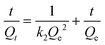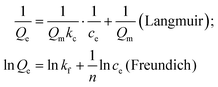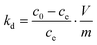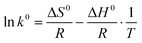Syntheses and applications of mesoporous hydroxyapatite: a review
Olaiya Peter
Oni
ab,
Yan
Hu
c,
Sijie
Tang
ab,
Honghan
Yan
ab,
Hao
Zeng
d,
Huimin
Wang
ab,
Liya
Ma
 e,
Changying
Yang
e,
Changying
Yang
 *ab and
Jiabing
Ran
*ab and
Jiabing
Ran
 *ab
*ab
aCollege of Biological & Pharmaceutical Sciences, China Three Gorges University, Yichang 443002, China. E-mail: changying.yang@ctgu.edu.cn; jiabingran@outlook.com; ranjiabing@ctgu.edu.cn
bHubei Key Laboratory of Natural Products Research and Development, China Three Gorges University, Yichang, 443002, China
cDepartment of Rehabilitation Medicine, Zhongnan Hospital, Wuhan University, Wuhan 430000, China
dThe State Key Laboratory Breeding Base of Basic Science of Stomatology (Hubei-MOST) & Key Laboratory of Oral Biomedicine Ministry of Education, School & Hospital of Stomatology, Wuhan University, 237 Luoyu Road, Wuhan 430079, China
eThe Centre of Analysis and Measurement of Wuhan University, Wuhan University, Wuhan, 430072, China. E-mail: maliya@whu.edu.cn
First published on 8th November 2022
Abstract
Mesoporous hydroxyapatite (MHAP), featuring high porosity, high surface area, and three-dimensional ordered passageways, has diverse applications in several fields. Thus, an in-depth understanding of the synthesis strategies and practical applications of MHAP is of great significance and has drawn tremendous attention. In this review work, we have firstly summarized and compiled all the strategies for MHAP synthesis and mainly classified them into three categories according to the physical–chemical properties of templates used in the process: hard template method, soft template method, and template-free method. In addition, we have systematically compared the advantages and disadvantages of the three categories of methods and given our comments in detail. Then, we have briefly introduced the applications of MHAP, mainly from the following aspects: wastewater treatment, drug delivery, and bone tissue engineering. Primarily, we have emphasized the applications of MHAP-based drug delivery systems in disease therapy, including cancer treatment, anti-depression, bone regeneration, etc. Finally, we have put forward our views on the long-term perspective and development trends of MHAP in the future.
1. Introduction
Nanotechnology has gained significant interest in the last few years due to its positive and fantastic contribution to several fields. The nanomaterials produced through the techniques made possible by nanotechnology have been of great importance in several fields of scientific research. Of the numerous available nanomaterials, hydroxyapatite (HAP) has received considerable attention owing to its multifunctionality and ability to be modified for different purposes. HAP has the general chemical formula Ca10(PO4)6(OH)2 and is a naturally occurring calcium phosphate mineral belonging to the apatite family.1,2 It has gained much popularity in many fields, including the biomedical sector, bio-ceramic, pharmaceuticals, tissue engineering, catalysis reactions, environmental pollution control, etc.3 For instance, HAP nanoparticles with various morphologies and surface properties have been extensively investigated as nano delivery devices, especially for proteins, drugs, and other biological factors, due to their biocompatible, non-toxic, and non-inflammatory nature.4,5 However, the natural HAP lacks some great features that will make it function well, such as high porosity, high surface area, and a highly porous network. This reality has led to further research towards the synthesis of hollow and porous HAP. Until now, porous HAP has not only been regarded as an excellent nano-carrier but has also been very useful in applications like tissue engineering, bioimaging, wastewater treatment, etc.4,5According to the International Union of Pure and Applied Chemistry (IUPAC) classification, pores can be roughly classified into three categories: macropores, mesopores, and micropores. Mesopores are pores that fall within the range of 2 and 50 nm,6 and mesoporous materials, i.e., materials with pore diameter between 2 and 50 nm, have been found to have some unique ability and usefulness. The mesopore lies in the middle of two other measures: the micropore having a pore size less than 2 nm and the macropore with a pore size greater than 50 nm. Materials with a pore size less than 100 nm are generally referred to as nanoporous materials with mesoporous sizes seeming to have received some great attention from researchers in recent times owing to their unique characteristics (e.g., high pore volume, high surface area, and ordered pore network). For example, the macroporous materials have failed over time, especially in drug delivery, where they exhibited a high burst release profile due to their lower porosity.7 In addition, mesoporous materials behave better in catalysis, adsorption, and other applications when compared to macroporous or microporous materials. This glaring usefulness of mesoporous materials and their relevance beyond the level of both microporous and macroporous materials have led to some exciting research into them, thus being one of the significant reasons why they are the center of this review work. In sum, we in this work have examined extensively and provided the various synthesis strategies as well as applications of mesoporous HAP (MHAP). Additionally, we have concluded with some of our views on the future of the nanoparticles as well as well-thought recommendations.
2. Hydroxyapatite
HAP has been one of the most interesting materials to study in recent times for biomedical, therapeutic and even environmental applications. It is nanocrystalline, which confers it the ability to be tough and withstand pressure.9 Stoichiometric HAP has the formula Ca10(PO4)6(OH)2 with a Ca/P ratio of 1.67.9,10 Its elemental composition is similar to that of bones and teeth, which is one reason it is advantageous in the dental and orthopedic fields.11,12 HAP has a hexagonal shape (space group P63/m) with crystallographic parameters of a = 9.432 Å, c = 6.881 Å, and β = 120°.13 This structure has two binding sites on its surface: the phosphate site, negatively charged, and the calcium site, positively charged.14 Tetrahedral PO4 groups, where P5+ ions are located at the center of the tetrahedron while 4 O atoms occupy the top, are compactly assembled, forming the crystalline network of HAP. Each PO4 tetrahedron is shared by a column and delimits two kinds of unconnected channels (Fig. 1A). The first type of channel demonstrates a diameter of 2.5 Å and is surrounded by four Ca2+ ions (Ca(I)) in each unit cell (Fig. 1B). Each Ca(I) ion is surrounded by three oxygen triangles, two of which are located above and below Ca(I) and the third larger triangle is almost at the same height along the c axis as Ca(I) (Fig. 1C).15 The remaining six calcium (Ca) ions (Ca(II)) in the unit cell constitute the second type of channel (diameter: 3–4.5 Å) and are located at the periphery of the channel (Fig. 1A). In the unit cell, these Ca(II) ions are located in two dimensions 1/4 and 3/4, and form alternate equilateral triangles around the helicoidal senary axis of the apatite lattice (Fig. 1D). In addition, they are in coordination with 7 O atoms, six of which belong to PO4 tetrahedra and the remaining one O atom comes from OH−. These OH− ions are present at the column and perpendicular to the unit cell face, and located at the center of the second type of channel. Their O atom is located at 0.4 Å out of the plane of Ca(II) while their H atom is located at 1 Å, almost on the triangle plane of Ca(II). These OH− ions also align along the c axis of the column to balance the positive charge of the HAP crystal.8 Owing to the dimension of the two channels, Ca2+, PO43−, and OH− could leave their lattice sites and move along the tunnel in the direction of the OZ axis (Fig. 1C). Simultaneously, the left vacancies can be substituted by various ions or cluster of ions but the crystallographic structure of HAP is unchanged. Thus, HAP is a highly non-stoichiometric compound with Ca/P molar ratio ranging from 1.50 to 1.67.16 The formula of non-stoichiometric HAP could be expressed as Me10(XO4)6(Y)2, where Me, X, and Y can be8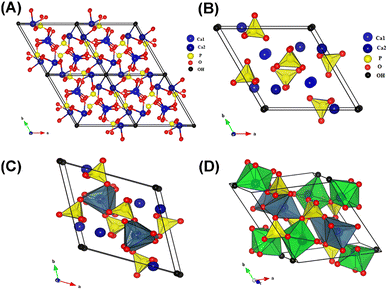 | ||
| Fig. 1 (A) Projection of the unit cell of HAP according to the plane (001); (B) projection showing the arrangement of octahedral [Ca(1)O6] in the HAP structure; (C) projection showing the sequence of octahedral [Ca(1)O6] and tetrahedral [PO4] in the HAP structure; and (D) projection showing the sequence of octahedral [Ca(1)O6] and [Ca(2)O6] and also tetrahedral [PO4] in the HAP structure. Reprinted with permission from ref. 8. Copyright 2017, Elsevier B. V. | ||
• M = Ca, Mg, Sr, Ba, Cd, Cu, Zn, Pb, etc.
• Z = P, V, As, S, Si, Ge, C, Cr, As, Mn, etc.
• X = F, Cl, OH, O, Br, I, Cl, etc.
Besides, Me could also be monovalent cations (e.g. Na+ and K+) and trivalent cations (e.g. Eu3+), PO43− could also be substituted by CO32− and HPO42−, and OH− could also be substituted by O2−, S2−, and CO32−. There have been records of various types of HAP, but the most reported are those with different morphologies such as plates, nanofibers, nanorods, nanotubes, etc.4,11,17,18 One of the significant focuses of researchers in recent times has been to look at the synthesis procedure to influence and regulate some parameters of the nanoparticles, including the structure, size, morphology, and crystallinity.18,19 Through these controlled processes, limitations like the low surface area, low mechanical strength, lesser biocompatibility, and bioactivity, which constantly affect the application of nanoparticles, can be easily eliminated.20 In the review “Synthesis methods for nanosized hydroxyapatite in diverse structures” by Shojai et al., synthesis strategies for HAP with different shapes (sphere, rod, needle, tube, fiber, prism, worm, platelet, strip, flake, dandelion, flower, dumbbell, bowknot, etc.), size (nanoscale and micrometer scale), size distribution, and phase composition (monocalcium phosphate monohydrate, octacalcium phosphate, α-tricalcium phosphate, amorphous calcium phosphate, etc.) are summarized in detail.21 Several factors, including synthesis steps, pH, temperature, templates used, etc., could influence the properties mentioned above of HAP. For instance, Shojai et al. prepared a series of HAP with different morphology, size, and phase composition by adjusting the pH, temperature, and duration of hydrothermal treatment (Fig. 2A).22
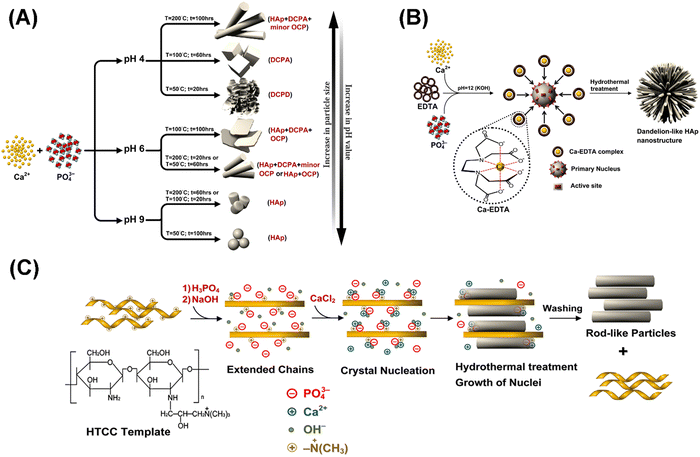 | ||
| Fig. 2 (A) Effect of pH, temperature, and duration of hydrothermal treatment on the phase, morphology, and particle size of the HAP powder. Reprinted with permission from ref. 22. Copyright 2012, Elsevier B. V.; nucleation and growth of dandelion-like and rod-like nano HAP synthesized through a hydrothermal method and using (B) EDTA (reprinted with permission from ref. 23. Copyright 2012, The American Ceramic Society) and (C) HTCC (reprinted with permission from ref. 24. Copyright 2010, Elsevier B. V.) as templates, respectively. | ||
As mentioned, HAP is the main inorganic component of bones and teeth. Thus, the synthesis of HAP could be considered a biomimetic mineralization process. Stephen Mann once reported that biomineralization relied on specific molecular interactions at the inorganic–organic interfaces (electrostatic binding or association, geometric matching, and stereochemical correspondence), which led to controlled nucleation and growth of inorganic crystals.25 In the process of mineralization of bones and teeth in vivo, collagen and highly acidic non-collagenous proteins serve as organic templates to mediate the nucleation and growth of HAP. Regarding collagen, it has two important functions: (I) serving as the structural template to provide a place (i.e., the less dense 40 nm long gap zone in the periodic 67 nm cross-striated pattern of the collagen fibril) for apatite crystals to nucleate and grow from an amorphous phase and (II) functioning in synergy with inhibitors of HAP to actively mediate mineralization (the positive net charge near the C-terminal end of collagen promotes the infiltration of the fibrils with amorphous calcium; the cluster of charged amino acids forms nucleation sites controlling the conversion of amorphous calcium into a parallel array of oriented apatite crystals). As for acidic non-collagenous proteins, they play a key role in directing the nucleation and growth of HAP.26 In this regard, it is generally acknowledged that organic templates are indispensable in the controlled synthesis of HAP.27 In the work of Zhu et al., EDTA was utilized as a template to synthesize HAP through a hydrothermal process (160 °C, low pH, Ca//EDTA = 1). The as-prepared HAP is prism-like and of micrometer scale.28 Similarly, Lak et al. used EDTA to prepare HAP through a hydrothermal method at a pH of 12. The as-obtained HAP is dandelion-like and of nanoscale.23 The rationale behind this phenomenon is as follows: calcium ions were wrapped by EDTA to form Ca-EDTA complexes. At the same time, plentiful OH− ions were absorbed on the planes of initial clusters. These absorbed OH− ions served as active sites for the adsorption of Ca-EDTA complexes. The space inhibitions of Ca-EDTA complexes with each other prevent the Ca atoms from being ordered according to HAP's intrinsic crystal structure. Instead, the absorbed Ca-EDTA molecules on the surface dictate a 3D pattern structure for further growth under hydrothermal conditions (Fig. 2B). In addition, researchers have also paid much attention to the synthesis of oriented nano-structured HAP (anisotropic growth along the (002) crystal plane) through the regulation of organic templates.29–33 In the work of Zhu et al., rod-like HAP nanoparticles were synthesized through a low-temperature hydrothermal method in the presence of N-[(2-hydroxy-3-trimethylammonium)propyl] chitosan chloride (HTCC) as a cationic polymer template.24 Through charge and stereochemical complementarity, PO43− and OH− were absorbed onto the HTTC molecules. As a result, the HTTC polymer molecules were converted to extended chains. The extended chains were firstly connected to form a 2D structure through the ion bonds of PO43−. Then the 2D HTTC/PO43−/OH− layers were self-assembled into a 3D rod-like morphology via hydrogen bond interactions. With the addition of the Ca precursor, crystal nucleation occurred and rod-like HAP was formed (Fig. 2C). Huang et al. used an etched polyamide membrane as the template to induce oriented growth of the HAP rod perpendicular to the polyamide substrate.34 They held that the etched polyamide membrane served as a calcium reservoir, which supplied continuous Ca2+ ions for the oriented growth of HAP on the polyamide substrate. HAP is highly hydrophilic, and this confers on it the ability to be used in dehydration reactions, especially as a direct heterogeneous catalyst; a typical example of the process involves the reaction of lactic acid to produce acrylic acid, which in itself is a very important immediate for several acrylate polymers as well as other related molecules and also very prominent in the Guerbet coupling of alcohols.12 Additionally, through isomorphous substitutions, HAP could also be utilized as an excellent catalyst support for various reactions.12 For instance, HAP has been applied as a metal support in different reactions, including oxidation of alcohols, methane, and CO; selective reduction of NOx over copper and silver-impregnated HAP; Claisen-Schmidt condensation; Michael addition; Heck reaction; Knoevenagel condensation; and the Diels–Alder reaction.2 In addition, HAP is also one of the top-ranking prospective materials as a reinforcing filler in composites. This is because of its adaptability making it useful in the field of tissue engineering (e.g., HAP/collagen,35 HAP/polymethyl methacrylate,36 HAP/polyamide,37 and HAP/polyethylene38,39). This has also given rise to the production of different enhanced versions of HAP, particularly MHAP which is of great interest to researchers.3–5,17,18,40,41
3. Mesoporous hydroxyapatite
MHAP is an enhanced version of HAP as it possesses a hollow mesoporous structure that differentiates it from other types of HAP.4,42 It belongs to engineered nanomaterials, which have been said to be very promising as they possess unique properties through some chemical or physical processes and are contributing massively to the growth of nanoscience and nanotechnology.19,20 MHAP has high porosity, high surface–volume ratio, and three-dimensional mesoporous passageways, increasing its abilities and usefulness compared to the pure HAP39,40,43,44. Fig. 3 demonstrates a typical TEM image of a mesoporous HAP nanoparticle (MHANP), showing a rod-like morphology with clear and distinct porosity, which confers the mesoporous structure. The image also shows that the particles are monodispersed with an average diameter of <100 nm, which shows the excellent distribution system of the material. They also possess uniform pore size distributions making them very relevant and applicable in fields such as gas sensing, catalysis, optics, polymer filling, and adsorption systems.45 A typical example is the reported enhanced protein adsorption capacity and its stabilized release.9 MHAP also has some other unique physical–chemical properties like solubility, biocompatibility, osteoconductivity, osteoinductivity, and lower toxicity, and these have rapidly increased its usefulness in the fields of orthopedics, drug delivery systems, bone tissue engineering as well as bioimaging.4,5,17,40,43–46 | ||
| Fig. 3 TEM image of typical MHANPs. Reprinted with permission from ref. 35. Copyright 2014, Marcelo F. Cipreste, Edesia M. B. Sousa et al. | ||
4. Syntheses of mesoporous hydroxyapatite
In the last few years, researchers have been exploring several methods and procedures to produce modified HAP with mesoporous nature leading to further improvements and enhanced abilities. Through the modified synthesis, problems like non-specific targeting, poor pharmacokinetics, low surface area, and the like associated with pure HAP have been eradicated in most of the synthesized MHAP.38,47–49 Since several researchers are working on different ways to get the best MHAP and its customizable types, various techniques have been used over time for the synthesis. They include the opposite ion core strategy (graft onto method), micro-emulsion method, microwave irradiation, sonochemistry-assisted microwave method, hydrothermal method, co-precipitation method (wet chemistry method), sol–gel method, etc. Though the approaches mentioned above have different names and operation procedures, some have something in common: the usage of templates.In most cases, templates are applied not only to mediate the nucleation and growth of HAP, therefore regulating its size, size distribution, and morphology, but also to serve as a sacrificing phase to generate hollow cavities or mesopores. In other words, the particle size, morphology, pore structure, pore size, and pore size distribution of as-prepared MHANPs are functions of the type of template used.50 Thus their physical–chemical properties can be delicately controlled by selecting different templates.51,52 Generally, templates could roughly be classified into soft and hard templates. Thus, this review systematically summarizes and compares the available strategies concerning MHAP or MHANP synthesis from three aspects: hard template synthesis, soft template synthesis, and template-free synthesis. Besides, we also briefly introduce some exceptional examples of MHANP or MHAP synthesis that can neither be classified as the template method nor the template-free method.
4.1. Hard template method
Hard templating synthesis, also known as nano casting, involves using the precursors of the desired inorganic hard materials (e.g., Si foam, carbon nanorods, CaCO3 nanoparticles) to fill mesoporous molds. The molds will be eliminated at the end of the process, leaving the final material to show the shape, size, and distribution of the void space left by the hard template. Standard methods to eliminate the hard templates include sintering, dissolution, and etching. Whichever of the three methods is used, it must be carefully carried out so that it will not alter the chemical properties of the as-prepared materials.Here, we take CaCO3 as an example for hard template, also known as opposite ion core synthesis. It can be defined as a triple approach methodology. The first step involves the synthesis of the inner CaCO3 nanoparticles, which are then used as the core for the further synthesis of hollow MHANPs.4 Then, the addition of phosphoric acid will make way for the replacement of CO32− by PO43−, a process that leads to the building up of the HANP shell, which will surround the CaCO3 core.4 This stage is allowed to proceed for a reaction time of at least 12 hours for good aging. Besides the strategy mentioned above, the CaCO3/HANP core/shell structure could also be constructed through hydrothermal processes.53
Moreover, the CaCO3 core can be hybridized with other functional components to endow the final MHANPs with specific properties. For instance, in Lin et al.'s work, the CaCO3/Fe3O4 core was utilized as the template for synthesizing magnetic MHANPs, which may find diverse applications in magnetic and pH-responsive drug delivery.54 The final stage of this opposite ion core strategy is using acetic acid to selectively wash out the CaCO3 core, which then releases pure hollow MHANPs.38 The ability of acetic acid to remove the CaCO3 core is due to the difference in the dissolution of CaCO3 and HAP in acetic acid solution.17
Fig. 4 shows the symmetrical overview of the process involved in the synthesis, as explained above, and the SEM/TEM of the as-prepared MHANP. It has an oval-shaped and ellipsoidal morphology with a large surface area and pore size. In addition to acetic acid, sucrose has also been applied to remove the CaCO3 core (Fig. 5A). Fig. 5B shows the TEM images of as-prepared MHANPs, and mesopores could be observed.55 This method features straightforwardness, simplicity in the mode of synthesis, lesser resources, and lesser cost of production. In addition, some researchers have further developed this method by adjusting the morphology of CaCO3 cores (Fig. 6); as a result, the morphology of as-prepared MHAP can be controlled [31]. However, the as-prepared MHANPs also have some fatal defects (e.g., non-uniform morphology, unequal size, inferior crystallinity, low phase purity), which inevitably limits their applications in many fields.
 | ||
| Fig. 4 (a) An illustration indicating the synthetic process of MHAP using the CaCO3 template; SEM images of (b) CaCO3 nanoparticles, (c) CaCO3/HA core/shell nanocomposites, and (d) MHANPs; and TEM images of (e) CaCO3 nanoparticles, (f) CaCO3/HA core/shell nanocomposites, and (g) MHANPs (inset: dark-field TEM image). Reprinted with permission from ref. 17. Copyright 2013, Royal Society of Chemistry. | ||
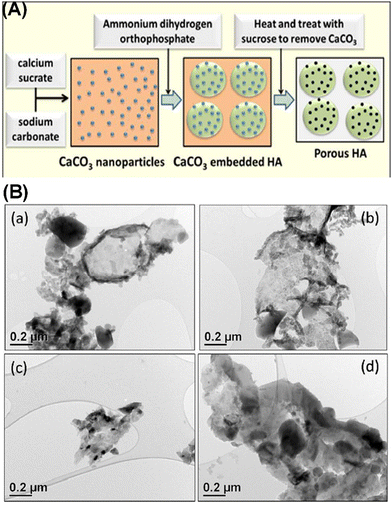 | ||
| Fig. 5 (A) Schematic diagram showing the process of using sucrose to remove CaCO3 and (B) TEM images of MHANPs synthesized using CaCO3 as a template and sucrose as an extraction solvent. Reprinted with permission from ref. 55. Copyright 2018, Royal Society of Chemistry. | ||
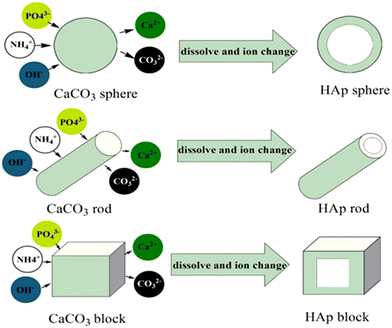 | ||
| Fig. 6 Schematic representation of the formation of different MHAP morphologies using the CaCO3 block, rod, and balls as hard templates. Reprinted with permission from ref. 50. Copyright 2018, The Society of Powder Technology Japan. Published by Elsevier B.V. and The Society of Powder Technology Japan. | ||
Besides CaCO3, carbon is another commonly used hard template for MHANP synthesis. Kamieniak et al. reported a novel strategy for fabricating MHANPs using carbon nanorods as a hard template, synthesized using mesoporous silica (SBA-15) and an acidified sucrose solution (Fig. 7A).56 The basic technique involves the addition of the precursors ((NH4)2HPO4 solution and Ca(NO3)2 solution) to the preformed carbon nanorod template as well as the subsequent template removal using the dissolution route. This method also ensures that the meso-channels are filled.12,50,51,57Fig. 7C shows the TEM image of as-prepared MHANPs using carbon nanorods as a template, and an apparent mesoporous structure with a pore size of around 5 nm could be observed. As mentioned before, the size and distribution of pores of MHANPs are functions of the type of template used. Kamieniak et al. also fabricated three carbon templates with different structures for synthesizing different MHANPs.56 They found that the kind of carbon template significantly affects the pore properties of MHANPs produced (Table 1).
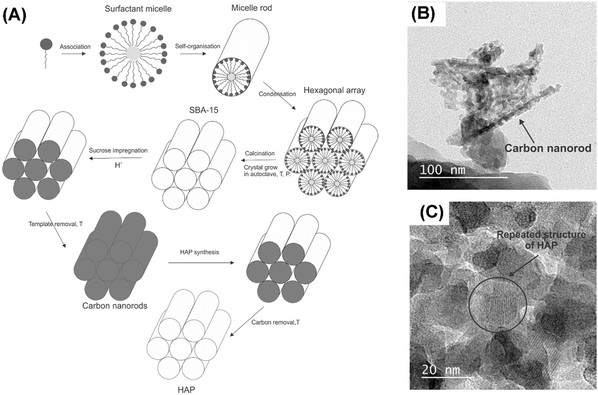 | ||
| Fig. 7 (A) The whole preparation process of the MHANPs using carbon nanorods as a hard template; TEM images of the as-prepared (B) carbon nanorod/HAP composite and (C) MHANP. Reprinted with permission from ref. 56. Copyright 2017, Elsevier Ltd and Techna Group S.r.l. | ||
| Template | Surface area (m2 g−1) | Pore shape | Pore diameter (nm) | Pore volume (cm3 g−1) | Micropore volume (cm3 g−1) | Mesopore volume (cm3 g−1) |
|---|---|---|---|---|---|---|
| MCF (mesostructured cellular foam) | 600 | Spherical mesopores in close packing, with large windows | 28 | 2.0 | 0.3 | 1.7 |
| CVD (carbon vapor deposition) | 380 | Spherical but irregular mesopores | 10–30 | 0.6 | 0.0 | 0.6 |
| Sucrose carbon replica | 740 | Irregular meso- and micropores | 5 | 0.6 | 0.1 | 0.5 |
From the high-resolution TEM image (Fig. 7C), it could also be found that the as-prepared MHANP had higher crystallinity than that prepared using CaCO3 as a hard template. However, it still demonstrated less irregular morphology and inhomogeneous size. More importantly, considering that the carbon-based templates are removed through solvent dissolution, the phase purity of the resultant MHANPs might be compromised to some extent owing to the residual carbon impurities.
4.2. Soft template method
Unlike the above-discussed hard templating methodology, the soft template method involves a system built on the principle of organic templates’ self-assembling ability, which can then produce porous but inorganic materials.45 Researchers have over the years explored several soft templates including cetyltrimethylammonium bromide (CTAB),43,59–62 pluronic P123,63–66 pluronic F127,67 mono-alkyl phosphate (MAP),18 sodium dodecyl sulfate (SDS),68 3,3′,5,5′-tetramethylbenzidine (TMB),68 dendrimer,39 casein,18 polyethylene glycol (PEG),37,69 EDTA,70 monododecyl phosphate,71 mono-alkyl phosphate,72 tetradecyltrimethylammonium bromide,73 vitamin C,73 tea polyphenol,74 chlorogenic acid,74etc. The produced MHANPs from these different templates differ as the template also tends to modify or determine the morphology of the produced materials, just like the hard templates do.75 In addition, these soft templates also serve as a sacrificing phase to generate mesopores. In a typical soft templating methodology, co-precipitation, hydrothermal, and sol–gel processes are frequently utilized for synthesizing HAP. At the same time, calcination and solvent extraction are frequently applied to remove soft templates. When considering hydrothermal, co-precipitation, or calcination processes, the size uniformity and crystallinity of as-prepared MHANPs are much higher than those fabricated using a hard template. However, in view of the smaller 3D particle size of a soft template than a hard template, the as-prepared MHANPs, compared to those from hard template synthesis, don’t have a hollow mesoporous cavity, and their pore size, in principle, is much smaller.Cetyltrimethylammonium bromide (CTAB) micelles are the most widely used soft template for fabricating MHANPs. In the work of Pramanik et al., CTAB was utilized to synthesize MHANPs through a successive hydrothermal process and calcination process (Fig. 8A). The as-prepared MHANPs demonstrated a length of 89 ± 5 nm, a diameter of 38 ± 6 nm, an aspect ratio of 2.34, a surface area of 62.58 m2 g−1, a pore volume of 0.19 cm3 g−1, and a pore size of 4.7 ± 0.9 nm (Fig. 8B).58 Compared to MHANPs fabricated without using templates, MHANPs synthesized using CTAB as a template demonstrated oriented growth along the c axis (Fig. 9). The poly(amido amine) dendrimer seems to have the same template effect as CTAB (Fig. 8A). The as-obtained MHANPs have a length of 82 ± 3 nm, a diameter of 36 ± 4 nm, an aspect ratio of 2.28, a surface area of 56.46 m2 g−1, a pore volume of 0.18 cm3 g−1, and a pore size of 5.5 ± 1.25 nm (Fig. 8B).58 However, compared to CTAB, PVP and ascorbic acid pose a more substantial influence on the morphology and size of resultant MHANPs. Kumar et al. prepared three kinds of MHANPs through a successive microwave-assisted hydrothermal/calcination process by using CTAB, PVP, and ascorbic acid as templates, respectively. They found that ascorbic acid, CTAB, and PVP induced oriented growth of HAP along the c-axis, forming needle-like, rod-like, and fiber-like MHANPs, respectively (Fig. 9).76
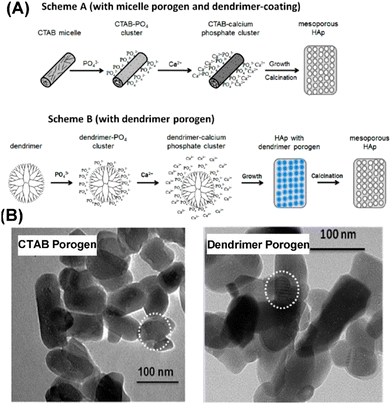 | ||
| Fig. 8 (A) Schematic diagram showing the preparation process of MHANPs synthesized using CTAB or dendrimer as a soft template through subsequent hydrothermal/calcination treatment and (B) TEM images of MHANPs synthesized using CTAB or dendrimer as a porogen. Reprinted with permission from ref. 58. Copyright 1997, American Chemical Society. | ||
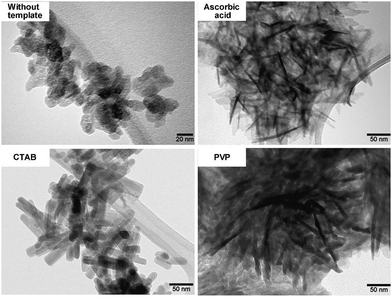 | ||
| Fig. 9 SEM images of MHANPs synthesized through a microwave-assisted hydrothermal/calcination process using three different soft templates. Reprinted with permission from ref. 76. Copyright 2018, Elsevier Ltd and Techna Group S.r.l. | ||
Mohammad et al. fabricated a carbonated MHANP through a co-precipitation process using non-ionic surfactant P123 as the soft template. They found that surfactant extraction methods could significantly affect the pore properties of as-prepared MHANPs. In Table 2, the influence of three solvent extraction strategies (i.e., water, ethanol, acetone) and calcination strategy (550 °C) on the porous structure of as-prepared MHANPs is demonstrated.78 Briefly, solvent extraction-generated MHANPs exhibited higher surface area, larger pore size, and larger pore volume than calcination-generated MHANPs.
| Template extraction strategy | Surface area (m2 g−1) | Pore size (nm) | Pore volume (cm3 g−1) |
|---|---|---|---|
| Ethanol | 86.67 | 21.08 | 0.45 |
| Water | 84.21 | 22.55 | 0.48 |
| Acetone | 57.25 | 31.18 | 0.47 |
| Calcination at 550 °C | 15.20 | 8.34 | 0.16 |
Lett et al. prepared a kind of MHANP through a sol–gel process using stearic acid as the soft template (Fig. 10A).77 They found that the pH environment of the sol–gel process could influence the porosity and pore size of the as-prepared MHANP (Fig. 10C). In Table 3, the average pore size and BET surface area of the fabricated MHANP with stearic acid as the soft template are demonstrated. Obviously, with the increase in the pH value of the sol–gel process, the surface area of as-prepared MHANPs was gradually enhanced. In addition to stearic acid, lauric acid was also utilized as a soft template for synthesizing MAHNPs, which featured a slightly lower surface area (47.567 ± 0.0595 m2 g−1) and smaller average pore size (3.87 nm) than those fabricated using stearic acid as the template.48 The pore size of as-prepared MHANPs has something to do with the alkyl chain length of the surfactant template.
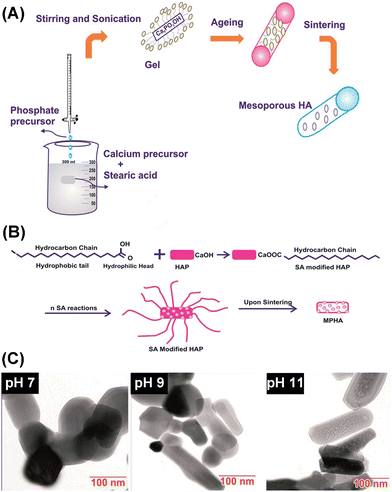 | ||
| Fig. 10 Schematic diagram of (A) the preparation process of MHANPs through a sol–gel method using stearic acid as a template and (B) the pore-forming mechanism; and (C) TEM images of MHANPs synthesized at different pH values. Reprinted with permission from ref. 77. Copyright 2011, Royal Society of Chemistry. | ||
| Sol–gel synthesis, pH | Surface area (m2 g−1) | Average pore size (nm) |
|---|---|---|
| pH 7 | 7.7138 ± 0.0474 | — |
| pH 9 | 10.5197 ± 0.0399 | — |
| pH 11 | 66.265 ± 0.0345 | 5.86 |
Among diverse soft templates, researchers have also found a category of organic compounds that could act as the template for generating mesopores and serve as the Ca source or P source for synthesizing MHANPs. Here, we call this method self-template synthesis. Zhou et al. reported a self-template method for synthesizing MHANPs, in which hexametaphosphate (P6) was utilized as a phosphorus source and simultaneously as a template. The whole process was carried out in a hydrothermal environment. Generally, hydrothermal treatment induced the hydrolysis of P6 to generate PO43−, which then reacted with Ca2+ to form HAP.
Meanwhile, the space occupied in the complex by P6 was partially preserved with HAP formed around, resulting in a mesoporous structure (surface area of 114 m2 g−1 and mean pore size in the range between 6 and 26 nm) (Fig. 11A). In addition, Zhou et al. further developed this strategy by introducing tea polyphenols to mediate the size and morphology of as-prepared MHANPs (Fig. 11B). The rationale behind this phenomenon could be ascribed to the high amount of OH groups of tea polyphenols binding to Ca2+, inducing anisotropic growth of HAP along the c axis.79 Bharath et al. utilized carboxymethylcellulose calcium salt as a template and simultaneously as a Ca source to synthesize MHANPs through a successive hydrothermal process and a calcination treatment. The as-prepared MHANPs demonstrated a spindle-like structure with a length of 20 nm and an average diameter of 10 nm, a high surface area of 180 m2 g−1, and pore size distribution between 20 and 60 nm.80
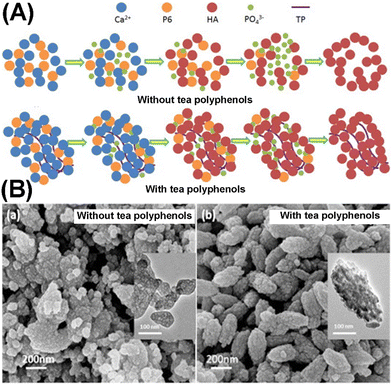 | ||
| Fig. 11 (A) Diagrams showing the preparation process of MHANPs using hexametaphosphate as the self-template (upper) and the process of tea polyphenols inducing anisotropic growth of HAP and (B) SEM images of MHANPs prepared using hexametaphosphate as the self-template. Reprinted with permission from ref. 79. Copyright 2014, Elsevier B. V. | ||
In addition to a single soft template, binary soft templates have also been applied to mediate the morphology and particle size of MHANPs or to expand the pore size of MHANPs. In the work of Bharath et al., CTAB and PEG were utilized as soft templates, and a hydrothermal process was applied to fabricate MHANPs. By changing the initial feeding ratio of CTAB/PEG, the morphology of as-prepared MHANPs could be modified from nanorods to spheres (Fig. 12A), influencing the surface area and pore size of MHANPs (Fig. 12B).81
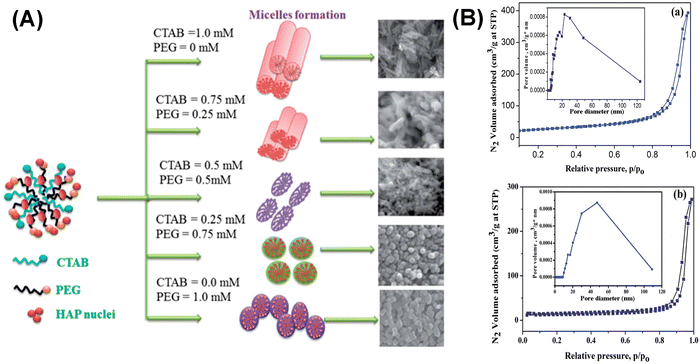 | ||
| Fig. 12 (A) Schematic diagram and SEM images showing the morphology change of as-prepared MHANPs by changing the CTAB/PEG ratio and (B) the N2 adsorption–desorption isotherms and pore size distributions of (a) rod-like and (b) sphere-like MHANPs. Reprinted with permission from ref. 81. Copyright 2011, Royal Society of Chemistry. | ||
Zhang et al. fabricated Sr-substituted MHANPs through a hydrothermal process utilizing CTAB and trisodium citrate as templates (Fig. 13A). The as-prepared Sr substituted MHANP demonstrated monodisperse nanorods with a length of 120–150 nm, a diameter of around 20 nm, a surface area of 70.4 m2 g−1, a pore volume of 0.373 cm3 g−1, and a mesopore size of 3–5 nm (Fig. 13B).82 Uota et al. successfully synthesized a kind of MHANP through a successive co-precipitation/calcination process, simultaneously utilizing nonaoxyethylene dodecyl ether (C12EO9) and polyoxyethylene (20) sorbitan monostearate (Tween 60) as templates. Herein, owing to much less adhesion of decapsulated HAP nanoparticles, which could be ascribed to the particle-separating effect of the C12EO9 molecules absorbed on the outer surface of the stearate-encapsulated HAP nanoparticles to restrict their accumulation or interfacial coordination (Fig. 14A), the as-prepared MHANPs demonstrated higher surface area (364 m2 g−1) than those prepared using single templates (Fig. 14B).83
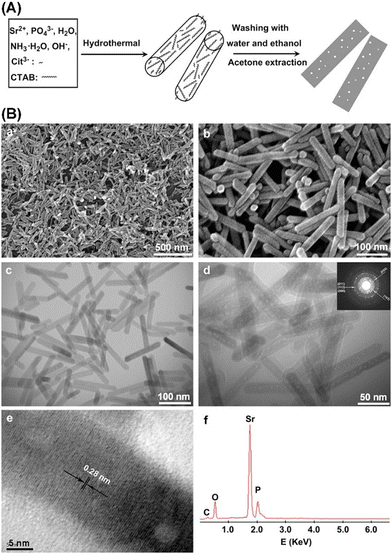 | ||
| Fig. 13 (A) Schematic diagram showing the preparation process of MHANPs using CTAB and trisodium citrate as templates and (B) SEM, TEM, and EDS of as-prepared MHANPs. Reprinted with permission from ref. 82. Copyright 2010, Elsevier B. V. | ||
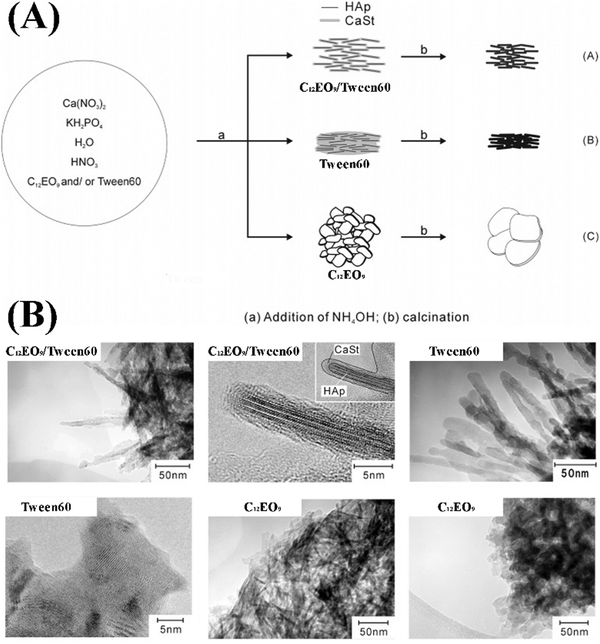 | ||
| Fig. 14 (A) Schematic diagram showing the preparation process of MHANPs using C12EO9 as a template and (B) TEM images of as-prepared MHANPs. Reprinted with permission from ref. 83. Copyright 2005, American Chemical Society. | ||
Zeng et al. reported a facile approach for fabricating pore-expanding MHANPs utilizing 1,3,5-trimethyl benzene (TMB) as an auxiliary solubilizer and the general soft template CTAB. TMB can enter the amphiphilic CTAB micelle to form the swelled TMB/CTAB-PO43− complex, and in the presence of Ca2+, Ca9(PO4)6 clusters were preferentially condensed on the micelle surface owing to the conformation compatibility between the identical hexagonal shapes of the micelles and Ca9(PO4)6 (Fig. 15A). Compared to MHANPs without TMB, the channels have enlarged dimensions of 5–15 nm and the space between the nanochannels is filled with an ordered crystalline HAP structure with hexagonal crystals (Fig. 15B). In addition, the surface area of as-prepared MHANPs was also improved.84
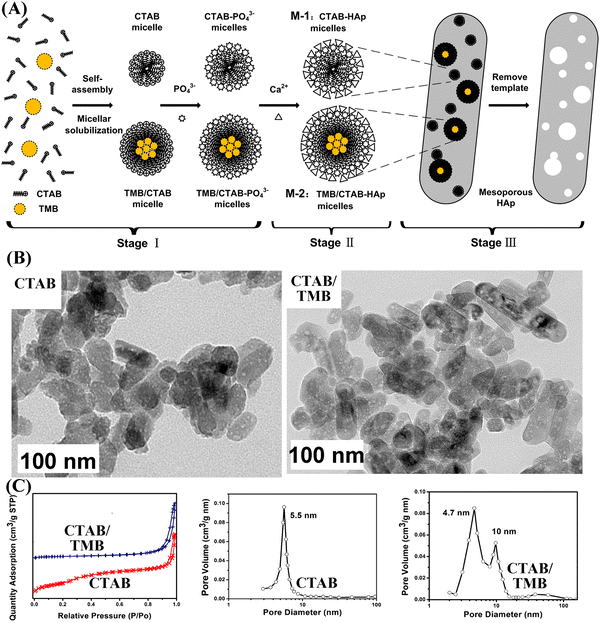 | ||
| Fig. 15 (A) The mechanism of pore size expansion of MHANPs through adding TMA as a pore expanding agent; (B) TEM images and (C) N2 adsorption–desorption isotherms and BJH pore size distribution of MHANPs synthesized using CTAB and CTAB/TMB as templates, respectively. Reprinted with permission from ref. 84. Copyright 2013, Elsevier B.V. Published by Elsevier B.V. | ||
In the work of Bakhtiari et al., CTAB was utilized as a soft template for preparing MHANPs, while 1-dodecanethiol was utilized as a swelling agent for broadening the pore size of as-obtained MHANPs. Briefly, the change in the degree of interaction between CTAB, the solvent, and the swelling agent causes the formation of different micelle structures and, consequently, different pore morphologies and pore sizes (Fig. 16A). In the case of low concentration, 1-dodecanethiol led to an increase in the pore size. In contrast, higher concentrations caused the formation of microemulsion and foam structures (Fig. 16B).85 In addition, they also found that an increase in the synthesis temperature also led to a decrease in particle size, an increase in the average pore diameter, and an improved ordering degree of the pore structure.86
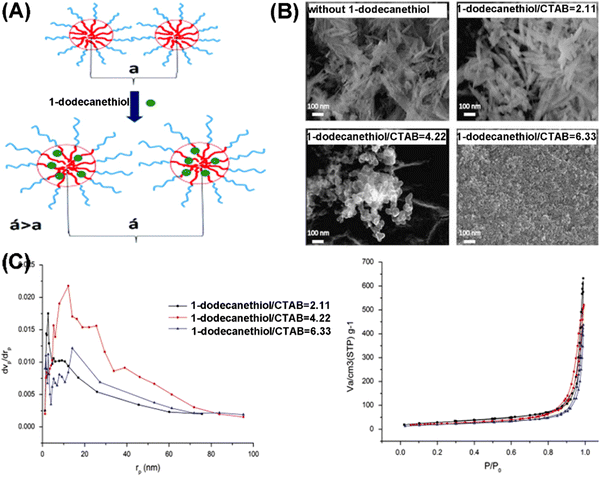 | ||
| Fig. 16 (A) Schematic diagram showing the effect of 1-dodecanethiol on the distance between the pore centers (a is the distance in the absence of the swelling agent, and a′ is the distance in the presence of the swelling agent); (B) SEM images showing the influence of 1-dodecanethiol on the morphology and pore structure of as-prepared MHANPs; and (C) BET curves and BJH pore size distribution showing the influence on the pore size and surface area of as-prepared MHANPs. Reprinted with permission from ref. 85. Copyright 2015, Chinese Materials Research Society. Production and hosting by Elsevier B.V. | ||
In addition to conventional soft templates, some specific organic templates have also been utilized for synthesizing MHAP. For instance, Sabu et al. employed an eggshell membrane as a bio-template to synthesize MHAP with the assistance of microwave heating. Briefly, calcium and phosphorus precursors were infiltrated into the eggshell membrane and heated in a domestic microwave oven. MHAP retaining the interwoven hierarchical structure of the eggshell membrane through this operation was formed.1Fig. 17 shows the hierarchical mesoporous structure of as-prepared MHAP.
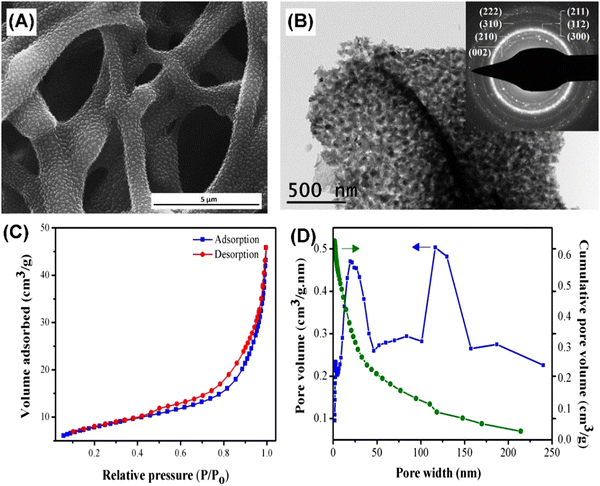 | ||
| Fig. 17 (A) SEM image, (B) TEM image, (C) BET curve, and (D) BJH pore size distribution of MHAP prepared using an eggshell membrane as a bio-template with the assistance of micro heating. Reprinted with permission from ref. 1. Copyright 2018, Elsevier Ltd and Techna Group S.r.l. | ||
4.3. Template-free method
The template-free method means neither soft nor hard templates are needed in the whole preparation process of MHANPs. As mentioned before, an essential operation for the template method is the template removal procedure, which inevitably increases the complexity of the preparation process of MHANPs. In addition, the remaining templates may negatively influence the porous structure of as-prepared MHANPs. However, owing to the absence of a template, the template-free method loses its advantage in mediating the morphology, size distribution, and size uniformity of MHANPs. In general, template-free synthesis is commonly realized with the assistance of microwave treatment. Liang et al. reported a template-free sonochemistry-assisted microwave method for synthesizing MHANPs (Fig. 18). For one thing, the quick heating caused by the microwave and the strong cavitation effect of ultrasonic waves generate a large number of bubbles in the precursor solution. Simultaneously, microwave irradiation-induced heating also accelerates HAP crystal formation and growth, preventing the bubbles’ breakup. In addition, volumetric and selective heating promotes the formation of high-power densities in reaction zones. For another thing, the quick cooling down of the system and the quick escape of gas from the precursor solution result in the formation of a mesoporous structure. Resultantly, holes are left in the interior or on the surface of MHANPs.87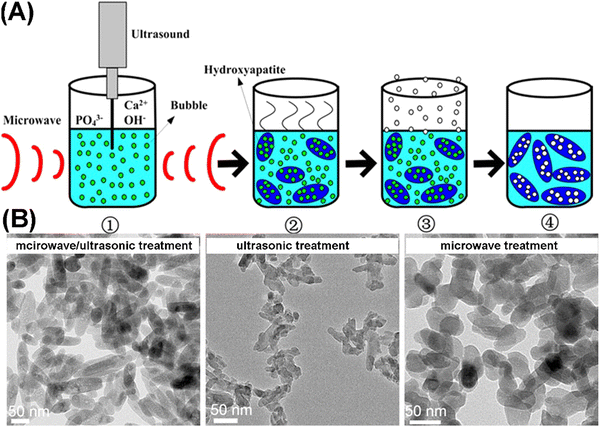 | ||
| Fig. 18 (A) Schematic diagram showing the rationale behind the sonochemistry-assisted microwave method for synthesizing MHANPs and (B) TEM images of as-prepared MHANPs. Reprinted with permission from ref. 87. Copyright 2013, Springer Science Business Media New York. | ||
Akram et al. also reported a continuous microwave flow method for synthesizing MHANPs without templates. It features a dramatic reduction in reaction time, higher product yields, higher purity products, and higher nucleation and growth rate. This system consists of two peristaltic pumps, which are applied to feed the reactants through the Teflon coil placed in the microwave oven (Fig. 19A). By changing the microwave power, retention time, and reactant concentration, the particle size (14–26 nm), surface area (80–105 m2 g−1), and pore size (mesopore) could be modified on demand (Fig. 19B).88
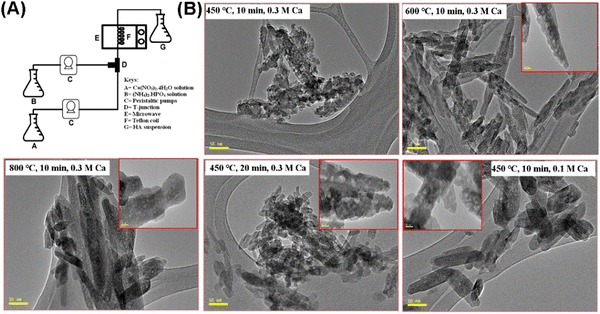 | ||
| Fig. 19 (A) Schematic diagram showing the preparation process of MHANPs through a continuous microwave flow method and (B) TEM images of MHANPs synthesized under different reaction conditions. Reprinted with permission from ref. 88. Copyright 2015, Elsevier B.V. | ||
Gu et al. also reported a novel gas–liquid chemical precipitation method for synthesizing MHANPs without using any templates (Fig. 20A). The as-prepared MHANPs were in the size range of 2–50 nm, and the mesopores were in the range of 2–32 nm (Fig. 20C). Their surface area reached up to 116.8 m2 g−1. Their total pore volume was around 0.508 mL g−1 (Fig. 20B).89
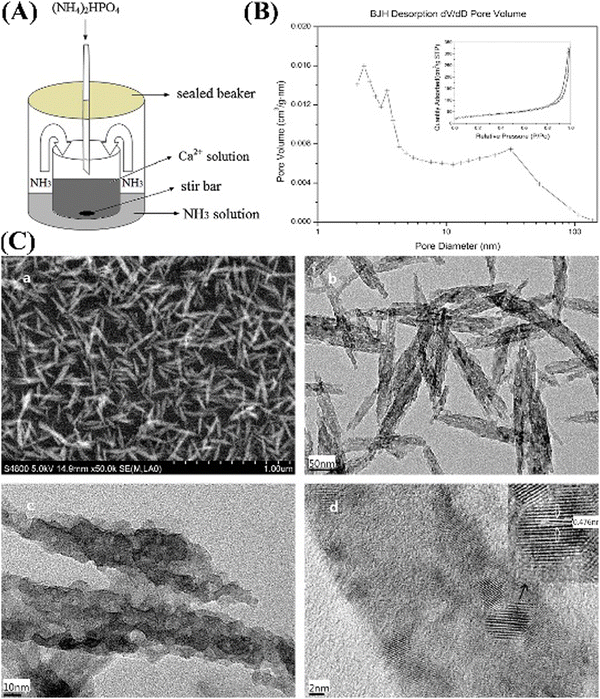 | ||
| Fig. 20 (A) Schematic diagram showing the gas–liquid chemical precipitation method; (B) N2 adsorption–desorption isotherm and BJH pore size distribution curve of as-prepared MHANPs; and (C) SEM and TEM images of as-prepared MHANPs. Reprinted with permission from ref. 89. Copyright 2014, Elsevier B.V. | ||
5. Applications of mesoporous hydroxyapatite
The MHANP has been one of the most valuable nanomaterials in recent times, as its application cuts across several fields of life. The various components and structures of the nanoparticle also make it a potential candidate for various biomedical and environmental applications. Some of the reported applications over the years are discussed below.5.2. Removal of toxic materials in wastewater
One of this generation's significant environmental challenges is the release of harmful materials into the environment. The various recurrent exposure of humans and animals to some toxic materials has been one of the critical causes of mortality in recent times, some of which might be accidental. Heavy metals and organic pollutants have been at the forefront of all these harmful materials, as established by researchers’ reports.69,91–94 For instance, water and soil contamination has been a significant threat to the life of humans and other entities in ecology.91,92 Additionally, the accumulation of harmful organic compounds in humans due to the breakdown of one organ or the other is also detrimental to the proper health of an individual. For example, p-cresol is commonly found in the gastrointestinal system of patients with kidney failure.92 Due to their harmful effects, several mechanisms have been explored to eliminate these materials. These include adsorption, flocculation, ion exchange, reverse osmosis, solvent extraction, chemical precipitation, electrochemical deposition, and membrane filtration.92,94 However, the most effective of all that is useful across the board is the adsorption technique, as it was reported to be cost-effective, easy to use, accurate, feasible, and sensitive.91,92,94 However, the choice of materials used in the adsorption technique will, in one way or the other, influence how accurate and precise the results will be. Works of literature over the years have reported some materials that have been used as adsorbents, including magnetic nanocomposites, activated carbon, zeolite-imidazolate, functionalized silica, NiO and Ni(OH)2 nanorods, graphene-based nanocomposites, etc.91,92 The high surface area, large pore size, stability, and surface modification ability of MHAP affirmed its adsorbent ability. It improved the surface's reactivity, making it easier for toxic materials in water to react with the new active sites.91 Herein, we briefly discuss the applications of MHANPs in wastewater treatment.Toxic heavy metal ions mostly discharged from the foundry and mining industries have underlined the high importance of wastewater treatment. Three main mechanisms could explain the heavy metal adsorption of MHANPs: ion exchange, formation of ion complexes on the surface, and precipitation.55 Aklil et al. found that the uptake amounts of Cu2+, Zn2+, and Pb2+ by calcium phosphate were merely 14.26 mg g−1, 12.83 mg g−1, and 84.80 mg g−1, respectively.95 The uptake amounts of Cu2+ and Ni2+ by magnetite-HAP were also only 10.31 and 7.14 mg g−1, respectively.96 Through hybridization with biochar, the adsorption capacity of HAP was slightly improved (49.99 mg g−1 for Pb2+, 49.50 mg g−1 for Cu2+, and 45.50 mg g−1 for Zn2+).97 Aklil et al. prepared nanosheet-shaped HAP and found that its adsorption capacity for heavy metal ions was greatly enhanced. The corresponding uptake amounts of Cd2+, Cu2+, and Pb2+ reached 152.9, 118.9, and 236.3 mg g−1, respectively.98 In this regard, some researchers held that the specific mesoporous structure of MHANPs might endow them with superior heavy metal ion adsorption capability. For instance, the highest uptake amounts of Pb2+, Cd2+, and Ni2+ by MHANPs developed by Wijesinghe et al. were up to 181, 122, and 81 mg g−1, respectively.97 Bharath et al. further found that the shape of MHANPs also affects their adsorption capability toward heavy metal ions. The uptake amounts of Pb2+ by rod-like MHANPs and sphere-like MHANPs were 714.14 mg g−1 and 526.31 mg g−1, respectively.81 Bharath et al. reported a mesoporous dendritic α-Fe2O3/HAP nanocomposite which consisted of a 1 μm long central trunk with secondary branches of 80 nm. Its maximum removal capacity for Pb2+ reached 754.14 mg g−1, and the experimental data fitted well with the pseudo-second-order mode.99
Organic contaminants are abundantly present in the aquatic environment due to their use as intermediates in the plastic, cosmetic and pharmaceutical industries. Chang et al. fabricated a kind of carbon dots/copper oxide/MHANP (CDs/CuO/MHANP) composite, in which CuO served as a catalyst, CDs functioned as an electron donor, and the MHANP provided a porous structure and high specific surface area, as well as interfacial bonding, being the efficient charge transport channels (Fig. 21A). In this formulation, the CuO phase underwent dynamic restructuring and therefore boosted the electron transport, which promoted its reduction capability against 4-nitrophenol (4-NP) under both dark condition and visible light illumination condition (Fig. 21C–F).90 Song et al. synthesized a carbon/MHANP (MHANP@C) composite by using MHANPs wrapped in uniform carbon layers. The MHANP@C nanocomposite could effectively remove Co2+ from aqueous solution (e.g., 83% Co2+ removal efficiency from 6 ppm aqueous solution). In addition, in Co2+ adsorption, Ca2+ replacement by Co2+ resulted in the ready formation of CoOH+, which served as the peroxymonosulfate (PMS) catalytic active center (Fig. 22). Co2+ substituted MHANP@C/PMS completely degraded methylene blue in 1 min. In comparison, it took 45 min in a homogeneous Co2+/PMS system.100
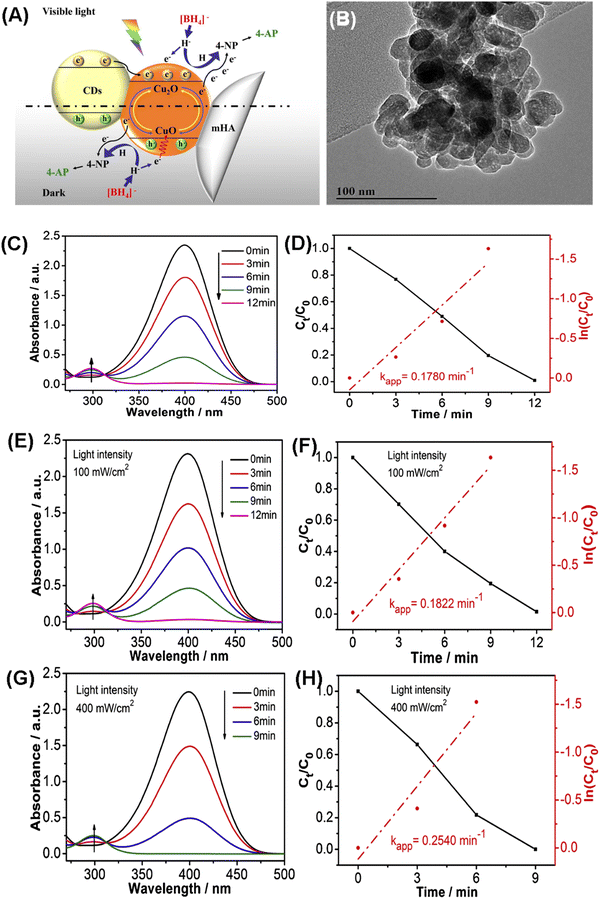 | ||
| Fig. 21 (A) Schematic diagram showing the catalytic reduction of 4-NP by the CDs/CuO/MHANP composite; (B) TEM image of the CDs/CuO/MHANP composite; (C) UV-vis absorption spectra of 4-NP and plots of Ct/C0 and ln(Ct/C0) versus time for 4-NP reduction over CDs/CuO/MHANP under the dark condition (C and D) and under illumination with visible light at (E and F) 100 and (G and H) 400 mW cm−2, respectively. Reprinted with permission from ref. 90. Copyright 2019, Elsevier B.V. | ||
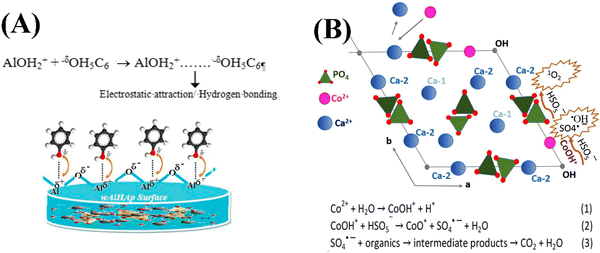 | ||
| Fig. 22 (A) Schematic description of electrostatic interactions between phenol and the surface of the Al/MHANP composite;101 (B) mechanism of Co2+ adsorption and radical production facilitated by CoOH+ and the nucleophile tetrahedral PO4. Reprinted with permission from ref. 100. Copyright 2018, Royal Society of Chemistry. | ||
5.2. Drug/protein/gene delivery and disease therapy
Biological factor delivery is the most reported application of MHANPs. Over the years, the biomedical world has faced a significant problem of not finding suitable carriers that could effectively deliver the cargo to the appropriate site of action without degradation on the way. Some important reasons why the MHANP has been explored for this application include its porosity, large surface area, biocompatibility, resistance to mechanical force, good loading capacity, and the ability to release the cargo sustainably.102 In addition to conventional drugs, the MHANP has also been used in the field of protein delivery as it possesses some excellent characteristics that can easily make that possible. HAP particles with various morphologies and surface properties have been considered ideal protein delivery devices due to their biocompatibility, nontoxicity, and noninflammatory properties. Protein loading on HAP is commonly realized by surface physical adsorption, and the loaded proteins usually demonstrate burst release behaviour due to weak bonds between protein and HAP. Ordered MHANPs, featuring high surface areas, small and tunable pore sizes, and large pore volumes, are considered better protein vector.103 Specifically, HAP possesses two binding sites, i.e., calcium and phosphate sites, which are well positioned to efficiently bind to the acidic group and basic group of proteins, respectively.102Additionally, MHANPs have a larger pore size than the average diameter of proteins, usually around 5.38 nm.102 Moreover, MHANPs have been applied for gene delivery for some unique disease treatments. Regarding this topic, Bakan et al. have systematically summarized the applications of MHANPs in DNA/siRNA/mRNA delivery and the main parameters which can influence the carrier properties and transfer efficiency in their review work entitled “Gene delivery by hydroxyapatite and calcium phosphate nanoparticles: a review of the novel and recent applications”.104 However, whether MHANPs have some advantages in gene delivery has not been reported and verified.
Yang et al. compared the doxorubicin (DOX) loading and delivery properties of HANPs and MHANPs. The DOX loading efficiency of HANPs and MHANPs was 17.9% and 93.7%, respectively, and the mesoporous structure of MHANPs delayed the release of loaded DOX compared to HANPs (Fig. 23A).105 Their average pore size and surface area greatly influenced the drug loading amount and drug release behaviors of MHANPs. Lett et al. prepared three kinds of MHANPs with different surface area and average pore size through a sol–gel method utilizing fatty acid (HAP, 7.7138 ± 0.0474 m2 g−1, no pores), lauric acid (HAP-LA, 47.567 ± 0.0595 m2 g−1, 3.87 nm), and stearic acid (HAP-SA, 66.265 ± 0.0345 m2 g−1, 5.86 nm) as templates, respectively. MHANPs with higher surface area and larger pore size demonstrated higher vancomycin loading capability and far more sustained vancomycin release behaviours.48 In addition, the drug delivery capability of MHANPs was pH dependent, which could be ascribed to the different dissolution rates of apatite under different pH conditions (Fig. 23B and C). Unlike free DOX, the DOX-loaded MHANP demonstrated better killing efficiency (Fig. 23D) and internalization efficiency (Fig. 23E) against BT-20 cells.105
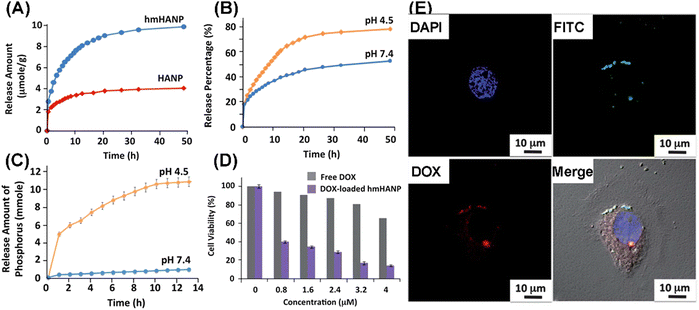 | ||
| Fig. 23 (A) Release profiles of DOX-loaded HANPs and MHANPs at pH 7.4; (B) release profiles of DOX-loaded MHANPs at different pH values; (C) release amount of PO43− at different pH values; (D) cell viability of BT-20 cells treated with different concentrations of DOX from free DOX and DOX loaded MHANPs; and (E) confocal images of BT-20 cells incubated with DOX loaded MHANPs for 24 h. Reprinted with permission from ref. 105. Copyright 2013, Royal Society of Chemistry. | ||
Gu et al. further investigated DOX's adsorption kinetics and thermodynamics on MHANPs, which were prepared through a gas–liquid co-precipitation method. They found that the adsorption kinetics of DOX on MHANPs could be described using Ho' pseudo-second-order rate equation (Fig. 24):
| Qt = ki·t1/2 |
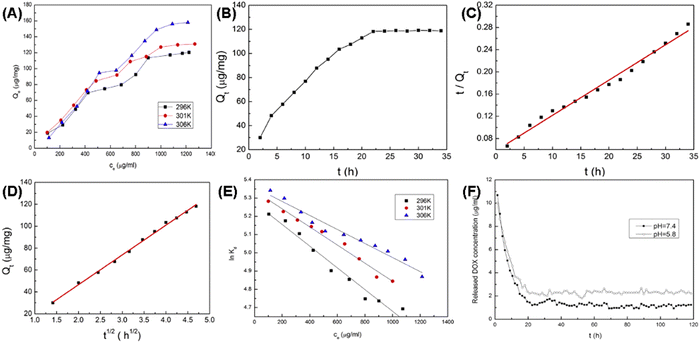 | ||
Fig. 24 (A) Adsorption isotherms of DOX on MHANPs at three different temperatures; plots of (B) Qtversus t, (C) t/Qtversus t, and (D) Qtversus t for adsorption of DOX on MHANPs; (E) linear plots of ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) kdversus ce of DOX adsorption on MHANPs; and (F) release profiles of DOX loaded MHANPs in PBS. Reprinted with permission from ref. 89. Copyright 2014, Elsevier B.V. kdversus ce of DOX adsorption on MHANPs; and (F) release profiles of DOX loaded MHANPs in PBS. Reprinted with permission from ref. 89. Copyright 2014, Elsevier B.V. | ||
To describe the adsorption equilibrium, they also simulated the relationship between the adsorbed amount (Qe) and its equilibrium concentration (Ce) using two standard models, Langmuir and Freundlich.
ΔG0 = −RT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) k0 k0 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k0 are obtained by plotting ln
k0 are obtained by plotting ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) kdversus ce and extrapolating ce to zero. The distribution coefficient kd is calculated using the following equation:
kdversus ce and extrapolating ce to zero. The distribution coefficient kd is calculated using the following equation:where m is the mass of MHANPs and V is the volume of the solution. The values of ΔG0 and ΔH0 have been obtained from the van’t Hoff equation:
Finally, they found that the adsorption was increased at high temperatures, the adsorption of DOX on MHANPs was an endothermic and increasing entropy process, and the adsorption of DOX on MHANPs could be considered as physical adsorption.89
Ibrahim et al. reported a supercritical CO2 method for controlling the loading of ibuprofen in or on MHANPs, mediating its release behavior. Fig. 25A shows the whole drug loading apparatus. By changing the drug loading pressure, the proportions of ibuprofen on the surface of MHANPs or in the mesopores of MHANPs could be adjusted on demand. Generally, the higher the loading pressure is, the deeper the loaded ibuprofen enters the pores, and the more complex the loaded drug would be released (Fig. 25B).106
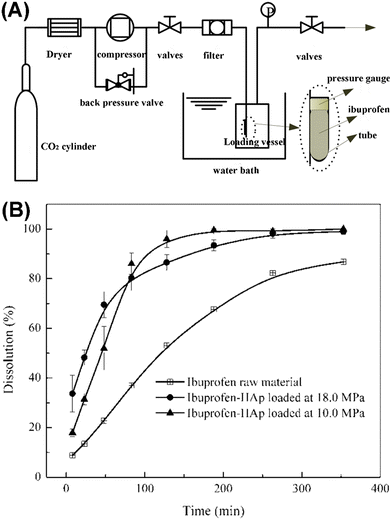 | ||
| Fig. 25 (A) Schematic diagram showing the drug loading process using a supercritical CO2 method and (B) ibuprofen release curves showing the influence of drug loading pressure on the drug release behavior.106 | ||
Surface modification has been applied to control the drug-loading capability and drug-release behaviors of MAHNPs. Verma et al. reported a kind of citrate-functionalized MHANP fabricated using a co-precipitation method and featuring a surface area of 182.9 m2 g−1. It demonstrated sustained pH-dependent DOX release behavior. This pH-responsive DOX release behavior might be due to the electrostatic binding of the positively charged DOX to the negatively charged citrate-functionalized MHANPs and the porous structure of MHANPs.107 Ansari et al. also reported a kind of MHANP with pH-responsive epirubicin delivery capability by modifying the MHANP with folic acid-conjugated polyethylene glycol (PEG) molecules. The release behavior of epirubicin from modified MHANPs demonstrated an increase in release rate in an acidic environment. The release amount of drug in a pH 5.5 environment was 2.5 fold more than in a pH 7.4 environment.108 Wu et al. developed a sustained delivery system of simvastatin (SIM) based on the poly(isopropyl acrylamide) (PNIPAAM) capped MHANP, which was prepared through surface-initiated atom transfer radical polymerization (Fig. 26A). The SIM loaded MHANP-PNIPAAM (MHANP-SIM-PNIPAAM) demonstrated a sustained release of SIM at 37 °C for 16 days. Compared to the control group (MHANP-SIM), MHANP-SIM-PNIPAAM exhibited better performance in terms of cell proliferation, ALP activity, and calcium deposition due to the sustained release of SIM (Fig. 26D).38
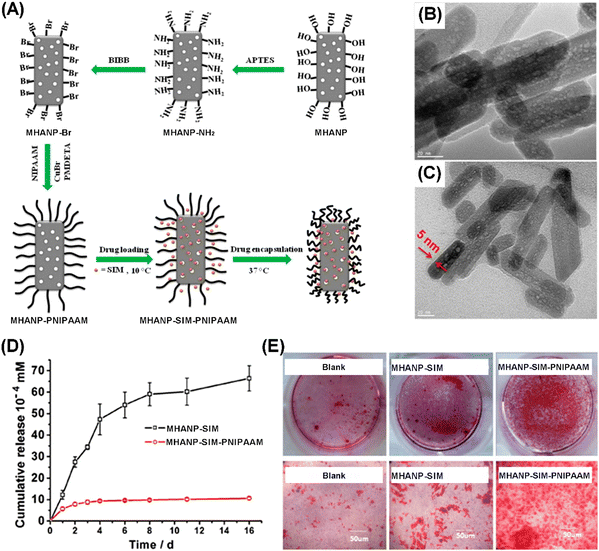 | ||
| Fig. 26 (A) Schematic diagram of the preparation process of MHANP-SIM-PNIPAAM; TEM images of (B) MHANP-SIM-PNIPAAM and (C) MHANP-SIM; (D) drug release curves of MHANP-SIM-PNIPAAM and MHANP-SIM; and (E) Alizarin Red staining of BMSCs cultured in the blank, MHANP-SIM, and MHANP-SIM-PNIPAAM in osteogenic medium for 14 days. Reprinted with permission from ref. 38. Copyright 2016, Elsevier B.V. | ||
Li et al. constructed a novel cell-targeting, pH-sensitive nanocarrier based on MHANPs for intracellular drug delivery (LA-BSA-CBA-MHANPs), utilizing lactobionic acid-conjugated bovine serum albumin (LA-BSA) molecules as end-caps and 4-carboxyphenylboronic acid (CBA) as an intermediate linker (Fig. 27A). In physiological environments (pH 7.4), DOX loaded LA-BSA-CBA-MHANPs demonstrated a slow drug release rate, while under acidic conditions (pH 5.0) the release rate was greatly enhanced due to the breakage of cyclic ester bond linkages between LA-BSA and MHANPs (Fig. 27B). In addition, the incorporation of lactose acid made the as-prepared MHANPs more easily being captured by HepG2 cells.109
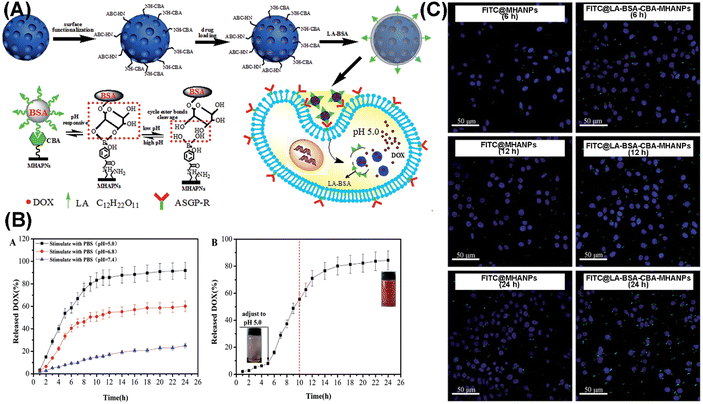 | ||
| Fig. 27 (A) Schematic diagram showing the preparation process of LA-BSA-CBA-MHANPs and the rationale behind their pH-responsiveness; (B) pH-dependent drug release profiles of DOX loaded LA-BSA-CBA-MHANPs; and (C) representative CLSM images of FITC@MHANPs and FITC@ LA-BSA-CBA-MHANPs, endocytosed by HepG2 cells. Reprinted with permission from ref. 109. Copyright 2015, Royal Society of Chemistry. | ||
Proteins have larger molecular sizes and more complex structural and surface compositions than drug molecules. Still, MHANPs have roughly similar loading and delivery behaviors to drug molecules. For instance, Zhang et al. compared the concentration/time-dependent adsorption behaviour of BSA on HANPs and MHANPs and investigated the protein release behaviour of BSA-loaded MHANPs. MHANPs demonstrated a higher loading capacity than HANPs (Fig. 28A–C). In addition, the loading amounts of BSA on MHANPs decreased as the pH increased, and the release behaviour could be controlled by the environmental pH of protein adsorption (Fig. 28D–F).103
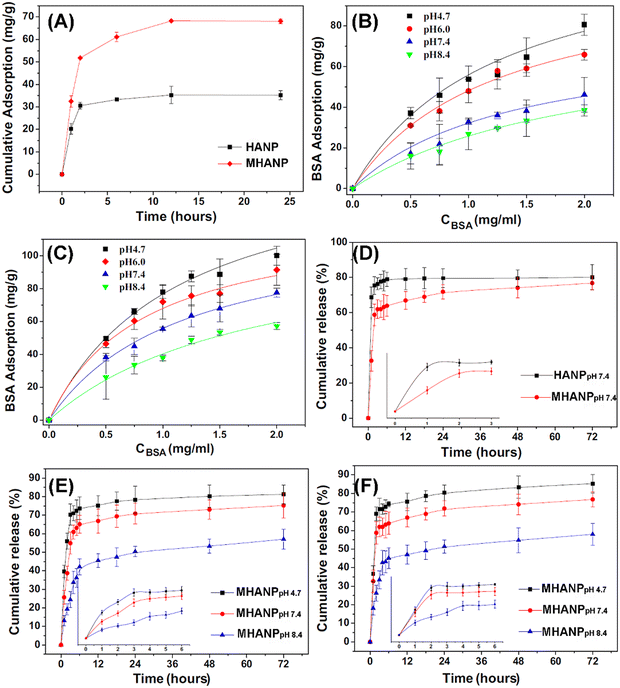 | ||
Fig. 28 (A) The kinetics of protein adsorption on HANPs and MHANPs in 1.5 mg mL−1 BSA solution at pH 7.4; adsorption isotherms of BSA over (B) HANPs and (C) MHANPs in working solutions of different pH (pH 4.7, 6.0, 7.4 and 8.4); (D) the cumulative release kinetics of BSA from HANPpH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.4 and MHANPpH 7.4 and MHANPpH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.4; release profiles from MHANPpH 7.4; release profiles from MHANPpH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.7, MHANPpH 4.7, MHANPpH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.4 and MHANPpH 7.4 and MHANPpH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8.4 at pH 7.4 (E) and pH 5.0 (F), respectively. MHANPpH 8.4 at pH 7.4 (E) and pH 5.0 (F), respectively. MHANPpH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.7, MHANPpH 4.7, MHANPpH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.4 and MHANPpH 7.4 and MHANPpH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8.4 mean three kinds of BSA-loaded MHANPs; the loading pH is 4.7, 7.4, and 8.4, respectively. Reprinted with permission from ref. 103. Copyright 2014, Published by Elsevier B.V. 8.4 mean three kinds of BSA-loaded MHANPs; the loading pH is 4.7, 7.4, and 8.4, respectively. Reprinted with permission from ref. 103. Copyright 2014, Published by Elsevier B.V. | ||
In addition, the release behaviours of MHANPs against different proteins are different. In the work of Zhang et al., MHANPs with a rod-shaped length of 75–125 nm, a diameter of about 25 nm, a surface area of 86.29 m2 g−1, a pore volume of 0.27 cm3 g−1, and a pore size of 3.8 nm were synthesized through a hydrothermal method using F127 as the template, and then utilized for BSA and lysozyme (LSZ) delivery (Fig. 29). Generally, the BSA-loaded MHANPs demonstrated a sustained release of BSA for up to 4 days, while the LSZ-loaded HANPs initially exhibited a burst release phenomenon (over 50% of the loaded proteins were released within the first seven hours) and then displayed a slow release. In addition, the two proteins' release curves fit well with the Higuchi model.110
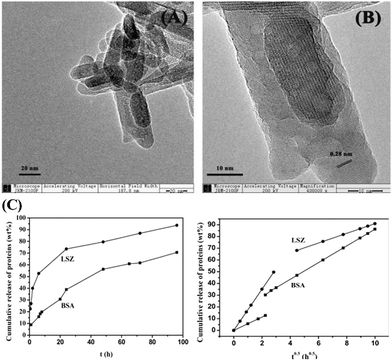 | ||
| Fig. 29 (A) Low-resolution and (B) high-resolution TEM images of as-prepared MHANPs; and (C) release profiles of BSA and LSZ from the HANP samples in SBF at 37 °C and Higuchi plot for the release of proteins from MHANP samples. Reprinted with permission from ref. 110. Copyright 2014, Elsevier B.V. | ||
Several typical examples are presented in the following concerning the applications of MHANP-based drug delivery systems in disease treatment. Feiz et al. fabricated a chitosan/folic acid-modified MHANP for pH-responsive delivery of adenosine 5′-triphosphate (ATP) (termed FA.Cs.ATP@HAP) (Fig. 30A). The FA.Cs.ATP@HAP nanoparticles showed satisfactory colloidal stability and controlled drug release behavior (Fig. 30B) and could be efficiently internalized into the tumor cells in a time-dependent manner and exhibited strong fluorescence within the cells (Fig. 30D). Compared to free ATP, the as-prepared nanoparticles exhibited better inhibition effect against the proliferation of tumor cells. In contrast, no significant cytotoxic effect was observed in the normal cells (Fig. 30C–E).111
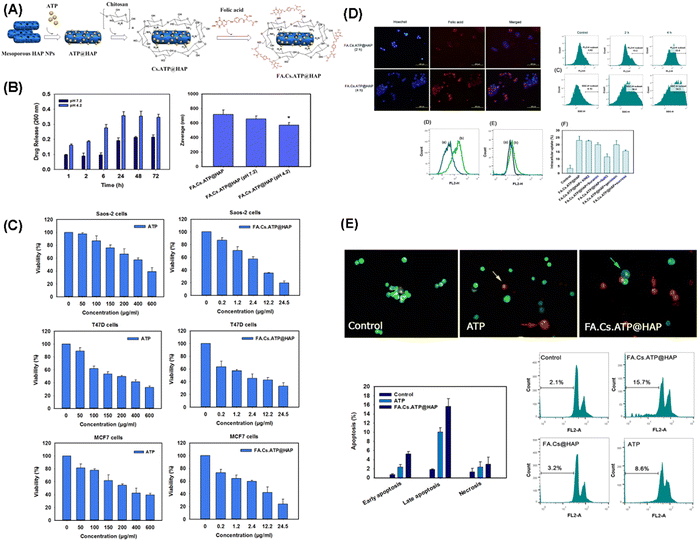 | ||
| Fig. 30 (A) Schematic diagram showing the synthesis of FA.Cs.ATP@HAP nanoparticles; (B) assessment of ATP release from the nanoparticles as a function of time at various pH values; (C) effect of free ATP and FA.Cs.ATP@HAP nanoparticles on the viability of tumor cells; (D) qualitative and quantitative evaluation of the cellular uptake of FA.Cs.ATP@HAP nanoparticles; and (E) induction of apoptosis by free ATP and FA.Cs.ATP@HAP nanoparticles. Reprinted with permission from ref. 111. Copyright 2018, Elsevier B.V. | ||
Meshkini et al. reported a pH-responsive drug delivery system termed MTX-F127@ZnHAP to overcome the resistance of cancer cells to chemotherapeutic agents. The MTX-F127@ZnHAP system is based on the F127 decorated Zn substituted MHANP, which is then conjugated with methotrexate (MTX) through a stable amide linkage (Fig. 31A). The pH-responsive release of Zn2+ and MTX from MTX-F127@ZnHAP effectively potentiates the antitumor efficacy of the drug-loaded nanoparticles, leading to caspase-mediated cell death (Fig. 31B–F).112
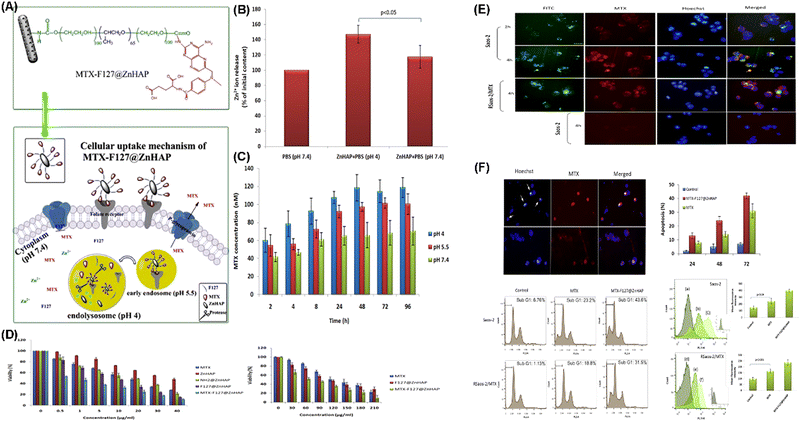 | ||
| Fig. 31 (A) Schematic diagram showing the composition and structure of MTX-F127@ZnHAP nanoparticles and their biological effects on osteosarcoma cells; (B) evaluation of Zn2+ release from Zn substituted MHANPs; (C) assessment of MTX release from the nanoparticles under the simulated lysosomal condition; (D) effect of Zn substituted MHANPs and functionalized nanoparticles on osteosarcoma cell viability; (E) qualitative evaluation of the cellular uptake of MTX-F127@ZnHAP nanoparticles; and (F) induction of cell death in osteosarcoma cells. Reprinted with permission from ref. 112. Copyright 2017, Elsevier B.V. | ||
Izadi et al. developed a superparamagnetic nanocarrier for DOX (F.P.D.MMHAP(2)) by incorporating Fe3O4 nanoparticles into MHANPs and modifying their surface with PEG and folic acid (Fig. 32A). Compared to control groups, the as-developed F.P.D.@MMHAP(2) under a static magnetic field (SMF) exhibited more substantial killing capability against saos-2 cells through the increased ROS level and caspase-3 level (Fig. 32B). In addition, the cellular uptake capability of F.P.D.@MMHAP by saos-2 cells under a SMF was also greatly enhanced (Fig. 32C).113
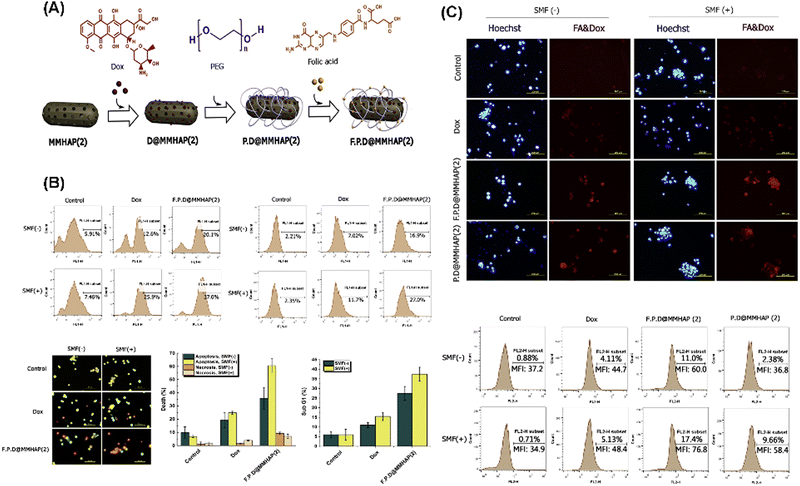 | ||
| Fig. 32 (A) Schematic diagram showing the composition and structure of as-prepared F.P.D.@MMHAP(2); (B) manifestation of the cytotoxic mechanism of F.P.D.@MMHAP(2); and (C) evaluation of the cellular uptake of F.P.D.@MMHAP(2) in the presence and absence of a SMF. Reprinted with permission from ref. 113. Copyright 2019, Elsevier B.V. | ||
Li et al. reported the cancer cell triggered controlled-release gadolinium doped luminescent and mesoporous hydroxyapatite nanorod (Gd: SrHap-aptamer) system utilizing cell-type-specific aptamers as caps (Fig. 33A). Owing to the aptamer, DOX loaded Gd: SrHap (Gd: SrHap-Dox-aptamer) could be internalized explicitly by MFC-7 cells but could not be uptaken by NIH3T3 cells (Fig. 33B); therefore the system has excellent cytocompatibility against normal cells but strong killing capability against cancer cells through nucleolin induced release of DOX (Fig. 33C). In addition, owing to the presence of Gd, Gd: SrHap demonstrated outstanding targeting fluorescence capability and magnetic resonance imaging capability (Fig. 33D).114 Shyong et al. loaded olanzapine into MHANPs (Fig. 34A) for treating depression (Fig. 34C). The olanzapine-loaded MHANPs demonstrated excellent biocompatibility (Fig. 34B) and released the loaded drug in a sustained manner for over 20 h (Fig. 34D). Through the systematic locomotor activity test (Fig. 34E), hippocampus activity test (Fig. 34F), swimming course test (Fig. 34G), and learning/memory test (Fig. 34H), it could be found that the olanzapine loaded MHANPs showed long-acting antidepression capability in rats with induced depression.115 In addition, Shyong et al. also used stearic acid-modified MHANPs for olanzapine loading and delivery for depression treatment. The as-prepared antidepressant carrier could maintain a longer continuous medication release period (over two weeks).116
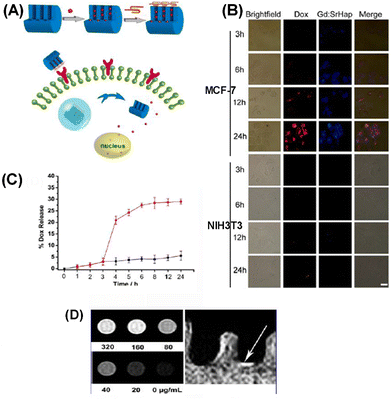 | ||
| Fig. 33 (A) Schematic diagram showing the cancer cell-triggered release of drugs from the pores of Gd: SrHap capped with an aptamer; (B) fluorescence microscope images of MCF-7 cells and NIH3T3 cells incubated with Gd: Sr-Dox-aptamer; (C) Dox release profiles from Gd: SrHap-Dox-aptamer without nucleolin (black) and until t = 3 h when nucleolin was added; and (D) phantom NMR images of Gd: SrHap dissolved in cell lysis solution showing T1-weighted bright contrast. Reprinted with permission from ref. 114. Copyright 2012, American Chemical Society. | ||
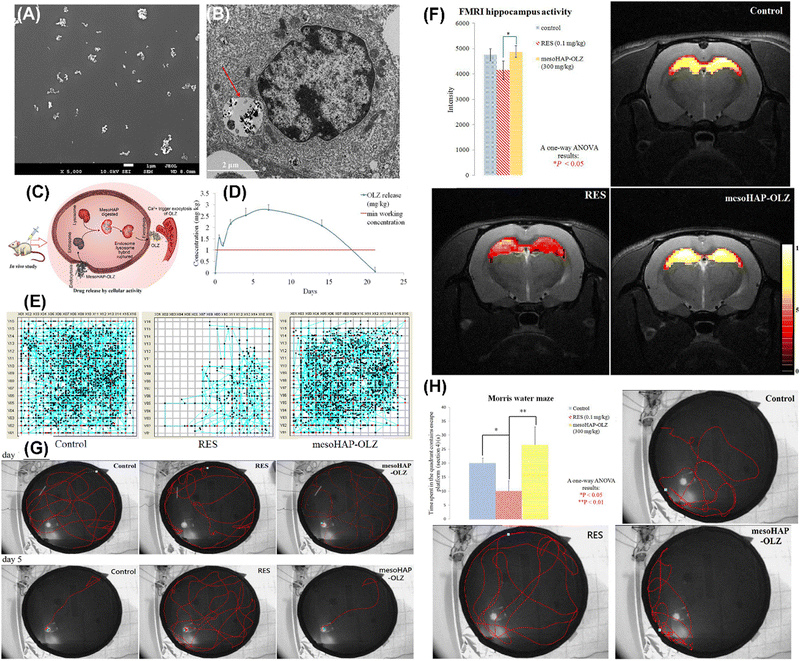 | ||
| Fig. 34 (A) SEM image of MHANPs; (B) TEM image of RAW-264.7 cultured with olanzapine MHANPs; (C) brief illustration of olanzapine loaded MHANPs’ in vivo delivery via intramuscular injection; (D) olanzapine in vivo release curve of olanzapine loaded MHANPs; (E) locomotor activity test, (F) hippocampus activity test, (G) swimming course test, and (H) learning/memory ability test. Reprinted with permission from ref. 116. Copyright 2017, Elsevier B.V. | ||
More examples of application of MHANPs or MHAP in drug delivery and disease therapy are summarized and compiled in Table 4.
| Drug | Functions | Properties of MHANPs | Drug load/release kinetics | Ref. |
|---|---|---|---|---|
| Rhodamine B | Model drug | Prepared by co-precipitation (soft template); 60–80 nm in length, 10–20 nm in width; surface area of 66 m2 g−1 and average pore size of 23 nm | A burst release of about 50% within 1 h, followed by sustained release and complete release after 55 h | 117 |
| Doxorubicin | Cancer treatment | MHANP coated Fe3O4 through a soft template method (F127); particle size smaller than 100 nm, pore diameter of about 2 nm; saturation magnetization value of 20–45 emu g−1 | 93% drug can be loaded into the Fe3O4@MHAP composite; only about 10% drug was released at pH 7.4 after 24 h, while 70% drug was released at pH 5.5 after 24 h | 118 |
| Doxorubicin | Cancer therapy | Prepared by a hydrothermal method (self-template); spherical-like particles with a size below 100 nm or spindle-like with a size of about 200 nm; surface area of 114 m2 g−1 and mean pore sizes between 6 and 26 nm | About 10% of the loaded drug was gradually released for 24 h in a sustained manner (pH 7.4); about 30% of the loaded drug was released for 24 h in a sustained manner (pH 5.5) | 79 |
| Doxorubicin | Cancer therapy | Prepared by a hydrothermal method (soft template); rod-like, surface area of 88 m2 g−1 and average pore size of 15.7 nm | Sustained release for 24 h | 9 |
| Ciprofloxacin | Antibacterial activity against Staphylococcus aureus and Escherichia coli | Prepared through opposite ion core synthesis (hard template); particle size of 500 nm, surface area of 105.3349 m2 g−1, pore size of 3.6 nm, pore volume of 0.533 cm3 g−1, and porosity of 63.45% | 80% drug was released at pH 4.5 within 5 days (Fickian diffusion); a sustained drug release was obtained, and only 48.73% of the drug was released after 9 days at pH 7.4 (anomalous diffusion) | 119 |
| Yttrium-90 | Potential agent to treat osteosarcoma | Prepared through a soft template method and functionalized with PVA and collagen; surface area of 56 m2 g−1, pore diameter of 17.5 nm, and pore volume of 0.086 cm3 g−1 | A capacity of adsorption of about 32% of yttrium element in the PVA/collagen/MHANP matrix, aiming at a concentration of 24 wt% of this element in MHANPs; no significant yttrium amount was released for 120 h | 120 |
| Methylprednisolone acetate | To treat rheumatoid arthritis | Prepared by the co-precipitation method; mean particle size of 70.45 nm, pore size of 2.71 n, surface area of 15.321 m2 g−1, pore volume of 3.520 cm3 g−1 | Drug loading of 44.53%, sustained drug release for 8 h (34%) | 121 |
| Methionine | Cartilage-forming substance | Prepared by a sol–gel method (soft template); Scherrer size of 10 nm, surface area of 66 m2 g−1, average pore size of 5.86 nm | Nearly 80% of the drug was released during the first 12 h, followed by a slow release of the remaining 20% of the drug | 77 |
| Ibuprofen | Model drug | Prepared through a soft template method, doped with Eu3+; rod-like morphology, 20–40 nm in width, 100–200 nm in length; surface area of 54.8 m2 g−1, pore volume of 0.253 cm3 g−1, and average pore size of 10.3 nm; emitting red luminescence under UV irradiation | 44 wt% loading rate of ibuprofen; a burst release of about 50% within 1 h followed by a relatively slow release and complete release after 24 h | 122 |
| Ibuprofen | Anti-inflammation | Prepared by a co-precipitation method (soft template); modified with alendronate; surface area of 215.18 m2 g−1, volume of 0.38 cm3 g−1, and particle size of 2–10 nm | Loading amount of 49.1 wt%; burst release within the first 30 min, and then sustained for 100 min | 43 |
| Ibuprofen | Model drug | Prepared using a hard template method (CaCO3); particle size of 1.169 ± 0.476 μm, surface area of 85 m2 g−1, pore volume of 0.4227 cm3 g−1, and pore size of 20.002 nm | Burst release within the first 3 h and then sustained release for 24 h | 123 |
| Ibuprofen | Model drug | Prepared through a hydrothermal method; substituted with Sr2+; monodisperse nanorods with a length of 120–150 nm, a diameter of around 20 nm, and a mesopore size of 3–5 nm; emitting intense bright blue light; surface area of 70.4 m2 g−1, pore volume of 0.373 cm3 g−1 | Sustained release for 35 h | 82 |
| Catechin | Antioxidant | Prepared through a soft template method (CTAB); surface area of 38.425 m2 g−1, total pore volume of 0.372 cm3 g−1, and average pore size of 3.872 nm; amine-functionalized with 3-aminopropyltriethoxysilane, and then conjugated with catechin through a stable amide linkage | 60 μg g−1 loading amount of catechin, sustained release (<30%) for 72 h | 124 |
| Vancomycin | Anti-infection | Prepared through a sol–gel method (fatty acid, lauric acid, and stearic acid as templates); particle size of 3–6 nm, surface area of 66 m2 g−1 | Rapid release of 50% of the loaded drug during the first 20 h and prolonged release that extended up to 50 h | 48 |
| Vancomycin | Model drug | Prepared through a hydrothermal method (CaCO3/Fe3O4 composite as a template); surface area in the range of 34–67 m2 g−1; pore size distributed from meso- to macro-scale in the range of about 2–100 nm; particle size over 1 μm | Drug delivery amount in the range of 28–35 mg g−1 and drug delivery efficiency in the range of 75–89%; sustained release for 48 h | 54 |
| Vancomycin | Treatment of bone inflammation | Prepared through a hydrothermal method (CTAB as the template); doped with Sr; maximum surface area 78 m2 g−1; pore size in the range of 25 to 40 nm | Sustained release for 70 h | 125 |
| Methotrexate | Inhibiting tumor cell growth and reproduction | Prepared through a hard template method (CaCO3), Au nanoparticles were conjugated onto the surface of as-prepared MHANPs; particle size of 500 nm, surface area of 137.6 m2 g−1, average pore size of 9.4 nm | Drug loading capacity of 14.7%, 30.88% (pH 7.4) and 54.14% (pH 5) of the loaded drug was gradually released within 500 min | 126 |
| Vincristine | Bone cancer therapy | Prepared via a hydrothermal method (soft template); rod-like with a mean size of 285.32 ± 10.29, superficial area of 103.5 m2 g−1, and pore size diameters ranging from 2 to 8 nm | NA | 127 |
| Methotrexate/andrographolide | Epidermoid skin cancer therapy | Prepared via a successive hydrothermal process and a calcination treatment (carboxymethylcellulose as a self-template); spindle-like with a length of 20 nm and an average diameter of 10 nm, surface area of 180 m2 g−1, and pore size distribution in the range of 20–60 nm | 50–55.5% drug loading efficiency at pH 7.0 in PBS; pH-responsive, drug release rate in an acidic environment is much higher than that in a neutral or alkaline environment; sustained release for over 24 h | 80 |
5.3. Applications in bone regeneration/treatment
HAP is one of the major inorganic components of bone tissue, while the main organic component is collagen. Due to this and many excellent characteristics of MHAP, like osteoconductivity, good bioactivity, large surface area, and biocompatibility, researchers’ attention has been drawn to the usage of MHAP in various applications regarding bone tissue.44,128–131 Specifically, MHAP has been employed in four essential areas of bone treatment: drug delivery carrier, osteoconductive filler for bone cement, coating for metallic orthopedic implants, and as a constituent of bone scaffolds.5,44,61,128,130One of the critical areas of application of MHAP in bone regeneration is its usage as a bone grafting material due to its excellent characteristics. However, it was reported that the use of pure MHAP as a bone grafting material has some flaws with respect to mechanical strength as it is hard to easily fit in with the bone tissue in the physical environment. The realization of this fact led to the development of composites of HAP where other materials to improve its grafting ability and fitting capability. For example, Zhang et al. fabricated a chitosan/MHAP composite and noted that the introduction the bioactive polymer enhanced osteoblasts' survival rate. They also reported that osteoblast differentiation and matrix mineralization led to excellent osteoinduction and osteoconduction.44 Li et al. homogeneously deposited MHANPs onto carbon nanotubes (CNTs) through a soft template method (Fig. 35A). By doing this, efficient load transfer between MHANPs and CNTs was realized, and the interfacial bonding was therefore greatly enhanced. In addition, the high surface area and large surface roughness promote cell adhesion and proliferation.128 In the work of Li et al., the MHANP was surface-modified with poly(γ-benzyl-L-glutamate) (PBLG) of different amounts (from 11 to 50 wt%) through the in situ ring-opening polymerization of γ-benzyl-L-glutamate N-carboxy anhydride (BLG-NCA) (Fig. 35B). Then, porous scaffolds of PBLG-g-MHANP/PLGA were fabricated via a solvent casting/particulate leaching operation. The micro-CT data showed that the as-prepared tissue engineering scaffold significantly accelerated the in situ bone regeneration (Fig. 35C).47
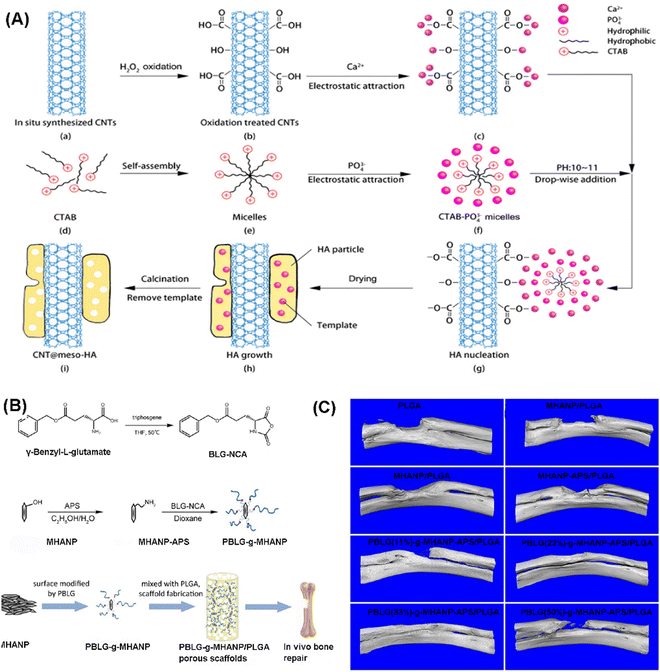 | ||
| Fig. 35 (A) Schematic diagram showing the deposition of MHANPs onto carbon nanotubes; (B) schematic diagram showing the preparation process of the PBLG-g-MHANP and pblg-g-MHANP/PLGA porous scaffold; (C) Micro-CT 3D reconstruction images of rabbit radius defects implanted with as-prepared scaffolds. Reprinted with permission from ref. 47. Copyright 2019, American Chemical Society. | ||
Furthermore, MHAP has been greatly useful as a drug and protein delivery system for bone cancer treatment and subsequent bone regeneration. Cipreste et al. developed a hybrid and functionalized system of polyvinyl alcohol, collagen, and MHNP that was prepared and used as a carrier system to load and release yttrium-90.59 The functionalization and incorporation of the radionuclides improved the fabricated system with enhanced permeability and retention. Coupled with the extremely slow release of harmful yttrium-90 and the excellent radioactivity result with no radionuclide losses in the matrix, the system has proven to be a superb radioisotope carrier material.59 This aligns with Maia et al.'s research, where they loaded MHANPs with vincristine for bone cancer treatment. The as-prepared nanoparticles have shown significant advantages as excellent drug carriers, including biocompatibility, non-immunogenic property, and proven ability to infuse bone tissues easily. Upon their incorporation, the vincristine loaded MHANPs showed an antiangiogenic effect which was revealed through the reduction in the number of vessels.60 Aside from the above-described two significant applications of MHAP in the field of bone engineering, there have been a few reports about its usage as a potential osteoconductive filler in the fabrication of bone cement. Bone cement has been used over the years to treat fractures and fix a functioning orthopedic prosthesis.131
5.4. Others
In addition to the abovementioned applications, MHAP and MHANPs have also been used in some particular scientific areas. For instance, there has been some research on the application of MHAP as a catalyst in some essential chemical processes. The ion exchangeability of the nanomaterial has made it one of the crucial catalytic supports, especially with some basic metal cations and anions. The availability of Ca2+ as a component of MHANPs made it easier for it to be substituted or exchanged with several transition metals. The use of two metals has been reported to enhance this catalytic activity.92 Radhakrishnan et al. reported their research where they produced MHAP. They infused it with other metals like Au, Ag, and Pd, leading to the production of either monometallic or bimetallic catalysts. These metallic doped MHANPs, alongside the bare MHAP, were subjected to catalytic reaction using TBHP as an oxidant and methanol as a solvent towards the esterification of furfural to methyl 2-furoate. They all proved to be effective catalysts; however, one of the bimetallic MHANPs doped with 80% Au and 20% Pd showed excellent catalytic actions with 94.2% conversion of furfural. They also reported proof that the catalysts are stable and can be easily reused.132 Bharath and Ponpandian also worked in a similar area and reported a novel MHAP/α-Fe2O3 composite, which showed great photocatalytic activities toward eliminating organic compounds from contaminated wastewater.133Bioimaging is a very distinct technique in the biomedical sciences involving processes like morphological analysis of anatomical structures, sensitive measurements of intracellular movements, and quantitative visualization of molecular processes, among many others.75,134 Kevin et al. reported that they successfully linked a fluorescent dye called fluorescein isothiocyanate (FITC) to the surface of hollow MHANPs for intracellular bioimaging in BT-20 cancer cells without showing any severe cytotoxicity.4 This was also corroborated by Loo et al. They reported that a fluorescent dye can be used as a tracking device for nanoparticles and that the encasement of the imaging molecules, like the dyes, only requires the presence of the molecules during the formation of the nanoparticle since it is a relatively straightforward reaction. Regarding far more information about the applications of MHANPs in bioimaging, the readers could refer to the review work “Multifunctional hydroxyapatite nanoparticles for drug delivery and multimodal molecular imaging” by Syamchand et al.135
MHANPs have also been successfully introduced into the dialysis system by Ooi et al. for the removal of p-cresol since they exhibit similar composition and functionalities to the blood-compatible tissue of the bone, and they were also noted to have no adverse effects on the patients.69,92
6. Conclusions and perspectives
This review work was carried out to cover two significant angles. The first one is compiling all the currently available strategies for MHAP synthesis, classifying them into three categories (i.e., hard template method, soft template method, and template-free method) and summarizing their basic features, and comparing the main advantages and disadvantages of the three types of strategies. The second introduces the main applications of MHAP from three aspects: wastewater treatment, disease therapy, and bone tissue engineering.As to the hard template method, the templates that could be used are scarce; the commonly used templates are CaCO3 and carbon materials. This method features straightforwardness, simplicity in the mode of synthesis, lesser resources, and lesser cost of production. By changing the structure and morphology of the hard templates used, the pore structure and shape of as-prepared MHANPs could be easily modified. In addition, in addition to tiny mesoporous passageways, the as-prepared MHANPs commonly have a large hollow central cavity. However, they also have some fatal defects, such as non-uniform morphology, unequal size, inferior crystallinity, and low phase purity, which are mainly caused by the template removal process. CaCO3 templates are commonly removed through an acid etching process. However, HAP is also dissolvable in aqueous acid solution, which inevitably undermines the crystallinity, morphology, phase purity, size distribution, and mesoporous structure of as-prepared MHANPs. Carbon templates are commonly removed through a facile dissolution route, so they are prone to be left within the interior of as-prepared MHANPs, which, therefore, poses a negative influence on the pore size and pore structure of the MHANPs. In this regard, advanced hard templates, which not only can be easily removed but also have negligible influence on the physical/chemical properties of MHANPs in the removal process, should be developed in the future. In our opinion, some hard substances which are not dissolvable in water or slightly dissolvable in water but could be quickly sublimated through heating (e.g., naphthalene spheres, sulfur powder, benzoic acid powder, anthracene powder) or could be wholly oxidized into CO2 and H2O through burning in the air (e.g., poly(styrene) sphere and its surface modified products) can serve as ideal hard templates for MHANP synthesis. Since MHANPs need to be in nanoscale, the size of templates used should also be in nanoscale or less. Thus, processing these hard substances into nanosized templates with narrow size distribution may be the main challenge of using these hard substances for MHANP synthesis. The smallest size of commercially available poly(styrene) sphere is close to 100 nm, but its fancy price is another concern that should be considered carefully. In addition to the above-mentioned hard templates, another category of materials, metal–organic frameworks (MOFs), are also potential candidates as hard templates for MHANP synthesis. MOFs are a rapidly growing class of hybrid crystalline porous materials and have been applied in diverse fields.136 They are in the nanoscale and have an extremely narrow size distribution. In addition, the size and morphology of MOFs could be adjusted on demand by changing the synthesis temperature, solvents, reaction time, etc. As mentioned before, the size and morphology of MHANPs are the functions of the size and shape of hard templates. Thus, diverse MHANPs with different morphologies and sizes, in principle, could be fabricated using MOFs as hard templates. For instance, the size of zeolitic imidazolate framework-8 (ZIF) ranges from dozens of nanometers to a few micrometers,137,138 and it demonstrates different morphologies, including octahedral, flower-like, flake-like, etc.139 Moreover, the surface chemistry of MOFs could be modified through a post-modification process. For instance, MOF-Leiden Institute of Chemistry (MOF-LIC-1) has large NH2 groups, while ZIF-90 has large CHO groups on its surface. Diverse molecules or molecular chains could be grafted onto their surface through Schiff's base reaction.140,141 These introduced molecules or molecular chains could serve as active sites to mediate the nucleation and growth of HAP crystals. The best way to remove MOFs might be sintering, during which the organic ligands are oxidized into CO2 and H2O, which could also serve as porogenic agents. At the same time, the metal ions are converted into metallic oxidizers and left within the as-prepared MHANPs.
Concerning the soft template method, rich templates could be applied (e.g., surfactants, polymers, and small molecules), and this method could be realized through diverse routes, including the hydrothermal method, sol–gel method, co-precipitation method, etc. The as-prepared MHANPs usually have ordered mesoporous passageways, and their size uniformity and crystallinity are much higher than those fabricated using a hard template method. By adjusting the composition of soft templates (either single template or binary template), the structure of final MHANPs could be controlled, and diverse morphologies, shapes, and sizes could be obtained. In addition, some soft templates could also serve as pore-expanding agents, broadening the pore size of final MHANPs—the advantages of the soft template route over other routes. However, because of the smaller 3D particle size of a soft template than a hard template, the as-prepared MHANPs, compared to those from hard template synthesis, don’t have a hollow mesoporous cavity, and their pore size, in principle, is much smaller. In this regard, we assumed that synergistically utilizing hard and soft templates for synthesizing MHANPs might be a good option. For instance, we can modify the surface of a CaCO3 template or a MOF template with a soft template through covalent grafting or non-covalent interaction and then use the as-prepared templates for MHAP synthesis. To obtain large hollow cavities in the center of MHANPs, large-sized supramolecular aggregates or polymer entanglements/crystallites could also be used as soft templates for MHAP synthesis. As to the template-free method, neither soft nor hard templates are needed in the whole preparation process of MHANPs. Therefore, no template extraction procedure is required, significantly simplifying the whole process. The template-free method commonly needs the assistance of microwave treatment; therefore, a large amount of energy consumption is indispensable. However, owing to the absence of a template, the template-free method loses its advantage in mediating the morphology, size distribution, and size uniformity of MHANPs. Since the mesopore-creating mechanism of the template-free method is entirely different from that of the template method, synergistically utilizing the template and template-free method for MHAP synthesis might endow them with a much more complex mesoporous structure and much richer physical/chemical properties. Currently, the soft template method might be a better strategy for MHAP synthesis compared to the other two categories of methods. For one thing, innumerable soft templates could be utilized for fabricating MHANPs. As mentioned before, the particle size, morphology, pore structure, pore size, and pore size distribution of as-prepared MHANPs are functions of the type of template used. Therefore, various MHANPs could be obtained by changing the soft template's composition. For another thing, the different operating procedures of the soft template method, in principle, could endow the final MHANPs with much richer structural features. However, based on our analysis, we held that synergistically utilizing the hard template method and the soft template method or synergistically utilizing the template method and the template-free method might be a more promising future strategy.
This review discusses three significant applications of MHANPs, including wastewater treatment, disease therapy, and bone tissue engineering. Two kinds of toxic or harmful substances in water could be dealt with by MHANPs: heavy metal ions and organic contaminants. Three main mechanisms could explain the adsorption of heavy metal ions in water by MHANPs: ion exchange, formation of ion complexes on the surface, and precipitation. The formation of heavy metal ion complexes on the surface of MHANPs commonly occurs by the interaction between heavy metal ions and phosphate and hydroxyl groups of MHANPs through hydrogen bonding. Removing heavy metal ions in water is less efficient because of the weak hydrogen bonding interactions. In addition, MHAP-induced precipitation of heavy metal ions is also far from satisfactory because their removal efficiency highly relies on the precipitation reaction between PO43− and heavy metal ions. However, MHANPs demonstrate poor dissolution capability in water. Therefore they could only release limited PO43−. Thus, limited progress, to the best of our knowledge, could be made to improve the adsorption capability of MHANPs against heavy metal ions by forming heavy ion complexes on the surface of MHANPs or precipitation in the future. The ion exchange capability of MHANPs against heavy metal ions is determined by the rate of vacant Ca lattice sites being substituted by heavy metal ions. Thus, the key points to enhance the exchange capability of MHANPs against heavy metal ions include increasing the number of vacant Ca lattice sites and exposing them to the dissociative heavy metal ions. Commonly, ion doping or hybridization with other metal oxides could change the crystal composition/structure of MHANPs, driving more calcium ions to leave their lattice sites. In addition, precise control over the morphology, crystallinity, size, and surface area of MAHPs through adjusting the templates used, synthesis conditions, etc., could expose more Ca lattice sites, enhancing their exchange capability against heavy metal ions in water. However, not all heavy metal ions could easily occupy the vacant Ca lattice sites of MHANPs. Due to their valence state and ionic radii differences, heavy metal ions may have different exchange capabilities against the Ca2+ of MHANPs (i.e., selectivity). Thus, the exchange selectivity of MHANPs against different heavy metal ions should be investigated in detail in the future. Removing organic contaminants from wastewater commonly depends on an oxidation/reduction process, through which the organic contaminants can be transformed into environmentally friendly substances. MHANPs have no oxidation/reduction capability, but their mesoporous structure could serve as the ideal support for diverse catalysts, forming efficient catalytic active centers for the degradation of organic contaminants. However, until now, only a few related materials have been reported. This might be because of their inferior catalytic degradation capability against organic contaminants compared to other advanced catalysts, including ZnO, TiO, MOF, etc. Therefore, using MHANPs to remove organic contaminants from wastewater isn’t a promising strategy in the future.
MHANPs feature nanosize, biocompatibility, biodegradability, and cargo delivery capability and could be easily internalized by cells. These characteristics promote the applications of MHANPs in disease treatment. In this work, we have reported the applications of MHANP-based cargo delivery systems in the therapy of bone-related diseases, cancer therapy, anti-oxidation, anti-infection, and anti-inflammation. MHANPs have been successfully applied for loading and delivering small-sized drug molecules. Generally, the loading of these drugs in MHANPs is through the physical adsorption of drugs in the mesoporous pores of MHANPs. In contrast, drug release is realized through the desorption of drug molecules from mesoporous pores. However, in some cases, the drug-release behavior should be stimulus-responsive or be controlled on demand. For instance, in cancer therapy, the drug delivery behavior should be acid-responsive. In the tissue regeneration process, the drug delivery time should be prolonged and fit well with the tissue regeneration period. Thus, in the future, one research focus is on MHANP-based drug delivery systems’ controlled or responsive release of drugs. Researchers have attempted various pre-synthesis modification or post-synthesis modification strategies to achieve this goal. For instance, they have incorporated magnetic nanoparticles into MHANPs to endow them with magnetic-responsive drug delivery capability or modified the surface of MHANPs with poly(N-isopropyl acrylamide) to endow them with heat-responsive drug delivery capability. In addition to the two examples mentioned above, we also assumed that modifying the surface of MHANPs with folic acid molecules would endow them with tumor-targeted drug delivery capability. In the future, we believe more advanced responsive or controlled drug delivery systems based on modified MHANPs could be developed, enabling them to find much more diverse and advanced applications in biomedical engineering.
Currently, large-sized biological factors (e.g., proteins, DNA, and RNA) have demonstrated huge treatment potential against diverse diseases. For instance, RNA vaccines have been successfully used for preventing COVID-19 in the USA. Because of the size limitation of the mesopores of MHANPs, large-sized biological factors can only be absorbed onto the surface of MHANPs instead of being incorporated into their mesoporous passages. Thus, another research focus related to MHANP-based drug delivery systems is efficient loading and release of large-sized biological factors. To achieve this goal, much attention should be paid to expanding the pore size of MHANPs in the future. Methods to increase the pore size of MHANPs are discussed in detail in the above paragraphs.
HAP is the main inorganic component of living bone and has excellent osteoconductivity and mechanical strength. Thus, HAP has been processed into diverse scaffolds or cement for bone regeneration and repair. However, HAP is not osteogenic, while bone regeneration still needs the assistance of osteogenic factors. Thus, as to the application of MHANPs in bone tissue engineering, the critical point lies in how to improve their osteogenic properties. Ion doping (Mg, Zn, Sr, Si, etc.) and surface modification with osteogenic molecules have been extensively reported to improve HAP's osteogenic properties. Owing to the mesoporous structure of MHANPs, growth factors (e.g., bone morphogenetic protein and vascular endothelial growth factor) and osteogenic drugs (e.g., icariin and dexamethasone) could be loaded to enhance their osteogenic properties. Besides, bone regeneration induced by biomaterial-mediated immunoregulation, which could be realized through cytokines or drugs, or by changing the size, morphology, mechanical strength, toughness, etc. of biomaterials, has drawn tremendous attention.142,143 We believe it would be interesting to investigate the immunoregulation capability of MHANPs or MHANP-based drug delivery systems and their subsequent immunoregulation-mediated bone regeneration properties.
Conflicts of interest
There are no conflicts to declare.References
- U. Sabu, G. Logesh, M. Rashad, A. Joy and M. Balasubramanian, Microwave assisted synthesis of biomorphic hydroxyapatite, Ceram. Int., 2019, 45, 6718–6722 CrossRef CAS.
- S. Hajimirzaee, S. Chansai, C. Hardacre, C. E. Banks and A. M. Doyle, Effects of surfactant on morphology, chemical properties and catalytic activity of hydroxyapatite, J. Solid State Chem., 2019, 276, 345–351 CrossRef CAS.
- W. Wenxin, I. Abdul-Rauf, Z. Deng, W. Hongtao and L. Jun, Conversion of waste eggshells to mesoporous hydroxyapatite nanoparticles with high surface area, Mater. Lett., 2013, 110, 195–197 CrossRef.
- Y.-H. Yang, C. W. Kevin, L. Yung-He, C. Hui-Yuan, S. Eric, Y. Yusuke and L. Feng-Huei, Facile Synthesis of Hollow Mesoporous Hydroxyapatite Nanoparticles for Intracellular Bio-imaging, Curr. Nanosci., 2011, 7, 926–931 CrossRef.
- T. Gao, Z. Ning, W. Tu, W. Zongliang, Z. Peibiao and L. Jianguo, Environmental pH-controlled loading and release of protein on mesoporous hydroxyapatite nanoparticles for bone tissue engineering, Mater. Sci. Eng., C, 2015, 46, 158–165 CrossRef.
- H. C. Wu, J. Y. Lin and T. W. Wang, Development of Mesoporous Magnetic Hydroxyapatite Nanocrystals, Mater. Sci. Forum, 2018, 916, 161–165 Search PubMed.
- N. F. Mohammad, R. Othman and F. Y. Yeoh, The Effect of Surfactant Extraction Method on Pore Characteristics of Mesoporous Carbonated Hydroxyapatite, Adv. Mater. Res., 2013, 858, 190–198 Search PubMed.
- A. Fihri, C. Len, R. S. Varma and A. Solhy, Hydroxyapatite: A review of syntheses, structure and applications in heterogeneous catalysis, Coord. Chem. Rev., 2017, 347, 48–76 CrossRef CAS.
- H. Zhou, Y. Yang, M. Yang, W. Wang and Y. Bi, Synthesis of mesoporous hydroxyapatite via a vitamin C templating hydrothermal route, Mater. Lett., 2018, 218, 52–55 CrossRef CAS.
- I. Khan, K. Saeed and I. Khan, Nanoparticles: Properties, applications and toxicities, Arabian J. Chem., 2019, 12, 908–931 CrossRef CAS.
- G. S. Kumar, G. Karunakaran, E. K. Girija, E. Kolesnikov, N. V. Minh, M. V. Gorshenkov and D. Kuznetsov, Size and morphology-controlled synthesis of mesoporous hydroxyapatite nanocrystals by microwave-assisted hydrothermal method, Ceram. Int., 2018, 44, 11257–11264 CrossRef CAS.
- A. M. Doyle, K. Joanna, J. K. Peter and E. B. Craig, Novel Synthesis of Mesoporous Hydroxyapatite using Carbon Nanorods as a Hard-Template, Ceram. Int., 2017, 43, 5412–5416 CrossRef.
- M. I. Kay, R. Young and A. Posner, Crystal structure of hydroxyapatite, Nature, 1964, 204, 1050–1052 CrossRef CAS.
- M. U. Munir, A. Ihsan, Y. Sarwar, S. Z. Bajwa, K. Bano, B. Tehseen, N. Zeb, I. Hussain, M. T. Ansari, M. Saeed, J. Li, M. Z. Iqbal, A. Wu and W. S. Khan, Hollow mesoporous hydroxyapatite nanostructures; smart nanocarriers with high drug loading and controlled releasing features, Int. J. Pharm., 2018, 544, 112–120 CrossRef CAS.
- N. Y. Mostafa and P. W. Brown, Computer simulation of stoichiometric hydroxyapatite: Structure and substitutions, J. Phys. Chem. Solids, 2007, 68, 431–437 CrossRef CAS.
- S. Koutsopoulos, Synthesis and characterization of hydroxyapatite crystals: a review study on the analytical methods, J. Biomed. Mater. Res., 2002, 62, 600–612 CrossRef CAS.
- Y. H. Yang, C. H. Liu, Y. H. Liang, F. H. Lin and K. C. Wu, Hollow mesoporous hydroxyapatite nanoparticles (hmHANPs) with enhanced drug loading and pH-responsive release properties for intracellular drug delivery, J. Mater. Chem. B, 2013, 1, 2447–2450 RSC.
- J. Chen, Z. Wang, Z. Wen, S. Yang, J. Wang and Q. Zhang, Controllable self-assembly of mesoporous hydroxyapatite, Colloids Surf., B, 2015, 127, 47–53 CrossRef CAS PubMed.
- W. P. S. L. Wijesinghe, M. M. M. G. P. G. Mantilaka, T. A. N. Peiris, R. M. G. Rajapakse, K. G. U. Wijayantha, H. M. T. G. A. Pitawala, T. N. Premachandra, H. M. T. U. Herath and R. P. V. J. Rajapakse, Preparation and characterization of mesoporous hydroxyapatite with non-cytotoxicity and heavy metal adsorption capacity, New J. Chem., 2018, 42, 10271–10278 RSC.
- I. P. Jalil, A. A. Negar, M. Hatamian, M. Arabfirouzjaei, J. Javadpour and M. Rashidi, Doxorubicin loaded large-pore mesoporous hydroxyapatite coated superparamagnetic Fe3O4 nanoparticles for cancer treatment, Int. J. Pharm., 2016, 509, 159–167 CrossRef.
- M. Sadat-Shojai, M.-T. Khorasani, E. Dinpanah-Khoshdargi and A. Jamshidi, Synthesis methods for nanosized hydroxyapatite with diverse structures, Acta Biomater., 2013, 9, 7591–7621 CrossRef CAS PubMed.
- M. Sadat-Shojai, M.-T. Khorasani and A. Jamshidi, Hydrothermal processing of hydroxyapatite nanoparticles—A Taguchi experimental design approach, J. Cryst. Grow., 2012, 361, 73–84 CrossRef CAS.
- A. Lak, M. Mazloumi, M. Mohajerani, A. Kajbafvala, S. Zanganeh, H. Arami and S. Sadrnezhaad, Self-Assembly of Dandelion-Like Hydroxyapatite Nanostructures Via Hydrothermal Method, J. Am. Ceram. Soc., 2008, 91, 3292–3297 CrossRef CAS.
- A. Zhu, Y. Lu, Y. Si and S. Dai, Frabicating hydroxyapatite nanorods using a biomacromolecule template, Appl. Surf. Sci., 2011, 257, 3174–3179 CrossRef CAS.
- S. Mann, D. D. Archibald, J. M. Didymus, T. Douglas, B. R. Heywood, F. C. Meldrum and N. J. Reeves, Crystallization at Inorganic-organic Interfaces: Biominerals and Biomimetic Synthesis, Science, 1993, 261, 1286–1292 CrossRef CAS.
- F. Nudelman, K. Pieterse, A. George, P. H. H. Bomans, H. Friedrich, L. J. Brylka, P. A. J. Hilbers, G. de With and N. A. J. M. Sommerdijk, The role of collagen in bone apatite formation in the presence of hydroxyapatite nucleation inhibitors, Nat. Mater., 2010, 9, 1004–1009 CrossRef CAS.
- K. Sato, in Biomineralization I: Crystallization and Self-Organization Process, ed. K. Naka, Springer Berlin Heidelberg, Berlin, Heidelberg, 2007, pp. 127–153 DOI:10.1007/128_075.
- R. Zhu, R. Yu, J. Yao, D. Wang and J. Ke, Morphology control of hydroxyapatite through hydrothermal process, J. Alloys Compd., 2008, 457, 555–559 CrossRef CAS.
- F. Ye, H. Guo and H. Zhang, Biomimetic synthesis of oriented hydroxyapatite mediated by nonionic surfactants, Nanotechnology, 2008, 19, 245605 CrossRef.
- X. Liu, K. Lin, R. Qian, L. Chen, S. Zhuo and J. Chang, Growth of Highly Oriented Hydroxyapatite Arrays Tuned by Quercetin, Chem. – Eur. J., 2012, 18, 5519–5523 CrossRef CAS PubMed.
- Q. Lin, D. Huang, J. Du, Y. Wei, Y. Hu, X. Lian, X. Xie, W. Chen and Y. S. Zhang, Nano-hydroxyapatite crystal formation based on calcified TiO2 nanotube arrays, Appl. Surf. Sci., 2019, 478, 237–246 CrossRef CAS.
- B. Cao and C. Mao, Oriented Nucleation of Hydroxylapatite Crystals on Spider Dragline Silks, Langmuir, 2007, 23, 10701–10705 CrossRef CAS PubMed.
- M. Xu, T. Liu, M. Qin, Y. Cheng, W. Lan, X. Niu, Y. Wei, Y. Hu, X. Lian and L. Zhao, Bone-like hydroxyapatite anchored on alginate microspheres for bone regeneration, Carbohydr. Polym., 2022, 287, 119330 CrossRef CAS PubMed.
- D. Huang, M. Yin, Q. Lin, Y. Qin, Y. Wei, Y. Hu, X. Lian, M. Guo, J. Du and W. Chen, Aligned hydroxyapatite nano-crystal formation on a polyamide surface, RSC Adv., 2017, 7, 43040–43046 RSC.
- M. F. Cipreste and E. M. B. Sousa, Poly(Vinyl Alcohol)/Collagen/Hydroxyapatite Nanoparticles Hybrid System Containing Yttrium-90 as a Potential Agent to Treat Osteosarcoma, J. Biomater. Nanobiotechnol., 2014, 05, 24–30 CrossRef.
- N. Maleki-Dizaji, J. Samira, B. Jaleh, B. J. Mohammad, R. Maryam and A. Khosro, Methylprednisolone acetate-loaded hydroxyapatite nanoparticles as a potential drug delivery system for treatment of rheumatoid arthritis: In vitro and in vivo evaluations, Eur. J. Pharm. Sci., 2016, 91, 225–235 CrossRef PubMed.
- T. Lei, W. Tao, C. Ning, Y. Qi, Z. Yu-Feng, L. Chuan-Jun and S. Bin, PNIPAAM modified mesoporous hydroxyapatite for sustained osteogenic drug release and promoting cell attachment, Mater. Sci. Eng., C, 2016, 62, 888–896 CrossRef PubMed.
- T. Wu, L. Tan, N. Cheng, Q. Yan, Y.-F. Zhang, C.-J. Liu and B. Shi, PNIPAAM modified mesoporous hydroxyapatite for sustained osteogenic drug release and promoting cell attachment, Mater. Sci. Eng., C, 2016, 62, 888–896 CrossRef CAS PubMed.
- J. A. Lett, M. Sundareswari, K. Ravichandran, M. B. Latha, S. Sagadevan and M. R. Bin Johan, Tailoring the morphological features of sol–gel synthesized mesoporous hydroxyapatite using fatty acids as an organic modifier, RSC Adv., 2019, 9, 6228–6240 RSC.
- X. Huang, L. Dalong, W. Yadong, L. Jiwel, C. Weilu, H. Jinmei, T. Huayu and H. Yudong, Preparation of pH-responsive mesoporous hydroxyapatite nanoparticles for ingtracellualr controlled release of an anticancer drug, J. Biomater. Sci., Polym. Ed., 2015, 1–7 Search PubMed.
- J. Qian, T. Liang and Y. Yuan, Synthesis of mesoporous hydroxyapatite nanoparticles using a template-free sonochemistry-assisted microwave method, J. Mater. Sci., 2013, 48, 5334–5341 CrossRef.
- Y. Mengmeng, Z. Huan, H. Saisai and D. Linhong, Mesoporous hydroxyapatite nanoparticles hydrothermally synthesized in aqueous solution with hexametaphosphate and tea polyphenols, Mater. Sci. Eng., C, 2016, 71, 439–445 Search PubMed.
- D. Li, Y. Zhu and Z. Liang, Alendronate functionalized mesoporous hydroxyapatite nanoparticles for drug delivery, Mater. Res. Bull., 2013, 48, 2201–2204 CrossRef CAS.
- J. Zhang, G. Liu, Q. Wu, J. Zuo, Y. Qin and J. Wang, Novel mesoporous hydroxyapatite/chitosan composite for bone repair, J. Bionic. Eng., 2012, 9, 243–251 CrossRef.
- L. Bakhtiari, J. Javadpour, H. R. Rezaie, M. Erfan and M. A. Shokrgozar, The effect of swelling agent on the pore characteristics of mesoporous hydroxyapatite nanoparticles, Prog. Nat. Sci.: Mater. Int., 2015, 25, 185–190 CrossRef CAS.
- E. Sistanipour, A. Meshkini and H. Oveisi, Catechin-conjugated mesoporous hydroxyapatite nanoparticle: A novel nano-antioxidant with enhanced osteogenic property, Colloids Surf., B, 2018, 169, 329–339 CrossRef CAS PubMed.
- L. Li, X. Shi, Z. Wang, M. Guo, Y. Wang, Z. Jiao and P. Zhang, Porous Scaffolds of Poly (lactic-co-glycolic acid) and Mesoporous Hydroxyapatite Surface Modified by Poly (γ-benzyl-l-glutamate)(PBLG) for in Vivo Bone Repair, ACS Biomater. Sci. Eng., 2019, 5, 2466–2481 CrossRef CAS PubMed.
- J. A. Lett, S. Sagadevan, J. J. Prabhakar, N. A. Hamizi, I. A. Badruddin, M. R. Johan, A. R. Marlinda, Y. Abdul Wahab, T. M. Yunus Khan and S. Kamangar, Drug leaching properties of vancomycin loaded mesoporous hydroxyapatite as bone substitutes, Processes, 2019, 7, 826 CrossRef CAS.
- S. Loo, T. Moore, B. Banik and F. Alexis, Biomedical applications of hydroxyapatite nanoparticles, Curr. Pharm. Biotechnol., 2010, 11, 333–342 CAS.
- X. Xia, J. Chen, J. Shen, D. Huang, P. Duan and G. Zou, Synthesis of hollow structural hydroxyapatite with different morphologies using calcium carbonate as hard template, Adv. Powder Technol., 2018, 29, 1562–1570 CrossRef CAS.
- P. Julien, M. S. Janina, A. Karine and V. G. Cathie, Mesoporous Hydroxyapatite by hard templating of silica and carbon foams for protein release, J. Mater. Sci., 2013, 48, 3722–3730 CrossRef.
- J. J. Leila Bakhtiari, H. Reza Rezaie, M. Erfan, B. Mazinani and A. Aminian, Pore size control in the synthesis of hydroxyapatite nanoparticles: The effect of pore expander content and the synthesis temperature, Ceram. Int., 2016, 42, 11259–11264 CrossRef.
- Y.-J. Guo, Y.-Y. Wang, T. Chen, Y.-T. Wei, L.-F. Chu and Y.-P. Guo, Hollow carbonated hydroxyapatite microspheres with mesoporous structure: hydrothermal fabrication and drug delivery property, Mater. Sci. Eng., C, 2013, 33, 3166–3172 CrossRef CAS PubMed.
- K. Lin, L. Chen, P. Liu, Z. Zou, M. Zhang, Y. Shen, Y. Qiao, X. Liu and J. Chang, Hollow magnetic hydroxyapatite microspheres with hierarchically mesoporous microstructure for pH-responsive drug delivery, CrystEngComm, 2013, 15, 2999–3008 RSC.
- W. Wijesinghe, M. Mantilaka, T. N. Peiris, R. Rajapakse, K. U. Wijayantha, H. Pitawala, T. Premachandra, H. Herath and R. Rajapakse, Preparation and characterization of mesoporous hydroxyapatite with non-cytotoxicity and heavy metal adsorption capacity, New J. Chem., 2018, 42, 10271–10278 RSC.
- J. Kamieniak, A. M. Doyle, P. J. Kelly and C. E. Banks, Novel synthesis of mesoporous hydroxyapatite using carbon nanorods as a hard-template, Ceram. Int., 2017, 43, 5412–5416 CrossRef CAS.
- Z.-L. Liu, Q.-Y. Jia, X.-D. Li, S.-P. Li, J. Shen, J. Lin and D.-X. Li, Synthesis of hollow mesoporous HAp-Au/MTX and its application in drug delivery, Colloids Surf., A, 2020, 586, 124231 CrossRef CAS.
- N. Pramanik and T. Imae, Fabrication and characterization of dendrimer-functionalized mesoporous hydroxyapatite, Langmuir, 2012, 28, 14018–14027 CrossRef CAS PubMed.
- M. F. Cipreste and E. M. Sousa, Poly(Vinyl Alcohol)/Collagen/Hydroxyapatite Nanoparticles Hybrid System Containing Yttrium-90 as a Potential Agent to Treat Osteosarcoma, J. Biomater. Nanobiotechnol., 2014, 5, 24–30 CrossRef.
- F. C. de Aguiar, A. L. Maia, L. B. André, T. M. Aline, A. R. Gilson, D. C. Armando, C. P. Diogo, F. Christian and D. C. Soares, Vincristine-loaded hydroxyapatite nanoparticles as a potential delivery system for bone cancer therapy, J. Drug Targeting, 2017, 26, 592–603 Search PubMed.
- N. Pramanik and T. Imae, Fabrication and characterization of dendrimer-functionalized mesoporous hydroxyapatite, Langmuir, 2012, 28, 14018–14027 CrossRef CAS PubMed.
- Y. F. Zhao and J. Ma., Co-synthesis and Drug Delivery Properties of Mesoporous Hydroxyapatite-silica Composites, J. Nanosci. Nanotechnol., 2009, 9, 3720–3727 CrossRef CAS PubMed.
- H. Jinmei, L. Dalong, H. Xin, L. Jiwei, T. Huayu, C. Xuesi and H. Yudong, Intracellular pH-responsive mesoporous hydroxyapatite nanoparticles for targeted release of anticancer drug, RSC Adv., 2015, 5, 30920–30928 RSC.
- Z. A. Ammar, A. Muhammad, G. Yi-Fan, W. A. Ibrahim, O. L. Hendrik and R. Hussain, Continuous Microwave Flow Synthesis of Mesoporous Hydroxyapatite, Mater. Sci. Eng., C, 2015, 56, 356–362 CrossRef PubMed.
- N. Abbasi Aval, J. Pirayesh Islamian, M. Hatamian, M. Arabfirouzjaei, J. Javadpour and M. R. Rashidi, Doxorubicin loaded large-pore mesoporous hydroxyapatite coated superparamagnetic Fe3O4 nanoparticles for cancer treatment, Int. J. Pharm., 2016, 509, 159–167 CrossRef CAS PubMed.
- J. Jafar, A. A. Negar and B. Alizera, Application of Sucrose as a cosurfactant in controlling the pore diameter in the synthesis of mesoporous Hydroxyapatite Nanoparticles, Ceram. Int., 2015, 59, 311–317 Search PubMed.
- C. Yamin, Z. Wandong, X. Xianghua, W. Yonglan and C. Nana, Rod-shaped hydroxyapatite with mesoporous structure as drug carriers for proteins, Appl. Surf. Sci., 2014, 322, 71–77 CrossRef.
- W. Jing, Z. Fanyan, W. Yi, Y. Yuanman, T. Wei, Y. Manli and L. Changseng, Preparation of pore expanded mesoporous hydroxyapatite via auxiliary solubilizing template method, Colloids Surf., A, 2014, 441, 737–743 CrossRef.
- A. Jagadeesh Kumar, G. Bhrath, K. Karthick, D. Mangalaraj, C. Viswanathan and N. Ponpandan, Shape evolution and size controlled synthesis of mesoporous hydroxyapatite nanostructures and their morphology dependent Pb(ii) removal from waste water, RSC Adv., 2014, 4, 37446–37458 RSC.
- R. P. Singh, J. P. Singh, A. Pal and T. Kaur, Encapsulation of vancomycin in copper doped hydroxyapatite mesoporous nanoparticles of different morphologies, J. Drug Delivery Sci. Technol., 2020, 55, 101441 CrossRef CAS.
- G. Haifeng, Z. Zhiqiang, Y. Feng, L. Guoping and Z. Zhiheng, Preparation of magnetic, luminescent and mesoporous hydroxyapatite nanospindles with high specific surface area, Rare Met. Mater. Eng., 2014, 43, 2647–2651 CrossRef.
- J. Chen, Z. Wang, Z. Wen, S. Yang, J. Wang and Q. Zhang, Controllable self-assembly of mesoporous hydroxyapatite, Colloids Surf., B, 2015, 127, 47–53 CrossRef CAS PubMed.
- P. M. S. Shanthi, R. Mangalaraja, A. Uthirakumar, S. Velmathi, T. Balasubramanian and M. Ashok, Synthesis and characterization of porous shell-like nano hydroxyapatite using Cetrimide as template, J. Colloid Interface Sci., 2010, 350, 39–43 CrossRef CAS PubMed.
- H. Zhou, M. Yang, S. Hou, X. Ni, Y. Bi, W. Wang, M. G. Kutty and L. Yang, Tailoring the morphological features of hydrothermally synthesized mesoporous hydroxyapatite using polyphenols and phosphate sources, Ceram. Int., 2017, 43, 12851–12856 CrossRef CAS.
- T. Moore, S. C. Loo, B. Banik and F. Alexis, Biomedical Applicatins of Hydroxyapatite Nanoparticles, Curr. Pharm. Biotechnol., 2010, 11, 333–342 Search PubMed.
- G. S. Kumar, G. Karunakaran, E. K. Girija, E. Kolesnikov, N. Van Minh, M. V. Gorshenkov and D. Kuznetsov, Size and morphology-controlled synthesis of mesoporous hydroxyapatite nanocrystals by microwave-assisted hydrothermal method, Ceram. Int., 2018, 44, 11257–11264 CrossRef CAS.
- J. A. Lett, M. Sundareswari, K. Ravichandran, M. B. Latha, S. Sagadevan and M. R. B. Johan, Tailoring the morphological features of sol–gel synthesized mesoporous hydroxyapatite using fatty acids as an organic modifier, RSC Adv., 2019, 9, 6228–6240 RSC.
- N. F. Mohammad, R. Othman and F. Y. Yeoh, The effect of surfactant extraction method on pore characteristics of mesoporous carbonated hydroxyapatite, Adv. Mater. Res., 2013, 858, 190–198 Search PubMed.
- H. Zhou, M. Yang, S. Hou and L. Deng, Mesoporous hydroxyapatite nanoparticles hydrothermally synthesized in aqueous solution with hexametaphosphate and tea polyphenols, Mater. Sci. Eng., C, 2017, 71, 439–445 CrossRef CAS PubMed.
- G. Bharath, K. Rambabu, F. Banat, S. Anwer, S. Lee, N. BinSaleh, S. Latha and N. Ponpandian, Mesoporous hydroxyapatite nanoplate arrays as pH-sensitive drug carrier for cancer therapy, Mater. Res. Express, 2019, 6, 085409 CrossRef CAS.
- G. Bharath, A. J. Kumar, K. Karthick, D. Mangalaraj, C. Viswanathan and N. Ponpandian, Shape evolution and size controlled synthesis of mesoporous hydroxyapatite nanostructures and their morphology dependent Pb (II) removal from waste water, RSC Adv., 2014, 4, 37446–37457 RSC.
- C. Zhang, C. Li, S. Huang, Z. Hou, Z. Cheng, P. Yang, C. Peng and J. Lin, Self-activated luminescent and mesoporous strontium hydroxyapatite nanorods for drug delivery, Biomaterials, 2010, 31, 3374–3383 CrossRef CAS PubMed.
- M. Uota, H. Arakawa, N. Kitamura, T. Yoshimura, J. Tanaka and T. Kijima, Synthesis of high surface area hydroxyapatite nanoparticles by mixed surfactant-mediated approach, Langmuir, 2005, 21, 4724–4728 CrossRef CAS PubMed.
- F. Zeng, J. Wang, Y. Wu, Y. Yu, W. Tang, M. Yin and C. Liu, Preparation of pore expanded mesoporous hydroxyapatite via auxiliary solubilizing template method, Colloids Surf., A, 2014, 441, 737–743 CrossRef CAS.
- L. Bakhtiari, J. Javadpour, H. Rezaie, M. Erfan and M. Shokrgozar, The effect of swelling agent on the pore characteristics of mesoporous hydroxyapatite nanoparticles, Prog. Nat. Sci.: Mater. Int., 2015, 25, 185–190 CrossRef CAS.
- L. Bakhtiari, J. Javadpour, H. R. Rezaie, M. Erfan, B. Mazinani and A. Aminian, Pore size control in the synthesis of hydroxyapatite nanoparticles: The effect of pore expander content and the synthesis temperature, Ceram. Int., 2016, 42, 11259–11264 CrossRef CAS.
- T. Liang, J. Qian, Y. Yuan and C. Liu, Synthesis of mesoporous hydroxyapatite nanoparticles using a template-free sonochemistry-assisted microwave method, J. Mater. Sci., 2013, 48, 5334–5341 CrossRef CAS.
- M. Akram, A. Z. Alshemary, Y.-F. Goh, W. A. W. Ibrahim, H. O. Lintang and R. Hussain, Continuous microwave flow synthesis of mesoporous hydroxyapatite, Mater. Sci. Eng., C, 2015, 56, 356–362 CrossRef CAS PubMed.
- L. Gu, X. He and Z. Wu, Mesoporous hydroxyapatite: Preparation, drug adsorption, and release properties, Mater. Chem. Phys., 2014, 148, 153–158 CrossRef CAS.
- Q. Chang, W. Xu, N. Li, C. Xue, Y. Wang, Y. Li, H. Wang, J. Yang and S. Hu, Dynamic restructuring of carbon dots/copper oxide supported on mesoporous hydroxyapatite brings exceptional catalytic activity in the reduction of 4-nitrophenol, Appl. Catal., B, 2020, 263, 118299 CrossRef CAS.
- B. Habiba, O. Abdeladim, L. Abdelaziz, N. Jean-Michel and S. Ahmed, Development of sulfonate-functionalized hydroxyapatite nanoparticles for calcium removal from aqueous solutions, Colloid Interface Sci. Commun., 2019, 30, 100178–100186 CrossRef.
- G. Bharath and N. Ponpandian, Hydroxyapatite nanoparticles on dendritic α Fe2O3 hierarchical architectures for heterogeneous photocatalyst and adsorption of Pb(II) ions from industrial wastewater, RSC Adv., 2015, 5, 84685–84693 RSC.
- X. Wei, C. Quing, L. Ning, X. Chaorui, W. Yanzhong, L. Ying, W. Huiqi, Y. Jinlong and H. Shengliang, Dynamic restructuring of carbon dots/copper oxide supported on mesoporous hydroxyapatite brings exceptional catalytic activity in the reduction of 4-nitrophenol, Appl. Catal., B, 2020, 263, 118299–118307 CrossRef.
- H. Zhang, F. Song, S. Wang, L. Liu, X. Tan and S. Liu, Atomic-level design of CoOH+ -hydroxyapatite@C catalysts for superfast degradation of organics by peroxymonosulfate activation, Chem. Commun., 2018, 54, 4919–4922 RSC.
- C. Santhosh, V. Velmurugan, G. Jacob, S. K. Jeong, A. N. Grace and A. Bhatnagar, Role of nanomaterials in water treatment applications: a review, Chem. Eng. J., 2016, 306, 1116–1137 CrossRef CAS.
- X.-Y. Zhao, Y.-J. Zhu, J. Zhao, B.-Q. Lu, F. Chen, C. Qi and J. Wu, Hydroxyapatite nanosheet-assembled microspheres: Hemoglobin-templated synthesis and adsorption for heavy metal ions, J. Colloid Interface Sci., 2014, 416, 11–18 CrossRef CAS PubMed.
- D. N. Thanh, P. Novák, J. Vejpravova, H. N. Vu, J. Lederer and T. Munshi, Removal of copper and nickel from water using nanocomposite of magnetic hydroxyapatite nanorods, J. Magn. Magn. Mater., 2018, 456, 451–460 CrossRef CAS.
- A. Aklil, M. Mouflih and S. Sebti, Removal of heavy metal ions from water by using calcined phosphate as a new adsorbent, J. Hazard. Mater., 2004, 112, 183–190 CrossRef CAS PubMed.
- G. Bharath and N. Ponpandian, Hydroxyapatite nanoparticles on dendritic α-Fe 2 O 3 hierarchical architectures for a heterogeneous photocatalyst and adsorption of Pb (II) ions from industrial wastewater, RSC Adv., 2015, 5, 84685–84693 RSC.
- F. Song, H. Zhang, S. Wang, L. Liu, X. Tan and S. Liu, Atomic-level design of CoOH + –hydroxyapatite@ C catalysts for superfast degradation of organics via peroxymonosulfate activation, Chem. Commun., 2018, 54, 4919–4922 RSC.
- K. Bouiahya, I. Es-saidi, C. El Bekkali, A. Laghzizil, D. Robert, J. M. Nunzi and A. Saoiabi, Synthesis and properties of alumina-hydroxyapatite composites from natural phosphate for phenol removal from water, Colloid Interface Sci. Commun., 2019, 31, 100188 CrossRef CAS.
- S. Xiaofeng, W. Xiadong, L. Dongsong, L. Jianguo, Z. Peibiao and C. Xuesi, Preparation of Mesoporous Nano-Hydroxyapatite Using a Surfactant Template Method for Protein Delivery, J Bionic Eng., 2012, 9, 224–233 CrossRef.
- N. Zhang, T. Gao, Y. Wang, Z. Wang, P. Zhang and J. Liu, Environmental pH-controlled loading and release of protein on mesoporous hydroxyapatite nanoparticles for bone tissue engineering, Mater. Sci. Eng., C, 2015, 46, 158–165 CrossRef CAS PubMed.
- F. Bakan, Gene delivery by hydroxyapatite and calcium phosphate nanoparticles: a review of novel and recent applications, Hydroxyapatite-Advances in Composite Nanomaterials, Biomedical Applications and Its Technological Facets, 2018, 157–176 Search PubMed.
- Y.-H. Yang, C.-H. Liu, Y.-H. Liang, F.-H. Lin and K. C.-W. Wu, Hollow mesoporous hydroxyapatite nanoparticles (hmHANPs) with enhanced drug loading and pH-responsive release properties for intracellular drug delivery, J. Mater. Chem. B, 2013, 1, 2447–2450 RSC.
- A.-R. Ibrahim, X. Li, Y. Zhou, Y. Huang, W. Chen, H. Wang and J. Li, Synthesis of spongy-like mesoporous hydroxyapatite from raw waste eggshells for enhanced dissolution of ibuprofen loaded via supercritical CO2, Int. J. Mol. Sci., 2015, 16, 7960–7975 CrossRef CAS PubMed.
- G. Verma, K. Barick, N. G. Shetake, B. Pandey and P. Hassan, Citrate-functionalized hydroxyapatite nanoparticles for pH-responsive drug delivery, RSC Adv., 2016, 6, 77968–77976 RSC.
- L. Ansari, M. Derakhshi, E. Bagheri, N. Shahtahmassebi and B. Malaekeh-Nikouei, Folate conjugation improved uptake and targeting of porous hydroxyapatite nanoparticles containing epirubicin to cancer cells, Pharm. Dev. Technol., 2020, 25, 601–609 CrossRef CAS PubMed.
- D. Li, J. He, X. Huang, J. Li, H. Tian, X. Chen and Y. Huang, Intracellular pH-responsive mesoporous hydroxyapatite nanoparticles for targeted release of anticancer drug, RSC Adv., 2015, 5, 30920–30928 RSC.
- W. Zhang, Y. Chai, X. Xu, Y. Wang and N. Cao, Rod-shaped hydroxyapatite with mesoporous structure as drug carriers for proteins, Appl. Surf. Sci., 2014, 322, 71–77 CrossRef CAS.
- M. S. Feiz and A. Meshkini, Targeted delivery of adenosine 5′-triphosphate using chitosan-coated mesoporous hydroxyapatite: a theranostic pH-sensitive nanoplatform with enhanced anti-cancer effect, Int. J. Biol. Macromol., 2019, 129, 1090–1102 CrossRef CAS PubMed.
- A. Meshkini and H. Oveisi, Methotrexate-F127 conjugated mesoporous zinc hydroxyapatite as an efficient drug delivery system for overcoming chemotherapy resistance in osteosarcoma cells, Colloids Surf., B, 2017, 158, 319–330 CrossRef CAS PubMed.
- A. Izadi, A. Meshkini and M. H. Entezari, Mesoporous superparamagnetic hydroxyapatite nanocomposite: A multifunctional platform for synergistic targeted chemo-magnetotherapy, Mater. Sci. Eng., C, 2019, 101, 27–41 CrossRef CAS PubMed.
- Z. Li, Z. Liu, M. Yin, X. Yang, Q. Yuan, J. Ren and X. Qu, Aptamer-Capped Multifunctional Mesoporous Strontium Hydroxyapatite Nanovehicle for Cancer-Cell-Responsive Drug Delivery and Imaging, Biomacromolecules, 2012, 13, 4257–4263 CrossRef CAS PubMed.
- Y.-J. Shyong, M.-H. Wang, L.-W. Kuo, C.-F. Su, W.-T. Kuo, K.-C. Chang and F.-H. Lin, Mesoporous hydroxyapatite as a carrier of olanzapine for long-acting antidepression treatment in rats with induced depression, J. Controlled Release, 2017, 255, 62–72 CrossRef CAS PubMed.
- Y.-J. Shyong, M.-H. Wang, H.-C. Tseng, C. Cheng, K.-C. Chang and F.-H. Lin, Mesoporous hydroxyapatite as olanzapine carrier provides a long-acting effect in antidepression treatment, J. Med. Chem., 2015, 58, 8463–8474 CrossRef CAS PubMed.
- H. C. Wu, J. Lin and T. W. Wang, Development of Mesoporous Magnetic Hydroxyapatite Nanocrystals, Materials Science Forum, Trans Tech Publ, 2018, 916,161–165 Search PubMed.
- N. A. Aval, J. P. Islamian, M. Hatamian, M. Arabfirouzjaei, J. Javadpour and M.-R. Rashidi, Doxorubicin loaded large-pore mesoporous hydroxyapatite coated superparamagnetic Fe3O4 nanoparticles for cancer treatment, Int. J. Pharm., 2016, 509, 159–167 CrossRef PubMed.
- M. U. Munir, A. Ihsan, Y. Sarwar, S. Z. Bajwa, K. Bano, B. Tehseen, N. Zeb, I. Hussain, M. T. Ansari and M. Saeed, Hollow mesoporous hydroxyapatite nanostructures; smart nanocarriers with high drug loading and controlled releasing features, Int. J. Pharm., 2018, 544, 112–120 CrossRef CAS PubMed.
- M. F. Cipreste and E. M. Sousa, Poly (vinyl alcohol)/collagen/hydroxyapatite nanoparticles hybrid system containing yttrium-90 as a potential agent to treat osteosarcoma, J. Biomater. Nanobiotechnol., 2014, 5, 24–30 CrossRef.
- S. Jafari, N. Maleki-Dizaji, J. Barar, M. Barzegar-Jalali, M. Rameshrad and K. Adibkia, Methylprednisolone acetate-loaded hydroxyapatite nanoparticles as a potential drug delivery system for treatment of rheumatoid arthritis: In vitro and in vivo evaluations, Eur. J. Pharm. Sci., 2016, 91, 225–235 CrossRef CAS PubMed.
- P. Yang, Z. Quan, C. Li, X. Kang, H. Lian and J. Lin, Bioactive, luminescent and mesoporous europium-doped hydroxyapatite as a drug carrier, Biomaterials, 2008, 29, 4341–4347 CrossRef CAS PubMed.
- S. Safi, F. Karimzadeh and S. Labbaf, Mesoporous and hollow hydroxyapatite nanostructured particles as a drug delivery vehicle for the local release of ibuprofen, Mater. Sci. Eng., C, 2018, 92, 712–719 CrossRef CAS PubMed.
- E. Sistanipour, A. Meshkini and H. Oveisi, Catechin-conjugated mesoporous hydroxyapatite nanoparticle: A novel nano-antioxidant with enhanced osteogenic property, Colloids Surf., B, 2018, 169, 329–339 CrossRef CAS PubMed.
- F. Jiang, D.-P. Wang, S. Ye and X. Zhao, Strontium-substituted, luminescent and mesoporous hydroxyapatite microspheres for sustained drug release, J. Mater. Sci.: Mater. Med., 2014, 25, 391–400 CrossRef CAS PubMed.
- Z.-L. Liu, Q.-Y. Jia, X.-D. Li, S.-P. Li, J. Shen, J. Lin and D.-X. Li, Synthesis of hollow mesoporous HAp-Au/MTX and its application in drug delivery, Colloids Surf., A, 2020, 586, 124231 CrossRef CAS.
- A. L. C. Maia, C. d A. Ferreira, A. L. B. d Barros, A. T. M. e Silva, G. A. Ramaldes, A. d Silva Cunha Júnior, D. C. d P. Oliveira, C. Fernandes and D. C. Ferreira Soares, Vincristine-loaded hydroxyapatite nanoparticles as a potential delivery system for bone cancer therapy, J. Drug Targeting, 2018, 26, 592–603 CrossRef CAS PubMed.
- S. Xiaoqing, L. Haipeng, L. Baoe, K. Jianli, L. Chunyong, W. Hongshui, Y. Zhenyang and Q. Zhijun, Carbon Nanotube-reinforced mesoporous hydroxyapatite composites with excellent mechanical and biological properties for bone replacement material application, Mater. Sci. Eng., C, 2017, 77, 1078–1087 CrossRef PubMed.
- K. Daizhi, L. Junhua, F. Yonglan, Z. Fuxing and L. Mengqui, Glucose biosensor based on glucose oxidase immobilized on a nanofilm composed of mesoporous hydroxyapatite, titanium dioxide, and modified with multi-walled carbon nanotubes, Microchim. Acta, 2012, 176, 73–80 CrossRef.
- S. Xincui, L. Linlong, W. Zongliang, G. Min, W. Yu, J. Zixue and Z. Peibiao, Porous Scaffolds of Poly(lactic-co-glycolic acid) and Mesoporous Hydroxyapatite Surface Modified by Poly(γ benzyl-L-glutamate) (PBLG) for in Vivo Bone Repair, ACS Biomater. Sci. Eng., 2019, 5, 2466–2481 CrossRef PubMed.
- M. Chiara Palmieri, G. Molino, G. Montalbano, S. Fiorilli and C. Vitale-Brovarone, Biomimetic and mesoporous nano-hydroxyapatite for bone tissue application; a short review, Biomed. Mater., 2020, 15, 022001–022020 CrossRef PubMed.
- R. Radhakrishnan, K. Kannan, S. Kumaravel and S. Thiripuranthagan, Oxidative esterification of furfural over Au–Pd/HAP-T and Au–Ag/HAP-T bimetallic catalysts supported on mesoporous hydroxyapatite nanorods, RSC Adv., 2016, 6, 45907–45922 RSC.
- K. Kathiravan, R. Ramakrishnan, K. Shathivel and T. Sivakumar, Oxidative esterification of furfural over Au-Pd/HAP-T and Au-Ag/HAP-T bimettalic catalysts supported on mesoporous hydroxyapatite nanorods, RSC Adv., 2016, 6, 45907–45922 RSC.
- S. S. Sasidharanpillai and G. Sony, Multifunctional Hydroxyapatite Nanoparticles for drug delivery and Multimodal Molecular Imaging, Microchim. Acta, 2015, 182, 1567–1589 CrossRef.
- S. S. Syamchand and G. Sony, Multifunctional hydroxyapatite nanoparticles for drug delivery and multimodal molecular imaging, Microchim. Acta, 2015, 182, 1567–1589 CrossRef CAS.
- J. Ran, H. Zeng, J. Cai, P. Jiang, P. Yan, L. Zheng, Y. Bai, X. Shen, B. Shi and H. Tong, Rational design of a stable, effective, and sustained dexamethasone delivery platform on a titanium implant: An innovative application of metal organic frameworks in bone implants, Chem. Eng. J., 2018, 333, 20–33 CrossRef CAS.
- Y.-R. Lee, M.-S. Jang, H.-Y. Cho, H.-J. Kwon, S. Kim and W.-S. Ahn, ZIF-8: A comparison of synthesis methods, Chem. Eng. J., 2015, 271, 276–280 CrossRef CAS.
- C.-W. Tsai and E. H. G. Langner, The effect of synthesis temperature on the particle size of nano-ZIF-8, Microporous Mesoporous Mater., 2016, 221, 8–13 CrossRef CAS.
- G. Zheng, Z. Chen, K. Sentosun, I. Pérez-Juste, S. Bals, L. M. Liz-Marzán, I. Pastoriza-Santos, J. Pérez-Juste and M. Hong, Shape control in ZIF-8 nanocrystals and metal nanoparticles@ZIF-8 heterostructures, Nanoscale, 2017, 9, 16645–16651 RSC.
- S. M. Cohen, Postsynthetic methods for the functionalization of metal–organic frameworks, Chem. Rev., 2012, 112, 970–1000 CrossRef CAS PubMed.
- W. Morris, C. J. Doonan, H. Furukawa, R. Banerjee and O. M. Yaghi, Crystals as Molecules: Postsynthesis Covalent Functionalization of Zeolitic Imidazolate Frameworks, J. Am. Chem. Soc., 2008, 130, 12626–12627 CrossRef CAS PubMed.
- O. R. Mahon, D. C. Browe, T. Gonzalez-Fernandez, P. Pitacco, I. T. Whelan, S. Von Euw, C. Hobbs, V. Nicolosi, K. T. Cunningham and K. H. Mills, Nano-particle mediated M2 macrophage polarization enhances bone formation and MSC osteogenesis in an IL-10 dependent manner, Biomaterials, 2020, 239, 119833 CrossRef CAS PubMed.
- J. Li, X. Jiang, H. Li, M. Gelinsky and Z. Gu, Tailoring materials for modulation of macrophage fate, Adv. Mater., 2021, 33, 2004172 CrossRef CAS PubMed.
| This journal is © the Partner Organisations 2023 |