Open Access Article
Open Access ArticleMetal-based-oxide nanoparticles assisted the in vitro culture growth of Populus alba as micronutrients: essential metabolic processes and genetic stability
Mohamed F. Ahmeda,
Mostafa A. Ibrahimb,
Ahmed S. Mansourcf,
Ahmed N. Emam *def,
Ashraf B. Abd El-Razikg and
Eman Tawfikh
*def,
Ashraf B. Abd El-Razikg and
Eman Tawfikh
aHorticulture Department, Faculty of Agriculture, Ain Shams University, P.O. Box 68, Hadayek Shobra, 11241, Cairo, Egypt
bProduction and R&D Unit, NanoFab Technology Company, 6th October City, Giza, Egypt
cDepartment of Laser Applications in Meteorology, Chemistry and Agriculture, National Institute of Laser Enhanced Sciences (NILES), Cairo University, Cairo, Egypt
dRefractories, Ceramics and Building Materials Department, Advanced Materials Technology and Mineral Resources Research Institute, National Research Centre (NRC), El Bohouth St., Dokki, 12622 Cairo, Egypt. E-mail: ahmed.gsc.ndp@gmail.com; an.emam@nrc.sci.eg; Tel: +20 1018232249
eNanomedicine & Tissue Engineering Lab., Medical Research Center of Excellence (MRCE), National Research Centre (NRC), Egypt
fFaculty of Postgraduate Studies for Nanotechnology, Cairo University, Zayed City, Giza, Egypt
gGenetics Department, Faculty of Agriculture, Ain Shams University, P.O. Box 68, Hadayek Shobra, 11241, Cairo, Egypt
hBotany and Microbiology Department, Faculty of Science, Helwan University, 11795, Cairo, Egypt
First published on 13th April 2023
Abstract
The present study evaluates the in vitro culture growth rate of Populus alba upon using nano metal-based-oxides such as hematite (Fe2O3 NPs), zinc oxide (ZnO NPs), and manganese oxide (Mn2O3 NPs) nanoparticles as analogues of three primary micronutrients such as iron (Fe), zinc (Zn), and manganese (Mn), which exist in soil as micronutrients. Herein, the in vitro culture growth rate was investigated using three different concentrations (i.e., 20, 40, and 60 mg L−1) of as-prepared metal oxide nanoparticles compared to the control. In addition, the as-prepared nanoparticles have been prepared via the co-precipitation method. Furthermore, the physicochemical properties were investigated using transmission electron microscopy, Fourier transform infrared spectroscopy, X-ray diffraction, and dynamic light scattering techniques. Overall, a significant difference in the biomass production-related parameters such as fresh weight, shoot length, and root length was observed compared to the control upon the treatment with micronutrient-based nano-metal-oxides (i.e., Mn2O3 > Fe2O3 > ZnO NPs, respectively). In addition, a significant increase in the root number of Populus alba plants upon their treatment with ZnO NPs was observed compared to other prepared nano-metal-oxides and the control. Also, a remarkable increase in the chlorophyll index was monitored upon the treatment with Fe2O3 NPs rather than the other commonly used Mn2O3 and ZnO NPs, respectively. Moreover, RAPD-PCR bioassays were applied, and the actual six primers showed a genetic variation percentage of 34.17%, indicating that Populus alba is highly genetically stable even in highly contaminated soil. As a result, our findings suggest an idea that indicates the ability to enhance the in vitro culture growth rate of Populus alba plants using metal oxide nanoparticles as analogous to essential micronutrients.
1. Introduction
Heavy metals are still one of the most hazardous inorganic contaminants for water and soil composition due to their potential toxicity and bio-accumulative nature. In addition, extended human activities such as mining, chemical, and metal processing industries, and other allied industries significantly contribute to heavy metals in the environment at higher-than-normal levels.1,2 Therefore, the development and survival of living organisms are threatened by the interference of these contaminants in the functioning of vital cellular components that exist in plant, animal, and human tissues.2–4 Copper (Cu), lead (Pb), cadmium (Cd), mercury (Hg), nickel (Ni), cobalt (Co), and chromium (Cr) are examples of naturally abundant heavy metals. Except for Pb, Cd, and Hg, these elements are essential to organisms in minute amounts, but excessive amounts of these elements can be harmful.5,6Other heavy metals include Fe, Mn, and Zn, considered essential micronutrient elements for plants, animals, and other living organism.7–9 However, toxic effects could be monitored upon uptake more than the plant's requirements and consequently harm the nutritional equilibrium in the soil.10–12 Iron (Fe) is primarily involved in photosynthesis in plants. The availability of micronutrients to plant roots is affected by soil pH, with Fe being more readily available in soils with a low pH.13–16 In addition, Fe is an essential component in the composition of heme proteins such as cytochromes, catalase, peroxidase, e.g., hemoglobin, and iron-sulfur proteins like ferredoxin aconitase and superoxide dismutase (SOD).17 At the same time, Zn aids in producing chlorophyll in plants. When the soil lacks Zn, the leaves darken, and plant growth is impeded.18 In addition, Mn is a vital plant mineral nutrient involved in various physiological activities, including photosynthesis. Manganese shortages are common in sandy soils, organic soils with a pH above 6, and highly weathered tropical soils. Mn is easily transferred from root to shoot via the transpiration stream, but it is not easily remobilized to other organs via phloem once it reaches the leaves.19
Engineered nanomaterials have received significant attention due to their unique properties, which provide an opportunity to be used in several applications, especially in the agricultural sector.20–23 Consequently, nanomaterials increase crop productivity through efficient regulation of micronutrient delivery to plants and targeted sites, ensuring that agrochemicals are used as little as possible, with efficient uptake of nutrients.24,25 Such nano-fertilizers or nano-encapsulated nutrients may have qualities that are beneficial to crops, such as the on-demand release of nutrients and the controlled release of chemical fertilizers to regulate plant growth and accelerate target activity.26–29
Phytoremediation is a renewable technology that restores contaminated soil and water sources using natural or genetically modified plant species. In various review articles examining the principles, types, and mechanisms of the phytoremediation process, the advantages of low cost, environmental friendliness, and safety are highlighted.30–33 Poplars are frequently used in phytoremediation because of their rapid growth, adaptability, and well-developed root system that reaches underground water and transports large amounts of water.34 However, P. alba could not accumulate heavy metals like other hyper-accumulators, which can be overcome through high biomass production and, consequently, a relatively high amount of extracted metal per plant.35–38 Particular white poplars, in particular, are interesting due to their high tolerance to arid conditions and their use in horticulture and landscaping, especially genotypes with a pyramidal tree shape.39 The chemical composition of Populus alba wood varies according to the species and age of trees.40 The following substances are present: mineral substances, extractives, cellulose, lignin, and holocellulose. Previous research found that trees cellulose and lignin content increases with age. The comparison of cellulose content in studied poplar wood, on the other hand, revealed that its content was unaffected by tree species, age, or growth environment.40 While the content of extractives in poplar wood varied according to tree species and growth environment. Regardless of age, wood from P. nigra and P. alba contained less holocellulose than wood from P. deltoides x maximowiczii and P. trichocarpa. Finally, the lignin content of Populus deltoides x maximowiczii and Populus trichocarpa wood didn't vary with age and was comparable to that of 30 year-old Populus nigra wood. Furthermore, P. alba grows well in various soil and site conditions and is relatively resistant to climate changes such as drought, temperature, and salt.41 It is primarily used in river lowland afforestation and is grown in specialised plantations to produce furniture logs, pallets, and other products.42 Pelleri et al. discovered that Populus alba L. attains a commercially acceptable diameter after 9 to 10 years (30 cm).43
The in vitro culture of tree species offers a rapid instrument to produce the clonal planting stock. Still, it may also facilitate studies on the effects of elevated levels of heavy metals on plant performance and the selection of metal-tolerant genotypes.38 In this regard, several reports have focused on the tolerance of in vitro cultured white poplars to heavy metals by monitoring the difference in their genotypes.44–46 Based on developmental and molecular data, such systems could be sensitive and reliable for studying heavy metal stress responses of white poplars.47
Herein, we aim to investigate the in vitro culture growth assay of Populus alba upon the treatment with nano-oxide particle forms of each of Fe, Mn, and Zn (e.g., Fe2O3, ZnO, and Mn2O3 NPs) as micronutrients through evaluation of growth parameters and photosynthetic index. In addition, test their genetic stability using RAPD-PCR techniques. The obtained results will help us track the phytoremediation activity of Populus alba (P. alba) via the plant's tolerance to growth in heavy metal contamination.
2. Experimental
2.1. Materials
Zinc sulfate heptahydrate (ZnSO4·7H2O, 99%), ferrous sulfate heptahydrate (FeSO4·7H2O, 98%), manganese sulfate (MnSO4, 99%), and sodium hydroxide (NaOH, 98%) were used without any further purification. All the chemicals used were of analytical reagent grade, obtained from LOBA chemicals.2.2. Preparation of nano-metal oxides for enhanced in vitro culture growth P. alba as phytoremediation of heavy metals
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 under vigorous stirring for 12 h. The precipitate was centrifuged and washed with deionized water several times until pH (6.5–7). The resultant residue was dried in an oven at 80 °C and ground to a fine powder using a mortar. The powder was calcined for 2 hours at 300 °C.
2 under vigorous stirring for 12 h. The precipitate was centrifuged and washed with deionized water several times until pH (6.5–7). The resultant residue was dried in an oven at 80 °C and ground to a fine powder using a mortar. The powder was calcined for 2 hours at 300 °C.2.3. Characterization
The morphological structure of the as-prepared nano-metal oxide particles (e.g., Fe2O3, ZnO, and Mn2O3-NPs) was investigated using Transmission Electron Microscope (TEM) JOEL-JEM 2100 to investigate the micrograph of obtained samples under an operating voltage of 200 kV. X-ray diffraction (XRD) measurements have been carried out using a Bruker D8 advanced X-ray Powder Diffractometer operating with a Cu target with Kα1 = 1.54060 Å, Kα2 = 1.5444 Å, with 2θ step 4° and recorded at the range from 20° to 80°. In addition, the size distribution of as-prepared nanoparticles was measured by the Malvern Zeta-sizer Nano ZS instrument with a He/Ne laser (633 nm) at an angle of 173° using backscatter optics. Finally, Fourier-transform infrared spectroscopy (FT-IR) has been used to explore the chemical groups functionalized on their surfaces. The Brunauer–Emmet–Teller (BET) method was applied to obtain the surface area (SBET) and non-local density functional theory (NL-DFT) for cylindrical pores was applied to evaluate the pore size distribution (PSD). N2 physisorption experiments were carried out on surface area and pore size distribution analyser (BELSORP MAX). Prior to analysis, the samples were outgassed under high vacuum at 350 °C for 3 h. The adsorption/desorption isotherms were obtained at −196 °C allowing 4 min for equilibration between successive points. Textural properties were determined from the isotherms by using the BELMaster Data Analysis software. The pore volume was evaluated on the adsorption branch of the isotherm at p/p0 = 0.97.2.4. Plant materials and the in vitro culture with nano-metal oxide
Explants of Populus alba Lauche (Populus alba L.) nodal segments were cultured and acclimatized on Murashige and Skoog medium (MS medium) as previously reported.51 Each nano-metal oxide (i.e., Fe, Zn, Mn) used as a micronutrient was prepared at three different concentrations (i.e., 20, 40, and 60 mg L−1). These different concentrations were inoculated in MS jars separately. The nodal segments of Populus alba were cultured in these jars compared to the control. The culture was conducted at “Vitro Plant Labs”. Each jar contains three explants. The culture conditions were conducted under white light with a light intensity of 38 μmol m−2 s−1. After that, these cultures were incubated at 25 °C for four weeks, and the measurements were estimated.2.5. Morphological measurements
This study measured seven morphological biometric parameters for all these treatments: plantlet fresh weight, shoot length, shoot number, root length, leaf number, number of roots, and rooting percentage. Seven to ten replicates for each parameter were measured for accurate mean results. The data obtained from all these parameters were applied to find out the relation among these different treatments.Fresh leaves from plantlets were used to measure chlorophyll a, b, carotenoids, and total pigmentation. They were determined in accordance with Metzner et al.52 These leaves (0.5 g) were homogenised in 85% acetone, centrifuged, and transferred to a new tube. The extract was then measured using a colorimeter against a blank of 85% pure acetone at three different wavelengths (i.e., 452, 645 and 664 nm). The concentrations of chlorophyll a, b, and carotenoids were calculated as g ml−1 using Metzner et al. equations;52
| Chl.a = (10.3 × A664) − (0.918 × A645) |
| Chl.b = (19.7 × A645) − (3.87 × A664) |
| Caroteniods = (4.3 × A452) × ((0.0265 × Chl.a) + (0.426 × AChl. b)) |
2.6. DNA isolation and RAPD-PCR bioassay
The genomic DNA of P. alba from different treatments was isolated according to Lu et al.56 According to Doyle and Doyle,57 the total genomic DNA of Populus alba individuals was extracted using the CTAB method. Half gramme of leaves were mixed with 800 μl of 2% CTAB buffer and incubated at 65 °C for 45 minutes (vortex each 10 min). Centrifuge the tubes for 12 minutes at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm, then transfer the supernatant to new tubes with an equal volume of chloroform
000 rpm, then transfer the supernatant to new tubes with an equal volume of chloroform![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) isoamyl alcohol (24
isoamyl alcohol (24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and set for 3 minutes at room temperature. The tubes were then centrifuged (12
1) and set for 3 minutes at room temperature. The tubes were then centrifuged (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm per 10 min per 4 °C). The upper aqueous layer was then transferred to new Eppendorf tubes with 800 μl of absolute ice-cold ethanol added and stored overnight at −20 °C. After centrifuging the tubes to precipitate the DNA pellets, they were washed with ice-cold 70% ethanol. Finally, resuspend pellets in 50 μl of TE buffer and keep at −20 °C till applying RAPD-PCR.
000 rpm per 10 min per 4 °C). The upper aqueous layer was then transferred to new Eppendorf tubes with 800 μl of absolute ice-cold ethanol added and stored overnight at −20 °C. After centrifuging the tubes to precipitate the DNA pellets, they were washed with ice-cold 70% ethanol. Finally, resuspend pellets in 50 μl of TE buffer and keep at −20 °C till applying RAPD-PCR.
A Random amplified polymeric DNA (RAPD-PCR) bioassay was applied using ten primers; only 6 of them gave clear, reproducible bands. The reaction mixture was prepared as follows; 12.5 μl of master mix (Bioline®), 1.5 μl of both forward and reverse primers; 2 μl genomic DNA, and diluted to 25 μl by adding 7.5 μl sterile ddH2O. We performed the reaction program in the thermocycler Biometra® (Germany). The amplification process was performed using 35 cycles of 30 s at 95 °C, 30 s at the specific primer's melting temperature (Tm) (Table 1), and 1 min at 72 °C. PCR products were screened on a 1.4% (wt/vol) agarose gel (Agagel Mini; Biometra®). Gel images were analyzed with BioRad Quantity One (4.6.2) software using the method described by Williamson and Campbell58 and Bio-Rad Laboratories (2005).
| Name | Seq. | Tm | Total no. of bands | Polymorphic bands | Polymorphism (%) | Size range bp |
|---|---|---|---|---|---|---|
| Deca-12 | 5′-CTTGCCCACG-3′ | 38.5 | 4 | 0 | 0 | 509–792 |
| Deca-13 | 5′-GTGGCAAGCC-3′ | 39 | 3 | 3 | 100 | 215–678 |
| Deca-11 | 5′-ATCGGCTGGG-3′ | 39.3 | 5 | 1 | 20 | 135–559 |
| Deca-7 | 5′-CCGCCCGGAT-3′ | 45 | 2 | 0 | 0 | 418–707 |
| Deca-4 | 5′-CGTTGGCCCG-3′ | 44 | 4 | 1 | 25 | 194–566 |
| Deca-10 | 5′-AGCCGGCCTT-3′ | 43.1 | 5 | 3 | 60 | 121–730 |
| Total | 23 | 8 | 34.17 | |||
In random-amplified polymorphic DNA (RAPD) analyses a short primer is designed and added to a PCR reaction with the target DNA. RAPD is a PCR based technique for identifying genetic variation. The RAPD-PCR protocol consisted of an initial denaturing step of 2 min at 95 °C, followed by 45 cycles at 95 °C for 1 min (denaturation), 36 °C for 1 min (annealing of primers), and 72 °C for 2 min (extension). Cycling was concluded with a final extension at 72 °C for 4 min, and then held indefinitely at 4 °C.
2.7. Statistical analysis
This work's statistical analysis of the morphology, physiology and RAPD-PCR against different treatment with nano-metal oxide particle data was performed using Minitab 19 with the following analysis: descriptive analysis, general linear model, grouping by Tukey pairwise comparison, and correlation. A combined phylogenetic tree and principal component analysis (PCA) correlation plots were obtained using the Community Analysis Package (CAP software) to assess the relationship between all Populus treatments based on morphological, physiological, and molecular data. In addition, PCA is a software used to group the similar groups of treatment based on certain algorithm (Pearson correlation in this case).3. Results
3.1. Preparation and characterization of nano-metal oxides particles
Metal-based oxide nanoparticles micronutrients including Fe2O3, ZnO, and Mn2O3 were prepared via co-precipitation methods.48–50 In such cases, metal precursors such as FeSO4, ZnSO4, and MnSO4 have been used, which chemically hydrolyse in an alkali medium to form metal-hydroxide. Then the metal-hydroxide precipitate was treated thermally to form metal-oxide nanoparticles. The formation mechanism for each of Fe2O3, ZnO, and Mn2O3 NPs was demonstrated as follows;| M − R + H2O(hydration) → M(aq.)+ + R−(aq.) | (1) |
| NaOH + H2O(hydration) → Na+(aq.) + OH−(aq.) | (2) |
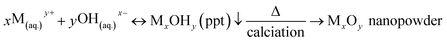 | (3) |
The photophysical properties such as UV-vis absorption spectra has been investigated as shown in Fig. 1. ZnO NPs exhibit a single narrow absorption band in UV region at 375 nm. While Fe2O3 showed a single and weak absorption band in a visible region at 415 nm and shoulder at 545 nm due to red colour characterized to hematite sample nature. Finally, a broad band at visible band at 530 nm.
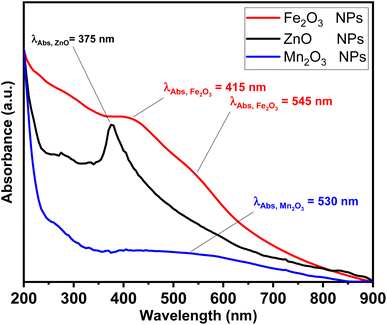 | ||
| Fig. 1 Absorption spectra for ZnO (black line), Fe2O3 (red line) and Mn2O3 (blue line) nanoparticles. | ||
The morphological structure has been confirmed via TEM and XRD measurements, as shown in Fig. 2 and 3, respectively. TEM micrographs showed that Fe2O3 NPs are quasi-spherical with a relatively narrow distribution. The average particle size was 20 ± 5 nm (Fig. 2a and b). While in the case of as-prepared ZnO-NPs, the average particle size was 30 ± 5 nm with a quasi-spherical shape (see Fig. 2c and d). Finally, the average particle size of Mn2O3 was 40 ± 10 nm, with agglomerated spherical nanoparticles in vesicles (see Fig. 2e and f).
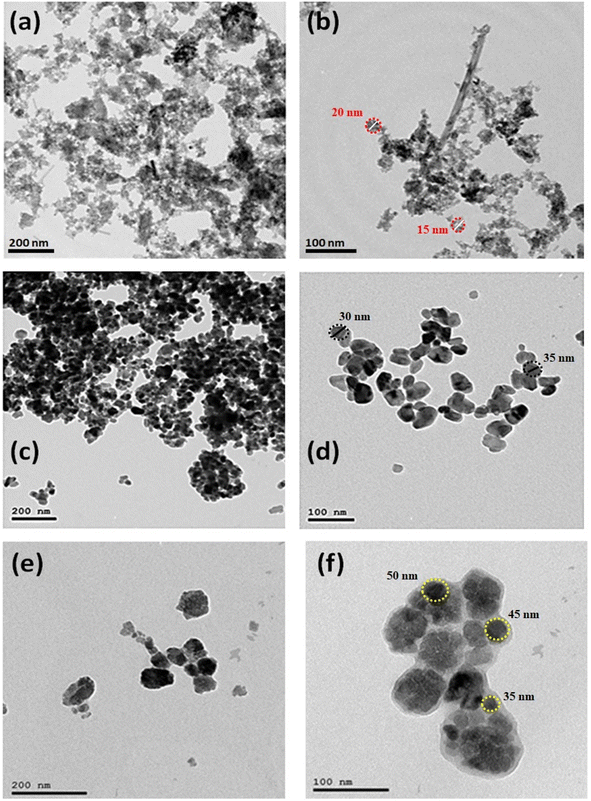 | ||
| Fig. 2 TEM micrographs of Fe2O3 (a and b), ZnO (c and d) and Mn2O3 (e and f) nanoparticles at different magnification powers. | ||
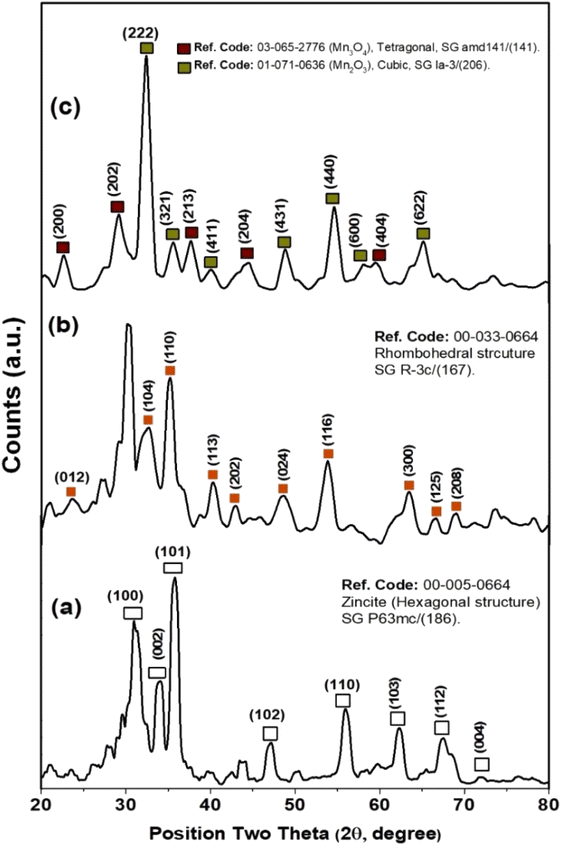
Furthermore, XRD measurements were carried out to investigate the crystallographic structure of as-prepared metal-based oxide nanoparticles of ZnO, Fe2O3, and Mn2O3-NPs, as shown in Fig. 3. XRD patterns of ZnO NPs indicated the formation of a hexagonal structure of ZnO nanoparticles (see Fig. 3a), where the three strongest lines have been obtained at 31.28°, 33.948°, and 35.81° due to the (100), (002), and (101) crystallographic plans of the hexagonal structure of ZnO NPs (i.e., Zincite), as reported by ICCD-PDF No. 00-005-0664. In addition, the existence of five diffraction reflections at 47.14°, 55.93°, 62.33°, 67.47°, and 71.92° due to (102), (110), (103), (112), and (004) reflection plans for the hexagonal crystallographic structure of ZnO NPs. In addition, the average crystalline size of ZnO nanocrystals via Debye Sherer equation was about 7.98 ± 1.2 nm. Fig. 3b shows the XRD patterns of as-prepared hematite nanoparticles (i.e., Fe2O3 NPs). Two of the strongest lines were observed at 32.6° and 35.16° due to (104) and (110) reflection plans of the rhombohedral crystallographic structure of hematite nanoparticles (i.e., Fe2O3 NPs. See Fig. 3b). Moreover, another five diffraction patterns were at 40.35°, 42.95°, 48.59°, 53.84°, and 63.48° due to (113), (202), (024), (116), and (300) reflection plans for the rhombohedral crystallographic structure of Fe2O3 NPs, respectively (see Fig. 3b). Also, the average crystalline size of Fe2O3 nanocrystals via Debye Sherer equation was about 6.22 ± 1 nm. XRD patterns of manganese oxide nanoparticles revealed a mixture of crystallographic reflection plans for both Mn(III) and Mn(IV) oxide nanoparticles (i.e., Mn2O3 and Mn3O4 NPs), respectively. As depicted in Fig. 3c, Mn(III) oxide nanoparticles (Mn2O3 NPs) exhibited the dominant crystallographic structure with a cubic structure (see Fig. 3c). The two strongest lines of Mn2O3 were observed at 32.41° and 54.64°, corresponding to (222) and (440) reflection plans for the cubic structure of Mn2O3 NPs. Another four diffraction patterns accompanied these diffraction patterns were recorded at 35.56°, 40.1°, 48.89°, and 65.12°, corresponding to (321), (441), (431), and (622) reflection plans of Mn2O3 NPs. Furthermore, the minor phase Mn3O4 NPs, exhibit diffraction patterns at 22.62°, 29.17°, 37.66°, 44.55°, and 59.53° for (200), (202), (213), (204), and (404) reflection plans, respectively (see Fig. 3c), and the average crystalline size was about 6.17 nm.
In addition, the colloidal properties based on DLS and zeta potential measurements for Fe2O3, ZnO, and Mn2O3 nanoparticles were investigated, as shown in Fig. 4. The hydrodynamic particle size of as-prepared nanoparticles is remarkably increased, agreeing with their agglomeration presented in TEM micrographs, as shown in Fig. 2. This aggregation is due to its hydrophilic nature. In addition, the strength of the steric forces of the functional groups on the surface of nanoparticles and a steric interaction caused by the formation of a layer of water around the material.59 In this regard, the hydrodynamic diameter (HD) of Fe2O3 nanoparticles was about 232 ± 29.24 nm (see Fig. 4a and Table 2). In addition, Fe2O3 nanoparticles exhibit good dispersion in water, where the polydispersity index (PDI) is about 0.586.
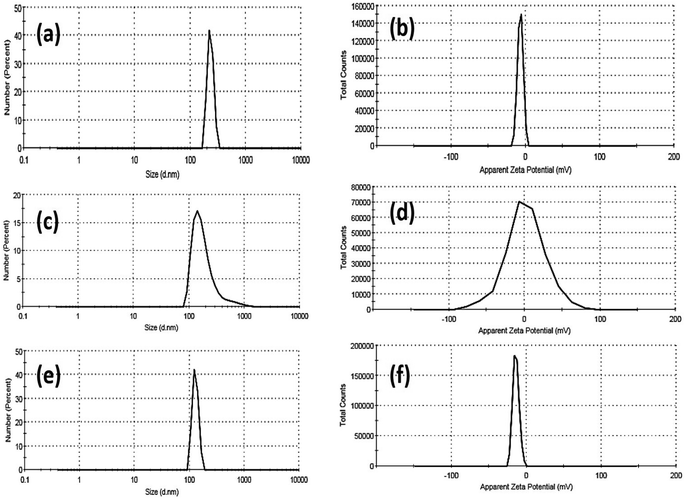 | ||
| Fig. 4 DLS and zeta-potential data of Fe2O3 (a and b), ZnO (c and d) and Mn2O3 (e and f) nanoparticles. | ||
| Sample | Dynamic light scattering (DLS) | Zeta potential (η, mV) | |
|---|---|---|---|
| Hydrodynamic diameter (HD, nm) | Polydispersity index (PDI) | ||
| Fe2O3-NPs | 232 ± 29.24 | 0.586 | −6.96 |
| Mn2O3-NPs | 201 ± 143 | 0.585 | +1.39 |
| ZnO-NPs | 129.1 ± 16.6 | 0.797 | −14 |
Moreover, the zeta potential (η) of Fe2O3 nanoparticles was about −6.96 mV. The hydrodynamic particle size of Mn2O3 nanoparticles was smaller than Fe2O3, about 201 ± 143 nm. In addition, Mn2O3 nanoparticles exhibit a good dispersion in water, where the polydispersity index (PDI) is about 0.585 (see Fig. 4c and Table 2). Moreover, the zeta potential (η) of Mn2O3 nanoparticles is positively charged at +1.39 mV. Finally, ZnO NPs exhibited a smaller HD than Fe2O3 and Mn2O3 NPs (see Fig. 4e and Table 2). The hydrodynamic particle size of ZnO NPs was 129.1 ± 16.6 nm, and the polydispersity index (PDI) was about 0.797, indicating their fine dispersion in water.
Moreover, the ZnO nanoparticles' zeta potential (η) exhibits more electronegative behaviour at about −14 mV. Based on our findings, the size acquired by DLS, as shown in Table 2, is frequently larger than that obtained by TEM images, as shown in Fig. 2. DLS measurements are based on an intensity-based approach for solvated samples. The hydrodynamic diameter (HD) is the size of the nanoparticle plus the liquid layer around the particle (i.e., the relative humidity of the scattered particles). In contrast, TEM measurements are based on a number-based system dealing with dry samples under ultra-high vacuum conditions and calculating the projected surface area based on how much the incoming electrons passed through the sample.
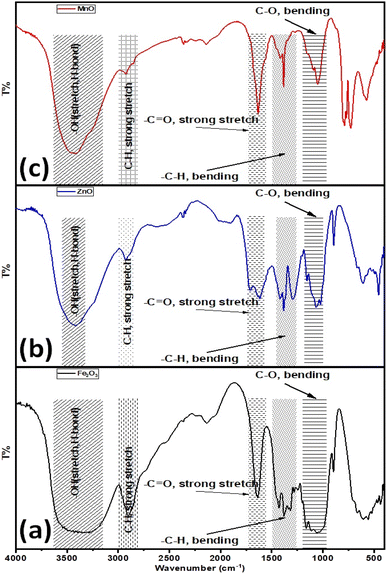
Furthermore, the surface properties of the as-prepared metal-based oxide nanoparticles were explored via their FT-IR spectra, as shown in Fig. 5. The FT-IR spectrum of Fe2O3 NPs exhibited several absorption bands as follows (see Fig. 5a), considering the broadband for the –OH stretching vibration in the range of 3550–3100 cm−1. Also, the absorption bands at 1640, 1430, and 1421 cm−1 are associated with the stretching vibration of the –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group and the bending vibration of adsorbed water, –CH group. The absorption band at 2890 cm−1 is attributed to the stretching vibration of the aliphatic saturated –CH group. The peaks for C–H and C–O bending vibrations are around 1157 and 1112 cm−1, respectively. Furthermore, the bending vibration of the –C–O group is indicated by the absorption peak at 1060 cm−1. While in the case of ZnO-NPs, the broadband for the –OH stretching vibration is in the range of 3420 cm−1. Also, the absorption bands at 1640, 1430, and 1421 cm−1 are associated with the stretching vibration of the –C
O group and the bending vibration of adsorbed water, –CH group. The absorption band at 2890 cm−1 is attributed to the stretching vibration of the aliphatic saturated –CH group. The peaks for C–H and C–O bending vibrations are around 1157 and 1112 cm−1, respectively. Furthermore, the bending vibration of the –C–O group is indicated by the absorption peak at 1060 cm−1. While in the case of ZnO-NPs, the broadband for the –OH stretching vibration is in the range of 3420 cm−1. Also, the absorption bands at 1640, 1430, and 1421 cm−1 are associated with the stretching vibration of the –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group and the bending vibration of adsorbed water, –CH group. The absorption band at 2925 cm−1 is attributed to the stretching vibration of the aliphatic saturated –CH group. The peaks for C–H and C–O bending vibrations are around 1157 and 1112 cm−1, respectively. Furthermore, the bending vibration of the –C–O group is indicated by the absorption peak at 1060 cm−1. The absorption band at 895 cm−1 is attributed to the strong bending vibration of –C
O group and the bending vibration of adsorbed water, –CH group. The absorption band at 2925 cm−1 is attributed to the stretching vibration of the aliphatic saturated –CH group. The peaks for C–H and C–O bending vibrations are around 1157 and 1112 cm−1, respectively. Furthermore, the bending vibration of the –C–O group is indicated by the absorption peak at 1060 cm−1. The absorption band at 895 cm−1 is attributed to the strong bending vibration of –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– in vinylidene (see Fig. 5b). Finally, the FT-IR spectrum for the as-prepared Mn2O3 NPs depicted in Fig. 4c demonstrates that the broadband for the –OH stretching vibration is 3420 cm−1. Also, the absorption bands at 1640, 1430, and 1421 cm−1 are associated with the stretching vibration of the –C
C– in vinylidene (see Fig. 5b). Finally, the FT-IR spectrum for the as-prepared Mn2O3 NPs depicted in Fig. 4c demonstrates that the broadband for the –OH stretching vibration is 3420 cm−1. Also, the absorption bands at 1640, 1430, and 1421 cm−1 are associated with the stretching vibration of the –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group and the bending vibration of adsorbed water, –CH group. The absorption band at 2925 cm−1 is attributed to the stretching vibration of the aliphatic saturated –CH group. The peaks for C–H and C–O bending vibrations are around 1157 and 1112 cm−1, respectively. Furthermore, the bending vibrations at 1060 and 895 cm−1 were attributed to the –C–O and –C
O group and the bending vibration of adsorbed water, –CH group. The absorption band at 2925 cm−1 is attributed to the stretching vibration of the aliphatic saturated –CH group. The peaks for C–H and C–O bending vibrations are around 1157 and 1112 cm−1, respectively. Furthermore, the bending vibrations at 1060 and 895 cm−1 were attributed to the –C–O and –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– in vinylidene functional groups, respectively (see Fig. 5c). Based on the FTIR data, the –OH functional group that revealed water content/absorption onto the surfaces of the nanoparticles could improve the hydrophilicity of the as-prepared nano metal oxides in aqueous systems. In addition, improves the water uptake and maintains better water status under drought stress conditions and then improves the nutrients uptake such as nitrogen (N), and potassium (K),60 and consequently improves seeds germination, plant growth, physiological functioning, photosynthetic efficiency, and hormonal activities.60
C– in vinylidene functional groups, respectively (see Fig. 5c). Based on the FTIR data, the –OH functional group that revealed water content/absorption onto the surfaces of the nanoparticles could improve the hydrophilicity of the as-prepared nano metal oxides in aqueous systems. In addition, improves the water uptake and maintains better water status under drought stress conditions and then improves the nutrients uptake such as nitrogen (N), and potassium (K),60 and consequently improves seeds germination, plant growth, physiological functioning, photosynthetic efficiency, and hormonal activities.60
Finally, N2 adsorption/desorption technique was used in measuring the surface area and pore size distribution of the prepared nano compounds. Surface area parameters were evaluated for all the prepared samples using BET surface area measurements, as listed in Table 3. The surface area of ZnO nanoparticles was about 49.16 m2 g−1, which higher than both Mn2O3 and Fe2O3 with BET surface area value of 33.834 and 33.383 m2 g−1, respectively. In contrast Fe2O3 NPs showed a highest pore distribution compared to Mn2O3 and ZnO NPs. In addition, as-prepared metal oxides nanoparticles exhibited narrow pore size distributions, and capillary condensation steps on nitrogen adsorption isotherms as shown in Fig. 6a and b.
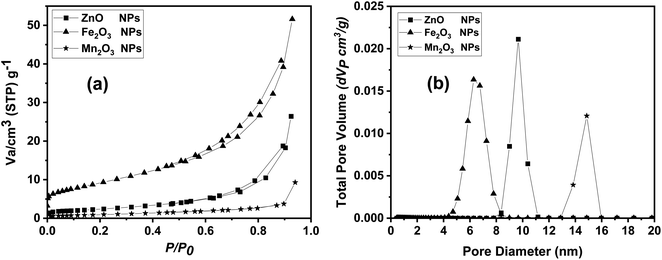 | ||
| Fig. 6 BET data including (a) adsorption/desorption isotherm and (b) pore size distribution analysis for ZnO, Fe2O3 and Mn2O3 nanoparticles. | ||
3.2. Morphological parameters and chlorophyll content
Seven morphological parameters, including one with a fixed rooting percentage of (100%) have been investigated for all treatments. The other six parameters varied and had significant differences in their treatment with three different levels of metal oxide nanoparticles compared to the control (Table 3). The chlorophyll index, reflecting the photosynthesis process, varied but had no significant difference (Table 4). Moreover, the correlation relationship among all the parameters (Table 5) illustrates that most of the parameters' ranges have positively affected each other, except rooting percentage, which negatively affected the other parameters in response to metal oxide nanoparticle treatments.| Sample | Surface area (SBET), m2 g−1 | BET analysis | |
|---|---|---|---|
| Total pore volume (Vp), cm3 g−1 | Mean pore diameter (D), nm | ||
| ZnO NPs | 49.1619 | 0.4087 | 17.844 |
| Fe2O3 NPs | 33.383 | 0.79839 | 9.4394 |
| Mn2O3 NPs | 33.834 | 0.14368 | 14.987 |
| Treatment | Fe2O3 NPs (mg L−1) | ZnO NPs (mg L−1) | Mn2O3 NPs (mg L−1) | Control | P-value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 20 | 40 | 60 | 20 | 40 | 60 | 20 | 40 | 60 | |||
| Fresh wt ‘g’ | 0.210 ± 0.00115d | 0.191 ± 0.00115e | 0.178 ± 0.00115g | 0.145 ± 0.00153h | 0.135 ± 0.002i | 0.185 ± 0.003f | 0.223 ± 0.00115c | 0.261 ± 0.0025b | 0.292 ± 0.0025a | 0.189 ± 0.002ef | 0.00 |
| Shoot length ‘cm’ | 7.033 ± 0.058b | 6.766 ± 0.116bcd | 6.366 ± 0.153e | 5.566 ± 0.058g | 6.033 ± 0.058f | 6.566 ± 0.153cde | 7.033 ± 0.153b | 6.533 ± 0.058de | 7.966 ± 0.058a | 6.833 ± 0.058bc | 0.00 |
| Shoot number | 1.00 ± 0.00b | 1.00 ± 0.00b | 1.00 ± 0.00b | 1.00 ± 0.00b | 1.00 ± 0.00b | 1.00 ± 0.00b | 2.00 ± 0.00a | 2.00 ± 0.00a | 2.00 ± 0.00a | 1.00 ± 0.00b | 0.10 |
| Leaf number | 8.333 ± 0.577c | 9.00 ± 0.00c | 10.0 ± 0.00c | 8.667 ± 0.577c | 9.0 ± 0.00c | 8.667 ± 1.155c | 14.33 ± 0.577ab | 15.66 ± 1.155a | 12.33 ± 1.155b | 9.00 ± 0.00c | 0.00 |
| Root length ‘cm’ | 4.133 ± 0.116cde | 4.433 ± 0.058c | 4.366 ± 0.153cde | 5.067 ± 0.058b | 4.067 ± 0.058de | 4.033 ± 0.058e | 8.033 ± 0.153a | 4.400 ± 0.173cd | 4.067 ± 0.116de | 5.133 ± 0.153b | 0.00 |
| Root number | 5.333 ± 0.577d | 7.00 ± 0.00bc | 7.00 ± 0.00bc | 7.667 ± 1.155ab | 6.00 ± 0.00cd | 8.667 ± 0.577a | 3.00 ± 0.00e | 5.00 ± 0.00d | 5.00 ± 0.00d | 5.667 ± 0.577cd | 0.00 |
| Chl index | 9.30 ± 8.20a | 10.90 ± 8.36a | 7.70 ± 5.49a | 3.333 ± 1.724a | 3.830 ± 1.80a | 4.330 ± 3.43a | 10.60 ± 7.02a | 5.130 ± 4.50a | 5.830 ± 4.87a | 5.630 ± 3.54a | 0.55 |
| Fresh wt | Shoot length | Shoot no. | Leaf no. | Root length | Root no. | |
|---|---|---|---|---|---|---|
| Shoot length | 0.818 | |||||
| Shoot no. | 0.827 | 0.540 | ||||
| Leaf no. | 0.697 | 0.343 | 0.917 | |||
| Root length | 0.060 | 0.068 | 0.412 | 0.432 | ||
| Root no. | −0.534 | −0.492 | −0.704 | −0.634 | −0.583 | |
| Chlorophyll | 0.131 | 0.170 | 0.092 | 0.031 | 0.198 | −0.237 |
3.3. Estimation of metal oxide nanoparticles uptake in Populus alba
Populus alba treated with different nano-metal oxides. The metal uptake by P. alba was nearly the same with no significant differences (Table 6). These results confirm plant uptake of equivalent nano-heavy metals-based oxides. Hence, the estimated morphological and chlorophyll parameters are related to the type of nano-metal oxides besides the concentration.| Treatment | Fe2O3 NPs (mg L−1) | ZnO NPs (mg L−1) | Mn2O3 NPs (mg L−1) | Control | P-value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 20 | 40 | 60 | 20 | 40 | 60 | 20 | 40 | 60 | |||
| Metal conc. ‘ppm’ | 0.297a | 0.407a | 0.377a | 0.557a | 0.637a | 0.787a | 0.430a | 0.467a | 0.750a | 0.410a | 0.138a |
3.4. DNA isolation and RAPD-PCR bioassay
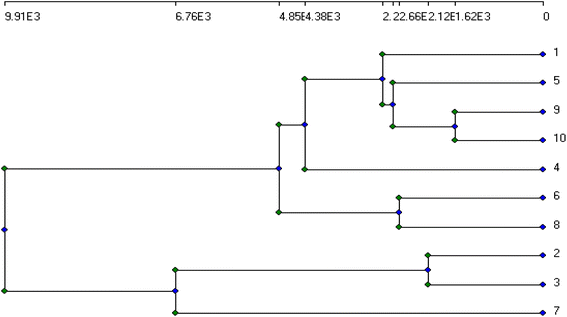
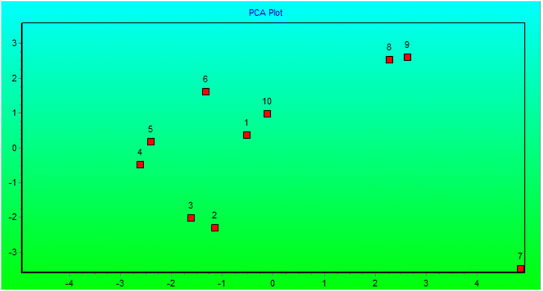
The genetic stability of Populus alba treatments involving nano metal-based oxides, nanoparticles, and micronutrients has significant practical and commercial implications. In this study, we estimated the fingerprinting profiles of the regenerated culture by RAPD to confirm if the plantlets were genetically stable or not. Ten random decamer-RAPD primers were tested for initial screening; only six primers gave clear and reproducible bands. The polymorphic bands ranged from 2 bands with the Deca-7 primer to 5 polymorphic bands with both the Deca-11 and Deca-10 primers (Table 1). These six primers produced 23 bands, with eight polymorphic bands, resulting in a total polymorphism percentage of 34.17% (see Fig. 7). The total molecular weights of all bands ranged from 121–792 bp. Fig. 8 shows a total phylogenetic tree that resulted from all the combined morphological parameters, chlorophyll index, and genetic variation. Fig. 9 showed the principal component analysis (PCA) plot, which showed the correlation between nano-metal oxide effects on the P. alba plant. The graph illustrated that responses to nano-Mn concentrations are separated, where control, nano-Fe, and nano-Zn are grouped. In addition, the Pearson correlation plot for the covariance ordination and correlation based on morphology and physiology among different nano-metal oxides treatments of Populus alba has been illustrated in Fig. 10.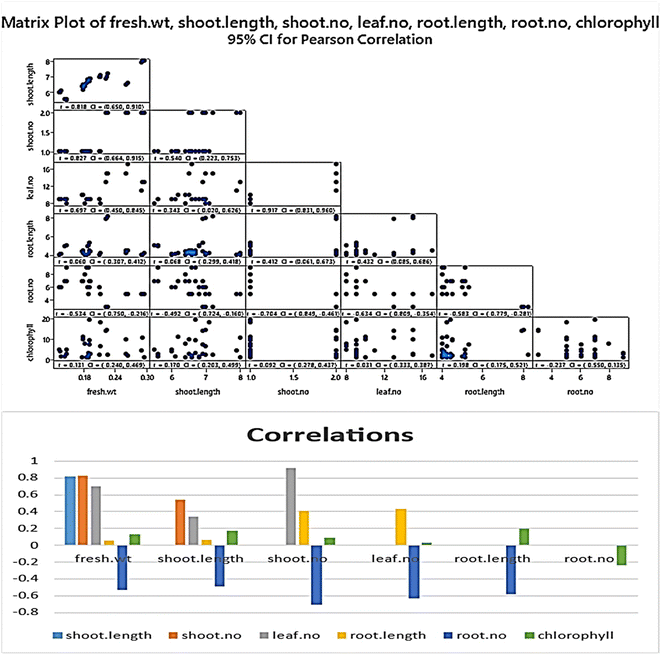 | ||
| Fig. 10 Pearson plot showing covariance ordination and correlation based on morphology and physiology among different nano-metal oxides treatments of Populus alba. | ||
4. Discussion
Recently, looking for economic and eco-friendly soil heavy metal remediation techniques instead of conventionally used physicochemical techniques has become an urgent need. This is because biological techniques based on phytoremediation do not have any side effects on the soil ecosystem; they are economically viable compared to other traditionally used techniques.33,61 The effect of Populus alba uptake on the nano-heavy metal micronutrients varied and was reflected in morphological and chlorophyll parameters. Some parameters were enhanced with exposure to heavy metal-based oxide nanoparticles-based micronutrients, while others were inhibited compared to control. The toxic effect of most heavy metals is caused by their bonding with –SH protein groups, leading to the inhibition of enzyme activity and compromising their structure. They also substitute essential elements in biomolecules, causing their deficiency.62 Metal ion concentrations in the soil limit plant assimilation of essential micro-and macronutrients.63The acclimatization of P. alba culture was performed by Ahmed et al.51 and El-Sayed et al.64 to obtain the best growth conditions. Gomes and co-workers explored the morphological, anatomical, and chlorophyll content of affected vegetation areas surrounding the mining company that produced heavy metals such as Zn, Cd, and Pb.65 Moreover, Sadak and Bakry suggested using ZnO as an example of metal oxide-based nanomaterials rather than bulk forms in treating plants.66 In such a study, ZnO-NPs exhibited an excellent effect and improved the biochemical, growth, and consequent biomass yield.
Our findings agreed with previous reports by Atteya et al.67 and Gheith et al.,68 respectively. In their studies, a remarkable increase in the biomass yield and plant growth parameters was observed upon the treatment of jojoba and maize with Zn precursors.67,68 However, Prasad and co-workers proved that ZnO-NPs have a remarkable effect on enhancing peanut plant growth and that biomass yields are higher than ordinary Zn precursors such as ZnSO4 at lower concentrations. This enhancement in peanut plant growth is attributed to the high adsorption of ZnO-NPs in the plant-based on their smaller size than ZnSO4.69 Finally, Tawfik and co-workers reported a significant increase in the growth parameters of Atriplex halimus upon treatment with ZnO-NPs in saline environments.70 Furthermore, the efficiency of nanoparticles can vary with different genotypes and applications for various reasons, including differences in nanoscale size, the likelihood that these structures will lose their properties during plant physiological processes, and the properties of nanoscale metals.71–73
5. Conclusion
This study evaluated the in vitro propagation ability, photosynthetic pigment contents, and genetic variation of P. alba if the growth media includes metal-based oxide nanoparticles as a source of micronutrients such as ZnO, Fe2O3 and Mn2O3 NPs. These nanoparticles cause variation in growth due to changes in their physiological and biochemical activities, especially when the heavy metal involved plays a beneficial role in plant growth and development. The genetic variation in the DNA content of polluted P. alba individuals adapts the plant to tolerate and adapt to nano-metal contamination. Overall, a significant difference in the biomass production-related parameters such as fresh weight, shoot length, root length, and root number was observed compared to control upon the treatment with micronutrients in terms of Mn2O3 NPs as a source of Mn3+. Whereas treatment with ZnO NPs showed a significant increase in the root number of Populus alba plants compared to control. Also, we monitored a remarkable increase in the chlorophyll index upon treatment with Fe2O3 NPs. Furthermore, RAPD-PCR bioassays demonstrated that metal oxide nanoparticles aided the in vitro culture of Populus alba and are highly genetically stable even in highly contaminated environments or soil.Author contributions
Mohamed Fathy Ahmed; performed the agricultural experiments on the P. alba. Mostafa Ahmed Ibrahim and Ahmed Sadek Mansour; synthesis of nanomaterials. Ahmed Nabile Emam (corresponding author); synthesis and performing characterization experiments for metal oxide nanomaterials, data analysis & discussion, and writing/formulating and revising the manuscript. Ashraf Bakry Abd El-Razik; performing molecular genetic experiments. Eman Tawfik; conceptualization, contribution in performing and analysis of the agricultural and molecular genetics data.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank Dr Aisha Mostafa and Dr Amal Farouk at the National Research Centre (NRC) X-Ray Crystallography Laboratory for performing XRD measurements of as-prepared metal oxide nanoparticles using the Bruker D8 advanced X-Ray Powder Diffractometer.Notes and references
- P. B. Tchounwou, C. G. Yedjou, A. K. Patlolla and D. J. Sutton, Exp. suppl., 2012, 101, 133–164 Search PubMed.
- H. Ali, E. Khan and I. Ilahi, J. Chem., 2019, 2019, 6730305 Search PubMed.
- M. Jaishankar, T. Tseten, N. Anbalagan, B. B. Mathew and K. N. Beeregowda, Interdiscip. Toxicol., 2014, 7, 60–72 CrossRef PubMed.
- A. Asati, M. Pichhode and K. Nikhil, Int. j. appl. innov. eng. manag., 2016, 5, 56–66 Search PubMed.
- J. Briffa, E. Sinagra and R. Blundell, Heliyon, 2020, 6, e04691 CrossRef CAS PubMed.
- I. Raskin, P. N. Kumar, S. Dushenkov and D. E. Salt, Curr. Opin. Biotechnol., 1994, 5, 285–290 CrossRef CAS.
- B. Singh and D. Schulze, Nat. Educ. Knowl., 2015, 6, 1 Search PubMed.
- D. G. Gomes, J. C. Pieretti, W. R. Rolim, A. B. Seabra and H. C. Oliveira, in Advances in Nano-Fertilizers and Nano-Pesticides in Agriculture, ed. S. Jogaiah, H. B. Singh, L. F. Fraceto and R. d. Lima, Woodhead Publishing, 2021, pp. 111–143, DOI:10.1016/B978-0-12-820092-6.00005-7.
- Z. Rengel, J. Soil Sci. Plant Nutr., 2015, 15, 397–409 Search PubMed.
- R. Djingova and I. Kuleff, in Trace metals in the Environment, Elsevier, 2000, vol. 4, pp. 137–185 Search PubMed.
- R. D. Reeves, Phytoremediation of toxic metals: using plants to clean up the environment, 2000 Search PubMed.
- H. Wintz, T. Fox and C. Vulpe, Biochem. Soc. Trans., 2002, 30, 766–768 CrossRef CAS PubMed.
- M. Becker and F. Asch, J. Plant Nutr. Soil Sci., 2005, 168, 558–573 CrossRef CAS.
- J. Morrissey and M. L. Guerinot, Chem. Rev., 2009, 109, 4553–4567 CrossRef CAS PubMed.
- G. R. Rout and S. Sahoo, Rev. Agric. Sci., 2015, 3, 1–24 CrossRef.
- D. Neina, Appl. Environ. Soil Sci., 2019, 2019, 5794869 Search PubMed.
- A. Gautam and R. S. Dubey, Molecular physiology of abiotic stresses in plant productivity, 2018, pp. 256–290 Search PubMed.
- B. Hafeez, Y. Khanif and M. Saleem, J. Exp. Agric. Int., 2013, 374–391 CAS.
- J. F. Loneragan, in Manganese in soils and plants, Springer, 1988, pp. 113–124 Search PubMed.
- H. Chhipa, Methods Microbiol., 2019, 46, 115–142 CAS.
- M. Usman, M. Farooq, A. Wakeel, A. Nawaz, S. A. Cheema, H. ur Rehman, I. Ashraf and M. Sanaullah, Sci. Total Environ., 2020, 721, 137778 CrossRef CAS PubMed.
- H. Chemingui, M. Smiri, T. Missaoui and A. Hafiane, Bull. Environ. Contam. Toxicol., 2019, 102, 477–485 CrossRef CAS PubMed.
- M. Naderi and A. Abedi, J. Nanotechnol., 2012, 11, 18–26 Search PubMed.
- D. Mishra and P. Khare, in Sustainable Agriculture Reviews 50: Emerging Contaminants in Agriculture, ed. V. Kumar Singh, R. Singh and E. Lichtfouse, Springer International Publishing, Cham, 2021, pp. 235–257, DOI:10.1007/978-3-030-63249-6_9.
- N. Sarkar, S. Chaudhary and M. Kaushik, in Plant-Microbes-Engineered Nano-particles (PM-ENPs) Nexus in Agro-Ecosystems: Understanding the Interaction of Plant, Microbes and Engineered Nano-particles (ENPS), ed. P. Singh, R. Singh, P. Verma, R. Bhadouria, A. Kumar and M. Kaushik, Springer International Publishing, Cham, 2021, pp. 153–163, DOI: DOI:10.1007/978-3-030-66956-0_10.
- P. Zhang, Z. Guo, S. Ullah, G. Melagraki, A. Afantitis and I. Lynch, Nat. Plants, 2021, 7, 864–876 CrossRef PubMed.
- K. Sampathkumar, K. X. Tan and S. C. J. Loo, iScience, 2020, 23, 101055 CrossRef CAS PubMed.
- M. C. DeRosa, C. Monreal, M. Schnitzer, R. Walsh and Y. Sultan, Nat. Nanotechnol., 2010, 5, 91 CrossRef CAS PubMed.
- R. Nair, S. H. Varghese, B. G. Nair, T. Maekawa, Y. Yoshida and D. S. Kumar, Plant Sci., 2010, 179, 154–163 CrossRef CAS.
- E. L. Arthur, P. J. Rice, P. J. Rice, T. A. Anderson, S. M. Baladi, K. L. Henderson and J. R. Coats, Crit. Rev. Plant Sci., 2005, 24, 109–122 CrossRef CAS.
- S. Parmar and V. Singh, J. Plant Sci. Res., 2015, 2, 135 Search PubMed.
- M. Laghlimi, B. Baghdad, H. El Hadi and A. Bouabdli, Open J. Ecol., 2015, 5(8), 375–388 CrossRef.
- M. M. Al-Khazan and R. M. Al-Zlabani, Egypt. J. Exp. Biol. (Bot.), 2019, 15, 87–97 Search PubMed.
- E. W. Aitchison, S. L. Kelley, P. J. Alvarez and J. L. Schnoor, Water Environ. Res., 2000, 72, 313–321 CrossRef CAS.
- I. Pulford and C. Watson, Environ. Int., 2003, 29, 529–540 CrossRef CAS PubMed.
- M. Laghlimi, B. Baghdad, H. El Hadi and A. Bouabdli, Open J. Obstet. Gynecol., 2015, 5, 375 Search PubMed.
- W. T. Liu, J. C. Ni and Q. X. Zhou, 2013.
- S. Di Lonardo, M. Capuana, M. Arnetoli, R. Gabbrielli and C. Gonnelli, Environ. Sci. Pollut. Res., 2011, 18, 82–90 CrossRef PubMed.
- B. Kovačević, S. Orlović, S. Rončević and D. Miladinović, Acta Hortic., 2010, 885, 197–202 CrossRef.
- D. Krutul, T. Klosinska, A. Antczak, M. Drozdzek, A. Radomski and J. Zawadzki, Ann. WULS Forest. Wood Technol., 2019, 105, 125–132 CrossRef.
- J. Richardson, J. Isebrands and J. Ball, Poplars and willows: Trees for society and the environment, pp. 92–123, 2014 Search PubMed.
- D. I. Dickmann and J. Kuzovkina, in Poplars and willows: Trees for society and the environment, CABI, Wallingford UK, 2014, pp. 8–91 Search PubMed.
- F. Pelleri, S. Ravagni, E. Bianchetto and C. Bidini, Ann. Silvic. Res., 2013, 37(1), 13–21 Search PubMed.
- I. Kališová-Špirochová, J. Punčochářová, Z. Kafka, M. Kubal, P. Soudek and T. Vaněk, Water, Air, Soil Pollut.: Focus, 2003, 3, 269–276 CrossRef.
- K. Bojarczuk, Pol. J. Environ. Stud., 2004, 13, 115–120 CAS.
- M. Katanic, A. Pilipovic, S. Orlovic, B. Krstic, B. Kovacevic and S. Pekec, 2008.
- E. Cherian, A. Rajan and G. Baskar, Int. J. Mod. Sci. Technol., 2016, 1(1), 17–22 Search PubMed.
- X.-C. Yang, Y.-L. Shang, Y.-H. Li, J. Zhai, N. R. Foster, Y.-X. Li, D. Zou and Y. Pu, J. Nanomater., 2014, 2014, 740856 Search PubMed.
- A. Shoba, B. Kavitha, H. Aswathaman, H. Ganesan and N. S. Kumar, Mater. Today: Proc., 2022, 48, 521–526 CAS.
- V. Koutu, L. Shastri and M. M. Malik, Mater. Sci., 2016, 34, 819–827 CAS.
- M. Hewidy, A. M. Hosni, A. Abdel Razik, M. F. Ahmed and M. Bahnasy, Arab. Univ. J. Agric. Sci., 2019, 27, 2273–2290 Search PubMed.
- H. Metzner, H. Rau and H. Senger, Planta, 1965, 65, 186–194 CrossRef CAS.
- R. A. Isaac and J. D. Kerber, Instrumental methods for analysis of soils and plant tissue, 1971, pp. 17–37 Search PubMed.
- Z.-Y. Hseu, Bioresour. Technol., 2004, 95, 53–59 CrossRef CAS PubMed.
- S. Shirani Bidabadi, Int. J. Phytorem., 2020, 22, 1259–1268 CrossRef CAS PubMed.
- Y. Lu, S. Chanroj, L. Zulkifli, M. A. Johnson, N. Uozumi, A. Cheung and H. Sze, Plant Cell, 2011, 23, 81–93 CrossRef CAS PubMed.
- J. J. Doyle, Focus, 1990, 12, 13–15 Search PubMed.
- A. M. Campbell, Am. Biol. Teach., 1997, 59, 164–170 CrossRef.
- W. Marimón-Bolívar and E. E. González, Dyna, 2018, 85, 19–26 CrossRef.
- A. Rasheed, H. Li, M. M. Tahir, A. Mahmood, M. Nawaz, A. N. Shah, M. T. Aslam, S. Negm, M. Moustafa, M. U. Hassan and Z. Wu, Front. Plant Sci., 2022, 13, 1–15 Search PubMed.
- M. T. Gómez-Sagasti, I. Alkorta, J. M. Becerril, L. Epelde, M. Anza and C. Garbisu, Water, Air, Soil Pollut., 2012, 223, 3249–3262 CrossRef.
- F. Van Assche and H. Clijsters, Plant, Cell Environ., 1990, 13, 195–206 CrossRef CAS.
- J. Oleksyn, P. Karolewski, M. Giertych, A. Werner, M. Tjoelker and P. Reich, Trees, 1996, 10, 135–144 Search PubMed.
- I. M. El-Sayed, S. A. Shaaban, L. S. Taha and M. H. Mahgoub, Plant Arch., 2019, 19, 2655–2663 Search PubMed.
- M. P. Gomes, T. C. L. L. d. S. Marques, M. d. O. G. Nogueira, E. M. d. Castro and Â. M. Soares, Sci. Agric., 2011, 68, 566–573 CrossRef CAS.
- M. S. Sadak and B. A. Bakry, Bull. Natl. Res. Cent., 2020, 44, 98 CrossRef.
- A. K. Atteya, E. A. Genaidy and H. A. Zahran, Biosci. Res., 2018, 15, 1528–1541 Search PubMed.
- E.-S. M. S. Gheith, M. M. Shafik, O. Z. El-Badry and B. M. A. Kareem, Biosci. Res., 2018, 15, 54–59 Search PubMed.
- T. Prasad, P. Sudhakar, Y. Sreenivasulu, P. Latha, V. Munaswamy, K. R. Reddy, T. Sreeprasad, P. Sajanlal and T. Pradeep, J. Plant Nutr., 2012, 35, 905–927 CrossRef CAS.
- M. Tawfik, G. Bakhoum, S. Sadak Mervat and M. Kabesh, Bull. Natl. Res. Cent., 2017, 41, 286–305 Search PubMed.
- O. B. Nalci, H. Nadaroglu, A. H. Pour, A. A. Gungor and K. Haliloglu, Plant Cell, Tissue Organ Cult., 2019, 136, 269–277 CrossRef CAS.
- Z. Wang, L. Xu, J. Zhao, X. Wang, J. C. White and B. Xing, Environ. Sci. Technol., 2016, 50, 6008–6016 CrossRef CAS PubMed.
- W.-M. Lee, Y.-J. An, H. Yoon and H.-S. Kweon, Environ. Toxicol. Chem., 2008, 27, 1915–1921 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2023 |