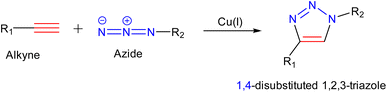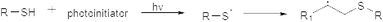Open Access Article
Open Access ArticleCuAAC ensembled 1,2,3-triazole linked nanogels for targeted drug delivery: a review
Gurleen Singha,
Ather Majeeda,
Riddima Singha,
Nancy Georgea,
Gurjaspreet Singhb,
Sofia Guptab,
Harminder Singh a,
Gurpreet Kaur*c and
Jandeep Singh
a,
Gurpreet Kaur*c and
Jandeep Singh *a
*a
aSchool of Chemical Engineering and Physical Sciences, Lovely Professional University, Phagwara 144411, Punjab, India. E-mail: singhjandeep@gmail.com
bDepartment of Chemistry and Centre of Advanced Studies in Chemistry, Panjab University, Chandigarh 160014, India
cDepartment of Chemistry, Gujranwala Guru Nanak Khalsa College, Civil Lines, Ludhiana 141001, Punjab, India. E-mail: chemgurpreet5@gmail.com
First published on 18th January 2023
Abstract
Copper(I) catalyzed alkyne azide cycloaddition (CuAAC), the quintessential example of ‘click chemistry’, provides an adaptable and adequate platform for the synthesis of nanogels for sustained drug release at targeted sites because of their better biocompatibility. The coupling of drugs, carried out via various synthetic routes including CuAAC, into long-chain polymeric forms like nanogels has exhibited considerable assurance in therapeutic advancements and intracellular drug delivery due to the progression of water solubility, evacuation of precocious drug release, and improved upthrust of the pharmacokinetics of the nanogels, thereby rendering them as better and efficient drug carriers. The inefficiency of drug transmission to the target areas due to the resistance of complex biological barriers in vivo is a major hurdle that impedes the therapeutic translation of nanogels. This review compiles the data of nanogels synthesized specifically via CuAAC ‘click’ methodology, as scaffolds for targeted drug delivery and their assimilation into nanomedicine. In addition, it elaborates the ability of CuAAC to graft specific moieties and conjugating biomolecules like proteins and growth factors, onto orthogonally functionalized polymer chains with various chemical groups resulting in nanogels that are not only more appealing but also more effective at delivering drugs, thereby enhancing their site-specific target approach and initiating selective therapies.
1. Introduction
In the domains of biomedicine, bio-nanotechnology, and pharmaceutics, polymer-based drug nanocarriers have always been fascinating drug therapies for targeted site-specific drug delivery.1–3 A wide range of diseases including neurological disorders, cardiovascular diseases, and malignancies can be treated and healed in a better and more efficient manner using polymeric drug delivery systems owing to the targeted and controlled release of therapeutics,4–6 thereby reducing the side effects of drugs, especially those for cancer which is a major threat to human health after cardiovascular diseases which are the leading cause of human fatality.7–10 It was reported by the International Agency for Research on Cancer (IARC), in 2018, that there were 18.1 million cancer diagnoses globally, with a projected increase to 29.5 million by 2040.10 Cancer therapy still has high side effects of anti-cancerous drugs, while damaging the fast-growing cells of the body as well as the tumor sites in adequate immune responses resulting in adverse side effects,11,12 To tackle such issues, researchers have focused on the development of nanocarriers to transport the drugs for high intracellular drug delivery.13,14The design and expansion of nanocarriers play a key role in the development of new advanced therapeutic ways as the nanotechnology implied for their synthesis additionally empowers the drugs of concern to enhance their vital properties like solubility and increased concentration at the target site for higher potency and reduced side effects.15 These desirable traits of drugs in consideration with the stealth behavior of the nanocarriers towards the immune system are being explored for the treatment of many chronic and acute disorders, as the nanoparticles potentially improve the therapeutic index, decrease off-target toxicity, and change the pharmacokinetic profile of pharmaceuticals16 so that the desirable drug gets incorporated into the body and accumulates at the required site while exhibiting pharmacophoric properties exclusively towards the target cells, known as ‘targeted drug delivery’. A variety of nanocarriers have been reported in the literature for applications in targeted drug delivery such as mesoporous nanocarriers, dendrimers, carbon nanotubes, gold nanoparticles, quantum dots, nanogels, etc., and are represented in Fig. 1.17
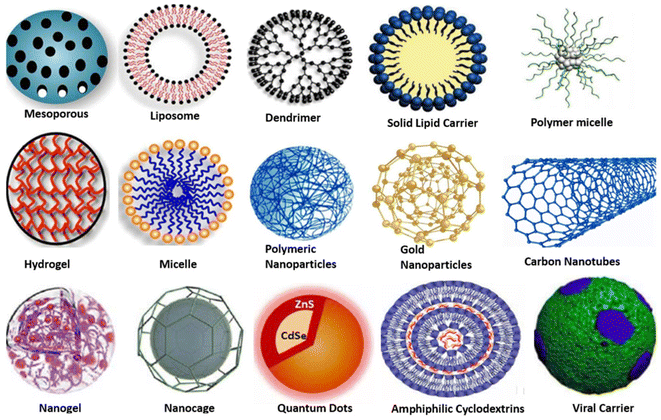 | ||
| Fig. 1 Structural representation of various nanocarriers, as potential agents, for targeted drug delivery. Adapted from ref. 17, used under Creative Common CC-BY license. | ||
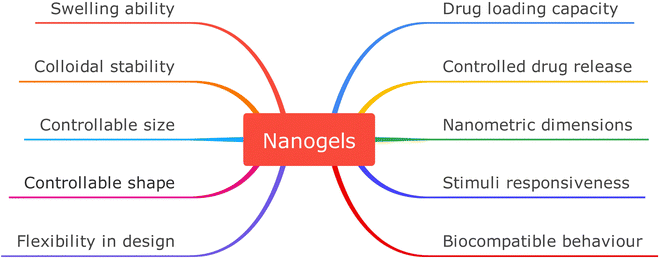
Nanogels have proved to be apt agents for targeted drug delivery due to their high biocompatibility and beneficial dimensions ranging from human organs, and cells to viruses.18,19 Nanogels essentially are submicron-sized hydrogels comprised of either physical or chemical crosslinked polymeric chains that make 3D customizable permeable organization having great limits with regards to water retention without dissolving in an aqueous solution.20–22 The vast array of nanogels and their significant potential to cross biological barriers23 due to their nanometric dimensions have highlighted them to act as carriers (Fig. 2)24 for the transport of bioactive species, resulting in the development of the best therapeutic strategy known as the ‘controlled drug delivery’ approach, whose aim is the controlled release of the administrated drug within the optimum range of concentration, thereby limiting the potential adverse effects.25,26
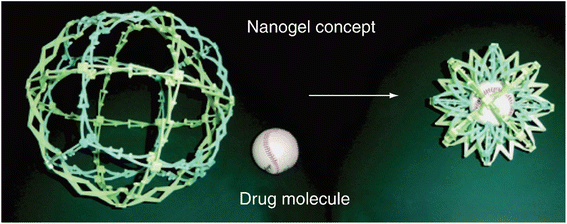 | ||
| Fig. 2 A pictorial depiction of drug loading by the nanogels.24 Adapted from Nanomedicine, 2010, 5(2), 165–168 with permission of Future Medicine Ltd. | ||
Due to the numerous advantages like stealth behavior, high drug loading ability, and sustained release presented by these nanogels as ‘targeted drug delivery’ agents, the researchers have upsurged their efforts to synthesize such polymeric systems via different efficient and cost-effective methodologies wherein Cu(I)-catalyzed alkyne–azide cycloaddition (CuAAC), the prototypical ‘Click Chemistry’ pathway27,28 has been extensively and successfully explored for the synthesis of tailor-made novel nanogels.29 This review enlists the diverse nanogel systems, as drug delivery agents, that have been synthesized via CuAAC. The structural characteristics, the type of reactants (monomers/polymers) used, and the functional behavior of the reported nanogels have been presented and discussed in the forthcoming sections following some general characteristic features, the classification, and the mechanism of action of these nanogels.
2. Characteristic aspects and classification of nanogels
2.1. Push for the requirement of nanogels
The conventional anti-cancerous drugs used in therapy have numerous side effects on the normal growing cells of the host body, apart from tumor sites like hair nails, skin, etc.30 To overcome these undesirable side effects, researchers have developed various polymer-based macromolecular drug delivery systems, including nanoparticulate drug delivery systems, wherein the drug is loaded on the target site by physical interaction.31,32 The conjugation of anti-cancer drugs to the polymer-based skeleton to form polymeric prodrug is an excellent strategy to improve the solubility of the specific hydrophobic drugs, thereby preventing the early and premature release of drugs with the improvement of drug pharmacokinetics.2.2. Physicochemical and stability aspects of nanogels
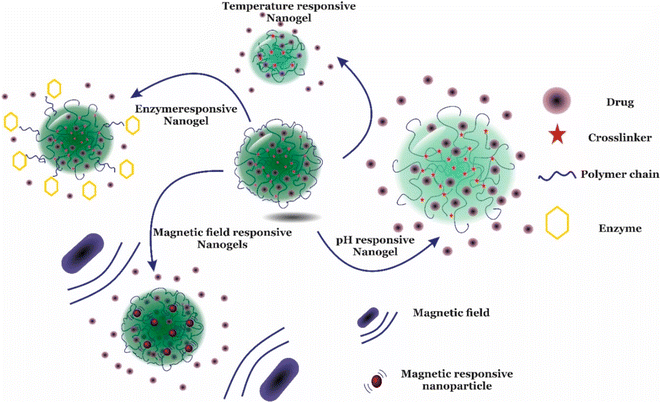 | ||
| Fig. 3 An illustrative representation of different modes of drug release by nanogels in response to different types of stimuli. Adapted from ref. 38, used under Creative Common CC-BY license. | ||
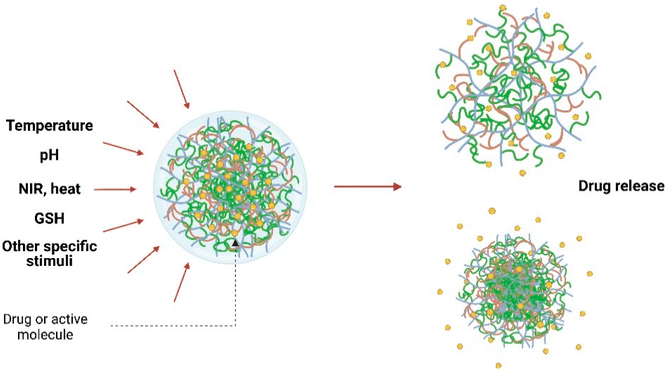 | ||
| Fig. 4 A representation of targeted drug release from nanogel via swell–shrink action stimulated by external factors. Adapted from ref. 42, used under Creative Common CC-BY license. | ||
• Nanometric dimensions: this property is attributed to surface area and volume ratio, so if the structure is well-created, these smaller dimensions enable nanoparticles to pass through different biological membranes and infiltrate tissues and cellular pathways.23,35 The nanoscale size of the nanogels is crucial for their ability to permeate and remain in the tumor, and the nanometric dimensions give them a higher surface area to the volume aspect ratio, making hydrophobic medicines more soluble. Quantum mechanical properties, biological reactivity, and the nanogels' intrinsic surface qualities are all controlled by their nanometric size.36,37
• Response to external stimuli: nanogel designing is done in such a way that they must respond to specific stimuli and must retain high drug encapsulation stability while circulating, but while reaching the targeting site, the drug should release readily in response to the appropriate stimulus. Recent advances have demonstrated that stimuli-responsive release of drugs in response to changes in pH, temperature, redox, light, enzymes, etc. (Fig. 3)38 is the most common release mechanism now in use regardless of physical embedding or covalent connection of the nanogels.39,40 As a result of these factors, the nanoparticle-composed gel undergoes several ancillary or conformational changes which also alter the hydrophilicity and hydrophobicity of the gels made of nanoparticles, thereby resulting in drug release.18,41
• Swelling behavior: nanogels possess this peculiar property due to solvent penetration which causes expansion of the polymeric framework, resulting in increased volume in the system. This property can be controlled with the help of external stimuli like pH, temperature, or particular molecules (Fig. 4)42 that can change the swelling/de-swelling qualities, which further can be regulated by different structural factors like the extent of crosslinking, the presence of different kinds of functional groups, charge concentration, etc.43,44
• Size and shape control: the size of the nanogels can be controlled chemically by limiting the rate of phagocytosis and helping in the passive transport of loaded drugs inside the intracellular cavity. Also, nanogel shapes can be manipulated with proper design to increase their quality and performance. The best example of this is the non-spherical type of nanogels which are used for drug delivery and circulate for a longer time in the circulatory system.45,46
• Drug loading: this characteristic of nanogel depends mainly on the properties of the polymeric system used to synthesize the nanogel, and the kind of functional groups attached to the polymeric unit. Physical entrapment, as well as covalent and noncovalent interactions, and passive/diffusion-based drug loading are the different pathways for incorporating drugs into the nanogels. The drug loading efficiency is established by the hydrophobic core, which permits cargo loading via hydrophobic or electrostatic interactions. The hydrogen bonding and van der Waal forces of interaction within the gel network are initiated by these dangling functional groups of polymeric chains, thereby making it easier to transport drugs.47–49
• Drug release: drug delivery at the targeted site is the main approach to the use of nanogels. This includes diffusion release, drug degradation, or response to the external environment. Drug release can be manipulated and controlled by mechanisms like framework degradation, diffusional release, or in response to external stimuli like pH, temperature, redox potential, etc. By suitable nanogel composition, the release may be regulated and prolonged, thereby making it possible to time and place the delivery of medicine so that it meets the body's physiological needs. In addition to the physicochemical properties of the nanogels, the process of drug release is also related to how the cargo are loaded into the nanogels. Swelling nanogels to increase mesh size or degradation unlocks a door for drug diffusion, but medicines need to be dissociated to be released.47–49
• Colloidal stability: in the cellular matrix and body fluids, nanogels possess higher colloidal stability, which helps to ensure good performances in drug delivery and drug stability. The polymeric chains utilized for nanogels synthesis form crosslinked networks that enclose the loaded molecule, increasing its colloidal stability. The colloidal stability of nanogels is also reported to get improved with active free radicals. The stabilizer or dispersion, typically a block or graft copolymer, binds covalently to the surface of the expanding particles to prevent coagulation and increase the nanogel system's colloidal stability.42,50
• Biocompatible behavior: although nanocarriers can have interactions with cells and tissues, they must not have any cytotoxic, apoptotic, or necrotic effect on the individual.51 Biocompatibility of nanogels gives the sophistication when in use must perform optimal and desired functions without provoking any kind of general or systemic destructive effects.52–54 Due to their composition of biocompatible polymers, nanogels when implemented at the molecular scale, do not cause any negative biological reactions like mutagenic or immunological activation. This biocompatibility of nanogels is an extremely desirable characteristic of any nanocarrier for drug delivery purposes.46
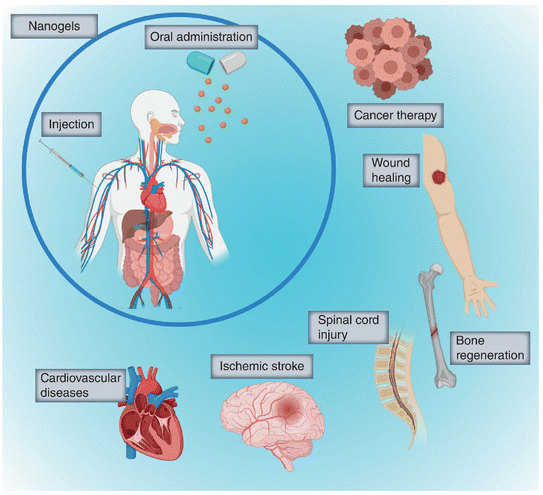 | ||
| Fig. 6 Target organs for implementation of nanogels as drug carriers in vivo.59 Adapted from Nanomedicine, 2020, 15(27), 2707–2727 with permission of Future Medicine Ltd. | ||
2.3. Mechanism of action, biodistribution, and toxicity
Besides the targeted drug delivery, nanogels are now a leading choice for scientists with the aim to reduce the adverse effects of drugs while keeping in view the health of the host.60 For the nanogel to reach the target site, the mode of circulation and the strategies of the drug delivery system (active and passive targeting) (Fig. 7) are the top consideration factors.37,61 While the passive targeting strategies work on the enhanced permeability, the retention effect of the cytosol in the cell is wholly chance-dependent and is based upon the circulation time in the host.62 The active mode of drug transportation involves more specific and efficient delivery of the drug to the target site63 as it is mediated by affinity ligands connected to a carrier, avoiding the accumulation of the drug in normal tissues and thus helps in enhancing the efficiency of therapeutic doses and helps in limiting the side effects on normal organs of the host.64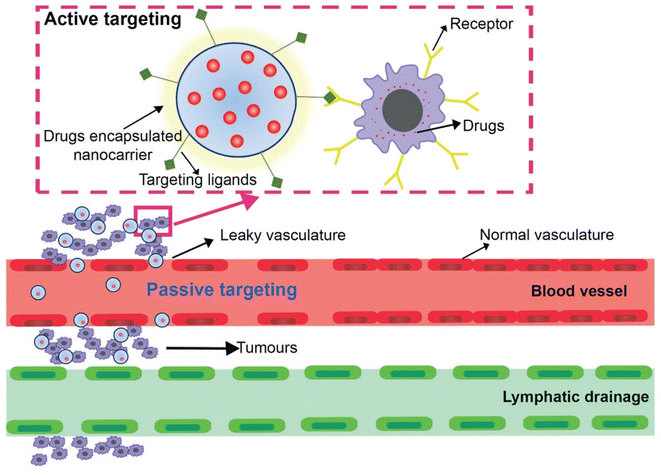 | ||
| Fig. 7 A schematic representation of the phenomena of active and passive targeting exhibited by a nanocarrier encapsulating the drug. Reproduced from ref. 65 with permission from the Royal Society of Chemistry. | ||
Nanogels' surface physicochemical qualities are directly linked to their biodistribution in vivo. Nanogels can have a wide range of morphologies, from core–shell to spherical, depending on the particles used and the assembly method. Chemical processes or physical crosslinking based on hydrogen bonds, ionic interactions, or other intermolecular connections can produce these varying morphologies. That being said, the biocompatibility of the surface embellishment is a crucial factor that can significantly affect biodistribution.66,67 To maintain their biodistribution, nanogels can be PEGylated, which makes them more hydrophilic, protects them from any charge their core may carry, and creates a steric hindrance for interaction with serum proteins. In addition, the biodistribution and clearance from the circulation of these ‘stealth’ nanogels are significantly affected by particle size.68,69 Researchers generally are of the opinion that smaller particles with a higher curvature can decrease opsonization responses and subsequent clearance by macrophages, resulting in longer circulation times and greater tumor accumulation.70 Apart from the biodistribution, the intrinsic toxicity of nanogels is also an important parameter for practical implementation. The polymeric ingredients used in nanogel synthesis must be either non-toxic in the first place or degrade into non-toxic byproducts during metabolism. For instance, polymeric systems based on methacrylates and acrylates can be hydrolyzed into small molecule alcohols and non-toxic poly-methacrylic acid and poly-acrylic acid, respectively. On the other hand, enormous surface/mass ratio, and interactions with cells including surface activation, uptake, intracellular breakdown or deposition, all of these factors impact possible toxicity and side effects by abnormally stimulating and/or harming the cells taking up such carriers.18,19,39,46
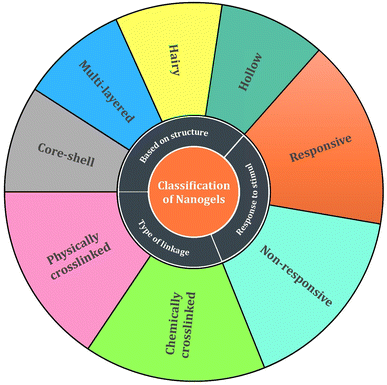
2.4. Classification of nanogels
Multiple factors influence the design and development of nanogels which include the tuning of nanogels for desired functions, point of action, polymeric constituents required for production, and the long-term releasing behavior of the loaded drugs. This classification of nanogels based on various factors is illustrated in Fig. 8.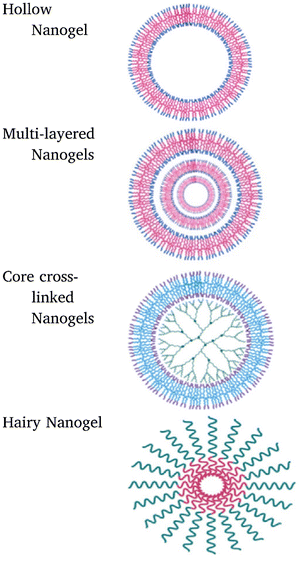 | ||
| Fig. 9 Representative classification of nanogels based on their structures. Reprinted from ref. 35. Copyright 2020, with permission from Elsevier. | ||
Nanogels can be categorized based on the linkage present in network chains of the gel structure into two categories primarily:
(a) Physically cross-linked nanogels: these types of nanogels are formed either by weaker linkages like van der Waals interactions, hydrophobic forces of attraction, or by hydrogen bonding but have a poor preference in comparison to chemically cross-linked nanogels owing to the stability factor.72,73 The nanogels can be those that are liposome modified, micellar type, and hybrid nanogels. These types of nanogels have the ability to form a complex with various substances like proteins, drugs, and DNA molecules36 and possess various desirable features such as reversibility, no chemical reactions, and/or toxic bioactive agents.35 When utilizing associating polymers, it might be challenging to create stable physically cross-linked nanogels with regulated sizes due to the relative weakness of their non-covalent contacts.74
(b) Chemically cross-linked nanogels: these nanogels are characterized by permanently joined polymer chains via strong covalent linkages at different junctions.75 The permanent chemical linkages across the gel networks in chemical cross-linked nanogels and the functional groups present in the gel networks determine their characteristics.76 These nanogels are of several types based on the mode of synthesis like inverse emulsion polymerization, photo-induced crosslinking polymerization, reversible addition–fragmentation chain transfer (RAFT) polymerization, click chemistry crosslinking polymerization, etc. (Fig. 10). Out of these, click chemistry crosslinking polymerization is the optimistic mode for the synthesis and formulation of nanogels due to its promising advantages:
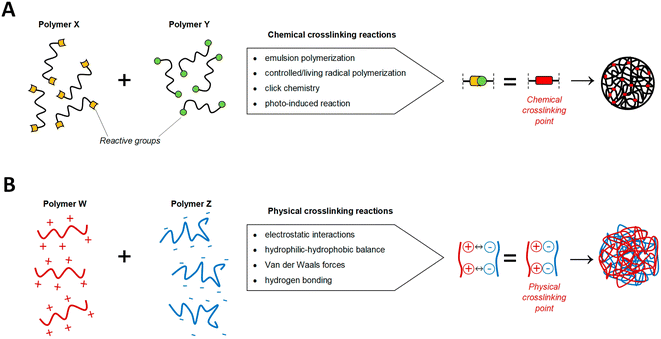 | ||
| Fig. 10 Synthetic strategy of nanogels using chemical and physical crosslinking approaches. (A) Different chemical processes utilizing the nature of the corresponding groups can be used to generate covalent crosslinking's covalent bonds (red) between the active reacting species (yellow and green) of polymers X and Y. (B) The self-assembly of a Nano-scaffold is enabled by the physical interactions between polymers W and Z. Adapted from ref. 79, used under Creative Common CC-BY license. | ||
• Developing robust nanocarrier products with high efficacy.
• Insertion of desirable moieties into the nanocarrier system with high precision.
• Imparting multi-functionality to the nanocarriers via different molecules/functional groups.
• Facile synthesis in aqueous conditions.
• Effortless affixation of high molecular weight-antibodies onto the nanoparticles etc.77,78
3. ‘Click chemistry’ as the synthetic route for nanogel synthesis
3.1. Current synthetic strategies for improved efficacy and payload for nanogels
As apparent from Fig. 10, the literature suggests that the synthesis of nanogels is formally achieved by crosslinking the polymeric units involved, for which there are broadly two categories, i.e. chemical crosslinking method including click chemistry, emulsion polymerization, controlled radical polymerization, and photo-induced reaction; and physical crosslinking methods.79 When compared to their covalently crosslinked counterparts, the comparatively lower mechanical strength, and more degradation-prone nature of physically crosslinked nanogels renders them more sensitive to the sol–gel transition caused by changes in environmental stimuli, thereby raising questions about their viability for use in harsh in vivo conditions like blood circulation. However, the therapeutic efficacy of a drug is significantly improved when the payload is contained within a nanogel that has been covalently crosslinked.80 As a result, more and more exploration of the chemical routes for crosslinking polymeric structures for nanogels synthesis is being currently undertaken to improve the performance and efficiency of the synthesized nanogels, some of which are discussed in Table 1.| Methodology | Description |
|---|---|
| Emulsion polymerization | The thrust behind this process is to control the dimensions of the end product which is achieved by the polymerization process within a small volume. This is done by the formation of monodisperse stable organic/aqueous droplets containing the monomeric/polymeric chains as well as the catalysts and crosslinking agents.79 Since bimolecular termination is inhibited by the spatial separation of propagating chains in emulsion polymerizations, it is possible to simultaneously achieve high rates of polymerization and create polymer with a high molecular weight,81 thereby making this methodology an effective contender for nanogel synthesis |
| Controlled/living radical polymerization | Adding crosslinking agents allows for the synthesis of crosslinked particles or gels with a controlled polymer molecular weight in this methodology. Through the incorporation of quiescent forms in the dispersing species, the rate of bimolecular termination and lengthening of the life of the expanding polymer chains can be reduced.82 By adjusting the size and molar mass of the nano scaffolds, CLRP has proven to be effective in nanogel design wherein tuning the final morphology and structural composition such as porosity of the nanogels is possible due to the wide range of molar mass and branching structure of the nanogel building blocks that can be achieved by varying the concentrations of monomers and crosslinking agent83,84 |
| Photo-induced crosslinking | This technique is environmentally friendly as no crosslinking agents or catalysts are required, and no waste products are generated. For crosslinking, light irradiation is used on reactants that have a photo-activatable group. A photoinitiator kickstarts the reaction by being broken down by light to produce reactive radical species39,80 |
| Click chemistry | This methodology promises rapidity, adaptability, regiospecificity, high yields, and purity under aqueous reaction conditions. Oxygen, water, common organic solvents, synthesis conditions, biological substances, and pH all show high tolerance. The researchers address this ‘click’ methodology to have the potential to alter the status quo of polymeric nanogels synthesis and inspire novel approaches to the creation of polymeric drug therapies85,86 |
3.2. ‘Clicking’ polymeric moieties to synthesize nanogels
Click chemistry,87 being one of the most apparent synthetic routes88,89 has arisen as a profoundly encouraging strategy since 2001, when Dr Karl Barry Sharpless presented this methodology to the global research community for which he was recently awarded the Nobel prize in chemistry, 2022. Due to its facile synthetic route and the significant advantage of no by-product formation, it is utilized for synthesizing and fine-tuning nanogels with incredible selectivity, gentle response conditions, and thermodynamically favorable and stereospecific product formation (Fig. 11). Further, click chemistry has found its importance in material science, bio-chemistry,90 and bio-medical fields,91 with emphasis on the production and synthesis of polymeric structures,92 cancer therapeutics,93 antimicrobial agents,94,95 and chemosensors for selective identification of biomolecules in the living cells96 as well as ion recognition.97–100 Thus, click chemistry is a convenient approach for the synthesis of drug-like compounds that use a few simple and repeatable processes to speed up the drug development process.101–104 The nanogels synthesized via click chemistry are extremely compatible with encapsulated bio-actives such as live cells, proteins, and medicines,29,105 owing to click re-unique action's biorthogonality. As a result, this methodology has an enormous influence on the growing era of medicinal chemistry, with the considerable development of nanogels and innovative techniques for screening compounds.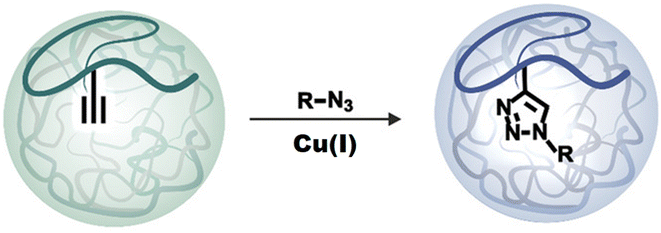 | ||
| Fig. 11 An illustration of the synthesis of nanogels following the ‘click’ reaction. Adapted from ref. 106, used under Creative Common CC BY-NC-ND license. | ||
3.3. Types of click reactions used for nanocarriers designing
There are a bunch of strategies that can effectively synthesize nanogels that require the use of polymers. These polymers can be of natural origin that include collagen, albumin, fibrin, and some polysaccharide polymers like hyaluronic acid, chitosan, agarose, and heparin whereas the synthetic precursors include poly-lactic acid, polyglycolic derivatives, polyacrylates, and polymethacrylates.67 The characterization of these starting materials is based on the behalf of target-specific functional groups responsible for the formation of covalent linkage and help in interlinkage for the development of a three-dimensional network of nanogels. Some of the ‘click-based synthetic routes followed for the synthesis of nanogels are listed below:79,803.3.1.1. Copper(I)-catalyzed alkyne azide cycloaddition. This is a quick method that ensures rapid reaction time, high yield and purity, regiospecificity, adaptability, and aqueous reaction conditions107 (Fig. 12). Both the azide and the triple bond can be grafted onto polymeric backbones with relative ease (through nucleophilic substitution, for instance) and a stable conjugate is formed.108 Since the reaction is typically unaffected by steric effects, therefore substituted primary, secondary, or tertiary aromatic azides can readily engage in this transformation with alkyne-derived components.79
Crescenzi and co-workers reported the synthesis of hyaluronic acid (HA)-sodium salt based ‘click’ hydrogels via sewing of water-soluble polysaccharide derivatives equipped with side chains bearing alkyne and azide moieties, as shown in Fig. 13. The polysaccharide-bearing nanogels were proved to act as a drug reservoir for doxorubicin after executing gelation in aqueous solutions of benzydamine and doxorubicin. These hydrogels were effective scaffolds for cells in tissue engineering as well as for the controlled drug release of therapeutically important macromolecules.109
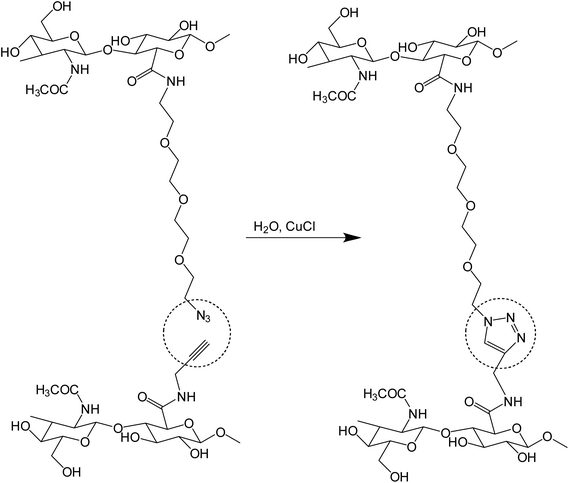 | ||
| Fig. 13 A schematic representation of the synthesis of HA-based hydrogels from alkyne and azide-bearing polysaccharides.109 | ||
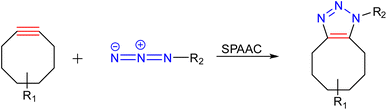
3.3.1.2. Strain-promoted alkyne azide cycloaddition (SPAAC)/copper-free reaction. The major advantage of strain promoted alkyne azide cycloaddition (SPAAC) is the absence of any metal atom/ion that can be toxic to cells and progression at ambient temperature. The highly efficient kinetics and selectivity of SPAAC render prevention of any undesirable side reactions that could occur during the conjugation process. Besides, the reaction is generally inert towards functional groups found in naturally occurring amino acids, nucleotides, and carbohydrates,110 which contributes to its attractiveness for bio-conjugation applications and selective alterations of biomolecules111 as well as nanocarrier synthesis (Fig. 14).
Hodgson research group reported the synthesis of hydrogels wherein SPAAC between PEG chains having aza-dibenzocyclooctynes and azide functionalities were used to create a range of PEG hydrogels (Fig. 15), which possessed mechanical and rheological qualities that could be adjusted by changing the polymer molecular weight and the number of cross-linking groups per chain. The rapid gelation was recorded upon the combination of the two components in an aqueous solution, with no further stimuli or catalysts required, suggesting the system may be useful as an injectable hydrogel.112
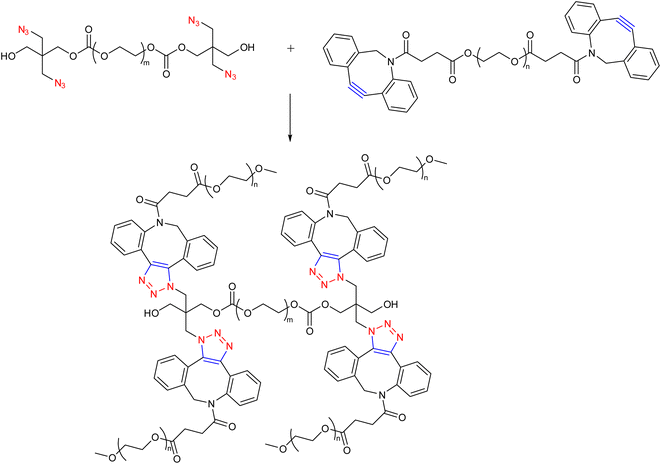 | ||
| Fig. 15 Combination of PEG chains having aza-dibenzocyclooctynes and azide functionalities via SPAAC to yield injectable hydrogel.112 | ||
3.3.2.1. Thiol–ene reaction and thiol–disulfide exchange. The thiol–ene photopolymerization reaction equips the researchers to synthesize homogeneous polymer networks with the advantages of oxygen inhibition insensitivity, diminished stress, and simplified polymerization kinetics.113,114 The overall rate, amount, and incidence of the thiol–ene click reaction (Fig. 16) have been successfully regulated through spatial and temporal control of the photoinitiation component, resulting in polymeric materials with an outstanding range of characteristics. Thiol–disulfide exchange (Fig. 17) also has received significant attention to stabilize hydrogels/nanogels since the oxidizing chemicals are routinely used in the production of macro-, micro-, and nano-scale disulfide-stabilized hydrogels/nanogels, which may have an adverse effect on the enclosed drug. This is significant for drug delivery since the blood's general redox potential is oxidative, which favors the development of disulfides, while the intracellular compartments are often reductive, leading to the deconstruction of disulfide bonds upon cellular internalisation.115
Zwitterionic nanogels are essentially polymers with stoichiometrically equal numbers of positively and negatively charged groups along the primary polymer backbone. Zwitterionic nanogels are superhydrophilic because of the presence of strong coulombic interactions along the polymer backbone; as a result, they do not behave as usual polyelectrolytes and exhibit excellent resistance to excessively saline conditions. Additionally, having neutrally charged serum or plasma on their backbones, they have been demonstrated to have great anti-fouling capabilities and also resist macrophage phagocytosis and prevent protein adsorption during drug delivery to tumor membranes, allowing for stable blood flow.116,117 Phan and co-workers presented the combination of cystamine and α,ω-functionalized poly(sulfobetaine)s (FPSBs) to synthesize the zwitterionic nanogels (Fig. 18). Aminolysis of cystamine crosslinkers resulted in the opening of thiolactone rings of PSB, and the liberated thiol-groups react with furan-maleimide double-bonds to produce nanogel networks by the thiol–ene ‘click’ reaction. Glutathione (GSH) as a reducing agent destroyed nanogels easily. Furthermore, the drug loading capacity of the nanogels was also demonstrated by loading them with doxorubicin (DOX) with drug loading capacity of 24.3%. The nanogels possessed low cytotoxicity against normal HEK293 cells but strong cytotoxicity against HeLa cancer cells.118
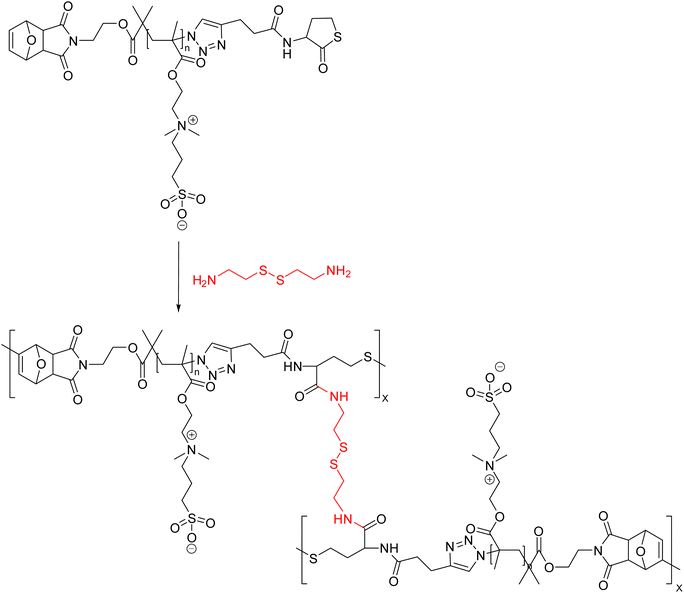 | ||
| Fig. 18 A schematic representation of combination of cystamine and α,ω-functionalized poly(sulfobetaine)s (FPSBs) to yield redox-responsive nanogels via thiol–ene reaction.118 | ||
4. CuAAC in nanocarriers and nanogels designing with 1,2,3-triazole linkers
CuAAC methodology in nanocarrier designing involves optimized bioconjugation, providing faster and more efficient reactions in the production of a variety of conjugated triazole-linked neoglycoconjugates, based on copper-catalyzed coupling reactions.119–123 Utilizing the click methodology, a variety of biomolecules including polyethylene glycol (PEG), lactose, DNA, biotin, and various recombinant proteins have been immobilized in a stable manner even without the generation of any side products. CuAAC in relation to the synthesis of nanocarriers and nanogels is classified under the chemical methods, as it results in the formation of covalently crosslinked polymeric nanogels. Besides the swelling nature, which is considered a ‘superior’ property of nanogels, the top aspects taken into consideration during the synthesis of nanocarriers are-high biocompatibility, high loading with high surface area, and colloidal stability ensuring targeted and adequate drug delivery via an active and passive mode of transportation and drug release due to quantified surface properties and particle size,68,124 all of which can be achieved via CuAAC pathway.Roberts and co-workers demonstrated the stitching of PAPMA4-stat-POEGMA40-N3 copolymer to cellulose nanocrystals (CNCs) to form a type of elongated colloidal nanoparticles via Cu(I) catalyzed alkyne–azide cycloaddition to subsequently use them for drug-delivery applications, as shown in Fig. 19. The amino groups of the copolymer allow for the attachment of diagnostic or therapeutic moieties, and the copolymer itself acts as a ‘stealth’ corona to reduce protein adsorption. The synthesized CNCs were revealed to be harmless in both human ovarian cancer (HEYA8) and human breast cancer (MDA-MB-436) cell lines. Further findings also provided credence to the use of polymer-coated CNCs in medicine and suggested the need for further in vitro and in vivo research to determine the extent to which they can function as drug-delivery agents.125
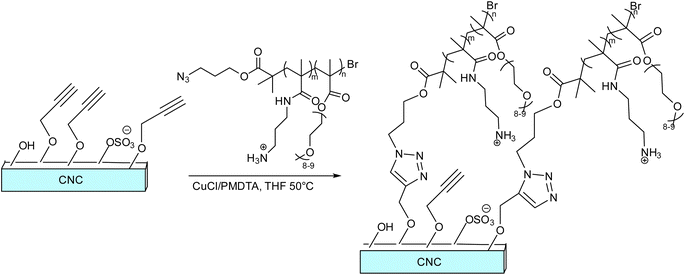 | ||
| Fig. 19 A schematic representation of conjugation of PAPMA4-stat-POEGMA40-N3 copolymer to cellulose nanocrystals (CNCs).125 | ||
Meer et al. reported the surface modification of azide-conjugated ultra-small gold nanoparticles with alkyne containing groups (Fig. 20) via CuAAC to yield strongly fluorescing nanoparticles that were traceable inside eukaryotic cells. The nanoparticles were subjected to functionalization with azide-functionalized tripeptide lysine(N3)-cysteine-asparagine and subsequently reacted with alkyne-terminated fluorescein (FAM) to give the FAM-clicked ultrasmall gold nanoparticles. These nanoparticles were taken up very efficiently by HeLa cells after 24 hours, thereby demonstrating their penetrating ability into the nuclear membrane to a significant extent. Each nanoparticle had about 8 fluorescein molecules covalently bonded to it.126
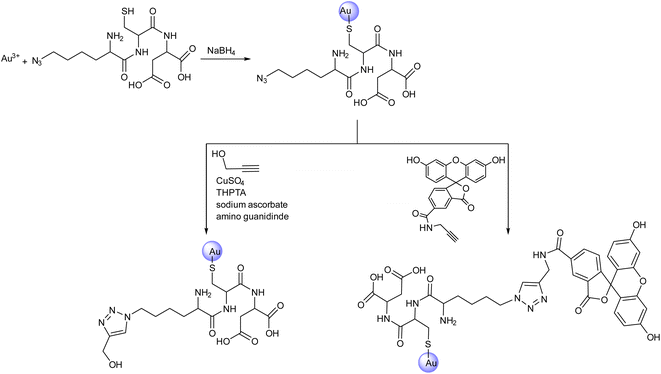 | ||
| Fig. 20 A schematic representation of azide conjugation of ultrasmall gold nanoparticles and their subsequent ‘click’ conversion by reacting with different alkyne moieties.126 | ||
Haring research group presented the synthesis of ‘click-TIA’ as shown in Fig. 21, which when subjected to sonication in water resulted in the production of supramolecular viscoelastic hydrogels. It was demonstrated that the substitution of 1,2,4-triazole ring with 1,2,3-triazole moiety in the compound induced the synthesis of hydrogelator. The hydrogel was implied as a drug carrier for oxytetracycline by encapsulation of the drug and exhibited controlled release.57
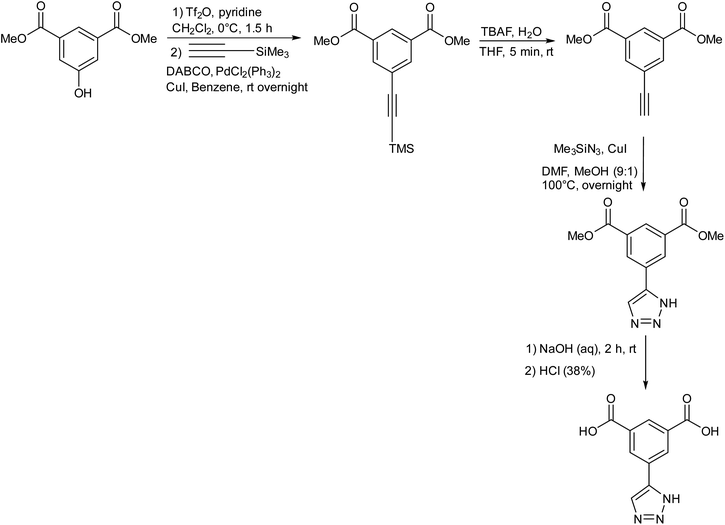 | ||
| Fig. 21 A schematic representation of the synthesis of click-TIA in multiple steps.57 | ||
Quadir et al. presented polypeptide-based block copolymers modified with click chemistry for the synthesis of pH-sensitive nanoscale drug delivery systems. N-carboxyanhydride-based ring-opening polymerization resulted in the production of PEG-polypeptide copolymers with pendant amine chains and subsequent functionalization was carried out via azide–alkyne cycloaddition. Self-assembly into stable polymersomes and disassembly in a pH-responsive manner were seen in the produced block copolymers that contain a polypeptide block with amine-functional side groups., as shown in Fig. 22. To encapsulate hydrophilic cytotoxic drugs like doxorubicin at physiological pH, these block copolymers self-assemble into vesicular nanometer-scale structures, which then spontaneously disassemble at endosomal pH levels following cellular uptake.127
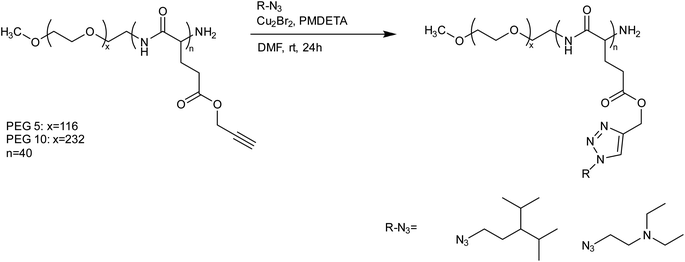 | ||
| Fig. 22 A schematic representation of the synthesis of PEG-polypeptide copolymer from N-carboxyanhydride using CuAAC.127 | ||
Abandansari research group reported a Boltorn®H40 and poly(vinylpyridine) based nanogel synthesized via CuAAC through mini-emulsion polymerization wherein poly(vinylpyridine) (PVP) was utilized as a pH sensitive crosslinker and H40-poly(ε-caprolactone) (H40-PCL) dendrimers were used as the core (Table 2, entry 1). The nanogel exhibited swelling on exposure to low pH environment, thereby releasing the loaded drug.128 Assali and coworkers developed poly(diacetylene) (PDA) based nanogel having diacetylenic hydrophobic part and oligoethyleneoxide based hydrophilic part connected through 1,2,3-triazole ring (Table 2, entry 2) Due to the presence of PDAs, the nanogel displayed intense blue colour, attributing to the absorption of the enyne moieties in the visible region.129
| Entry | Nanogel material | Basic structure | Aspect(s) of nanogel | Key function(s) | Reference |
|---|---|---|---|---|---|
| 1 | Boltorn® H40 and poly(vinylpyridine) nanogel | 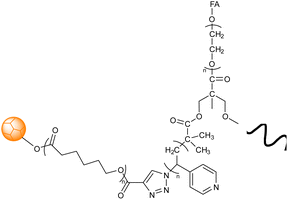 |
CuAAC assisted in covalent bond formation resulting in the chemically cross-linked nanogels comprising hyperbranched dendrimer (H40-PCL) with PVP as a crosslinker through mini emulsion polymerization | pH-sensitive nanogel utilized in the release of a hydrophobic anti-cancer medication | 128 |
| 2 | Nanogel formed through diacetylenic-based glycolipids | 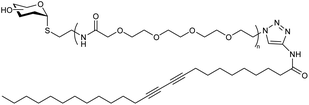 |
The presence of a 1,2,3-triazole ring between the hydrophobic and hydrophilic part of the diacetylene-based glycolipids resulted in their self-organization into 1D-nanotubes, and at higher concentrations, these bundled nanotubes crosslinked to form the nanogels | Nanocontainer for cytotoxic topotecan drug incorporation and its site-specific release | 129 |
| 3 | Poly(ethylene glycol) (PEG) monomers-based nanogel | 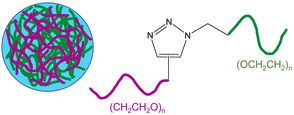 |
Alkyne and azide functionalized PEG monomers were crosslinked via CuAAC to synthesize the nanogel and the polymerization was subsequently ceased by the addition of a Cu-chelating agent | The nanogel coatings efficiently exhibit short-term protein resistance, encompassing superior surfaces which could be well utilized for in vitro diagnostics | 130 |
| 4 | Ga-porphyrin-OH nanogel based on PEG | 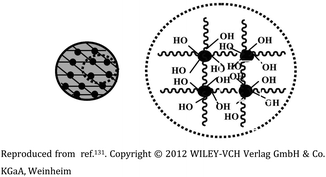 |
Fluorescent nanogel synthesized via reverse (mini)emulsion-CuAAC having uniform size | Applicability in near-infrared (NIR) imaging due to their fluorescence and cell affinity | 131 |
| 5 | Ga-porphyrin-FA nanogel based on PEG | 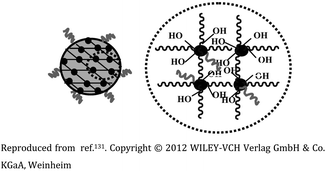 |
The diameter of nanogels ranged between 30–120 nm, with an emission maximum between 700 and 800 nm. Applicability in near-infrared (NIR) imaging due to their fluorescence and cell affinity | Folate immobilization on the surface to target tumor cells for cell imaging and drug release | 131 |
| 6 | Hydroxyethyl methacrylate (HEMA), acrylated methyl ether poly (ethylene glycol) (ACMPEG), and Fe3O4 based nanogel | 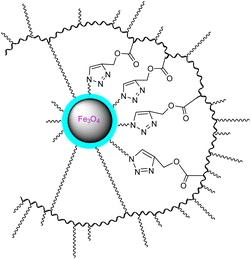 |
Click chemistry and surfactant-free emulsion photopolymerization in combination to produce amphiphilic nanogels from vinylated SPIONs/HEMA/PEG | Carrier for the anti-cancer drug quercetin | 132 |
| 7 | Polyethylene glycol (PEG), polyethyleneimine (PEI), and rhodamine-based nanogel | 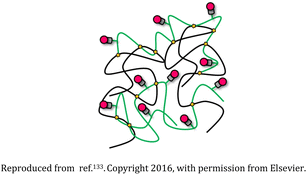 |
The nanostructures were biodegradable and appropriate for transporting drugs or genetic material and also could be tracked as they interacted with the cells | Drug delivery devices for sustained release of the succinimide anticonvulsant drug ethosuximide | 133 |
| 8 | Polyethylene glycol (PEG), polyethyleneimine (PEI), and rhodamine-based nanogel | 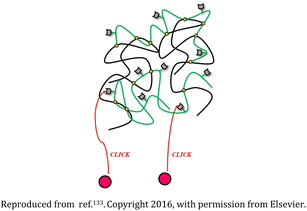 |
The nanostructures were biodegradable and appropriate for transporting drugs or genetic material and also could be tracked as they interacted with the cells | Drug delivery devices for sustained release of the succinimide anticonvulsant drug ethosuximide | 133 |
| 9 | Polyethylene glycol (PEG), and polyethyleneimine (PEI) based nanogel framed via rhodamine | 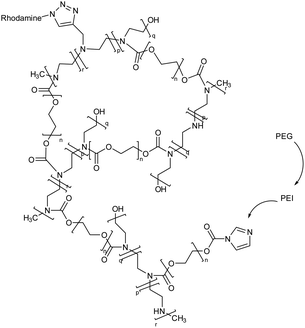 |
The potential of the nanogels to enhance or decrease microglia internalization was evaluated after they were coated with various concentrations of PEG monomethyl ether | Transportation of drugs or genes into the microglia environment, enhancing their therapeutic efficacy for reduction of microglia internalization | 134 |
| 10 | Polyethylene glycol and polyethyleneimine based nanogel | 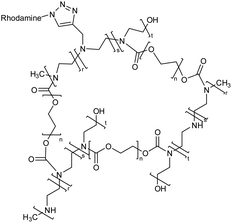 |
The nanogel loaded with the anti-hepatic stetosis properties bearing polyphenol i.e., hydroxytyrosol (HT) on in vitro administration exhibit the reduction of intracellular triglyceride levels | Potential pharmacological treatment of non-alcoholic fatty liver disease (NAFLD) | 135 |
| 11 | Polyethylene glycol and polyethyleneimine-based nanogels with high colloidal stability and biocompatibility | 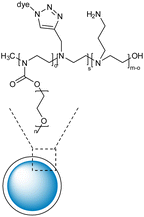 |
RhB and Cy5 fluorescent dyes bearing azide groups were used for the synthesis of the nanogels and utilizing the unreacted amines in the PEI chains, the primary amines linkers were grafted around the nanogel surface | Drug carrier for rolipram to reduce astrogliosis | 136 |
| 12 | Amine and pyridine-functionalized PEG-PEI based nanogel | 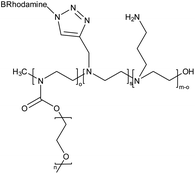 |
The nanogel was synthesized via emulsification-evaporation method and a microscopic molecular examination of the surface features of these systems and an evaluation of their cytocompatibility were also performed | Efficient drug delivery devices with prolonged sustained release | 137 |
| 13 | Polyethylene glycol (PEG) and polyethylene-imine (PEI) based nanogel coated with primary amines | 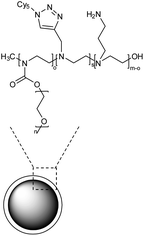 |
The nanogel was synthesized by emulsification-evaporation method and experimental analysis indicated that activated astrocytes were more likely to be targeted by NG in vitro than microglia or neurons | Targeted delivery of rolipram to quench the pro-inflammatory effects mediated by astrocyte activation | 138 |
| 14 | Poly(ε-caprolactone)-b-poly(glycidyl methacrylate) (PCL-b-PGMA) block copolymers based nanogel | 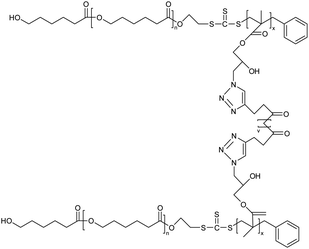 |
A combination of ring-opening polymerization and reversible addition–fragmentation chain transfer polymerization was implied for the synthesis of copolymers for nanogel production | Potential carrier for low molecular weight drugs | 124 |
| 15 | Poly-amino acid-based nanogel | 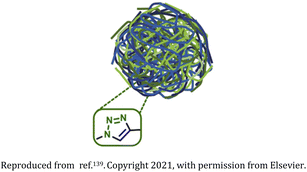 |
Biodegradable and biocompatible cross-linked nanogels synthesized from polyglutamic acid ∼100 nm in size | Hydrophilic drug carriers for the chemotherapeutic agent doxorubicin for triple-negative breast cancer treatment | 139 |
| 16 | Anisotropic gold nanoparticles incorporated-polymeric thermoresponsive nanogel | 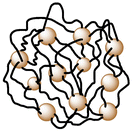 |
Nanogel was thermoresponsive in nature and synthesized via click chemistry and thermo-nanoprecipitation with gold nanoparticles as the crosslinking agent | High NIR light-to-heat conversion efficiency for photothermal therapy | 140 |
| 17 | Photoresponsive oligonucleotide-based nanogel | 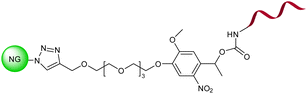 |
The photoirradiation of nanogel led to hyperchromic shift at 260 nm and a simultaneous redshift of the absorption in the 350–400 nm region | Control of protein release via managing the dissociation of a protein's DNA complex | 141 |
| 18 | Thiacalix[4]arene functionalized chitosan-based nanogel (CS-g-TC4A) | 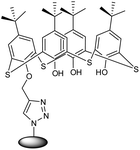 |
The unique multifunctional nano adsorbent nanogel was an efficient sorption agent with controlled super-paramagnetic and sorption capabilities | The synthesized CS-g-TC4A nanogel having a triazole ring was used as an effective adsorbent for metal ions | 142 |
| 19 | Hyaluronan and riboflavin derivatives based nanohydrogel | 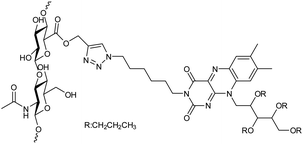 |
The nanohydrogels formulation could be freeze-dried when dextrose was added as a cryoprotectant since they were extremely stable in water solutions | Drug carriers for the delivery of hydrophobic drugs | 143 |
| 20 | Thermoresponsive poly(N-vinylcaprolactam) nanogels functionalized with glucose and maltose | 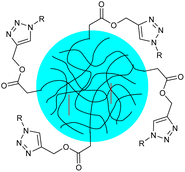 |
The nanogels were synthesized via semibatch precipitation polymerization which shrank upon heating and was equipped with a thermoresponsive structure and surface functionalities that allow for biorelevant interactions | Enhanced affinity for biomolecules due to the presence of carbohydrates results in greater potential for therapeutic uses as drug-delivery agents | 144 |
| 21 | Biodegradable dendritic polyglycerol nanogel | 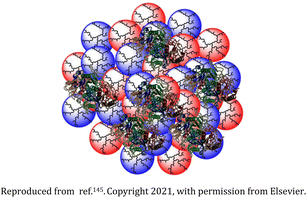 |
The dendritic polyglycerol nanogels were synthesized by inverse nanoprecipitation wherein the introduction of benzacetal bonds induced the biodegradability | Effective protein encapsulation and release among other biomacromolecules | 145 |
| 22 | Bisphosphonate ligand-functionalized dextran nanogel |  |
Controlled functionalization of the nanogel with bisphosphonate ligand through astoichiometric click-chemistry in-emulsion method | Hepatic avoidance, binding to the bone on the inside of marrow cavities, and anti-osteoporotic actions | 146 |
| 23 | Alkynopoly(acrylic acid), diazido-poly(ethylene glycol), and diazido-poly(butylene succinate) based nanogel | 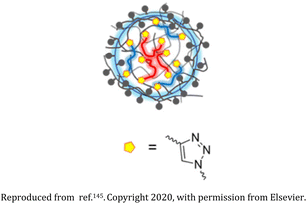 |
Amphiphilicity of the nanogel is controlled via variations in the hydrophobic diazido-poly(butylene succinate) and the hydrophilic diazido-poly(ethylene glycol) | Interaction with multi-walled carbon nanotubes (MWCNTs), thereby acting as a potential dispersant of MWCNTs in hydrophilic media | 147 |
| 24 | Nanogel based on (adamantly (AD)-benzoic imine-conjugated poly [poly(ethylene glycol) monomethyl ether methacrylate]-co-poly(2-hydroxyethyl methacrylate) (PPEGMA-co-PHEMA-AD) as well as doxorubicin (DOX)-hydrazone and b-cyclodextrin(b-CD) | 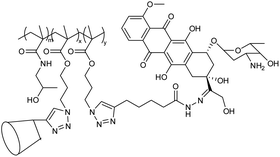 |
The polymeric nanogels were synthesized via host–guest interactions, for dual pH-triggered multistage drug delivery, and nanogels were reorganized into smaller nanoparticles in response to tumor acidity | Deep penetration of the drug at the tumor site, the release of doxorubicin in endosomal or lysosomal acidity (pH ∼5) | 148 |
| 25 | A pH and reduction dual-sensitive prodrug nanogel | 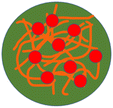 |
The nanogel displayed excellent size stability against large-volume dilution, high salt concentration, and prolonged incubation in phosphate-buffered saline | Release doxorubicin in the intracellular acidic and reducible environment after being successfully absorbed by cancer cells | 149 |
Donahoe group presented the synthesis of nanogels via CuAAC from poly(ethyleneglycol) (PEG) monomers functionalized separately with alkyne and azide moieties (Table 2, entry 3). An exceptional aspect of this procedure was the closure of nanogel synthesis by the addition of copper-chelating agent.130 Fu et al. reported PEG based fluorescent nanogels having –OH groups and Ga–porphyrin complex; and Ga–porphyrin complex with folate functional groups (Table 2, entry 4, 5). Due to their fluorescent nature, the nanogels were proposed to be applicable in near infrared (NIR) imaging.131 Khoee and Abedini reported the synthesis of Fe3O4 nanoparticles based nanogel from vinylated SPIONs/HEMA/PEG (Table 2, entry 6) which was utilized as a carrier for the anticancer drug quercetin.132 Mauri et al. reported two different synthetic routes for a fluorescent-labelled nanogel using PEG, alkyne functionalized polyethyleneimine (PEI) and rhodamine azide via CuAAC which possessed therapeutic applications (Table 2, entry 7, 8)133 and utilized PEG, alkyne functionalized polyethyleneimine (PEI) and rhodamine azide to synthesize nanogels which were coated with PEG monomethyl ether (mPEG) for the treatment of microglia (Table 2, entry 9).134 The group also developed polyethylene glycol and polyethyleneimine-based nanogel systems (Table 2, entry 10) for the treatment of hepatic steatosis in an in vitro model wherein the developed nanogel technology outperforms more traditional drug administration routes and was therefore a potential technique for the management of non-alcoholic fatty liver disease (NAFLD).135 Papa research group in 2020 synthesized nanogels from PEG and PEI polymers and fluorescent-labelled them with Cy5 and RhB dyes (Table 2, entry 11) to subsequently imply them for the treatment of activated astrocytes in spinal cord injury.136 Pinelli et al. in 2021 demonstrated different functionalization methodologies for PEG-PEI nanogels which resulted in specific body tissue targeting (Table 2, entry 12).137 Vismara et al. in 2019 developed nanogels from PEG activated with carbonyldiimidazole (CDI) and PEI (Table 2, entry 13) which were used as a vector for the anti-inflammatory drug rolipram.138
Cao et al. in 2015 synthesized nanogels from poly(ε-caprolactone)-b-poly(glycidyl methacrylate) (PCL-b-PGMA) block copolymers functionalized with azide groups and the crosslinking agent dipropargyl adipate (DPA) in a facile one-step procedure (Table 2, entry 14).124 Castano research group in 2021 synthesized biocompatible and biodegradable poly-amino acid-based nanogels from polyglutamic acid (PGA) (Table 2, entry 15). The nanogels were utilized as a drug carrier for doxorubicin (DOX) for the treatment of triple negative breast cancer (TNBC).139 Glitscher and coworkers in 2022 synthesized polymeric thermoresponsive nanogels by integrating anisotropic gold nanoparticles which acted as crosslinkers as well as light-to-heat transducers (Table 2, entry 16).140
Lai group in 2017 reported photoresponsive nanogel functionalized with DNA (Table 2, entry 17) which exhibited enhanced absorption at 260 nm and a simultaneous bathochromic shift at 350–400 nm in UV-vis region.141 Lakouraj and coworkers presented chitosan-g-thiacalix[4]arene based nanogel synthesized via CuAAC using monopropargyl thiacalix[4]arene and chitosan-azide (Table 2, entry 18).142 Manzi research group in 2017 combined amphiphilic derivatives of hyaluronic acid (HA) and riboflavin (Rfv), via CuAAC, to develop a biocompatible polysaccharide nanohydrogel (Table 2, entry 19) as a potent drug carrier for hydrophobic drugs.143
Siirilä and coworkers in 2020 developed poly(N-Vinylcaprolactam) (PNVCL) based nanogel (Table 2, entry 20) which exhibited thermoresponsive behavior and was functionalized with glucose and maltose moieties for drug delivery.144 Steinhilber group reported the synthesis of dendritic polyglycerol nanogels (Table 2, entry 21) by crosslinking of the precipitated nanoparticles by bioorthogonal CuAAC wherein benzacetal bonds were incorporated to obtain biodegradability.145 Heller et al. in 2013 presented clickable groups bearing nanogels synthesized through astoichiometric click-chemistry in-emulsion method wherein functionalization of the nanogel was done with bisphosphonate ligand which subsequently resulted in enhanced and efficient binding to the inner walls of marrow cavities (Table 2, entry 22).146 Kertsomboon research group developed amphiphilic nanogels using alkyno-poly(acrylic acid) (alkyno-PAA), diazido-poly(ethylene glycol), and diazido-poly(butylene succinate) (Table 2, entry 23) which were implemented to be dispersants of multi-walled carbon nanotubes (MWCNTs) in hydrophilic media.147 Zan research group in 2014 synthesized nanogels based on the copolymer poly[N-(2-hydroxypropyl) methacrylamide]-co-poly(3-azidopropyl methacrylate) (PHPMA-co-PAzPMA) conjugated with doxorubicin (DOX)-hydrazone and b-cyclodextrin (b-CD) (PHPMA-co-PPMA-DOX-CD) (Table 2, entry 24).148 Additional research showed that the nanoparticles could easily break through collagen hydrogels designed to simulate the thick matrix of tumor tissue. Zhang and colleagues in 2016 created a nanoscale drug formulation (CLP) by conjugating DOX with an acid-sensitive hydrazone bond and cross-linking with a reduction-responsive disulfide-containing linkage in the core (Table 2, entry 25). CLP was round and had a diameter of 60.6 ± 13.7 nm; it retained its uniform size even after being diluted many times, exposed to high salt concentrations, and incubated for extended periods of time in phosphate-buffered saline. CLP had considerable promise for optimum anti-tumor therapy due to its simple production, favorable stability, controlled release qualities, superior biodistribution, and enhanced suppression of tumor growth.149
5. Polymer functionalization: a fine-tuning process for tailor-made nanogels
The fundamental component of a functional polymer is its synthesis, which can aid in functionalization with the increase in the efficiency of drug delivery. The considerable efforts to develop novel polymerization routes owe to the exploration possibilities of material in addition to the scientific research interests.92 Post nanogel synthesis, the primary aim is the qualitative and selective modification of synthesized polymer under mild conditions with none or negligible side reactions.129,150,151 To use synthetic polymer for drug delivery, it is important to design a polymer system that must mimic the action of living tissues, so that it is suitable for drug delivery.152 The process of polymer functionalization provides a way to tackle the modification process by performing specific grafting, in the desired manner, to the functional group of long-chain polymers, condensing the macromolecules via coupling to elaborate the nanogels to get the required shape and size for preferred drug transport, and productive interaction with the biological environment.134 Therefore, cross-linking, mechanical properties, degradation, and the tuneable property must be undertaken to obtain final nanogels of desired biocompatibility.153Approach through click chemistry ensures that molecules and polymers are activated by introducing a suitable click functional group and then repeating the process for stable conjugation of the polymer. This technique is normally carried through copper(I) catalyzed azide–alkyne cycloaddition but it may be alternately carried out using a copper-free strain-promoted azide–alkyne approach to avoid the CuAAC reaction cytotoxity.154,155 The main advantage of this reaction is the moderate environment in presence of simple conditions like aqueous conditions which usually give high yields. This method leads to a stereospecific way of reaction completion and provides non-toxic side products which can be eliminated without chromatographic separation techniques, as shown in Fig. 23.38,153 In addition, thiol–base reactions,156,157 ester bond activation,66 ring opening, and closing reactions,134,158 oxime linkage,136–140 and multicomponent reactions159 also lead to effective post-polymerization depending upon the substituent required.
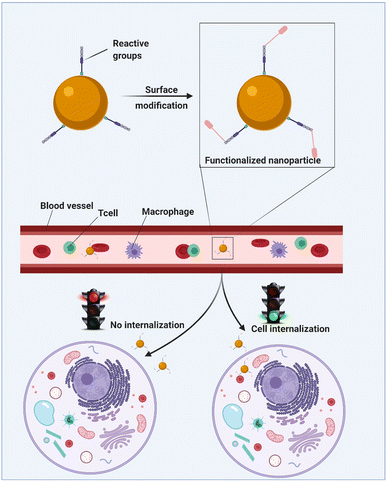 | ||
| Fig. 23 An illustrative representation of the polymer functionalization process resulting in cell internalization. Adapted from ref. 160, used under Creative Common CC-BY license. | ||
6. Conclusion and challenges
This review describes the summarised property of CuAAC-mediated ‘click’ nanogels as robust and efficient tools for drug delivery. Nanogels can hold a wide range of moiety combinations like water and biological fluids, making them extremely adaptable carriers for targeted drug delivery, and have advantages over conventional carrier systems because of their greater drug loading, swelling, biocompatibility, and colloidal stability. Click reactions are helpful to perform the strategies for the development of tailor-made nanogels via polymer functionalization under moderate conditions which enable the researchers to graft the desired alkyne and azide functionalities on suitable polymeric systems and subsequently combine them to synthesize the nanogels with high yields, purity and short reaction time. Also, click chemistry results in the increased binding between nanogels and target cells and hence enhances targeting focusing, thereby overcoming the weak points of nonspecific drug administration.However, certain issues regarding this methodology need to be addressed, the most prominent one being the use of Cu(I) catalyst in the synthesis, and its associated in vivo toxicity. Furthermore, polydispersity is also an issue related to the nanogels, which ought to be addressed by using suitable synthetic methodologies and optimum polymer and crosslinker concentrations. Also, the utilization of fillers like graphene, carbon nanotubes, and synthetic polymers can cause toxic by-products following the degradation of the nanogels inside the body. As a result, it becomes imperative to design more biocompatible drug delivery systems within appropriate concentrations to overcome the toxic aspects. These valuable features of nanogels and a better understanding of their behavior in vivo can guide our future efforts to improve the pharmacokinetic profile, improved drug loading capacity, and effectual delivery of nanogels to the desired target site. In light of this, we anticipate that the potential and usefulness of CuAAC ‘click’ chemistry in the production of nanogels will be substantially higher in the future.
Abbreviations
| CDI | Carbonyldiimidazole |
| CuAAC | Copper(I) catalyzed alkyne azide cycloaddition |
| CNCs | Cellulose nanocrystals |
| CNS | Central nervous system |
| DOX | Doxorubicin |
| DPA | Dipropargyl adipate |
| EPR | Enhanced permeability and retention |
| GSH | Glutathione |
| H40-PCL | H40-poly(ε-caprolactone) |
| HA | Hyaluronic acid |
| IARC | International agency for research on cancer |
| mPEG | PEG monomethyl ether |
| MCRs | Multi-component reactions |
| MWCNTs | Multi-walled carbon nanotubes |
| NAFLD | Non-alcoholic fatty liver disease |
| NIR | Near infrared |
| PAA | Poly(acrylic acid) |
| PCL | Poly(ε-caprolactone) |
| PCL-b-PGMA | Poly(ε-caprolactone)-b-poly(glycidyl methacrylate) |
| PDA | Poly(diacetylene) |
| PEG | Polyethylene glycol |
| PEI | Polyethyleneimine |
| PGA | Polyglutamic acid |
| PNVCL | Poly(N-vinylcaprolactam) |
| PVP | Poly(vinylpyridine) |
| RAFT | Reversible addition–fragmentation chain transfer |
| TNBC | Triple negative breast cancer |
Conflicts of interest
There are no conflicts to declare.References
- J. Kim, J. Shim, Y. J. Kim, K. Char, K. Do Suh and J. W. Kim, Macromol. Biosci., 2010, 10, 1171–1176 CrossRef PubMed.
- W. Yuan and H. Li, in Nanostructures for Drug Delivery, ed. E. Andronescu and A. Grumezescu, Elsevier Inc., Amsterdam, 1st edn, 2017, ch. 14, vol. 1, pp. 445–460 Search PubMed.
- J. Nicolas, S. Mura, D. Brambilla, N. Mackiewicz and P. Couvreur, Chem. Soc. Rev., 2013, 42, 1147–1235 RSC.
- A. M. Tsimberidou, Cancer Chemother. Pharmacol., 2015, 76, 1113–1132 CrossRef CAS PubMed.
- R. M. Sharkey and D. M. Goldenberg, Ca-Cancer J. Clin., 2006, 56, 226–243 CrossRef PubMed.
- Y. T. Lee, Y. J. Tan and C. E. Oon, Eur. J. Pharmacol., 2018, 834, 188–196 CrossRef CAS PubMed.
- P. S. Hegde and D. S. Chen, Immunity, 2020, 52, 17–35 CrossRef CAS PubMed.
- R. A. DePinho, Nature, 2000, 408, 248–254 CrossRef CAS PubMed.
- P. A. Jones and S. B. Baylin, Cell, 2007, 128, 683–692 CrossRef CAS PubMed.
- J. Ferlay, M. Colombet, I. Soerjomataram, C. Mathers, D. M. Parkin, M. Piñeros, A. Znaor and F. Bray, Int. J. Cancer, 2019, 144, 1941–1953 CrossRef CAS PubMed.
- M. Goodman, Semin. Oncol. Nurs., 1989, 5, 29–52 CrossRef CAS PubMed.
- C. C. Sun, D. C. Bodurka, C. B. Weaver, R. Rasu, J. K. Wolf, M. W. Bevers, J. A. Smith, J. T. Wharton and E. B. Rubenstein, Support Care Cancer, 2005, 13, 219–227 CrossRef PubMed.
- R. Tong, L. Tang, L. Ma, C. Tu, R. Baumgartner and J. Cheng, Chem. Soc. Rev., 2014, 43, 6982–7012 RSC.
- A. Rösler, G. W. M. Vandermeulen and H. A. Klok, Adv. Drug Delivery Rev., 2012, 64, 270–279 CrossRef.
- J. H. Adair, M. P. Parette, E. I. Altinoǧlu and M. Kester, ACS Nano, 2010, 4, 4967–4970 CrossRef CAS PubMed.
- Z. Cheng, A. Al Zaki, J. Z. Hui, V. R. Muzykantov and A. Tsourkas, Science, 2012, 338, 903–910 CrossRef CAS PubMed.
- A. A. Yaqoob, H. Ahmad, T. Parveen, A. Ahmad, M. Oves, I. M. I. Ismail, H. A. Qari, K. Umar and M. N. Mohamad Ibrahim, Front. Chem., 2020, 8, 1–23 CrossRef PubMed.
- E. S. Anooj, M. Charumathy, V. Sharma, B. V. Vibala, S. T. Gopukumar, S. I. B. Jainab and S. Vallinayagam, J. Mol. Struct., 2021, 1239, 130446 CrossRef CAS.
- R. T. Chacko, J. Ventura, J. Zhuang and S. Thayumanavan, Adv. Drug Delivery Rev., 2012, 64, 836–851 CrossRef CAS PubMed.
- P. N. Kendre and T. S. Satav, Polym. Bull., 2019, 76, 1595–1617 CrossRef CAS.
- M. M. Yallapu, M. Jaggi and S. C. Chauhan, Drug Discovery Today, 2011, 16, 457–463 CrossRef CAS PubMed.
- S. Hajebi, N. Rabiee, M. Bagherzadeh, S. Ahmadi, M. Rabiee, H. Roghani-Mamaqani, M. Tahriri, L. Tayebi and M. R. Hamblin, Acta Biomater., 2019, 92, 1–18 CrossRef CAS PubMed.
- J. C. Cuggino, E. R. O. Blanco, L. M. Gugliotta, C. I. Alvarez Igarzabal and M. Calderón, J. Controlled Release, 2019, 307, 221–246 CrossRef CAS PubMed.
- S. V. Vinogradov, Nanomedicine, 2010, 5, 165–168 CrossRef CAS PubMed.
- L. Zha, B. Banik and F. Alexis, Soft Matter, 2011, 7, 5908–5916 RSC.
- J. K. Oh, R. Drumright, D. J. Siegwart and K. Matyjaszewski, Prog. Polym. Sci., 2008, 33, 448–477 CrossRef CAS.
- O. Z. Fisher, T. Kim, S. R. Dietz and N. A. Peppas, Pharm. Res., 2009, 26, 51–60 CrossRef CAS PubMed.
- A. V. Kabanov and S. V. Vinogradov, Angew. Chem., Int. Ed., 2009, 48, 5418–5429 CrossRef CAS PubMed.
- Y. Jiang, J. Chen, C. Deng, E. J. Suuronen and Z. Zhong, Biomaterials, 2014, 35, 4969–4985 CrossRef CAS PubMed.
- C. T. Moody, S. Palvai and Y. Brudno, Acta Biomater., 2020, 112, 112–121 CrossRef CAS PubMed.
- P. Y. Liyanage, S. D. Hettiarachchi, Y. Zhou, A. Ouhtit, E. S. Seven, C. Y. Oztan, E. Celik and R. M. Leblanc, Biochim. Biophys. Acta, Rev. Cancer, 2019, 1871, 419–433 CrossRef CAS PubMed.
- M. M. J. Kamphuis, A. P. R. Johnston, G. K. Such, H. H. Dam, R. A. Evans, A. M. Scott, E. C. Nice, J. K. Heath and F. Caruso, J. Am. Chem. Soc., 2010, 132, 15881–15883 CrossRef CAS PubMed.
- T. López-León, E. L. S. Carvalho, B. Seijo, J. L. Ortega-Vinuesa and D. Bastos-González, J. Colloid Interface Sci., 2005, 283, 344–351 CrossRef PubMed.
- A. Khan, M. B. H. Othman, B. P. Chang and H. M. Akil, Iran. Polym. J., 2015, 24, 317–328 CrossRef CAS.
- S. Shah, N. Rangaraj, K. Laxmikeshav and S. Sampathi, Int. J. Pharm., 2020, 581, 119268 CrossRef CAS PubMed.
- L. Kumari and H. R. Badwaik, in Polysaccharide Carriers for Drug Delivery, ed. S. Maiti and S. Jana, Elsevier Ltd, Amsterdam, 1st edn, 2019, ch. 18, vol. 1, pp. 497–557 Search PubMed.
- M. F. Attia, N. Anton, J. Wallyn, Z. Omran and T. F. Vandamme, J. Pharm. Pharmacol., 2019, 71, 1185–1198 CrossRef CAS PubMed.
- S. G. Hesaroeiye, H. R. Bagtash, S. Boddohi, E. V. Farahani and E. Jabbari, Gels, 2020, 6, 1–32 Search PubMed.
- I. Neamtu, A. G. Rusu, A. Diaconu, L. E. Nita and A. P. Chiriac, Drug Delivery, 2017, 24, 539–557 CrossRef CAS PubMed.
- X. Du, Y. Gao, Q. Kang and J. Xing, Front. Bioeng. Biotechnol., 2021, 9, 1–17 Search PubMed.
- F. Pinelli, M. Saadati, E. N. Zare, P. Makvandi, M. Masi, A. Sacchetti and F. Rossi, Int. Mater. Rev., 2022, 1–25 Search PubMed.
- T. Kaewruethai, C. Laomeephol, Y. Pan and J. A. Luckanagul, Gels, 2021, 7, 1–18 CrossRef PubMed.
- M. Matusiak, S. Kadlubowski and J. M. Rosiak, Radiat. Phys. Chem., 2020, 169, 108099 CrossRef CAS.
- S. V. Vinogradov, T. K. Bronich and A. V. Kabanov, Adv. Drug Delivery Rev., 2002, 54, 135–147 CrossRef CAS PubMed.
- M. A. Qureshi and F. Khatoon, J. Sci.: Adv. Mater. Devices, 2019, 4, 201–212 Search PubMed.
- D. M. Eckmann, R. J. Composto, A. Tsourkas and V. R. Muzykantov, J. Mater. Chem. B, 2014, 2, 8085–8097 RSC.
- L. Xing, Y. T. Fan, L. J. Shen, C. X. Yang, X. Y. Liu, Y. N. Ma, L. Y. Qi, K. H. Cho, C. S. Cho and H. L. Jiang, Int. J. Biol. Macromol., 2019, 141, 85–97 CrossRef CAS PubMed.
- H. Zhang, Y. Zhai, J. Wang and G. Zhai, Mater. Sci. Eng., C, 2016, 60, 560–568 CrossRef CAS PubMed.
- A. Setia and P. Ahuja, in Organic Materials as Smart Nanocarriers for Drug Delivery, ed. A. M. Grumezescu, Elsevier Inc., Amsterdam, 1st edn, 2018, ch. 8, vol. 1, pp. 293–368 Search PubMed.
- C. Li, S. R. Obireddy and W. F. Lai, Drug Delivery, 2021, 28, 1594–1602 CrossRef CAS PubMed.
- S. F. Medeiros, J. O. C. Filizzola, P. F. M. Oliveira, T. M. Silva, B. R. Lara, M. V. Lopes, B. Rossi-Bergmann, A. Elaissari and A. M. Santos, Mater. Lett., 2016, 175, 296–299 CrossRef CAS.
- J. M. Anderson, A. Rodriguez and D. T. Chang, Semin. Immunol., 2008, 20, 86–100 CrossRef CAS PubMed.
- A. A. Kulkarni and P. S. Rao, in Nanomaterials in Tissue Engineering, ed. A. K. Gaharwar, S. Sant, M. J. Hancock and S. A. Hacking, Woodhead Publishing Limited, 1st edn, 2013, ch. 1, vol. 1, pp. 27–63 Search PubMed.
- K. Huang, Y. He, Z. Zhu, J. Guo, G. Wang, C. Deng and Z. Zhong, ACS Appl. Mater. Interfaces, 2019, 11, 22171–22180 CrossRef CAS PubMed.
- Z. E. Yilmaz and C. Jérôme, Macromol. Biosci., 2016, 16, 1745–1761 CrossRef CAS PubMed.
- F. Zhan, W. Chen, Z. Wang, W. Lu, R. Cheng, C. Deng, F. Meng, H. Liu and Z. Zhong, Biomacromolecules, 2011, 12, 3612–3620 CrossRef CAS PubMed.
- M. Häring, J. Rodríguez-López, S. Grijalvo, M. Tautz, R. Eritja, V. S. Martín and D. Díaz Díaz, Mol. Pharm., 2018, 15, 2963–2972 CrossRef PubMed.
- S. Vandghanooni and M. Eskandani, Int. J. Biol. Macromol., 2019, 141, 636–662 CrossRef CAS PubMed.
- F. Pinelli, Ó. F. Ortolà, P. Makvandi, G. Perale and F. Rossi, Nanomedicine, 2020, 15, 2707–2727 CrossRef CAS PubMed.
- E. Lallana, A. Sousa-Herves, F. Fernandez-Trillo, R. Riguera and E. Fernandez-Megia, Pharm. Res., 2012, 29, 1–34 CrossRef CAS PubMed.
- K. Ulbrich, K. Holá, V. Šubr, A. Bakandritsos, J. Tuček and R. Zbořil, Chem. Rev., 2016, 116, 5338–5431 CrossRef CAS PubMed.
- F. Masood, Mater. Sci. Eng., C, 2016, 60, 569–578 CrossRef CAS PubMed.
- T. D. Clemons, R. Singh, A. Sorolla, N. Chaudhari, A. Hubbard and K. S. Iyer, Langmuir, 2018, 34, 15343–15349 CrossRef CAS PubMed.
- X. Wang, S. Li, Y. Shi, X. Chuan, J. Li, T. Zhong, H. Zhang, W. Dai, B. He and Q. Zhang, J. Controlled Release, 2014, 193, 139–153 CrossRef CAS PubMed.
- P. Sharmiladevi, K. Girigoswami, V. Haribabu and A. Girigoswami, Mater. Adv., 2021, 2, 2876–2891 RSC.
- A. Aykaç, M. Noiray, M. Malanga, V. Agostoni, J. M. Casas-Solvas, É. Fenyvesi, R. Gref and A. Vargas-Berenguel, Biochim. Biophys. Acta, Gen. Subj., 2017, 1861, 1606–1616 CrossRef PubMed.
- C. Vasile, D. Pamfil, E. Stoleru and M. Baican, Molecules, 2020, 25, 1539 CrossRef CAS PubMed.
- K. S. Soni, S. S. Desale and T. K. Bronich, J. Controlled Release, 2016, 240, 109–126 CrossRef CAS PubMed.
- D. E. Owens and N. A. Peppas, Int. J. Pharm., 2006, 307, 93–102 CrossRef CAS PubMed.
- M. R. Gordon, J. Zhuang, J. Ventura, L. Li, K. Raghupathi and S. Thayumanavan, Mol. Pharm., 2018, 15, 1180–1191 CrossRef CAS PubMed.
- A. Sharma, T. Garg, A. Aman, K. Panchal, R. Sharma, S. Kumar and T. Markandeywar, Artif. Cells, Nanomed., Biotechnol., 2016, 44, 165–177 CrossRef CAS PubMed.
- M. F. Akhtar, M. Hanif and N. M. Ranjha, Saudi Pharm. J., 2016, 24, 554–559 CrossRef PubMed.
- A. J. Sivaram, P. Rajitha, S. Maya, R. Jayakumar and M. Sabitha, Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol., 2015, 7, 509–533 CrossRef CAS PubMed.
- Y. Sasaki and K. Akiyoshi, Chem. Rec., 2010, 10, 366–376 CAS.
- H. Wang, Q. Chen and S. Zhou, Chem. Soc. Rev., 2018, 47, 4198–4232 RSC.
- F. Sultana, Manirujjaman, Md. Imran-Ul-Haque, M. Arafat and S. Sharmin, J. Appl. Pharm. Sci., 2013, 3, 95–105 Search PubMed.
- P. K. Avti, D. Maysinger and A. Kakkar, Molecules, 2013, 18, 9531–9549 CrossRef CAS PubMed.
- G. Yi, J. Son, J. Yoo, C. Park and H. Koo, Biomater. Res., 2018, 22, 1–8 CrossRef PubMed.
- E. Mauri, S. M. Giannitelli, M. Trombetta and A. Rainer, Gels, 2021, 7, 1–23 CrossRef PubMed.
- X. Zhang, S. Malhotra, M. Molina and R. Haag, Chem. Soc. Rev., 2015, 44, 1948–1973 RSC.
- P. A. Lovell and F. J. Schork, Biomacromolecules, 2020, 21, 4396–4441 CrossRef CAS PubMed.
- S. Beyazit, B. Tse Sum Bui, K. Haupt and C. Gonzato, Prog. Polym. Sci., 2016, 62, 1–21 CrossRef CAS.
- H. Gao, W. Li and K. Matyjaszewski, Macromolecules, 2008, 41, 2335–2340 CrossRef CAS.
- S. Kim and H. D. Sikes, Polym. Chem., 2020, 11, 1424–1444 RSC.
- C. D. Hein, X. M. Liu and D. Wang, Pharm. Res., 2008, 25, 2216–2230 CrossRef CAS PubMed.
- Z. Huang, Y. Zhou, Z. Wang, Y. Li, W. Zhang and N. Zhou, Chin. J. Polym. Sci., 2017, 35, 317–341 CrossRef CAS.
- H. C. Kolb, M. G. Finn and K. B. Sharpless, Angew. Chem., Int. Ed., 2001, 40, 2004–2021 CrossRef CAS PubMed.
- P. Chaturvedi, N. Chaturvedi, S. Gupta, A. Mishra, M. Singh and T. Siddhartha, Int. J. Pharm. Sci. Rev. Res., 2011, 10, 111–117 CAS.
- N. M. Meghani, H. H. Amin and B. J. Lee, Drug Discovery Today, 2017, 22, 1604–1619 CrossRef CAS PubMed.
- M. Meldal and F. Diness, Trends Chem., 2020, 2, 569–584 CrossRef CAS.
- E. Kim and H. Koo, Chem. Sci., 2019, 10, 7835–7851 RSC.
- K. Wang, K. Amin, Z. An, Z. Cai, H. Chen, H. Chen, Y. Dong, X. Feng, W. Fu, J. Gu, Y. Han, D. Hu, R. Hu, D. Huang, F. Huang, F. Huang, Y. Huang, J. Jin, X. Jin, Q. Li, T. Li, Z. Li, Z. Li, J. Liu, J. Liu, S. Liu, H. Peng, A. Qin, X. Qing, Y. Shen, J. Shi, X. Sun, B. Tong, B. Wang, H. Wang, L. Wang, S. Wang, Z. Wei, T. Xie, C. Xu, H. Xu, Z. K. Xu, B. Yang, Y. Yu, X. Zeng, X. Zhan, G. Zhang, J. Zhang, M. Q. Zhang, X. Z. Zhang, X. Zhang, Y. Zhang, Y. Zhang, C. Zhao, W. Zhao, Y. Zhou, Z. Zhou, J. Zhu, X. Zhu and B. Z. Tang, Mater. Chem. Front., 2020, 4, 1803–1915 RSC.
- K. Mashayekh and P. Shiri, ChemistrySelect, 2019, 4, 13459–13478 CrossRef CAS.
- D. Dheer, V. Singh and R. Shankar, Bioorg. Chem., 2017, 71, 30–54 CrossRef CAS PubMed.
- A. Rani, G. Singh, A. Singh, U. Maqbool, G. Kaur and J. Singh, RSC Adv., 2020, 10, 5610–5635 RSC.
- A. M. Gazzali, L. Colombeau, P. Arnoux, H. A. Wahab, C. Frochot, R. Vanderesse and S. Acherar, Tetrahedron, 2017, 73, 532–541 CrossRef CAS.
- G. Singh, Priyanka, A. Singh, P. Satija, Sushma, Pawan, Mohit, J. Singh and J. Singh, New J. Chem., 2021, 45, 7850–7859 RSC.
- P. Kaur, B. Lal, N. Kaur, G. Singh and A. Singh, J. Photochem. Photobiol., A, 2019, 382, 111847 CrossRef CAS.
- P. Saini, Sonika, G. Singh, G. Kaur, J. Singh and H. Singh, Mol. Catal., 2021, 504, 111432 CrossRef CAS.
- G. Singh, Sushma, A. Singh, P. Satija, Shilpy, Mohit, Priyanka, J. Singh and A. Khosla, Inorg. Chim. Acta, 2021, 514, 120028 CrossRef CAS.
- P. Thirumurugan, D. Matosiuk and K. Jozwiak, Chem. Rev., 2013, 113, 4905–4979 CrossRef CAS PubMed.
- S. Naja, Z. Talebpour and A. Mehdi, Tetrahedron, 2019, 118, 556–573 Search PubMed.
- N. Seda, J. El-gindi, H. Galla and L. De Cola, Microporous Mesoporous Mater., 2011, 144, 9–14 CrossRef.
- A. Croisy and P. Maillard, Tetrahedron, 2011, 67, 4924–4932 CrossRef.
- S. Wang, W. J. Placzek, J. L. Stebbins, S. Mitra, R. Noberini, M. Koolpe, Z. Zhang, R. Dahl, E. B. Pasquale and M. Pellecchia, J. Med. Chem., 2012, 55, 2427–2436 CrossRef CAS PubMed.
- A. Gruber, L. Navarro and D. Klinger, Adv. Mater. Interfaces, 2019, 7, 1901676 CrossRef.
- J. Kaur, M. Saxena and N. Rishi, Bioconjugate Chem., 2021, 32, 1455–1471 CrossRef CAS PubMed.
- N. K. Obhi, D. M. Peda, E. L. Kynaston and D. S. Seferos, Macromolecules, 2018, 51, 2969–2978 CrossRef CAS.
- V. Crescenzi, L. Cornelio, C. Di Meo and S. Nardecchia, Biomacromolecules, 2007, 8, 1844–1850 CrossRef CAS PubMed.
- L. Nuhn, E. Bolli, S. Massa, I. Vandenberghe, K. Movahedi, B. Devreese, J. A. Van Ginderachter and B. G. De Geest, Bioconjugate Chem., 2018, 29, 2394–2405 CrossRef CAS PubMed.
- N. J. Agard, J. A. Prescher and C. R. Bertozzi, J. Am. Chem. Soc., 2004, 126, 15046–15047 CrossRef CAS PubMed.
- S. M. Hodgson, E. Bakaic, S. A. Stewart, T. Hoare and A. Adronov, Biomacromolecules, 2016, 17, 1093–1100 CrossRef CAS PubMed.
- C. E. Hoyle and C. N. Bowman, Angew. Chem., Int. Ed., 2010, 49, 1540–1573 CrossRef CAS PubMed.
- C. E. Hoyle, A. B. Lowe and C. N. Bowman, Chem. Soc. Rev., 2010, 39, 1355–1387 RSC.
- S. F. Chong, R. Chandrawati, B. Städler, J. Park, J. Cho, Y. Wang, Z. Jia, V. Bulmus, T. P. Davis, A. N. Zelikin and F. Caruso, Small, 2009, 5, 2601–2610 CrossRef CAS PubMed.
- P. Saha, R. Ganguly, X. Li, R. Das, N. K. Singha and A. Pich, Macromol. Rapid Commun., 2021, 42, 1–23 CrossRef PubMed.
- M. Lei, W. Zhang, C. Yi, L. Yan and Y. Tian, Colloids Surf., B, 2021, 206, 111959 CrossRef CAS PubMed.
- Q. T. Phan, M. P. Patil, T. T. K. Tu, G. Do Kim and K. T. Lim, React. Funct. Polym., 2020, 147, 104463 CrossRef CAS.
- K. Nwe and M. W. Brechbiel, Cancer Biother.Radiopharm., 2009, 24, 289–302 CrossRef CAS PubMed.
- M. Meldal, Macromol. Rapid Commun., 2008, 29, 1016–1051 CrossRef CAS.
- C. G. Parker and M. R. Pratt, Cell, 2020, 180, 605–632 CrossRef CAS PubMed.
- M. Tarnowska and T. Krawczyk, Biosens. Bioelectron., 2020, 169, 112614 CrossRef CAS PubMed.
- L. Mei, Y. Liu, J. Rao, X. Tang, M. Li, Z. Zhang and Q. He, ACS Appl. Mater. Interfaces, 2018, 10, 17582–17593 CrossRef CAS PubMed.
- X. T. Cao, A. M. Showkat and K. T. Lim, Eur. Polym. J., 2015, 68, 267–277 CrossRef CAS.
- M. G. Roberts, Q. Yu, R. Keunen, J. Liu, E. C. Ngae Wong, C. K. Rastogi, R. M. Reilly, R. M. Reilly, C. Allen, M. A. Winnik and M. A. Winnik, Biomacromolecules, 2020, 21, 2014–2023 CrossRef CAS PubMed.
- S. B. Van Der Meer, K. Loza, K. Wey, M. Heggen, C. Beuck, P. Bayer and M. Epple, Langmuir, 2019, 35, 7191–7204 CrossRef CAS PubMed.
- M. A. Quadir, S. W. Morton, Z. J. Deng, K. E. Shopsowitz, R. P. Murphy, T. H. Epps and P. T. Hammond, Mol. Pharm., 2014, 11, 2420–2430 CrossRef CAS PubMed.
- H. S. Abandansari, M. R. Nabid, S. J. T. Rezaei and H. Niknejad, Polymer, 2014, 55, 3579–3590 CrossRef CAS.
- M. Assali, J. J. Cid, I. Fernández and N. Khiar, Chem. Mater., 2013, 25, 4250–4261 CrossRef CAS.
- C. D. Donahoe, T. L. Cohen, W. Li, P. K. Nguyen, J. D. Fortner, R. D. Mitra and D. L. Elbert, Langmuir, 2013, 29, 4128–4139 CrossRef CAS PubMed.
- G. D. Fu, H. Jiang, F. Yao, L. Q. Xu, J. Ling and E. T. Kang, Macromol. Rapid Commun., 2012, 33, 1523–1527 CrossRef CAS PubMed.
- S. Khoee and N. Abedini, Polymer, 2014, 55, 5635–5647 CrossRef CAS.
- E. Mauri, I. Moroni, L. Magagnin, M. Masi, A. Sacchetti and F. Rossi, React. Funct. Polym., 2016, 105, 35–44 CrossRef CAS.
- E. Mauri, P. Veglianese, S. Papa, A. Mariani, M. De Paola, R. Rigamonti, G. M. F. Chincarini, S. Rimondo, A. Sacchetti and F. Rossi, Eur. Polym. J., 2017, 94, 143–151 CrossRef CAS.
- E. Mauri, M. Gori, S. M. Giannitelli, A. Zancla, P. Mozetic, F. Abbruzzese, N. Merendino, G. Gigli, F. Rossi, M. Trombetta and A. Rainer, Mater. Sci. Eng., C, 2021, 124, 112080 CrossRef CAS PubMed.
- S. Papa, V. Veneruso, E. Mauri, G. Cremonesi, X. Mingaj, A. Mariani, M. De Paola, A. Rossetti, A. Sacchetti, F. Rossi, G. Forloni and P. Veglianese, J. Controlled Release, 2021, 330, 218–228 CrossRef CAS PubMed.
- F. Pinelli, F. Pizzetti, A. Rossetti, Z. Posel, M. Masi, A. Sacchetti, P. Posocco and F. Rossi, Colloids Surf., A, 2021, 614, 126164 CrossRef CAS.
- I. Vismara, S. Papa, V. Veneruso, E. Mauri, A. Mariani, M. De Paola, R. Affatato, A. Rossetti, M. Sponchioni, D. Moscatelli, A. Sacchetti, F. Rossi, G. Forloni and P. Veglianese, ACS Nano, 2020, 14, 360–371 CrossRef CAS PubMed.
- A. Duro-Castano, A. Sousa-Herves, A. Armiñán, D. Charbonnier, J. J. Arroyo-Crespo, S. Wedepohl, M. Calderón and M. J. Vicent, J. Controlled Release, 2021, 332, 10–20 CrossRef CAS PubMed.
- E. A. Glitscher, J. Bergueiro and M. Calderón, Eur. Polym. J., 2022, 175, 111342 CrossRef CAS.
- J. Lai, P. Jiang, E. R. Gaddes, N. Zhao, L. Abune and Y. Wang, Chem. Mater., 2017, 29, 5850–5857 CrossRef CAS PubMed.
- M. M. Lakouraj, F. Hasanzadeh and E. N. Zare, Iran. Polym. J., 2014, 23, 933–945 CrossRef CAS.
- G. Manzi, N. Zoratto, S. Matano, R. Sabia, C. Villani, T. Coviello, P. Matricardi and C. Di Meo, Carbohydr. Polym., 2017, 174, 706–715 CrossRef CAS PubMed.
- J. Siirilä, S. Hietala, F. S. Ekholm and H. Tenhu, Biomacromolecules, 2020, 21, 955–965 CrossRef PubMed.
- D. Steinhilber, M. Witting, X. Zhang, M. Staegemann, F. Paulus, W. Friess, S. Küchler and R. Haag, J. Controlled Release, 2013, 169, 289–295 CrossRef CAS PubMed.
- D. A. Heller, Y. Levi, J. M. Pelet, J. C. Doloff, J. Wallas, G. W. Pratt, S. Jiang, G. Sahay, A. Schroeder, J. E. Schroeder, Y. Chyan, C. Zurenko, W. Querbes, M. Manzano, D. S. Kohane, R. Langer and D. G. Anderson, Adv. Mater., 2013, 25, 1449–1454 CrossRef CAS PubMed.
- T. Kertsomboon and S. Chirachanchai, Polym. Degrad. Stab., 2020, 179, 109266 CrossRef CAS.
- M. Zan, J. Li, S. Luo and Z. Ge, Chem. Commun., 2014, 50, 7824–7827 RSC.
- Y. Zhang, J. Ding, M. Li, X. Chen, X. Zhuang, Y. Huang and X. Chen, ACS Appl. Mater. Interfaces, 2016, 8, 10673–10682 CrossRef CAS PubMed.
- Y. Oz, A. Barras, R. Sanyal, R. Boukherroub, S. Szunerits and A. Sanyal, ACS Appl. Mater. Interfaces, 2017, 9, 34194–34203 CrossRef CAS PubMed.
- A. Ashfaq, M. C. Clochard, X. Coqueret, C. Dispenza, M. S. Driscoll, P. Ulański and M. Al-Sheikhly, Polymers, 2020, 12, 1–67 CrossRef PubMed.
- B. Aktan, L. Chambre, R. Sanyal and A. Sanyal, Biomacromolecules, 2017, 18, 490–497 CrossRef CAS PubMed.
- F. Pinelli, G. Perale and F. Rossi, Gels, 2020, 6, 1–16 CrossRef PubMed.
- V. O. Rodionov, S. I. Presolski, D. D. Díaz, V. V. Fokin and M. G. Finn, J. Am. Chem. Soc., 2007, 129, 12705–12712 CrossRef CAS PubMed.
- J. Tummatorn, P. Batsomboon, R. J. Clark, I. V. Alabugin and G. B. Dudley, J. Org. Chem., 2012, 77, 2093–2097 CrossRef CAS PubMed.
- C. Boyer, M. Whittaker and T. P. Davis, J. Polym. Sci., Part A: Polym. Chem., 2011, 49, 5245–5256 CrossRef CAS.
- M. O. Pehlivaner Kara and A. K. Ekenseair, Biomacromolecules, 2017, 18, 1473–1481 CrossRef CAS PubMed.
- A. Pikabea and J. Forcada, J. Polym. Sci., Part A: Polym. Chem., 2017, 55, 3573–3586 CrossRef CAS.
- M. A. Mironov, I. D. Shulepov, V. S. Ponomarev and V. A. Bakulev, Colloid Polym. Sci., 2013, 291, 1683–1691 CrossRef CAS.
- P. Makvandi, S. Iftekhar, F. Pizzetti, A. Zarepour, E. N. Zare, M. Ashrafizadeh, T. Agarwal, V. V. T. Padil, R. Mohammadinejad, M. Sillanpaa, T. K. Maiti, G. Perale, A. Zarrabi and F. Rossi, Environ. Chem. Lett., 2021, 19, 583–611 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2023 |