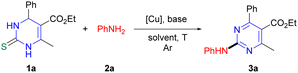Open Access Article
Open Access ArticleCopper-promoted dehydrosulfurative carbon–nitrogen cross-coupling with concomitant aromatization for synthesis of 2-aminopyrimidines†
Ngoc Son Le Pham a,
Yujeong Kwona,
Hyunik Shinb and
Jeong-Hun Sohn
a,
Yujeong Kwona,
Hyunik Shinb and
Jeong-Hun Sohn *a
*a
aDepartment of Chemistry, Chungnam National University, Daejeon 34134, Republic of Korea. E-mail: sohnjh@cnu.ac.kr
bYonsung Fine Chemicals R&D Center, Suwon 16675, Republic of Korea
First published on 20th December 2022
Abstract
Copper-promoted dehydrosulfurative C–N cross-coupling of 3,4-dihydropyrimidin-1H-2-thione with amine accompanied by concomitant aromatization to generate 2-aryl(alkyl)aminopyrimidine derivatives is described. The reaction proceeded well with a wide range of thiono substrates and aryl/aliphatic amines as the coupling partners, offering efficient access to biologically and pharmacologically valuable 2-aryl(alkyl)aminopyrimidines with rapid diversification.
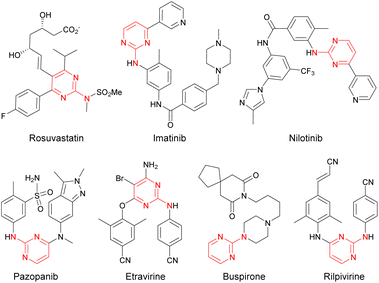
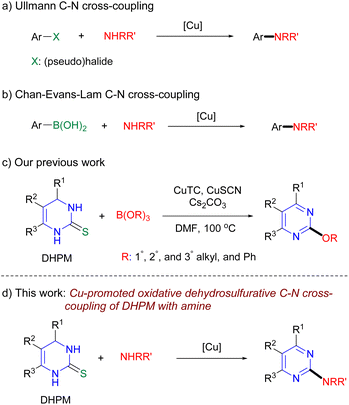
Owing to their roles as a critical binding fragment toward biological targets in many drugs and natural products, pyrimidine motifs have been of great interest in the field of drug discovery and development.1 In particular, 2-aminopyrimidine structures have been incorporated into various commercialized drugs, such as the hypocholesterolemic agent rosuvastatin, antileukemic agents imatinib and nilotinib, the antiangiogenetic agent pazopanib, anti-HIV agents rilpivirine and etravirine, and the anxiolytic agent buspirone (Fig. 1).2 In addition, 2-aminopyrimidine derivatives are known to be useful molecular probes in the research on chemical/biological interfaces.3 The biological significance of this privileged scaffold has naturally generated much interest from organic and medicinal chemists for the efficient synthesis of 2-aminopyrimidine derivatives. In general, syntheses of 2-aryl(alkyl)aminopyrimidines have been performed by nucleophilic aromatic substitution or metal-catalyzed C–N cross-coupling of 2-(pseudo)halopyrimidine with amine.4–6 However, the synthesis of many 2-(pseudo)halopyrimidine partners, especially for densely substituted partners, requires tedious multi-steps, which limits the rapid diversification of 2-aminopyrimidine derivatives by these reaction protocols. In this regard, we previously developed Liebeskind–Srogl-type Pd-catalyzed/Cu-mediated oxidative dehydrosulfurative C–N cross-coupling to offer 2-aminopyrimidines.7 The reaction conditions of this method, however, required large amounts of metals (3 equivalents of Cu and 0.2 equivalents of Pd) for acceptable yields of the desired products. The requirement of the large amounts of metals directed us to envision an alternative method using a stoichiometric amount of Cu with no Pd.
Copper-promoted carbon–carbon and carbon-heteroatom cross-coupling reactions have been extensively investigated8 and are increasingly used in synthetic chemistry with advantages over other common transition-metals, such as palladium or nickel, which are costly, toxic, or air- and moisture-sensitive, and often require a specially tailored and expensive ligand.9 To form carbon-heteroatom bonds, such as C–N, C–O, and C–S bonds, in the Cu-promoted couplings, organo(pseudo)halide substrates have been commonly used as the electrophilic partner (arylating agent) to react with the nucleophilic partner (Scheme 1a).10 Since independent reports by Chan,11 Evans,12 and Lam13 regarding Cu-mediated arylation of amines or alcohols in 1998, arylboronic acids and derivatives have become another standard arylating agent of nucleophiles (Scheme 1b).14 Other organometallics, such as organobismuth, –lead, –tin, and –siloxane, or hypervalent iodonium salt, were also studied as the arylating agents, but their preparations are generally nontrivial.15 Recently, we developed Cu-promoted C–O cross-coupling of DHPM with boric ester (borate) or alcohol accompanying concomitant oxidative dehydrogenation (aromatization) to produce 2-alkoxypyrimidines (Scheme 1c).16 Albeit large amounts of Cu (4.5 equivalents) as in the case of the Liebeskind–Srogl-type oxidative dehydrosulfurative C–N cross-coupling, the results demonstrated that the thiono substrate is a suitable electrophilic partner for Cu-promoted C–O cross-coupling, which inspired us to explore Cu-promoted oxidative dehydrosulfurative C–N cross-coupling of DHPM with amine to provide 2-aminopyrimidine compounds (Scheme 1d). As a surrogate for the 2-(pseudo)halopyrimidines, DHPMs bearing various C4–C6 substituents can be readily produced by the Biginelli three component reaction with versatile aldehyde, β-ketoester, and thiourea.17 Thus, Cu-promoted oxidative dehydrosulfurative C–N cross-coupling of DHPM with amine could offer a shortcut and general access to a wide range of 2-aryl(alkyl)aminopyrimidine derivatives. To our knowledge, no reports describing Cu-promoted direct C–N cross-coupling of thiono substrate with amine have been published.18–20
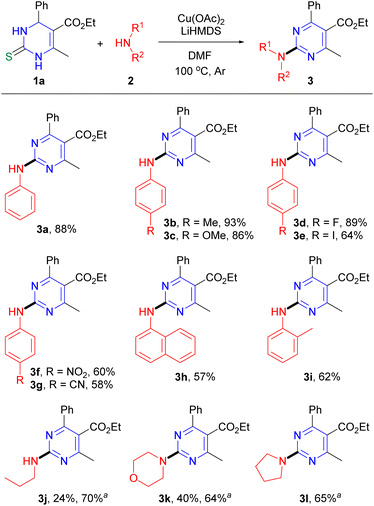
Initial studies of the reaction were carried out under conditions similar to the previous C–O coupling of DHPM with borate.16a When DHPM 1a (ref. 21) (0.25 mmol) was reacted with aniline 2a (1.5 equiv.) in the presence of a mixture of Cu(I)-thiophene-2-carboxylate (CuTC, 3 equiv.) and CuSCN (1.5 equiv.), and Cs2CO3 (3 equiv.) in N,N-dimethylformamide (DMF) at 100 °C for 18 h under Ar atmosphere, the desired aminated pyrimidine 3a (ref. 4b) was produced in 22% yield (entry 1, Table 1). We found that the replacement of base Cs2CO3 with K2CO3 slightly increased the reaction yield (entry 2) and the use of CuSCN alone (3 equiv.), instead of the mixture of the two Cu salts, gave the desired product in 68% yield (entry 3). These results encouraged us to perform further optimization studies by varying the reaction parameters. Solvents such as toluene (PhMe) and 1,4-dioxane were less effective than DMF (entries 4 and 5). With respect to base, we observed that lithium hexamethyldisilazide (LiHMDS), which provided the desired product in 90% yield, was superior to other bases examined in the studies, such as Cs2CO3, K2CO3, potassium t-butoxide (t-BuOK), K3PO4, and lithium diisopropylamide (LDA) (entries 6–9). Since the amount of Cu salt used in the reaction was 1.5 equivalents less than in the previous C–O coupling with borate, we expected that further reduction of the amount of Cu salt could be achieved. We found that 2 equivalents of CuSCN exhibited no significant decrease in the reaction yield (entry 10), while less than 2 equivalents of CuSCN significantly reduced the yield (entries 11 and 12). Next, we investigated the reaction with respect to the Cu source; we found that copper(II) acetate (Cu(OAc)2) provided the desired product in slightly higher yield (88%), compared with CuSCN (entry 17). No desired product was produced in the absence of either Cu salt or base (entries 18 and 19) and lower yields of the desired product were observed at other temperatures (entries 20 and 21). Instead of Ar atmosphere, the reaction under air did not give the desired product (entry 22). Because of the higher stability, lower cost, and easier isolation of the desired product, we decided to use Cu(OAc)2 rather than CuSCN to investigate the reaction scope.22
| Entry | Cu (equiv.) | Base | Solvent | Yield (%) |
|---|---|---|---|---|
| a Reaction conditions: DHPM 1a (0.25 mmol), aniline (0.38 mmol), base (1.5–2.0 equiv.), and solvent (1.5 mL) at 100 °C for 18 h under Ar.b The reaction at 120 °C.c The reaction at 80 °C.d The reaction under air. | ||||
| 1 | CuTC (3.0)/CuSCN (1.5) | Cs2CO3 | DMF | 22 |
| 2 | CuTC (3.0)/CuSCN (1.5) | K2CO3 | DMF | 25 |
| 3 | CuSCN (3.0) | K2CO3 | DMF | 68 |
| 4 | CuSCN (3.0) | K2CO3 | PhMe | Trace |
| 5 | CuSCN (3.0) | K2CO3 | Dioxane | Trace |
| 6 | CuSCN (3.0) | tBuOK | DMF | 75 |
| 7 | CuSCN (3.0) | K3PO4 | DMF | 57 |
| 8 | CuSCN (3.0) | LDA | DMF | 63 |
| 9 | CuSCN (3.0) | LiHMDS | DMF | 90 |
| 10 | CuSCN (2.0) | LiHMDS | DMF | 86 |
| 11 | CuSCN (1.5) | LiHMDS | DMF | 61 |
| 12 | CuSCN (1.0) | LiHMDS | DMF | 41 |
| 13 | CuI (2.0) | LiHMDS | DMF | 80 |
| 14 | CuCl (2.0) | LiHMDS | DMF | 23 |
| 15 | CuBr (2.0) | LiHMDS | DMF | 21 |
| 16 | Cu2O (2.0) | LiHMDS | DMF | 0 |
| 17 | Cu(OAc)2 (2.0) | LiHMDS | DMF | 88 |
| 18 | — | LiHMDS | DMF | 0 |
| 19 | Cu(OAc)2 (2.0) | — | DMF | 0 |
| 20b | Cu(OAc)2 (2.0) | LiHMDS | DMF | 65 |
| 21c | Cu(OAc)2 (2.0) | LiHMDS | DMF | 38 |
| 22d | Cu(OAc)2 (2.0) | LiHMDS | DMF | 0 |
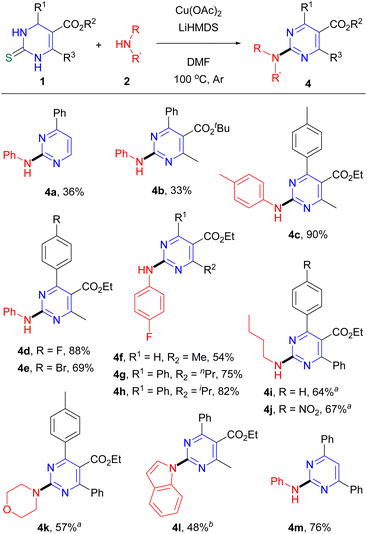
Under optimal reaction conditions, the scope of the reaction with various DHPMs and amines was assessed. With respect to aryl amines, we observed that various functional groups on the phenyl were compatible with the reaction method (Scheme 2). Electron-donating methyl and methoxy groups present at the para position on aniline provided the desired products 3b (ref. 23) and 3c,4b respectively, in high yields. Halide groups F and I at the para position gave 3d (ref. 7) (89%) and 3e (ref. 7) (64%), respectively, and other electron-withdrawing groups, such as nitro and cyano groups, yielded 3f (ref. 7) and 3g,7 respectively, in 58–60% yields. Bicyclic 1-naphthylamine and sterically hindered o-methylaniline were also suitable as the coupling partner to produce the desired products 3h (ref. 6) and 3i (ref. 6) in 57% and 62% yields, respectively. We also investigated the reaction with aliphatic amines as the coupling partners. When the reaction of DHPM 1a with n-PrNH2 was performed the desired product 3j was produced in only 24% yield. This unacceptable low yield motivated us to determine the reaction conditions using Cu(OAc)2, K3PO4, ethylene glycol and 4 Å molecular sieves in i-PrOH, under which the desired product 3j was obtained in 57% yield. Thus, the reaction conditions were applied to the reactions with other aliphatic amines. When secondary amines, morpholine and pyrrolidine, were reacted, the corresponding products 3k (ref. 4e) and 3l (ref. 4b) were produced in 64% and 65% yields, respectively.24
We assessed the scope of the reaction with respect to the DHPM substrate by varying substituents at the C4 to C6 positions (Scheme 3). The reaction of the DHPM possessing no substituents at the C5 and C6 positions with aniline gave the desired product 4a (ref. 25) in 36% yield. A t-butoxycarbonyl group at the C5 position resulted in pyrimidine 4b (ref. 7) in 33% yield in the reaction with aniline; much lower yield compared with ethoxycarbonyl group (3a, 88% yield) might be attributed to decomposition of the t-butoxycarbonyl group under the reaction conditions. We also investigated the effects of substituents at C4 aryl by varying the para substituent and observed no distinct preference of the reaction toward either electron-donating or electron-withdrawing substituents. When tolyl, 4-fluorophenyl, and 4-bromophenyl groups at the C4 position of DHMP were examined for the reaction with p-toluidine or aniline, the corresponding products 4c–e (ref. 6) were obtained in good yields. DHPM with no substituents at the C4 position yielded the desired product 4f in 54% yield in the reaction with 4-fluoroaniline. Substituent n-propyl or i-propyl group at the C6 position, which gave pyrimidine 4g or 4h, respectively, in good yield in the reaction with 4-fluoroaniline, was compatible with the reaction method. The phenyl group at the C6 position also gave the desired product 4i (ref. 26) or 4j in 64–67% yields when coupling with n-BuNH2 under the conditions for aliphatic amines. Tolyl and phenyl groups at the C4 and C6 positions, respectively, were also compatible substituents in the reaction with morpholine to generate 4k. Next, we attempted the reaction of DHPM with indole to produce 2-indolylpyrimidine, which was not successful in the previous Pd-catalyzed/Cu-mediated reaction.7 We found that the reaction of 1a with indole in the presence of CuTC and Cs2CO3 in 1,4-dioxane at 100 °C under air provided the desired product 4l (ref. 27) in 48% yield.
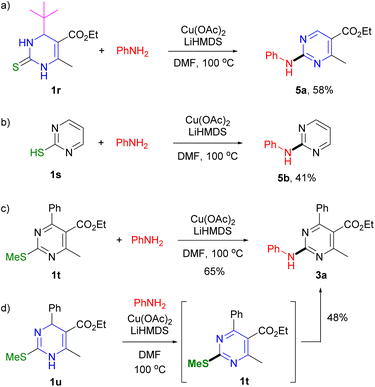
To understand the mode of the reaction, control experiments were performed. When DHPM 1r containing t-butyl group at the C4 position was reacted with aniline, the debutylated product 5a,28 was produced as the major product (Scheme 4a). As described in previous reports regarding the oxidative dehydrogenation (aromatization) and the oxidative dehydrosulfurative cross-coupling reactions of DHPM derivatives possessing the C4 t-Bu group, published by others and us, these results support the involvement of a radical intermediate in the aromatization process.16,19,29,30 When the reaction of 2-mercaptopyrimidine 1s with aniline was performed, the desired pyrimidine 5b (ref. 31) was obtained (Scheme 4b), consistent with the proposition that the C–S single bond generated after deprotonation is involved in the reaction of DHPM. Pyrimidinyl thioether 1t (ref. 32) and dihydropyrimidinyl thioether 1u,4b which provide the same product 3a, were also compatible with the reaction method (Scheme 4c and d). We found that the reaction of 1u proceeded via aromatization to yield pyrimidine 1t as an intermediate in advance of C–N coupling.
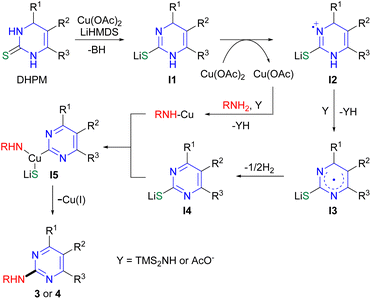
Based on the results, a plausible mechanism of the reaction is proposed as shown in Scheme 5. The reaction could proceed via the generation of dihydropyrimidine I1 possessing C–S single bond after deprotonation and coordination to Cu, which then undergoes aromatization to yield pyrimidine I4 likely via the generation of radical species I3.29b Radical I3 could arise from nitrogen radical cation I2 formed from I1 by single electron transfer. Oxidative addition of pyrimidine I4 to Cu(I)NHR produced from the amine and Cu(I) species could afford Cu(III) complex I5,20 which is then transformed to 2-aminopyrimidine 3 or 4 by reductive elimination.
In summary, we have developed a Cu-promoted oxidative dehydrosulfurative C–N cross-coupling reaction of DHPMs with amines to produce 2-aryl(alkyl)aminopyrimidine derivatives. To our knowledge, this is the first use of thiono substrate as an arylating partner in the Cu-promoted C–N cross-coupling reaction. The reaction proceeded well with various DHPMs and aryl amines or aliphatic amines as coupling partners, providing efficient access to biologically and pharmacologically important 2-aryl(alkyl)aminopyrimidines with rapid diversification.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2022R1A2C1009730).Notes and references
-
(a) M. Lagoja, Chem. Biodiversity, 2005, 2, 1–50 CrossRef
; (b) Pharmaceutical Substances: Synthesis, Patents, Applications, ed. A. Kleemann and J. Engel, Thieme, Stuttgart, Germany, 2001 Search PubMed
.
-
(a) L. Yet. Privileged Structures in Drug Discovery: Medicinal Chemistry and Synthesis, John Wiley & Sons, Inc., New Jersey, USA, 2018, ch. 7, pp. 237–283 Search PubMed
; (b) S. Kumar and B. Narsimhan, Chem. Cent. J., 2018, 12, 38 CrossRef
.
-
(a) S. D. Gilbert, S. J. Mediatore and R. T. Batey, J. Am. Chem. Soc., 2006, 128, 14214–14215 CrossRef CAS
; (b) B. Puffer, C. Kreutz, U. Rieder, M. O. Ebert, R. Konrat and R. Micura, Nucleic Acids Res., 2009, 37, 7728–7740 CrossRef CAS
.
- For selected examples, see:
(a) F.-A. Kang, J. Kodah, Q. Guan, X. Li and W. V. Murray, J. Org. Chem., 2005, 70, 1957–1960 CrossRef CAS PubMed
; (b) M. Matloobi and C. O. Kappe, J. Comb. Chem., 2007, 9, 275–284 CrossRef CAS PubMed
; (c) X.-C. Wang, G.-J. Yang, Z.-J. Quan, P.-Y. Ji, J.-L. Liang and R.-G. Ren, Synlett, 2010, 1657–1660 CrossRef CAS
; (d) X.-C. Wang, G.-J. Yang, X.-D. Jia, Z. Zhang, Y.-X. Da and Z.-J. Quan, Tetrahedron, 2011, 67, 3267–3272 CrossRef CAS
; (e) K. Singh, K. Singh, B. Wan, S. Franzblau, K. Chibale and J. Balzarini, Eur. J. Med. Chem., 2011, 46, 2290–2294 CrossRef CAS
; (f) Q. Yang, Z. Quan, S. Wu, B. Du, M. Wang, P. Li, Y. Zhang and X. Wang, Tetrahedron, 2015, 71, 6124–6134 CrossRef CAS
; (g) L. A. Ho, C. L. Raston and K. A. Stubbs, Eur. J. Org. Chem., 2016, 2016, 5957–5963 CrossRef CAS
; (h) T.-T. Li, C. Pannecouque, E. De Clercq, C.-L. Zhuang and F.-E. Chen, Molecules, 2020, 25, 1581 CrossRef CAS
; (i) M. Huang, Y. Huang, J. Guo, L. Yu, Y. Chang, X. Wang, J. Luo, Y. Huang, Z. Tu, X. Lu, Y. Xu, Z. Zhang, Z. Zhang and K. Ding, Eur. J. Med. Chem., 2021, 211, 113023 CrossRef CAS
; (j) J. Y. Lee, Y. S. Shin, S. Jeon, S. I. Lee, S. Noh, J.-E. Cho, M. S. Jang, S. Kim, J. H. Song, H. R. Kim and C. M. Park, Bioorg. Med. Chem. Lett., 2021, 39, 127885 CrossRef CAS
.
- Other strategy to 2-aminopyrimidines using three-component reaction with aldehydes, β-ketonitriles and substituted guanidines: C. Val, A. Crespo, V. Yaziji, A. Coelho, J. Azuaje, A. E. Maatougui, C. Carbajales and E. Sotelo, ACS Comb. Sci., 2013, 15, 370–378 CrossRef CAS
.
- The route involving 2-NH2-pyrimidine intermediates for the C-N cross-coupling: Y. Zhang, Z.-J. Quan, H.-P. Gong, Y.-X. Da and Z. Zhang, Tetrahedron, 2015, 71, 2113–2118 CrossRef CAS
.
- N. H. T. Phan, H. Kim, H. Shin, H.-S. Lee and J.-H. Sohn, Org. Lett., 2016, 18, 5154–5157 CrossRef PubMed
.
-
(a) G. Evano, N. Blanchard and M. Toumi, Chem. Rev., 2008, 108, 3054–3131 CrossRef CAS
; (b) Copper Catalysis in Organic Synthesis, ed. G. Anilkumar and S. Saranya, Wiley-VCH Verlag GmbH & Co., Weinheim, Germany, 2020 Search PubMed
.
-
(a) H. Rao and H. Fu, Synlett, 2011, 745–769 CAS
; (b) C. Sambiagio, S. P. Marsden, A. J. Blacker and P. C. McGowan, Chem. Soc. Rev., 2014, 43, 3525–3550 RSC
; (c) S. Thapa, B. Shrestha, S. K. Gurung and R. Giri, Org. Biomol. Chem., 2015, 13, 4816–4827 RSC
; (d) J. Jiang, L. Du and Y. Ding, Mini-Rev. Org. Chem., 2020, 17, 26–46 CrossRef CAS
.
-
(a) S. U. Tekale, V. B. Jadhav, V. P. Pagore, S. S. Kauthale, D. D. Gaikwad and R. P. Pawar, Mini-Rev. Org. Chem., 2013, 10, 281–301 CrossRef CAS
; (b) P. Bichler and J. A. Love, Top. Organomet. Chem., 2010, 31, 39–64 CrossRef CAS
; (c) H. Lin and D. Sun, Org. Prep. Proced. Int., 2013, 45, 341–394 CrossRef CAS
; (d) Q. Zeng, L. Zhang and Y. Zhou, Chem. Rec., 2018, 18, 1278–1291 CrossRef CAS
.
- D. M. T. Chan, K. L. Monaco, R.-P. Wang and M. P. Winters, Tetrahedron Lett., 1998, 39, 2933–2936 CrossRef CAS
.
- D. A. Evans, J. L. Katz and T. R. West, Tetrahedron Lett., 1998, 39, 2937–2940 CrossRef CAS
.
- P. Y. S. Lam, C. G. Clark, S. Saubern, J. Adams, M. P. Winters, D. M. T. Chan and A. Combs, Tetrahedron Lett., 1998, 39, 2941–2944 CrossRef CAS
.
-
(a) J. X. Qiao and P. Y. S. Lam, Synthesis, 2011, 829–856 CrossRef CAS
; (b) K. S. Rao and T.-S. Wu, Tetrahedron, 2012, 68, 7735–7754 CrossRef
; (c) I. Munir, A. F. Zahoor, N. Rasool, S. A. R. Naqvi, K. M. Zia and R. Ahmad, Mol. Diversity, 2019, 23, 215–259 CrossRef CAS PubMed
; (d) J.-Q. Chen, J.-H. Li and Z.-B. Dong, Adv. Synth. Catal., 2020, 362, 3311–3331 CrossRef CAS
; (e) A. Vijayan, D. N. Rao, K. V. Radhakrishnan, P. Y. S. Lam and P. Das, Synthesis, 2021, 53, 805–847 CrossRef CAS
.
-
(a) G. I. Elliott and J. P. Konopelski, Tetrahedron, 2001, 57, 5683–5705 CrossRef CAS
; (b) J.-P. Finet, A. Y. Fedorov, S. Combes and G. Boyer, Curr. Org. Chem., 2002, 6, 597–626 CrossRef CAS
; (c) S. V. Ley and A. W. Thomas, Angew. Chem., Int. Ed., 2003, 42, 5400–5449 CrossRef CAS
.
-
(a) H. Kim, J. Lee, H. Shin and J.-H. Sohn, Org. Lett., 2018, 20, 1961–1965 CrossRef CAS PubMed
; (b) J. Lee, Y. Kwon, D.-C. Lee and J.-H. Sohn, RSC Adv., 2021, 11, 36821–36825 RSC
.
- For recent selected reviews, see:
(a) K. Singh and K. Singh, Adv. Heterocycl. Chem., 2012, 105, 223–308 CrossRef CAS
; (b) Suresh and J. S. Sandhu, ARKIVOC, 2012, 66–133 Search PubMed
; (c) R. V. Patil, J. U. Chavan, D. S. Dalal, V. S. Shinde and A. G. Beldar, ACS Comb. Sci., 2019, 21(3), 105–148 Search PubMed
.
- Since Kappe's seminal work, five- or six-membered thiono substrates, such as thioamides, thiourethanes, and thioureas, have been utilized as electrophilic partners in Pd-catalyzed/Cu-mediated dehydrosulfurative C–C cross-couplings with boronic acids, stannanes, siloxanes, or terminal alkynes, as a notable extension of the Liebeskind–Srogl reaction. For the representative examples, see:
(a) A. Lengar and C. O. Kappe, Org. Lett., 2004, 6, 771–774 CrossRef CAS
; (b) H. Prokopcova and C. O. Kappe, J. Org. Chem., 2007, 72, 4440–4448 CrossRef CAS PubMed
; (c) S. Silva, B. Sylla, F. Suzenet, A. Tatibouët, A. P. Rauter and P. Rollin, Org. Lett., 2008, 10, 853–856 CrossRef CAS PubMed
; (d) S. Silva, S. Tardy, S. Routier, F. Suzenet, A. Tatibouët, A. P. Rauter and P. Rollin, Tetrahedron Lett., 2008, 49, 5583–5586 CrossRef CAS
; (e) N. Arshad, J. Hashim and C. O. Kappe, J. Org. Chem., 2009, 74, 5118–5121 CrossRef CAS PubMed
; (f) X. Guinchard and E. Roulland, Org. Lett., 2009, 11, 4700–4703 CrossRef CAS PubMed
; (g) Q. Sun, F. Suzenet and G. Guillaumet, J. Org. Chem., 2010, 75, 3473–3476 CrossRef CAS
; (h) Q. Sun, F. Suzenet and G. Guillaumet, Tetrahedron Lett., 2012, 53, 2694–2698 CrossRef CAS
; (i) Z.-J. Quan, W. H. Hu, X. D. Jia, Z. Zhang, Y.-X. Da and X.-C. A. Wang, Adv. Synth. Catal., 2012, 354, 2939–2948 CrossRef CAS
; (j) Z. F. Yan, Z.-J. Quan, Y.-X. Da, Z. Zhang and X.-C. Wang, Chem. Commun., 2014, 50, 13555–13558 RSC
; (k) Y. Wu, Y. Xing, J. Wang, Q. Sun, W. Kong and F. Suzenet, RSC Adv., 2015, 5, 48558–48562 RSC
; (l) W. Zou, Z. Huang, K. Jiang, Y. Wu, Y. Xue, F. Suzenet, Q. Sun and G. Guillaumet, Tetrahedron, 2017, 73, 5485–5492 CrossRef CAS
; (m) O. V. Maltsev, A. Pöthig and L. Hintermann, Org. Lett., 2014, 16, 1282–1285 CrossRef CAS
.
- We previously demonstrated that DHPMs are suitable substrates for Liebeskind–Srogl-type Pd-catalyzed/Cu-mediated dehydrosulfurative C–C, C–N and C–O cross-couplings:
(a) H. Kim, N. H. T. Phan, H. Shin, H.-S. Lee and J.-H. Sohn, Tetrahedron, 2017, 73, 6604–6613 CrossRef CAS
; (b) H. Yang, N. S. L. Pham, H. Shin and J.-H. Sohn, Bull. Korean Chem. Soc., 2020, 41, 881–883 CrossRef CAS
; (c) N. S. L. Pham, H. Shin, J. Y. Kang and J.-H. Sohn, J. Org. Chem., 2020, 85, 5087–5096 CrossRef PubMed
; (d) N. H. T. Phan, J. Lee, H. Shin and J.-H. Sohn, J. Org. Chem., 2021, 86, 5423–5430 CrossRef PubMed
.
- A Cu-promoted C–N cross-coupling of disulfide substrate prepared from DHPM was reported by Quan and Wang: K.-J. Wei, Z. Quan, Z. Zhang, Y. Da and X. Wang, Org. Biomol. Chem., 2016, 14, 2395–2398 RSC
.
- C. K. Khatri, S. M. Potadar and G. U. Chaturbhuj, Tetrahedron Lett., 2017, 58, 1778–1780 CrossRef CAS
.
- The reaction of 1.00 g of 1a with aniline provided the desired product 3a in 79% yield.
- Z. Quan, F. Jing, Z. Zhang, Y. Da and X. Wang, Chin. J. Chem., 2013, 31, 1495–1502 CrossRef CAS
.
- The reaction with acyclic secondary amine such as dibutylamine or dicyclohexylamine provided only a trace amount of the desired 2-aminopyrimidine product.
- Y. F. Liu, Y. J. Bai, J. Zhang, Y. Y. Li, J. P. Jiao and X. L. Qi, Eur. J. Org. Chem., 2007, 36, 6084–6088 CrossRef
.
- J. J. V. Eynde, N. Labuche, Y. V. Haverbeke and L. Tietze, ARKIVOC, 2003, 15, 22–28 Search PubMed
.
- H. P. Gong, Z. J. Quan and X. C. Wang, Appl. Organomet. Chem., 2016, 30, 949–953 CrossRef CAS
.
- A. Porcheddu, G. Giacomelli, L. De Luca and A. M. A. Ruda, J. Comb. Chem., 2004, 6, 105–111 CrossRef CAS PubMed
.
-
(a) K. Yamamoto, Y. G. Chen and F. G. Buono, Org. Lett., 2005, 7, 4673–4676 CrossRef CAS
; (b) B. Han, R. F. Han, Y. W. Ren, X. Y. Duan, Y. C. Xu and W. Zhang, Tetrahedron, 2011, 67, 5615–5620 CrossRef CAS
.
- We attempted to trap the radical species using radical scavengers, such as TEMPO, BHT, or 1,1-diphenylethylene but could not obtain the pyrimidine-radical scavenger adduct, presumably due to the faster aromatization than the intermolecular adduct formation.
- L. B. Delvos, J. M. Begouin and C. Gosmini, Synlett, 2011, 16, 2325–2328 Search PubMed
.
- N. N. Karade, S. V. Gampawar, N. P. Tale and S. B. Kedar, J. Heterocycl. Chem., 2010, 47, 740–744 CAS
.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, compound characterization data, NMR spectra. See DOI: https://doi.org/10.1039/d2ra05180j |
| This journal is © The Royal Society of Chemistry 2023 |