Open Access Article
Open Access ArticleResearch progress of bio-slurry remediation technology for organic contaminated soil
Jing Sun a,
Fujia Wangab,
Xiaohan Jiab,
Xiaowei Wang
a,
Fujia Wangab,
Xiaohan Jiab,
Xiaowei Wang *b,
Xinxin Xiaob and
Huaijin Dongb
*b,
Xinxin Xiaob and
Huaijin Dongb
aEnvironmental Science and Engineering, Qilu University of Technology, Jinan 250353, China
bEnvironmental Testing and Experiment Center, Chinese Research Academy of Environmental Sciences, Beijing 100012, China. E-mail: x.w.wang@outlook.com
First published on 6th April 2023
Abstract
Bio-slurry remediation technology, as a controllable bioremediation method, has the significant advantage of high remediation efficiency and can effectively solve the problems of high energy consumption and secondary pollution of traditional organic pollution site remediation technology. To further promote the application of this technology in the remediation of organically polluted soil, this paper summarizes the importance and advantages of bio-slurry remediation technology compared with traditional soil remediation technologies (physical, chemical, and biological). It introduces the technical infrastructure and its technological processes. Then, various factors that may affect its remediation performance are discussed. By analyzing the applications of this technology to the remediation of typical organic pollutant-(polycyclic aromatic hydrocarbons(PAHs), polychlorinated biphenyls(PCBs), total petroleum hydrocarbons(TPH), and pesticide) contaminated sites, the following key features of this remediation technology are summarised: (1) the technology has a wide range of applications and can be used in a versatile way in the remediation projects of various types of organic-contaminated soil sites such as in clay, sand, and high organic matter content soil; (2) the technology is highly controllable. Adjusting environmental parameters and operational conditions, such as nutrients, organic carbon sources (bio-stimulation), inoculants (bio-augmentation), water-to-soil ratio, etc., can control the remediation process, thus improving the restoration performance. To sum up, this bio-slurry remediation technology is an efficient, controllable and green soil remediation technology that has broad application prospects.
1 Introduction
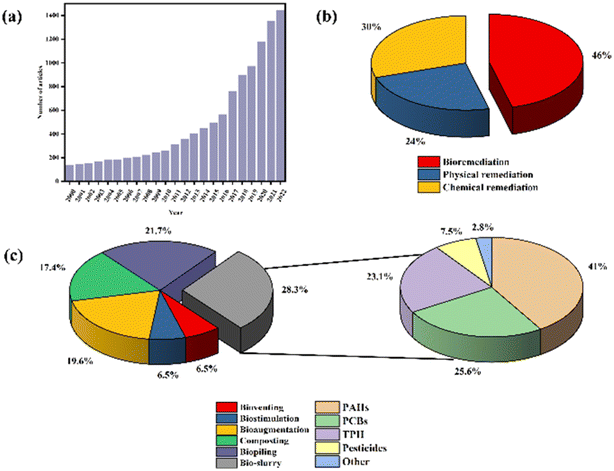
The problem of environmental pollution generated and accumulated during industrial development has become increasingly prominent.1 Organic pollutants such as polycyclic aromatic hydrocarbons (PAHs), polychlorinated biphenyls (PCBs), petroleum hydrocarbons (TPH), and pesticides are constantly being discharged from various industries such as steel, coking, petrochemical, and coal. Chemicals are released into the environment. The long-term adsorption of hydrophobic organic pollutants in soil and sediments can lead to severe soil and groundwater pollution problems.2–4 Thus the development of remediation technologies for organic pollutant-contaminated soils has become a significant global research hotspot.During the past two decades, the number of papers related to the remediation of organic-pollutant contaminated soils has been dramatically increasing worldwide (Fig. 1a). Remediation techniques for soils contaminated with organic pollutants can be classified by their natures (physical, chemical, or biological remediation) or application types (in situ, ex situ). Physical remediation technology is efficient but suffers from high cost and energy consumption.5 Chemical remediation technology requires the addition of chemicals. Although this can shorten the remediation duration and improve the efficiency, it may cause serious secondary pollution.6 The versatile bioremediation technology is different; although its remediation time is relatively long, its high selectivity, low energy consumption, and environmental friendliness make this technology an emerging method for remediation of organic-pollutant contaminated soils7 (Fig. 1b).
Bioremediation describes using microorganisms to degrade organic pollutants in soil into products that are less toxic or harmless to the environment.8 Among them, the in situ bioremediation technology is to add nutrients or foreign microorganisms to the polluted soil in situ for remediation. Still, due to the complex composition of the contaminated soil, the microorganisms are susceptible to death due to adverse environmental effects. The effect of the in situ bioremediation technology is not ideal.9 Ectopic bioremediation technology transfers contaminated soil and mixes it with microorganisms for remediation. The current ectopic bioremediation technologies mainly include bioventing, biostimulation, bioaugmentation, composting, bio-piling, and bio-slurry remediation technology10 (Fig. 1c).
Most ex situ bioremediation technologies adopt an “online remediation-offline research” model, which only considers the influence of external factors on the final degradation effect, while the self-fermentative activity of microorganisms has not been intensely studied in the field of soil remediation.11 But in many other areas, the study of the behavior and fate of the microbes themselves have matured. For example, in the medical field, Ackermann et al. induced a microbiota in a bioreactor to produce therapeutic phagocytes with the same functions as pluripotent stem cells and professional phagocytes and successfully treated acute lower respiratory tract infections in mice.12 In sewage treatment, Chen et al. successfully degraded high-concentration ammonia nitrogen wastewater using Nitrosomonas for denitrification in a sequencing batch reactor.13 In food processing, Kumar et al. used the fermentation of Penicillium viridans in agricultural waste substrates to efficiently and economically produced pullulanase by fermenting Penicillium viridicatum in agricultural waste substrates and successfully used it in the food industry.14
Bio-slurry remediation technology can consider both energy consumption and duration. Still, the limitations of this technology are that the availability of microorganisms is low, and the remediation process is difficult to intervene.15 One way to address this problem is to combine slurry remediation technology with microbial fermentation technology. The rational use of fermentation technology can provide a suitable growth environment for microorganisms, such as enhancing solid–liquid mass transfer to improve the utilization rate of microorganisms and adding water to reduce the initial.
Thus, the concentration of pollutants reduces the pressure of microbial remediation and adjusts key parameters to precisely control and optimize the microbial remediation process.16 It proves the great potential of bio-slurry remediation technology combining microbial fermentation technology and traditional slurry remediation technology in the field of soil remediation in the future.
By comparing the advantages and disadvantages of physical, chemical, traditional biological, and bio-slurry remediation technologies in the soil remediation field, this paper clarifies the advantages and necessity of bio-slurry remediation technology. Further, it introduces the infrastructure and its technological processes. Subsequently, this paper discusses the possible factors that may affect the remediation performance of bio-slurry remediation technology and conducts a more in-depth discussion on the different bio-slurry remediation technologies corresponding to different types of organic pollutants. This work proposes future directions for developing this biological slurry remediation technology and promoting its practical application in organic pollutant-contaminated soil remediation.
2 Physical, chemical, and biological remediation techniques for organic pollutant-contaminated soils
The process of researchers exploring the remediation of different types of organic pollutant-contaminated soils has led to the development of several remediation techniques, including physical remediation,17 chemical remediation,18 and bioremediation.192.1 Physical remediation
Physical remediation uses physical methods to extract organic pollutants from the soil into gas or liquid phase and separate them out.20 Physical remediation techniques mainly include thermal desorption and vapor extraction.21,222.2 Chemical remediation
Chemical remediation uses chemicals to extract organic contaminants from the soil or convert them into less harmful or toxic products.31 Chemical remediation mainly includes chemical oxidation,32 drenching,33 curing,34 and electrochemical remediation.352.3 Bioremediation
Bioremediation uses microorganisms to degrade organic contaminants in soil into non-toxic or low-toxic small molecule organic or inorganic substances.46 Bioremediation mainly includes bioventing,47 biostimulation,48 bioaugmentation,49 composting,50 bio-piling,51 bio-slurry remediation.15Volume is several hundred cubic meters, which can remediate large-scale contaminated soil, and it has already been applied under pilot scale.60 Liu et al. conducted a 42 day pilot bio-piling remediation of diesel-contaminated soil and obtained a 79% removal rate.51 The advantage of bio-piling is the efficient transfer of water, nutrients, and oxygen. Still, some vapor organic compounds may migrate into the environment if not properly controlled, and the cost of bio-piling increases compared to composting.61
| Technology | Principle | Advantages | Disadvantages | |
|---|---|---|---|---|
| Physical remediation | Thermal desorption | High temperature enhances evaporation and soil separation of organic pollutants | 1. Easy to operate. 2. Wide range of remediation targets | 1. Generate toxic exhaust gas. 2. Needs to be used in conjunction with a gas purification unit. 3. Not suitable for large-scale soil remediation |
| Vapor extraction | Extraction of volatile organic pollutants from soil into the gas phase using steam for further processing | 1. High remediation efficiency. 2. Highly adaptable. 3. Highly actionable | 1. Weakly volatile substances do not remediate well. 2. Not suitable for large-scale soil remediation | |
| Chemical remediation | Chemical oxidation | Remediation of organic pollutants by reactive radicals generated by oxidation reactions | 1. High remediation efficiency. 2. Remediation can be done in situ in the soil | 1. Chemical reactions are susceptible to interference. 2. Some chemicals are toxic to the soil. 3. Chemical agents are more expensive |
| Drenching | Organic contaminants are removed by reacting with the drench solution | High applicability to different depths of soil | 1. Requirement for soil permeability. 2. Leaching fluid composition and content are difficult to determine | |
| Electrochemical remediation | Immobilization and removal of organic contaminants using binders | More complete soil removal | Single type of binder | |
| Anion and cation migration processes change the physicochemical properties of the soil so that contaminants are adsorbed on the electrode and removed | 1. High remediation efficiency. 2. No harm to the soil itself | 1. Reactions are susceptible to pH and conductivity. 2. More expensive. 3. Difficult to apply practically | ||
| Bioremediation | Bioventing | Injection of air or pure oxygen into the unsaturated zone of the soil to stimulate microbial degradation of organic pollutants | 1. Can adjust soil temperature. 2. Do not change the physical and chemical properties of the soil itself. 3. Low cost | 1. Longer restoration time. 2. Difficult to remove high molecular weight organics |
| Biostimulation | Adding nutrients to stimulate microorganisms | 1. Promotes microorganisms growth. 2. Do not change the physical and chemical properties of the soil itself. 3. Low cost | 1. Longer remediation time. 2. Difficult to remove high molecular weight organics | |
| Bioaugmentation | Addition of exogenous microorganisms | 1. Co-degradation of organic matter with indigenous microorganisms. 2. Do not change the physical and chemical properties of the soil itself. 3. Targeted for pollutants. 4. Low cost | Exogenous microorganisms may inhibit the growth of indigenous microorganisms | |
| Composting | Addition of organic improvers to enhance microbial activity | Aeration on top of composting to enhance remediation efficiency. | 1. Longer restoration time. 2. Difficult to remove high molecular weight organics | |
| Bio-piling | Aeration on the basis of composting | Use of aeration to improve remediation efficiency | Generate additional costs | |
| Bio-slurry remediation | Soil mixed with water and stirred | 1. Promotes microorganisms growth. 2. Co-degradation of organic matter with indigenous microorganisms. 3. Use of stirring to enhance mass transfer. 4. High remediation efficiency. 5. Effective removal of high molecular weight organics. 6. Do not contaminate the surrounding environment of the remediation site | 1. Additional costs compared to other bioremediation technologies, but still economically beneficial compared to physical technologies. 2. The remediation process needs to be studied in depth. 3. Large-scale adoption still faces challenges |
Bio-slurry remediation is a fusion of various bioremediation technologies.62 Unlike composting, where contaminated soil contains only a tiny amount of water, bio-slurry remediation artificially mixes contaminated soil with water to form a slurry where the volume of water is always several times the magnitude of the soil, which not only reduces the initial concentration of contaminants to reduce microbial pressure but also enhances the transfer of organic contaminants to improve the chances of microbial contact with the contaminants.63 The bio-slurry remediation is carried out in a reactor, which avoids the release of volatile organic compounds and protects the atmosphere.2 The reactor is equipped with a stirring device, effectively saving human resources consumption.64 The researchers can also monitor or change key parameters such as pH and temperature in the contaminated soil at any time in the control system accompanying the reactor to guarantee the survival of microorganisms.65
In 1988, Bachmann et al. first used bio-slurry to remediate lindane-contaminated soil. They discussed the conditions that may impact the efficiency of remediation, which is the first study to use bio-slurry for remediation.66 In 1997, Ramaswami et al. first discovered that bio-slurries could remediate PAHs in coal tar-contaminated soils.67 In 1998, two studies by Fava et al. demonstrated the efficient remediation capacity of the bio-slurry for PCB-contaminated soil.68,69 After 2000, a large number of studies on bio-slurry remediation were generated, most of which were on PAHs,70–74 which may be related to the change in concern about pollutants. In 2017, Pino-Herrera et al. first reviewed the mass transfer processes that may occur during bio-slurry remediation.63 Until today, the bio-slurry remediation technology has been proven to be an effective tool for the remediation of various types of organic pollutant-contaminated soils.15,65,75–77
3 Overview of bio-slurry remediation technology
3.1 Bio-slurry remediation technology process flow
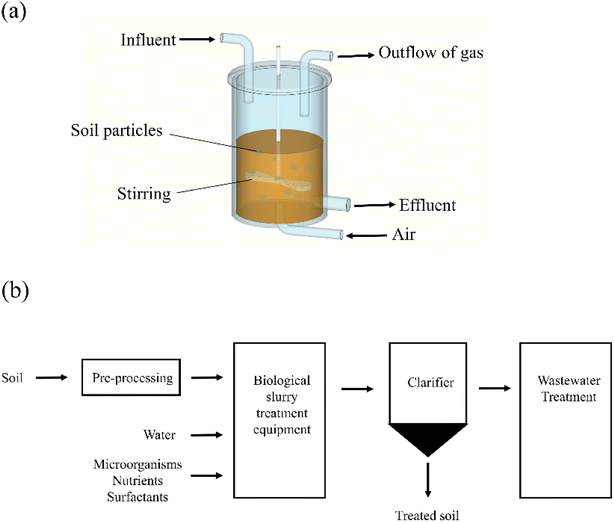
Typical bio-slurry remediation equipment and process are shown in Fig. 2. Bio-slurry remediation is an integrated biological treatment process. The process equipment usually consists of four parts: contaminated soil pretreatment and conditioning unit, bio-slurry treatment unit, power supply system, and additional equipment.15 Additional equipment includes mud sedimentation, sewage treatment equipment, and exhaust gas treatment equipment.63 Bio-slurry remediation equipment can be divided into three operating modes: batch, semi-continuous and continuous, among which the batch is the mainstream.63 Ongoing bio-slurry treatment, although feasible in principle, but not typical.78 Batch and semi-continuous processing equipment are more suitable for contaminated soil remediation.15 According to the different electron acceptors in the bioremediation process, it can also be divided into: aerobic (molecular oxygen), anoxic (nitrate and some metal cations), anaerobic (sulfate reduction, methanogenic fermentation), and mixed modes.62 Currently, anaerobic bio-slurry remediation is an emerging research area, while aerobic bio-slurry remediation dominates in practical applications.79A vital feature of the bio-slurry remediation process is the biological treatment of contaminated soil in a near-homogeneous suspension undersaturated conditions.80 After the pretreatment process of adding nutrients, inoculating bacteria, and adjusting pH, the contaminated soil enters the bio-slurry reactor.81 The bio-slurry remediation process uses mechanical or pneumatic mixing to keep the soil in suspension, and the soil-to-water ratio is usually maintained at 10–30% w/v.63
Compared with other bioremediation technologies, bio-slurry remediation technology has some unique advantages, mainly reflected in: (1) high mass transfer rate, which can significantly improve the contact efficiency of microorganisms/pollutants/nutrients;82 (2) the biodegradation rate of organic pollutants is significantly higher than in situ bioremediation; (3) multiple electron acceptors can be used: O2, SO42−, CO2, NO3−;83 (4) controllable and adjustable environmental parameters, such as temperature, pH, etc.84,85 (5) make full use of biotechnology to improve treatment capacity by inoculating exogenous bacteria, etc.72,84,86 (6) enhanced desorption and solubility of organic pollutants in soil by adding surfactants.65
However, since the process of bio-slurry remediation involves excavation and pretreatment of contaminated soil, as well as the construction and operation of slurry remediation equipment, the overall remediation cost will be higher than most other simple bioremediation techniques.87 Nonetheless, bio-slurry remediation remains more cost-effective than physicochemical remediation techniques such as incineration, leaching, and thermal desorption.88
3.2 Main influencing factors of bio-slurry remediation
Bio-slurry remediation technology has higher requirements for the soil pretreatment process. Since organic pollutants are primarily concentrated in the fine particles of the soil,83 the coarser parts of the soil (pebbles and sand, 0.85–4 mm) after the contaminated soil is crushed and screened are separated and discarded or treated separately. In contrast, the finer fractions (clay and organics, <0.85 mm) are retained and loaded into the bio-slurry reactor.63The solids concentration in the bio-slurry is a key variable that determines the power of the mixing equipment required for the bio-slurry treatment device, the efficiency of the bio-slurry aeration, and the size of the split post-treatment unit, hence together mixing the soil fines with water to form a slurry with a concentration between 15–60% w/v.63 Recycling separated water for slurry preparation is a common method to reduce subsequent wastewater treatment and disposal.89
Mixing strength is another key factor in the design of the bio-slurry remediation processes, which directly affects the degradation performance of organic matter.81 Continuous mixing during remediation not only maintains the homogeneity of the slurry during the remediation process but also enhances turbulence and improves mass transfer rates.75 Appropriate mixing strength can keep solid particle suspension and slurry homogeneity. Especially for hydrophobic organic pollutants such as PAHs and PCBs, adequate aeration of the aerobic slurry bioremediation process can significantly improve the biodegradation rate.81 There are various types of mixing devices, and the aerobic bio-slurry remediation device is mainly mechanical mixing and pneumatic mixing device.90 Hybrid modes include batch and continuous. Although batch mixing is less intensive, it can significantly save electricity consumption. The choice of mixing power is based on various considerations, such as contaminants' remediation requirements and operating costs. The higher the slurry density, the higher the required equipment power and the more difficult it is to achieve oxygen transfer.85 Therefore, the small-scale and pilot-scale experiments can be used to determine the optimal slurry concentration and mixing power in the early stage to control equipment procurement and operating costs.84
In addition, immediate adjustment of the external environmental conditions during the bio-slurry remediation process can significantly increase the biodegradation efficiency of pollutants.65 Typically, the bio-slurry remediation process is controlled by real-time monitoring of the pH, dissolved oxygen concentration, and inorganic nutrient concentration of the slurry.84 Standard operation parameters are as follows: using alkali (e.g., NaOH) or acid (e.g., H2SO4) to adjust the pH of the slurry and maintain it between 6.75 to 7.25, the slurry temperature is controlled within the range of 25–30 °C, the dissolved oxygen concentration is 90% of the saturated dissolved oxygen concentration, and using nitrogen and phosphorus salts (NH4Cl, KH2PO4, Na2HPO4) as inorganic nutrient sources to ensure no microbial nutrient limitations.85
4 Application case study of bio-slurry remediation technology
Limited by the high comprehensive cost of large-scale bio-slurry treatment equipment and the complexity of microbial activities during operation, most relevant remediation studies are at the laboratory pilot or pilot level. Bio-slurry remediation is mainly for PAHs, PCBs, TPH, and pesticide-contaminated soils.4.1 Remediation of PAHs contaminated soil
PAHs are a class of common organic pollutants produced in large quantities during the combustion of fossil fuels and the production of chemicals in industrial production. PAHs are carcinogenic, teratogenic, and mutagenic, posing a severe threat to human health and the ecological environment.91 PAHs are easily deposited in the soil, and their hydrophobicity limits their bioavailability in the natural environment, making them difficult to treat by common bioremediation techniques.76 Therefore, there is an urgent need to develop effective in situ or ex situ remediation technologies to achieve the degradation and remediation of PAHs in soils. Bio-slurry remediation technology provides an economical and environmentally friendly strategy for the remediation of PAHs contaminated soils (Table 2).| Contaminant | Soil (w/v) | Operational conditions | Removal efficiency | Ref. |
|---|---|---|---|---|
| N.R: not reported; LSLR: low substrate loading ratio; HSLR: high substrate loading ratio. | ||||
| Pyrene | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Equipment volume: 5 L, stirring speed: 200 rpm, running time: 42 days | 99% | 70 |
| Pyrene | N.R | Temperature: 28 ± 2 °C, substrate loading ratio: 0.12, 0.24, 0.36 g pyrene per kg soil-day, running time: 120 h | Sterile: 6%, native bacteria: 34%, LSLR: 90%, HSLR: 50% | 73 |
| Phenanthrene | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Equipment volume: 1 L, pollutant concentration: 100 mg kg−1, running time: 60 days | Sterile: 5%, native bacteria: 17%, Pseudomonas: 87.8%, Pseudomonas aeruginosa: 85.5%, Pseudomonas and Pseudomonas aeruginosa: 92.8% | 86 |
| Pyrene, benzanthracene | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Aeration volume: 60 L h−1, temperature: 20–25 °C, running time: 34 days | Fusarium: 90% pyrene, 33.3% benzanthracene, Dictyostelium: 81.5% pyrene, 49.2% benzanthracene | 74 |
| 2–6 ring PAHs | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Temperature: 28 °C | 2–3 ring PAHs: 80%, 4–6 ring PAHs: 70% | 72 |
| Phenanthrene | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Aerobic run: 60 days, anaerobic operation: 30 days | Aerobic: 95%, anaerobic: 95% | 92 |
| Naphthalene | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Reactor volume: 1 L, running time: 6 h | Pseudomonas putida M8: 90% | 93 |
| Fluoranthracene, phenanthrene | 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
N.R | Fluoranthracene: 56.94%, phenanthrene: 63.16% | 94 |
| N.R | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Running time: 210 days | Test: 80.5% pilot test: 93.4% | 64 |
| Naphthalene | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Running time: 45 days | 86.5% | 65 |
| Naphthalene | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Running time: 49 days | 99.84% | 75 |
| Phenanthrene, pyrene, benzo[a]anthracene | N.R | Running time: 7 days | Phenanthrene: 63%, pyrene: 92%, benzo[a]anthracene: 94% | 95 |
In the bio-slurry system, the microbial community exists in both dissolved and attached states, and the dissolved microorganisms are involved in the biodegradation of PAHs. In contrast, most attached strains do not appear involved in the biodegradation of PAHs.70 Fig. 3 shows the change in pyrene concentration in pyrene-contaminated soil during a 42 day bio-slurry remediation process. In this process, the systemic lysate flora: Mycobacterium spp., Pseudomonas spp., Rhodobacter spp., and Burkholderia spp. dominated the biodegradation of pyrene at a rate of 0.0696 mg per day.
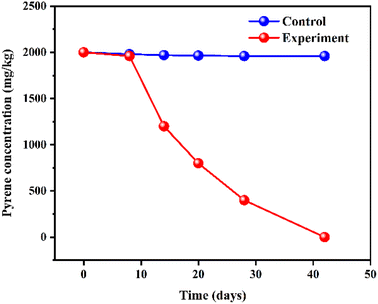 | ||
| Fig. 3 Degradation of pyrene during bio-slurry remediation.70 Reproduced from ref. 70 with permission from MDPI, copyright [2019]. | ||
Yu and coworkers used gas chromatography-mass spectrometry (GC-MS) to study the microbial degradation pathway of pyrene during this bio-slurry remediation process, as shown in Fig. 4.
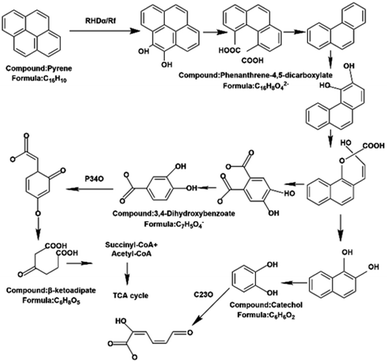 | ||
| Fig. 4 PYR biodegradation pathway in bio-slurry reactors. RHDα: PYR dioxygenase is responsible for transferring electrons to catalyze PYR degradation. Rf: larger dioxygenase indicator unit at the coding end of the Rf gene. C23O: catalyzes endocytic cleavage of catechol. P34O: not detected.70 Reproduced from ref. 70 with permission from MDPI, copyright [2019]. | ||
The biodegradation of pyrene is through pyrene dioxygenase attacking the benzene ring to form cis-4,5-dihydroxypyrene. It is metabolized to catechol via the phenanthrene-4,5-dicarboxylate, phenanthrene, cis-1,2-dihydroxyphenanthrene, and cis-1,2-dihydroxynaphthalene pathways, which are further metabolized by endocytosis. Another possible degradation pathway is that cis-1,2-dihydroxyphenanthrene enters the TCA cycle via the 3,4-dihydroxybenzoate, β-adipate pathway.
The degradation efficiency of PAHs in soil depends on the initial concentration of PAHs in the bio-slurry and the inoculation of bacteria species. The researchers set up six parallel bio-slurry treatment equipment (reactors A, B, C, D, E, and F) to remediate pyrene-contaminated soil through bio-slurry technology (Fig. 5). Sterilized reactor A showed a 6% pyrene degradation rate due to the natural volatilization of pyrene under agitation. Reactor B, which was not inoculated with exotic strains, showed a pyrene-degradation rate of 34% due to the presence of native microbial strains in the pyrene-contaminated soil. The pyrene degradation rate in reactor C, inoculated with exotic strains, was increased by nearly 90% compared with reactor B, which proved the effectiveness of exogenous pressure inoculation (bio-augmentation).
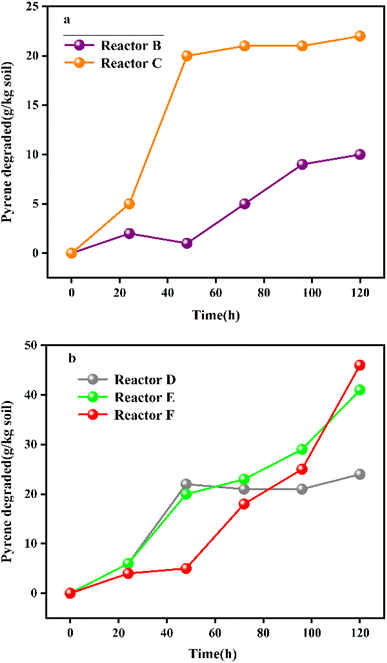 | ||
| Fig. 5 (a) Degradation of pyrene in reactors B and C; reproduced from ref. 96 with permission from Elsevier, copyright [2008]. (b) Degradation of pyrene in reactors D, E, and F.96 Reproduced from ref. 96 with permission from Elsevier, copyright [2008]. | ||
Similarly, the initial concentration of pyrene had a significant effect on its degradation rate, and the initial concentrations of pyrene in reactors D, E, and F were 0.12 pyrene per kg soil-day, 0.24 pyrene per kg soil-day, and 0.36 pyrene per kg soil-day, respectively. The degradation rate was slow in the early stage (24 hours) and gradually increased in the later stage, and the final degradation rate was stable at about 51%. This indicated that the initial concentration of pyrene in the contaminated soil directly affected the treatment effect of bio-slurry remediation and clarified the linear correlation between the efficiency of pyrene degradation and the number of colonies.96
Therefore, for PAH-contaminated soil with high pollutant concentration and long contamination time, the injection of exogenous strains of bacteria can significantly improve the degradation efficiency of PAHs by bio-slurry remediation technology. Nasseri et al. added Pseudomonas aeruginosa, Pseudomonas aeruginosa, and complex bacteria into phenanthrene-contaminated soils to investigate the biodegradation mechanism of phenanthrene (100 mg kg−1) in soil.86 As shown in Fig. 6, the concentration of phenanthrene decreased by only 5% in the control group (B1, sterilized condition); in the experiment group without exogenous bacterial strains injected (B2), the biodegradation rate of phenanthrene by endogenous microorganisms was about 17% (B2); and in bio-augmentation experiments with the addition of Pseudomonas aeruginosa and Pseudomonas spp. (S3), the degradation efficiencies of phenanthrene were 87.8%, 85.5%, and 92.8%. The experimental results indicated that targeted injection of exogenous strains could significantly improve the effect of bio-slurry remediation. Jiang and colleagues added a bacterial strain named MZJ_21 to phenanthrene-contaminated soil and compared its treatment efficiency with sterilized soil. Only 8.6% of phenanthrene was removed from the sterilized soil after 48 hours. In natural soil, the degradation rate of phenanthrene is already 54.38%. In contrast, the degradation rate of phenanthrene in the bio-slurry reactor with MZJ_21 was as high as 95.41%, which was 1.75 times higher than that without the addition of MZJ_21, which once again proved the effectiveness of bio-augmentation (injection of exogenous bacterial strains) on bio-slurry remediation.78
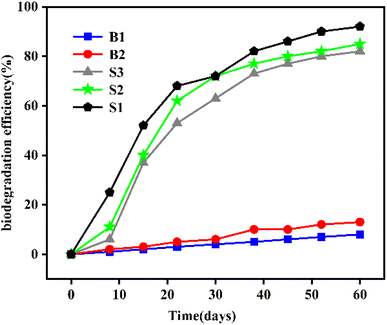 | ||
| Fig. 6 Phenanthrene (PHE) degradation profile during the treatment of bioaugmented and non-bioaugmented bio-slurry remediation.86 Reproduced from ref. 86 with permission from Springer, copyright [2010]. | ||
The physical and chemical properties of PAHs are also a key factor affecting bio-slurry remediation technology's degradation effect. PAHs with fewer benzene rings and smaller molecular weights are easier to biodegrade.72 As shown in Fig. 7, the PAH concentrations showed a significant downward trend after 15 days of bio-slurry remediation, among which the bicyclic and tricyclic PAHs decreased by more than 80%, respectively, while the four to six-cyclic PAHs decreased by about 70%.
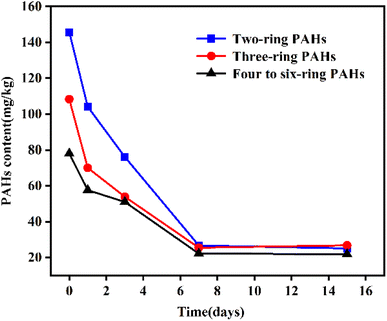 | ||
| Fig. 7 Degradation of two to six-ring PAHs during the bio-slurry remediation.72 Reproduced from ref. 72 with permission from MDPI, copyright [2020]. | ||
Process operating parameters such as bio-slurry temperature, water-to-soil ratio, and mixing strength are also essential factors in the bio-slurry remediation of PAHs contaminated soil.74 Gong et al. monitored the effects of parameters such as temperature, water–soil ratio, and aeration volume on the remediation effect during the pilot-scale bio-slurry treatment. The best remediation effect was achieved when the process conditions were a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 water–soil ratio, 20–25 °C temperature, and 60 L h−1 aeration rate. Using purified and complex cultured fungi from the contaminated soil as the degradation flora, after 34 days of bioremediation, 90% of pyrene and 33.3% of benzanthracene were degraded by Fusarium; Mucor degraded 81.5% of pyrene and 49.2% of benzanthracene, and Penicillin degraded 52% of pyrene and 46% of benzanthracene.
1 water–soil ratio, 20–25 °C temperature, and 60 L h−1 aeration rate. Using purified and complex cultured fungi from the contaminated soil as the degradation flora, after 34 days of bioremediation, 90% of pyrene and 33.3% of benzanthracene were degraded by Fusarium; Mucor degraded 81.5% of pyrene and 49.2% of benzanthracene, and Penicillin degraded 52% of pyrene and 46% of benzanthracene.
4.2 Remediation of PCBs contaminated soil
PCBs are a group of persistent organic pollutants widely distributed in soil and sediment.97 Traditional remediation techniques such as extraction, landfill, and thermal desorption are ineffective in separating and removing PCBs from the soil due to their strong adsorption to soil particles.98 Bio-slurry remediation utilizes a soil/water system to enhance the desorption of PCB from the soil by controlling environmental and process conditions, ultimately separating and removing PCBs from contaminated soil with lower viscosity.99 Bio-slurry degradation of PCBs usually results from a combination of exogenous biphenyl-like substrates and aerobic microorganisms, where PCBs are microbially degraded to chlorobenzoic acid, which is mineralized to complete degradation.68Cyclodextrins can not only enhance the desorption of PCBs from soil particles in bio-slurry, but also act as an additional carbon source to promote the colonization of aerobic microbial and enhance the bio-slurry remediation effect. Fava et al. used an aerobic bio-slurry method to remediate high-concentration PCB-contaminated landfill soil by adding inorganic nutrients and exogenous biphenyl-like organic matter as auxiliary substrates to cultivate native aerobic bacteria in the contaminated soil (Fava et al., 1998). 10 g L−1 of hydroxypropyl-β-cyclodextrin and γ-cyclodextrin were added to the slurry system on day 39 and day 100 during remediation. By monitoring the changes of intermediates during the remediation process, such as the concentration of the produced metabolite chlorobenzoic acid, and the concentration of chloride ions in the degradation system, it was found that cyclodextrins significantly enhanced the biodegradation process of PCBs in soil. Furthermore, both added cyclodextrins were confirmed to be utilized by microorganisms as carbon sources during the remediation process.
The above study investigated the bio-slurry remediation effect of two other surfactants, Triton X-100 and Quillaya Saponin, on PCBs-contaminated soils. The concentration of PCBs in contaminated soil was about 350 mg kg−1, and the above process was used for bioremediation. 10 g L−1 Triton X-100 and Quillaya Saponin were added to the treatment device on day 43 and day 100, respectively. The results showed that Triton X-100 negatively affected the bioremediation process of PCBs in contaminated soil by inhibiting the natural bacteria that degrade chlorobenzoic acid without being metabolically utilized by the microbial community. In contrast, Quillaya Saponin slightly facilitated the biodegradation and dechlorination rate of PCBs and was readily available for microbial degradation.69
4.3 Remediation of total petroleum hydrocarbons (TPH) contaminated soil
TPH is also a type of widespread soil contaminant. Due to their natural occurrence in the soil environment, hundreds of microorganisms in the soil have evolved the ability to use TPH as a carbon source.100 Therefore, bio-slurry remediation technology has become one of the popular remediation techniques for treating TPH-contaminated soils (Table 3).101| Concentration | Soil (w/v) | Operational conditions | Removal efficiency | Ref. |
|---|---|---|---|---|
| N.R: not reported. | ||||
67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 mg kg−1 500 mg kg−1 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Equipment volume: 6 L, reaction temperature: 21 °C, stirring speed: 400 rpm, running time: 90 days | 95% | 89 |
| 888.57 mg kg−1 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Equipment volume: 4 L, running time: 75 days | 99% | 102 |
| 200 g kg−1 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Equipment volume: 3 L, running time: 60 days | 88% | 103 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mg kg−1 000 mg kg−1 |
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Equipment volume: 1 L, running time: 9 days | 94% | 85 |
| 2243 mg kg−1 | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Equipment volume: 2 L, running time: 28 days | Test: 57%, pilot test: 65% | 104 |
| 2500 mg kg−1 | N.R | Equipment volume: 5 L, running time: 6 days | 94% | 71 |
13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mg kg−1 000 mg kg−1 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Equipment volume: 24 L, running time: 105 days | 40% | 15 |
The positive effect of carbon source addition during bio-slurry remediation on the treatment performance of TPH-contaminated soil.102 Two sets of parallel bio-slurry remediation experiments were conducted to study the effect of carbon sources on the degradation of TPH in marine sediments. The TPH content in the marine sediment was 888.57 mg kg−1, as shown in Fig. 8. The bio-slurry remediation process was divided into three stages, which lasted about 75 days in total. In the third stage, researchers changed the salinity of the bio-slurry system to simulate the slurry composition from seawater instead of clean water. The concentration of TPH in the sediment of both sets of experiments increased to a certain extent, which may be due to the microorganisms. The flocs produced by microorganisms or extracellular polymers on biofilm increased the adsorption of TPH in water by sediment particles, leading to an increase in the concentration of TPH in the sediment.
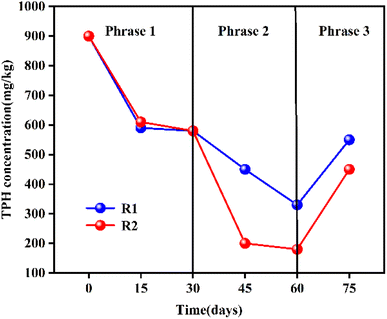 | ||
| Fig. 8 Degradation of total petroleum hydrocarbons (TPH) in the bio-slurry reactor.102 Reproduced from ref. 102 with permission from Italian Association of Chemical Engineering – AIDIC, copyright [2020]. | ||
Adding exogenous strains of bacteria to the anaerobic bio-slurry remediation process can also significantly improve the efficiency of bio-slurry remediation.103 In the process of anaerobic bio-slurry remediation of petroleum hydrocarbon-contaminated soil (maximum total petroleum hydrocarbon concentration of 200 g kg−1, the average composition of 64% aliphatic compounds, 25% aromatic compounds, 8% heterocyclic compounds, and 3% tarry asphaltic substances) from high-concentration oily waste storage sites, three parallel groups of bio-slurry remediation experiments were carried out, namely C1 (TPH-contaminated soil concentration of 46.1 g kg−1 without biofertilizer), C2 (TPH soil concentration of 46.0 g kg−1 with biofertilizer), and C3 (TPH contaminated soil concentration of 14.0 g kg−1 with biofertilizer). The effects of different initial TPH concentrations were also investigated. The changes in soil TPH concentrations after ten weeks are shown in Fig. 9. C1 and C2 curves indicated that the biofertilizer application could significantly improve the bio-slurry remediation performance. Compared with C3, the initial remediation rate of C2 decreased slightly, which might be due to the toxic effect of a high concentration of TPH-contaminated soil in C2, which inhibited the growth and reproduction of microbial, resulting in a lower remediation rate.
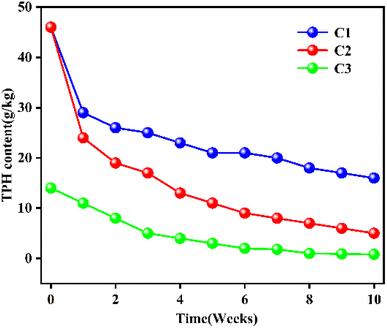 | ||
| Fig. 9 Effects of adding exogenous bacteria to the TPH residual concentration of the TPH-contaminated soil C1, C2, and C3. C1-no added exogenous bacteria; C2, C3- adding exogenous bacteria.103 Reproduced from ref. 103 with permission from Taylor and Francis Ltd, copyright [2003]. | ||
Bio-stimulation and bio-augmentation can effectively promote the degradation of TPH in soil and sediment. In the four sets of bio-slurry reactors operated in parallel, A (addition of water and contaminated soil only), B (applying a carbon source, with N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P ratio of 4
P ratio of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), C (bio-stimulation: salt-tolerant biomass input), and D (bio-stimulation: salt-tolerant bacterial consortium input), the native microorganisms in the reactor A native microorganisms had a lower degradation rate due to initial inhibition of the transition from anaerobic to aerobic environment. The degradation rate in reactor B was higher than that in reactor A, which demonstrated the effectiveness of bio-stimulation. In the bio-augmentation groups, the degradation rate in reactor C was lower than that in reactor D due to the competing effect of each salt-tolerant bacteria in reactors (Fig. 10).
1), C (bio-stimulation: salt-tolerant biomass input), and D (bio-stimulation: salt-tolerant bacterial consortium input), the native microorganisms in the reactor A native microorganisms had a lower degradation rate due to initial inhibition of the transition from anaerobic to aerobic environment. The degradation rate in reactor B was higher than that in reactor A, which demonstrated the effectiveness of bio-stimulation. In the bio-augmentation groups, the degradation rate in reactor C was lower than that in reactor D due to the competing effect of each salt-tolerant bacteria in reactors (Fig. 10).
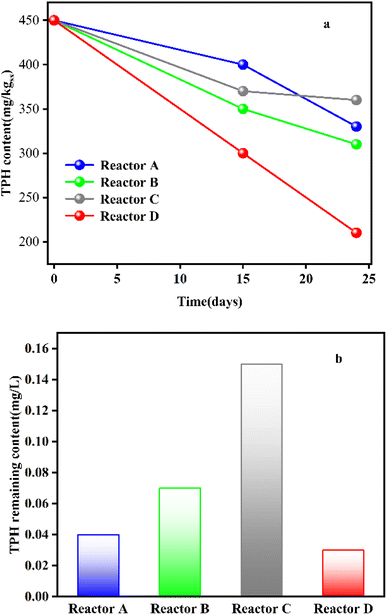 | ||
| Fig. 10 (a) TPH content in reactors A, B, C, D; (b) TPH remaining content in reactors A, B, C, D.15.Reproduced from ref. 15 with permission from Elsevier, copyright [2022]. | ||
Long-term continuous disposal of petroleum hydrocarbon-contaminated soils can be achieved by the semi-continuous operation.89 They used an 8 L bio-slurry remediation device operating at 10% solids concentration (0.1 kg dry soil per kg slurry). During operation, 10% of the slurry was withdrawn from the bio-slurry and replaced with untreated slurry every two weeks, and operated in this manner for 90 days, resulting in 95% of the TPH being degraded.
4.4 Remediation of pesticide-contaminated soil
Due to the long-term accumulation of pesticides in soils and their difficult degradation characteristics, the production and use of pesticides such as fungicides, insecticides, and herbicides have caused serious site pollution problems.105 Since it is difficult to remove the pesticides via traditional physicochemical remediation techniques,106 bio-slurry remediation method has become one of the popular technologies for pesticide-contaminated soil remediation (Table 4).73| Contaminant | Soil (w/v) | Operational conditions | Removal efficiency | Ref. |
|---|---|---|---|---|
| N.R: not reported. | ||||
| Hexachlorocyclohexane | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Aerobic equipment volume: 14 L, anaerobic equipment volume: 3 L, running time: 10 days | Aerobic: 50%, anaerobic: 70% | 83 |
| 2,4-Dichlorophenoxyacetic acid, 4-chloro-2-methylphenoxyacetic acid, alachlor, melamine carbosulfan | N.R | Running time: 14 days | 90% | 107 |
| Hexachlorocyclohexane | N.R | Running time: 42 days | 90–100% | 66 |
| g-Hexachlorocyclohexane, a-hexachlorocyclohexane, d-hexachlorocyclohexane | N.R | Equipment volume: 5 L, running time: 30 days | g-Hexachlorocyclohexane: 94.5%, a-hexachlorocyclohexane: 78.5%, d-hexachlorocyclohexane: 66.1% | 108 |
| Pendimethalin | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5–1 5–1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 25 |
Equipment volume: 0.5 L, running time: 5 days | N.R | 109 |
| S-ethyl dipropylthiocarbamate | N.R | N.R | Biodegradation rate: 2.5 L min−1 | 110 |
Aerobic bio-slurry remediation can effectively treat soils contaminated with pesticide.107 After two weeks of treatment, the total pesticide concentration in the contaminated soil (pollutant: the mixture of 2,4-dichlorophenoxyacetic acid (2,4-D), 4-chloro-2-methylphenoxyacetic acid, alachlor, melamine and carbofuran) was reduced from 800 mg kg−1 to 20 mg kg−1. At the same time, temperature also plays a significant role in the bioremediation process of some pesticides.66 In the bio-slurry in the temperature range of 20–30 °C, the degradation rate of HCH in contaminated soil could reach 90–100% after 42 days of degradation. However, HCH did not degrade when the slurry temperature was below 4 °C or above 40 °C.
Different water-soil ratios during remediation can also significantly affect the bio-slurry remediation efficiency of some pesticides.109 Fig. 11 shows six parallel groups of bio-slurry remediation experiments were carried out to remediate dimethoate-contaminated soil (RSW1-RSW6), with the water-soil ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, 1
5, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, 1
7, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, 1
10, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15, 1
15, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 and 1
20 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25, respectively. After 120 hours of treatment, the highest bio-slurry remediation rate of dimethoate-contaminated soil was 95.35% in the reactor with a water–soil ratio of 1
25, respectively. After 120 hours of treatment, the highest bio-slurry remediation rate of dimethoate-contaminated soil was 95.35% in the reactor with a water–soil ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20. The water-soil ratio directly affected the desorption rate of dimethoate from the soil matrix to the liquid phase during the slurry remediation process. The highest desorption rate of dimethoate and the best remediation performance of pesticide-contaminated soil were achieved.
20. The water-soil ratio directly affected the desorption rate of dimethoate from the soil matrix to the liquid phase during the slurry remediation process. The highest desorption rate of dimethoate and the best remediation performance of pesticide-contaminated soil were achieved.
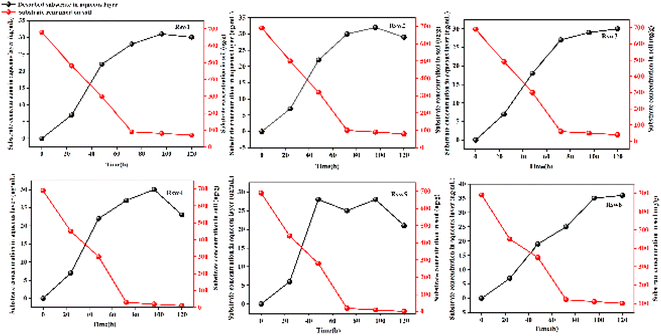 | ||
| Fig. 11 Degradation of pendimethalin and its solid–liquid desorption.109 Reproduced from ref. 109 with permission from Elsevier, copyright [2007]. | ||
5 Conclusion and outlook
As an efficient, controllable, economical, green, and low-carbon soil remediation technology, bio-slurry remediation technology can be used for the bioremediation of various textures of polluted soils such as clay, sandy soils and soils, and high organic matter content soils. The bio-slurry remediation process and efficiency can be adjusted by controlling the environmental parameters and operation conditions, such as: reasonably regulating the nitrogen, phosphorus, organic carbon source (bio-stimulation), inoculant (bio-enhancement), water-soil ratio, temperature, etc. Given the current development trend and requirements of the soil remediation industry, bio-slurry remediation not only has the remediation efficiency of conventional physical and chemical remediation technologies but also has the characteristics of cost-and environmental friendliness. It is a soil remediation technology with excellent promotion potential.Although bio-slurry remediation technology has made certain progress, there are still some areas that need to be improved in future development: (1) most of the domestic and international research focuses on the entry of microorganisms into bio-slurry treatment equipment or the influence of external environmental parameters on the final degradation rate of organic pollutants. However, this typical “black-box” research mode ignores the activity mechanism of microbial communities inside the bio-slurry treatment equipment. With the development of molecular biology toolkits, it is now feasible to monitor, regulate, and promote the activity of microbial communities inside the slurry by utilizing state-of-art molecular biology tools during bioremediation, to improve the bioremediation ability of target organic matters. (2) The research process of bio-slurry remediation technology primarily focuses on the effect of bio-slurry remediation. There is a lack of horizontal comparison with related physical and chemical remediation technologies, and demonstrations of the remediation effect and discussions of its advantages are not convincing. Therefore, the future research direction needs to pay more attention to horizontal technology comparison in the laboratory-test stage to demonstrate and highlight the technical advantages of bio-slurry remediation technology. (3) Most of the current research on bio-slurry remediation technology is limited to the laboratory trial stage, which is not conducive to the promotion and development of the technology. With the changes in technical concepts and remediation needs in the actual remediation projects, the bio-slurry remediation technology requires expansion of the experimental scales. It needs to shift the research focus to the pilot scale and field trials to promote the practical application of this technology. (4) Although there is a lack of systematic research on the secondary use of soil after bio-slurry remediation, from the perspective of other bioremediation technologies, the physicochemical properties of remediated soils will not be affected by the bioremediation process, so we have reason to believe that bio-slurry remediated soil can better maintain the organic matter content in the soil such as humic acid, which can be subsequently used for agricultural activities such as horticulture, tillage, planting, etc. Therefore, bio-slurry remediation is a technology to improve the value of recycling and the economical use of contaminated soil. (5) In addition, for bio-slurry remediation of complex contaminated soils, there is a risk that heavy metals may be adsorbed rather than removed, resulting in their constant presence in the slurry system. Researchers need to pay close attention to the subsequent treatment of the slurry to prevent the risk of leaking heavy metals into the soil.
Author contributions
Jing Sun conceived the review and collected the literature. All authors were involved in writing and revising the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Key R&D Program of China (No. 2020YFC1807903).References
- Z. Qin, J. Hazard. Mater., 2022, 11 Search PubMed.
- W. Zeng, J. Qiu, D. Wang, Z. Wu and L. He, Sci. Total Environ., 2022, 810, 151294 CrossRef CAS PubMed.
- L. Fei, M. Bilal, S. A. Qamar, H. M. Imran, A. Riasat, M. Jahangeer, M. Ghafoor, N. Ali and H. M. N. Iqbal, Environ. Res., 2022, 211, 113060 CrossRef CAS PubMed.
- T. G. Ambaye, A. Chebbi, F. Formicola, S. Prasad, F. H. Gomez, A. Franzetti and M. Vaccari, Chemosphere, 2022, 293, 133572 CrossRef CAS PubMed.
- D. Cudjoe and P. M. Acquah, Chemosphere, 2021, 265, 129186 CrossRef PubMed.
- P. O. Oladoye, O. M. Olowe and M. D. Asemoloye, Chemosphere, 2022, 288, 132555 CrossRef CAS PubMed.
- C. Zhang, D. Wu and H. Ren, Sci. Rep., 2020, 10, 9188 CrossRef CAS PubMed.
- L. Wang, X. Zhang, C. Tang, P. Li, R. Zhu, J. Sun, Y. Zhang, H. Cui, J. Ma, X. Song, W. Zhang, X. Gao, X. Luo, L. You, Y. Chen and Z. Dai, Nat. Commun., 2022, 13, 3879 CrossRef CAS PubMed.
- J. A. Sáez, M. D. Pérez-Murcia, A. Vico, M. R. Martínez-Gallardo, F. J. Andreu-Rodríguez, M. J. López, M. A. Bustamante, J. C. Sanchez-Hernandez, J. Moreno and R. Moral, J. Hazard. Mater., 2021, 402, 123481 CrossRef PubMed.
- J. D. Aparicio, E. E. Raimondo, J. M. Saez, S. B. Costa-Gutierrez, A. Álvarez, C. S. Benimeli and M. A. Polti, J. Environ. Chem. Eng., 2022, 10, 107141 CrossRef CAS.
- M. Jain, S. A. Khan, K. Sharma, P. R. Jadhao, K. K. Pant, Z. M. Ziora and M. A. T. Blaskovich, Bioresour. Technol., 2022, 344, 126305 CrossRef CAS PubMed.
- M. Ackermann, H. Kempf, M. Hetzel, C. Hesse, A. R. Hashtchin, K. Brinkert, J. W. Schott, K. Haake, M. P. Kühnel, S. Glage, C. Figueiredo, D. Jonigk, K. Sewald, A. Schambach, S. Wronski, T. Moritz, U. Martin, R. Zweigerdt, A. Munder and N. Lachmann, Nat. Commun., 2018, 9, 5088 CrossRef PubMed.
- W. Chen, X. Dai, D. Cao, S. Wang, X. Hu, W. Liu and D. Yang, Sci. Rep., 2016, 6, 35693 CrossRef CAS PubMed.
- V. Kumar, B. Naik, M. Choudhary, A. Kumar and N. Khanduri, Sci. Rep., 2022, 12, 12661 CrossRef CAS PubMed.
- A. Avona, M. Capodici, D. Di Trapani, M. G. Giustra, P. Greco Lucchina, L. Lumia, G. Di Bella and G. Viviani, Sci. Total Environ., 2022, 806, 150708 CrossRef CAS PubMed.
- J. Luo, Y. Li, H. Li, Y. Li, L. Lin, Y. Li, W. Huang, J. Cao and Y. Wu, Bioresour. Technol., 2022, 344, 126318 CrossRef CAS PubMed.
- H. Guo, Y. Wang, L. Liao, Z. Li, S. Pan, C. Puyang, Y. Su, Y. Zhang, T. Wang, J. Ren and J. Li, Chem. Eng. J., 2022, 436, 135239 CrossRef CAS.
- S. Bolan, L. P. Padhye, C. N. Mulligan, E. R. Alonso, R. Saint-Fort, T. Jasemizad, C. Wang, T. Zhang, J. Rinklebe, H. Wang, K. H. M. Siddique, M. B. Kirkham and N. Bolan, J. Hazard. Mater., 2023, 443, 130189 CrossRef CAS PubMed.
- M. A. Tufail, J. Iltaf, T. Zaheer, L. Tariq, M. B. Amir, R. Fatima, A. Asbat, T. Kabeer, M. Fahad, H. Naeem, U. Shoukat, H. Noor, M. Awais, W. Umar and M. Ayyub, Sci. Total Environ., 2022, 850, 157961 CrossRef CAS PubMed.
- Y. Chen, D. Liu, J. Ma, B. Jin, J. Peng and X. He, Sci. Total Environ., 2021, 781, 146773 CrossRef CAS PubMed.
- B. Wu, S. Guo, M. Zhang, C. Chen and Y. Zhang, Environ. Pollut., 2022, 310, 119905 CrossRef CAS PubMed.
- J. Miller de Melo Henrique, J. Isidro, C. Sáez, R. López-Vizcaíno, A. Yustres, V. Navarro, E. V. Dos Santos and M. A. Rodrigo, Chemosphere, 2022, 296, 134052 CrossRef CAS PubMed.
- S. Wang, F. Cheng, Z. Shao, B. Wu and S. Guo, Sci. Total Environ., 2023, 857, 159405 CrossRef CAS PubMed.
- R. Yue, X. Zhang, Y. Zhong, Z. Chen, Y. Zhao, D. Wang, Z. Wang and X. Mao, J. Hazard. Mater., 2022, 423, 126937 CrossRef CAS PubMed.
- L. Zhan, Z. Xia and Z. Xu, Environ. Res., 2022, 211, 113101 CrossRef CAS PubMed.
- G. Hu, H. Liu, C. Chen, H. Hou, J. Li, K. Hewage and R. Sadiq, J. Hazard. Mater., 2021, 410, 124570 CrossRef CAS PubMed.
- C. Liang and S.-Y. Yang, Chemosphere, 2021, 285, 131471 CrossRef CAS PubMed.
- M. M. Al-Hadhrami, A. S. Alkindi and A. H. Muggeridge, Fuel, 2014, 135, 413–426 CrossRef CAS.
- Y. Yu, L. Liu, C. Yang, W. Kang, Z. Yan, Y. Zhu, J. Wang and H. Zhang, Chemosphere, 2019, 236, 124319 CrossRef CAS PubMed.
- C. Qin, Y. Zhao, W. Zheng and Y. Li, J. Hazard. Mater., 2010, 176, 294–299 CrossRef CAS PubMed.
- K.-H. Wei, J. Ma, B.-D. Xi, M.-D. Yu, J. Cui, B.-L. Chen, Y. Li, Q.-B. Gu and X.-S. He, J. Hazard. Mater., 2022, 432, 128738 CrossRef CAS PubMed.
- C. Li, L. He, X. Yao and Z. Yao, Chemosphere, 2022, 295, 133868 CrossRef CAS PubMed.
- E. E. Woodward, M. L. Hladik, A. R. Main, M. Cahn, J. L. Orlando and J. Teerlink, Environ. Pollut., 2022, 315, 120325 CrossRef CAS PubMed.
- F. Wang, Z. Shen, R. Liu, Y. Zhang, J. Xu and A. Al-Tabbaa, Chemosphere, 2020, 239, 124738 CrossRef CAS PubMed.
- J. Liu, Y. Yang, Q. Zheng, X. Su, J. Liu and Z. Zhou, Sci. Total Environ., 2022, 834, 154991 CrossRef CAS PubMed.
- Y.-C. Chang, Y.-P. Peng, K.-F. Chen, T.-Y. Chen and C.-T. Tang, Process Saf. Environ. Prot., 2022, 163, 105–115 CrossRef CAS.
- V. M. A. Magalhães, G. P. Mendes, J. D. B. Costa-Filho, R. Cohen, C. S. M. Partiti, M. M. G. R. Vianna and O. Chiavone-Filho, J. Environ. Chem. Eng., 2020, 8, 103568 CrossRef.
- M. Yurderi, A. Bulut, İ. E. Ertas, M. Zahmakiran and M. Kaya, Appl. Catal., B, 2015, 165, 169–175 CrossRef CAS.
- X. Xin, J. Nepal, A. L. Wright, X. Yang and Z. He, J. Cleaner Prod., 2022, 363, 132630 CrossRef CAS.
- E. Agathokleous, E. Paoletti, C. J. Saitanis, W. J. Manning, T. Sugai and T. Koike, Sci. Total Environ., 2016, 573, 1053–1062 CrossRef CAS PubMed.
- N. Tabassum, T. Hashino, B. N. Phan, K. Hayano and H. Yamauchi, Soils Found., 2022, 62, 101183 CrossRef.
- S. Chaiyaput, N. Arwaedo, N. Kingnoi, T. Nghia-Nguyen and J. Ayawanna, Case Stud. Constr. Mater., 2022, 16, e01082 Search PubMed.
- Y. Xie, L. Lu and B. Chen, J. Hazard. Mater., 2022, 437, 129088 CrossRef CAS PubMed.
- X. Chen, T. Han, X. Miao, X. Zhang, L. Zhao, Y. Sun, H. Ye, X. Li and Y. Li, Chem. Eng. J., 2022, 446, 136901 CrossRef CAS.
- X. Yang, L. Liu, Y. Wang and G. Qiu, Sci. Total Environ., 2022, 817, 153042 CrossRef CAS PubMed.
- P. R. Yaashikaa and P. S. Kumar, Environ. Pollut., 2022, 312, 120031 CrossRef CAS PubMed.
- F. J. G. Frutos, O. Escolano, S. García, M. Babín and M. D. Fernández, J. Hazard. Mater., 2010, 183, 806–813 CrossRef CAS PubMed.
- Y. Lv, J. Bao, Y. Dang, D. Liu, T. Li, S. Li, Y. Yu and L. Zhu, J. Hazard. Mater., 2022, 130209 Search PubMed.
- B. Muthukumar, S. Surya, K. Sivakumar, M. S. AlSalhi, T. N. Rao, S. Devanesan, P. Arunkumar and A. Rajasekar, Chemosphere, 2023, 310, 136826 CrossRef CAS PubMed.
- Y. Liu, K. Zhang, H. Zhang, K. Zhou, Y. Chang, Y. Zhan, C. Pan, X. Shi, H. Zuo, J. Li and Y. Wei, J. Environ. Manage., 2023, 325, 116553 CrossRef CAS PubMed.
- P.-F. Liu, Z.-H. Yang, Y.-L. Chen, K.-H. Lo and C.-M. Kao, Sci. Total Environ., 2021, 786, 147395 CrossRef CAS.
- I. M. S. Anekwe and Y. M. Isa, Water-Energy Nexus, 2021, 4, 134–140 CrossRef CAS.
- S. M. C. Magalhães, R. M. Ferreira Jorge and P. M. L. Castro, J. Hazard. Mater., 2009, 170, 711–715 CrossRef PubMed.
- S. Curiel-Alegre, B. Velasco-Arroyo, C. Rumbo, A. H. A. Khan, J. A. Tamayo-Ramos, C. Rad, J. L. R. Gallego and R. Barros, Chemosphere, 2022, 307, 135638 CrossRef CAS PubMed.
- Y.-Z. Gong, Q.-Y. Niu, Y.-G. Liu, J. Dong and M.-M. Xia, Environ. Pollut., 2022, 314, 120232 CrossRef CAS PubMed.
- W. Ren, H. Liu, T. Mao, Y. Teng, R. Zhao and Y. Luo, J. Hazard. Mater., 2022, 433, 128793 CrossRef CAS PubMed.
- G. Colombini, C. Rumpel, S. Houot, P. Biron and M.-F. Dignac, Environ. Pollut., 2022, 315, 120369 CrossRef CAS PubMed.
- J. Xiao, G. Wang, H. Liu and X. Dai, Chemosphere, 2022, 306, 135637 CrossRef CAS PubMed.
- A. Pervaiz, Q. Zhong, S. A. U. Rehman, C. Ma, Y. Jiao and M. He, Sci. Total Environ., 2023, 856, 159213 CrossRef CAS PubMed.
- L.-C. Olivia, G.-C. Minerva, P.-J. Rocío and M.-P. Francisco José, Environ. Pollut., 2021, 275, 116642 CrossRef CAS PubMed.
- J. van Dorst, D. Wilkins, S. Crane, K. Montgomery, E. Zhang, T. Spedding, G. Hince and B. Ferrari, Environ. Pollut., 2021, 290, 117977 CrossRef CAS PubMed.
- I. V. Robles-González, F. Fava and H. M. Poggi-Varaldo, Microb. Cell Fact., 2008, 7, 5 CrossRef PubMed.
- D. O. Pino-Herrera, Y. Pechaud, D. Huguenot, G. Esposito, E. D. van Hullebusch and M. A. Oturan, J. Hazard. Mater., 2017, 339, 427–449 CrossRef CAS PubMed.
- S. Geng, W. Qin, W. Cao, Y. Wang, A. Ding, Y. Zhu, F. Fan and J. Dou, Chemosphere, 2022, 287, 132183 CrossRef CAS PubMed.
- F. A. Bezza and E. M. Nkhalambayausi Chirwa, Chemosphere, 2016, 144, 635–644 CrossRef CAS PubMed.
- A. Bachmann, W. de Bruin, J. C. Jumelet, H. H. Rijnaarts and A. J. Zehnder, Appl. Environ. Microbiol., 1988, 54, 548–554 CrossRef CAS PubMed.
- A. Ramaswami and R. G. Luthy, Environ. Sci. Technol., 1997, 31, 2260–2267 CrossRef CAS.
- F. Fava, D. Di Gioia and L. Marchetti, Biotechnol. Bioeng., 1998, 58, 345–355 CrossRef CAS PubMed.
- F. Fava and D. D. Gioia, Appl. Microbiol. Biotechnol., 1998, 50, 623–630 CrossRef CAS.
- C.-C. Yu, T.-C. Chang, C.-S. Liao and Y.-T. Chang, Sustainability, 2019, 11, 1088 CrossRef CAS.
- Y. Miyoshi, J. Okada, T. Urata, M. Shintani and K. Kimbara, Microorganisms, 2020, 8, 291 CrossRef CAS PubMed.
- R. Forján, I. Lores, C. Sierra, D. Baragaño, J. L. R. Gallego and A. I. Peláez, Appl. Sci., 2020, 10, 2837 CrossRef.
- S. Venkata Mohan, K. Sirisha, R. Sreenivasa Rao and P. N. Sarma, Ecotoxicol. Environ. Saf., 2007, 68, 252–262 CrossRef CAS PubMed.
- Z. Gong, P. Li, S. Guo, X. Jing, X. Wang and H. Zhang, Huan Jing Ke Xue, 2001, 22, 112–116 CAS.
- A. Tuhuloula, A. Altway, S. R. Juliastuti and S. Suprapto, IOP Conf. Ser.: Earth Environ. Sci., 2018, 175, 012014 CrossRef.
- S.-H. Liu, G.-M. Zeng, Q.-Y. Niu, Y. Liu, L. Zhou, L.-H. Jiang, X. Tan, P. Xu, C. Zhang and M. Cheng, Bioresour. Technol., 2017, 224, 25–33 CrossRef CAS PubMed.
- S. Lundstedt, P. Haglund and L. Öberg, Environ. Toxicol. Chem., 2003, 22, 1413–1420 CrossRef CAS PubMed.
- X. Jiang, Z. Mao, L. Zhong, J. Yu and Y. Tang, Int. J. Environ. Res. Public Health, 2022, 19, 5515 CrossRef CAS PubMed.
- P. I. Murungi and A. A. Sulaimon, Environ. Sci. Pollut. Res., 2022, 29(27), 40358–40372 CrossRef CAS PubMed.
- Y.-T. Chang, H.-C. Chen, H.-L. Chou, H. Li and S. A. Boyd, Environ. Sci. Pollut. Res., 2021, 28, 6078–6089 CrossRef CAS PubMed.
- F. Scargiali, A. Brucato, G. Micale and A. Tamburini, Ind. Eng. Chem. Res., 2020, 59, 8037–8045 CrossRef CAS.
- T. Kebede, Y. Gonfa, A. F. Senbeta and G. Sime, Effects of Bio-slurry and Chemical Fertilizers on Soil Physico-chemical Properties and Food Safety of Maize (Zea Mays L.) Grain, 18 May, 2022, PREPRINT (Version 1) available at Research Square Search PubMed.
- I. V. Robles-González, E. Ríos-Leal, I. Sastre-Conde, F. Fava, N. Rinderknecht-Seijas and H. M. Poggi-Varaldo, Process Biochem., 2012, 47, 1640–1648 CrossRef.
- M. R. Samaei, S. B. Mortazavi, B. Bakhshi, A. J. Jafari, N. Shamsedini, H. Mehrazmay and M. Ansarizadeh, J. Environ. Chem. Eng., 2022, 10, 106914 CrossRef CAS.
- A. Partovinia, F. Naeimpoor and P. Hejazi, J. Hazard. Mater., 2010, 181, 133–139 CrossRef CAS PubMed.
- S. Nasseri, R. R. Kalantary, N. Nourieh, K. Naddafi, A. H. Mahvi and N. Baradaran, J. Environ. Health Sci. Eng., 2010, 7(3), 199–208 CAS.
- H. Wu, Y. Yu and K. Yip, Energy Fuels, 2010, 24, 5652–5659 CrossRef CAS.
- M. A. Haque, M. Jahiruddin, M. M. Rahman and M. A. Saleque, Res. Agric., Livest. Fish., 2015, 2(1), 27–33 CrossRef.
- S. M. Khezri, S. H. Fatemi, M. K. Poshtegal and S. Hasanlou, J. Food, Agric. Environ., 2010, 8(3/4 part 2), 1156–1157 Search PubMed.
- S. Rashidi, A. Halaji Sani and S. Razavi, Biosci., Biotechnol. Res. Asia, 2017, 14, 1371–1385 Search PubMed.
- A. T. Lawal, Cogent Environ. Sci., 2017, 3, 1339841 CrossRef.
- H. S. Kim and W. J. Weber, Environ. Sci. Technol., 2005, 39, 2274–2279 CrossRef CAS PubMed.
- E. Collina, G. Bestetti, P. Di Gennaro, A. Franzetti, F. Gugliersi, M. Lasagni and D. Pitea, Environ. Int., 2005, 31, 167–171 CrossRef CAS PubMed.
- B. Wang, X. Xu, X. Yao, H. Tang and F. Ji, J. Environ. Manage., 2019, 249, 109388 CrossRef CAS PubMed.
- F. Moscoso, F. J. Deive, M. A. Longo and M. A. Sanromán, Int. J. Environ. Sci. Technol., 2015, 12, 1243–1252 CrossRef CAS.
- S. Venkata Mohan, D. Prasanna, B. Purushotham Reddy and P. N. Sarma, Int. Biodeterior. Biodegrad., 2008, 62, 162–169 CrossRef.
- S. H. Safe, Crit. Rev. Toxicol., 1994, 24, 87–149 CrossRef CAS PubMed.
- J. Borja, D. M. Taleon, J. Auresenia and S. Gallardo, Process Biochem., 2005, 40, 1999–2013 CrossRef CAS.
- A. J. Hudak and D. P. Cassidy, Biotechnol. Bioeng., 2004, 88, 861–868 CrossRef CAS PubMed.
- R. M. Atlas, Microbiol. Rev., 1981, 45, 30 CrossRef PubMed.
- A. Akbari, C. David, A. A. Rahim and S. Ghoshal, Water Res., 2021, 202, 117424 CrossRef CAS PubMed.
- L. Lumia, G. Rabbeni, M. G. Giustra, S. Giumento, G. Gallo and G. D. Bella, Chem. Eng. Trans., 2020, 79, 391–396 Search PubMed.
- M. S. Kuyukina, I. B. Ivshina, M. I. Ritchkova, J. C. Philp, C. J. Cunningham and N. Christofi, Soil Sediment Contam., 2003, 12, 85–99 CrossRef CAS.
- F. J. G. Frutos, R. Pérez, O. Escolano, A. Rubio, A. Gimeno, M. D. Fernandez, G. Carbonell, C. Perucha and J. Laguna, J. Hazard. Mater., 2012, 199–200, 262–271 CrossRef CAS PubMed.
- Y. Sun, M. Kumar, L. Wang, J. Gupta and D. C. W. Tsang, in Bio-Based Materials and Biotechnologies for Eco-Efficient Construction, Elsevier, 2020, pp. 261–283 Search PubMed.
- M. Rafique, A. Rehman, M. F. H. Munis, S. ur Rehman and H. J. Chaudhary, J. Basic Microbiol., 2016, 56, 105–119 CrossRef PubMed.
- J. T. Cookson, Bioremediation engineering: design and application, McGraw-Hill, 1995 Search PubMed.
- J. C. Quintero, M. T. Moreira, J. M. Lema and G. Feijoo, Chemosphere, 2006, 63, 1005–1013 CrossRef CAS PubMed.
- S. Venkatamohan, M. Ramakrishna, S. Shailaja and P. Sarma, Bioresour. Technol., 2007, 98, 2584–2589 CrossRef CAS PubMed.
- H. Znad, N. Kasahara and Y. Kawase, Process Biochem., 2006, 41, 1124–1128 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2023 |