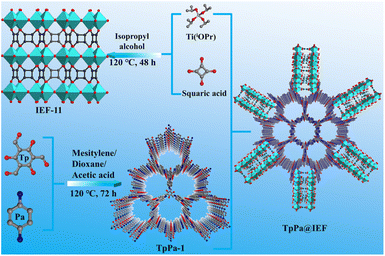
Novel COF@Ti–MOF hybrid photocatalysts enabling enhanced photocatalytic CO2 reduction in a gas–solid system without additives†
Rui-Gang
Yang
a,
Yao-Mei
Fu
b,
Xing
Meng
 a,
Li
Xue
a,
Zhen
Zhou
a,
Li
Xue
a,
Zhen
Zhou
 a,
Yu-Ou
He
a,
Jian-Xin
Qu
a,
Hai-Ning
Wang
a,
Yu-Ou
He
a,
Jian-Xin
Qu
a,
Hai-Ning
Wang
 *a and
Zhong-Min
Su
*a and
Zhong-Min
Su
 bc
bc
aSchool of Chemistry and Chemical Engineering, Shandong University of Technology, Zibo 255049, People's Republic of China. E-mail: wanghn913@foxmail.com
bShandong Engineering Research Center of Green and High-value Marine Fine Chemical, Weifang University of Science and Technology, Shouguang, 262700, China
cSchool of Chemistry and Environmental Engineering, Changchun University of Science and Technology, Changchun, 130022, China
First published on 18th May 2023
Abstract
With the increasing global energy demand and environmental problems, the photoreduction of CO2 into valuable chemical materials is considered to be an ideal way to recycle carbon resources. Naturally, the design and synthesis of efficient photocatalysts used for the photocatalytic reduction of CO2 still face great challenges. Herein, a series of stable hybrid photocatalysts TpPa@IEF has been produced by integrating the two-dimensional covalent organic framework TpPa-1 with the Ti-based metal–organic framework IEF-11. The photocatalytic performance of TpPa@IEF has been evaluated via gas–solid reaction mode under visible light as well as no sacrificial agents and photosensitizers. The experimental results show that TpPa@IEF exhibits excellent photocatalytic activity in pure and dilute CO2 (10%) environments. The introduction of IEF-11 has resulted in a significant improvement in photocatalytic performance; TpPa@IEF achieved a great CO generation rate of 78.87 μmol g−1 h−1 in pure CO2, notably, and obtained another excellent CO generation rate of 116.47 μmol g−1 h−1 in dilute CO2 (10%), which are 3.63 and 4.93 times higher than that of TpPa-1, respectively. The excellent photocatalytic performance of TpPa@IEF hybrids stems from the high charge separation efficiency and CO2 capture ability, as well as the abundant catalytic active sites. The preparation of TpPa@IEF hybrid materials presents a novel perspective for the design and synthesis of efficient photocatalysts.
Introduction
The excessive consumption of fossil fuels leads to the increasing concentration of carbon dioxide (CO2) in the atmosphere, which leads to a series of environmental problems, for example, the greenhouse effect. Therefore, developing new technologies for the enrichment and conversion of CO2 and realizing closed-loop utilization of CO2 can alleviate the energy and environmental crisis. The strategies for the conversion of CO2 include photochemical conversion,1,2 biological conversion,3,4 CO2 hydroconversion5 and electrochemical conversion.6,7 Compared with other methods, the reduction of CO2 into renewable fuels using photocatalysts has many advantages and is considered to be an environmentally friendly and efficient method to realize the carbon cycle. Therefore, there is an urgent need to develop an efficient photocatalyst that can capture visible light and effectively suppress the recombination of photogenerated carriers, converting CO2 into chemical materials effectively.As a booming family of porous materials, covalent organic frameworks (COFs) composed of organic molecules assembled through covalent bonds have been the subject of wide-ranging research in various areas such as catalysis, gas storage, sensing, etc.8,9 With long-range ordered structures, designable band gaps, and large surface areas, COFs have appeared as a new family of photoactive materials for photocatalytic reduction of CO2.10 In addition, most COFs have extensive light trapping in the visible region due to the absorption properties of functional groups and large conjugation systems. Based on these advantages, Lostch et al.11 employed TFPT–COF as a photocatalytic platform to achieve an efficient catalytic activity for the first time. Although COFs show many advantages in the photocatalysis field,12 severe charge carrier recombination remains the main obstacle to further optimizing their photocatalytic performance. Therefore, various approaches, including ligand functionalization, metal doping, and heterostructure construction, have been developed to address the above problem.13–15 Until now, there have been several examples of COF-based photocatalysts, such as Au/TAPB–DMTP–COF, α-Fe2O3/TpPa–2-COF and MoS2/TpPa–1-COF, which could effectively solve the problem of low carrier utilization efficiency, resulting in enhanced photocatalytic activity.16–18 The valuable achievements provide an important idea for the design of new composite catalysts.
Metal–organic frameworks (MOFs), which are another class of crystalline porous materials, exhibit the advantages of large surfaces, bountiful active catalytic sites, and tunable and designed structures.19 Up to now, some famous frameworks such as the UIO, PCN, ZIFs and MIL series have been widely studied and are extensively applied in the fields of gas adsorption and separation,20,21 energy storage,22,23 drug transport24,25 and catalysis,26 especially in the field of photocatalysis.27–30 Among the reported MOFs, the inherent advantages of Ti-based MOFs, such as high chemical stability, good photocatalytic activity, and being clean and pollution-free, are widely studied and applied.31,32 However, there are a few reports on Ti-based MOFs explored in the area of photocatalytic reduction of CO2.33
The reported works on COF-based photocatalysts inspire us to develop new hybrid photocatalysts. MOFs have been demonstrated to be able to form heterojunctions with COFs well,34,35 and the photocatalytic performances of the generated hybrid materials suppress those of each monomer. Due to their advantages of large surface areas, available porosity and sufficient active sites, as well as their good visible light trapping ability, MOFs and COFs can be used as excellent substitutes for building hybrid photocatalysts. Therefore, IEF-11 as a Ti-based MOF with abundant catalytic active sites and good electron transport ability and TpPa-1 as a Schiff base COF with good visible light capture ability are selected to construct hybrid photocatalysts to achieve great photocatalytic performances. Moreover, IEF-11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 36 and TpPa-1
36 and TpPa-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 37 have good thermal and chemical stability, which is very beneficial for building a stable photocatalyst.
37 have good thermal and chemical stability, which is very beneficial for building a stable photocatalyst.
Based on this background, a class of heterostructured hybrid photocatalysts TpPa@IEF based on 3D Ti-based MOF IEF-11 and 2D COF TpPa-1 have been successfully proposed in this work, which have a core–shell structure with TpPa-1 as the core and IEF-11 as the shell and exhibit great chemical stability. Their photocatalytic performances towards CO2 have been evaluated driven by visible light under both pure CO2 and low CO2 concentration (10%). These heterogeneous photocatalysts, TpPa@IEF, are able to achieve CO generation rates of 78.87 μmol g−1 h−1 and 116.47 μmol g−1 h−1 in pure CO2 and 10% CO2 environments, respectively. Furthermore, a series of characterization studies demonstrates that excellent photocatalytic performance is ascribed to the high charge separation and transfer efficiency and CO2 capture ability, as well as the abundant catalytic active sites.
Experimental section
The chemical reagents and solvents used in this work are commercially available and were used directly. They are listed in the ESI.† The detailed synthesis process, photochemical test methods and photocatalytic performance tests of all materials are given in the ESI.†Results and discussion
Herein, a series of hybrid photocatalytic materials based on TpPa-1 and IEF-11 were designed and synthesized with the help of the solvothermal method (Scheme 1). 2D COF TpPa-1 was pre-synthesized through the classical Schiff base reaction. Then, the as-synthesized TpPa-1 was placed in the mixed system containing titanium isopropoxide and 3,4-dihydroxycyclobut-3-ene-1,2-dione, which were heated in an oven, in order to obtain the target hybrid photocatalysts TpPa@IEF. The obtained hybrid photocatalytic materials were named TpPa@IEF-X (X is 50, 100 and 150) according to the mass of TpPa-1 used.The chemical structure and the purity of the as-synthesized samples were evaluated via powder X-ray diffraction (PXRD) analysis. The PXRD pattern of the IEF-11 displays real evidence, consistent with the corresponding simulation (Fig. 1a), demonstrating the successful construction of IEF-11. All the diffraction peaks of TpPa@IEF-X are similar to those of IEF-11 and TpPa-1 in the PXRD patterns, indicating that the successful integration of IEF-11 and TpPa-1 does not affect their structures. Meanwhile, the as-synthesized TpPa@IEF-150 was selected to demonstrate the chemical stability of the prepared photocatalysts. The samples were immersed in different pH solutions for 24 h, and then, the obtained samples were subjected to PXRD and FT-IR measurements. The test results certify the great chemical stability of the obtained composite photocatalysts (Fig. S1 and S2†). It should be mentioned that the XPS measurements of TpPa@IEF-150 after treatment with different pH solutions have demonstrated that the elemental valence states and binding energy magnitudes remain unchanged (Fig. S3–S7†), and the SEM images of TpPa@IEF-150 after treatment in different pH solutions further demonstrate the structural stability of the material (Fig. S8–S10†). Moreover, the thermogravimetric analysis shows that the samples are capable of maintaining good thermal stability until 300 °C (Fig. S11†).
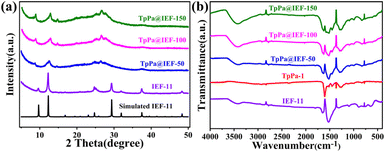 | ||
| Fig. 1 (a) The PXRD patterns of IEF-11 and TpPa@IEF-X, and (b) the FT-IR spectra of TpPa-1, IEF-11 and TpPa@IEF-X. | ||
Fourier transform infrared (FT-IR) spectroscopic analysis was employed to reveal the characteristic chemical bonds of the prepared samples, which was used to confirm the structure of the composite photocatalysts. Fig. 1b shows that the characteristic absorption peak at 640 cm−1 belongs to the stretching convulsion of the Ti–O bonds in IEF-11.36 The strong characteristic peaks at 1521 cm−1 and 1251 cm−1 are attributed to the stretching vibrations of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond and the β-ketamine bond C–N, respectively.38 The appearance of the characteristic peak at 1632 cm−1 is due to the vibration of the C
C bond and the β-ketamine bond C–N, respectively.38 The appearance of the characteristic peak at 1632 cm−1 is due to the vibration of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds of the 3,4-dihydroxycyclobut-3-ene-1,2-dione and Tp. The FT-IR spectra demonstrate that TpPa-1 and IEF-11 have been successfully synthesized and the vibrations of the characteristic peaks of TpPa-1 and IEF-11 can be shown in the hybrid material.
O bonds of the 3,4-dihydroxycyclobut-3-ene-1,2-dione and Tp. The FT-IR spectra demonstrate that TpPa-1 and IEF-11 have been successfully synthesized and the vibrations of the characteristic peaks of TpPa-1 and IEF-11 can be shown in the hybrid material.
The apparent morphology of the as-synthesized samples is observed by the scanning electron microscopy (SEM) characterization (Fig. 2a–d). The SEM image shows that the morphology of IEF-11 exhibits a regular structure of hexagonal prisms, whose sizes are less than 100 nm. In the hybrid photocatalysts TpPa@IEF-X, IEF-11 grows on the surface of TpPa-1, where IEF-11 particles become smaller and more irregular as the mass of TpPa-1 increases. In TpPa@IEF-50 and TpPa@IEF-100, the particles of IEF-11 grow on the surface of TpPa-1 thickly, while IEF-11 particles are thinly distributed on the surface of TpPa-1 in TpPa@IEF-150. The difference in the surface morphology of TpPa@IEF-X may have an explicit effect on the photocatalytic CO2 reduction. The TEM images show that TpPa-1 has a petal-like structure with a smooth surface. Therefore, the images of TpPa@IEF-X reveal smaller IEF-11 particles covering the surface of the rod-like structure from TpPa-1, resulting in a rough and irregular surface, compared to that of TpPa-1, demonstrating that IEF-11 is successfully distributed at the surface of TpPa-1. In a word, TpPa@IEF-X exhibit a core–shell structure, where TpPa-1 serves as the core and IEF-11 as the shell.
 | ||
| Fig. 2 SEM images of (a) IEF-11, (b) TpPa@IEF-50, (c) TpPa@IEF-100, and (d) TpPa@IEF-150, and TEM images of (e) TpPa-1 and (f) TpPa@IEF-100. | ||
The chemical state of each element in TpPa@IEF-150 has been further investigated by X-ray photoelectron spectroscopy (XPS) measurements. Through full spectrum scanning (Fig. S12†), the binding energies of the C, N, Ti and O elements were determined to be 283.0 eV, 398.0 eV, 457.0 eV and 530 eV, respectively. The high-resolution XPS spectrum of C 1s is shown in Fig. 3a, where the binding energies of 283.14 eV, 287.15 eV and 284.8 eV correspond to the characteristic peaks of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, C–N and C
N, C–N and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds, respectively. As shown in Fig. 3b, the high-resolution spectrum of N 1s indicates that the characteristic peak (398.23 eV) is due to the C
C bonds, respectively. As shown in Fig. 3b, the high-resolution spectrum of N 1s indicates that the characteristic peak (398.23 eV) is due to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond and the peak with a binding energy of 399.86 eV is derived from the C–N bond (Fig. 3b). The binding energy of 530.25 eV in the high-resolution spectrum of O 1s corresponds to the characteristic peak of the Ti–O bond, while the characteristic peak at the binding energy of 531.53 eV is attributed to the C
N bond and the peak with a binding energy of 399.86 eV is derived from the C–N bond (Fig. 3b). The binding energy of 530.25 eV in the high-resolution spectrum of O 1s corresponds to the characteristic peak of the Ti–O bond, while the characteristic peak at the binding energy of 531.53 eV is attributed to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds of IEF-11 (Fig. 3c). The high-resolution spectrum of Ti 2p shows the existence of binding energies of 457.20 eV and 462.94 eV, corresponding to the two characteristic peaks of Ti 2p3/2 and Ti 2p1/2 (Fig. 3d), respectively.
O bonds of IEF-11 (Fig. 3c). The high-resolution spectrum of Ti 2p shows the existence of binding energies of 457.20 eV and 462.94 eV, corresponding to the two characteristic peaks of Ti 2p3/2 and Ti 2p1/2 (Fig. 3d), respectively.
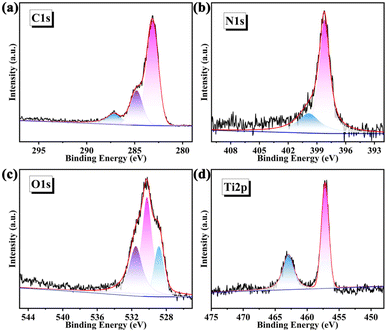 | ||
| Fig. 3 High-resolution XPS energy spectra of TpPa@IEF-150: (a) C 1s, (b) N 1s, (c) O 1s, and (d) Ti 2p. | ||
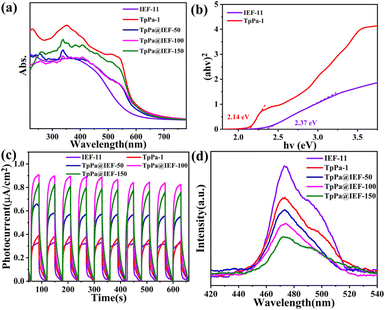
The CO2 adsorption curves of TpPa-1, IEF-11 and TpPa@IEF-X samples are recorded at room temperature. The calculated results show that the CO2 adsorption capacities of IEF-11, TpPa@IEF-50, TpPa@IEF-100, TpPa@IEF-150 and TpPa-1 increased sequentially, corresponding to 9.16 cm3 g−1, 24.32 cm3 g−1, 26.84 cm3 g−1, 29.06 cm3 g−1 and 42.44 cm3 g−1, respectively (Fig. S13†). Therefore, the composite photocatalysts TpPa@IEF-X inherit the adsorption ability of TpPa-1 toward the substrate CO2, and the introduction of TpPa-1 could be beneficial for the CO2 photocatalytic reduction reaction. To evaluate the light absorption capacity of the catalyst, their optical absorption ability was investigated by UV-vis diffuse reflectance spectroscopy (DRS). The absorption edge of IEF-11 locates at about 570 nm, which is narrower than that of TpPa-1. The optical absorption edges of the hybrid materials TpPa@IEF-X are similar to that of TpPa-1 and red-shifted (approximately 630 nm) compared to that of IEF-11 (Fig. 4a). The composite photocatalysts TpPa@IEF-X inherit the excellent light capture ability of TpPa-1 and can achieve a light absorption range similar to that of TpPa-1. Through analyzing the DRS spectra, the correlation band gaps with the values of 2.37 eV and 2.14 eV have been calculated for IEF-11 and TpPa-1 with the help of the K–M formula, respectively (Fig. 4b). Furthermore, the band structures of IEF-11 and TpPa-1 could be established with assistance from the Mott–Schottky (M–S) experiment. The flat band potentials of IEF-11 (Fig. S14†) and TpPa-1 (Fig. S15†) are −1.08 eV and −0.81 eV vs. the Ag/AgCl electrode, corresponding to −0.88 eV and −0.61 eV (vs. NHE), respectively, according to the M–S plots. Based on the positive slope in the M–S plots of IEF-11 and TpPa-1, it is concluded that both of them are n-type semiconductors. Naturally, the conduction bands of IEF-11 and TpPa-1 are determined to be −1.08 eV and −0.81 eV, respectively. Naturally, their valence bands are further calculated to be 1.29 eV (IEF-11) and 1.33 eV (TpPa-1), respectively, based on the energy band relationship.
With the help of photoelectrochemical tests, the synthesized photocatalytic materials TpPa@IEF-X are characterized for their photoelectrochemical properties. The photoluminescence (PL) spectrum is employed to evaluate the recombination rates of photo-generated electron and hole pairs.
The PL intensities of TpPa-1 and IEF-11 are significantly higher than those of the hybrid material TpPa@IEF-X, and the PL intensities of TpPa@IEF-150, TpPa@IEF-100 and TpPa@IEF-50 decrease sequentially (Fig. 4d), which proves that the introduction of IEF-11 effectively reduces the recombination of photogenerated carriers in the hybrid materials TpPa@IEF-X and the photoluminescence process is suppressed, providing more photogenerated electrons for the photocatalytic reduction of CO2. In addition, transient photocurrent response tests further reveal the ability of the sample to separate photogenerated electrons and holes. The transient photocurrent response shows that the photocurrent density of the hybrid materials TpPa@IEF-X is much higher than those of the original TpPa-1 and IEF-11, demonstrating that the introduction of IEF-11 indeed improves the separation efficiency of photogenerated electrons and holes. In the electrochemical impedance spectrum, the composite photocatalysts TpPa@IEF-X exhibit a smaller Nyquist semicircle radius compared to TpPa-1 and IEF-11, which indicates that TpPa@IEF-X have faster interfacial charge transfer rates (Fig. S16†).
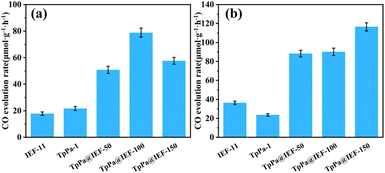
Under the illumination of an LED light source, the homemade photocatalytic reactor was filled with different concentrations of CO2 gas and a few drops of water to conduct the photocatalytic CO2 reduction reaction experiment. The photocatalytic reduction procedure is a more advanced gas–solid reaction with water as the reducing agent under the condition of no photosensitizer and sacrificial agent, where the main product is CO as well as a little H2. The results of the photocatalytic reduction of CO2 show that the prepared photocatalysts display high selectivity, in which TpPa@IEF-X possess >90% selectivity (Table S1†). The CO generation rates of these composites TpPa@IEF-50, TpPa@IEF-100 and TpPa@IEF-150 are 46.48 μmol g−1 h−1, 78.87 μmol g−1 h−1 and 57.61 μmol g−1 h−1, respectively, and their CO generation rates increase by 2.14, 3.63, and 2.65 times, compared to that of TpPa-1. It can be seen that the photocatalytic activity of TpPa@IEF-X in a pure CO2 environment is higher than those of TpPa-1 (21.72 μmol g−1 h−1) and IEF-11 (17.84 μmol g−1 h−1) (Fig. 5a). Moreover, a controlled experiment was also carried out. The photocatalytic capability of the physical mixture based on TpPa-1 and IEF-11 was evaluated, and it should be mentioned that its photocatalytic performance is lower than that of the hybrid materials TpPa@IEF-X. Additionally, the formation rates of CO by TpPa@IEF-100 at certain times are shown in Fig. S17.† Notably, the same photocatalytic CO2 reduction experiment was also performed under solar illumination, and the formation rate of CO by TpPa@IEF-100 was only 1.57 μmol g−1 h−1. Therefore, to determine the key factors in the photocatalytic CO2 conversion process, a series of controlled experiments was conducted. When the steps of the CO2 reduction reaction are taken in the dark, the reaction system is completely inactive and no products are produced. If the photocatalyst is removed, only water and CO2 are present in the reaction system, and no products appear. When replacing CO2 in the reaction system with N2, the photocatalytic reaction is performed under the same test conditions, and no reaction products are detected, indicating that the generated CO indeed results from the introduced CO2.
Nowadays, the concentration of CO2 in anthropogenic emissions from thermal power plants and flue gases is relatively low.39 Therefore, there is an urgent need to convert CO2 into useful chemicals at low concentrations because the uptake and activation of CO2 at low concentrations are very difficult.40 Although photocatalysts exhibit superior activity in pure CO2, it is a huge challenge to the continue good catalytic performance at low CO2 concentrations. Based on this background, the photocatalytic performances of samples are evaluated in a dilute CO2 gas (10% CO2 and 90% N2) environment. It is surprising that TpPa@IEF-X hybrid materials show excellent photocatalytic performances with high product selectivity (>90% Table S1†). The CO generation rates of TpPa@IEF-50, TpPa@IEF-100 and TpPa@IEF-150 are 88.32 μmol g−1 h−1, 90.21 μmol g−1 h−1 and 116.47 μmol g−1 h−1, respectively (Fig. 5b). TpPa@IEF exhibit excellent photocatalytic performances in both pure CO2 and dilute CO2 (10%) environments. It should be mentioned that the O2 generation rates of the composite samples were also calculated, as given in Fig. S18 and S19.† Subsequently, two samples, TpPa@IEF-100 and TpPa@IEF-150, were selected to test the durability of photocatalytic CO2 reduction, and the samples were still able to maintain high catalytic activity after four cycles of testing (Fig. S20 and S21†). Notably, the photocatalytic effect in the dilute CO2 (10%) environment is superior compared to that in the pure CO2 system due to the advantage of CO2 enrichment resulting from –OH in TpPa-1,41 which allows the photocatalytic reduction of the CO2 reaction to proceed fully, resulting in an excellent photocatalytic effect. In summary, the prepared photocatalysts surpass the catalytic activity of the parent TpPa-1 and IEF-11 in pure CO2 and dilute CO2 (10%) environments. Driven by visible light, TpPa@IEF provide a constant source of energy for the occurrence of photocatalytic reduction reaction with their excellent light absorption ability, and the reasonable combination can effectively separate and transfer photogenerated charges, thus improving the conversion efficiency of CO2 into CO. Up to now, TpPa@IEF has outperformed some other heterogeneous photocatalysts. Table S2† summarizes and shows the results of the studies on the reduction of CO2 to CH4 or CO using COF and its composites as photocatalysts. Compared with the published research results, the photocatalysts TpPa@IEF obtain good CO yields using water as the reducing agent in the CO2 photoreduction process without adding any additives.
The photocatalytic reaction mechanism
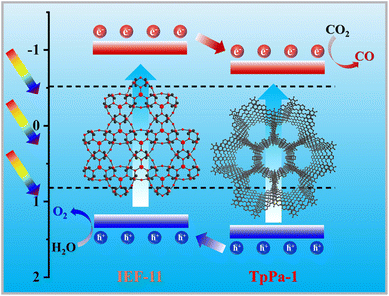
A possible mechanism for the photoreduction of CO2 is presented based on the synergistic effect of TpPa-1 and IEF-11 (Fig. 6). Because after photoexcitation, the photogenerated electrons of IEF-11 leap from the VB to the CB, and then transfer to TpPa-1 for reducing CO2. The ordered arrangement of Ti–O–Ti bonds in IEF-11 is beneficial for carrier transport. The large specific surface area, abundant catalytically active sites and good CO2 capture ability of TpPa@IEF-X provide sufficient conditions for photocatalytic CO2 reduction. Under the irradiation of solar light, the photogenerated CB of TpPa-1 converts CO2 into CO, and the remaining holes of IEF-11 are consumed by H2O in the reaction system. In the type-II heterojunction formed by TpPa-1 and IEF-11, the reverse migration of photogenerated electrons and holes achieves effective separation and promotes the photocatalytic CO2 reduction reaction.Conclusions
In summary, a series of stable hybrid materials TpPa@IEF-X based on TpPa-1 and IEF-11 have been prepared and employed for achieving a visible light-driven, sacrificial agent-free and photosensitizer-free photoreduction of CO2via the gas–solid reaction mode, where TpPa@IEF-100 achieves great CO formation (78.87 μmol g−1 h−1) in a pure CO2 environment, and TpPa@IEF-150 obtains an excellent CO generation rate of up to 116.47 μmol g−1 h−1 in a dilute CO2 environment. The photochemical measurements show that the rational coupling of TpPa-1 and IEF-11 has the natural advantage of broad light absorption ability, rich active sites and efficient photogenerated electron and hole pair separation, finally improving the photoreduction performance of CO2. This work provides an effective strategy for the preparation of new high-performance photocatalysts, indicating that COF@MOF hybrid materials have promising applications in solar-driven photocatalytic CO2 reduction systems.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the Natural Science Foundation of Shandong Province (No. ZR2021MB005 and ZR2021MB074) and the National Natural Science Foundation of China (No. 52176193).References
- E. Gong, S. Ali, C. B. Hiragond, H. S. Kim, N. S. Powar, D. Kim, H. Kim and S.-I. In, Solar fuels: Research and development strategies to accelerate photocatalytic CO2 conversion into hydrocarbon fuels, Energy Environ. Sci., 2022, 15, 880–937 RSC.
- S. C. Shit, I. Shown, R. Paul, K.-H. Chen, J. Mondal and L.-C. Chen, Integrated nano-architectured photocatalysts for photochemical CO2 reduction, Nanoscale, 2020, 12, 23301–23332 RSC.
- F. Gong, H. Zhu, Y. Zhang and Y. Li, Biological carbon fixation: From natural to synthetic, J. CO2 Util., 2018, 28, 221–227 CrossRef CAS.
- P. Gupta, M. T. Noori, A. E. Núñez and N. Verma, An insight into the bioelectrochemical photoreduction of CO2 to value-added chemicals, iScience, 2021, 24, 102294 CrossRef CAS PubMed.
- J. Wei, R. Yao, Y. Han, Q. Ge and J. Sun, Towards the development of the emerging process of CO2 heterogenous hydrogenation into high-value unsaturated heavy hydrocarbons, Chem. Soc. Rev., 2021, 50, 10764–10805 RSC.
- G. Wang, J. Chen, Y. Ding, P. Cai, L. Yi, Y. Li, C. Tu, Y. Hou, Z. Wen and L. Dai, Electrocatalysis for CO2 conversion: From fundamentals to value-added products, Chem. Soc. Rev., 2021, 50, 4993–5061 RSC.
- S. Das, J. Pérez-Ramírez, J. Gong, N. Dewangan, K. Hidajat, B. C. Gates and S. Kawi, Core–shell structured catalysts for thermocatalytic, photocatalytic, and electrocatalytic conversion of CO2, Chem. Soc. Rev., 2020, 49, 2937–3004 RSC.
- M. Lu, M. Zhang, J. Liu, Y. Chen, J.-P. Liao, M.-Y. Yang, Y.-P. Cai, S.-L. Li and Y.-Q. Lan, Covalent organic framework based functional materials: important catalysts for efficient CO2 utilization, Angew. Chem., Int. Ed., 2022, 61, e202200003 CAS.
- S. Mallakpour, E. Azadi and C. M. Hussain, Emerging new-generation hybrids based on covalent organic frameworks for industrial applications, New J. Chem., 2021, 45, 7014–7046 RSC.
- F.-M. Zhang, J.-L. Sheng, Z.-D. Yang, X.-J. Sun, H.-L. Tang, M. Lu, H. Dong, F.-C. Shen, J. Liu and Y.-Q. Lan, Rational design of MOF/COF hybrid materials for photocatalytic H2 evolution in the presence of sacrificial electron donors, Angew. Chem., Int. Ed., 2018, 57, 12106–12110 CrossRef CAS PubMed.
- L. Stegbauer, K. Schwinghammer and B. V. Lotsch, A hydrazone-based covalent organic framework for photocatalytic hydrogen production, Chem. Sci., 2014, 5, 2789–2793 RSC.
- J.-X. Cui, L.-J. Wang, L. Feng, B. Meng, Z.-Y. Zhou, Z.-M. Su, K. Wang and S. Liu, A metal-free covalent organic framework as a photocatalyst for CO2 reduction at low CO2 concentration in a gas–solid system, J. Mater. Chem. A, 2021, 9, 24895–24902 RSC.
- A. T. Partho, M. Tahir and B. Tahir, Recent advances in covalent organic framework (COF) nanotextures with band engineering for stimulating solar hydrogen production: A comprehensive review, Int. J. Hydrogen Energy, 2022, 47, 34323–34375 CrossRef CAS.
- C. Xia, K. O. Kirlikovali, T. H. C. Nguyen, X. C. Nguyen, Q. B. Tran, M. K. Duong, M. T. Nguyen Dinh, D. L. T. Nguyen, P. Singh, P. Raizada, V.-H. Nguyen, S. Y. Kim, L. Singh, C. C. Nguyen, M. Shokouhimehr and Q. V. Le, The emerging covalent organic frameworks (COFs) for solar-driven fuels production, Coord. Chem. Rev., 2021, 446, 214117 CrossRef CAS.
- R. Shah, S. Ali, F. Raziq, S. Ali, P. M. Ismail, S. Shah, R. Iqbal, X. Wu, W. He, X. Zu, A. Zada, Adnan, F. Mabood, A. Vinu, S. H. Jhung, J. Yi and L. Qiao, Exploration of metal organic frameworks and covalent organic frameworks for energy-related applications, Coord. Chem. Rev., 2023, 477, 214968 CrossRef CAS.
- M.-Y. Gao, C.-C. Li, H.-L. Tang, X.-J. Sun, H. Dong and F.-M. Zhang, Boosting visible-light-driven hydrogen evolution of covalent organic frameworks through compositing with MoS2: A promising candidate for noble-metal-free photocatalysts, J. Mater. Chem. A, 2019, 7, 20193–20200 RSC.
- X. Shi, Y. Yao, Y. Xu, K. Liu, G. Zhu, L. Chi and G. Lu, Imparting catalytic activity to a covalent organic framework material by nanoparticle encapsulation, ACS Appl. Mater. Interfaces, 2017, 9, 7481–7488 CrossRef CAS PubMed.
- Y.-P. Zhang, H.-L. Tang, H. Dong, M.-Y. Gao, C.-C. Li, X.-J. Sun, J.-Z. Wei, Y. Qu, Z.-J. Li and F.-M. Zhang, Covalent-organic framework based Z-scheme heterostructured noble-metal-free photocatalysts for visible-light-driven hydrogen evolution, J. Mater. Chem. A, 2020, 8, 4334–4340 RSC.
- J. Meng, X. Liu, C. Niu, Q. Pang, J. Li, F. Liu, Z. Liu and L. Mai, Advances in metal–organic framework coatings: Versatile synthesis and broad applications, Chem. Soc. Rev., 2020, 49, 3142–3186 RSC.
- J. Ren, N. M. Musyoka, H. W. Langmi, B. C. North, M. Mathe and X. Kang, Fabrication of core–shell MIL-101(Cr)@UiO-66(Zr) nanocrystals for hydrogen storage, Int. J. Hydrogen Energy, 2014, 39, 14912–14917 CrossRef CAS.
- A. Ebadi Amooghin, H. Sanaeepur, R. Luque, H. Garcia and B. Chen, Fluorinated metal–organic frameworks for gas separation, Chem. Soc. Rev., 2022, 51, 7427–7508 RSC.
- Y. Zheng, S. Zheng, H. Xue and H. Pang, Metal–organic frameworks for lithium–sulfur batteries, J. Mater. Chem. A, 2019, 7, 3469–3491 RSC.
- N. Makhanya, B. Oboirien, J. Ren, N. Musyoka and A. Sciacovelli, Recent advances on thermal energy storage using metal–organic frameworks (MOFs), J. Energy Storage, 2021, 34, 102179 CrossRef.
- L. Chen, X. Zhang, X. Cheng, Z. Xie, Q. Kuang and L. Zheng, The function of metal–organic frameworks in the application of MOF-based composites, Nanoscale Adv., 2020, 2, 2628–2647 RSC.
- M. Moharramnejad, A. Ehsani, M. Shahi, S. Gharanli, H. Saremi, R. E. Malekshah, Z. S. Basmenj, S. Salmani and M. Mohammadi, MOF as nanoscale drug delivery devices: Synthesis and recent progress in biomedical applications, J. Drug Delivery Sci. Technol., 2023, 81, 104285 CrossRef CAS.
- A. Ahmad, S. Khan, S. Tariq, R. Luque and F. Verpoort, Self-sacrifice MOFs for heterogeneous catalysis: Synthesis mechanisms and future perspectives, Mater. Today, 2022, 55, 137–169 CrossRef CAS.
- Y. Chen, D. Wang, X. Deng and Z. Li, Metal–organic frameworks (MOFs) for photocatalytic CO2 reduction, Catal. Sci. Technol., 2017, 7, 4893–4904 RSC.
- H.-N. Wang, Y.-H. Zou, H.-X. Sun, Y. Chen, S.-L. Li and Y.-Q. Lan, Recent progress and perspectives in heterogeneous photocatalytic CO2 reduction through a solid-gas mode, Coord. Chem. Rev., 2021, 438, 213906 CrossRef CAS.
- M. Tahir, B. Ajiwokewu, A. A. Bankole, O. Ismail, H. Al-Amodi and N. Kumar, MOF based composites with engineering aspects and morphological developments for photocatalytic CO2 reduction and hydrogen production: A comprehensive review, J. Environ. Chem. Eng., 2023, 11, 109408 CrossRef CAS.
- S. Gulati, S. Vijayan, Mansi, S. Kumar, B. Harikumar, M. Trivedi and R. S. Varma, Recent advances in the application of metal–organic frameworks (MOFs)-based nanocatalysts for direct conversion of carbon dioxide (CO2) to value-added chemicals, Coord. Chem. Rev., 2023, 474, 214853 CrossRef CAS.
- S. He, Q. Rong, H. Niu and Y. Cai, Platform for molecular-material dual regulation: A direct Z-scheme MOF/COF heterojunction with enhanced visible-light photocatalytic activity, Appl. Catal., B, 2019, 247, 49–56 CrossRef CAS.
- H. Assi, G. Mouchaham, N. Steunou, T. Devic and C. Serre, Titanium coordination compounds: From discrete metal complexes to metal–organic frameworks, Chem. Soc. Rev., 2017, 46, 3431–3452 RSC.
- S.-M. Liu, Z. Zhang, X. Li, H. Jia, M. Ren and S. Liu, Ti-Substituted Keggin-type polyoxotungstate as proton and electron reservoir encaged into metal–organic framework for carbon dioxide photoreduction, Adv. Mater. Interfaces, 2018, 5, 1801062 CrossRef.
- M. Zhang, J.-N. Chang, Y. Chen, M. Lu, T.-Y. Yu, C. Jiang, S.-L. Li, Y.-P. Cai and Y.-Q. Lan, Controllable synthesis of COFs-based multicomponent nanocomposites from core–shell to yolk-shell and hollow-sphere structure for artificial photosynthesis, Adv. Mater., 2021, 33, 2105002 CrossRef CAS PubMed.
- C. Guo, F. Duan, S. Zhang, L. He, M. Wang, J. Chen, J. Zhang, Q. Jia, Z. Zhang and M. Du, Heterostructured hybrids of metal–organic frameworks (MOFs) and covalent–organic frameworks (COFs), J. Mater. Chem. A, 2022, 10, 475–507 RSC.
- P. Salcedo-Abraira, A. A. Babaryk, E. Montero-Lanzuela, O. R. Contreras-Almengor, M. Cabrero-Antonino, E. S. Grape, T. Willhammar, S. Navalón, E. Elkäim, H. García and P. Horcajada, A novel porous Ti-squarate as efficient photocatalyst in the overall water splitting reaction under simulated sunlight irradiation, Adv. Mater., 2021, 33, 2106627 CrossRef CAS PubMed.
- S. Kandambeth, A. Mallick, B. Lukose, M. V. Mane, T. Heine and R. Banerjee, Construction of crystalline 2D covalent organic frameworks with remarkable chemical (acid/base) stability via a combined reversible and irreversible route, J. Am. Chem. Soc., 2012, 134, 19524–19527 CrossRef CAS PubMed.
- D. Shang, D. Li, B. Chen, B. Luo, Y. Huang and W. Shi, 2D–2D SnS2/covalent organic framework heterojunction photocatalysts for highly enhanced solar-driven hydrogen evolution without cocatalysts, ACS Sustainable Chem. Eng., 2021, 9, 14238–14248 CrossRef CAS.
- K. Li, H. Yu, P. Feron, M. Tade and L. Wardhaugh, Technical and energy performance of an advanced, aqueous ammonia-based CO2 capture technology for a 500 MW coal-fired power station, Environ. Sci. Technol., 2015, 49, 10243–10252 CrossRef CAS PubMed.
- G. V. Last and M. T. Schmick, A review of major non-power-related carbon dioxide stream compositions, Environ. Earth Sci., 2015, 74, 1189–1198 CrossRef CAS.
- R.-G. Yang, Y.-M. Fu, H.-N. Wang, D.-P. Zhang, Z. Zhou, Y.-Z. Cheng, X. Meng, Y.-O. He and Z.-M. Su, ZIF-8/covalent organic framework for enhanced CO2 photocatalytic reduction in gas-solid system, Chem. Eng. J., 2022, 450, 138040 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Materials and methods, experimental details including synthesis, experimental procedure, supporting data, and FTIR spectra of photocatalysts, PXRD patterns, CO2 adsorption, Mott–Schottky plots, XPS spectrum, PL spectra, time-yield graph and table of CO on TpPa@IEF-X, UV-visible absorption spectrum. See DOI: https://doi.org/10.1039/d3qi00217a |
| This journal is © the Partner Organisations 2023 |