Open Access Article
Open Access ArticleResponses of aquatic vegetables to biochar amended soil and water environments: a critical review
Xiangjun Wanga,
Yaming Zhaoa,
Guangwei Yaob,
Zhizhong Linb,
Laiyuan Xub,
Yunli Jianga,
Zewen Jina,
Shengdao Shana and
Lifeng Ping *a
*a
aKey Laboratory of Recycling and Eco-Treatment of Waste Biomass of Zhejiang Province, Zhejiang University of Science and Technology, Hangzhou, 310023, PR China. E-mail: lfping2005@aliyun.com
bKaihua Agricultural and Rural Bureau, Quzhou, Zhejiang Province 324399, PR China
First published on 1st February 2023
Abstract
Aquatic vegetables, including lotus root, water spinach, cress, watercress and so on, have been cultivated as commercial crops for a long time. Though aquatic vegetables have great edible and medicinal values, the increasing demands for aquatic vegetables with high quality have led to higher requirements of their soil and water environments. Unfortunately, the soil and water environment often face many problems such as nutrient imbalance, excessive fertilization, and pollution. Therefore, a new cost-effective and eco-friendly solution for addressing the above issues is urgently required. Biochars, one type of pyrolysis product obtained from agricultural and forestry waste, show great potential in reducing fertilizer application, upgrading soil quality and remediating pollution. Application of biochars in aquatic vegetable cultivation would not only improve the yield and quality, but also reduce its edible risk. Biochars can improve the soil micro-environment, soil microorganism and soil enzyme activities. Furthermore, biochars can remediate the heavy metal pollution, organic pollution and nitrogen and phosphorus non-point source pollution in the water and soil environments of aquatic vegetables, which promotes the state of cultivation conditions and thereby improves the yield and quality of aquatic vegetables. However, the harmful substances such as heavy metals, PAHs, etc. derived from biochars can cause environmental risks, which should be seriously considered. In this review, the application of biochars in aquatic vegetable cultivation is briefly summarized. The changes of soil physicochemical and biological properties, the effects of biochars in remediating water and soil environmental pollution and the impacts of biochars on the yield and quality of aquatic vegetables are also discussed. This review will provide a comprehensive overview of the research progress on the effects of biochars on soil and water environments for aquatic vegetable cultivation.
1. Introduction
Aquatic vegetables are traditional vegetables in China. The artificial planting area of aquatic vegetables in China is more than 733![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 hectares, which is mainly distributed in the middle and lower regions of the Yangtze River Basin,1 and the common aquatic vegetables include lotus root (Nelumbo nucifera Gaertn), Zizania aquatica (Zizania latifolia Griseb. Turcz.), cress (Oenanthe stoloni fera DC.), water spinach (Ipomoea aquatic Forsk), water-shield (Brasenia schreberi J.F. Gmel.), watercress (Nasturtium officinale R. Br.), etc. With the development of the economy, aquatic vegetables with good edible qualities have become more favorable, which has led to growing demands and expanding scales for cultivating aquatic vegetables.2 The cultivation of aquatic vegetables with high quality requires good soil and water environments. However, recent studies indicated that the soil of aquatic vegetables is facing problems due to application of excessive fertilizer that has led to nutrient imbalance and soil acidification, which give rise to nitrogen (N) and phosphorus (P) non-point source pollution.3–6 Besides, heavy metal pollution7–9 and organic pollution10,11 have also been observed in aquatic vegetable soils. Therefore, to improve the fertility of the soil, remediating polluted water and soil environments and maintaining the ecological balance of the water and soil environment for producing high quality aquatic vegetables have become more and more urgent. It is reported that application of biochars in the cultivation of aquatic vegetables could improve the flooded soil physicochemical properties and fertilities,12,13 purify the water quality, and alleviate the extent of pollutants,14–18 which would promote the growth of aquatic vegetables and enhance their yields and qualities.
000 hectares, which is mainly distributed in the middle and lower regions of the Yangtze River Basin,1 and the common aquatic vegetables include lotus root (Nelumbo nucifera Gaertn), Zizania aquatica (Zizania latifolia Griseb. Turcz.), cress (Oenanthe stoloni fera DC.), water spinach (Ipomoea aquatic Forsk), water-shield (Brasenia schreberi J.F. Gmel.), watercress (Nasturtium officinale R. Br.), etc. With the development of the economy, aquatic vegetables with good edible qualities have become more favorable, which has led to growing demands and expanding scales for cultivating aquatic vegetables.2 The cultivation of aquatic vegetables with high quality requires good soil and water environments. However, recent studies indicated that the soil of aquatic vegetables is facing problems due to application of excessive fertilizer that has led to nutrient imbalance and soil acidification, which give rise to nitrogen (N) and phosphorus (P) non-point source pollution.3–6 Besides, heavy metal pollution7–9 and organic pollution10,11 have also been observed in aquatic vegetable soils. Therefore, to improve the fertility of the soil, remediating polluted water and soil environments and maintaining the ecological balance of the water and soil environment for producing high quality aquatic vegetables have become more and more urgent. It is reported that application of biochars in the cultivation of aquatic vegetables could improve the flooded soil physicochemical properties and fertilities,12,13 purify the water quality, and alleviate the extent of pollutants,14–18 which would promote the growth of aquatic vegetables and enhance their yields and qualities.
Biochars, rich in organic carbon, are a kind of carbon sequestration substance obtained from pyrolysis of organic biomass materials under oxygen-limited conditions.19 Biochars can be made from agricultural and forest residues, sludge and other raw materials.20 Biochars have prominent properties with condensed aromatic structures, abundant pores and large specific surface areas, and importantly, most biochars are alkaline,21 which is conducive to improving the acidity of soil and in situ passivation of heavy metal contaminated soil. In addition, the –COOH, –OH, –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, –CHO, phenolic –OH and other oxygen-containing functional groups of biochars can promote the immobilization and binding efficiency of inorganic contaminants in the soil, which is beneficial to improving soil fertility.22 As one of important soil amendments, biochars can sorb and improve the soil nutrients, which can enhance the soil mineral carbon and nitrogen contents.23 Besides, biochars can affect soil microbial biomass and the growth, diversity and community compositions of microbes by directly providing growth promoters for soil biota and indirectly changing soil properties.24 With the porous structures, biochars can be shelter for soil microorganisms by providing habitats. In addition, biochars can influence soil nutrient and carbon availability, soil pH, the sorption of toxins and chemical signals, soil water holding capacity against desiccation, which affect microbial abundance.25 Furthermore, changes in soil microbial growth, diversity and community compositions can regulate the cycle of N and P nutrients in the soil and promote the soil nutrient utilization rate.23 Additionally, biochars can also improve the microbial carbon and nitrogen contents as well as soil sucrase, phosphatase, catalase, and urease activities.26,27 Moreover, biochars can not only remediate heavy metal pollution, and N and P non-point source pollution, but also facilitate the degradation of organic pollutants (such as polycyclic aromatic hydrocarbons, PAHs).28,29 Biochars have been widely used in the sequestration of atmospheric carbon, reduction of water erosion and improvement of crop yields.30–32
O, –CHO, phenolic –OH and other oxygen-containing functional groups of biochars can promote the immobilization and binding efficiency of inorganic contaminants in the soil, which is beneficial to improving soil fertility.22 As one of important soil amendments, biochars can sorb and improve the soil nutrients, which can enhance the soil mineral carbon and nitrogen contents.23 Besides, biochars can affect soil microbial biomass and the growth, diversity and community compositions of microbes by directly providing growth promoters for soil biota and indirectly changing soil properties.24 With the porous structures, biochars can be shelter for soil microorganisms by providing habitats. In addition, biochars can influence soil nutrient and carbon availability, soil pH, the sorption of toxins and chemical signals, soil water holding capacity against desiccation, which affect microbial abundance.25 Furthermore, changes in soil microbial growth, diversity and community compositions can regulate the cycle of N and P nutrients in the soil and promote the soil nutrient utilization rate.23 Additionally, biochars can also improve the microbial carbon and nitrogen contents as well as soil sucrase, phosphatase, catalase, and urease activities.26,27 Moreover, biochars can not only remediate heavy metal pollution, and N and P non-point source pollution, but also facilitate the degradation of organic pollutants (such as polycyclic aromatic hydrocarbons, PAHs).28,29 Biochars have been widely used in the sequestration of atmospheric carbon, reduction of water erosion and improvement of crop yields.30–32
Nonetheless, the aquatic environment is different from terrestrial environments, thus it is urgent to strengthen studies on the environmental impact of biochars on aquatic environments such as the carbon (C)/N/P cycle and so on. Besides, biochars contain harmful substances such as heavy metals, PAHs, etc., which have potential negative effects on the biomass of aquatic phytoplankton and aquatic animals' normal activities.33,34
A large number of studies and reviews so far have focused on the impact of biochars on terrestrial vegetables. However, there are few systematic studies or reviews on the application of biochars in aquatic vegetable cultivation. Here, we summarize the application of biochar in aquatic vegetable cultivation by studying the effects of biochars on soil physicochemical and biological properties, highlighting the effects of pristine and modified biochars on water and soil environmental pollution, discussing the effects of biochars on the yield and quality of aquatic vegetables and providing useful suggestions for future research. This review will provide a comprehensive overview of the research progress on the effects of biochars on the soil and water environments for aquatic vegetable cultivation.
2. Effects of biochars on soil fertility of aquatic vegetables
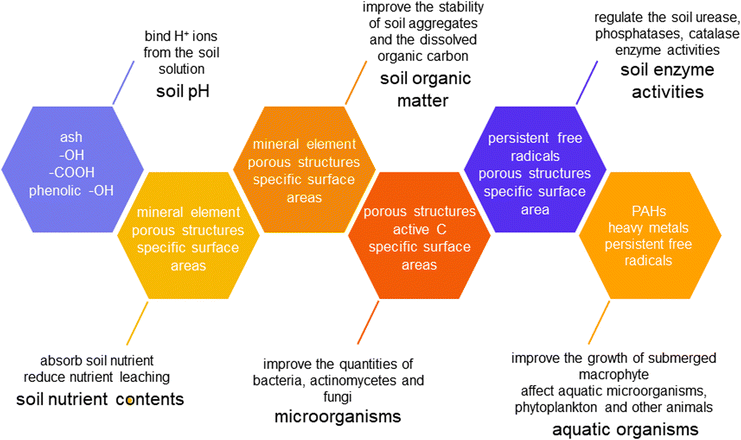
Biochars can improve the soil fertility of aquatic vegetables. In order to better understand the connections between biochars and soil fertility, the effects of biochars on soil physicochemical and biological properties for aquatic vegetable cultivation are described (Fig. 1).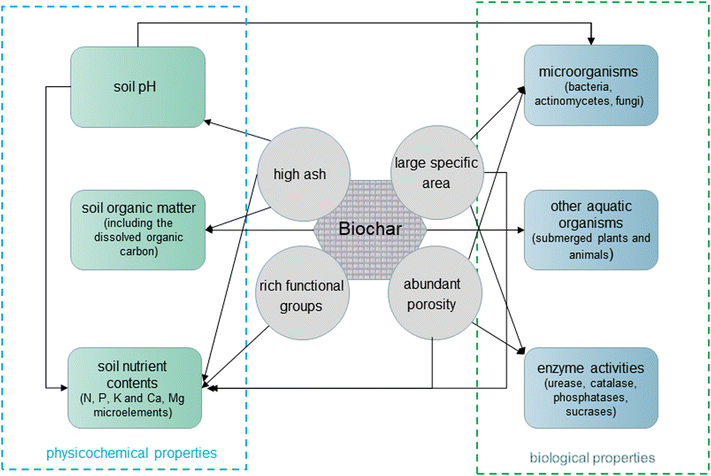 | ||
| Fig. 1 Effects of biochars on soil physicochemical and biological properties for aquatic vegetable cultivation. | ||
2.1 Effects of biochars on soil physicochemical properties for aquatic vegetable cultivation
Besides, biochar addition can also increase soil nutrition retention by improving soil physical structures. Juriga et al.48 found that the content of water-stable macro-aggregates in soil was increased after 20 t ha−1 biochar was added. This study indicated that the mean weight diameter and the index of water-stable aggregates of soil increased, and the coefficient of vulnerability of the soil decreased. The addition of livestock and poultry biochar to soil can improve the soil physical properties including porosity and permeability, thus further enhancing soil water and nutrient retention.
Moreover, biochars with porous structures and large specific surface areas can adsorb and retain nutrients in soil, which can reduce nutrient leaching and improve nutrient utilization for aquatic vegetables. The surface of biochar is capable of adsorbing ions in soil solutions, and the electrostatic and capillary forces on the surface reduce the leaching loss of nutrients in the soil.49,50 In terms of N sources, there are two main mechanisms for the effects of biochars on soil N availability: biological (fixation, mineralization, denitrification, and plant absorption) and abiotic (adsorption, volatilization, and leaching).51,52 Biochars with porous structures have the ability to adsorb NH4+–N, which plays an important role in slowing down soil N release. NO3−–N, with high mobility, is the main form of N leached from vegetable fields.53 The modified biochars applied to soil can absorb NO3− through anion exchange sites, thus improving the retention of NO3−.49 Biochars also have the ability to retain inorganic N ions.54 Zhao et al.55 suggested that application of coconut shell biochar could reduce soil N leaching by 17.3–34.0% in water spinach soil, which could be attributed to negatively charged functional groups on the surface of the biochar, thus the added biochar could absorb positively charged NH4+.56,57 The ammonia N in soil was fixed with biochar particles, therefore preventing the conversion of ammonia N into nitrate N to reduce the leaching of N. Another report indicated that the amount of TN and NH4+ in the underground seepage of water cress soil decreased after treatment with wheat straw biochar pyrolyzed at 500 °C and 700 °C.58 There is a relative lack of research on nutrients in the surface water of aquatic vegetable fields except for paddy fields. Wang et al.59 stated that the mean values of all sampling days of NH4+, NO3−, NO2−, TN, and total P (TP) concentrations in the surface water of paddy plots supplemented with biochar were 15.2%, 55.6%, 26.6%, 22.3%, and 52.6%, which were lower than those of the controls.
In summary, biochars could improve the soil nutrient contents and elevate nutrient effectiveness and reduce nutrient leaching by absorbing and fixing nutrients in soil. Besides, biochars could improve soil physical structures to increase soil nutrition retention (Fig. 2).
2.2 Effects of biochars on soil biological properties for aquatic vegetable cultivation
To date, there are few studies that have investigated the effects of biochar application on microorganisms in the soil and water environment and its cellular and molecular mechanisms. With the aid of incubation experiments on microorganisms, Zhang et al.61 investigated the effects of biochars on soil bacterium (Paracoccus denitrificans) denitrification by physiological, proteomic and metabolomics analyses. The results showed that the enhancement effect on denitrification was positively correlated with the pyrolysis temperature (300–500 °C) and dosage (0.1–1%), regardless of the raw material (corn straw or wheat straw). Besides, the study also found that bulk particles of corn straw biochar pyrolyzed at 500 °C might directly modulate carbon metabolism by the adsorption of extracellular metabolites rather than direct contact with cells, which reduced power distribution, improved energy utilization efficiency and enhanced the growth rate of Pc. denitrifificans.
Biochars show inhibition or activation functions on different enzymes, thus regulating the soil enzyme activities. A previous study showed that the combination of Fe fertilizer and PB increased soil urease activity of water spinach soil, while CB combined with Fe fertilizer increased the activities of ureases, sucrases and catalases in water spinach soil.18 Application of peanut shell biochar in water spinach soil increased the activities of soil ureases, phosphatases, catalases, and sucrases by 29.06%, 119.16%, 14.98%, and 189.18%.12 Jiang et al.43 reported that biochar and exogenous Ca could enhance the soil enzymatic activity of urease and catalase, which improved soil health. Nevertheless, the previous report confirmed that adding rice straw biochar to water spinach soil with Cd contamination reduced soil acid phosphatase activities.15 Biochar can directly affect soil pH and adsorb both enzymes themselves and their substrates,63 which regulates soil urease, sucrase, phosphatase, and catalase activity. Furthermore, the persistent free radicals on the surface of biochar can participate in electron transport and interact with redox enzymes to enhance oxidoreductase activity (Fig. 2).63 As far as we know, the aquatic system is different from the soil system. To date, few studies have explored how enzyme activity will work in the aquatic system and how enzymes interact with the soil system, which are worth further investigation.
Biochars may introduce potential ecological risks to soil-water systems. Bastos et al.68 found that Vibrio fischeri, a marine bioluminescent bacterium, was most sensitive to the aqueous extract of soil-biochar. Zhang et al.69 reported that the maximum obstruction rate of bamboo biochar to Pseudokirchneriella subcapitata and Limnodrilus hoffmeisteri was 6.47% and 29.87%. When the pyrolysis temperature of biochar exceeded 600 °C, the biotoxicity of Phyllostachys pubescens was low and it had little effect on its biomass, reproduction, and lipid contents. Similarly, Smith et al.70 reported that water-soluble organic compounds from pinewood biochar (pyrolysis temperature of 500 °C) had no obvious toxicity towards blue–green algae growth. Nevertheless, Zhang et al.71 suggested that pine needle biochar inhibited the luminescence rate of Photobacterium phosphoreum and the cell growth and chlorophyll-A synthesis of Scenedesmus obliquus, and the inhibition rate was elevated with the increase of biochar amount added. Due to the intracellular reactive oxygen species (ROS) in aquatic organisms formed from the high concentration of free radicals in biochar particles, the activities of ROS were improved in P. phosphoreum and S. obliquus, which in turn increased the activities of superoxide dismutase (SOD). It is well known that SOD and POD activities are effective biomarkers to determine toxicological performance.72–74
The studies mentioned above indicated that application of biochars to soil and aquatic environments would introduce toxic substances such as polycyclic aromatic hydrocarbons (PAHs), heavy metals, and free radicals, which could be inevitably formed during biochar production.69,75–77 Consequently, this could cause potential adverse effects on aquatic microorganisms, phytoplankton, and aquatic animals (Fig. 2). When biochar is applied to aquatic environments, the possible safety risks and stability effects should be comprehensively evaluated.
3. Effects of biochars for remediating water and soil environment pollution
3.1 Effects of biochars on heavy metal pollution in soil and water environments
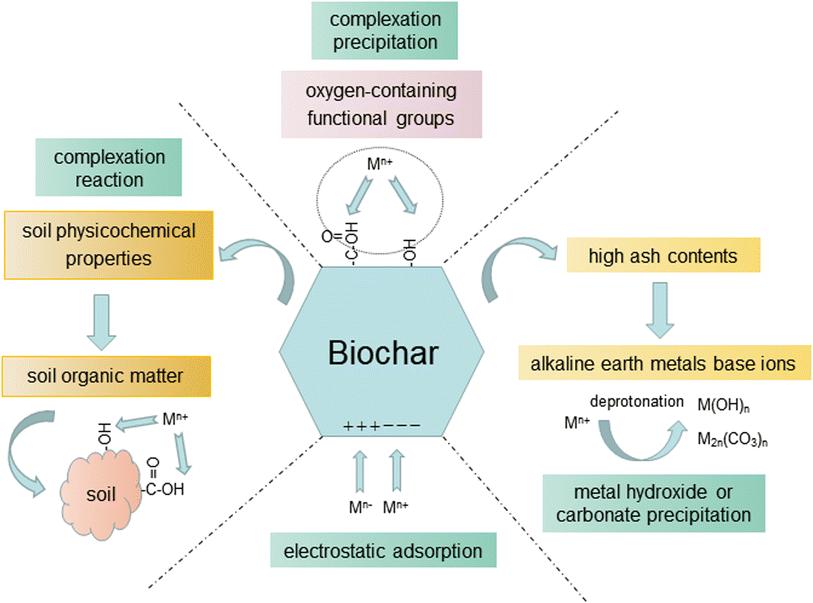
Heavy metals, derived from anthropogenic activities such as mining, irrigation with wastewater, waste disposal, and addition of fertilizers to agricultural land, are common pollutants in soil and water environments, which can reduce the quality of aquatic vegetables78,79 and become a threat to the edible safety of aquatic vegetables. Aquatic vegetables were grown in lakes, wetlands and paddy fields along the Yangtze River.1,80 The concentrations of copper (Cu), Cd, zinc (Zn), and Pb in the Yangtze Plain sediments were above the safe value, and the pollution levels of heavy metals in the sediments were: Zn > arsenic (As) > Cd > Cu > Pb > mercury (Hg).8 Cui et al.7 reported that the Hazard Quotients (HQs) of As, Cd, chromium (Cr), and Cu in the water body of Taihu Lake were 2.8, 1.6, 1.4, and 4.86, respectively, which exceeded the safe value (HQ = 1) and had a negative impact on water shield.Heavy metals have different states. The acid soluble state is highly mobile and easily utilized by plants, while the residual state with weak migration ability is the most stable state hardly used by plants. The reducible and oxidizable state can convert into the acid soluble state in the appropriate environment. Biochars can reduce the availability of heavy metals by directly changing the state of heavy metals in soil by increasing the soil pH. Guo et al.12 applied biochars, Se fertilizer and their combination to two cultivars of water spinach soil with heavy metal pollution. It was found that all three application methods could reduce the contents of Cr(VI) in soil, but biochars played a major role. The contents of total Cr in the soil did not change obviously, which may be due to the conversion of Cr from the available state to residual state after application of biochar. Chen et al.81 showed that application of biochars and pig manure could increase the contents of carbonate-bound Cu and organic matter-bound Cu in soil, while the contents of exchangeable Cu decreased, thus the bioavailability of Cu was decreased as well. Bashir et al.14 suggested that the soil pH increased when rice straw biochar was applied to Cd contaminated soil of water spinach, and the Cd in soil was immobilized by rice straw biochar through adsorption and precipitation, therefore reducing the Cd stress of water spinach (Fig. 3). Similar effects of biochar from Camellia oleifera shell were reported under submerged conditions in soil. The coupling of submerged conditions with biochar could promote the transformation of the acid soluble state of Cd into the residue state of Cd, and compared with the control treatment, the contents of acid soluble Cd decreased by 45% in the treated group after 60 days of flooding.82
Methylmercury is a toxic substance that can be accumulated in the aquatic food chain, and is mainly derived from the microbial methylation of inorganic Hg2+ in anoxic sediments or photomethylation in the water environment.83 Bussan et al.84 examined the effects of activated carbon and pinewood biochar on Hg methylation in wetland sediments. The methylation rate of inorganic 200Hg2+ was 0.37%/d in untreated sediment, but it decreased to 0.08%/d in treated sediment. The methylation rate of activated carbon and biochar treated sediment decreased by 80% and 88%, respectively. Activated carbon and biochar can be used to remediate polluted sediment in aquatic environments and reduce the content of bioavailable Hg in the sediment.
Biochars can also adsorb and immobilize heavy metals in soil and water environments, and the adsorption capacity of heavy metals in water environments is improved after modification. After application of 2% Mg oxide biochar–chitosan composite materials, the content of bioavailable Cd, acid soluble Cd, and residual Cd was reduced by 22.32%, 24.77%, and 22.24%.85 Biochars can also cooperate with submerged plants to remediate heavy metal polluted water environments. Chen et al.86 applied rice husk biochar to Vallisneria spinulosa in copper-polluted water, and the results indicated that the addition of rice husk biochar reduced the copper enrichment content of V. spinulosa, and then reduced the copper stress on V. spinulosa. Addition of biochar could also increase the chlorophyll content of V. spinulosa leaves and the height of the aboveground part, and promote the growth of V. spinulosa. With the increase of addition amount of biochar, dissolved oxygen and redox potential decreased, as well as Cu, nitrate nitrogen, and ammoniacal nitrogen contents, while the pH and electrical conductivity (EC) increased, as well as P content. The combined application of biochar and V. spinulosa was superior to V. spinulosa alone in the remediation of Cu-polluted water, but the increase of P content may have potential risks to water. The mechanisms of biochars for remediating heavy metal pollution in the soil and water environment are shown in Fig. 3.
3.2 Effects of biochar on nitrogen and phosphorus non-point source pollution in aquatic vegetable fields
In order to improve crop yield, excess N and P fertilizers are applied to farmland, which gives rise to N and P non-point source pollution from agriculture.87,88 It is reported that TN and TP load from agricultural non-point sources accounted respectively for 46.52% and 67.22% according to the national pollution census in 2020. N and P loss from paddy fields is easier than from dryland, which has become a problem in the prevention and control of water pollution.87,88N and P non-point source pollution promotes eutrophication in the aquatic environment, which causes water quality decline. Some aquatic vegetables are sensitive to changes of water quality, such as water shield. It has become an endangered plant due to anthropogenic impacts and habitat loss.64 Water quality is an important factor affecting the normal growth, yield and quality of aquatic vegetables. Because of their porous structures, extensive surface areas, negatively charged surface and abundant surface functional groups, biochars can retain both cationic and anionic nutrients, and are used as an adsorbent to purify sewage. Besides, the superior adsorption performance of biochars can also be used to control N and P non-point source pollution.
Biochars have excellent potential to decrease N leaching risks in the soil and water environments of aquatic vegetables (Fig. 4). N leaching is the main pathway of N loss in open vegetable fields. Compared with conventional fertilizer and partial substitution of inorganic fertilizer with organic fertilizer, biochar-based fertilizer had a positive effect on N leaching during rainfall.53 Li et al.67 applied rice husk biochar to the soil of submerged plants, and found that a low dose of 10 mg g−1 rice husk biochar reduced the contents of TN in aquatic environments by 5.81%, compared with the control. The results showed that the low dose of rice husk biochar applied to the soil and water environment could improve water quality and reduce N non-point source pollution in water environments. Besides, biochar application can increase the water-holding capacity of soil to reduce N leaching.89
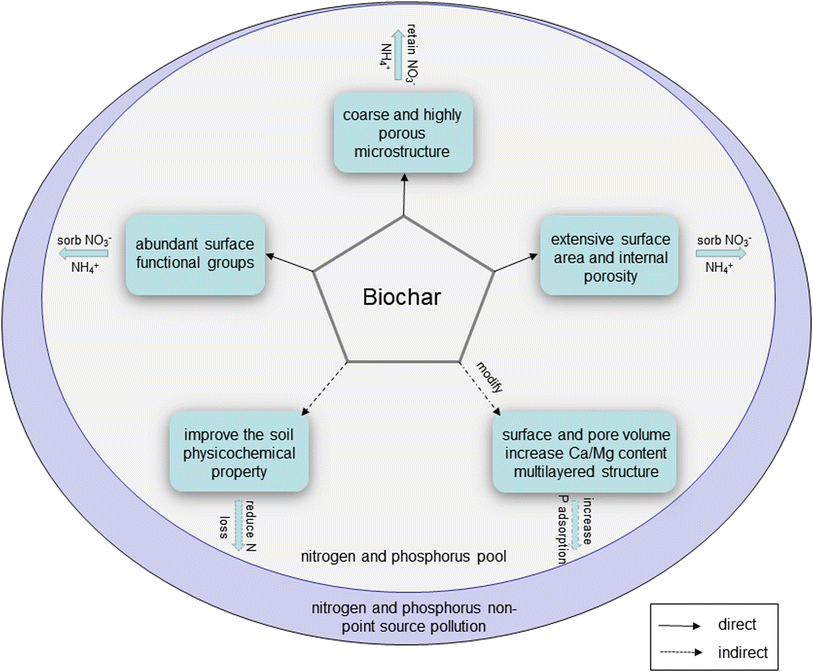 | ||
| Fig. 4 The mechanisms of biochars for remediating nitrogen and phosphorus non-point source pollution. | ||
Removal of P from water is a necessary measure to control eutrophication and reduce N and P non-point source pollution. Biochars can also be used to capture and reuse P in aquatic vegetable cultivation. Duan et al.58 applied 1% rice straw biochar (pyrolyzed at 500 °C) to the soil of cress. The study indicated that TP concentration in water environments decreased after biochar was added. Li et al.67 applied rice husk biochar to the soil of submerged plants and found that a low dose of 10 mg g−1 rice husk biochar reduced the total P content by 95.0%.
In addition, biochars were modified by adding some components to further improve their physicochemical properties. After modification, the number of functional groups, the specific surface area and pore volume of biochar increased (Fig. 4). The modified biochar has better characteristics for N and P adsorption in the water environment, and it is able to improve the water quality of aquatic vegetables and P recycling in the water environment. Bolbol et al.90 enhanced P adsorption capacity by embedding Mg–Fe layered dihydroxide (LDH) particles into the biochar matrix, and the maximum P adsorption capacity was increased from 1.39 mg g−1 of the original biochar to 17.46 mg g−1 of the LDH biochar. Meanwhile, the equilibrium time was reduced from 8 hours of the original biochar to 1 hour of LDH biochar. Melia et al.91 used correlation analysis to find out that using biochar with higher Ca and Mg contents is more conducive to P adsorption in water environments. Zhu et al.92 reported that the surface of a prepared nano-MgO biochar (pyrolyzed at 750 °C) was uniformly dispersed with MgO particles with a particle size of 3–10 nm, and the adsorption capacity of P reached 452.752 mg g−1. The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond formed by the introduction of Mg2+ into the metazoan carbon interacted with HPO42−/H2PO4− in the water environment under the action of hydrogen bonding, which improved its adsorption ability for P. The results of these two studies are consistent with a previous report.91
O bond formed by the introduction of Mg2+ into the metazoan carbon interacted with HPO42−/H2PO4− in the water environment under the action of hydrogen bonding, which improved its adsorption ability for P. The results of these two studies are consistent with a previous report.91
3.3 Effects of biochars on organic pollution in soil and water environments
Due to the long-term application of pesticides, fertilizer, plastic film, wastewater irrigation, sewage application and other activities, organic pollution in soil and water environments was investigated.93 The migration and transmission of emerging organic pollutants such as pharmaceuticals and personal care products in the soil and water environment has been a hot issue and drawn extensive attention. It was found that the high content of ofloxacin and erythromycin-H2O in the sediment of the Jiangsu section of the lower Yangtze River in China caused a serious threat to aquatic organisms.11 Sun et al.94 investigated agricultural land in the Yangtze River Delta and found that the total concentrations of antibiotics in dry soil ranged from 4.55 to 2010 mg kg−1, and the average tetracycline content was 34.9 mg kg−1. Xiong et al.95 reported the presence of antibiotic resistance genes and integrase-like genes (intI1) in 129 vegetables (including 6 watercress samples) in aquatic meat from Guangzhou and Xiamen in southern China. The study also found that tetracycline TetA genes could be detected in all samples. The Yangtze River and its tributaries as irrigation sources for aquatic vegetables, including lakes, wetlands, and paddy fields,1,80 are affected by the spread of antibiotics and drug-resistant genes in water and soil environments. This will eventually cause edible safety risks to aquatic vegetables and other agricultural products thus threatening human health. Bao et al.96 reported that water contaminated with antibiotics had a greater risk to cress than soil contaminated with antibiotics. Under the same polluted conditions, the physiological effects of oxytetracycline and enrofloxacin in water culture treatment were significantly higher than those in soil culture treatment, and oxytetracycline had a stronger inhibition effect on the growth of cress. Whereas, enrofloxacin had a higher accumulation capacity in cress, and the enrichment index of the two antibiotics in cress was higher in water culture treatment than in soil. Therefore, it is crucial to control antibiotics in water for the quality and safety of aquatic vegetables.The pore structures and large specific surface areas of biochars contribute to the adsorption of antibiotics in the water environment. Arun et al.97 found that sludge biochar can adsorb amoxicillin in water. Compared with pristine biochars, the adsorption capacity of modified biochars for organic pollutants was improved. Li et al.98 reported that the maximum adsorption capacity of tetracycline by magnetic porous tea waste biochar (MKHBC) reached 236.93 mg g−1, which was 14 times that of the control. The adsorption effect of MKHBC on tetracycline was slightly affected by the water pH and coexisting ions. Compared with the single antibiotic system, a ternary system with norfloxacin (NOR), oxytetracycline (OTC) and sulfadiazine (SMR) had a higher adsorption efficiency for NOR by pristine biochar. The adsorption efficiencies of pristine biochars and KOH-modified biochars for OTC increased, while the adsorption efficiencies of pristine biochars and KOH modified biochars for SMR were inhibited.99
Antibiotic resistance genes (ARGs) easily spread in the water environment, and extracellular DNA encoding ARGs play an important role in the transmission of ARGs. Biochars can absorb extracellular DNA to inhibit the proliferation and propagation of antibiotic resistance genes in the water environment. Fu et al.100 reported that the extracellular DNA adsorption capacity of magnetic biochar modified by quaternary phosphonate increased by about 9 times, and more than 92.7% of the resistance genes in the extracellular DNA of water were removed as compared with unmodified biochars.
PAHs, composed of two or more aromatic rings with different arrangements, are toxic persistent organic pollutants, and PAHs with more than 4 or 5 rings are carcinogenic to humans.101 A report investigated the organic pollution status of aquatic vegetables from Guangxi in China and found that the contents of PAHs in hydroponic soil were higher than that in terrestrial soil. Besides, the content of PAHs with 5 or 6 rings in aquatic vegetables was positively correlated with the content of PAHs with 6 rings in soil. The aquatic vegetables had more 6-ring PAHs than terrestrial vegetables as well.102 Biochars have great potential to remediate the soil and water environment polluted by PAHs, since biochars have abundant functional groups in their pore structures and large specific surface areas, which can bind PAHs through adsorption and electrostatic action. Cheng et al.103 reported a hydrophobic, high-nitrogen-doped macroalgae biochar, which can be used as an adsorbent to remove PAHs in water environments, and the adsorption capacity of this biochar was up to 90 mg g−1. This study provided a new way for the utilization of algae resources to remove PAHs in soil and water environments. On the other hand, biochars can be used as catalysts to degrade PAHs. Hung et al.104 reported that sludge biochar with Fe and manganese pyrolyzed at 700 °C could activate percarbonates and showed a significant oxidative removal function on PAHs in polluted aquatic sediments, with a maximum removal rate of 87%.
4. Effects of biochars on the yield and quality of aquatic vegetables
4.1 Effects of biochar on the yield of aquatic vegetables
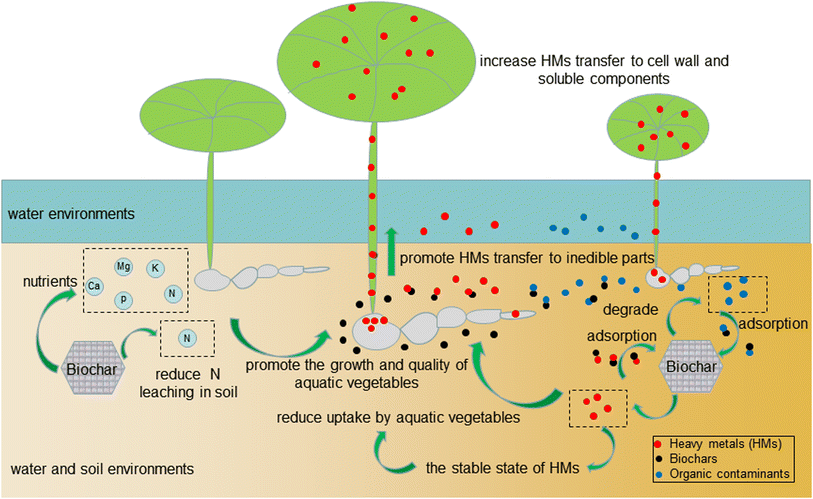
The addition of biochars to soil can improve the nitrogen use efficiency (NUE) of aquatic vegetables, increase the nutrient supply for crop growth and further improve the soil quality,55 which promote the growth of aquatic vegetables. The lasting effect of biochars on increasing crop yields depends on the amount of N in the soil.49 The early effect of biochars on crop yields may be due to nutrient input and a liming effect, while the later effect may be caused by the oxidation of biochar that is beneficial to nutrient retention in soil.105 Repeated application of biochars can maintain the liming effect and increase fertilizer function.49Application of biochars to aquatic vegetables can also promote growth and enhance yields. The growth parameters of water spinach and nutrient uptake by shoots and roots increased with the increasing addition amount of biochars.89 The dry biomass yield of water spinach treated with coconut shell biochar was 13.3–40.1% higher than that treated with chemical fertilizer. At a high HBC application rate, the NUE of water spinach was about 6% higher than that of chemical fertilizer treatment.55 Similarly, Zhou et al.53 reported that a biochar-based fertilizer (BF) enhanced the NUE of water spinach by minimizing N loss via decreasing leaching and increasing N uptake by water spinach, and BF enabled the highest N uptake of the plant. The aboveground biomass of cress treated with 1% wheat straw biochar pyrolyzed at 500 °C was the highest, reaching 1192.9 g m−2. Besides, N and K nutrient accumulation of cress with wheat straw biochar treatment was higher than that of the control.58 PB and CB combined with Fe fertilizer could improve the shoot height and root length of watercress, which can be attributed to the biochar improving the soil texture structure, decreasing bulk densities, enhancing the utilization of Fe fertilizer, and synergistically promoting crop growth.18 Rice straw biochar can increase soil EC and available P concentration, thus increasing the yield of water spinach.15 Because biochar can change soil properties and Se fertilizer affects physiological regulation in plant leaves, the combined application of peanut shell biochar and Se fertilizer was conducive to improving the accumulation of shoot and root dry weight of water spinach.12
When biochars are applied to soil, they can improve the physiological and biochemical characteristics of aquatic vegetables, which is conducive to increasing vegetable yield. The relative chlorophyll contents of lotus root leaves were significantly increased after applying pinewood biochar, and the addition of 10% (volume ratio of pinewood biochar to soil) pinewood biochar could promote the formation of chlorophyll. After applying 32% pinewood biochar to lotus root for 35 days, the number of floating leaves, leaf height, leaf diameter, and leaf area of lotus root increased and reached the maximum. The total fresh weight of lotus root, leaf, and dry weight of rhizome increased with increasing the application amount of biochar.16 Malondialdehyde (MDA), a final decomposition product of membrane lipid peroxidation under conditions of aging or stress, can reflect the degree of harm suffered by a plant.106 Jiang et al.43 reported that the application of biochar and Ca can increase chlorophyll content and decrease MDA content in water spinach seedlings, which indicated that Pb-induced oxidative stress is alleviated by the application of biochar and Ca. The shoot and root dry weight of water spinach increased with the increase of exogenous Ca and biochar. The biochar exhibits a larger effect on the dry weight of roots and shoots than Ca. However, too much biochar added would inhibit the growth of aquatic vegetables. Chen et al.81 reported that the application of 5% biochars in Cu-polluted soil inhibited the growth of water spinach. One possible reason was that the amount of biochar added was too high, and the biochars adsorbed available nutrients in the soil, which inversely affected the nutrition absorption of water spinach.
Application of biochars to aquatic vegetable cultivation could not only improve soil physicochemical properties such as decreasing bulk densities and improving the soil texture structure and soil EC, but also decrease N leaching and increase the NUE of aquatic vegetables. Due to the above effects, the physiological and biochemical characteristics of aquatic vegetables improved, which is propitious to the growth of aquatic vegetables. Consequently, the yield of aquatic vegetables is improved.
4.2 Effects of biochars on quality of aquatic vegetables
Under the pollution condition of Cd, the contents of vitamin A and vitamin C in water lettuce (Limnocharis flava), water spinach, and watercress were decreased.78 Biochars can alleviate the stress of heavy metals on aquatic vegetables, which indirectly improves the vitamin contents of aquatic vegetables. Soluble sugars and soluble proteins are important indicators affecting the taste of vegetables. The application of biochars can increase the contents of soluble sugars and soluble proteins in aquatic vegetables, which is beneficial to improving the quality of vegetables. A study on Cr-polluted aquatic environments with the combined application of biochars and Se fertilizer indicated that the contents of soluble sugars and soluble proteins in the leaves of water spinach were increased.12 PB and CB combined with Fe fertilizer also showed similar effects on watercress qualities.18Biochars can change the state of heavy metals in soil and water environments, which decreases the toxicity and transferability of heavy metals. Consequently, the absorption of heavy metals by aquatic vegetables was reduced, as well as the edible safety risk of aquatic vegetables. Hu et al.15 found that adding rice straw biochar to Cd contaminated soil could reduce the content of Cd in the aboveground and underground parts of water spinach, which may be attributed to the application of biochar increasing soil pH thus reducing the DTPA-extractable (phytoavailable) and the bioavailable Cd content. When exogenous Cu pollution was 200 mg kg−1 and 400 mg kg−1, the application of biochars to Cu-contaminated soil reduced Cu content in the roots of water spinach by 18.4% and 4.3%, respectively.81 There are several parameters characterizing the toxicity and transferability of heavy metals. The bioconcentration factor reflects the degree of plant absorption of heavy metals from soil or water environments, while the biological transfer factor refers to the transference of heavy metals among different organs of the plant. Compared to a control treatment, the addition of 32% pinewood biochar reduced the content of Cd in rhizomes, petioles, and leaves by 69%, 81%, and 55%, respectively. The bioconcentration factor of Cd in lotus root decreased by 71% and the biological transfer factor of Cd from underground to ground increased by 1.3 times, which suggested that biochars can alleviate the influence of heavy metals on lotus root and reduce the accumulation of Cd in the edible parts of lotus root.16
Biochars have large specific surface areas and are protonated, which can also enable adsorption and immobilization of heavy metals in soil and water environments. Therefore, the absorption of heavy metals by aquatic vegetables was reduced. Bashir et al.14 reported that compared with phosphate rock and zeolites, rice straw biochar was the best amendment to decrease Cd enrichment of water spinach. This study indicated that rice straw biochar with larger specific surface areas had a stronger adsorption ability towards heavy metals. The addition of 1.5% and 3% rice straw biochar reduced the aboveground and underground Cd contents of water spinach, the bio-concentration factor, the translocation factor, and the extraction coefficient factor. Antioxidant enzymatic activities, such as SOD and peroxidase (POD), have been proven to be effective biomarkers to determine the toxicological performance.72 The activities of SOD and POD in soil decreased under the action of rice straw biochar, which suggested that biochars could reduce the oxidative damage of Cd in polluted soil.
Biochars combined with other fertilizers (including Fe and Se fertilizer) can also reduce the safety risks of heavy metals to aquatic vegetables. Fe plays an important role in the complexation between heavy metals and humus,107 and low concentrations of Se fertilizer can improve the resistance of plants to various adverse factors. Yu et al.18 found that PB and CB could reduce the accumulation of Cd/Pb in the roots and aboveground parts of watercress. The combined application of biochars and Fe fertilizer also increased the content of soil organic matter. Under the action of Fe, the ternary complex of Fe, organic matter and Cd/Pb (Fe–OM–Cd/Pb) might form, which can reduce the content of available heavy metals. Studies by Xu et al.40 indicated that biochar application in flooded soil contaminated by heavy metals showed a significant negative correlation between organic matter content and exchangeable Zn content. Guo et al.12 used subcellular microscopic experiments to study Cr enrichment in water spinach. The results showed that peanut shell biochar increased the proportion of Cr in the cell wall and soluble components such as cytoplasmic vacuoles in the roots, stems and leaves of water spinach, and reduced the proportion of Cr in organelles, which might be the reason why biochar reduced Cr content in water spinach. At the same time, Se alleviates the cytotoxicity of Cr by changing the subcellular distribution of Cr in roots and leaves, chelating and separating metal ions.
The application of both Litchi branch biochar and exogenous Ca could alleviate Pb phytotoxicity and minimize its accumulation in water spinach. Biochar can cause hydroxyl ions to deprotonate the soil surface and elevate the soil pH to form precipitation such as Pb5(PO4)3OH and Pb3(CO3)2(OH)2, which can immobilize Pb. Ca-dependent inactivation of Pb2+ can occur in the form of plant cell wall deposits, which can alleviate the stress of Pb2+ on water spinach. The combination of biochar and exogenous Ca manifested synergistic effects in remediating Pb pollution and reduced the edible risk of water spinach.43
A previous report showed that the accumulation concentration of As in the aboveground part of water spinach increased by 1.30–2.55 times under flooding conditions, which increased the edible risk of water spinach.108 Biochars can reduce the enrichment index of As in water spinach under flooding conditions and improve its quality. Qin et al.109 reported that water spinach had a high bioaccumulation coefficient of As. Then, they examined the effect of rice straw biochar on As absorption in water spinach under acidic conditions, and the results suggested that the application of biochars inhibited the absorption of As by water spinach, which can be attributed to the protonated surface of biochars exhibiting enhanced adsorption capacity for As. The bioaccessibility of As in the edible part of water spinach was also reduced. As uptake by the same plant is related to the ratio of P to As in the soil solution, with the increased ratio of soluble P to soluble As, the absorption of As by the plants tended to decrease. The mechanisms of the effects of biochars on the yields and qualities of aquatic vegetables are shown in Fig. 5.
5. Conclusions and future perspective
Biochars can be used in aquatic vegetable cultivation to improve the physicochemical and biological properties of the cultivation conditions, which can promote the growth and elevate the yield of aquatic vegetables. Biochars have an excellent remediation effect on the heavy metal pollution, N and P non-point source pollution, and organic pollution in water and soil environments. Furthermore, biochars can reduce the accumulation of heavy metals, antibiotics and PAHs in aquatic vegetables, which can reduce the edible safety risk of aquatic vegetables and improve the quality of aquatic vegetables. Therefore, biochars can be used as a cost-effective and eco-friendly soil amendment for aquatic vegetable fields, showing superior application prospects. In addition, the mechanisms of biochar effects on the C/N/P cycle in aquatic environments and biochar–soil–water–crop interactions should be paid more and more attention, opening new windows for applications of biochar in soil and water remediation.However, there are still some aspects regarding the potential of biochar to improve soil and water ecosystems that need further investigation. Especially, little attention has been paid to the effects of biochars on microorganisms and aquatic organisms in the locality of aquatic vegetables with physiological, proteomic, and metabolomics analyses. Therefore, more research efforts are needed to identify the micro-mechanisms by which biochar application affects microorganisms and aquatic organisms.
Author contributions
Xiangjun Wang, Yaming Zhao, Guangwei Yao, Zhizhong Lin, Laiyuan Xu, Yunli Jiang, Zewen Jin, Shengdao Shan, and Lifeng Ping delineated and conducted the literature survey. All listed authors wrote, read, and approved the submitted version.Conflicts of interest
The authors have no conflict of interest to declare.Acknowledgements
The authors would like to thank the Major Projects for Science and Technology Development of Zhejiang Province, China (2019C02061) and Major Projects for Science and Technology Development of Zhejiang Province (2020C01017). We also thank the anonymous reviewers for their constructive comments that improved the quality of this manuscript greatly.References
- H. Guo, Cultivation of lotus (Nelumbo nucifera Gaertn. ssp. nucifera) and its utilization in China, Genet. Resour. Crop Evol., 2009, 56, 323–330 CrossRef.
- M. Wu, Y. Zong, B. Zhao and H. Zhu, Development status, problems and development ideas of aquatic vegetables industry in China (in Chinese), Journal of Changjiang Vegetables, 2019, 35–41 CAS.
- J. Han, J. Shi, L. Zeng, J. Xu and L. Wu, Impacts of continuous excessive fertilization on soil potential nitrification activity and nitrifying microbial community dynamics in greenhouse system, J. Soils Sediments, 2017, 17, 471–480 CrossRef CAS.
- X. Ju, G. Xing, X. Chen, S. Zhang, L. Zhang, X. Liu, Z. Cui, B. Yin, P. Christie, Z. Zhu and F. Zhang, Reducing environmental risk by improving N management in intensive Chinese agricultural systems, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 3041–3046 CrossRef CAS PubMed.
- L. Liang, X. Zhao, X. Yi, Z. Chen, X. Dong, R. Chen and R. Shen, Excessive application of nitrogen and phosphorus fertilizers induces soil acidification and phosphorus enrichment during vegetable production in Yangtze River Delta, China, Soil Use Manage., 2013, 29, 161–168 CrossRef.
- D. C. Seo, R. D. DeLaune, M. J. Han, Y. C. Lee, S. B. Bang, E. J. Oh, J. H. Chae, K. S. Kim, J. H. Park and J. S. Cho, Nutrient uptake and release in ponds under long-term and short-term lotus (Nelumbo nucifera) cultivation: Influence of compost application, Ecol. Eng., 2010, 36, 1373–1382 CrossRef.
- L. Cui, J. Li, X. Gao, B. Tian, J. Zhang, X. Wang and Z. Liu, Human health ambient water quality criteria for 13 heavy metals and health risk assessment in Taihu Lake, Front. Environ. Sci. Eng., 2022, 16, 41 CrossRef CAS.
- R. Li, X. Tang, W. Guo, L. Lin, L. Zhao, Y. Hu and M. Liu, Spatiotemporal distribution dynamics of heavy metals in water, sediment, and zoobenthos in mainstream sections of the middle and lower Changjiang River, Sci. Total Environ., 2020, 714, 136779 CrossRef CAS PubMed.
- Y. Luo, X. Zhao, T. Xu, H. Liu, X. Li, D. Johnson and Y. Huang, Bioaccumulation of heavy metals in the lotus root of rural ponds in the middle reaches of the Yangtze River, J. Soils Sediments, 2017, 17, 2557–2565 CrossRef CAS.
- W. Li, Z. Liu, B. Hu and L. Zhu, Co-occurrence of crAssphage and antibiotic resistance genes in agricultural soils of the Yangtze River Delta, China, Environ. Int., 2021, 156, 106620 CrossRef PubMed.
- G. Zhang, S. Lu, Y. Wang, X. Liu, Y. Liu, J. Xu, T. Zhang, Z. Wang and Y. Yang, Occurrence of antibiotics and antibiotic resistance genes and their correlations in lower Yangtze River, China, Environ. Pollut., 2020, 257, 113365 CrossRef CAS PubMed.
- X. Guo, Q. Ji, R. Muhammad, H. Li, D. Li and G. Chen, Effects of biochar and foliar application of selenium on the uptake and subcellular distribution of chromium in Ipomoea aquatica in chromium-polluted soils, Ecotoxicol. Environ. Saf., 2020, 206, 111184 CrossRef CAS PubMed.
- C. Yu, S. Wang, P. Tongsiri, M. Cheng and H. Lai, Effects of poultry-litter biochar on soil properties and growth of water spinach (Ipomoea aquatica Forsk.), Sustainability, 2018, 10, 2536 CrossRef CAS.
- S. Bashir, J. Zhu, Q. Fu and H. Hu, Cadmium mobility, uptake and anti-oxidative response of water spinach (Ipomoea aquatic) under rice straw biochar, zeolite and rock phosphate as amendments, Chemosphere, 2018, 194, 579–587 CrossRef CAS PubMed.
- J. Hu, F. Wu, S. Wu, C. Lam, X. Lin and M. Wong, Biochar and Glomus caledonium influence Cd accumulation of upland Kangkong (Ipomoea aquatica Forsk.) intercropped with Alfred Stonecrop (Sedum alfredii Hance), Sci. Rep., 2014, 4, 4671 CrossRef PubMed.
- A. Liu, D. Tian, Y. Xiang and H. Mo, Effects of biochar on growth of Asian lotus (Nelumbo nucifera Gaertn.) and cadmium uptake in artificially cadmium-polluted water, Sci. Hortic., 2016, 198, 311–317 CrossRef CAS.
- X. Qin, Y. Liu, Q. Huang, L. Zhao and Y. Xu, Effects of sepiolite and biochar on enzyme activity of soil contaminated by Cd and atrazine, Bull. Environ. Contam. Toxicol., 2020, 104, 642–648 CrossRef CAS PubMed.
- B. Yu, D. Li, Y. Wang, H. He, H. Li and G. Chen, The compound effects of biochar and iron on watercress in a Cd/Pb-contaminated soil, Environ. Sci. Pollut. Res., 2020, 27, 6312–6325 CrossRef CAS PubMed.
- S. P. Sohi, E. Krull, E. Lopez-Capel and R. Bol, A review of biochar and its use and function in soil, Adv. Agron., 2010, 105, 47–82 CAS.
- M. Ahmad, A. U. Rajapaksha, J. E. Lim, M. Zhang, N. Bolan, D. Mohan, M. Vithanage, S. S. Lee and Y. S. Ok, Biochar as a sorbent for contaminant management in soil and water: a review, Chemosphere, 2014, 99, 19–33 CrossRef CAS PubMed.
- D. Pandey, A. Daverey and K. Arunachalam, Biochar: production, properties and emerging role as a support for enzyme immobilization, J. Cleaner Prod., 2020, 255, 120267 CrossRef CAS.
- S. Mandal, S. Pu, S. Adhikari, H. Ma, D. Kim, Y. Bai and D. Hou, Progress and future prospects in biochar composites: application and reflection in the soil environment, Crit. Rev. Environ. Sci. Technol., 2021, 51, 219–271 CrossRef CAS.
- Y. Ding, Y. Liu, S. Liu, Z. Li, X. Tan, X. Huang, G. Zeng, L. Zhou and B. Zheng, Biochar to improve soil fertility. A review, Agron. Sustainable Dev., 2016, 36, 36 CrossRef.
- Z. Dai, X. Xiong, H. Zhu, H. Xu, P. Leng, J. Li, C. Tang and J. Xu, Association of biochar properties with changes in soil bacterial, fungal and fauna communities and nutrient cycling processes, Biochar, 2021, 3, 239–254 CrossRef CAS.
- J. Lehmann, M. C. Rillig, J. Thies, C. A. Masiello, W. C. Hockaday and D. Crowley, Biochar effects on soil biota – a review, Soil Biol. Biochem., 2011, 43, 1812–1836 CrossRef CAS.
- Y. Jiang, X. Wang, Y. Zhao, C. Zhang, Z. Jin, S. Shan and L. Ping, Effects of biochar application on enzyme activities in tea garden soil, Front. Bioeng. Biotechnol., 2021, 9, 728530 CrossRef PubMed.
- Z. Jin, X. Zhang, X. Chen, Z. Du, L. Ping, Z. Han and P. Tao, Dynamics of soil organic carbon mineralization and enzyme activities after two months and six years of biochar addition, Biomass Convers. Biorefin., 2023, 13, 1153–1162 CrossRef CAS.
- L. Kong, B. Song, T. Zhang, K. Gao and J. Liu, Effects of soil organic matter on biochar application in developing the biodegradation potentials of polycyclic aromatic hydrocarbons (PAHs), Appl. Soil Ecol., 2021, 167, 104046 CrossRef.
- N. Ni, F. Wang, Y. Song, Y. Bian, R. Shi, X. Yang, C. Gu and X. Jiang, Mechanisms of biochar reducing the bioaccumulation of PAHs in rice from soil: degradation stimulation vs. immobilization, Chemosphere, 2018, 196, 288–296 CrossRef CAS PubMed.
- S. H. Sadeghi, Z. Hazbavi and M. K. Harchegani, Controllability of runoff and soil loss from small plots treated by vinasse-produced biochar, Sci. Total Environ., 2016, 541, 483–490 CrossRef CAS PubMed.
- V. Vijay, S. Shreedhar, K. Adlak, S. Payyanad, V. Sreedharan, G. Gopi, S. v. d. VoortTessa, P. Malarvizhi, S. Yi, J. Gebert and P. Aravind, Review of large-scale biochar field-trials for soil amendment and the observed influences on crop yield variations, Front. Energy Res., 2021, 9, 710766 CrossRef.
- H. Xu, A. Cai, D. Wu, G. Liang, J. Xiao, M. Xu, G. Colinet and W. Zhang, Effects of biochar application on crop productivity, soil carbon sequestration, and global warming potential controlled by biochar C
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N ratio and soil pH: a global meta-analysis, Soil Tillage Res., 2021, 213, 105125 CrossRef.
N ratio and soil pH: a global meta-analysis, Soil Tillage Res., 2021, 213, 105125 CrossRef. - W. H. Clements, R. G. Stahl Jr and R. C. Landis, Ecological effects of biochar on the structure and function of stream benthic communities, Environ. Sci. Technol., 2015, 49, 14649–14654 CrossRef CAS PubMed.
- P. Godlewska, Y. S. Ok and P. Oleszczuk, The dark side of black gold: ecotoxicological aspects of biochar and biochar-amended soils, J. Hazard. Mater., 2021, 403, 123833 CrossRef CAS PubMed.
- DB42/T298.2—2022, Code of practice for aquatic vegetable cultivation—Part 2: Oudai.
- DB42/T298.3—2022, Code of practice for aquatic vegetable cultivation—Part 3: Water bamboo.
- DB42/T 298.1—2022, Code of practice for aquatic vegetable cultivation—Part 1: Seed lotus.
- S. Gul, J. K. Whalen, B. W. Thomas, V. Sachdeva and H. Deng, Physico-chemical properties and microbial responses in biochar-amended soils: mechanisms and future directions, Agric., Ecosyst. Environ., 2015, 206, 46–59 CrossRef CAS.
- X. Yang, A. D. Igalavithana, S. E. Oh, H. Nam, M. Zhang, C. H. Wang, E. E. Kwon, D. C. W. Tsang and Y. S. Ok, Characterization of bioenergy biochar and its utilization for metal/metalloid immobilization in contaminated soil, Sci. Total Environ., 2018, 640, 704–713 CrossRef PubMed.
- C. Xu, J. Zhao, W. Yang, L. He, W. Wei, X. Tan, J. Wang and A. Lin, Evaluation of biochar pyrolyzed from kitchen waste, corn straw, and peanut hulls on immobilization of Pb and Cd in contaminated soil, Environ. Pollut., 2020, 261, 114133 CrossRef CAS PubMed.
- Y. Lee, J. Jo, I. T. Kim and Y. Yoo, Chemical characteristics and NaCl component behavior of biochar derived from the salty food waste by water flushing, Energies, 2017, 10, 1555 CrossRef.
- H. Gu, H. Qiu, T. Tian, S. Zhan, T. Deng, R. L. Chaney, S. Wang, Y. Tang, J. Morel and R. Qiu, Mitigation effects of silicon rich amendments on heavy metal accumulation in rice (Oryza sativa L.) planted on multi-metal contaminated acidic soil, Chemosphere, 2011, 83, 1234–1240 CrossRef CAS PubMed.
- S. Jiang, Y. Liu and Y. Shu, Biochar and exogenous calcium assisted alleviation of Pb phytotoxicity in water spinach (Ipomoea aquatica Forsk) cultivated in Pb-spiked soil, Environ. Geochem. Health, 2022, 44, 207–219 CrossRef CAS PubMed.
- R. Jarosz, M. H. Monika, K. Gondek, M. Kopec, T. Losak and M. M. Lidia, Changes in quantity and quality of organic matter in soil after application of poultry litter and poultry litter biochar-5-year field experiment, Biomass Convers. Biorefin., 2022, 12, 2925–2934 CrossRef CAS.
- D. Wang, S. J. Fonte, S. J. Parikh, J. Six and K. M. Scow, Biochar additions can enhance soil structure and the physical stabilization of C in aggregates, Geoderma, 2017, 303, 110–117 CrossRef CAS PubMed.
- Z. Lv, H. Ma, H. Wang, W. Chen, X. Liu and G. Pan, Effects of biochar and organic fertilizer application on yield of Artemisia selengensis and soil properties (in Chinese), Chin. Agric. Sci. Bull., 2018, 34, 32–35 Search PubMed.
- B. R. Kiran and M. N. V. Prasad, Biochar and rice husk ash assisted phytoremediation potentials of Ricinus communis L. for lead-spiked soils, Ecotoxicol. Environ. Saf., 2019, 183, 109574 CrossRef CAS PubMed.
- M. Juriga, V. Šimanský, J. Horák, E. Kondrlová, D. Igaz, N. Polláková, N. Buchkina and E. Balashov, The effect of different rates of biochar and biochar in combination with N fertilizer on the parameters of soil organic matter and soil structure, Biologia, 2018, 19, 153–161 Search PubMed.
- H. Blanco-Canqui, Does biochar improve all soil ecosystem services?, GCB Bioenergy, 2021, 13, 291–304 CrossRef CAS.
- H. Cheng, D. L. Jones, P. Hill, M. S. Bastami and C. Tu, Influence of biochar produced from different pyrolysis temperature on nutrient retention and leaching, Arch. Agron. Soil Sci., 2018, 64, 850–859 CrossRef CAS.
- T. J. Clough, L. M. Condron, C. Kammann and C. Müller, A review of biochar and soil nitrogen dynamics, Agronomy, 2013, 3, 275–293 CrossRef CAS.
- T. T. N. Nguyen, C. Xu, I. Tahmasbian, R. Che, Z. Xu, X. Zhou, H. Wallace and S. Bai, Effects of biochar on soil available inorganic nitrogen: a review and meta-analysis, Geoderma, 2017, 288, 79–96 CrossRef CAS.
- M. Zhou, S. Ying, J. Chen, P. Jiang and Y. Teng, Effects of biochar-based fertilizer on nitrogen use efficiency and nitrogen losses via leaching and ammonia volatilization from an open vegetable field, Environ. Sci. Pollut. Res., 2021, 28, 65188–65199 CrossRef CAS PubMed.
- C. Steiner, B. Glaser, W. Geraldes Teixeira, J. Lehmann, W. E. H. Blum and W. Zech, Nitrogen retention and plant uptake on a highly weathered central Amazonian Ferralsol amended with compost and charcoal, J. Plant Nutr. Soil Sci., 2008, 171, 893–899 CrossRef CAS.
- F. Zhao, G. Zou, Y. Shan, Z. Ding, M. Dai and Z. He, Coconut shell derived biochar to enhance water spinach (Ipomoea aquatica Forsk) growth and decrease nitrogen loss under tropical conditions, Sci. Rep., 2019, 9, 20291 CrossRef CAS PubMed.
- S. H. Bai, F. Reverchon, C. Xu, Z. Xu, T. J. Blumfield, H. Zhao, L. Van Zwieten and H. M. Wallace, Wood biochar increases nitrogen retention in field settings mainly through abiotic processes, Soil Biol. Biochem., 2015, 90, 232–240 CrossRef CAS.
- B. P. Singh, B. J. Hatton, B. Singh, A. L. Cowie and A. Kathuria, Influence of biochars on nitrous oxide emission and nitrogen leaching from two contrasting soils, J. Environ. Qual., 2010, 39, 1224–1235 CrossRef CAS PubMed.
- J. Duan, L. Xue, A. Yin, Y. Feng and L. Yang, Rural low-pollution wastewater purification in Oenanthe Javanica wetland with biochar addition (in Chinese), J. Agro-Environ. Sci., 2017, 36, 353–361 Search PubMed.
- W. Wang, C. Zeng, J. Sardans, C. Wang, D. Zeng and J. Penuelas, Amendment with industrial and agricultural wastes reduces surface-water nutrient loss and storage of dissolved greenhouse gases in a subtropical paddy field, Agric., Ecosyst. Environ., 2016, 231, 296–303 CrossRef CAS.
- M. Z. Hossain, M. M. Bahar, B. Sarkar, S. W. Donne, Y. S. Ok, K. N. Palansooriya, M. B. Kirkham, S. Chowdhury and N. Bolan, Biochar and its importance on nutrient dynamics in soil and plant, Biochar, 2020, 2, 379–420 CrossRef.
- Y. Zhang, Z. Zhang and Y. Chen, Biochar mitigates N2O emission of microbial denitrification through modulating carbon metabolism and allocation of reducing power, Environ. Sci. Technol., 2021, 55, 8068–8078 CrossRef CAS PubMed.
- Y. Zhang and Y. Wang, Soil enzyme activities with greenhouse subsurface irrigation, Pedosphere, 2006, 16, 512–518 CrossRef CAS.
- A. V. Gorovtsov, T. M. Minkina, S. S. Mandzhieva, L. V. Perelomov, G. Soja, I. V. Zamulina, V. D. Rajput, S. N. Sushkova, D. Mohan and J. Yao, The mechanisms of biochar interactions with microorganisms in soil, Environ. Geochem. Health, 2020, 42, 2495–2518 CrossRef CAS PubMed.
- C. Xie, J. Li, F. Pan, J. Fu, W. Zhou, S. Lu, P. Li and C. Zhou, Environmental factors influencing mucilage accumulation of the endangered Brasenia schreberi in China, Sci. Rep., 2018, 8, 17955 CrossRef CAS PubMed.
- E. Jeppesen, M. Søndergaard, M. Søndergaard and K. Christoffersen, The structuring role of submerged macrophytes in lakes, Springer, New York, 1998 Search PubMed.
- H. Liu, W. Zhou, X. Li, Q. Chu, N. Tang, B. Shu, G. Liu and W. Xing, How many submerged macrophyte species are needed to improve water clarity and quality in Yangtze floodplain lakes?, Sci. Total Environ., 2020, 724, 138267 CrossRef CAS PubMed.
- W. Li, J. Zhou, H. Ding, H. Fu, J. Liu, Y. Chen, T. Dai, Q. Lou, X. Zhong, H. Fan and J. Zhong, Low-dose biochar added to sediment improves water quality and promotes the growth of submerged macrophytes, Sci. Total Environ., 2020, 742, 140602 CrossRef CAS PubMed.
- A. C. Bastos, M. Prodana, N. Abrantes, J. J. Keizer, A. M. V. M. Soares and S. Loureiro, Potential risk of biochar-amended soil to aquatic systems: an evaluation based on aquatic bioassays, Ecotoxicology, 2014, 23, 1784–1793 CrossRef CAS PubMed.
- C. Zhang, B. Shan, S. Jiang and W. Tang, Effects of the pyrolysis temperature on the biotoxicity of Phyllostachys pubescens biochar in the aquatic environment, J. Hazard. Mater., 2019, 376, 48–57 CrossRef CAS PubMed.
- C. R. Smith, P. G. Hatcher, S. Kumar and J. W. Lee, Investigation into the sources of biochar water-soluble organic compounds and their potential toxicity on aquatic microorganisms, ACS Sustainable Chem. Eng., 2016, 4, 2550–2558 CrossRef CAS.
- Y. Zhang, R. Yang, X. Si, X. Duan and X. Quan, The adverse effect of biochar to aquatic algae-the role of free radicals, Environ. Pollut., 2019, 248, 429–437 CrossRef CAS PubMed.
- Y. Ji, P. Wu, J. Zhang, J. Zhang, Y. Zhou, Y. Peng, S. Zhang, G. Cai and G. Gao, Heavy metal accumulation, risk assessment and integrated biomarker responses of local vegetables: a case study along the Le'an river, Chemosphere, 2018, 199, 361–371 CrossRef CAS PubMed.
- K. Panngom, T. Chuesaard, N. Tamchan, T. Jiwchan, K. Srikongsritong and G. Park, Comparative assessment for the effects of reactive species on seed germination, growth and metabolisms of vegetables, Sci. Hortic., 2018, 227, 85–91 CrossRef CAS.
- V. Baskar, S. Nayeem, S. P. Kuppuraj, T. Muthu and S. Ramalingam, Assessment of the effects of metal oxide nanoparticles on the growth, physiology and metabolic responses in in vitro grown eggplant (Solanum melongena), 3 Biotech, 2018, 8, 362 CrossRef PubMed.
- T. Dutta, E. Kwon, S. S. Bhattacharya, B. H. Jeon, A. Deep, M. Uchimiya and K. H. Kim, Polycyclic aromatic hydrocarbons and volatile organic compounds in biochar and biochar-amended soil: a review, GCB Bioenergy, 2017, 9, 990–1004 CrossRef CAS.
- J. Yang, B. Pan, H. Li, S. Liao, D. Zhang, M. Wu and B. Xing, Degradation of p-nitrophenol on biochars: role of persistent free radicals, Environ. Sci. Technol., 2016, 50, 694–700 CrossRef CAS PubMed.
- Y. Qin, G. Li, Y. Gao, L. Zhang, Y. S. Ok and T. An, Persistent free radicals in carbon-based materials on transformation of refractory organic contaminants (ROCs) in water: a critical review, Water Res., 2018, 137, 130–143 CrossRef CAS PubMed.
- H. Widowati, The influence of cadmium heavy metal on vitamins in aquatic vegetables, Makara J. Sci., 2012, 16, 33–38 Search PubMed.
- A. Kumar, E. Singh, R. Mishra and S. Kumar, Biochar as environmental armour and its diverse role towards protecting soil, water and air, Sci. Total Environ., 2022, 806, 150444 CrossRef CAS PubMed.
- H. Guo, S. Li, J. Peng and D. Ke, Zizania latifolia Turcz. cultivated in China, Genet. Resour. Crop Evol., 2007, 54, 1211–1217 CrossRef.
- X. Chen, X. Guo and G. Chen, Effects of biochar and pig manure on Ipomoea aquatica Forsk growth and copper forms in copper-polluted soil (in Chinese), J. Agro-Environ. Sci., 2016, 35, 915–918 Search PubMed.
- T. Cai, H. Du, X. Liu, B. Tie and Y. Yang, Adsorption properties of Oiltea Camellia shell-modified biochar and effects of coupled waterlogging on soil Cd morphology (in Chinese), Environ. Sci., 2021, 42, 2522–2530 Search PubMed.
- S. Haldar and A. Ghosh, Microbial and plant-assisted heavy metal remediation in aquatic ecosystems: a comprehensive review, 3 Biotech, 2020, 10, 205 CrossRef PubMed.
- D. D. Bussan, R. F. Sessums and J. V. Cizdziel, Activated carbon and biochar reduce mercury methylation potentials in aquatic sediments, Bull. Environ. Contam. Toxicol., 2016, 96, 536–539 CrossRef CAS PubMed.
- J. Xiang, Q. Lin, X. Yao and G. Yin, Removal of Cd from aqueous solution by chitosan coated MgO-biochar and its in situ remediation of Cd-contaminated soil, Environ. Res., 2021, 195, 110650 CrossRef CAS PubMed.
- L. Chen, W. Li, J. Zhou, J. Zhong, G. Gao and H. Fan, Remediation of copper-polluted water using both biochar and a submerged macrophyte, Vallisneria spinulosa (in Chinese), Environ. Eng., 2018, 36, 54–58 Search PubMed.
- F. Li, Y. Jin, S. He, J. Jin, Z. Wang, S. Khan, G. Tian and X. Liang, Use of polyacrylamide modified biochar coupled with organic and chemical fertilizers for reducing phosphorus loss under different cropping systems, Agric., Ecosyst. Environ., 2021, 310, 107306 CrossRef CAS.
- L. H. Xue, P. F. Hou, Z. Y. Zhang, M. X. Shen, F. X. Liu and L. Z. Yang, Application of systematic strategy for agricultural non-point source pollution control in Yangtze River basin, China, Agric., Ecosyst. Environ., 2020, 304, 107148 CrossRef CAS.
- R. Fan, J. Luo, S. Yan, Y. Zhou and Z. Zhang, Effects of biochar and super absorbent polymer on substrate properties and water spinach growth, Pedosphere, 2015, 25, 737–748 CrossRef CAS.
- H. Bolbol, M. Fekri and M. Hejazi-Mehrizi, Layered double hydroxide-loaded biochar as a sorbent for the removal of aquatic phosphorus: behavior and mechanism insights, Arabian J. Geosci., 2019, 12, 503 CrossRef.
- P. M. Melia, R. Busquets, P. S. Hooda, A. B. Cundy and S. P. Sohi, Driving forces and barriers in the removal of phosphorus from water using crop residue, wood and sewage sludge derived biochars, Sci. Total Environ., 2019, 675, 623–631 CrossRef CAS PubMed.
- D. Zhu, H. Yang, X. Chen, W. Chen, N. Cai, Y. Chen, S. Zhang and H. Chen, Temperature-dependent magnesium citrate modified formation of MgO nanoparticles biochar composites with efficient phosphate removal, Chemosphere, 2021, 274, 129904 CrossRef CAS PubMed.
- J. Sun, L. Pan, D. C. W. Tsang, Y. Zhan, L. Zhu and X. Li, Organic contamination and remediation in the agricultural soils of China: a critical review, Sci. Total Environ., 2018, 615, 724–740 CrossRef CAS PubMed.
- J. Sun, L. Jin, T. He, Z. Wei, X. Liu, L. Zhu and X. Li, Antibiotic resistance genes (ARGs) in agricultural soils from the Yangtze River Delta, China, Sci. Total Environ., 2020, 740, 140001 CrossRef CAS PubMed.
- L. Xiong, Y. Sun, L. Shi and H. Yan, Characterization of antimicrobial resistance genes and class 1 integrase gene in raw meat and aquatic product, fresh vegetable and fruit, and swine manure in southern China, Food Control, 2019, 104, 240–246 CrossRef CAS.
- C. Bao, G. Gu and M. Zhang, Effects of veterinary antibiotics stress on growth and antibiotics accumulation of Oenanthe javanica DC (in Chinese), Chin. J. Soil Sci., 2016, 47, 164–172 CAS.
- S. Arun, K. Kothari, D. Mazumdar, M. Mukhopadhyay and P. Chakraborty, Biochar production from domestic sludge: a cost-effective, recycled product for removal of amoxicillin in wastewater, IOP Conf. Ser.: Mater. Sci. Eng., 2017, 225, 012164 Search PubMed.
- B. Li, Y. Zhang, J. Xu, Z. Xie, J. Tang, X. Li and S. Fan, Simultaneous carbonization, activation, and magnetization for producing tea waste biochar and its application in tetracycline removal from the aquatic environment, J. Environ. Chem. Eng., 2021, 9, 105324 CrossRef CAS.
- J. Luo, X. Li, C. Ge, K. Muller, H. Yu, P. Huang, J. Li, D. C. W. Tsang, N. S. Bolan, J. Rinklebe and H. Wang, Sorption of norfloxacin, sulfamerazine and oxytetracycline by KOH-modified biochar under single and ternary systems, Bioresour. Technol., 2018, 263, 385–392 CrossRef CAS PubMed.
- Y. Fu, F. Wang, H. Sheng, F. Hu, Z. Wang, M. Xu, Y. Bian, X. Jiang and J. M. Tiedje, Removal of extracellular antibiotic resistance genes using magnetic biochar/quaternary phosphonium salt in aquatic environments: a mechanistic study, J. Hazard. Mater., 2021, 411, 125048 CrossRef CAS PubMed.
- J. Lee, J. Jeong, S. Park and K. Lee, Monitoring and risk assessment of polycyclic aromatic hydrocarbons (PAHs) in processed foods and their raw materials, Food Control, 2018, 92, 286–292 CrossRef CAS.
- T. Zhao, M. Long, S. Qiao, Q. Sun, G. He and Y. Liang, Characteristics of polycyclic aromatic hydrocarbons (PAHs) pollution in aquatic and terrestrial vegetables in Guangxi (in Chinese), J. Ecol. Rural Environ., 2020, 36, 505–514 CAS.
- H. Cheng, R. Ji, Y. Bian, X. Jiang and Y. Song, From macroalgae to porous graphitized nitrogen-doped biochars – using aquatic biota to treat polycyclic aromatic hydrocarbons-contaminated water, Bioresour. Technol., 2020, 303, 122947 CrossRef CAS PubMed.
- C. Hung, C. Huang, C. Chen, C. Wu, Y. Lin and C. Dong, Activation of percarbonate by water treatment sludge-derived biochar for the remediation of PAH-contaminated sediments, Environ. Pollut., 2020, 265, 114914 CrossRef CAS PubMed.
- K. A. Spokas, K. B. Cantrell, J. M. Novak, D. W. Archer, J. A. Ippolito, H. P. Collins, A. A. Boateng, I. M. Lima, M. C. Lamb, A. J. McAloon, R. D. Lentz and K. A. Nichols, Biochar: a synthesis of its agronomic impact beyond carbon sequestration, J. Environ. Qual., 2012, 41, 973–989 CrossRef CAS PubMed.
- Z. S. Siddiqui, X. Wei, M. Umar, Z. Abideen, F. Zulfiqar, J. Chen, A. Hanif, S. Dawar, D. A. Dias and R. Yasmeen, Scrutinizing the application of saline endophyte to enhance salt tolerance in rice and maize plants, Front. Plant Sci., 2022, 12, 770084 CrossRef PubMed.
- X. Wang, H. Yu, F. Li, T. Liu, W. Wu, C. Liu, C. Liu and X. Zhang, Enhanced immobilization of arsenic and cadmium in a paddy soil by combined applications of woody peat and Fe(NO3)3: possible mechanisms and environmental implications, Sci. Total Environ., 2019, 649, 535–543 CrossRef CAS PubMed.
- Y. Liao, C. Syu and D. Lee, Comparison of As accumulation and speciation in water spinach (Ipomoea aquatica Forssk.) grown in As-elevated soils under flooding versus upland conditions, J. Hazard. Mater., 2021, 415, 125711 CrossRef CAS PubMed.
- J. Qin, A. Niu, Y. Liu and C. Lin, Arsenic in leafy vegetable plants grown on mine water-contaminated soils: uptake, human health risk and remedial effects of biochar, J. Hazard. Mater., 2021, 402, 123488 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2023 |