silva, Diruthenium (II, III) metallodrugs of ibuprofen and naproxen encapsulated in intravenously injectable polymer–lipid nanoparticles exhibit enhanced activity against breast and prostate cancer cells, Nanoscale, 2017, 9, 10701–10714, 10.1039/C7NR01582H.
- E. Barresi, I. Tolbatov, A. Pratesi, V. Notarstefano, E. Baglini, S. Daniele, S. Taliani, N. Re, E. Giorgini, C. Martini, F. Da Settimo, T. Marzo and D. La Mendola, A mixed-valence diruthenium (II, III) complex endowed with high stability: From experimental evidence to theoretical interpretation, Dalton Trans., 2020, 49, 14520–14527, 10.1039/D0DT02527E.
- E. Barresi, I. Tolbatov, T. Marzo, E. Zappelli, A. Marrone, N. Re, A. Pratesi, C. Martini, S. Taliani, F. Da Settimo and D. La Mendola, Two mixed valence diruthenium (II, III) isomeric complexes show different anticancer properties, Dalton Trans., 2021, 50, 9643–9647, 10.1039/D1DT01492G.
- J. A. Miyake, M. Benadiba, G. Ribeiro, D. D. Silva and A. Colquhoun, Novel ruthenium–gamma-linolenic acid complex inhibits C6 rat glioma cell proliferation in vitro and in the orthotopic C6 model in vivo after osmotic pump infusion, Anticancer Res., 2014, 34, 1901–1911 CAS.
- G. Ribeiro, M. Benadiba, D. de Oliveira Silva and A. Colquhoun, The novel ruthenium—γ–linolenic complex [Ru2(aGLA)4Cl] inhibits C6 rat glioma cell proliferation and induces changes in mitochondrial membrane potential, increased reactive oxygen species generation and apoptosis in vitro, Cell. Biochem. Funct., 2010, 28, 15–23, DOI:10.1002/cbf.1626.
- J. Rautio, H. Kumpulainen, T. Heimbach, R. Oliyai, D. Oh, T. Järvinen and J. Savolainen, Prodrugs: Design and clinical applications, Nat. Rev. Drug Discovery, 2008, 7, 255–270, DOI:10.1038/nrd2468.
- A. Ibrahim-Hashim and V. Estrella, Acidosis and cancer: From mechanism to neutralization, Cancer Metastasis Rev., 2019, 38(1), 149–155, DOI:10.1007/s10555-019-09787-4.
- I. Böhme and A. K. Bosserhoff, Acidic tumor microenvironment in human melanoma, Pigm. Cell Melanoma Res., 2016, 29, 508–523, DOI:10.1111/pcmr.12495.
- B. A. Webb, M. Chimenti, M. P. Jacobson and D. L. Barber, Dysregulated pH: A perfect storm for cancer progression, Nat. Rev. Cancer, 2011, 11, 671–677, DOI:10.1038/nrc3110.
- X. Zhang, Y. Lin and R. J. Gillies, Tumor pH and its measurement, J. Nucl. Med., 2010, 51, 1167–1170, DOI:10.2967/jnumed.109.068981.
- I. Coloma, M. Cortijo, I. Fernández-Sánchez, J. Perles, J. L. Priego, C. Gutiérrez, R. Jiménez-Aparicio, B. Desvoyes and S. Herrero, pH-and time-dependent release of phytohormones from diruthenium complexes, Inorg. Chem., 2020, 59, 7779–7788, DOI:10.1021/acs.inorgchem.0c00844.
- I. Tolbatov, N. Re, C. Coletti and A. Marrone, An insight on the gold(I) affinity of golB protein via multilevel computational approaches, Inorg. Chem., 2019, 58, 11091–11099, DOI:10.1021/acs.inorgchem.9b01604.
- I. Tolbatov, N. Re, C. Coletti and A. Marrone, Determinants of the lead(II) affinity in pbrR protein: a computational study, Inorg. Chem., 2019, 59, 790–800, DOI:10.1021/acs.inorgchem.9b03059.
- S. Todisco, M. Latronico, V. Gallo, N. Re, A. Marrone, I. Tolbatov and P. Mastrorilli, Double addition of phenylacetylene onto the mixed bridge phosphinito–phosphanido Pt (i) complex [(PHCy2)Pt(μ-PCy2){κ2P, O-μ-P(O)Cy2}Pt(PHCy2)](Pt–Pt), Dalton Trans., 2020, 49, 6776–6789, 10.1039/D0DT00923.
- I. Tolbatov, L. Storchi and A. Marrone, Structural reshaping of the zinc-finger domain of the SARS-CoV-2 nsp13 protein using bismuth(III) ions: A multilevel computational study, Inorg. Chem., 2022, 61(39), 15664–15677, DOI:10.1021/acs.inorgchem.2c02685.
- I. Tolbatov and A. Marrone, Computational strategies to model the interaction and the reactivity of biologically-relevant transition metal complexes, Inorg. Chim. Acta, 2022, 530, 120686, DOI:10.1016/j.ica.2021.120686.
- R. Paciotti, I. Tolbatov, A. Marrone, L. Storchi, N. Re and C. Coletti, Computational investigations of bioinorganic complexes: the case of calcium, gold and platinum ions, in AIP Conference Proceedings, AIP Publishing LLC, 2019, vol. 2186, no. 1, p. 030011, DOI:10.1063/1.5137922.
- I. Tolbatov, A. Marrone, R. Paciotti, N. Re and C. Coletti, Multilayered modelling of the metallation of biological targets, in International conference on computational science and its applications, Springer, Cham, 2021, pp. 398–412. DOI:10.1007/978-3-030-87016-4_30.
- I. Tolbatov and A. Marrone, Reactivity of N-heterocyclic carbene half-sandwich Ru-, Os-, Rh-, and Ir-based complexes with cysteine and selenocysteine: a Computational Study, Inorg. Chem., 2021, 61, 746–754, DOI:10.1021/acs.inorgchem.1c03608.
- D. Cirri, T. Marzo, I. Tolbatov, A. Marrone, F. Saladini, I. Vicenti, F. Dragoni, A. Boccuto and L. Messori, In vitro anti-SARS-CoV-2 activity of selected metal compounds and potential molecular basis for their actions based on computational study, Biomolecules, 2021, 11, 1858, DOI:10.3390/biom11121858.
- R. L. S. R. Santos, R. N. F. Sanches and D. de Oliveira Silva, Spectroscopic studies on interactions of the tetrakis(acetato)chloridodiruthenium(II,III) complex and the Ru2(II,III)-NSAID-derived metallodrugs of ibuprofen and ketoprofen with human serum albumin, J. Coord. Chem., 2015, 68, 3209–3228, DOI:10.1080/00958972.2015.1074684.
- B. Köberle, M. T. Tomicic, S. Usanova and B. Kaina, Cisplatin resistance: Preclinical findings and clinical implications, Biochim. Biophys. Acta, 2010, 1806, 172–182, DOI:10.1016/j.bbcan.2010.07.004.
- D. de Oliveira Silva, Ruthenium compounds targeting cancer therapy, Front. Anti-Cancer Drug Discovery, 2014, 4, 88–156 Search PubMed.
| This journal is © the Partner Organisations 2023 |