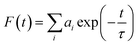Open Access Article
Open Access ArticleA new fluorescent probe for sensing Al3+ ions in the solution phase and CH3COO− in the solid state with aggregation induced emission (AIE) activity†
Rahul Bhowmick,
Payel Mondal and
Pabitra Chattopadhyay *
*
Department of Chemistry, The University of Burdwan, Golapbag, Burdwan-713104, West Bengal, India. E-mail: pabitracc@yahoo.com
First published on 24th January 2023
Abstract
An AIE (aggregation induced emission) active probe DFP–AMQ was designed and synthesized as a hexa-coordinated N2O donor chelator for the selective sensing of Al3+ colorimetrically as well as fluorimetrically with a 27-fold fluorescence enhancement for CH3CN–H2O (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v, pH 7.2, HEPES buffer). The fluorescence enhancement occurred through the blocking of ESIPT, chelation enhanced fluorescence effect (CHEF) arose, and as a result fluorescence enhancement was observed through 1
1, v/v, pH 7.2, HEPES buffer). The fluorescence enhancement occurred through the blocking of ESIPT, chelation enhanced fluorescence effect (CHEF) arose, and as a result fluorescence enhancement was observed through 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
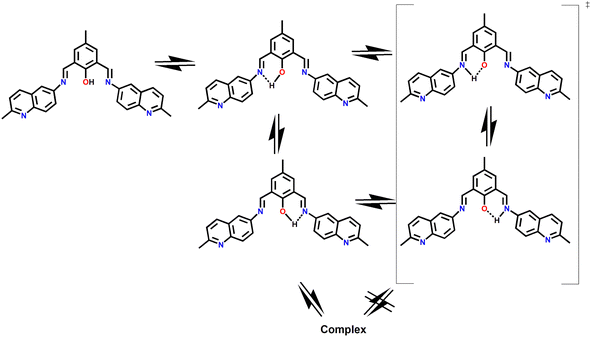
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexation with Al3+ ions. Detailed spectroscopic studies including UV-Vis, FTIR, 1H NMR, and HRMS studies were carried out to characterize the probable structure of DFP–AMQ including the complexation of DFP–AMQ with Al3+ ions. The spectrophotometric and spectrofluorimetric titrations revealed strong binding towards Al3+ and the Kd values were obtained from UV-Vis (3.26 × 10−5 M−1) and fluorescence titration (2.02 × 10−5 M−1). The limit of detection of Al3+ by DFP–AMQ was 1.11 μM. The quantum yields of DFP–AMQ and [DFP–AMQ–Al]+ were calculated to be 0.008 and 0.211, respectively. Dynamic light scattering (DLS) studies showed that the sizes of the particles increased with increasing water percentage due to aggregation. SEM (scanning electron microscopy) studies revealed interesting morphological changes in microstructures in which DFP–AMQ demonstrated a rod-like shape, which was converted to a spherical-like shape in the presence of Al3+ and when DFP–AMQ aggregated in H2O it showed aggregated block-like shape. In the solid phase, DFP–AMQ with nitrate has no particular shape, but in the presence of acetate, it converts to stone-like shape. This probe (DFP–AMQ) could be employed for on-site Al3+ ion detection in the solid state.
1 complexation with Al3+ ions. Detailed spectroscopic studies including UV-Vis, FTIR, 1H NMR, and HRMS studies were carried out to characterize the probable structure of DFP–AMQ including the complexation of DFP–AMQ with Al3+ ions. The spectrophotometric and spectrofluorimetric titrations revealed strong binding towards Al3+ and the Kd values were obtained from UV-Vis (3.26 × 10−5 M−1) and fluorescence titration (2.02 × 10−5 M−1). The limit of detection of Al3+ by DFP–AMQ was 1.11 μM. The quantum yields of DFP–AMQ and [DFP–AMQ–Al]+ were calculated to be 0.008 and 0.211, respectively. Dynamic light scattering (DLS) studies showed that the sizes of the particles increased with increasing water percentage due to aggregation. SEM (scanning electron microscopy) studies revealed interesting morphological changes in microstructures in which DFP–AMQ demonstrated a rod-like shape, which was converted to a spherical-like shape in the presence of Al3+ and when DFP–AMQ aggregated in H2O it showed aggregated block-like shape. In the solid phase, DFP–AMQ with nitrate has no particular shape, but in the presence of acetate, it converts to stone-like shape. This probe (DFP–AMQ) could be employed for on-site Al3+ ion detection in the solid state.
1. Introduction
Currently, for the detection of very low levels of molecules/ions, the development of fluorescent sensors has great importance, and in the appraisement of targetable species over fluorescent sensors in solution or in the solid state, several photophysical processes such as CHEF, PET, FRET, and ICT have been considered enormously.1–5 Many chemosensors present in the solution state compared to the fluorescent materials in solid states have been reported in the literature. Furthermore in the development of chemical as well as biological sensors due to wide-ranging applications, solid-state fluorescent materials have generated extensive interest ranging from the biomedical to optoelectronic fields in almost all phases of human life.6–10 Aggregation induced emission (AIE), used for the development of efficient fluorescent materials in the solid state is a distinctive photophysical phenomenon, which was first observed in the year 2001 in luminogen aggregation, demonstrating intense emission.11–13 Furthermore, for the development of fluorescent materials, aggregation induced emission (AIE) has opened a new area. Organic photo-luminescent probes show high fluorescence emissions through aggregation induced emission (AIE) such that the resulting material can bind with molecules via strong interaction. The AIE phenomenon occurs from the non-radiative decay when the aggregate state blocks through the control of intra-molecular motions. Likewise, the π–π interactions are prevented by the non-planar conformations, and AIE molecules are fixed when incorporated into solid matrixes, demonstrating strong emission. These special applications make AIE a good candidate for constructing fluorescent materials.14–17 In dilute solutions, AIE-active sensors show very weak emission and with gradually increasing water percentage, their emission increases because of constrained vibration and rotation.Aluminium (Al) is the 3rd most abundant element, which plays crucial roles in extensive range of applications including cooking utensils, cosmetics, and potable water supplies.18,19 Al3+ come into the body through the ecosystem and associate with increased incidences that could be responsible for neurotoxicity causing Alzheimer's and Parkinson's diseases, gastrointestinal and colic problems, bone deformation, anaemia, interruption in reproduction, and lung and breast cancer; furthermore, to some extent, certain amount of aluminium is also deadly to plants, fishes, and other living species.20–24 As such, for these reasons, there is a social as well as biological significance of Al3+ ions, and it is very much important to monitor trace levels of Al3+ ions. But it is very difficult to find a technique to sense the aluminium ions as it is not in coloured form. Hence, special attention is necessary to develop a chemosensor for aluminium, though strong hydration of Al3+ ions with interfering ions and weak coordination is problematic behaviour. Considering these facts, there are several fluorescent sensors for Al3+ ions in the solution state based on different photophysical processes.25–30 However, there are very few probes for the detection of Al3+ ions based on the AIE effect.31,32
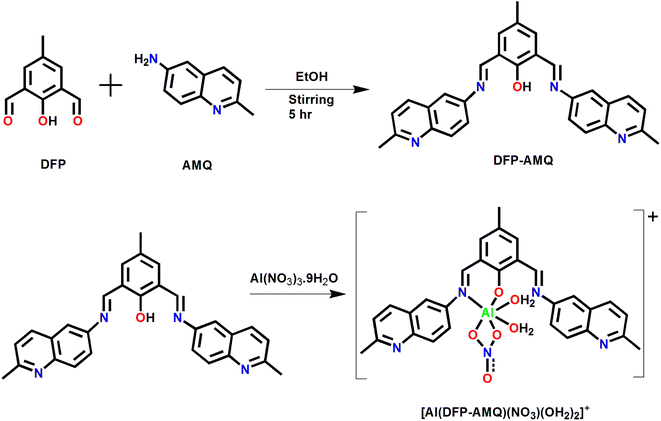
Here we report diformyl phenol and quinoline-based probe DFP–AMQ, which is a fluorescent sensor selective on Al3+ in the organo-aqueous medium (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, CH3CN
1, CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O, v/v, in HEPES buffer). Through blocking of ESIPT, chelation-enhanced fluorescence effect (CHEF) occurs and as a result, the fluorescence enhancement was observed. Aggregation induced emission enhancement (AIEE) is active in the presence of water. Dynamic light scattering (DLS) studies show the sizes of the particles, which increase with increasing water percentage due to aggregation. In the solid phase, DFP–AMQ shows strong fluorescence but in the presence of nitrate anion, fluorescence emission decreases and again fluorescence emission increased selectively under the presence of acetate anions. Scanning electron microscopy (SEM) shows different microstructures in different conditions. Fluorescence microscope images show the different morphologies in different water percentages.
H2O, v/v, in HEPES buffer). Through blocking of ESIPT, chelation-enhanced fluorescence effect (CHEF) occurs and as a result, the fluorescence enhancement was observed. Aggregation induced emission enhancement (AIEE) is active in the presence of water. Dynamic light scattering (DLS) studies show the sizes of the particles, which increase with increasing water percentage due to aggregation. In the solid phase, DFP–AMQ shows strong fluorescence but in the presence of nitrate anion, fluorescence emission decreases and again fluorescence emission increased selectively under the presence of acetate anions. Scanning electron microscopy (SEM) shows different microstructures in different conditions. Fluorescence microscope images show the different morphologies in different water percentages.
2. Experimental section
2.1 Materials and methods
The initial materials such as 2,6-diformyl phenol (DFP, prepared in the laboratory), 6-amino-2-methyl quinoline (AMQ) (Sigma Aldrich), were used to develop the ligand DFP–AMQ and to prepare Al3+ complexes, Al(NO3)3·9H2O (Merck, India) was used. Solvents such as CH3CN, CH3OH, and dimethyl formamide (DMF) (Merck, India) were of the reagent grade.2.2 Physical measurements
PerkinElmer 240 elemental analyzer was used for elemental analysis. Infrared spectra (400–4000 cm−1) were recorded using KBr pellets on a Nicolet Magna IR 750 series-II FTIR spectrophotometer. A Bruker 300 MHz NMR spectrometer was used for 1H-NMR recorded in the DMSO-d6 solvent and using tetramethylsilane (δ = 0) as an internal standard. An Agilent diode-array spectrophotometer (model, Agilent 8453) was used for recording UV-Vis spectra, a Hitachi-7000 spectrofluorimeter was used for recording steady-state fluorescence, ESI-MS+ (m/z) of the ligand and Al(III)-complex were recorded on a Waters' HRMS spectrometer (model: XEVOG2 QTOF). To analyse the morphology of DFP–AMQ in the solid state, FESEM images were taken using an EVO LS10 scanning electron microscope (SEM). For the pH study, a systronics digital pH meter (model 335) was used and 50 mM HCl or NaOH solution was used for the adjustment of pH. The particle size distribution was measured by dynamic light scattering (DLS) on a Malvern Zetasizer Nano ZS90 instrument. Optical fluorescence microscopy images were taken using a LEICA DM1000 LED upright microscope.2.3 Preparation of DFP–AMQ
2,6-Diformyl phenol (DFP) was prepared by following a literature procedure.33 2,6-Diformyl-phenol (DFP) (0.82 g, 5 mmol) was dissolved in 15 ml EtOH. To this solution, 6-amino-2-methylquinoline (1.58 g, 10 mmol) was added and the solution was refluxed for 5 h. After that, the reaction mixture was filtered and the filtrate was kept at room temperature. After 2 days a red colour crystalline product was formed (yield, 85.57%). 1H-NMR (in DMSO-d6) (δ, ppm): 2.50 (s, 3H, methyl), 2.66 (s, 6H, methyl), 7.44 (s, 2H, aromatic), 7.80 (m, 2H, aromatic), 7.84 (m, 4H, aromatic), 7.99 (d, 2H, aromatic), 8.24 (d, 2H, aromatic), 14.21 (s, 1H, OH) (please see Fig. S1†). ESI-MS+ (m/z): 445.26 (DFP–AMQ + H+) [Fig. S2†]. FTIR: 1626 cm−1 (CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) [Fig. S3†]. Melting point: 98 ± 1 °C. Elemental analysis: expected: C, 78.36%; H, 5.44%; N, 12.60%; calculated: C, 78.31%; H, 5.37%; N, 12.52%.
N) [Fig. S3†]. Melting point: 98 ± 1 °C. Elemental analysis: expected: C, 78.36%; H, 5.44%; N, 12.60%; calculated: C, 78.31%; H, 5.37%; N, 12.52%.
2.4 Synthesis of [DFP–AMQ–Al]+
DFP–AMQ (0.444 g, 1 mmol, was dissolved in a few drops of DMF), then, 25 ml CH3CN was added to Al(NO3)3·9H2O (0.375 g, 1 mmol in 5 ml MeOH solution) dropwise with continuous stirring. After 1.5 hours, the solution was filtered off and the filtrate was kept aside undisturbed. After one day, [(DFP–AMQ)Al)]+ was crystallised. It was collected by filtration, washed several times with MeCN, and dried in air. Several trials to grow single crystals failed. 1H-NMR (in DMSO-d6) (δ, ppm): 2.50 (s, 3H, methyl), 2.67 (s, 3H, methyl), 2.83 (s, 3H, methyl), 7.48–8.30 (12H, aromatic), 9.10 (s, 1H, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 9.20 (s, 1H, CH
N), 9.20 (s, 1H, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) (Fig. S4†). ESI-MS+ (m/z): 568.52[Al(DFP–AMQ)(H2O)2]+, 596.55 [Al(DFP–AMQ)(CH3OH)2]+ (Fig. S5†). FTIR: 1613 cm−1 (CH
N) (Fig. S4†). ESI-MS+ (m/z): 568.52[Al(DFP–AMQ)(H2O)2]+, 596.55 [Al(DFP–AMQ)(CH3OH)2]+ (Fig. S5†). FTIR: 1613 cm−1 (CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) [Fig. S6†].
N) [Fig. S6†].
2.5 Solution preparation for UV-Vis and fluorescence studies
A stock solution of DFP–AMQ was prepared (1.0 × 10−3 M) by dissolving the DFP–AMQ in 10 ml CH3CN for both UV-visible and fluorescence emission titrations. Similarly, another stock solution of Al3+, 1.0 × 10−3 M was prepared in de-ionised water. 10 mM HEPES buffer (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CH3CN
1 CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O) was prepared and the solution of pH was adjusted to 7.2 by using HCl and NaOH. 2.5 ml of the prepared buffer solution was pipetted out into a 5 ml cuvette to which 20 μM of the probe was added and Al3+ ion was added gradually starting from 0 to 30 μM in a regular interval of volume and UV-visible and fluorescence emission spectra were recorded for each solution. Path lengths of the cells used for absorption and emission studies were 1 cm. Fluorescence measurements were performed using a 5 nm × 5 nm slit width.
H2O) was prepared and the solution of pH was adjusted to 7.2 by using HCl and NaOH. 2.5 ml of the prepared buffer solution was pipetted out into a 5 ml cuvette to which 20 μM of the probe was added and Al3+ ion was added gradually starting from 0 to 30 μM in a regular interval of volume and UV-visible and fluorescence emission spectra were recorded for each solution. Path lengths of the cells used for absorption and emission studies were 1 cm. Fluorescence measurements were performed using a 5 nm × 5 nm slit width.
2.6 Job's study
Changing molar concentrations of two reactants Job's study was performed but their sum remains constant. The fluorescence emission of each solution is measured at a suitable wavelength and plotted against the mole fraction of one reactant. A maximum in fluorescence emission occurs at the mole ratio corresponding to the combined ratio of the reactants (Fig. S7†).2.7 Time-correlated single photon counting measurements
Time-correlated single photon counting (TCSPC) measurements were performed in pure acetonitrile and in 10 mM HEPES buffer (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CH3CN
1 CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O) of pH 7.2. The fluorescence decay rates of DFP–AMQ and the [DFP–AMQ–Al]+ complex were measured at 25 °C. TCSPC was performed by photoexcitation at 400 nm using a Hamamatsu MCP photomultiplier (R3809). The resulting data were analyzed by using the following equation with the help of the IBH DAS6 software.
H2O) of pH 7.2. The fluorescence decay rates of DFP–AMQ and the [DFP–AMQ–Al]+ complex were measured at 25 °C. TCSPC was performed by photoexcitation at 400 nm using a Hamamatsu MCP photomultiplier (R3809). The resulting data were analyzed by using the following equation with the help of the IBH DAS6 software.where ai represents the ith pre-exponential factor and t is the decay time.
3. Results and discussion
3.1 Synthesis and characterization
2,6-Diformyl-phenol (DFP, 1)–6-amino-2methylquinoline (AMQ, 2) based (DFP–AMQ) was produced by a Schiff base condensation in the ethanolic medium, which showed reddish colour, as shown in Scheme 1. This ligand has N2O donor atoms which are potentially hexa-coordinated binding to one metal ion leading to the formation of a mononuclear complex. The DFP–AMQ ligand and its Al3+ complex were isolated and characterized using various physicochemical (NMR, IR, and ESI-MS+ (m/z)) (Fig. S1–S6†) methods.3.2 UV-Vis absorption studies
The ESIPT phenomena are often exhibited by intramolecular hydrogen-bonded organic chromophores. In the route of ESIPT, which is an intramolecular form of photo acids and photo bases in chemistry where the molecule environs acidic as well as basic groups; upon excitation, the migration of acidic proton to the basic site occurs. Mostly, ESIPT processes are assisted by either five-or six-membered intramolecular hydrogen bonds in the ground state and commonly require a very minute movement of nuclei. The solvent is not required in intrinsic ESIPT to mediate such a proton transfer process and is barrierless in non-polar solvents occurring in the sub-picoseconds time region.34 In methyl salicylate, the first reported ESIPT reaction was observed.35 The UV-visible absorption spectral properties of DFP–AMQ were measured in 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CH3CN–H2O at pH 7.2 HEPES buffer. The UV-visible absorption spectrum of DFP–AMQ showed two important transitions with strong absorbance at 333 and 369 nm. As per Scheme 2, due to ESIPT the enol and keto tautomers of DFP–AMQ are in equilibrium. At 369 nm, the band is assigned to the transition to keto (quinoid) form. The absorption bands of DFP–AMQ at 369 and 333 nm decrease gradually upon the gradual addition of Al3+; however, two new peaks appeared at 432 and 486 nm, thereby, the formation of one isosbestic point at 400 nm, which clearly designates a clean transformation of the free ligand to its metal-bound state (Fig. 1).
1 CH3CN–H2O at pH 7.2 HEPES buffer. The UV-visible absorption spectrum of DFP–AMQ showed two important transitions with strong absorbance at 333 and 369 nm. As per Scheme 2, due to ESIPT the enol and keto tautomers of DFP–AMQ are in equilibrium. At 369 nm, the band is assigned to the transition to keto (quinoid) form. The absorption bands of DFP–AMQ at 369 and 333 nm decrease gradually upon the gradual addition of Al3+; however, two new peaks appeared at 432 and 486 nm, thereby, the formation of one isosbestic point at 400 nm, which clearly designates a clean transformation of the free ligand to its metal-bound state (Fig. 1).
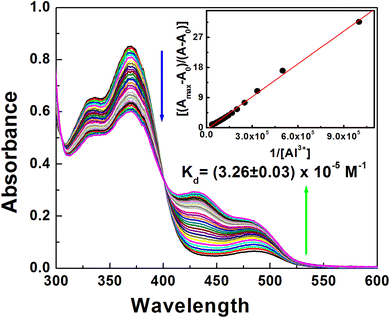 | ||
Fig. 1 Absorption titration of DFP–AMQ (20 μM) with Al3+ (0–30 μM) in CH3CN–H2O (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v, pH 7.20, 10 mM HEPES buffer). Inset shows Benesi–Hildebrand fitting of the data. 1, v/v, pH 7.20, 10 mM HEPES buffer). Inset shows Benesi–Hildebrand fitting of the data. | ||
This indicates the chelation of Al3+ by the hexadentate ligand moiety giving [Al(DFP–AMQ)]+. The spectral change (decrease in absorption spectra at 369 nm and increase at 432 and 486 nm) obviously indicates that the addition of Al3+ ions induces the decrease in the concentration of free ligands both in keto and enol forms. Here, the nitrogen atom takes part in the coordination with Al3+ and the formation of the complex inhibits the ESIPT (Scheme 2). UV-Vis absorption studies were performed to investigate the cation binding affinities towards aluminium ions, and when a change in the absorbance at 432 and 486 nm was plotted against [Al3+], with increasing [Al3+] a gradual increase in the absorbance was observed.
A linear form of the Benesi–Hildebrand equation (eqn (1)) was used to obtain the dissociation constant and stoichiometry of the complexation, where, A0 and Amax are the absorbances of the ligand in the absence and in the presence of excess metal ions, respectively, and n is the stoichiometry (DFP–AMQ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al3+) of the reaction. A linear least square-fit of (Amax − A0)/(A − A0) against 1/[M] clearly demonstrates a 1
Al3+) of the reaction. A linear least square-fit of (Amax − A0)/(A − A0) against 1/[M] clearly demonstrates a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry of the reaction (n = 1), which also gives an apparent dissociation constants Kd = (3.26 ± 0.03) × 10−5 M.
1 stoichiometry of the reaction (n = 1), which also gives an apparent dissociation constants Kd = (3.26 ± 0.03) × 10−5 M.
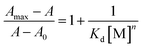 | (1) |
3.3 Fluorescence emission studies
The fluorescence emission spectra of DFP–AMQ and its titration with Al3+ were recorded in CH3CN–H2O (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v, pH 7.20, HEPES buffer) (Fig. 2) at λex = 400 nm. The binding between the free DFP–AMQ and Al3+ leads to the chelation-enhanced fluorescence (CHEF) effect causing ∼27-fold enhancement due to the increased structural rigidity of the formed complexes (Fig. 2). Upon excitation at 400 nm, the isosbestic point in the absorption spectra, in the beginning, there is a gradual increase in the fluorescence intensity (FI) at λem = 561 nm, then a blue shift occurs to λem = 535 nm with the increase in [Al3+] (Fig. 2). This shifting occurs due to the intramolecular charge transfer (ICT).36
1, v/v, pH 7.20, HEPES buffer) (Fig. 2) at λex = 400 nm. The binding between the free DFP–AMQ and Al3+ leads to the chelation-enhanced fluorescence (CHEF) effect causing ∼27-fold enhancement due to the increased structural rigidity of the formed complexes (Fig. 2). Upon excitation at 400 nm, the isosbestic point in the absorption spectra, in the beginning, there is a gradual increase in the fluorescence intensity (FI) at λem = 561 nm, then a blue shift occurs to λem = 535 nm with the increase in [Al3+] (Fig. 2). This shifting occurs due to the intramolecular charge transfer (ICT).36
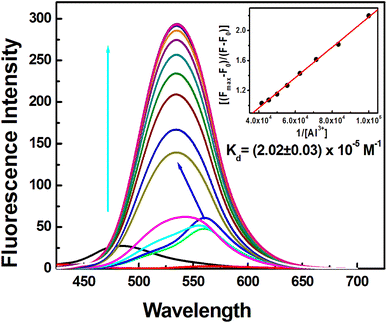 | ||
Fig. 2 Fluorescence titration of DFP–AMQ (20 μM) with Al3+ (0–30 μM) in CH3CN–H2O (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v, pH 7.20, 10 mM HEPES buffer), λex = 400 nm. Inset shows Benesi–Hildebrand fitting of the data. 1, v/v, pH 7.20, 10 mM HEPES buffer), λex = 400 nm. Inset shows Benesi–Hildebrand fitting of the data. | ||
To analyse the binding constant from the fluorescence titration data, a linear form of the Benesi–Hildebrand equation (eqn (1)) was used, which gives the apparent dissociation constant Kd = (20.2 ± 0.03) × 10−5 M. The binding constants value obtained from UV-Vis and the fluorescence titration data clearly indicate the agreement between two Kd values.
3.4 Selectivity study
The selectivity study showed that the probe could selectively detect Al3+ ions, which were not disturbed by other metal ions such as biologically rich K+, Na+, Mg2+, and Ca2+ metal ions, various transition metal ions, namely Fe2+, Fe3+, Mn2+, Ni2+, Cr3+, Co2+, and Cu2+, and heavy metal ions such as Hg2+, Cd2+, Zn2+, and Pb2+, and there was no interference (Fig. S8†). The fluorescent chemosensor was found to bind Al3+ reversibly when it reacted with excess EDTA (Fig. S9†). Moreover, no major change in the emission spectra was found even on excess addition of the above-mentioned interfering metal ions (Fig. S8†).3.5 Calculation of LOD
To determine the detection limit, fluorescence titration of DFP–AMQ with Al3+ was carried out by adding a micromolar concentration of Al3+ in DFP–AMQ (20 μM) and by the 3σ method. The LOD was calculated as follows:| LOD = 3 × Sd/S |
3.6 pH study
For real applications, the appropriate pH conditions required for the successful action of the sensor were estimated. The probe DFP–AMQ showed very weak fluorescence emission between pH 2 and 8, while the DFP–AMQ–Al complex showed very strong fluorescence emission between pH 5 and 8 in 10 mM HEPES buffer, clearly indicating that this pH range is suitable for fluorescence studies for the complexation with Al3+ (Fig. S11†). The fluorescence emission of free DFP–AMQ increases beyond pH 10.0 due to the acid dissociation of the proton.3.7 Aggregation induced emission (AIE) of DFP–AMQ
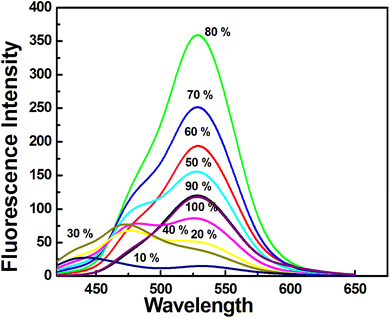
AIE was observed when the percentage of water was varied. Here, CH3CN and H2O were used as good and poor solvents, respectively. This study revealed that with increasing water percentages (0–100) the fluorescence emission increases towards the red region and at 80% of water content, it showed a maximum emission at 528 nm (Fig. 3). This observation clearly indicates the J-type aggregation induced emission due to the presence of a large π-conjugated planar structure. In the aggregates, however, DFP–AMQ molecules face strong face-to-face π–π interactions between two DFP–AMQ molecules, and these are expressed directly by the overlaps in π-conjugated systems. In CH3CN medium DFP–AMQ showed a typical AIE phenomenon having very weak fluorescence.Upon increasing the volume of water percentages up to 80%, fluorescence emission enhancement occurred due to the emissive TICT state (quasi-TICT*). Upon further increase of water from 80 to 99 in vol%, the fluorescence emission intensity decreased, which is known. Due to the restriction of intramolecular motion (RIM) in the aggregated state ACQ effect occurs. Consequently, the formation of a planar structure from the twisted structure in the excited state is accountable for the solution state quenching.
3.8 DLS measurements
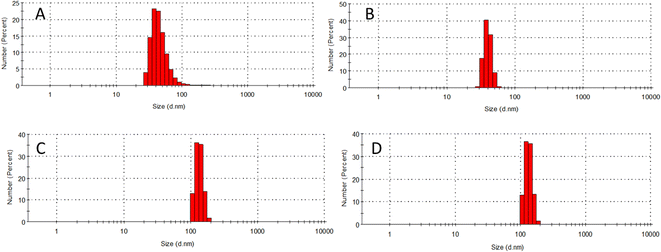
To realise the aggregation behaviour of DFP–AMQ, dynamic light scattering (DLS) experiments were performed in the presence of varying water percentages (Fig. 4). DLS studies revealed that the average size of the aggregated DFP–AMQ in CH3CN/H2O (fw = 10%) was ca. 40.48 nm, as shown in Fig. 4A. This also signifies that DFP–AMQ can be well-dissolved and dispersed in CH3CN/H2O solution. However, an increase in the volume of the water percentage (up to 80%) led to a significant increase in the average size of aggregates (Fig. 4D), which also supported the observed AIE activity of the probe DFP–AMQ. While, the average size of the aggregates became 154.62 nm in 80% volume percentage of water (Fig. 4D), which is in good agreement with the spectroscopic studies.Now to prove the aggregation behavior of DFP–AMQ in the presence of Al3+ ions, we also carried out the DLS measurements in the presence of 0%, 20%, and 50% water. However, an increase in the volume of water percentage led to a significant increase in the average size of aggregates (Fig. S12†), which also supported the observed AIE activity of the probe DFP–AMQ in the presence of Al3+. The average sizes of the above solutions are 204.04 nm, 297.41 nm, and 315.27 nm, respectively.
3.9 Solid state emission
The solid-state emission spectra of DFP–AMQ were measured at λex = 450 nm and its fluorescence intensity (FI) was found at λem = 628 nm. DFP–AMQ showed fluorescence emission due to the aggregation, which is clearly indicated by the scanning electron microscope studies. When the solid DFP–AMQ was ground in a mortar pastel with solid sodium nitrate, the emission decreased and after that, this material was ground with solid sodium acetate, and again the emission spectra were observed with very high intensity, almost double that of the solid DFP–AMQ–NO3. We also recorded the emission spectra with other anion salts, but only the acetate ion responded (Fig. S13†). Hence, this study revealed that in the solid state after grinding with sodium nitrate, it recognized only acetate ions (Fig. 5).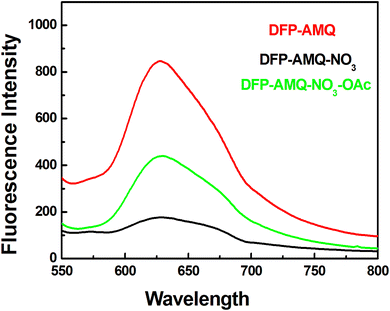 | ||
| Fig. 5 Fluorescence emission of DFP–AMQ with NO3− and both NO3−–OAc− in the solid state (at λex = 450 nm). | ||
3.10 SEM study
The morphologies of DFP–AMQ, its Al-complex, DFP–AMQ in 80% water, and DFP–AMQ with nitrate and DFP–AMQ–nitrate–acetate were studied using the scanning electron microscopy (SEM) analysis, which provided the evidence for the changes in the morphology of DFP–AMQ (rod-like shape) to DFP–AMQ–Al (spherical-like shape) aggregate formation of DFP–AMQ–H2O (aggregated-block-like shape), DFP–AMQ with nitrate (no particular shape) and DFP–AMQ–nitrate–acetate (stone-like shape) (Fig. 6).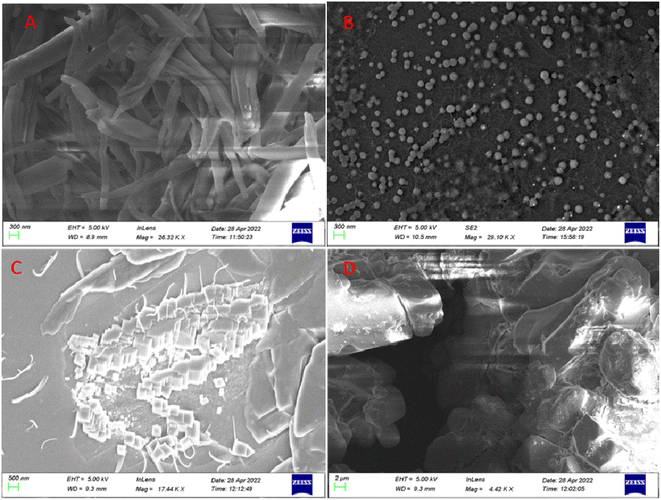 | ||
| Fig. 6 SEM images of (A) DFP–AMQ (solid sample), (B) [DFP–AMQ–Al]+ (solid sample), (C) DFP–AMQ in the presence of H2O, (D) DFP–AMQ–nitrate–acetate. | ||
3.11 Fluorescence microscope imaging
Fluorescence microscope images show that the solid DFP–AMQ showed fluorescent images after treatment with different percentages of water in the solution state and after drop casting on the slide, needle-like aggregated shapes were found (Fig. 7).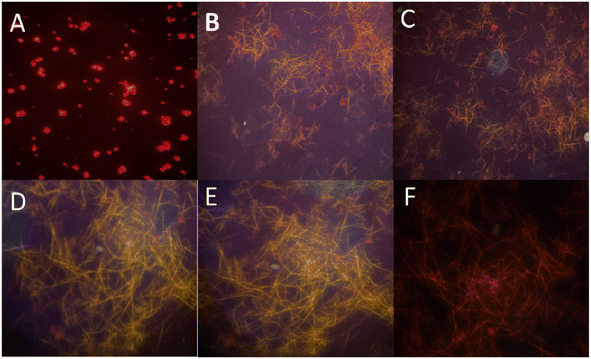 | ||
| Fig. 7 Fluorescence microscope images of (A) DFP–AMQ, DFP–AMQ in the presence of (B) 20% H2O, (C) 50% H2O, (D) 70% H2O, (E) 80% H2O and (F) 100% H2O. | ||
3.12 Time-correlated single photon counting measurements
Time-correlated single-photon counting measurements for the lifetime experiments for the DFP–AMQ in CH3CN and CH3CN–H2O and DFP–AMQ–Al3+ were performed. For the pure CH3CN, the lifetime of DFP–AMQ was 0.09 nS but in the organic aqueous medium, the lifetime increased to 4.59 nS due to its aggregation behavior, while the complex showed a lifetime of 9.58 nS (Fig. S14†).4. Conclusion
In summary, we have successfully designed and synthesized a DFP–AMQ-based hexa-coordinating N2O donor ligand that selectively recognizes Al3+ colorimetrically and fluorimetrically with ∼27-fold fluorescence enhancement in the presence of a number of various interfering metal ions, and it was found to undergo 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexation in MeCN–H2O (9
1 complexation in MeCN–H2O (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v, pH 7.2, HEPES buffer). By blocking ESIPT, chelation-enhanced fluorescence effect (CHEF) arose and the resulting fluorescence enhancement was observed. The complexation of DFP–AMQ to Al3+ was confirmed by 1H NMR and HRMS studies. The Kd values measured were 3.26 × 10−5 M−1 and 2.02 × 10−5 M−1 obtained from the UV-Vis and fluorescence titrations, respectively, revealing the strong binding towards Al3+ ions. The limit of detection of Al3+ by DFP–AMQ was 1.11 μM. The quantum yields of the DFP–AMQ and [DFP–AMQ–Al]+ were 0.008 and 0.211, respectively. Dynamic light scattering (DLS) studies showed an increase in the sizes of the particles with increasing water percentage and this was due to aggregation. Under the different reaction conditions, SEM studies revealed interesting microstructures. The rod-like shape of DFP–AMQ was converted to a spherical-like shape in the presence of Al3+ ions, whereas the block-like shape of the aggregated DFP–AMQ occurring in H2O was observed. In the solid phase, DFP–AMQ with nitrate had no particular shape, but in the presence of acetate, it was converted to a stone-like shape. The observed results indicate that this probe (DFP–AMQ) is very much useful for on-site Al3+ ions detection in the solid state through a fast, simple, and eco-friendly process; it may be useful in OLED and to develop biomedicine for Alzheimer's and Parkinson's diseases.
1, v/v, pH 7.2, HEPES buffer). By blocking ESIPT, chelation-enhanced fluorescence effect (CHEF) arose and the resulting fluorescence enhancement was observed. The complexation of DFP–AMQ to Al3+ was confirmed by 1H NMR and HRMS studies. The Kd values measured were 3.26 × 10−5 M−1 and 2.02 × 10−5 M−1 obtained from the UV-Vis and fluorescence titrations, respectively, revealing the strong binding towards Al3+ ions. The limit of detection of Al3+ by DFP–AMQ was 1.11 μM. The quantum yields of the DFP–AMQ and [DFP–AMQ–Al]+ were 0.008 and 0.211, respectively. Dynamic light scattering (DLS) studies showed an increase in the sizes of the particles with increasing water percentage and this was due to aggregation. Under the different reaction conditions, SEM studies revealed interesting microstructures. The rod-like shape of DFP–AMQ was converted to a spherical-like shape in the presence of Al3+ ions, whereas the block-like shape of the aggregated DFP–AMQ occurring in H2O was observed. In the solid phase, DFP–AMQ with nitrate had no particular shape, but in the presence of acetate, it was converted to a stone-like shape. The observed results indicate that this probe (DFP–AMQ) is very much useful for on-site Al3+ ions detection in the solid state through a fast, simple, and eco-friendly process; it may be useful in OLED and to develop biomedicine for Alzheimer's and Parkinson's diseases.
Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
Rahul Bhowmick gratefully acknowledges UGC New Delhi, India for Dr D. S. Kothari Postdoctoral Fellowship (award letter no. F.4-2/2006 (BSR)/CH/20-21/0201) and Payel Mondal is grateful to CSIR New Delhi for fellowship (award letter no. 09/0025(11972)/2021-EMR-I).References
- K. Dhara, S. Lohar, A. Patra, P. Roy, S. K. Saha, G. C. Sadhukhan and P. Chattopadhyay, Anal. Chem., 2018, 90, 2933–2938 CrossRef CAS PubMed.
- S. Pal, M. Mukherjee, B. Sen, S. Lohar and P. Chattopadhyay, RSC Adv., 2014, 4, 21608–21611 RSC.
- S. Pal, S. Lohar, M. Mukherjee, P. Chattopadhyay and K. Dhara, Chem. Commun., 2016, 52, 13706–13709 RSC.
- Y. Ma, S. Huang, M. Deng and L. Wang, ACS Appl. Mater. Interfaces, 2014, 6, 7790–7796 CrossRef CAS PubMed.
- R. S. Singh, R. K. Gupta, R. P. Paitandi, A. Misra and D. S. Pandey, New J. Chem., 2015, 39, 2233–2239 RSC.
- C. T. Chen, Chem. Mater., 2004, 16, 4389–4400 CrossRef CAS.
- W. Z. Yuan, Y. Gong, S. Chen, X. Y. Shen, J. W. Y. Lam, P. Lu, Y. Lu, Z. Wang, R. Hu, N. Xie, H. S. Kwok, Y. Zhang, J. Z. Sun and B. Z. Tang, Chem. Mater., 2012, 24, 1518–1528 CrossRef CAS.
- S. Reineke, F. Lindner, G. Schwartz, N. Seidler, K. Walzer, B. Lussem and K. Leo, Nature, 2009, 459, 234–238 CrossRef CAS PubMed.
- Y. Sun, N. Giebink, C. H. Kanno, B. Ma and M. E. Thompson, Nature, 2006, 440, 908–912 CrossRef CAS PubMed.
- S. Tonzani, Nature, 2009, 459, 312–314 CrossRef CAS PubMed.
- Z. He, C. Ke and B. Z. Tang, ACS Omega, 2018, 3, 3267–3277 CrossRef CAS.
- M. Shyamal, P. Mazumdar, S. Maity, G. P. Sahoo, G. Salgado-Morán and A. Misra, J. Phys. Chem. A, 2016, 120, 210–220 CrossRef CAS.
- X. Zhao, C. Ji, L. Ma, Z. Wu, W. Cheng and M. Yin, ACS Sens., 2018, 3, 2112–2117 CrossRef CAS.
- Z. Zhao and F. C. Spano, J. Chem. Phys., 2005, 122, 114701 CrossRef PubMed.
- A. Eisfeld and J. S. Briggs, Chem. Phys., 2006, 324, 376–384 CrossRef CAS.
- Y. Hong, J. W. Y. Lam and B. Z. Tang, Chem. Soc. Rev., 2011, 40, 5361–5388 RSC.
- J. Gierschner, L. Lüer, B. M. Medina, D. Oelkrug and H. J. Egelhaaf, J. Phys. Chem. Lett., 2013, 4, 2686–2697 CrossRef CAS.
- P. D. Darbre, F. Mannello and C. Exley, J. Inorg. Biochem., 2013, 128, 257–261 CrossRef CAS PubMed.
- V. Rondeau, D. Commenges, H. Jacqmin-Gadda and J. F. Dartigues, Am. J. Epidemiol., 2000, 152, 59–66 CrossRef CAS PubMed.
- S. Pal, B. Sen, M. Mukherjee, M. Patra, S. Lahiri (Ganguly) and P. Chattopadhyay, RSC Adv., 2015, 5, 72508–72514 RSC.
- E. Alvarez, M. L. F. Marcos, C. Monterroso and M. J. F. Sanjurjo, For. Ecol. Manage., 2005, 211, 227–239 CrossRef.
- J. Barcelo and C. Poschenrieder, Environ. Exp. Bot., 2002, 48, 75–92 CrossRef CAS.
- T. P. Flaten and M. Odegard, Food Chem. Toxicol., 1988, 26, 959–960 CrossRef CAS PubMed.
- R. A. Yokel, Neurotoxicology, 2000, 21, 813–828 CAS.
- K. Tiwari, M. Mishra and V. P. Singh, RSC Adv., 2013, 3, 12124–12132 RSC.
- M. Mukherjee, B. Sen, S. Pal, S. Banerjee, S. Lohar and P. Chattopadhyay, RSC Adv., 2014, 4, 64014–64020 RSC.
- M. Mukherjee, A. Maji, S. Pal, B. Sen and P. Chattopadhyay, Spectrochim. Acta, Part A, 2016, 157, 11–16 CrossRef CAS PubMed.
- B. Sen, S. Pal, S. Banerjee, S. Lohar and P. Chattopadhyay, Eur. J. Inorg. Chem., 2015, 1383–1389 CrossRef CAS.
- B. Sen, M. Mukherjee, S. Banerjee, S. Pal and P. Chattopadhyay, Dalton Trans., 2015, 44, 8708–8717 RSC.
- M. Mukherjee, S. Pal, S. Lohar, B. Sen, S. Sen, S. Banerjee, S. Banerjee and P. Chattopadhyay, Analyst, 2014, 139, 4828–4835 RSC.
- S. Dey, R. Purkait, K. Pal, K. Jana and C. Sinha, ACS Omega, 2019, 4, 8451–8464 CrossRef CAS PubMed.
- M. Shyamal, P. Mazumdar, S. Maity, S. Samanta, G. P. Sahoo and A. Misra, ACS Sens., 2016, 1, 739–747 CrossRef CAS.
- R. Bhowmick, R. Alam, T. Mistri, K. K. Das, A. Katarkar, K. Chaudhuri and M. Ali, RSC Adv., 2016, 6, 11388–11399 RSC.
- S. Scheiner, J. Phys. Chem. A, 2000, 104, 5898–5909 CrossRef CAS.
- A. Weller, Zeitschrift für Elektrochemie, 1956, 60, 1144–1147 CAS.
- J. Y. Choi, D. Kimb and J. Yoon, Dyes Pigm., 2013, 96, 176–179 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ra06978d |
| This journal is © The Royal Society of Chemistry 2023 |