[C5H12N]2SnBr6: a lead-free phase transition compound with switchable quadratic nonlinear optical properties†
Xinxin
Hu
ab,
Haojie
Xu
ab,
Wuqian
Guo
ab,
Shiguo
Han
a,
Yi
Liu
a,
Yu
Ma
ab,
Qingshun
Fan
ab,
Junhua
Luo
 a and
Zhihua
Sun
a and
Zhihua
Sun
 *ac
*ac
aState Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou, Fujian 350002, China. E-mail: sunzhihua@fjirsm.ac.cn
bUniversity of Chinese Academy of Sciences, Beijing 100049, China
cFujian Science & Technology Innovation Laboratory for Optoelectronic Information of China, Fuzhou, Fujian 350108, P. R. China
First published on 8th February 2023
Abstract
Solid-state phase transition materials with tunable quadratic nonlinear optical (NLO) properties under different states hold great potential applications in a wide range of fields. Despite great efforts, lead-free organic–inorganic hybrid NLO switchable materials are still scarce. Here, we report a new lead-free organic–inorganic hybrid compound (NMP)2SnBr6 (1, where NMP is N-methylpyrrolidinium) which adopts the zero-dimensional perovskite-like motif. It demonstrates a structural phase transition at Tc = 352 K, as confirmed by differential scanning calorimetry and specific heat measurements. Single-crystal structure analyses at different temperatures indicate the occurrence of symmetry breaking from Pbam (at 360 K, > Tc) to Pba2 (at 300 K, < Tc), which can be attributed to the configuration changes of organic N-methylpyrrolidinium cations. In particular, 1 shows remarkable NLO signal contrast up to ∼25 and excellent switching stability after multiple heating–cooling cycles, suggesting its potential application as a switchable NLO material. This work paves the way for exploring new organic–inorganic hybrid functional materials.
Introduction
Stimuli-responsive materials are those substances which can respond to external stimuli, such as temperature, light, and external magnetic and electric fields.1–7 The physical properties of these materials can be changed significantly between different states. These switching properties have been applied in a wide range of applications such as lasers, memorizers and switches, thereby attracting great interest.8–11 Among them, switchable NLO properties have become an important research topic in condensed matter science.12 Previous studies revealed that the switchable NLO properties can be realized by various strategies, in which one of the efficient ways is to introduce flexible organic cations into inorganic frameworks.13 The flexible organic counterparts in the organic–inorganic hybrid system are favorable to molecular motions, including order–disorder transformation, reorientational and rotational motion, which are tightly associated with the switchable NLO properties of phase transition materials.14–18 For example, in (N-methylpyrrolidinium)PbBr3,19 phase transition can be mainly attributed to the order–disorder transformation of flexible N-methylpyrrolidinium cations. Other reported studies on bis(cyclohexylaminium)-tetrabromo lead and [N-methylpiperidinium]PbI3 also show order–disorder phase transition and corresponding excellent tunable NLO properties, which benefit from the reorientation of organic moieties and inorganic parts.20,21 These findings disclose that phase transition compounds with tunable NLO properties could be feasibly tailored through incorporation of flexible organic molecules into the framework of hybrid compounds.22,23Organic–inorganic hybrid compounds combine advantages of both organic and inorganic components at the molecular level.24–26 Within this system, organic moieties endow structural diversity and dynamic characteristics, which are favorable to the design of phase transition materials with tunable NLO properties.22,27 As an important family of organic components, annular organic cations play a key role in the design of phase transition materials with tunable NLO properties.28,29 For example, organic–inorganic hybrids of (CHA)2PbBr4−4xI4x, (benzylammonium)2PbCl4 and (pyrrolidinium)CdCl3 show excellent switchable NLO properties.30–32 Despite great efforts, reports on phase transition materials with tunable NLO properties in the family of organic–inorganic hybrids are still scarce, and most of these organic–inorganic hybrid dielectric switchable compounds contain metal lead, which can cause damage to the environment and limit their commercial applications. Moreover, the majority of tunable NLO materials in the organic–inorganic hybrid system are formed by order–disorder type phase transformation. Therefore, it is highly desirable to explore new phase transition materials with tunable NLO properties by virtue of a complementary strategy.
Here, we report a new zero-dimensional lead-free hybrid compound by incorporating organic N-methylpyrrolidinium (NMP) with the Sn–Br inorganic framework, ([NMP]2SnBr6, 1), showing a reversible structural phase transition at T1 = 352 K upon heating as confirmed by differential scanning calorimetry. Emphatically, it shows a remarkable NLO signal contrast up to ∼25, suggesting its potential application as a switchable NLO material. After multiple heating–cooling cycles, 1 still shows great switchable NLO and dielectric stability. Such switchable responses of 1 can be mainly attributed to the configuration changes of organic cations. This work paves the way for exploring new functional materials with NLO switching properties.
Experimental section
Synthesis
All chemical ingredients are commercially purchased without any purification. 10 mL HBr solution and 0.5 g SnO were put in a 50 mL round-bottom beaker. After that, the mixture was stirred for 1 hour to form a transparent solution. 0.5 mL N-methylpyrrodine was then added into the beaker. Next, the mixture was heated and stirred for 30 minutes to form a clear solution. Crystals of 1 were obtained after two weeks by slowly evaporating the solution at ambient temperature. The purity of crystals was confirmed through powder X-ray diffraction analyses, and the measured data were in accordance with the simulated patterns obtained from its single-crystal structure.Thermal measurements
Pure crystals of 1 were chosen to carry out thermal measurements using differential scanning calorimetry (DSC) (NETZCHSCH DSC 3500). Both the heating and cooling rates were fixed at 5 K per minute in the temperature range between 315 K and 360 K. The measurement was performed under atmospheric pressure with nitrogen gas. Specific heat (Cp) was measured upon a heating mode based on the sapphire standard.Single-crystal structure analyses
Single-crystal X-ray diffraction data of 1 at 300 K was obtained using a Rigaku CCD diffractometer with Ga-Kα radiation of wavelength 1.3405 Å. A Bruker D8 diffractometer with Mo-Kα of wavelength 0.71073 Å was used to obtain the structural data of 1 at 360 K. All the crystal structures were solved using the Olex2 software package using the intrinsic phasing method of the ShelXT program. All atoms except H were refined using an anisotropic method. All hydrogen atoms in the structures were geometrically generated using the software of Olex2. A detailed description of crystal structures of 1 is presented in Table S1 (ESI†). And all crystallographic data were uploaded to The Cambridge Crystallographic Data Center (CCDC) with numbers 2210039 and 2210040,† respectively.Dielectric measurements
Silver was covered on the surface of single crystals of 1 to fabricate electrodes. Dielectric experiments were then carried out to measure the complex dielectric permittivity in the temperature range of 330–370 K. The frequency was set at 1 MHz and the AC voltage was fixed at 1 V.Second harmonic generation (SHG) signal measurements
Variable-temperature measurements of SHG signals were performed using an Nd:YAG laser with incident wavelength of 1064 nm. Standard KH2PO4 was used as a reference and treated using the same procedure. The ground powder of 1 was compressed to tablets for the SHG signal measurements.Powder X-ray diffraction measurement (PXRD)
The powder X-ray diffraction pattern of 1 was recorded on a Powder X-Ray Diffractometer (MiniFlex 600). The measurement was performed in the 2-theta range between 5° to 40° at room temperature.Theoretical calculation analyses
Theoretical calculation analyses of single-crystal structure of 1 were performed using Cambridge Sequential Total Energy Package (CASTEP) under the DFT method. The exchange-correlation potential was calculated using Perdew–Burke–Ernzerhof method for solid functions using the approximation method of GGA. The norm-conserving pseudopotential was used to characterize the interactions between ionic cores and electrons. The energy cutoff for the plane-wave basis was fixed at 500 eV.Results and discussion
Phase transition properties
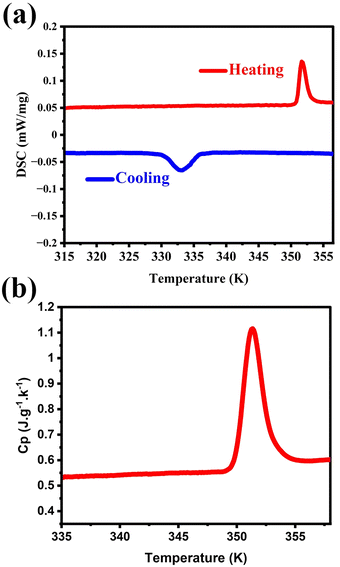
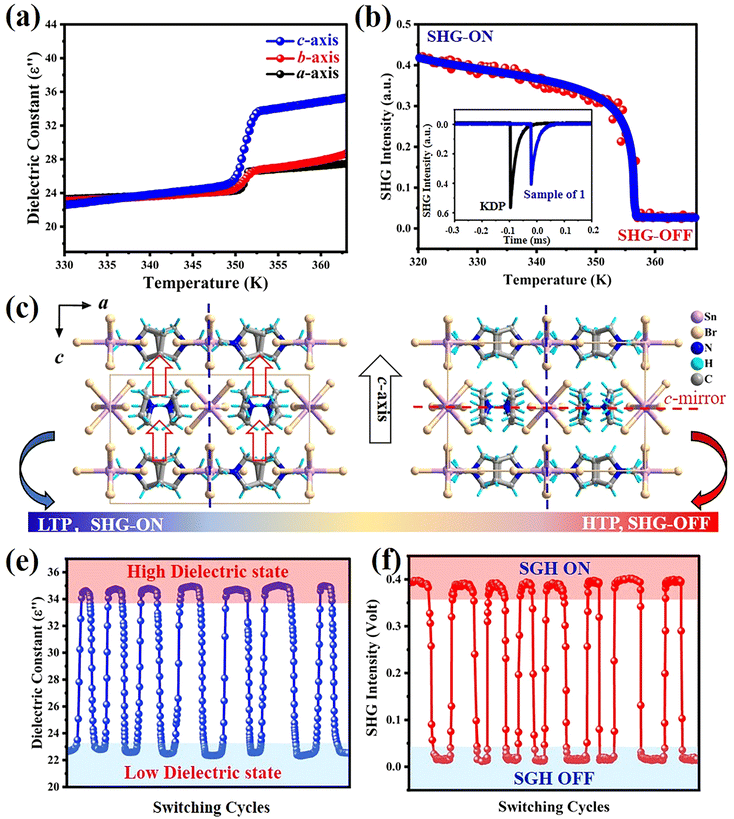
DSC and Cp measurements were effective ways used to investigate the thermal properties of phase transition materials. The structure phase transition of compounds was generally accompanied by thermal entropy changes, which can be confirmed in the heating and cooling process of DSC measurements. Here, the reversible endothermic and exothermic anomalies appeared at 352 K upon heating mode and 333 K upon cooling, which reveal the phase transition properties of 1 (Fig. 1a). In the DSC curves, the large thermal hysteresis (19 K) and sharp anomalies indicate its first order phase transition. The peak-like anomaly in Cp also corresponds well with the results of DSC (Fig. 1b). The enthalpy change (ΔH) and entropy change (ΔS) in the heating mode can be calculated from the Cp–T curve as 890.7 J mol−1 and 2.53 J mol−1 K−1, respectively. According to the Boltzmann equation ΔS = RInN, in which N is the ratio of orientations between different phases and R is the molar gas constant, N is calculated as 1.36, revealing the complexity of the phase transition of 1, instead of simple order–disorder transition (calculation is provided in the ESI†).Crystal structure analyses
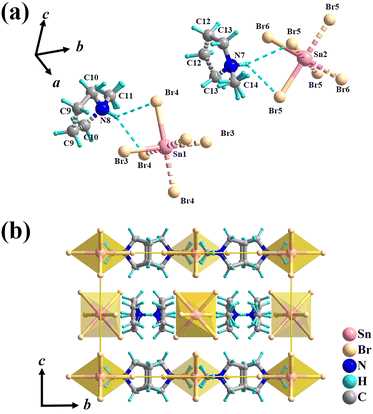
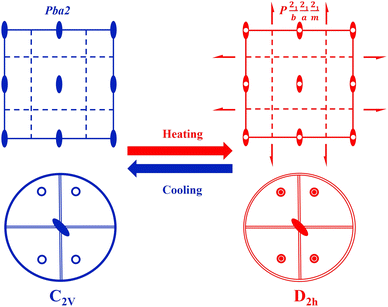
X-ray single-crystal diffraction analyses were performed to determine the microscopic mechanism of structural changes of 1 at two different temperatures, i.e., low temperature phase (LTP) at 300 K (below Tc) and high temperature phase (HTP) at 360 K (above Tc). The results reveal that 1 crystallizes in the same orthorhombic system but different space groups in different phases. In HTP, 1 crystallizes in a centric space group of Pbam. The cell parameters at HTP are α = γ = β = 90°, a = 13.9302 (10), b = 15.5956 (9), c = 10.1118 (8), V = 2196.8 (3) and Z = 4, as given in Table S1 (ESI†). The asymmetric unit of 1 consists of protonated NMP cations and anionic [SnBr6]2− octahedra in the HTP, as shown in Fig. 2a. Partial hydrogen atoms of NMP anions are disordered with atoms located at two possible equilibrium positions. The disorder characteristics can also be revealed by the thermal ellipsoids of atoms of NMP cation, in which the large thermal ellipsoids of C and N atoms indicate the average effect of the disordered atoms (Fig. S3, ESI†).33 Intermolecular H-bonding distances between protonated N atoms of NMP cations and Br atoms of [SnBr6] 2− anions are measured as 2.736 Å and 2.900 Å (Fig. S4, ESI†). All components of 1 show symmetric configurations with respect to their mirror planes due to highly crystallographic symmetry (Fig. 2b). In both phases, no significant changes for SnBr6 octahedrons are observed, whereas the distorted configuration of NMP cations can be observed in LTP. As the temperature decreased below Tc, crystal structure of 1 transforms into the non-centrosymmetric phase with the space group of Pba2 and point group of C2v. The cell parameters for this acentric structure are α = γ = β = 90°, a = 15.5317 (4), b = 13.8674 (3), c = 10.0184, V = 2157.39 (10) and Z = 4 (Table S1, ESI†). The changes in the cell volume and axes lengths suggest the emergence of phase transition. Meanwhile, the distorted NMP cations with N atoms moving toward the same direction of the crystallographic c-axis results in the disappearance of c-mirror plane and symmetry breaking. This structural transition means that the number of symmetric elements of 1 is halved from eight in the HTP to four in the LTP, as illustrated in Fig. 3.34 The asymmetric unit of 1 at LTP comprises two protonated NMP cations and two half-[SnBr6] 2− anions, as shown in Fig. 4a. Each Sn atom is coordinated with six Br atoms, forming an octahedral geometry. The organic cations are linked with the octahedral [SnBr6] 2− anions by N–H⋯Br hydrogen bonds (Fig. S4, ESI†). The bond lengths of Sn–Br are in the range of 2.593 to 2.607 Å, almost the same as those (2.5936 to 2.602 Å) in the HTP (Tables S2 and S3, ESI†). The angles of Br–Sn–Br are in the range of 88.05° to 91.4°, exhibiting slight deviation from the standard octahedral angle of 90°. In LTP, the hydrogen bond lengths of N–H⋯Br are 2.642 Å to 2.930 Å. The difference between the structure at HTP and LTP is that organic NMP cations show distorted configurations and orient along the crystallographic c-axis, which contribute to the acentric structure, as shown in Fig. 4b.35 The acentric structure was further confirmed by the measurement of SHG signals (Fig. 6b).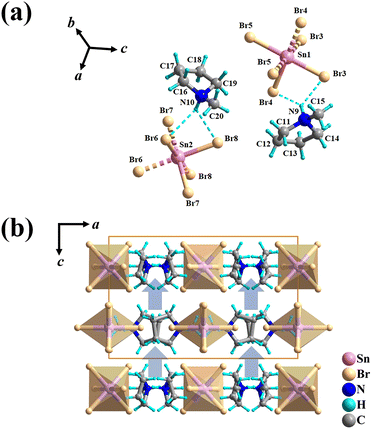 | ||
| Fig. 4 (a) Asymmetric unit and (b) packing view of the unit cell along the b-axis at 300 K. Dashed lines were used to link equivalent atoms except cyan dashed lines used to represent hydrogen bonds. | ||
The above studies reveal that between the HTP and LTP, no significant change was discovered in the inorganic SnBr6 octahedra, whereas the N atoms of NMP cations show obvious displacements. In detail, the N atoms of NMP cations deviate its c-mirror planes existing in HTP and reorientate toward the c-axis direction in LTP, leading to the structure phase transition.
Band structure analyses
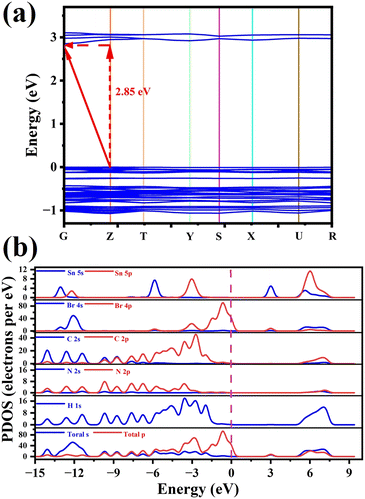
First principles DFT calculation was performed to analyze the electronic properties of 1. As shown in Fig. 5a, the lowest point of the conduction band and the highest point of the valence band are located at different points (G and Z, respectively), revealing the indirect bandgap characteristics of 1. Its bandgap is calculated as 2.76 eV, corresponding well with the experimental value (2.68 eV) obtained from the UV-vis absorption spectrum as shown in Fig. S2 (ESI†). The calculated distribution of partial density of states (PDOS) indicates the overlapped energy regions of C2s/p, N2s/p and H1s states are far away from the Fermi level due to strong covalent interactions of C–H and N–H bonds (Fig. 5b). However, the density states of Br4s/p and Sn5s/p are distributed near the Fermi level, determining the minimum of the conduction band and the maximum of the valence band. Those results denote that its band gap mainly stems from the electron distribution of inorganic components, resembling some previously reported materials such as (PEA)2PbI4.36 This is also revealed by the calculated charge-density distribution of 1 as shown in Fig. S5 (ESI†).Switchable dielectric and NLO properties
Generally, abrupt changes of physical properties of phase transition materials can be observed in the vicinity of Tc due to the structural changes. Here, variable-temperature dielectric properties of 1 were measured along different axes using single crystals. As shown in Fig. 6a, the real parts (ε′) of dielectric constants along three crystallographic axes keep virtually stable below Tc, matching with the low-dielectric state of 1. With temperature increasing to its Tc, obvious step-like changes in dielectric constant emerge along different crystallographic axes. Interesting, the ε′ value measured along c-axis is the highest in the high-dielectric state, in contrast with the lowest ε′ value along its a-axis. The difference in dielectric changes probably ascribes to the configuration changes of NMP cations, that is, the disappearance of the c-mirror and related symmetry breaking along the crystallographic c-direction (Fig. 6c). This dielectric transformation between different states resembles that of some switchable NLO materials, such as (hexamethyleneimine)2BiBr5 and (C3H8N2)2SbBr5.37,38 With the increasing temperature, 1 shows a dramatic variation of quadratic SHG signals, indicating the structural change of 1 from a non-centrosymmetric state (SHG-on) to a centrosymmetric one (SHG-off), as shown in Fig. 6b. The remarkable NLO signal contrast of ∼25 can be calculated between its SHG-on and SHG-off states, suggesting its potential applications in a switchable NLO field.39 The contrast figure of ∼25 is much higher than that of most NLO switching materials (liquids and solids), and is comparable with (Hdabco+)(CF3COO−) and NH4[(CH3)4N]SO4·H2O.40,41 Stability is an important factor for evaluating the potentials of functional materials. Therefore, the reversible properties of 1 were measured using crystal samples of 1. As shown in Fig. 6e, the dielectric responses can recover after at least 7 heating–cooling cycles without any attenuation. This reveals its great stability after repeating structure transformation. Furthermore, variable-temperature SHG measurements were carried out to confirm the switchable SHG responses. Similarly, the SHG signal responses show great anti-fatigue merit between its SHG-on and SHG-off states after multiple heating–cooling runs (Fig. 6f). Our further measurements reveal its SHG signals still retain rapid responses without any obvious suppression, even under long-time irradiation. These results suggest the great stability of 1, which affords its great potential applications as a NLO switching material.Conclusions
In summary, we have successfully explored a new lead-free organic–inorganic hybrid compound [NMP]2SnBr6. It demonstrates a structural phase transition along with the occurrence of symmetry breaking from Pbam to Pba2. The mechanism is ascribed to the symmetry configuration changes of organic cations, which differs from the majority of known NLO-switching systems. In particular, it shows distinct switchable NLO properties with a remarkable NLO signal contrast of up to ∼25. The contrast is larger than those of many other NLO switching materials. It also exhibits great dielectric and NLO stability after multiple cycles. These properties suggest its great potential application as a switchable NLO material. This work paves the way for exploring new organic–inorganic hybrid functional materials.Author contributions
Xinxin Hu carried out the experiments and wrote the manuscript. Haojie Xu and Wuqian Guo performed the characterization of compounds. Yi Liu performed theoretical calculations, and Shiguo Han analyzed the result of theoretical calculations. Yu Ma and Qingshun Fan measured the SHG signals. Junhua Luo provided suggestions on the manuscript writing. Zhuhua Sun conceived the idea and supervised the project.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank Aurang Zeb for help with experiments. This work was financially supported by the National Natural Science Foundation of China (22125110, 21875251, 21833010, and U21A2069), the Fujian Science & Technology Innovation Laboratory for Optoelectronic Information of China (2021ZR126), the Key Research Program of Frontier Sciences of the Chinese Academy of Sciences (ZDBS-LY-SLH024), the National Postdoctoral Program for Innovative Talents (BX2021315), and the National Key Research and Development Program of China (2019YFA0210402).References
- Y. Q. Shi, C. Zhang, H. Zhang, J. H. Bechtel, L. R. Dalton, B. H. Robinson and W. H. Steier, Low (Sub-1-Volt) Halfwave Voltage Polymeric Electro-Optic Modulators Achieved by Controlling Chromophore Shape, Science, 2000, 288, 119–122 CrossRef CAS PubMed
.
- M. Lee, H. E. Katz, C. Erben, D. M. Gill, P. Gopalan, J. D. Heber and D. J. McGee, Broadband Modulation of Light by Using An Electro-Optic Polymer, Science, 2002, 298, 1401–1403 CrossRef CAS PubMed
.
- F. Castet, V. Rodriguez, J. L. Pozzo, L. Ducasse, A. Plaquet and B. Champagne, Design and Characterization of Molecular Nonlinear Optical Switches, Acc. Chem. Res., 2013, 46, 2656–2665 CrossRef CAS PubMed
.
- M. A. C. Stuart, W. T. S. Huck, J. Genzer, M. Muller, C. Ober, M. Stamm, G. B. Sukhorukov, I. Szleifer, V. V. Tsukruk, M. Urban, F. Winnik, S. Zauscher, I. Luzinov and S. Minko, Emerging Applications of Stimuli-Responsive Polymer Materials, Nat. Mater., 2010, 9, 101–113 CrossRef PubMed
.
- S. Horike, S. Shimomura and S. Kitagawa, Soft Porous Crystals, Nat. Chem., 2009, 1, 695–704 CrossRef CAS PubMed
.
- H. J. Xu, W. Q. Guo, J. Q. Wang, Y. Ma, S. G. Han, Y. Liu, L. Lu, X. Pan, J. H. Luo and Z. H. Sun, A Metal-Free Molecular Antiferroelectric Material Showing High Phase Transition Temperatures and Large Electrocaloric Effects, J. Am. Chem. Soc., 2021, 143, 14379–14385 CrossRef CAS PubMed
.
- Y. Y. Tang, J. C. Liu, Y. L. Zeng, H. Peng, X. Q. Huang, M. J. Yang and R. G. Xiong, Optical Control of Polarization Switching in a Single-Component Organic Ferroelectric Crystal, J. Am. Chem. Soc., 2021, 143, 13816–13823 CrossRef CAS PubMed
.
- Y. L. Yu, M. Nakano and T. Ikeda, Directed Bending of a Polymer Film by Light - Miniaturizing A Simple Photomechanical System Could Expand Its Range of Applications, Nature, 2003, 425, 145 CrossRef CAS PubMed
.
- J. A. Delaire and K. Nakatani, Linear and Nonlinear Optical Properties of Photochromic Molecules and Materials, Chem. Rev., 2000, 100, 1817–1845 CrossRef CAS PubMed
.
- M. Samoc, N. Gauthier, M. P. Cifuentes, F. Paul, C. Lapinte and M. G. Humphrey, Electrochemical Switching of the Cubic Nonlinear Optical Properties of an Aryldiethynyl-Linked Heterobimetallic Complex Between Three Distinct States, Angew. Chem., Int. Ed., 2006, 45, 7376–7379 CrossRef CAS PubMed
.
- M. Wuttig and N. Yamada, Phase-Change Materials for Rewriteable Data Storage, Nat. Mater., 2007, 6, 824–832 CrossRef CAS PubMed
.
- W. Zhang, Y. Cai, R. G. Xiong, H. Yoshikawa and K. Awaga, Exceptional Dielectric Phase Transitions in a Perovskite-Type Cage Compound, Angew. Chem., Int. Ed., 2010, 49, 6608–6610 CrossRef CAS PubMed
.
- L. Li, Z. Sun, P. Wang, W. Hu, S. Wang, C. Ji, M. Hong and J. Luo, Tailored Engineering of An Unusual (C4H9NH3)2(CH3NH3)2Pb3Br10 Two-Dimensional Multilayered Perovskite Ferroelectric for A High-Performance Photodetector, Angew. Chem., Int. Ed., 2017, 56, 12150–12154 CrossRef CAS PubMed
.
- M. Ptak, M. Maczka, A. Gagor, A. Sieradzki, A. Stroppa, D. Di Sante, J. M. Perez-Mato and L. Macalik, Experimental and Theoretical Studies of Structural Phase Transition in A Novel Polar Perovskite-Like C2H5NH3 Na0.5Fe0.5(HCOO)(3) Formate, Dalton Trans., 2016, 45, 2574–2583 RSC
.
- P. Zhou, Z. H. Sun, S. Q. Zhang, C. M. Ji, S. G. Zhao, R. G. Xiong and J. H. Luo, A Sequentially Switchable Molecular Dielectric Material Tuned by the Stepwise Ordering in Diisopropylammonium Trifluoromethanesulfonate, J. Mater. Chem. C, 2014, 2, 2341–2345 RSC
.
- S. Y. Zeng, Z. H. Sun, C. M. Ji, S. Q. Zhang, C. Song and J. H. Luo, Dibenzylammonium trichloroacetate: an above-room-temperature order-disorder switchable dielectric material, CrystEngComm, 2016, 18, 3606–3611 RSC
.
- Z. X. Wang, W. Q. Liao, H. Y. Ye and Y. Zhang, Sequential Structural Transitions with Distinct Dielectric Responses in A Layered Perovskite Organic-Inorganic Hybrid Material: (C4H9N)2 PbBr4, Dalton Trans., 2015, 44, 20406–20412 RSC
.
- W. Zhang, H. Y. Ye, R. Graf, H. W. Spiess, Y. F. Yao, R. Q. Zhu and R. G. Xiong, Tunable and Switchable Dielectric Constant in an Amphidynamic Crystal, J. Am. Chem. Soc., 2013, 135, 5230–5233 CrossRef CAS PubMed
.
- Q. Fan, Y. Ma, H. Xu, Y. Song, Y. Liu, J. Luo and Z. Sun, Near-Room-Temperature Reversible Switching of Quadratic Optical Nonlinearities in A One-Dimensional Perovskite-Like Hybrid, Microstructures, 2022, 2, 2022013 CrossRef
.
- Z. H. Sun, X. T. Liu, T. Khan, C. M. Ji, M. A. Asghar, S. E. Zhao, L. N. Li, M. C. Hong and J. H. Luo, A Photoferroelectric Perovskite-Type Organometallic Halide with Exceptional Anisotropy of Bulk Photovoltaic Effects, Angew. Chem., Int. Ed., 2016, 55, 6545–6550 CrossRef CAS PubMed
.
- H. Y. Zhang, X. G. Chen, Z. X. Zhang, X. J. Song, T. Zhang, Q. Pan, Y. Zhang and R. G. Xiong, Methylphosphonium Tin Bromide: A 3D Perovskite Molecular Ferroelectric Semiconductor, Adv. Mater., 2020, 32, e2005213 CrossRef PubMed
.
- W. Q. Guo, X. T. Liu, S. G. Han, Y. Liu, Z. Y. Xu, M. C. Hong, J. H. Luo and Z. H. Sun, Room-Temperature Ferroelectric Material Composed of A Two-Dimensional Metal Halide Double Perovskite for X-ray Detection, Angew. Chem., Int. Ed., 2020, 59, 13879–13884 CrossRef CAS PubMed
.
- W. Zhang and R. G. Xiong, Ferroelectric Metal-Organic Frameworks, Chem. Rev., 2012, 112, 1163–1195 CrossRef CAS PubMed
.
- B. Saparov and D. B. Mitzi, Organic-Inorganic Perovskites: Structural Versatility for Functional Materials Design, Chem. Rev., 2016, 116, 4558–4596 CrossRef CAS PubMed
.
- Z. Y. Du, T. T. Xu, B. Huang, Y. J. Su, W. Xue, C. T. He, W. X. Zhang and X. M. Chen, Switchable Guest Molecular Dynamics in A Perovskite-Like Coordination Polymer Toward Sensitive Thermoresponsive Dielectric Materials, Angew. Chem., Int. Ed., 2015, 54, 914–918 CrossRef CAS PubMed
.
- W. Q. Liao, Y. Y. Tang, P. F. Li, Y. M. You and R. G. Xiong, Large Piezoelectric Effect in A Lead-Free Molecular Ferroelectric Thin Film, J. Am. Chem. Soc., 2017, 139, 18071–18077 CrossRef CAS PubMed
.
- S. Han, G.-E. Wang, G. Xu, J. Luo and Z. Sun, Ferroelectric Perovskite-Type Films with Robust in-Plane Polarization Toward Efficient Room-Temperature Chemiresistive Sensing, Fundam. Res., 2022 DOI:10.1016/j.fmre.2022.01.015
.
- L. Mao, C. C. Stoumpos and M. G. Kanatzidis, Two-Dimensional Hybrid Halide Perovskites: Principles and Promises, J. Am. Chem. Soc., 2019, 141, 1171–1190 CrossRef CAS PubMed
.
- C. R. Huang, X. Z. Luo, X. G. Chen, X. J. Song, Z. X. Zhang and R. G. Xiong, A Multiaxial Lead-Free Two-Dimensional Organic-Inorganic Perovskite Ferroelectric, Natl. Sci. Rev., 2021, 8, nwaa232 CrossRef CAS PubMed
.
- H. Y. Ye, W. Q. Liao, C. L. Hu, Y. Zhang, Y. M. You, J. G. Mao, P. F. Li and R. G. Xiong, Bandgap Engineering of Lead-Halide Perovskite-Type Ferroelectrics, Adv. Mater., 2016, 28, 2579 CrossRef CAS PubMed
.
- W. Q. Liao, Y. Zhang, C. L. Hu, J. G. Mao, H. Y. Ye, P. F. Li, S. D. Huang and R. G. Xiong, A Lead-Halide Perovskite Molecular Ferroelectric Semiconductor, Nat. Commun., 2015, 6, 7338 CrossRef PubMed
.
- Y. Y. Tang, Y. Ai, W. Q. Liao, P. F. Li, Z. X. Wang and R. G. Xiong, H/F-Substitution-Induced Homochirality for Designing High-Tc Molecular Perovskite Ferroelectrics, Adv. Mater., 2019, 31, e1902163 CrossRef PubMed
.
- Y. Zhang, W. Zhang, S. H. Li, Q. Ye, H. L. Cai, F. Deng, R. G. Xiong and S. D. Huang, Ferroelectricity Induced by Ordering of Twisting Motion in A Molecular Rotor, J. Am. Chem. Soc., 2012, 134, 11044–11049 CrossRef CAS PubMed
.
-
H. Fuess, T. Hahn, H. Wondratschek, U. Müller, U. Shmueli, E. Prince, A. Authier, V. Kopský, D. B. Litvin, M. G. Rossmann, E. Arnold, S. R. Hall, B. McMahon and T. Hahn, The International Tables for Crystallography, Springer, Dordrecht, 2006 Search PubMed
.
- X. Hu, H. Xu, Y. Liu, L. Lu, W. Guo, S. Han, J. Luo and Z. Sun, Incorporating an Aromatic Cationic Spacer to Assemble 2D Polar Perovskite Crystals Toward Self-Powered Detection of Quite Weak Polarized Light, J. Phys. Chem. Lett., 2022, 13, 6017–6023 CrossRef CAS PubMed
.
- Y. Liu, H. Ye, Y. Zhang, K. Zhao, Z. Yang, Y. Yuan, H. Wu, G. Zhao, Z. Yang, J. Tang, Z. Xu and S. Liu, Surface-Tension-Controlled Crystallization for High-Quality 2D Perovskite Single Crystals for Ultrahigh Photodetection, Matter, 2019, 1, 465–480 CrossRef
.
- J. Zhang, S. G. Han, X. T. Liu, C. M. Ji, K. W. Tao, M. A. Asghar, J. H. Luo and Z. H. Sun, Successive Near-Room-Temperature Dielectric Phase Transitions in a Lead-Free Hybrid Perovskite-Like Compound, Inorg. Chem. Front., 2019, 6, 233–237 RSC
.
- M. Li, B. Teng, S. Han, T. Yang, Y. Li, Y. Liu, X. Zhang, X. Liu, J. Luo and Z. Sun, Near-Room-Temperature Tunable Dielectric Response Induced by Dual Phase Transitions in a Lead-Free Hybrid: (C3H8N)2SbBr5, CrystEngComm, 2019, 21, 3740–3744 RSC
.
- P. Serra-Crespo, M. A. van der Veen, E. Gobechiya, K. Houthoofd, Y. Filinchuk, C. E. Kirschhock, J. A. Martens, B. F. Sels, D. E. De Vos, F. Kapteijn and J. Gascon, NH2-MIL-53(Al): A High-Contrast Reversible Solid-State Nonlinear Optical Switch, J. Am. Chem. Soc., 2012, 134, 8314–8317 CrossRef CAS PubMed
.
- Z. Sun, J. Luo, S. Zhang, C. Ji, L. Zhou, S. Li, F. Deng and M. Hong, Solid-State Reversible Quadratic Nonlinear Optical Molecular Switch with an Exceptionally Large Contrast, Adv. Mater., 2013, 25, 4159–4163 CrossRef CAS PubMed
.
- S. Liu, Z. Sun, C. Ji, L. Li, S. Zhao and J. Luo, Exceptional Bi-Step Switching of Quadratic Nonlinear Optical Properties in a One-Dimensional Channel Compound, Chem. Commun., 2017, 53, 7669–7672 RSC
.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2210039 and 2210040. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2qm01340a |
| This journal is © the Partner Organisations 2023 |