“Turn-on” fluorescence sensor for organic amines fabricated via sustainable processing†
Renjian
Hu
a,
Shiyun
Lin
b,
Danning
Hu
a,
Hongye
Huang
a,
Mengshi
Wang
a,
Ruoxin
Li
a,
Mei
Tian
c,
Zhigang
Shuai
 b and
Yen
Wei
b and
Yen
Wei
 *ad
*ad
aThe Key Laboratory of Bioorganic Phosphorus Chemistry & Chemical Biology, Department of Chemistry, Tsinghua University, Beijing 100084, China. E-mail: weiyen@tsinghua.edu.cn
bMOE Key Laboratory of Organic Optoelectronics and Molecular Engineering, Department of Chemistry, Tsinghua University, Beijing 100084, China
cDepartment of Nuclear Medicine and PET Center, The Second Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou 310009, China
dDepartment of Chemistry, Center for Nanotechnology and Institute of Biomedical Technology, Chung-Yuan Christian University, Chung-Li 32023, Taiwan, China
First published on 23rd November 2022
Abstract
The detection of organic amines is a pivotal issue in chemistry. Here we present the synthesis of a novel fluorescence probe with “turn-on” greenish emissive (486 nm) response towards a series of organic amines for the first time with di-catechol moiety-based sensing materials. This sensor was fabricated from a 1,5-disubstituted naphthalene scaffold that was prepared via a sustainable process without the use of column chromatography or recrystallization. We reveal its dual-mode sensing capability covering visual and quantitative recognition for organic amines with a detection limit down to sub-ppm level. This system may find a broad spectrum of potential applications for monitoring organic amines in various fields, such as bioscience, biomedicine, pharmaceuticals, food science and environmental chemistry.
Introduction
Organic amines are some of the most common compounds, whose basic structure could be ascribed to ammonia substituted with organic groups, and they have a highly significant role in biomedicine, pharmaceuticals, food science and environmental chemistry. The formation of volatile organic amines has proved to be associated with metabolic disorders, lung lesions, and kidney disease.1,2 Small-molecule drugs containing amino groups such as Revlimid®, Imbruvica®, and Eliquis® are among the most consumed drugs in recent years. From another aspect, biogenic amine is always handled from toxicological viewpoints in food science, as ingesting excess amount of biogenic amine might induce foodborne diseases.3,4 Monitoring of organic amines within the atmosphere, soil and water bodies deserves continuous research efforts in environmental science.5,6 Thus, the development of an efficient detection method for organic amines in both gaseous and solution phases is of great significance.Numerous methodologies, mainly covering chromatographic,7–9 electrochemical,10–13 colorimetric14–17 and fluorescent approaches,18–20 have been put forward to date for organic amine detection. Among these feasible protocols, the fluorescent sensor is remarkably appreciated for its high sensitivity and convenience in avoiding sophisticated pretreatments or expensive instrument operation. We could generally classify the fluorescent organic amine sensors into “turn-off” and “turn-on” type according to the reinforcement or attenuation of fluorescence signal upon contacting with the specific analyte. In most cases, signal transduction is realized through the analyte-induced quenching process.21–25 Organic amines are regarded as electron-rich species which tend to interact with the photoexcited state of the specific luminogen and trigger a fluorescence quenching effect. However, these “turn-off” type fluorescence sensing procedures always suffer from low signal-to-noise ratio,26 and it seems difficult for us to balance the requirements of high sensitivity and large detection range within these protocols. Thus, the fabrication of a “turn-on” type fluorescence organic amine sensor is fascinating and challenging. To date, rationally designed sensors based on MOF structures27–30 or AIE molecules31,32 have been sporadically reported. However, cumbersome preparation procedures and limited analyte scopes restrict their practical applications.
Here, we developed the novel fluorescent probe 4,4′-(naphthalene-1,5-diyl)bis(benzene-1,2-diol), abbreviated as DiCat, based on the 1,5-disubstituted naphthalene skeleton for the first time. As illustrated in Scheme 1, synthetic procedures and post-treatment are conducted under the guidance of green chemistry principles. DiCat, with its integrated catechol moiety, exhibits a broad-spectrum “turn-on” fluorescence sensing capability towards organic amines in both solution and gaseous phases. The detection limit using the spectroscopic method reaches sub-ppm level, and the resolution limit of the visual detection mode approaches ppm level. This protocol offers huge potential in organic amine monitoring in multidisciplinary fields.
Results and discussion
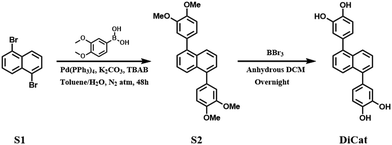
The catechol moiety and relevant interactions based on this molecular substructure have raised extensive research interest within the academic world, starting with the most famous hormones dopamine, adrenaline and noradrenaline, to the functional materials designed based on the catechol motif.33 Catechol and its derivatives have been revealed to undergo specific interaction with special structural domains of target proteins and play specific roles in biochemistry processes, such as anti-oxidative capability,34 anti-microbial activity,35 anti-inflammatory activity36etc. Based on the coordinative property of the 1,2-diol motif, catechol-decorated materials could interact with various metal surfaces and act as effective adhesive for advanced applications.37–39 In recent years, our group focused on the fabrication of polyphenol fluorophores and attempted to explore application scenarios in fluorescence imaging and sensing. We have published a series of research articles based on the luminogens with novel hydroquinone or catechol motif. Innovative sensing mechanism and remarkable detection capability have been achieved towards numerous analytes, including organic boron compound,40 oxidative species41 and lithium metal.42 These species are of great interest in organic synthetic chemistry, analytical chemistry and material chemistry.With the increasing realization of the versatility of polyphenol functionalized fluorophores, especially catechol moiety–decorated fluorophores, the development of novel molecular designs and feasible synthetic protocols has been an urgent demand. Here, we proposed the central symmetric bis-catechol fluorescent probe based on the di-substituted naphthalene scaffold. The synthetic procedure is briefly illustrated in Fig. 1, which involves a classical Suzuki-Miyaura reaction followed by BBr3 deprotection, adopted to obtain the target molecule. The high molecular symmetry of DiCat and the precursor renders them with low solubility in common organic solvents.43–45 Thus, we could improve the purification process by straightforward washing with small amounts of organic solvents instead of utilizing the exhausting column chromatography or recrystallization method. Table 1 shows a comparison of the purification process in our protocol with the conventional column chromatography method. Organic solvent consumption, isolation yield and product purity were comprehensively considered to evaluate the sustainability of these two processes. We find that under this rational molecular design, the bis-catechol fluorophore DiCat could be synthesized and feasibly purified by washing with lower solvent use. At the same time, the desired products could be obtained with satisfactory purity, and the total yield was determined as 74.4%. Detailed 1H-NMR, 13C-NMR, and MALDI-TOF-MS results are found in ESI.† As suggested by the mainstream scientific community, procedures with lower solvent usage could be considered as preferred choices, consistent with the basic green chemistry principles.46–48 From this aspect, our synthetic protocol exhibits huge potential in the development and construction of catechol-decorated fluorophores.
| Isolated yield (%) | Purity (%) | Usage amount of solvent (mL mmol−1) | |||
|---|---|---|---|---|---|
| EA | PE | DCM | |||
a Purified with simple washing and drying under the green chemistry principle. Step 1: washing with EA/PE = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 mobile phase first, then washing with DCM/PE = 1 50 mobile phase first, then washing with DCM/PE = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 to afford the crude product. Step 2: washing with EA several times to afford the target product.
b Purified with conventional column chromatography process. Step 1: flushing with EA/DCM/PE = 1 4 to afford the crude product. Step 2: washing with EA several times to afford the target product.
b Purified with conventional column chromatography process. Step 1: flushing with EA/DCM/PE = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 to obtain the crude product (Rf = 0.75). Step 2: for the low-solubility DiCat, column chromatography could not afford the reaction product as expected. Note: solvent used in the extraction process is not included. 2 to obtain the crude product (Rf = 0.75). Step 2: for the low-solubility DiCat, column chromatography could not afford the reaction product as expected. Note: solvent used in the extraction process is not included.
|
|||||
| Step 1 | 90.0a | 98.2 | 0.1 | 9.0 | 1.0 |
| 53.9b | 80.1 | 17.5 | 35.0 | 17.5 | |
| Step 2 | 82.7a | 99.1 | 20.0 | 0.0 | 0.0 |
| Traceb | |||||
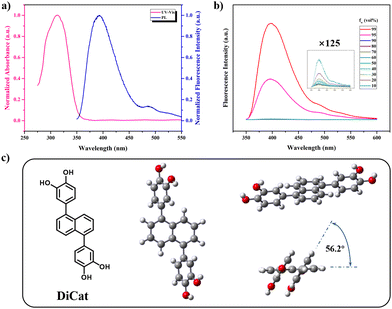
Fig. 2a shows the ground-state electron structure and conjugation length of DiCat, determined with UV-Vis spectra in THF solution. Maximum absorption wavelength is found at 311 nm, which shows a conspicuous bathochromic shift compared with naphthalene.40 This indicates that effective conjugation might exist between two catechol moieties and the naphthalene core. This inference is verified with theoretical calculations based on the B3LYP functional and 6-31G* basis set.49 As illustrated in Fig. 2c, we depict the optimal geometrical configuration from orthogonal perspectives. Torsion angle of 2.8° is identified between the linkage C–C bond and the naphthalene ring. The dihedral angle between the catechol moiety and naphthalene plane is determined to be 56.2°, which suggests that a certain degree of conjugation does exist, as mentioned above.
Next, we focused on the excited-state property of DiCat and recorded the fluorescence spectra in THF solution. The maximum emission wavelength is found to be located at 393 nm, with a mild Stokes shift of 82 nm. DI water was introduced as poor solvent in increasing fraction; the photoluminescence (PL) intensity first undergoes a sluggish decrease and then a steep rise upon surpassing the water fraction of 95%. This behavior agrees with our fundamental understanding of the AIE effect. We could ascribe this abnormal aggregate-state emissive property to the restriction of intramolecular motion (RIM) in solid state according to our previous work40 and generally accepted theory.50–52 And we expect this property could contribute to the detection process in the binary solvent with the specific water content.
Based on the tetra-coordinated boron-intermediated three-component sensing system proposed by our group, the combination of a fluorescent catechol-type molecule and boronic acid with electron-rich ligand garners a fresh emissive species at the long-wavelength region.40 We construct the di-catechol moiety integrated luminogen DiCat with intrinsic functionalization strategy41 in this work. Involving triethylamine (Et3N) as an efficient ligand with DiCat/PBA (phenylboronic acid) solution stimulates the onset of greenish fluorescence under UV excitation. The response process could be finished within a few seconds by mixing of the DiCat/PBA ensemble and triethylamine. This phenomenon inspires us to exploit other novel sensing systems for organic amines with rational design.
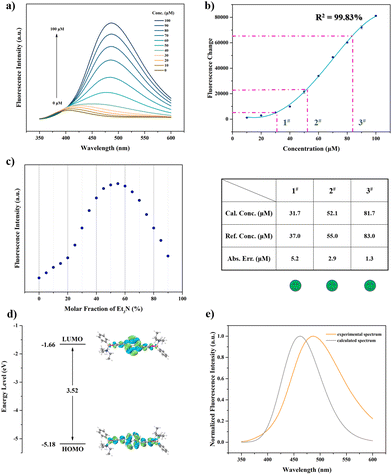
After systematic optimization, we determined the optimal solvent as THF/DI H2O (water fraction of 70%, vol%). The molar ratio of DiCat and PBA is fixed at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. (Detailed optimization procedure can be found in ESI†). As shown in Fig. 3a, gradually increasing the concentration of Et3N from 10 μM to 100 μM facilitates brighter emission and higher PL intensity at 486 nm. Focusing on the fluorescence change at 486 nm compared with pure DiCat in THF solution, a positive correlation between PL change and Et3N concentration is disclosed. Deviating beyond the linear relationship induced us to attempt polynomial regression for the mathematic expression of the standard curve. A four-order polynomial fitting yields a satisfactory fitting curve with a correlation coefficient of 99.83%, as shown in Fig. 3b. The specific functional relation is depicted below:
2. (Detailed optimization procedure can be found in ESI†). As shown in Fig. 3a, gradually increasing the concentration of Et3N from 10 μM to 100 μM facilitates brighter emission and higher PL intensity at 486 nm. Focusing on the fluorescence change at 486 nm compared with pure DiCat in THF solution, a positive correlation between PL change and Et3N concentration is disclosed. Deviating beyond the linear relationship induced us to attempt polynomial regression for the mathematic expression of the standard curve. A four-order polynomial fitting yields a satisfactory fitting curve with a correlation coefficient of 99.83%, as shown in Fig. 3b. The specific functional relation is depicted below:
| ΔFL = 4646 − 415.8c1 + 10.66c2 + 0.1636c3 − 0.001530c4 |
Aiming at deepening our understanding of the sensing mechanism dominating the abovementioned process, a series of control experiments was conducted. As shown in Fig. S14 (ESI†), the absence of DiCat diminishes the fluorescence feature of the sensing mixture, and no discriminative emission peak could be identified. Just a combination with PBA brings about mild influence on the emissive property of DiCat, while complexation of DiCat and Et3N yields another emission peak at 466 nm. Et3N is susceptible to induce the construction of a cross-linking network with DiCat through hydrogen bonding interaction, which suppresses intramolecular motion of DiCat. This blocking effect might engender the enhancement of PL intensity and bathochromic-shifted maximum emission wavelength. The extra addition of PBA boosts PL intensity and triggers further bathochromic shift of the emission wavelength. We could attribute this phenomenon to the synergistic effect, including formation of tetra-coordinated boron species and hydrogen-bonding-mediated cross-linking interaction, which renders restriction of intramolecular motion and extension of conjugation length. The stoichiometric ratio investigation depicted in Fig. 3c reveals that complexation of DiCat and Et3N exists beyond the typical chemical stoichiometric compound, which agrees with our hypothesized plausible sensing mechanism as mentioned above. Theoretical calculation also supports our viewpoint, as shown in Fig. 3d and 3e. Using Et3N as the typical analyte in our discussion, the energy level and electronic structure of the proposed tetra-coordinated boron species was acquired in Fig. 3d with TDDFT method. The calculated fluorescence spectrum depicted in Fig. 3e matches well with experimental results.
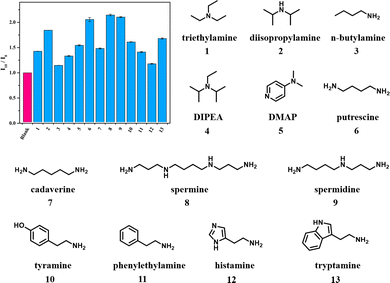
Success in Et3N detection with this protocol raised our enthusiasm for exploiting the detection capability of DiCat towards other organic amines. Primary amine and secondary amine with simple chemical structures, such as n-butylamine and diisopropylamine, are scrutinized with DiCat/PBA under the optimal detection condition identified above. A similar positive correlation of PL intensity versus amine concentration is observed, which could likewise be constrained with polynomial regression. Detailed fitting parameters are summarized in Table 2, while fluorescence spectrum and fitting curves are provided in ESI.† It is quite inspiring to notice that our sensing method seems to be compatible with primary, secondary, and tertiary amines. We continued our research by examining more frequently used organic amines such as N,N-diisopropylethylamine (DIPEA) and 4-dimethylaminopyridine (DMAP) and acquired positive results from both of them. Detection methods for eight organic amines commonly discussed in food science, including 1,4-diaminobutane (putrescine), 1,5-pentanediamine (cadaverine), spermine, spermidine, tyramine, phenylethylamine, histamine, and tryptamine,53 were further established. The versatility of this detection method based on DiCat/PBA is overviewed in Fig. 4, and we find the detection limit of this broad-spectrum protocol down to several μM (sub-ppm level). Outstanding selectivity towards amine substances, including primary amine, secondary amine, and tertiary amine, is developed, while other common nitrogen-containing species such as quaternary ammonium salt or amide induce minor change in PL intensity (Fig. S40, ESI†). Meanwhile, we checked other water-soluble organic compounds bearing electron-rich functional groups with our DiCat/PBA system (Fig. S41, ESI†). As shown in Fig. S42 (ESI†), selectivity towards organic amines is achieved with remarkable difference in relative intensity of the response compared with carbonyl, hydroxyl, carboxyl, and cyano containing compounds.
| Organic amine | P 0 (μM) | P 1 (μM−1) | P 2 (μM−2) | P 3 (μM−3) | P 4 (μM−4) | R 2 (%) |
|---|---|---|---|---|---|---|
| Triethylamine | 4646 | −415.8 | 10.66 | 0.1636 | −0.001530 | 99.83 |
| Diisopropylamine | −1657 | 470.2 | −15.70 | 0.3309 | −0.001700 | 99.98 |
| n-Butylamine | 279.3 | 96.56 | −10.12 | 0.3328 | −0.001950 | 99.89 |
| DIPEA | 4154 | −511.1 | 19.26 | −0.09951 | −0.00006874 | 99.91 |
| DMAP | 2232 | −214.9 | 11.06 | −0.09656 | 0.0002764 | 99.77 |
| Putrescine | 2222 | −327.3 | 29.45 | −0.2854 | 0.0008314 | 99.95 |
| Cadaverine | 5380 | −975.5 | 57.42 | −0.6772 | 0.002630 | 99.87 |
| Spermine | 5763 | −1342 | 113.8 | −1.527 | 0.006420 | 99.70 |
| Spermidine | 8091 | −1312 | 86.07 | −1.056 | 0.004220 | 99.96 |
| Tyramine | 9268 | −1233 | 55.20 | −0.5385 | 0.001720 | 99.90 |
| Phenylethylamine | 6510 | −1023 | 53.42 | −0.6015 | 0.002200 | 99.99 |
| Histamine | 4954 | −648.2 | 24.61 | −0.1843 | 0.0003816 | 99.71 |
| Tryptamine | 8371 | −1049 | 45.93 | −0.3973 | 0.001060 | 99.88 |
The solid-state emission behavior of DiCat inspires us to study its sensing capability under aggregation state. Diverse sensing patterns as illustrated in Fig. 5a could be fabricated on silica gel plates or paper-based substrate without any difficulty. The solid-state sensing capability of DiCat towards gaseous organic amines is demonstrated with primitive homemade equipment. Et3N atmosphere is created via vaporization of Et3N solution in MeOH with varying concentrations within sealed vessels. DiCat is dissolved in THF with an equal molarity of PBA and cast onto silica gel plates. Customized plates are put into sealed vessels after totally drying in the air for further observation. As shown in Fig. 5b, it is easy for us to distinguish the color change of solid-state sensing materials within the environment containing 10 ppm of Et3N vapor with the naked eye under hand-held UV lamp. “Turn-on” greenish fluorescence of DiCat/PBA complex occurs and tends to be brighter as the concentration of Et3N vapor increased. Taking out the sensing materials into the atmosphere would recover the blue emission. We are dedicated to the further design and fabrication of a sensing device with in situ fluorescence spectrum collection capability, and we expect it could on-line determine the precise concentration of specific gaseous-phase organic amines. Recently, based on this homemade equipment, we could semi-quantitatively determine Et3N vapor concentration through the atmosphere. The visual resolution limit is estimated to be 10 ppm, which is lower than the OSHA standard level for Et3N vapor. It indicates that a sensing array based on DiCat offers an effective monitoring protocol for organic amines to avoid inhalation injury in the industry.
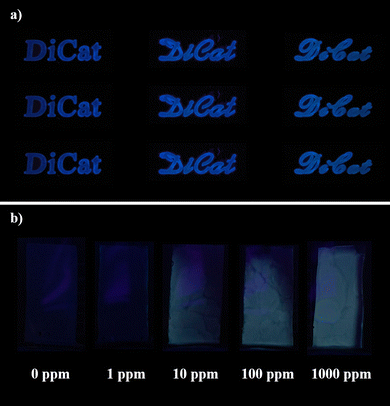 | ||
| Fig. 5 Emission behavior of DiCat in the solid state. (a) Diverse sensing patterns fabricated with DiCat, (b) solid-state sensing capability of DiCat/PBA towards gaseous substrates. | ||
Conclusions
Functional fluorophores decorated with catechol moiety have shown great promise in the development of future fluorescent chemical sensors. The feasible synthesis of these molecular skeletons is of vital importance as we consider the large-scale synthesis and application of catechol-based luminogens. Herein, we proposed the molecular design of the central symmetric bis-catechol fluorophore DiCat bearing a 1,5-disubstituted naphthalene scaffold. The unique molecular structure makes it possible to be fabricated with sustainable processing steps. Through avoiding the operation of conventional chromatography or recrystallization method, the entire purification process involves less solvent utilization and minor environmental disruption. We would further enrich viable fluorophore structures integrated with bis-catechol motif in the future, which could be synthesized with similar sustainable processing. Improvements on the agent use and catalyst selection of the whole synthetic procedure are also ongoing in our laboratory.This fluorescence probe DiCat exhibits a rapid “turn-on” fluorescence response towards a broad spectrum of organic amines under warm conditions. The average detection limit is estimated as sub-ppm level with mathematic treatments. These inspiring results reveal the huge potential of the bis-catechol fluorophores, with sensing applications in bioscience, biomedicine and pharmaceutical fields, as it could respond to a wide range of amino-containing analytes in the liquid phase, which could be a key intermediate of metabolism or common amino-containing drugs. The gaseous phase–sensing capability also renders the possibility of atmospheric detection towards biogenic amines or volatile organic amines, which will be valuable for real-time monitoring in food science and environmental chemistry.
Author contributions
This project was designed by R. H., S. L., Z. S. and Y. W.; synthesis and characterization were conducted by R. H.; theoretical calculation was performed by S. L. and Z. S.; constructive suggestions on the writing of the manuscript were put forward by D. H., H. H., M. W., R. L. and M. T.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 21788102). We are most grateful to Dr. Liucheng Mao, Mr. Wensheng Xie and Prof. Lei Tao of Tsinghua University for their valuable suggestions.Notes and references
- M. L. Simenhoff, J. F. Burke, J. J. Saukkonen, A. T. Ordinario, R. Doty and S. Dunn, Biochemical Profile of Uremic Breath, N. Engl. J. Med., 1977, 297, 132–135 CrossRef CAS PubMed.
- G. Preti, J. N. Labows, J. G. Kostelc, S. Aldinger and R. Daniele, Analysis of Lung Air from Patients with Bronchogenic Carcinoma and Controls Using Gas Chromatography-Mass Spectrometry, J. Chromatogr. B: Biomed. Sci. Appl., 1988, 432, 1–11 CrossRef CAS PubMed.
- A. K. Omer, R. R. Mohammed, P. S. M. Ameen, Z. A. Abas and K. Ekici, Presence of Biogenic Amines in Food and Their Public Health Implications: A Review, J. Food Prot., 2021, 84, 1539–1548 CrossRef PubMed.
- W. Wójcik, M. Łukasiewicz and K. Puppel, Biogenic Amines: Formation, Action and Toxicity: A Review, J. Sci. Food Agric., 2021, 101, 2634–2640 CrossRef PubMed.
- A. Fekete, A. K. Malik, A. Kumar and P. Schmitt-Kopplin, Amines in the Environment, Crit. Rev. Anal. Chem., 2010, 40, 102–121 CrossRef CAS.
- X. Ge, A. S. Wexler and S. L. Clegg, Atmospheric Amines – Part I. A Review, Atmos. Environ., 2011, 45, 524–546 CrossRef CAS.
- J. B. Noffsinger and N. D. Danielson, Liquid Chromatography of Aliphatic Trialkylamines with Post-Column Chemiluminescent Detection Using Tris(2,2′-Bipyridine)Ruthenium(III), J. Chromatogr. A, 1987, 387, 520–524 CrossRef CAS.
- W. G. Stillwell, M. S. Bryant and J. S. Wishnok, GC/MS Analysis of Biologically Important Aromatic Amines. Application to Human Dosimetry, Biomed. Environ. Mass Spectrom., 1987, 14, 221–227 Search PubMed.
- M. T. Salazar, T. K. Smith and A. Harris, High-Performance Liquid Chromatographic Method for Determination of Biogenic Amines in Feedstuffs, Complete Feeds, and Animal Tissues, J. Agric. Food Chem., 2000, 48, 1708–1712 CrossRef CAS PubMed.
- N. J. Tremblay, B. J. Jung, P. Breysse and H. E. Katz, Digital-Inverter Amine Sensing via Synergistic Responses by n and p Organic Semiconductors, Adv. Funct. Mater., 2011, 21, 4314–4319 CrossRef CAS PubMed.
- H. Huang, D. E. Gross, X. Yang, J. S. Moore and L. Zang, One-Step Surface Doping of Organic Nanofibers to Achieve High Dark Conductivity and Chemiresistor Sensing of Amines, ACS Appl. Mater. Interfaces, 2013, 5, 7704–7708 CrossRef CAS PubMed.
- S. Pandey and K. K. Nanda, Au Nanocomposite Based Chemiresistive Ammonia Sensor for Health Monitoring, ACS Sens., 2016, 1, 55–62 CrossRef CAS.
- H. Li, Y. Shi, G. Han, J. Liu, J. Zhang, C. Li, J. Liu, Y. Yi, T. Li, X. Gao, C. Di, J. Huang, Y. Che, D. Wang, W. Hu, Y. Liu and L. Jiang, Monolayer Two-Dimensional Molecular Crystals for an Ultrasensitive OFET-Based Chemical Sensor, Angew. Chem., Int. Ed., 2020, 59, 4380–4384 CrossRef CAS PubMed.
- T. Soga, Y. Jimbo, K. Suzuki and D. Citterio, Inkjet-Printed Paper-Based Colorimetric Sensor Array for the Discrimination of Volatile Primary Amines, Anal. Chem., 2013, 85, 8973–8978 CrossRef CAS PubMed.
- X. Zhong, D. Huo, H. Fa, X. Luo, Y. Wang, Y. Zhao and C. Hou, Rapid and Ultrasensitive Detection of Biogenic Amines with Colorimetric Sensor Array, Sens. Actuators, B, 2018, 274, 464–471 CrossRef CAS.
- H. Shibata, Y. Ikeda and D. Citterio, Inkjet-Printed Colorimetric Paper-Based Gas Sensor Arrays for the Discrimination of Volatile Primary Amines with Amine-Responsive Dye-Encapsulating Polymer Nanoparticles, in Biomimetic Sensing: Methods and Protocols, ed. J. E. Fitzgerald and H. Fenniri, Springer, New York, NY, 2019, pp. 101–114 Search PubMed.
- A. Sousaraei, C. Queirós, F. G. Moscoso, A. M. G. Silva, T. Lopes-Costa, J. M. Pedrosa, L. Cunha-Silva and J. Cabanillas-Gonzalez, Reversible Protonation of Porphyrinic Metal-Organic Frameworks Embedded in Nanoporous Polydimethylsiloxane for Colorimetric Sensing, Adv. Mater. Interfaces, 2021, 8, 2001759 CrossRef CAS.
- L. Pu, Fluorescence of Organic Molecules in Chiral Recognition, Chem. Rev., 2004, 104, 1687–1716 CrossRef CAS PubMed.
- Y. Fu, W. Xu, Q. He and J. Cheng, Recent Progress in Thin Film Fluorescent Probe for Organic Amine Vapour, Sci. China: Chem., 2016, 59, 3–15 CrossRef CAS.
- T. Skorjanc, D. Shetty and M. Valant, Covalent Organic Polymers and Frameworks for Fluorescence-Based Sensors, ACS Sens., 2021, 6, 1461–1481 CrossRef CAS PubMed.
- H. Peng, L. Ding, T. Liu, X. Chen, L. Li, S. Yin and Y. Fang, An Ultrasensitive Fluorescent Sensing Nanofilm for Organic Amines Based on Cholesterol-Modified Perylene Bisimide, Chem. – Asian J., 2012, 7, 1576–1582 CrossRef CAS PubMed.
- S. Rochat and T. M. Swager, Fluorescence Sensing of Amine Vapors Using a Cationic Conjugated Polymer Combined with Various Anions, Angew. Chem., Int. Ed., 2014, 53, 9792–9796 CrossRef CAS PubMed.
- E. Yue, X. Ma, Y. Zhang, Y. Zhang, R. Duan, H. Ji, J. Li, Y. Che and J. Zhao, Fluorescent Bilayer Nanocoils Assembled from an Asymmetric Perylene Diimide Molecule with Ultrasensitivity for Amine Vapors, Chem. Commun., 2014, 50, 13596–13599 RSC.
- Y. Fu, J. Yao, W. Xu, T. Fan, Q. He, D. Zhu, H. Cao and J. Cheng, Reversible and “Fingerprint” Fluorescence Differentiation of Organic Amine Vapours Using a Single Conjugated Polymer Probe, Polym. Chem., 2015, 6, 2179–2182 RSC.
- S. Dalapati, E. Jin, M. Addicoat, T. Heine and D. Jiang, Highly Emissive Covalent Organic Frameworks, J. Am. Chem. Soc., 2016, 138, 5797–5800 CrossRef CAS PubMed.
- W.-Q. Zhang, Q.-Y. Li, J.-Y. Cheng, K. Cheng, X. Yang, Y. Li, X. Zhao and X.-J. Wang, Ratiometric Luminescent Detection of Organic Amines Due to the Induced Lactam–Lactim Tautomerization of Organic Linker in a Metal–Organic Framework, ACS Appl. Mater. Interfaces, 2017, 9, 31352–31356 CrossRef CAS PubMed.
- X. Shen and B. Yan, A Novel Fluorescence Probe for Sensing Organic Amine Vapors from a Eu3+ β-Diketonate Functionalized Bio-MOF-1 Hybrid System, J. Mater. Chem. C, 2015, 3, 7038–7044 RSC.
- P. Mani, A. A. Ojha, V. S. Reddy and S. Mandal, Turn-on” Fluorescence Sensing and Discriminative Detection of Aliphatic Amines Using a 5-Fold-Interpenetrated Coordination, Inorg. Chem., 2017, 56, 6772–6775 CrossRef CAS PubMed.
- A. Mallick, A. M. El-Zohry, O. Shekhah, J. Yin, J. Jia, H. Aggarwal, A.-H. Emwas, O. F. Mohammed and M. Eddaoudi, Unprecedented Ultralow Detection Limit of Amines Using a Thiadiazole-Functionalized Zr(IV)-Based Metal–Organic Framework, J. Am. Chem. Soc., 2019, 141, 7245–7249 CrossRef CAS PubMed.
- J. Ren, Z. Niu, Y. Ye, C.-Y. Tsai, S. Liu, Q. Liu, X. Huang, A. Nafady and S. Ma, Second-Sphere Interaction Promoted Turn-On Fluorescence for Selective Sensing of Organic Amines in a TbIII-Based Macrocyclic Framework, Angew. Chem., Int. Ed., 2021, 60, 23705–23712 CrossRef CAS PubMed.
- M. Gao, S. Li, Y. Lin, Y. Geng, X. Ling, L. Wang, A. Qin and B. Z. Tang, Fluorescent Light-Up Detection of Amine Vapors Based on Aggregation-Induced Emission, ACS Sens., 2016, 1, 179–184 CrossRef CAS.
- Y. Hu, T. Han, N. Yan, J. Liu, X. Liu, W.-X. Wang, J. W. Y. Lam and B. Z. Tang, Visualization of Biogenic Amines and In Vivo Ratiometric Mapping of Intestinal PH by AIE-Active Polyheterocycles Synthesized by Metal-Free Multicomponent Polymerizations, Adv. Funct. Mater., 2019, 29, 1902240 CrossRef.
- J. Yang, M. A. C. Stuart and M. Kamperman, Jack of All Trades: Versatile Catechol Crosslinking Mechanisms, Chem. Soc. Rev., 2014, 43, 8271–8298 RSC.
- T. Miura, S. Muraoka and Y. Fujimoto, Inactivation of Creatine Kinase Induced by Quercetin with Horseradish Peroxidase and Hydrogen Peroxide: Pro-Oxidative and Anti-Oxidative Actions of Quercetin, Food Chem. Toxicol., 2003, 41, 759–765 CrossRef CAS PubMed.
- E.-Y. Jeong, J.-H. Jeon, C.-H. Lee and H.-S. Lee, Antimicrobial Activity of Catechol Isolated from Diospyros Kaki Thunb. Roots and Its Derivatives toward Intestinal Bacteria, Food Chem., 2009, 115, 1006–1010 CrossRef CAS.
- K. V. Dileep, I. Tintu, P. K. Mandal, P. Karthe, M. Haridas and C. Sadasivan, Binding to PLA2 May Contribute to the Anti-Inflammatory Activity of Catechol, Chem. Biol. Drug Des., 2012, 79, 143–147 CrossRef CAS PubMed.
- J. H. Waite and M. L. Tanzer, Polyphenolic Substance of Mytilus Edulis: Novel Adhesive Containing L-Dopa and Hydroxyproline, Science, 1981, 212, 1038–1040 CrossRef CAS PubMed.
- Q. Lyu, N. Hsueh and C. L. L. Chai, The Chemistry of Bioinspired Catechol(Amine)-Based Coatings, ACS Biomater. Sci. Eng., 2019, 5, 2708–2724 CrossRef CAS PubMed.
- G. Yan, G. Chen, Z. Peng, Z. Shen, X. Tang, Y. Sun, X. Zeng and L. Lin, The Cross-Linking Mechanism and Applications of Catechol–Metal Polymer Materials, Adv. Mater. Interfaces, 2021, 8, 2100239 CrossRef CAS.
- R. Hu, S. Lin, M. Wang, R. Li, Z. Shuai and Y. Wei, Catechol Moiety Integrated Tri-Aryl Type AIEgen for Visual and Quantitative Boronic Acid Detection, Chem. – Eur. J., 2022, 28, e202103351 CAS.
- M. Wang, Y. Wang, R. Hu, J. Yuan, M. Tian, X. Zhang, Z. Shuai and Y. Wei, Intrinsic Hydroquinone-Functionalized Aggregation-Induced Emission Core Shows Redox and PH Sensitivity, Commun. Chem., 2021, 4, 1–9 CrossRef.
- M. Wang, H. Liang, L. Wang, H. Zhang, J. Wang, Y. Wei, X. He and Y. Yang, First AIE Probe for Lithium-Metal Anodes, Matter, 2022, 5, 3530 CrossRef CAS.
- R. Pinal, Effect of Molecular Symmetry on Melting Temperature and Solubility, Org. Biomol. Chem., 2004, 2, 2692–2699 RSC.
- A. Gavezzotti, A. Molecular Symmetry, Melting Temperatures and Melting Enthalpies of Substituted Benzenes and Naphthalenes, J. Chem. Soc., Perkin Trans., 1995, 2, 1399–1404 RSC.
- S. H. Yalkowsky and S. C. Valvani, Solubility and Partitioning I: Solubility of Nonelectrolytes in Water, J. Pharm. Sci., 1980, 69, 912–922 CrossRef CAS PubMed.
- P. Anastas and N. Eghbali, Green Chemistry: Principles and Practice, Chem. Soc. Rev., 2009, 39, 301–312 RSC.
- H. C. Erythropel, J. B. Zimmerman, T. M. de Winter, L. Petitjean, F. Melnikov, C. H. Lam, A. W. Lounsbury, K. E. Mellor, N. Z. Janković, Q. Tu, L. N. Pincus, M. M. Falinski, W. Shi, P. Coish, D. L. Plata and P. T. Anastas, The Green ChemisTREE: 20 Years after Taking Root with the 12 Principles, Green Chem., 2018, 20, 1929–1961 RSC.
- R. A. Sheldon, Green Chemistry: Principles and Case Studies. By Felicia A. Etzkorn, Angew. Chem., Int. Ed., 2021, 60, 538–539 CrossRef CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson, H. Nakatsuji, X. Li, M. Caricato, A. V. Marenich, J. Bloino, B. G. Janesko, R. Gomperts, B. Mennucci, H. P. Hratchian, J. V. Ortiz, A. F. Izmaylov, J. L. Sonnenberg, D. Williams-Young, F. Ding, F. Lipparini, F. Egidi, J. Goings, B. Peng, A. Petrone, T. Henderson, D. Ranasinghe, V. G. Zakrzewski, J. Gao, N. Rega, G. Zheng, W. Liang, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, K. Throssell, J. A. Montgomery Jr., J. E. Peralta, F. Ogliaro, M. J. Bearpark, J. J. Heyd, E. N. Brothers, K. N. Kudin, V. N. Staroverov, T. A. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. P. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, J. M. Millam, M. Klene, C. Adamo, R. Cammi, J. W. Ochterski, R. L. Martin, K. Morokuma, O. Farkas, J. B. Foresman and D. J. Fox, Gaussian 16, Revision C.01, Gaussian, Inc., Wallingford CT, 2016 Search PubMed.
- J. Mei, N. L. C. Leung, R. T. K. Kwok, J. W. Y. Lam and B. Z. Tang, Aggregation-Induced Emission: Together We Shine, United We Soar!, Chem. Rev., 2015, 115, 11718–11940 CrossRef CAS PubMed.
- Y. Tu, Z. Zhao, J. W. Y. Lam and B. Z. Tang, Mechanistic Connotations of Restriction of Intramolecular Motions (RIM), Natl. Sci. Rev., 2021, 8, nwaa260 CrossRef PubMed.
- Q. Peng and Z. Shuai, Molecular Mechanism of Aggregation-Induced Emission, Aggregate, 2021, 2, e91 Search PubMed.
- M. H. S. Santos, Biogenic Amines: Their Importance in Foods, Int. J. Food Microbiol., 1996, 29, 213–231 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2qm01065h |
| This journal is © the Partner Organisations 2023 |