Open Access Article
Open Access ArticleThe recent development of nanozymes for targeting antibacterial, anticancer and antioxidant applications
Huimin Zhong†
,
Cong Jiang†
and
Yanyan Huang
 *
*
College of Light Industry and Food Engineering, Nanjing Forestry University, Nanjing 210037, China. E-mail: huangyanyan0819@njfu.edu.cn
First published on 6th January 2023
Abstract
Nowadays, nanozymes have not only been used as biosensors in the detection field, but also their application prospects in disease treatment have been explored. Numerous nanomaterials have similar catalytic activities such as peroxidase, oxidase, catalase and superoxide dismutase, and they can be used for antibacterial, anticancer and antioxidant therapy. Although there have been many studies on the application of nanozymes in the therapeutic field, the current nanozyme-based systems often lack targeting and ignore the harm to the surrounding normal tissues. Although promising, the biosafety of nanomaterials has always been the concern of researchers. To improve the treatment effect and reduce toxic and side effects, precision treatment has become the key. At present, a few studies have modified targeted molecules on nanozymes to achieve precise targeting through specific interaction with surface overexpression factors of bacteria or cells. Combined with the catalysis of nanozymes, the targeted treatment of diseases can be achieved. This review summarizes the current research of nanozyme systems in targeted antibacterial, anticancer and antioxidant applications. At the same time, the challenges and development prospects of nanozyme-based targeted therapy system are summarized. It is expected that this work will provide new ideas and new directions for the precise treatment of nanozymes.
Huimin Zhong received her B.E. degree from Zhaoqing University, China, in 2021. In the same year, she has been a postgraduate and joined associate professor Yanyan Huang's group at college of Light Industry and Food Engineering, Nanjing Forestry University. Her work is mainly focused on the design of nanozymes and their application in biosensing. |
Cong Jiang received his B.E. degree in Bioengineering from Huaiyin Institute of Technology, China, in 2021. In the same year, he joined associate professor Yanyan Huang's group at college of Light Industry and Food Engineering at Nanjing Forestry University. His work is mainly focused on the design of nanozymes and their applications for antibacteria. |
Yanyan Huang received her B.S. degree in Applied Chemistry from Nanjing Normal University, China, in 2012. In the same year, she joined Professor Jinsong Ren's group at Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, and received her PhD in 2018. Since 2019, she has been an associate professor at Nanjing Forestry University. Her work is mainly focused on the self-assembly of nanozymes and their potential biological applications. |
1. Introduction
As potential natural enzyme candidates, nanozymes overcome many disadvantages of natural enzymes such as high price, complex preparation and purification, poor stability and low recycling efficiency.1,2 Nanozymes have been gradually applied to the fields of biological detection, environmental protection, disease diagnosis and treatment, etc.3,4 In the early stage, nanozymes mainly focused on the detection fields.5,6 With the development of nanotechnology and researchers' understanding, nanozymes have been gradually extended to the therapeutic field.1 For example, nanomaterials with peroxidase-like activity can be used to catalyze the conversion of overexpressed hydrogen peroxide (H2O2) in tumor issues into highly active hydroxyl radicals, killing cancer cells effectively.7 Besides, with the assistance of catalase (CAT)-like activity, nanozymes can be used to enhance the photodynamic effect of a photosensitizer in a hypoxic system. In addition, using the antioxidant enzyme-like catalytic activity, excess H2O2 or superoxide anions in inflammatory tissue can be transformed into non-toxic products by CAT, superoxide dismutase (SOD) or glutathione peroxidase (GPx) mimics.8,9 In this way, the oxidative damage can be effectively alleviated.Although possessing broad prospects, the majority of nanozyme-based therapy systems lack targeting.2 They may be absorbed by normal cells, inducing toxic side effects to normal surrounding tissues in varying degrees. To achieve better therapy efficiency, large dose of nanoagents may be needed which may cause greater toxicity to tissues. For the above reasons, it is an urgent issue to establish a novel nanozyme system for efficient and precise disease treatment. In the life system, the interaction between receptors and ligands can be used to design targeted reagents. Based on that, relevant work has gradually emerged. Although there have been a lot of reviews referring to the application of nanozymes in disease treatment,1,2 the targeted therapy for disease by nanozymes has not attracted much attention. This paper systematically summarized the current research progress of nanozymes in targeted antibacterial, anticancer and antioxidation. The catalytic pathways of nanozymes for therapy and the widely studied targeting system were introduced (Fig. 1 and Table 1). Furthermore, the current challenges and future development of nanozyme therapy system with targeted function was also discussed. It is expected to provide support for the precise treatment of nanozymes through this review.
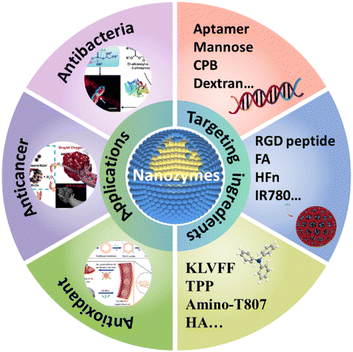 | ||
| Fig. 1 The development of nanozymes for targeted antibacterial (reproduced from ref. 12 with permission of 2020 American Chemical Society), anticancer (reproduced from ref. 30 with permission of 2018 American Chemical Society) and antioxidant applications (reproduced from ref. 35 with permission of 2020 American Chemical Society) and the targeting molecules involved in the corresponding targeted therapy system. | ||
| Application | Nanozyme | Targeting ingredient | Target | References |
|---|---|---|---|---|
| Antibacteria | PtCo@G | CPB | H. pylori | 14 |
| AuNPs-FeTPP | Mannose | Salmonella | 13 | |
| IONP-GOx | Dextran | S. mutans | 16 | |
| PtNZ@GOx@HA | Aptamer | S. aureus | 15 | |
| PAA-coated Cnp | The hydrolysis of phospholipids | Gram-negative and Gram-positive bacteria | 12 | |
| Anticancer | HMONs–MnOx | RGD peptide | U87 cancer cells | 30 |
| Lipo-Pd-pHCPT | RGD peptide | MDA-MB-231 breast cancer cells | 25 | |
| PNCNzyme@IAA | FA | HeLa cells | 26 | |
| N-PCNSs | HFn | HepG2 cells | 27 | |
| Sm-TCPP-Pt | Triphenylphosphine | MCF-7 cells | 32 | |
| Antioxidant | MPBzyme | NCM | BV2 cells, SH-SY5Y cells, and bEnd.3 cells | 38 |
| CeO2 | TPP | SH-SY5Y cells | 37 | |
| CeNCs/IONC/MSN-MB | Amino-T807 | SH-SY5Y cells | 36 | |
| CuxO@EM | KLVFF | HL-7702 cells | 35 | |
| CeO2 | MMT | HT-29 cells | 42 | |
| PBzyme | Inflammation mediated 8pyroptosis | BV2 and SH-SY5Y cells | 44 | |
| CSPQ | CM | SH-SY5Y cells | 45 | |
| MSe | HA | RAW 264.7 cells | 47 | |
| CeO2 | AntagomiR-26a | HUVECs | 49 | |
| Pt | TPP | HRECs | 50 |
2. Targeted antibacterial therapy
Bacterial infection,10 such as Helicobacter pylori (H. pylori), Salmonella, Staphylococcus aureus (S. aureus), etc., is an acute systemic infection caused by pathogenic bacteria or conditional pathogens. Clinically, it is mainly manifested as shivering, high fever, rash, etc., and some may have septic shock or migratory lesions. The elderly, children, those with chronic diseases or low immune function, untimely treatment and complications can develop sepsis or pyemia. The treatment of bacterial infection is mainly antibiotics, but the drug resistance of bacteria is an important challenge for antibiotics at present.With the development of nanotechnology, nanozymes provide the possibility for the treatment of bacterial infection. As we all know, the antibacterial mechanism of nanozymes is mainly divided into the following categories: peroxidase or oxidase mimics can transfer corresponding substrate H2O2 or oxygen (O2) into reactive oxygen species (ROS) such as hydroxyl radical (·OH) or singlet oxygen, thereby achieving antibacterial effect. In addition, using bioorthogonal techniques, prodrugs can be transformed into antibiotic drugs in the present of nanozymes, so as to obtain satisfactory antibacterial effect.11 Furthermore, with the phosphatase-like activity of nanozymes, the phospholipid structure of bacterial cell membrane will be decomposed, leading to the death of bacteria. Until now, the first antibacterial pathway has been studied most. Although nanozymes have been studied by a variety of researchers for antibacterial applications, most nanozyme-based systems lack targeting. Therefore, there may be problems of side effect risk and therapeutic effect. Considering these factors, some studies have modified some small molecular groups with the function of targeting bacteria on the surface of nanozymes. The targeted antibacterial system based on nanozymes mainly includes: aptamers and specific small molecules, such as mannose, C18-PEGn-benzeneboronic acid (CPB), dextran and so on.12–16 These substances can specifically bind with bacteria and then kill bacteria through the catalysis of nanozymes. This part mainly introduces the application of nanozymes with targeting effect in antibacterial system.
H. pylori, a Gram-negative bacterium, is mainly colonized in the human stomach. It is the main pathogenic factor of many gastric diseases. The common treatment of H. pylori infection is triple therapy. However, this treatment is gradually reduced due to the insufficient residence time of antibiotics in the stomach, the degradation of antibiotics by gastric acid, the drug resistance of bacteria and the side effects on other symbiotic bacteria.17,18 Inspired by the catalytic activities of nanozymes and the bacterial targeting effects of some molecules, Chen's group synthesized one nanozyme system to selectively kill H. pylori (Fig. 2).14 In their work, a PtCo nanocrystal core encapsulated in a graphitic shell was prepared. The obtained core–shell PtCo@G nanomaterials exhibited excellent oxidase-like property to transform O2 to highly active ROS under acidic environment. It was the modification of CPB that allowed PtCo@G nanozyme to target H. pylori. Together with the ROS generation of PtCo@G nanozyme, the PtCo@G@CPB nanocomposites could selectively kill H. pylori under stomach acidic environment. CCK-8 assay illustrated that the inhibition of PtCo@G@CPB on the proliferation of GES-1 cells was negligible, and the nanocomposite had no significant effect on other symbiotic initiating bacteria under relatively neutral conditions. These data indicated that PtCo@G@CPB nanocomposites possessed good biocompatibility. Additionally, it was found that PtCo@G@CPB remained in the stomach of H. pylori-infected mouse for a long time, and its ability to kill H. pylori was not inferior to that of triple-therapy. In the antibacterial research of nanozymes, the most studied are E. coli, S. aureus, and drug resistant S. aureus. The authors blazed a new trail and paid attention to H. pylori involved in gastrointestinal diseases, and ingeniously constructed a nanozyme system that can target H. pylori.
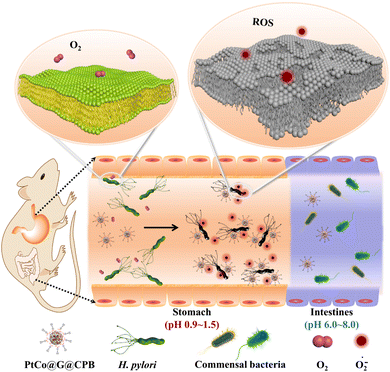 | ||
| Fig. 2 PtCo@graphene nanomaterials with oxidase-like property for selective killing of H. pylori. Reproduced from ref. 14 with permission of 2021 Springer Nature. | ||
Salmonella can trigger systemic disease in a vulnerable host through invading macrophage. Antibiotic therapy is often affected by factors such as poor intracellular permeability and easy degradation in cells, so it can't achieve the expected therapeutic effect. Bioorthogonal catalysis provides a promising strategy for intracellular infection. Recently, Rotello's group designed one targeted nanozyme to catalyse the conversion of pro-antibiotics to antibiotics in the intracellular environment to eliminate intracellular pathogens effectively.13 In their system, AuNPs used as the scaffolds were embedded by Iron tetraphenylporphyrin (FeTPP) after modification of mannose. With the targeting effect of mannose to the mannose receptor expressed on the surface of macrophages,19 the obtained nanozyme could be internalized selectively. Under this condition, the FeTTP used as the bioorthogonal catalyst could catalyse the pro-antibiotics into antibiotics. The generated ciprofloxacin could kill pathogenic Salmonella specifically. For biocompatibility study, Alamar Blue assay data demonstrated that under the same concentration of pro-antibiotics, antibiotics, and Man-AuNPs-FeTPP + pro-ciprofloxacin, non-toxic was found to the RAW 264.7 macrophages. Compared with others group in a Salmonella-macrophage infection model, Man-AuNPs-FeTPP + pro-ciprofloxacin treated group achieved a decreased bacteria viability. The targeted therapy of mannose is often used in anticancer systems. The author successfully combined the targeted function of mannose with the antibacterial properties of nanozyme.
Dental caries, a biofilm-induced disease, may lead to systemic complications in severe cases. The current antibiofilm methods are inefficient and based on broad-spectrum antimicrobial agents, which poses a challenge to target cariogenic pathogens without destroying symbiotic bacteria. To solve this problem, Cormode and Koo's groups constructed one bi-functional nanozyme to achieve the selective antibacterial effect in dental caries.16 They coated iron oxide nanoparticles with dextran (Dex-IONP) and linked glucose oxidase (GOx) covalently to obtain bi-functional nanozyme Dex-IONP-GOx. Under the high sugar level and acidic condition in biofilm environment, glucose could transform to ·OH with the catalysis of GOx and IONP. Since Streptococcus mutans (S. mutans) can express several glucan-binding proteins related to cell membrane, dextran can provide specific binding sites for S. mutans.20 In this way, Dex-IONP-GOx preferentially bound to S. mutans. Combined with the catalysis of IONP component, the selective sterilization of Dex-IONP-GOx system was realized without affecting Streptococcus oralis (symbiont). MTS assay demonstrated that Dex-IONP-GOx had no adverse effect on the cell viability of human primary oral gingival cells and fibroblast cells. Besides, after topical applications of Dex-IONP-GOx for 3 weeks, no deleterious effects on rats were received. Moreover, Dex-IONP-GOx treated group could achieve potently decrease of dental caries in a vivo rodent mode. The authors constructed a new antibacterial model of nanozymes which might not only broaden the application of nanozymes, but also provide a new scheme for the effective treatment of dental caries.
Recently, Qing and Yang's groups successfully designed one glucose-activated nanozyme for the treatment of diabetic infections (Fig. 3).15 In their work, the aptamer21 of S. aureus was modified on the surface of platinum nanoparticles to form Apt-PtNZ. The obtained nanomaterials and GOx were then encapsulated into hyaluronic acid (HA) shells. The generated nanocomposites were noted as APGH. Hyaluronidase secreted by bacteria could degrade HA effectively. The inner Apt-PtNZ and GOx were then released. With the targeting effect of aptamer on S. aureus,21 the Apt-PtNZ could bind to bacteria. Under diabetic infections, overexpressed glucose was catalyzed to gluconic acid and H2O2 by GOx, and subsequent acid environment activated peroxidase-like property of PtNZ to produce free radicals that can kill bacteria. MTT assay exhibited that negligible effect was monitored when incubating normal epithelial cells with Apt-PtNZs or APGH. Bacterial experiments demonstrated that compared with Apt-PtNZ@HA or GOx@HA, APGH treated S. aureus exhibited higher antibacterial ability, which was demonstrated by the destruction of bacterial morphology as well as the reduction of bacterial colonies. Further experiments illustrated that the wounds of mice treated with APGH healed rapidly compared with other treatments. Considering the high glucose concentration in diabetes infection system, the authors successfully constructed a self-activating therapeutic system with natural glucose oxidase and nanozyme, combined with the specific recognition of aptamer and bacteria, this “smart” targeted antibacterial effect could be successfully achieved.
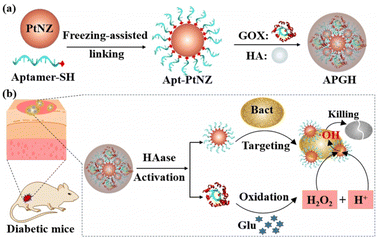 | ||
| Fig. 3 Glucose-activated Apt-PtNZ for the selective treatment of S. aureus infection in diabetic mice. Reproduced from ref. 15 with permission of 2021 Wiley-VCH. | ||
Furthermore, nanozyme-based nanoagents can also achieve significant antibacterial effect by targeting bacterial cell membranes. Recently, Chakravortty and Mugesh's group designed used polyacrylic acid (PAA)-coated nanoceria (Cnp) as antibacterial agents (Fig. 4).12 Owning to the Ce3+ and Ce4+ oxidation states in Cnp, the Cnp nanozyme could serve as phospholipase mimics to bind and hydrolysis phospholipid components on the surface of bacterial cell membrane. In this way, the antibacterial effect was achieved by destroying the cell membrane. This antibacterial strategy is different with other nanozyme-based system by catalysing the production of ROS. Since phospholipids are widely present in the extracellular membrane, the hydrolysis cell membrane activated by phospholipase can be used for targeting Gram-negative and Gram-positive bacteria. MTT experiment indicated that different from the cytotoxicity of silver nanoparticles, the PAA-coated Cnp nanozyme did not show toxic effect to HeLa cells. Further experimental data demonstrated that with the phospholipase-like property, PAA-coated Cnp could increase the contact effect with the lipophilic cell membrane and exhibited significant antibacterial efficiency against numerous human pathogens such as gastrointestinal pathogens as well as respiratory pathogens. Different from other nanozyme-based systems, this work used phospholipase mimics to destroy the cell membrane structure, providing a new idea for the construction of novel antibacterial agents. However, the authors paid more attention to the role of nanozymes and weakened the study of targeting effect. The mechanism of targeting and the anti-interference effect on complex systems have not been discussed in detail.
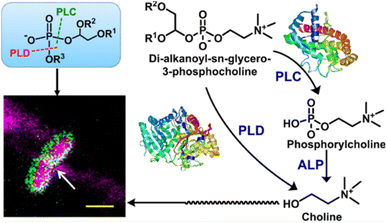 | ||
| Fig. 4 Polymer-coated nanoceria as a phospholipase mimic for targeting and destroying the cell membrane of bacteria. Reproduced from ref. 12 with permission of 2020 American Chemical Society. | ||
Based on the targeting effect of aptamers, carbohydrates, phenylboric acid and other molecules and the catalysis of nanozymes, the constructed composite systems can achieve precise antibacterial performance. However, the current system mainly uses peroxidase and oxidase to generate ROS, whether nanozymes can achieve other antibacterial pathways needs further research.
3. Targeted cancer therapy
Nanozymes can also be used for cancer therapy.7,22 At present, the nanozyme-based cancer treatment system mainly adopts two ways. The first way is peroxidase or oxidase mimics can respectively convert overexpressed H2O2 or O2 in tumor environment into high active ROS. The produced ROS may cause oxidative damage to cell components, leading to the death of cancer cells.1 The second way is utilizing catalase-like activity of nanozymes to catalyse overexpressed H2O2 into O2. The increased O2 level will improve the therapeutic effect of photodynamics for tumor tissues, especially the hypoxic tumor microenvironment. At present, most of nanozyme-based cancer therapy systems are short of targeting function. These nanozymes may be absorbed by normal cells, causing side effects on normal surrounding tissues in different levels. At the same time, it will also affect the treatment effect. Therefore, constructing novel nanozyme systems for effective and precise cancer treatment is an urgent challenge to be addressed. In view of the overexpression of some receptors on the surface of cancer cells, such as folic acid (FA) receptors and transferrin receptor 1, the targeted therapy of cancer cells can be realized by modifying the corresponding targeted factors on nanozymes. The current targeted factors were arginine–glycine–aspartic (RGD) peptide, FA, H-ferritin (HFn) or mitochondrial targeting molecule.23,24 Through these targeting approaches, effective catalytic treatment can be achieved.3.1. Transformation into ROS
Since increased lysosomes may provide more nutriment to cancer cells and is relevant to drug resistance, targeting lysosomes is considered as an original cancer treatment. Whereas, drugs can't specifically target lysosomes of tumor cells and they are stagnated in lysosomes, resulting in potency weakening. Nanozymes can be stagnated in lysosomes as well as nanozymes possess higher stability and safety. To this end, Wang, Zhuang and Huang's groups constructed a model system for screening ferritin nanocages (FTn)-based nanozyme with mutant P450-like and peroxidase-like activities and protecting group pairings.25 The selected Pd nanozyme and hydroxycamptothecin (HCPT) which assembled with the obtained caged fluorophores 1 (F1) (pro-HCPT), were merged into a liposome delivery system (Lipo-Pd-pHCPT). The Lipo-Pd-pHCPT modified with RGD and polyethylene glycol (PEG) to acquire tumor targeting and lengthen blood circulation respectively. Pd nanozyme was capable for splitting the propargylic ether bond of F1 selectively. Lipo-Pd-pHCPT targeted to tumor cells by FTn and RGD corresponding receptors mediating, subsequently entering cells by endocytosis and trapping in lysosomes. Lower pH in lysosomes and free radicals generated by peroxidase-like catalysis of Pd nanozymes prompted degradation of the liposome. In this way, Pd nanozymes activated the transformation of pro-HCPT into HCPT which released throughout the cell through free radicals-induced leakage of lysosomal membrane, resulting in tumor cells death. Their study offers an avenue to utilize nanozyme to target lysosomes for anticancer drugs design.The activation of prodrug mediated by enzymes in cancer cells has been considered as one potential method for targeted cancer treatment. Indole-3-acetic acid (IAA) is produced by tryptophan metabolism and exists in human body. Low concentration of IAA is harmless to human beings. However, under the catalysis of peroxidase, the single electron oxidation of IAA will produce free radical intermediates, which can induce the apoptosis of cells. Therefore, peroxidase and IAA can be used for cancer therapy. Inspired by this, Yan, Gao and Fan's groups prepared a metal-free phosphorous and nitrogen dual-doped porous hollow carbon nanosphere (PNCNzyme) as the peroxidase mimic (Fig. 5).26 Then, IAA and targeting ingredient FA were modified on the nanosphere to form FA-PNCNzyme@IAA. In this way, the nanocomposites could selectively bind with FA receptors overexpressed by HeLa cells, leading to their enrichment in HeLa cells. After localizing into lysosomes, the acidic microenvironment promoted the peroxidase-like property of PNCNzyme and catalyze the transformation of IAA to free radicals. The formed highly active ROS could kill HeLa cells through mitochondrial apoptosis. CCK-8 assay, blood test, blood biochemistry analysis and HeLa cells inoculated BALB/c nude mice model further verified the excellent biosafety and the tumor inhibition efficiency of FA-PNCNzyme@IAA system. At the tumor site, the catalytic transformation of prodrugs to anticancer drugs, combined with the targeting effect of FA, the nanocomposites can effectively reduce the side effects of drugs on normal tissues while achieving precise treatment. This work broad a new way for designing nanozyme-mediated prodrug system for targeted cancer therapy.
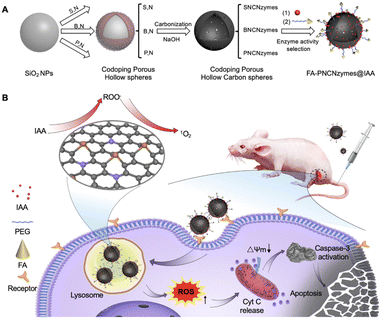 | ||
| Fig. 5 FA-functionalized nanozyme can activate prodrug for targeted tumor catalytic therapy. Reproduced from ref. 26 with permission of 2020 Elsevier. | ||
Yan and Gao's group designed N-doped porous carbon nanospheres (N-PCNSs) for tumor therapy (Fig. 6).27 Since HFn can specifically recognize HFn receptor (transferrin receptor 1, TfR1) overexpressed by tumor cells,28 in this way, the authors combined N-PCNSs with HFn to achieve the targeted cancer therapy. Fluorescence microscope data illustrated that with the assistance of HFn, N-PCNSs nanozyme could be transported to lysosome. This acidic environment could effectively activate the oxidase-like and peroxidase-like activities of nanozymes, catalysing the formation of ROS. It was found that this consuming oxygen reaction could suppress HepG2 and HT-29 cell viability through vitro tests. Body weight monitoring and pathological analysis of mice showed that the nanozyme-based system had good biocompatibility. The good effects of reducing tumor volumes and inhibiting tumor growth were achieved through HepG2 or HT-29 xenograft tumor models. Using the specific binding of HFn and TfR1, combined with the oxidase and peroxidase activities of N-PCNSs in the tumor microenvironment, the author successfully realized the targeted therapy. Different from the traditional targeting approaches, this specific protein modification may open up a new way for nanozymes to achieve targeted cancer therapy.
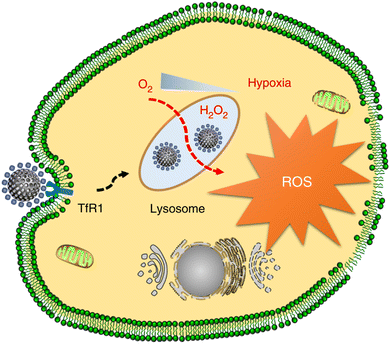 | ||
| Fig. 6 Ferritin-mediated targeting delivery of N-PCNSs for cancer therapy. Reproduced from ref. 27 with permission of 2018 Springer Nature. | ||
3.2. Transformation into O2
Photodynamic therapy (PDT) requires photosensitizers to convert the O2 at the tumor microenvironment into ROS under specific lighting conditions, resulting to oxidative damage and inhibited growth of tumor cells. However, in hypoxic environment, the therapeutic effect of photodynamic therapy will be affected significantly. How to increase the oxygen content in hypoxic environment is the key to improve the therapeutic effect of photodynamic therapy. The current methods include the direct delivery of O2 or using CAT to catalyse the decomposition of H2O2 into O2.29 Although promising, these systems also have some defects. For example, they often need high technical and equipment requirements and the stabilities of enzymes are also worthy of attention. With the development of nanotechnology and the wide applications of nanozymes, it provides a new idea for the photodynamic therapy in hypoxic environment.Chen and Shi's groups constructed a multifunctional nanozyme system for enhanced sonodynamic efficiency for cancer therapy (Fig. 7).30 In their work, hollow mesoporous organosilica nanoparticles (HMONs) were used as the templates for the growth of MnOx nanomaterials. The obtained HMONs–MnOx nanocomposites were then modified with protoporphyrin and RGD pentapeptide to form PMR nanocomposites. U87 cancer cells could overexpress integrin αvβ3 on their surface which had strong binding ability with the RGD peptide31 on the surface of PMR nanocomposites. In this way, PMR nanocomposites would be enriched in tumor tissues. Furthermore, the overexpressed glutathione under tumor microenvironment could decompose MnOx to form Mn2+. The released Mn2+ could be used for T1-weighted magnetic resonance (MR) imaging. In vitro cell experiments demonstrated that with the modification of RGD peptide, the endocytosis of nanocomposites increased. With the CAT-like property of MnOx component, the sonodynamic therapy (SDT) therapy effect of protoporphyrin was enhanced, leading to a significant decrease of U87 cancer cell viability. CCK-8 assay, hematochemistry, physiochemistry studies and organ and tissue staining assay of mice and the excretion assay of nanozymes all conformed that the PMR exhibited good biosafety. Animal experiments illustrated that the biocompatibility PMR nanocomposites could realize MR-guided SDT therapy for inhibiting the growth of tumor. This work provides novel strategies for designing cancer cells-targeted nanozyme systems for sonodynamic therapy. It should be noted that whether ultrasonic treatment will influence the morphology, size, catalytic activity, etc. of nanozyme has not been mentioned.
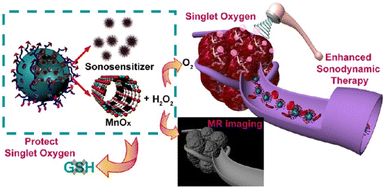 | ||
| Fig. 7 RGD peptide-modified nanozyme system for enhanced sonodynamic efficiency for cancer therapy. Reproduced from ref. 30 with permission of 2018 American Chemical Society. | ||
Similarly, You, Zhu, Sun and colleagues designed nanozyme-based system with mitochondrion-targeting effect for PDT.32 In their system, platinum nanoparticles were grown on the surface of two-dimensional metal–organic framework consisting of Sm-tetrakis(4-carboxyphenyl)porphyrin (TCPP) nanosheets. The formed nanocomposites were noted as Sm-TCPP-Pt. Triphenylphosphine was then modified on the nanocomposites for targeting mitochondrion.33 With the CAT-like property of platinum component, overexpressed H2O2 could be catalysed into O2 under hypoxic tumor environment. This was conducive to the therapeutic effect of photodynamic therapy. Cytotoxicity assessment, body weights and tumor and major organ staining assay of mice proved the biocompatibility of nanomaterials. Both in vitro and in vivo data indicated that compared with Sm-TCPP and Sm-TCPP-Pt, Sm-TCPP-Pt/triphenylphosphine exhibited remarkable PDT therapeutic effect whether in normal tumor environment or in hypoxic tumor environment. Based on the catalysis property of nanozymes, combined with the targeting effect of triphenylphosphine, this Sm-TCPP-Pt nanocomposite could effectively improve the photodynamic therapeutic effect. It may provide reference ways for the construction of mitochondrion-targeted nanozyme system for cancer therapy under hypoxic environment. Despite its potential applications, the biosafety of the nanomaterials has not been studied thoroughly.
In general, few targeted molecules have been selected for nanozyme-based precise cancer treatment systems currently. The most common targeting substrates are RGD peptides and folic acid molecules. However, in tumor issues, there are many overexpressed factors on the surface of cancer cells. Exploring novel nanozyme-based system for targeted cancer therapy is of great significance.
4. Targeted antioxidant therapy
Different from antibacterial and anticancer, the antioxidant mechanisms of nanozymes mainly depend their antioxidant enzyme-like properties such as CAT-, SOD- or GPx-like property to decompose the overexpressed ROS in inflammatory tissues, such as superoxide anion and hydrogen peroxide, into non-toxic products, so as to effectively protect tissues from oxidative damage.1,2 Nanomaterials with antioxidant enzyme-like activities can be used for the treatment of some neurological diseases, such as Alzheimer's disease (AD), Parkinson's disease (PD), ischemic stroke, sepsis and so on.34 The targeted molecules vary according to the particular model of inflammation, such as amyloid β (Aβ) targeting peptide, mitochondrial targeting molecule, Tau targeting molecule and so forth.35–37 These targeted molecules meet targeting function, combined with the free radical scavenging ability of nanozymes, the composite system can achieve a better anti-inflammatory effect.A mass of ROS and excessive inflammation after ischemic stroke will critically affect the prognosis of patients. Up to now, the poor efficacy of therapeutic drugs and their insufficient ability to target the damaged brain remain urgent problems. Aiming at the above problems, Cheng, Cai and colleagues successfully prepared neutrophil-like cell-membrane-coated mesoporous Prussian blue nanozyme (MPBzyme@NCM) for noninvasive treatment after ischemic stroke.38 The MPBzyme was wrapped with a differentiated HL-60 cell membrane, which with neutrophil-like property could bind to brain microvascular endothelial cells specifically by targeting effect, permitting MPBzyme into brain injury region. PB nanoparticles could mimic antioxidant enzymes for scavenge ROS and the electron spin resonance data conformed the ROS-scavenging activities of MPBzyme@NCM. CCK-8 assays, organ staining, biofluorescence imaging and ICP-OES of nanozyme biodistribution in mice indicated that the nanozyme could be further applied to the study of biological systems. In the treatment system of neurological diseases, compared with other nanozymes, the hydrodynamic diameter of the PB was around 106 nm and the size was relatively large.
Hyeon and Mook-Jung's group successfully designed ceria (CeO2) nanoparticles with triphenylphosphonium (TPP) for AD.37 In their system, CeO2 possessed excellent ROS scavenging ability. As a lipophilic cation, TPP that can target mitochondria since the mitochondrion membrane potential was negative charged. With this property, this TPP-modified CeO2 could selectively localized to mitochondria compared with single CeO2. MTT assay was carried out by incubated SH-SY5Y neuronal cells with TPP-modified CeO2, which show that the nanocomposite was nontoxic under an appropriate concentration range. TPP-modified CeO2 could inhibit Aβ-induced mitochondrial ROS in SH-SY5Y neuronal cells and HT22 mouse hippocampal cells significantly. It was evident that the administration of TPP-ceria NPs could ameliorate the neuronal damage as well as reduce reactive glial activation of 5XFAD mice. Taken together, this mitochondria-targeting CeO2 could efficiently reduce oxidative damage and suppress neuronal death in an AD mouse model.
Tau hyperphosphorylation as well as hyperphosphorylated tau aggregation are closely related to AD development. In this way, tau may serve as a potential therapeutic target for AD treatment.39 Recently, Ling, Tian and coworkers36 used mesoporous silica nanoparticles (MSNs) as the template, modified with ceria nanocrystals (CeNCs), iron oxide nanocrystals (IONCs) and amino-T807 (Fig. 8). The obtained nanocomposites were then loaded with methylene blue (MB). CCK-8 assay showed that the nanocomposite possessed excellent biocompatibility for further study. In their system, T807 could serve as an active hyperphosphorylated tau targeting agent in the neuron.40 The IONCs components could serve as high-resolution magnetic resonance imaging agents during AD treatment. When macrocyclic chelator 1,4,7-triazacyclononane-1,4,7-triacetic acid was labelled with Ga, the nanocomposites could further achieve positron emission tomography imaging. Furthermore, Due to the ultrasmall size of CeNCs could achieve remarkable ROS remove ability while MB molecules could efficiently inhibit the aggregation of tau. More importantly, both in vitro and in vivo experiment demonstrated that the nanocomposite could eventually be degraded in a physiological environment. Since the accumulated findings of weak correlation between Aβ deposition and cognition, as well as the failures of Phase III clinical trial on Aβ targeted therapy, this hyperphosphorylated tau-targeted multifunctional nanozymes may provide a new type of AD therapy.
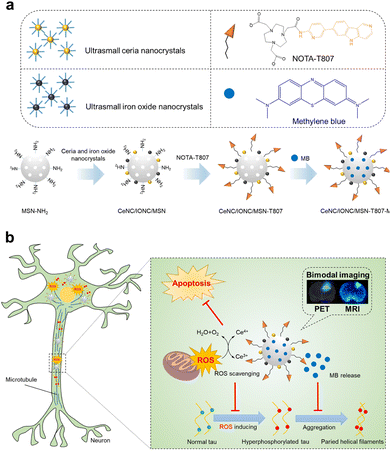 | ||
| Fig. 8 Tau-targeted nanocomposite with SOD-like property for the treatment of Alzheimer's disease. Reproduced from ref. 36 with permission of 2018 American Chemical Society. | ||
Recently, Qu, Ren and coworkers integrated CuxO nanozyme with erythrocyte membrane and Aβ-targeting pentapeptide KLVFF for AD therapy (Fig. 9).35 The obtained nanocomposites were noted as CuxO@EM-K. Pentapeptide KLVFF derived from Aβ could specifically target to Aβ.41 CuxO as multi-antioxidant enzyme mimics could relieve Aβ-induced oxidative stress of membrane and erythrocyte membrane that derived from 3xTg-AD mouse minimized immunogenicity. MTT assay and vitro hemolytic test of cell combined with the body weight, blood biochemistry test, and histopathology analysis of mice illustrated the good biocompatibility of nanozyme system both in vitro and in vivo. In vivo experimental data showed that CuxO@EM-K could effectively clear away Aβ and alleviate cognitive deficits in 3xTg-AD mice. In this work, the study of multienzyme-like activities is not systematic, and whether the multi enzyme activities interference with each other in the biological system has not been considered. Nevertheless, the Aβ-targeted CuxO@EM-K nanozymes are considered to be of prodigious potential for AD treatment.
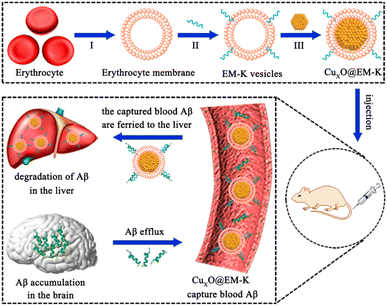 | ||
| Fig. 9 CuxO@EM-K nanozyme with Aβ-targeting effect for selectively clearing Aβ in an Alzheimer's disease model. Reproduced with permission. Reproduced from ref. 35 with permission of 2020 American Chemical Society. | ||
By adding CeNO3 and montmorillonite (MMT) into alkaline environment, Butch and Wei's group successfully prepared CeO2@MMT nanozymes for inflammatory bowel disease (IBD) therapy.42 By targeting effect, MMT components with negatively charge could transport the CeO2@MMT system to positively-charged inflammatory colon tissue. Then CeO2 components, as antioxidant enzyme mimics, could effectively remove free radicals in inflammatory sites. After orally administering CeO2@MMT in murine models, the nanomaterials could preferentially adhere to inflamed mucosa. Compared with other treatments, the group administered with CeO2@MMT could efficiently downregulate the levels of inflammatory factors as well as the free radical levels, and improve the activity of SOD. In this way, the colon-targeted CeO2@MMT nanozymes could effectively alleviate the damage of inflammatory tissue. Taken together, the authors have constructed a novel inflammatory model of nanozymes for antioxidation treatment. But compared with the specific binding of aptamer, RGD peptide, the targeting selectivity of electrostatic action is poor.
Inflammation mediated pyroptosis is a potential therapeutic target to alleviate neurodegenerative diseases such as PD.43 Therefore, evaluating novel and efficient pyroptosis inhibitors is of great significance for PD treatment. Recently, Cai and Zheng's group discovered that Prussian blue nanozyme (PBzyme) with excellent SOD and CAT mimics could serve as a powerful pyroptosis inhibitor for alleviating neurological diseases.44 CCK-8 assay, biochemistry assays and hematoxylin and eosin staining of major organs demonstrated that PBzyme possessed good biocompatibility and biosafety for biological research. To explore the efficiency of PBzyme in neurodegenerative diseases, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced PD mouse model was designed. Through intra-cerebroventricular injection of PBzyme, the MPTP-induced mitochondrial ROS level was decreased obviously due to its' outstanding antioxidant enzyme-like properties. Furthermore, PBzyme could prevent the loss of dopaminergic neurons in PD mouse by inhibiting the activation of NLRP3 inflammasome and pyroptosis of microglial cells. In a word, this work could remarkably alleviate the neurodegeneration of PD and might present a novel and effective therapeutic method for the treatment of PD.
Recently, Li and coworkers assembled ultrasmall PVP coated Cu2−xSe nanoparticles (CSP) with quercetin (Qe).45 The obtained nanocomposites were noted as CSPQ. In their work, CSP could serve as SOD, CAT and peroxidase mimics. The CSP with around 3 nm size could benefit the nanosystem to penetrate the blood–brain barrier. With these multienzyme-like properties, overexpressed ROS in inflammatory tissues could be removed successfully. As a kind of flavonoids, Qe has important physiological effects such as antioxidant, antitumor activity and radio sensitization.46 The modification of Qe could remarkably promote the therapeutic effect of CSP for the treatment of PD. In order to improve the therapeutic effect, the author further wrapped CSPQ into the cell membrane of neuron cells (CM). As we all know, microglia can express molecule-1 and α4β1 integrin. Due to the specific interaction between CM and molecule-1 and α4β1 integrin, the CSPQ@CM could achieve specific targeting effect on microglia. In this way, this microglia-targeted CSPQ@CM would eliminate overexpressed reactive oxygen species and promote the differentiation of microglia into anti-inflammatory M2 like phenotype effectively, leading to the remission of neuroinflammation in the MPTP-induced PD mouse (Fig. 10). CCK-8 assay showed that CSPQ nanoparticles had no significant cytotoxicity to any cell line under a certain concentration. No obvious histopathological damage was observed in any tissue of all mice, indicating the good biocompatibility and biosafety of nanozyme system. Combined cell membrane with ultrasmall size nanozymes, the obtained nanocomposites could serve as antioxidant as well as targeting agents for Parkinson's disease. The above works provide novel and potential strategies for the targeted treatment of neurological diseases by nanozymes. Although promising, there were few studies on the biosafety of CSPQ@CM in the work. In addition, the mechanism of multienzyme-like activities in cell environment has not been studied in depth.
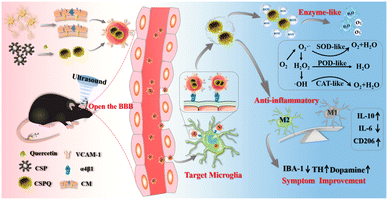 | ||
| Fig. 10 CSPQ@CM nanozymes can target microglia and treat Parkinson's disease efficiently. Reproduced from ref. 45 with permission of 2020 American Chemical Society. | ||
ROS produced by macrophages is an important way for the regulation of inflammatory immune response. The effective scavenging of reactive oxygen species plays an important role in alleviating inflammatory response. GPx as antioxidant enzymes can convert the overexpressed H2O2 into harmless products. Inspired by this, Liu and Ye's group combined HA with mesoporous selenium (MSe) to form MSe-HA nanocomposites.47 The MSe component could serve as GPx mimics to eliminate overexpressed ROS. Experiment data demonstrated that the HA component on the surface of MSe-HA nanocomposites could specifically bind to CD44 on the surface of macrophages,48 resulting in the selective aggregation of nanocomposites in the inflammation issue. Meanwhile, HA also possess good free radical scavenging ability which further promote the ROS scavenging ability of MSe-HA nanocomposites. The cell relative activity experiment showed that the toxicity of MSe NP and MSe-HA to RAW 264.7 cells, 3T3 cells, HUVE cells and red blood cells was negligible. Meanwhile, hemolysis did not occur. In vivo, MSe NPs and MSe-HA also exhibited low toxicity to mouse organs at a dose of 2 mg kg−1. The above results indicate that the nanomaterials possessed good biocompatibility and could be used for further study. For inflammation model, after intravenous injection of MSe-HA nanocomposites, the ROS level could be decreased remarkably. Further studies illustrated that the treatment of MSe-HA nanozymes could efficiently improve the survival rate and the organic dysfunction of mice compared with other treatments. Therefore, this system provides a new strategy for targeted therapy of inflammation by applying nanozymes.
It is a potentially effective therapeutic strategy to target disease related miRNAs. Inspired by that, Gao, Ling and coworkers designed a nanozyme-based hydrogel to achieve diabetic wound healing.49 In their work, polyethylenimine functionalized ceria nanoclusters were used as antioxidant enzyme mimics for ROS scavenging. In order to achieve the effect of precise treatment, AntagomiR-26a was modified. AntagomiR-26a was used to inhibit the antiangiogenic miR-26a which is a recognized target for hyperglycemia induction and can lead to impaired angiogenesis in diabetes wounds. MTS cell proliferation assay demonstrated that the nanocomposite exhibited no obvious cytotoxicity to HUVECs cells. Due to the properties of ceria and AntagomiR-26a components, diabetes wounds showed significantly faster wound closure and improved wound healing quality.
Besides, Jiang, Jiang, Yang and coworkers designed TPP-modified Pt nanozymes for mitochondrial targeting to eliminate oxidative damage in retinal neovascular diseases.50 Although there has been some work related to targeted antioxidation, it mainly focuses on the treatment of Alzheimer's disease and Parkinson's.
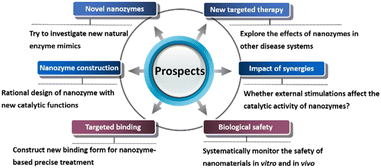
5. Conclusions and prospects
The unique catalytic properties of nanozymes have opened new ways for disease treatment. Meanwhile, the introduction of targeted peptides, proteins, carbohydrate molecules and other components can achieve precise disease treatment. Although some researchers have begun to construct targeted nanozyme systems for antibacterial, anticancer and antioxidant applications, there are still some problems in these systems (Fig. 11).(1) Since Fe3O4 nanoparticles with horseradish peroxidase-like activity was reported in 2007, studies have been mainly focused on peroxidase, oxidase, catalase and superoxide dismutase mimics in the past 15 years. However, these enzymes are only a small part of oxidoreductases. In nature, enzymes can be divided into seven categories according to catalytic reactions, including oxidoreductases, hydrolases, transferases, lyases, isomerases, synthetases and translocases. All these enzymes participate in important life processes. Researchers should not only focus on familiar enzymes and conduct research on the original basis. They need to overcome difficulties to explore unknown areas and find more novel nanozymes.
(2) In nature, a variety of enzymes participate in life activities. For example, antibacterial enzymes mainly include two categories: lysozyme and oxidoreductase, of which lysozyme includes bacterial lysozyme and fungal lysozyme, and oxidoreductase mainly includes glucose oxidase, lactoperoxidase and myeloperoxidase. However, recent studies only focus on horseradish peroxidase or oxidase mimics for antibacterial applications. Whether new nanozymes, for example, lysozyme mimics, can be constructed by introducing active groups, mimicking catalytic active centers as well as mimicking the microenvironment of catalytic sites and substrate binding sites may be the future research direction. Exploring novel nanozymes for antibacterial, anticancer and antioxidant application may promote the development of nanozymes for therapeutic application.
(3) In the life system, there are many specific binding effects on the surface of cell membrane or in the cell. At present, the research on nanozyme-based targeted therapy has not received widespread attention, and there are few reported systems with targeting effect. Meanwhile, the current nanozyme-based system is mainly focused on the interactions between the receptors and ligands on the cell membrane, and only several interactions are studied. For example, the cancer targeting system, which is still the conventional RGD peptide and folic acid. While other binding systems such as the interaction between polysaccharide and protein has not attracted attention. Exploring new targeting substances, combined with the catalytic effect of nanozymes, to achieve accurate and efficient treatment is the direction of future research. In addition, can the researchers build a nanozyme-based multi-targeted system to achieve more accurate treatment?
(4) Although nanozymes overcome the defects of poor stability of natural enzymes, their biological safety has always been an obstacle to the clinical application of nanomaterials. At present, many systems are applied to metal-based nanozymes, and the diffusion of metal ions needs attention. Although the biosafety of carbon-based nanomaterials is better than that of metal-based nanomaterials, there are still reports that carbon-based nanomaterials can cause cytotoxicity.51 Upon to now, many therapeutic systems pay little attention to the biological safety of nanomaterials. Most of the mentioned work only did a MTT experiment to verify their biocompatibility. Researchers should more systematically monitor the safety of nanomaterials in vitro and in vivo. In addition, rational design of efficient and safe nanozymes as therapeutic reagents is the focal point for the futural research.
(5) Many targeted therapeutic systems often introduce other synergistic effects, such as ultraviolet light, near-infrared light, ultrasound, etc. Whether these external factors affect the catalytic activity of nanozymes has not been discussed. Researchers should make a profound study the role and catalytic mechanism of nanozyme in the therapy system, rather than simply adding a series of actions for therapeutic effect. After all, in conventional life systems, the treatment system in which enzymes participate does not involve the above near-infrared light, ultrasound, etc.
(6) The current nanozyme-based system for targeted therapy is relatively single. For antioxidant system, more studies are focused on Alzheimer's disease and Parkinson's disease, while other inflammatory systems such as arthritis and dermatitis have not been studied. Researchers need to invest more energy to explore the effects of nanozymes in other disease systems. In addition to traditional antibacterial, anticancer and antioxidation applications, can nanozymes achieve other targeted therapy effects? Researchers can brainstorm to explore new areas of nanozymes.
Conflicts of interest
There are no conflicts to declare.Abbreviations
| Aβ | amyloid β |
| AD | Alzheimer's disease |
| CAT | catalase |
| CeNCs | ceria nanocrystals |
| CeO2 | ceria |
| CM | cell membrane of neuron cells |
| Cnp | nanoceria |
| CPB | C18-PEGn-benzeneboronic acid |
| CSP | PVP coated Cu2−xSe nanoparticles |
| Dex-IONP | dextran coated iron oxide nanoparticles |
| DSS | dextran sulfate sodium |
| F1 | fluorophores 1 |
| FA | folic acid |
| FeTPP | iron tetraphenylporphyrin |
| FTn | ferritin |
| GOx | glucose oxidase |
| GPx | glutathione peroxidase |
| HA | hyaluronic acid |
| HCPT | hydroxycamptothecin |
| HFn | H-ferritin |
| HMONs | hollow mesoporous organosilica nanoparticles |
| H2O2 | hydrogen peroxide |
| H. pylori | Helicobacter pylori |
| IBD | inflammatory bowel disease |
| IONCs | iron oxide nanocrystals |
| Lipo-Pd-pHCPT | a liposome delivery system merged with Pd nanozyme and pro-HCPT |
| MB | methylene blue |
| MMT | montmorillonite |
| MPBzyme@NCM | neutrophil-like cell-membrane-coated mesoporous Prussian blue nanozyme |
| MPTP | 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine |
| MR | magnetic resonance |
| MSe | mesoporous selenium |
| MSNs | mesoporous silica nanoparticles |
| N-PCNSs | N-doped porous carbon nanospheres |
| O2 | oxygen |
| ·OH | hydroxyl radical |
| PAA | polyacrylic acid |
| PB | Prussian blue |
| PBzyme | Prussian blue nanozyme |
| PD | Parkinson's disease |
| PDT | photodynamic therapy |
| PNCNzyme@IAA | porous hollow carbon nanosphere modified with Indole-3-acetic acid |
| pro-HCPT | hydroxycamptothecin assembled with caged fluorophores 1 |
| PVP | polyvinylpyrrolidone |
| Qe | quercetin |
| RGD | arginine–glycine–aspartic |
| ROS | reactive oxide species |
| S. aureus | Staphylococcus aureus |
| S. mutans | Streptococcus mutans |
| SDT | sonodynamic therapy |
| SOD | superoxide dismutase |
| TCPP | tetrakis(4-carboxyphenyl)porphyrin |
| TfR1 | transferrin receptor 1 |
| TPP | triphenylphosphonium |
Acknowledgements
Financial support was provided by the Natural Science Foundation of Jiangsu (BK20200764).Notes and references
- Y. Y. Huang, J. S. Ren and X. G. Qu, Chem. Rev., 2019, 119, 4357 CrossRef CAS PubMed.
- J. J. Wu, X. Y. Wang, Q. Wang, Z. P. Lou, S. R. Li, Y. Y. Zhu, L. Qin and H. Wei, Chem. Soc. Rev., 2019, 48, 1004 RSC.
- D. W. Jiang, D. L. Ni, Z. T. Rosenkrans, P. Huang, X. Y. Yan and W. B. Cai, Chem. Soc. Rev., 2019, 48, 3683 RSC.
- Z. W. Chen, Z. Z. Wang, J. S. Ren and X. G. Qu, Acc. Chem. Res., 2018, 51, 789 CrossRef CAS PubMed.
- X. L. Zhang, D. Wu, X. X. Zhou, Y. X. Yu, J. C. Liu, N. Hu, H. L. Wang, G. L. Li and Y. N. Wu, Trends Anal. Chem., 2019, 121, 115668 CrossRef CAS.
- X. Q. Tao, X. Wang, B. W. Liu and J. W. Liu, Biosens. Bioelectron., 2020, 168, 112537 CrossRef CAS PubMed.
- S. S. Ding, L. He, X. W. Bian and G. Tian, Nano Today, 2020, 35, 100920 CrossRef CAS.
- Q. Wang, C. Q. Cheng, S. Zhao, Q. Y. Liu, Y. H. Zhang, W. L. Liu, X. Z. Zhao, H. Zhang, J. Pu, S. Zhang, H. G. Zhang, Y. Du and H. Wei, Angew. Chem., Int. Ed., 2022, 61, e202201101 CAS.
- R. Hou, T. X. Lu, W. Gao, J. Shen, Z. Y. Yu, D. T. Li, R. H. Zhang, Y. Y. Zheng and X. J. Cai, ACS Nano, 2022, 16, 9559 CrossRef CAS PubMed.
- T. R. McCulloch, T. J. Wells and F. S. F. Guimaraes, Trends Microbiol., 2022, 30, 158 CrossRef CAS PubMed.
- R. Cao-Milán, S. Gopalakrishnan, L. D. He, R. Huang, L. S. Wang, L. Castellanos, D. C. Luther, R. F. Landis, J. M. V. Makabenta, C. H. Li, X. Z. Zhang, F. Scaletti, R. W. Vachet and V. M. Rotello, Chem, 2020, 6, 1113 Search PubMed.
- K. Khulbe, K. Karmakar, S. Ghosh, K. Chandra, D. Chakravortty and G. Mugesh, ACS Appl. Bio Mater., 2020, 3, 4316 CrossRef CAS PubMed.
- J. Hardie, J. M. Makabenta, A. Gupta, R. Huang, R. Cao-Milan, R. Goswami, X. Zhang, P. Abdulpurkar, M. Farkas and V. Rotello, Mater. Horiz., 2020, 9, 1089 Search PubMed.
- L. F. Zhang, L. Zhang, H. Deng, H. Li, W. T. Tang, L. Y. Guan, Y. Qiu, M. J. Donovan, Z. Chen and W. H. Tan, Nat. Commun., 2021, 12, 2002 CrossRef CAS PubMed.
- L. F. Chen, S. H. Xing, Y. L. Lei, Q. S. Chen, Z. Zou, K. Quan, Z. H. Qing, J. W. Liu and R. H. Yang, Angew. Chem., Int. Ed., 2021, 60, 23534 CrossRef CAS PubMed.
- Y. Huang, Y. Liu, S. Shah, D. Y. Kim, A. S. Soro, T. Ito, M. Hajfathalian, Y. Li, J. C. Hsu, L. M. Nieves, F. Alawi, P. C. Naha, D. P. Cormode and H. Koo, Biomaterials, 2021, 268, 120581 CrossRef CAS PubMed.
- L. X. Yan, B. B. Wang, X. Zhao, L. J. Chen and X. P. Yan, ACS Appl. Mater. Interfaces, 2021, 13, 60955 CrossRef CAS PubMed.
- Y. Qin, Y. H. Lao, H. X. Wang, J. B. Zhang, K. Yi, Z. G. Chen, J. Han, W. T. Song, Y. Tao and M. Q. Li, J. Mater. Chem. B, 2021, 9, 9826 RSC.
- X. Y. Gao, D. Mao, X. G. Zuo, F. Hu, J. Cao, P. Zhang, J. Z. Sun, J. Z. Liu, B. Liu and B. Z. Tang, Anal. Chem., 2019, 91, 6836 CrossRef CAS PubMed.
- G. R. Germaine and C. F. Schachtele, Infect. Immun., 1976, 13, 365 CrossRef CAS PubMed.
- H. Zhang, X. Y. Ma, Y. Liu, N. Duan, S. J. Wu, Z. P. Wang and B. C. Xu, Biosens. Bioelectron., 2015, 74, 872 CrossRef CAS PubMed.
- X. L. Zhang, G. L. Li, D. Wu, X. L. Li, N. Hu, J. Chen, G. Chen and Y. N. Wu, Biosens. Bioelectron., 2019, 137, 178 CrossRef CAS PubMed.
- H. Dong, G. X. Yang, X. Zhang, X. B. Meng, J. L. Sheng, X. J. Sun, Y. J. Feng and F. M. Zhang, Chem.–Eur. J., 2018, 24, 17148 CrossRef CAS PubMed.
- S. Y. Qin, A. Q. Zhang and X. Z. Zhang, Small, 2018, 14, 1802417 CrossRef PubMed.
- Z. Y. Sun, Q. Q. Liu, X. Y. Wang, J. Wu, X. Y. Hu, M. M. Liu, X. Y. Zhang, Y. H. Wei, Z. J. Liu, H. J. Liu, R. Chen, F. Wang, A. C. Midgley, A. Li, X. Y. Yan, Y. M. Wang, J. Zhuang and X. L. Huang, Theranostics, 2022, 12, 1132 CrossRef CAS PubMed.
- Q. Liang, J. Q. Xi, X. J. J. Gao, R. F. Zhang, Y. L. Yang, X. F. Gao, X. Y. Yan, L. Z. Gao and K. L. Fan, Nano Today, 2020, 35, 100935 CrossRef CAS.
- K. L. Fan, J. Q. Xi, L. Fan, P. X. Wang, C. H. Zhu, Y. Tang, X. D. Xu, M. M. Liang, B. Jiang, X. Y. Yan and L. Z. Gao, Nat. Commun., 2018, 9, 1440 CrossRef PubMed.
- K. L. Fan, X. H. Jia, M. Zhou, K. Wang, J. Conde, J. Y. He, J. Tian and X. Y. Yan, ACS Nano, 2018, 12, 4105 CrossRef CAS PubMed.
- S. Z. F. Phua, G. B. Yang, W. Q. Lim, A. Verma, H. Z. Chen, T. Thanabalu and Y. L. Zhao, ACS Nano, 2019, 13, 4742 CrossRef CAS PubMed.
- P. Zhu, Y. Chen and J. L. Shi, ACS Nano, 2018, 12, 3780 CrossRef CAS PubMed.
- A. R. M. Dias, L. Bodero, A. Martins, D. Arosio, S. Gazzola, L. Belvisi, L. Pignataro, C. Steinkühler, A. D. Corso, C. Gennari and U. Piarulli, ChemMedChem, 2019, 14, 938 CrossRef PubMed.
- Z. G. Gao, Y. J. Li, Y. Zhang, K. W. Cheng, P. J. An, F. H. Chen, J. Chen, C. Q. You, Q. Zhu and B. W. Sun, ACS Appl. Mater. Interfaces, 2020, 12, 1963 CrossRef CAS PubMed.
- J. Sun, K. Du, J. J. Diao, X. T. Cai, F. D. Feng and S. Wang, Angew. Chem., Int. Ed., 2020, 59, 12122 CrossRef CAS PubMed.
- F. F. Cao, L. Zhang, Y. W. You, L. R. Zheng, J. S. Ren and X. G. Qu, Angew. Chem., Int. Ed., 2020, 59, 5106 Search PubMed.
- M. M. Ma, Z. Q. Liu, N. Gao, Z. F. Pi, X. B. Du, J. S. Ren and X. G. Qu, J. Am. Chem. Soc., 2020, 142, 21702 CrossRef CAS PubMed.
- Q. Chen, Y. Du, K. Zhang, Z. Y. Liang, J. Q. Li, H. Yu, R. Ren, J. Feng, Z. M. Jin, F. Y. Li, J. H. Sun, M. Zhou, Q. G. He, X. L. Sun, H. Zhang, M. Tian and D. S. Ling, ACS Nano, 2018, 12, 1321 CrossRef CAS PubMed.
- H. J. Kwon, M. Y. Cha, D. Y. Kim, D. K. Kim, M. Soh, K. S. Shin, T. H. Hyeon and I. M. Jung, ACS Nano, 2016, 10, 2860 CrossRef CAS PubMed.
- L. S. Feng, C. R. Dou, Y. G. Xia, B. H. Li, M. Y. Zhao, P. Yu, Y. Y. Zheng, A. M. El-Toni, N. F. Atta, A. Galal, Y. S. Cheng, X. J. Cai, Y. Wang and F. Zhang, ACS Nano, 2021, 15, 2263 CrossRef CAS PubMed.
- C. X. Gong and K. Iqbal, Curr. Med. Chem., 2008, 15, 2321 CrossRef CAS PubMed.
- C. H. Gao, X. Y. Chu, W. Gong, J. P. Zheng, X. Y. Xie, Y. L. Wang, M. Y. Yang, Z. P. Li, C. S. Gao and Y. Yang, J. Nanobiotechnol., 2020, 18, 71 CrossRef CAS PubMed.
- C. W. Wei, Y. Peng, L. Zhang, Q. Y. Huang, M. Cheng, Y. N. Liu and J. Li, Bioorg. Med. Chem. Lett., 2011, 21, 5818 CrossRef CAS PubMed.
- S. Zhao, Y. X. Li, Q. Y. Liu, S. R. Li, Y. Cheng, C. Q. Cheng, Z. Y. Sun, Y. Du, C. J. Butch and H. Wei, Adv. Funct. Mater., 2020, 30, 2004692 CrossRef CAS.
- M. E. H. MSc, M. A. MSc, M. J. MSc, I. Kim, S. A. MSc and D. K. Choi, Mov. Disord., 2020, 35, 20 CrossRef PubMed.
- X. X. Ma, J. N. Hao, J. R. Wu, Y. H. Li, X. J. Cai and Y. Y. Zheng, Adv. Mater., 2022, 34, 2106723 CrossRef CAS PubMed.
- H. H. Liu, Y. B. Han, T. T. Wang, H. Zhang, Q. Xu, J. X. Yuan and Z. Li, J. Am. Chem. Soc., 2020, 142, 21730 CrossRef CAS PubMed.
- D. Xu, M. J. Hu, Y. Q. Wang and Y. L. Cui, Molecules, 2019, 24, 1123 CrossRef CAS PubMed.
- X. Chen, X. F. Zhu, Y. C. Gong, G. L. Yuan, J. Q. Cen, Q. S. Lie, Y. D. Hou, G. Ye, S. M. Liu and J. Liu, Appl. Mater. Today, 2021, 22, 100929 CrossRef.
- J. M. R. de la Rosa, A. Tirella, A. Gennari, I. J. Stratford and N. Tirelli, Adv. Healthcare Mater., 2017, 6, 1601012 CrossRef PubMed.
- H. B. Wu, F. Y. Li, W. Shao, J. Q. Gao and D. S. Ling, ACS Cent. Sci., 2019, 5, 477 CrossRef CAS PubMed.
- B. Xue, M. Y. Ge, K. L. Fan, X. L. Huang, X. Y. Yan, W. Jiang, B. Jiang and Z. L. Yang, J. Controlled Release, 2022, 350, 271 CrossRef CAS PubMed.
- X. Yuan, X. X. Zhang, L. Sun, Y. Q. Wei and X. W. Wei, Part. Fibre Toxicol., 2019, 16, 18 CrossRef PubMed.
Footnote |
| † These authors contribute equal to this work. |
| This journal is © The Royal Society of Chemistry 2023 |