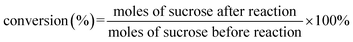Open Access Article
Open Access ArticleCatalytic production of 1,2-propanediol from sucrose over a functionalized Pt/deAl-beta zeolite catalyst†
Shizhuo Wang a,
Jikang Jianga,
Minyan Gua,
Feng Gao*b and
Zheng Shen*a
a,
Jikang Jianga,
Minyan Gua,
Feng Gao*b and
Zheng Shen*a
aNational Facility Agriculture Engineering Technology Research Center, Institute of New Rural Development, Tongji University, Shanghai 201804, China. E-mail: shenzheng@tongji.edu.cn; Tel: +86-21-65985811
bState Key Laboratory of Pollution Control and Resource Reuse, College of Environmental Science and Engineering, Tongji University, Shanghai 200092, China. E-mail: gaofeng_1111@126.com; Tel: +86-21-65985811
First published on 3rd January 2023
Abstract
To eliminate the dependence on fossil fuels and expand the applications of biomass conversion, an efficient Pt/deAl-beta@Mg(OH)2 catalyst was designed, with dealuminated beta zeolite loaded with Pt as the core and Mg(OH)2 as the shell. The catalyst was used to produce 1,2-propanediol (1,2-PDO) from sucrose. The preparation and reaction conditions of the catalyst were optimized. The optimal yield of 1,2-PDO was 33.5% when the conditions were 20 h of dealumination, 3.0 wt% Pt loading, 5.0 wt% Mg(OH)2, 200 mg of catalyst, 10 mL (11.25 mg mL−1) of sucrose solution, an initial H2 pressure of 6 MPa, 200 °C, and 3 h. The core–shell structure of the modified beta zeolite shows good stability, yielding more than 30.0% after three cycles of reuse. Firstly, the molecular zeolite can host more acid sites after dealumination by concentrated nitric acid and this can prolong the catalyst's service life. Secondly, the loading of Pt increases the distribution of acid sites and improves the shape selectivity of the catalyst. The introduction of alkali produces many alkaline sites, inhibits the occurrence of side reactions, and increases the product yield. The above modification methods increase the production of 1,2-PDO by promoting isomerization between glucose and fructose from sucrose hydrolysis and the reverse aldol condensation (RAC) reaction. This paper provides a theoretical basis and reference route for applying biomass conversion technology in practical production, which is of great significance for developing biomass resources into high-value-added chemical products.
1. Introduction
Biomass, as a clean and renewable resource, has been widely investigated to solve the problems of energy shortages and fossil fuel pollution. With its widely distributed nature, sucrose is one of the ideal raw materials for biomass resources.1,2 At present, scholars have made some achievements in the research of sucrose utilization and transformation methods. Saxena prepared diatomite catalysts loaded with Ni, Mo, and Cu to obtain 28% glycerol, 22% ethylene glycol (EG), and 13% 1,2-PDO at high temperature and pressure by using sucrose as a substrate.3 Length used copper aluminum oxide as a catalyst for the catalytic hydrogenolysis of a sucrose methanol suspension.4 About 60% of the distillable polyols can be gathered, of which approximately 60% are 1,2-PDO. Recently, researchers have made some progress in studying the sucrose hydrogenolysis to 1,2-PDO conversion path. When exploring the conversion path of sucrose on a bifunctional Ru-POM (polyoxometalate)/AC catalyst, Garcia-Bosch found that the catalyst needs to have both a metal and an acidic function. Fructose is an essential intermediate product in the synthesis process.5 As an important platform compound, 1,2-PDO is also a research hotspot in chemical synthesis. Previous studies on catalytic sucrose hydrogenolysis focused on the production of glycerol, EG, and 1,2-PDO and their separation, leading to a low yield of 1,2-PDO. Therefore, efficiently producing 1,2-PDO from sucrose has become of extremely high research value.Because of their acid catalytic and structure selection ability, zeolite-modified catalysts are widely used in producing platform compounds.6,7 Furthermore, dealumination and metal modification are commonly applied among the modification methods of beta zeolites. The acid treatment modification, as a reasonable dealumination modification, can regulate the distribution of Brønsted acid sites and Lewis acid sites in zeolites and remove non-framework aluminum from the pores of the beta zeolite.8 Metal modification can change the acidity site distribution and pore volume of the catalysts, among which Pt loading has been a standard modification method for beta zeolite in recent years.9–11 Under acidic conditions, glucose and fructose from sucrose hydrolysis are dehydrated to 5-hydroxymethylfurfural (HMF), and then HMF is hydrolyzed to produce formic acid and levopropionic acid. These side reactions reduce the yield of 1,2-PDO. Besides, acidic conditions convert fructose into lactic acid.7 Therefore, basicity is introduced into the reaction system to inhibit the side reactions and to change the reaction path.12 Recently, researchers have reported that the effect of using NaOH and MgO alone is limited, e.g., MgO alone produced fructose with a selectivity of only 38–44% and a yield of 25%.13 Alkali metal ion exchange treated zeolites, and metal heteroatom molecular sieves (Sn-beta molecular sieve, Ti/SiO2, etc.) can efficiently play the role of alkali metals and molecular sieves, with fructose selectivities of 60–86%.14,15 Zhu introduced WOx into the Pd/Al2O3 catalyst, which enhanced the process of glucose isomerization to fructose.16 The yield of 1,2-PDO increased to 56.1%, and the ratio of 1,2-PDO to ethylene glycol (EG) reached 10.6. They also introduced WOx into the Cu/Al2O3 catalyst. Under the combined action of the catalyst, the yield of 1,2-PDO was 52.6%.17 Many reports have focused on the study of biomass based one-step production of high value-added platform compounds. Polysaccharides are hydrolyzed into monosaccharides (e.g., glucose and fructose), then converted into hexitol after isomerization and retro-aldol condensation (RAC) reaction, and finally, further dehydrated to form target compounds.18,19 There are many studies on the preparation of lactic acid in this catalytic reaction system. Besides, lactic acid can be hydrogenated to 1,2-PDO in a hydrogen atmosphere.9
To improve on the traditional production methods of 1,2-PDO, we constructed a catalytic system to convert biomass into 1,2-PDO by a one-step hydrogenolysis efficiently. In this paper, a Pt/deAl-beta@Mg(OH)2 core–shell catalyst was designed and prepared by dealumination, metal modification, and alkali treatment. Efficient hydrogenolysis from sucrose to 1,2-PDO is achieved by promoting isomerization and retro-aldol reaction (RAC). Pt/deAl@Mg(OH)2 was synthesized by an in situ hydrothermal method using sucrose as the substrate and Mg(OH)2 as a base. The synthesis and catalytic reaction conditions were optimized to improve the yield of 1,2-PDO catalyzed by sucrose. Then, the catalytic activities and parameters of the reaction were explored. Finally, the stability of the core–shell structure in the process of catalyst reuse was investigated. Promoting the industrialization of biomass to prepare high-value-added chemicals provides a technical route reference for research on reducing cost and increasing production.
2. Materials and methods
2.1 Materials
Sucrose (C12H22O11, 99%), 1,2-PDO (C3H8O2, 99.5%), n-propyl alcohol (NPA, C3H8O, 99.5%), ethylene glycol (EG, C2H6O2, 99.5%), chloro-platinic acid (H2PtCl6·6H2O, Pt ≥ 37.5%), and a Pt standard volumetric solution were supplied by Beijing Innochem Science & Technology Co., Ltd. Nitric acid (HNO3, GR, 65–68%), glucose (C6H12O6, 99.5%), fructose (C6H12O6, 99%), 1,2-hexanediol (1,2-HDO, C6H14O2, 98%), magnesium oxide (MgO, 98.5%), and beta zeolite (commercial grade) were purchased from China National Pharmaceutical Group Co., Ltd.2.2 Catalyst preparation
Beta zeolite and nitric acid were added to a three-necked round-bottom flask at a ratio of 1 g![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 mL and mixed evenly. After the round-bottomed flask was placed in an oil bath at 80 °C, the aluminum was removed by condensation reflux (200 rpm) for 20 h. The resulting mixture (high-speed centrifuge, 3000 rpm, 20 min) was centrifuged and filtered, and the beta zeolite was rinsed with deionized water until the supernatant was neutral. The solid component (150 °C, 6 h) was dried in the oven, and the obtained powder was recorded as deAl-beta.2
20 mL and mixed evenly. After the round-bottomed flask was placed in an oil bath at 80 °C, the aluminum was removed by condensation reflux (200 rpm) for 20 h. The resulting mixture (high-speed centrifuge, 3000 rpm, 20 min) was centrifuged and filtered, and the beta zeolite was rinsed with deionized water until the supernatant was neutral. The solid component (150 °C, 6 h) was dried in the oven, and the obtained powder was recorded as deAl-beta.2
1 g of dried deAl-beta powder was added into a 1–10 wt% Pt chloroplatinic acid solution. The mixture was stirred uniformly and then ultrasonically treated for 15 min. The sample was left to stand at room temperature and dry at 105 °C for 6 h. It was then calcinated at 450 °C for 4 h (tube furnace, air atmosphere, heating rate of 2 °C min−1). After hydrothermal treatment (5 M Pa hydrogen pressure, 200 °C, 4 h), the PtO2 supported on the catalyst was reduced to Pt. The preparation of a metal-modified dealuminated beta (deAl-beta) zeolite was completed, and denoted as Pt/deAl-beta.
50–300 mg of catalyst, 0–80 wt% MgO, and 10 mL of deionized water were added to the reactor for hydrothermal reaction (200 °C, 600 rpm, 4 h). After the reaction, the mixture was cooled to room temperature, centrifuged and filtered, and the solid was then dried in an oven to a constant weight (150 °C). In this way, the preparation of the Pt/deAl-beta@Mg(OH)2 catalyst was completed.3
2.3 Catalytic reaction
The catalytic reaction of sucrose was carried out in a 50 mL batch reactor (Fig. S1†). It mainly comprises a control system, heating system, and reaction kettle. The reaction kettle is made of stainless steel and is equipped with a pressure gauge, temperature sensor, an inlet valve, and an outlet valve. 112.5 mg of sucrose was added to the reaction system. After three cycles of purging with 4 MPa H2 (clean air), 6 MPa H2 was added again and the reactor was sealed. The reaction was carried out at 180–240 °C for 1–4 h and then cooled to room temperature. The liquid-phase products and solid-phase catalysts were collected, respectively. The solid catalyst in the reactor was taken out, rinsed with excess deionized water, and dried in an oven to a constant weight (180 °C, 3 h) for reuse.2.4 Identification of catalytic products
2.5 Catalyst characterization
2.6 Calculation
Sucrose conversion (%) was defined as:The yield of products (%) was defined as:
3. Results and discussion
3.1 Synthesis and characterization of the catalyst
| Catalyst | SBETa (m2 g−1) | Smesob (m2 g−1) | Vmicroc (cm3 g−1) | Vmesod (cm3 g−1) | Davee (nm) | Dmesof (nm) |
|---|---|---|---|---|---|---|
| a BET surface area.b BJH adsorption cumulative surface area of pores.c Single point adsorption total pore volume of pores at P/P0 = 0.95.d BJH adsorption cumulative volume of pores.e Adsorption average pore diameter by BET.f BJH adsorption average pore diameter. * The number represents the mass fraction of Mg(OH)2 loading. | ||||||
| Beta | 428 | 134 | 0.36 | 0.36 | 3 | 11 |
| deAl-beta | 412 | 138 | 0.37 | 0.38 | 4 | 11 |
| Pt/deAl-beta | 302 | 116 | 0.33 | 0.41 | 4 | 14 |
| Pt/deAl-beta@5.0Mg(OH)2* | 139 | 102 | 0.18 | 0.25 | 5 | 10 |
| Pt/deAl-beta@7.5Mg(OH)2* | 230 | 177 | 0.26 | 0.37 | 5 | 8 |
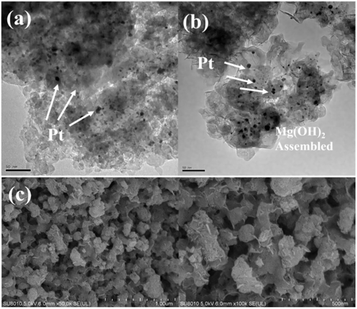
The crystal structures of beta, deAl-beta, Pt/deAl-beta, and Pt/deAl-beta@Mg(OH)2 were analyzed by using XRD (Fig. 2). The diffraction peaks of Pt completely matched the Pt (111), (200), and (220) crystal planes in the JCPDS no. 70-2057 card, indicating that the modified beta zeolites do not undergo severe skeleton collapse. Pt was successfully loaded onto the catalyst. After loading Mg(OH)2, the half-peak widths of Pt (111) and (200) increased, confirming that the Mg(OH)2 shell increases the dispersion of Pt nanoparticles under hydrothermal conditions and effectively inhibits the agglomeration of Pt nanoparticles. There was no peak of Mg(OH)2 in the XRD pattern, and it was inferred that Mg(OH)2 covered the surface of the beta zeolite in an amorphous manner. Comparing the catalysts with different loadings of Mg(OH)2, it can be found that the diffraction peak intensity decreases to a certain extent with the increase of Mg(OH)2 loading. It is speculated that a high Mg(OH)2 loading will form a thicker shell structure on the surface of the catalyst, which will strengthen the shielding effect on the crystal.
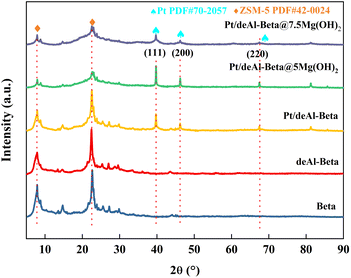
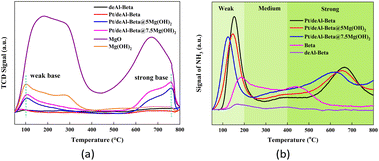
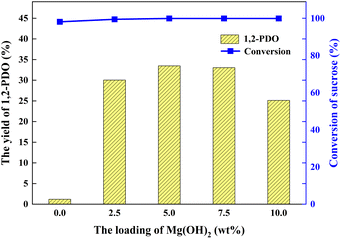
The Pt/deAl-beta@Mg(OH)2 catalyst has a unique shell–core structure, which combines the characteristics of Pt-based materials and Mg(OH)2, so it has excellent acidic sites and basic sites.20 Pyridine adsorption infrared (Py-IR) can be used to characterize the type and number of acid sites. Changes in the acid sites of the catalysts were analyzed using Py-IR, and the proportion of Lewis and strong acid sites and the total acid amount gradually increased with increasing alkaline loading (Table S1† and Fig. 3(b)). When the loading amount of Mg(OH)2 was 5.0 wt%, the number of weak acid sites and total acid amount of the catalyst decreased compared to Pt/deAl-beta. Appropriate alkaline loading can adjust the distribution of acidic sites in the catalyst. When the catalyst loading of Mg(OH)2 is 5.0 wt%, the lower Lewis acid amount and total acid amount are beneficial to converting sucrose to 1,2-PDO because only half of the glucose is produced after sucrose hydrolysis.5
3.2 Catalytic properties
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 10 h (molar ratio of SiO2/Al2O3 is more than 1700
1), 10 h (molar ratio of SiO2/Al2O3 is more than 1700![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
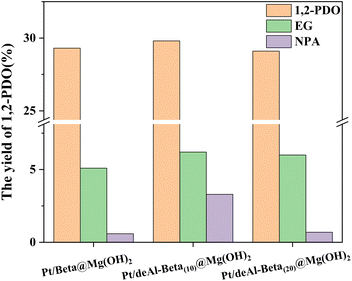
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), and 20 h were tested, respectively. Fig. 4 shows the yield results of 1,2-PDO, EG and NPA catalyzed by Pt/beta@Mg(OH)2 (control group), Pt/deAl-beta(10)@Mg(OH)2, and Pt/deAl-beta(20)@Mg(OH)2. The yields of 1,2-PDO and EG were 29.3%, 29.8%, and 29.1%, and 5.1%, 6.2%, and 6.0%, respectively. Under the catalytic effect of Pt/deAl-beta(10)@Mg(OH)2, the yield of NPA (main by-product) was 3.3%, which was much higher than 0.6% and 0.7% of the other two groups, suggesting that 1,2-PDO was more easily dehydrogenated to NPA under the catalysis of Pt/deAl-beta(10)@Mg(OH)2. In light of the yields of the target product, 1,2-PDO, and the by-product NPA, we used Pt/deAl-beta(20)@Mg(OH)2 for the experiment, and abbreviated it as Pt/deAl-beta@Mg(OH)2.
1), and 20 h were tested, respectively. Fig. 4 shows the yield results of 1,2-PDO, EG and NPA catalyzed by Pt/beta@Mg(OH)2 (control group), Pt/deAl-beta(10)@Mg(OH)2, and Pt/deAl-beta(20)@Mg(OH)2. The yields of 1,2-PDO and EG were 29.3%, 29.8%, and 29.1%, and 5.1%, 6.2%, and 6.0%, respectively. Under the catalytic effect of Pt/deAl-beta(10)@Mg(OH)2, the yield of NPA (main by-product) was 3.3%, which was much higher than 0.6% and 0.7% of the other two groups, suggesting that 1,2-PDO was more easily dehydrogenated to NPA under the catalysis of Pt/deAl-beta(10)@Mg(OH)2. In light of the yields of the target product, 1,2-PDO, and the by-product NPA, we used Pt/deAl-beta(20)@Mg(OH)2 for the experiment, and abbreviated it as Pt/deAl-beta@Mg(OH)2.
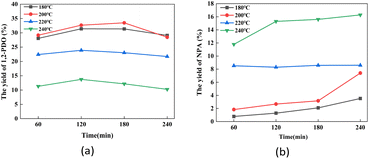 | ||
| Fig. 7 The effect of reaction time on sucrose hydrogenolysis into (a) 1,2-PDO and (b) NPA. Reaction conditions: catalyst (200 mg), sucrose (11.25 mg mL−1, 10 mL), and H2 (initial 6 MPa). | ||
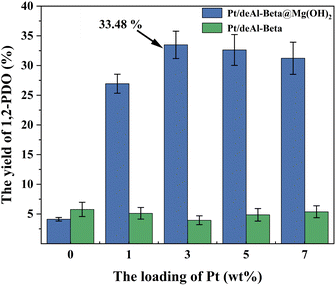
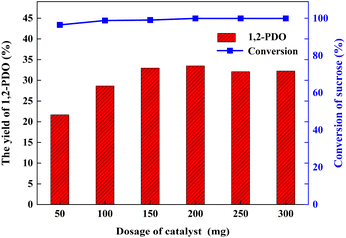
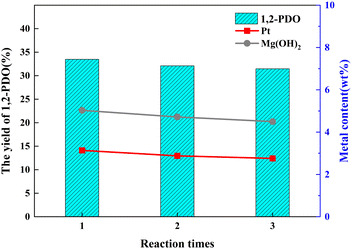
During the 3 h process, when the catalyst dosage increased from 50 mg to 200 mg, the yield of 1,2-PDO rose significantly from 21.7% to 33.5%. With the continuous improvement of catalyst dosage, the yield of 1,2-PDO is generally improved. From the perspective of economic feasibility, it can be determined that the optimal dosage of Pt/deAl-beta@Mg(OH)2 catalyst in this study is 200 mg (Fig. 8).
3.3 Analysis of the catalytic reaction mechanism
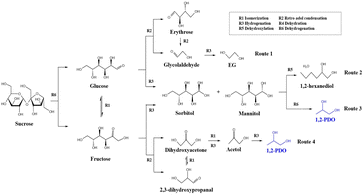
Sucrose is first converted to fructose and glucose by hydrolysis. Glucose can be converted to fructose by isomerization (Fig. 10).24 On the one hand, glucose produces erythrose and glycolaldehyde by RAC reaction. As the reaction proceeds, EG will eventually be generated (Route 1). On the other hand, fructose is converted to dihydroxyacetone (DHA) and 2,3-dihydroxypropanal (2,3-DHA) through the RAC reaction. In a hydrogen atmosphere, dihydroxyacetone finally produced 1,2-PDO by hydrogenation (Route 4). Besides, glucose and fructose can produce sorbitol and mannitol directly by hydrogenolysis. Then, hexitol is converted to 1,2-PDO by isomerization and RAC reaction (Route 3).25,26 The improvement caused by Mg(OH)2 in producing 1,2-PDO is due to three factors: the promotion of isomerization, RAC reaction, and the reconversion of hexitol.27,284. Conclusions
In this paper, based on the existing modified beta zeolite one-step catalytic process, to increase the production of 1,2-PDO from sucrose, the catalyst preparation parameters (dealumination time, Pt loading, and Mg(OH)2 loading) were adjusted. At the same time, the catalyst dosage, reaction temperature, and reaction time were optimized for the catalytic process. The optimal conditions were determined: Pt loading: 3.0 wt%, Mg(OH)2 loading: 5.0 wt%, catalyst: 200 mg, sucrose solution: 10 mL (11.25 mg mL−1), H2 initial pressure: 6 MPa, reaction temperature: 200 °C, and reaction time: 3 h. The optimal yield of 1,2-PDO was 33.5%. From the characterization results of CO2-TPD and NH3-TPD, it can be reported that after Mg(OH)2 loading, the acid sites are converted to medium and strong acid sites, and weak and strong alkaline sites are produced in large quantities. The characterization results of XRD show that the shell structure of Mg(OH)2 does not entirely block the reaction sites of Pt nanoparticles, which explains why the catalytic system can have both acid and alkali catalytic ability. Finally, the recycling performance of the catalyst was explored, and the structural advantages of the Pt/deAl-beta@Mg(OH)2 catalyst were verified: the unique shell–core structure can effectively prevent the loss of supported metals and increase the structural stability of the catalyst.Author contributions
Conceptualization, Zheng Shen and Feng Gao; methodology, Shizhuo Wang, Jikang Jiang, and Minyan Gu; validation, Zheng Shen and Feng Gao; data curation, Shizhuo Wang and Jikang Jiang; writing—original draft preparation, Shizhuo Wang; writing—review and editing, Shizhuo Wang , Zheng Shen and Feng Gao; formal analysis, Shizhuo Wang; supervision, Zheng Shen and Feng Gao; project administration, Zheng Shen and Feng Gao.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 21978224, U21A20322), Shanghai Science & Technology Committee (No. 21dz1202400, 22dz1209300) and Key Projects of Intergovernmental International Scientific and Technological Innovation Cooperation (2022YFE0120600).Notes and references
- M. Morales, P. Y. Dapsens, I. Giovinazzo, J. Witte, C. Mondelli, S. Papadokonstantakis, K. Hungerbühler and J. Pérez-Ramírez, Energy Environ. Sci., 2015, 8, 558–567 RSC.
- M. S. Holm, S. Saravanamurugan and E. Taarning, Science, 2010, 328, 602–605 CrossRef CAS.
- U. Saxena, N. Dwivedi and S. R. Vidyarthi, Ind. Eng. Chem. Res., 2005, 44, 1466–1473 CrossRef CAS.
- C. Lenth and R. DuPuis, Ind. Eng. Chem., 1945, 37, 152–157 CrossRef CAS.
- N. García-Bosch, C. Especel, A. G. Ruiz and I. Rodríguez-Ramos, Catal. Today, 2020, 357, 113–121 CrossRef.
- M. Xia, W. Dong, M. Gu, C. Chang, Z. Shen and Y. Zhang, RSC Adv., 2018, 8, 8965–8975 RSC.
- W. Dong, Z. Shen, B. Peng, M. Gu, X. Zhou, B. Xiang and Y. Zhang, Sci. Rep., 2016, 6, 1–8 CrossRef.
- M. D. González, Y. Cesteros and P. Salagre, Microporous Mesoporous Mater., 2011, 144, 162–170 CrossRef.
- S. Wang, J. Jiang, M. Gu, Y. Song, J. Zhao, Z. Shen, X. Zhou and Y. Zhang, Nanomaterials, 2022, 12, 3771 CrossRef CAS PubMed.
- C. Liu, J. M. Carraher, J. L. Swedberg, C. R. Herndon, C. N. Fleitman and J.-P. Tessonnier, ACS Catal., 2014, 4, 4295–4298 CrossRef CAS.
- H. W. Lee, J.-K. Jeon, K.-E. Jeong, C.-U. Kim, S.-Y. Jeong, J. Han and Y.-K. Park, Chem. Eng. J., 2013, 232, 111–117 CrossRef CAS.
- X. Wang, Y. Song, C. Huang, F. Liang and B. Chen, Green Chem., 2014, 16, 4234–4240 RSC.
- A. A. Marianou, C. M. Michailof, A. Pineda, E. F. Iliopoulou, K. S. Triantafyllidis and A. A. Lappas, ChemCatChem, 2016, 8, 1100–1110 CrossRef CAS.
- J. M. Carraher, C. N. Fleitman and J.-P. Tessonnier, ACS Catal., 2015, 5, 3162–3173 CrossRef CAS.
- I. Delidovich and R. Palkovits, ChemSusChem, 2016, 9, 547–561 CrossRef CAS PubMed.
- C. Liu, C. Zhang, S. Sun, K. Liu, S. Hao, J. Xu, Y. Zhu and Y. Li, ACS Catal., 2015, 5, 4612–4623 CrossRef CAS.
- C. Liu, C. Zhang, S. Hao, S. Sun, K. Liu, J. Xu, Y. Zhu and Y. Li, Catal. Today, 2016, 261, 116–127 CrossRef CAS.
- M. Xia, W. Dong, Z. Shen, S. Xiao, W. Chen, M. Gu and Y. Zhang, Sustainable Energy Fuels, 2020, 4, 5327–5338 RSC.
- M. Xia, Z. Shen, S. Xiao, B.-y. Peng, M. Gu, W. Dong and Y. Zhang, Appl. Catal., A, 2019, 583, 117126 CrossRef CAS.
- B. Y. Peng, Y. Su, Z. Chen, J. Chen, X. Zhou, M. E. Benbow, C. S. Criddle, W. M. Wu and Y. Zhang, Environ. Sci. Technol., 2019, 53, 5256–5265 CrossRef CAS.
- B. Y. Peng, Z. Chen, J. Chen, H. Yu, X. Zhou, C. S. Criddle, W. M. Wu and Y. Zhang, Environ. Int., 2020, 145, 106106 CrossRef CAS.
- B. Y. Peng, Z. Chen, J. Chen, X. Zhou, W. M. Wu and Y. Zhang, J. Hazard. Mater., 2021, 416, 125803 CrossRef CAS PubMed.
- V. Paixão, A. P. Carvalho, J. Rocha, A. Fernandes and A. Martins, Microporous Mesoporous Mater., 2010, 131, 350–357 CrossRef.
- M. Gu, Z. Shen, W. Zhang, M. Xia, J. Jiang, W. Dong, X. Zhou and Y. Zhang, ChemCatChem, 2020, 12, 3447–3452 CrossRef CAS.
- H. Chen, H. Yang, O. Omotoso, L. Ding, Y. Briker, Y. Zheng and Z. Ring, Appl. Catal., A, 2009, 358, 103–109 CrossRef CAS.
- G. Liang, L. He, H. Cheng, W. Li, X. Li, C. Zhang, Y. Yu and F. Zhao, J. Catal., 2014, 309, 468–476 CrossRef CAS.
- M. Gu, Z. Shen, L. Yang, B. Peng, W. Dong, W. Zhang and Y. Zhang, Ind. Eng. Chem. Res., 2017, 56, 13572–13581 CrossRef CAS.
- M. Gu, L. Liu, Y. Nakagawa, C. Li, M. Tamura, Z. Shen, X. Zhou, Y. Zhang and K. Tomishige, ChemSusChem, 2021, 14, 642–654 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ra07097a |
| This journal is © The Royal Society of Chemistry 2023 |