 Open Access Article
Open Access ArticleStability of carbon quantum dots: a critical review
Shweta Dua ab,
Pawan Kumar†
ab,
Pawan Kumar†
 b,
Balaram Pani
b,
Balaram Pani c,
Amarjeet Kaur
c,
Amarjeet Kaur d,
Manoj Khannaf and
Geeta Bhatt
d,
Manoj Khannaf and
Geeta Bhatt *e
*e
aDepartment of Electronic Science, University of Delhi, South Campus, India
bBhaskaracharya College of Applied Sciences, University of Delhi, India
cDean of Colleges, University of Delhi, India
dDepartment of Physics and Astrophysics, University of Delhi, India
eNon-Collegiate Women's Education Board, University of Delhi, Second Floor, Guru Tegh Bahadur Rd University Enclave, New Delhi, Delhi 110007, India. E-mail: geeta.bhatt@bcas.du.ac.in
fFaculty of Interdisciplinary and Applied Sciences, University of Delhi, India
First published on 9th May 2023
Abstract
Carbon quantum dots (CQDs) are fluorescent carbon nanomaterials with unique optical and structural properties that have drawn extensive attention from researchers in the past few decades. Environmental friendliness, biocompatibility and cost effectiveness of CQDs have made them very renowned in countless applications including solar cells, white light-emitting diodes, bio-imaging, chemical sensing, drug delivery, environmental monitoring, electrocatalysis, photocatalysis and other related areas. This review is explicitly dedicated to the stability of CQDs under different ambient conditions. Stability of CQDs is very important for every possible application and no review has been put forth to date that emphasises it, to the best of our knowledge. This review's primary goal is to make the readers cognizant of the importance of stability, ways to assess it, factors that affect it and proposed ways to enhance the stability for making CQDs suitable for commercial applications.
1. Introduction
The term “CQDs” refers to discrete, quasi-spherical, extremely fluorescent nanomaterials that are between 2 and 8 nm in size.1–3 CQDs feature a core–shell structure with a carbon core (2–3 nm with 0.2 nm lattice spacing)4 and self-passivated shell (comprising functional groups).5 The core (intrinsic states), according to studies, can either be graphitic crystalline (sp2) or amorphous (mixed sp2/sp3).6–8 The structure of CQDs depends on various parameters like the type of precursor, synthesis method, pH, solvent type, synthesis duration, etc.9 Due to their similarities to inorganic QDs, they were initially called “carbon nanoparticles” but later changed their name to “carbon dots”. They are also called carbon nanodots or fluorescent carbon nanoparticles.10Researchers' attention has been drawn ever since the unintentional discovery of CQDs by Xu et al. in 2004 while purifying single-walled carbon nanotubes made from arc-discharge soot by gel electrophoresis.11 CQDs mainly comprise carbon; an abundant and nontoxic element in the universe which has always captivated researchers since ages.
CQDs are a true competitor to their traditional inorganic counterparts due to their exceptional nature and distinctive structure, which are evident in their optical, chemical, physical and electronic properties, as well as their low cytotoxicity, good water solubility, excellent stability, and ease of synthesis.12,13
From both a theoretical and practical standpoint, fluorescence is one of the most attractive characteristics of CQDs. The vast majority of studies document the excitation-dependent emission behaviour of CQDs, however independent excitation-emission has also occasionally been observed in some cases. There are many theories that have been put forth to explain the emission in CQDs, but three have received the most consideration. These theories are: (1) size-dependent emission (quantum confinement effect), which is associated to the carbon core's conjugated p-domains; (2) the surface states, which are associated with the existence of functional groups linking carbon backbone; and (3) the molecular state in which fluorescent molecules are either free or bound and produce the emission.14–17
Earlier heavy metals like cadmium or mercury were considered to be the major components in the synthesis of traditional QDs. They were utilised in several applications and were acknowledged as a template for all other QDs.18 However, the use of them in biological systems has been seriously questioned due to their possible toxicity. Recent investigations have demonstrated that the degradation of heavy metals-based QDs in biological settings is caused by the release of extremely cytotoxic ions.19 With the development of CQDs (Cd-free QDs) which were considered superior to conventional QDs in terms of photo stability, thermal stability, aqueous stability, biocompatibility, chemical stability and cytotoxicity, the problem of degradation of cellular environment by Cd was reduced.20,21 This leads to the development of CQDs and consequently, CQDs have turned out to be excellent alternatives to classical metal-based semiconductor quantum dots (SQDs).22
The synthesis method, precursors employed, and other synthetic parameters (such as temperature, time, pH, etc.) affect the properties of CQDs (structure, fluorescence, solubility, etc.).23,24 Consequently, CQDs with diverse features can be synthesised by altering the said parameters which made them useful in myriad of applications including drug delivery, bioimaging, biosensors, chemical sensing, nanomedicine, light-emitting diodes, environmental monitoring, disease diagnosis, electrocatalysis, photocatalysis and white light-emitting diodes, etc.25,26
Although stability of CQDs is one of the prerequisites for implementing CQDs in practical applications, no review has been reported till date to the best of our knowledge emphasizing the stability of CQDs under different ambient conditions. Thus, this review systematically discusses various types of stability including photostability, thermal stability, time stability, stability under high salt conditions and different pH values. The current research on CQDs has mostly concentrated on two aspects: (i) establishing a more simple, affordable, and environmentally friendly synthetic approach; and (ii) broadening the CQDs' field of use. The aspect of stability is somewhat missing knowingly or unknowingly which needs significant attention, if CQDs are to be used in diverse applications. So, the purpose of this present review is to make the reviewers cognizant about the importance of stability, factors which influence it and possible ways to improve the CQD's stability.
2. Stability of CQDs
It has been observed that properties of CQDs are affected by various factors like UV light irradiation, temperature, salts (NaCl, KCl, etc.), pH, etc. thus the performance of CQDs should be evaluated exclusively in light of these parameters.2.1. Photostability of CQDs
Photostability is the property of a material which indicates the stability of material under the exposure to radiation (UV, visible, etc.). Usually, fluorescent materials tend to bleach with time when exposed to light. Therefore, high photostability is required in applications where materials are exposed to light for long duration.In 2014, Tak H. Kim et al. synthesised organosilane functionalized blue luminescent carbon dots (CDs) by utilising precursors such as citric acid (carbon source) and N-(β-aminoethyl)-γ-aminopropylmethyl-dimethoxysilane (AAPMS) (solvent). The synthesised CDs had an average diameter of around 0.9 nm with 47% QY The prepared CDs were then utilised for (i) thin film fabrication in silica matrix (CD in silica) and (ii) embedded with salt crystals (S-CDs) to perform the stability test. Different salts (KBr, KCl and NaCl) were used separately to form different types of S-CDs.
For the photostability test, a UV lamp (365 nm, 5 W) with an energy intensity of 30 mW cm−2 was used to expose the sample for 264 hours at a distance of 20 cm. A comparison was made among CDs in silica and S-CDs as shown in Fig. 1.
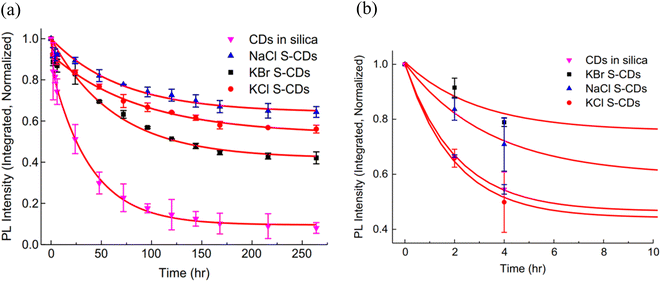 | ||
| Fig. 1 (a) UV stability of CD in silica and S-CDs in NaCl, KCl and KBr salts and (b) a detailed graphic showing the thermal stability of all samples throughout the first 8 hours (this figure has been adapted/reproduced from ref. 27 with permission from Elsevier, copyright 2014). | ||
A rapid degradation of PL intensity was observed when CDs in silica were directly exposed to UV over the testing period. 20% PL loss was observed after 4 hours exposure, stabilising at 10% of the original intensity after 150 hours exposure as shown in Fig. 1(a). PL degradation in S-CDs after 4 hours of exposure was less than 10%. i.e. much improved stability was observed by them under the same conditions as shown in Fig. 1.
The most stable specimen, S-CDs in NaCl, degraded 20% of the PL's original intensity in 60 hours, which is 15 times slower than CDs in silica. After stabilising at around 200 hours, it kept over 70% of the initial PL intensity level, which is 9 times greater than that of CDs in silica. When compared to CdTe quantum dots with salt crystal implanted, NaCl S-CDs were shown to have extraordinarily good PL stability. The three S-CDs samples' PL decay rates were also examined, and it was found that they were similar for the first four hours before becoming noticeably different. S-CDs in NaCl were found to be the most stable, while SCDs in KBr were the least stable. The water and oxygen trapped in the salt crystals during crystallisation resulted in oxidation degradation processes, which reduced the PL intensity by 30 to 40%. At the initial stage in all S-CD samples, fast rate PL decay was likely caused by the degradation of the adsorbed CD particles on the crystal surface.
The prepared S-CDs were used as a colour-converting low cost phosphor component for GaN UV light emitting diodes (LEDs). In order to evaluate the CDs stability, the synthesised LEDs were run nonstop for a week. In contrast to other S-CD-LEDs and the LED made from CDs in silica, the NaCl S-CD-LED demonstrated a stability level of 77% of PL intensity retention throughout the course of the testing period of one week, as shown in Fig. 2. Additionally, it was found that salt-embedded CDs offer a practical and efficient way to shield CDs against heat and UV deterioration. Comparing them to CDs in silica-LED, they showed a considerable improvement in PL stability as well as enhanced processability, proving that salt encapsulation has converted CDs into thermally and UV-stable color-converting phosphors for UV LEDs.27
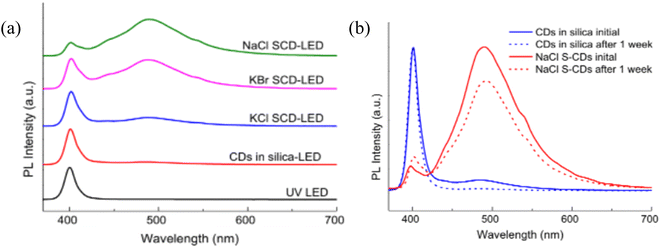 | ||
| Fig. 2 (a) Spectra (b) a week-long test of LED PL intensity (this figure has been adapted/reproduced from ref. 27 with permission from Elsevier, copyright 2014). | ||
In 2014, C. Wang et al. used the hydrothermal approach to synthesize blue luminescent carbon dots (CDs) utilising glucose as the carbon source and glutathione (GSH) at 180 °C for 22 hours. The QY of the synthesized CDs particles, which were amorphous in form and had a diameter of 2.6 ± 0.2 nm, was determined to be 7.2% at ambient temperature.
A xenon (Xe) lamp (450 W) was used to expose the synthesised CDs for varying lengths of time in order to test their photo stability. The fluorescence spectra revealed that even after two hours (120 minutes) of irradiation, the synthesized CDs maintained 92% of their original fluorescence intensity. This is illustrated in Fig. 3. According to the findings, the prepared CDs have high photostability.28
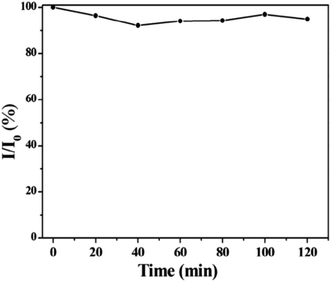 | ||
| Fig. 3 Photostability of the synthesized CQDs exposed to 450 W Xe light at different times (this figure has been adapted/reproduced from ref. 28 with permission from Elsevier, copyright 2015). | ||
In 2015, W. Wang et al., produced Carbon Nanodots (CNDs) from citric acid and urea by using solvothermal technique at 200 °C for 8 hours. According to the Stokes–Einstein relations, the produced CNDs had a diameter of 0.921 ± 0.005 nm and were transparent and brownish in colour. The production yield of prepared CNDs was about 60% and quantum efficiency around 35%. UV irradiation (365 nm, 8 W) was applied using a UV lamp with incidence intensity of 950 μW cm−2 to test the photostability of synthesised CNDs. To avoid any UV leakage, a black box was employed to house the UV bulb and CNDs were placed in it for up to 48 hours. Degeneration in fluorescence was observed which was caused by a photochemical process when CNDs were exposed to UV light. This decay in fluorescence intensity was caused by oxygen and the surface condition of CNDs. Fluorescence photobleaching of the synthesised CNDs was studied exhaustively along with the various ways for its prevention. As can be seen in Fig. 4, photobleaching caused a more than ten-fold reduction in fluorescence intensity, but no apparent wavelength shift was seen. They came to the following conclusions based on their observations and findings: (i) the length and intensity of exposure governed photobleaching (Fig. 4), the CNDs concentration (Fig. 5), the combined presence of solvent and oxygen (Fig. 6), and (ii) a slight loss in mass and size of CNDs due to photobleaching indicated a loss of molecular structure.
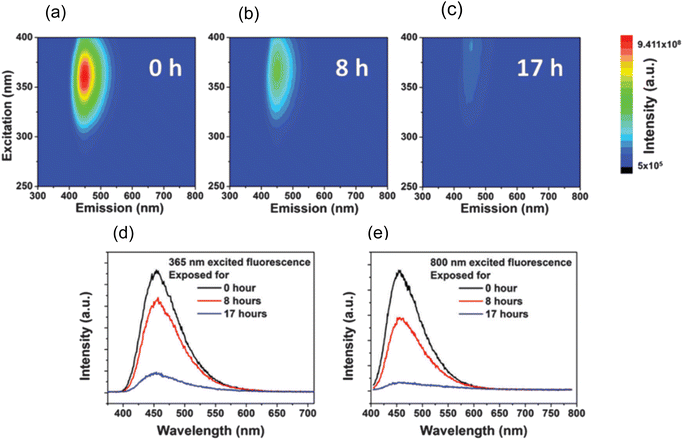 | ||
| Fig. 4 3D luminescence plots of CNDs in aqueous solution at ambient temperature after (a) 0 hour, (b) 8 hours, and (c) 17 hours of UV irradiation. From blue to green to red, the intensity rises. The luminescence spectra of CNDs in aqueous solutions upon excitation at (d) 365 nm and (e) 800 nm, respectively at ambient temperature (this figure has been adapted/reproduced from ref. 29 with permission from Royal Society of Chemistry, copyright 2016). | ||
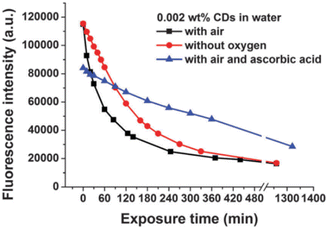 | ||
| Fig. 5 Kinetics of UV-induced fluorescence photobleaching of a 0.002 wt% CNDs solution in water in the presence and absence of oxygen and in the presence of ascorbic acid (this figure has been adapted/reproduced from ref. 29 with permission from Royal Society of Chemistry, copyright 2016). | ||
 | ||
| Fig. 6 (a) Fluorescence intensity as a function of concentration, normalised to CND concentration. The relationship shows that the fluorescence quantum yield of CNDs declines with increasing CND concentration greater than 0.0082 wt%. (b) The graphic shows how CND fluorescence self quenching occurs (this figure has been adapted/reproduced from ref. 29 with permission from Royal Society of Chemistry, copyright 2016). | ||
The research team discovered that encapsulating CNDs in a polymer matrix, like PMMA, results in the prolonged photostability of the prepared CNDs as shown in Fig. 7, thus opening many possibilities for upcoming applications.29
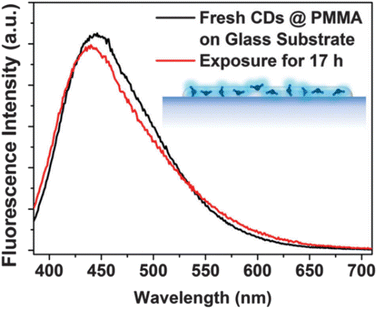 | ||
| Fig. 7 Before-and-after UV luminescence spectra of CND@PMMA on a glass substrate (this figure has been adapted/reproduced from ref. 29 with permission from Royal Society of Chemistry, copyright 2016). | ||
In 2016, Y. Guo et al., synthesized blue emitting CQDs via a novel, facile and low-cost hydrothermal treatment of human hair. They were cleaned with water and ethanol, added to an autoclave, and then heated in an oven at 200 °C for 24 hours to produce a black solid. The resulting CQDs were on average 4.56 nm in size. They exhibited strong excitation-dependent emission behaviour, with a quantum yield of 10.75%. To check the photostability of CQDs, continuous UV radiation (365 nm, 16 W) was shone upon CQDs. Even after 80 min of illumination, no decrease in fluorescence intensity was observed, demonstrating the good photostability of CQDs as shown in Fig. 8.30
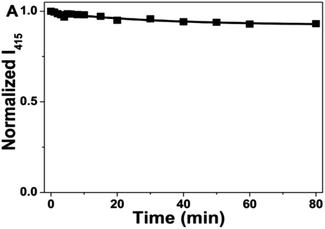 | ||
| Fig. 8 Test of the photostability of CQDs under continuous 365 nm illumination (this figure has been adapted/reproduced from ref. 30 with permission from Nature, copyright 2016). | ||
In the same year 2016, J. Zhao et al., prepared CQDs using precursors like sodium citrate and carbamide via the microwave method. The produced CQDs had a QY of 15% and a diameter of 3–5 nm on average. These CQDs were mixed with polyurethane acrylate (PUA) to form thin film (CQDs/PUA composite). As shown in Fig. 9, after 24 hours of nonstop UV light exposure, the QY of the CQDs in the films remains approximately 15%. Even after the constant 120 hours UV excitation, CQDs in the membrane were kept stable and the QY was still around 14%, indicating extraordinary photostability of CQDs. The findings demonstrate that the CQDs' fluorescence feature is stable in the polymer matrix and validates their potential as a material for flexible electronics.31
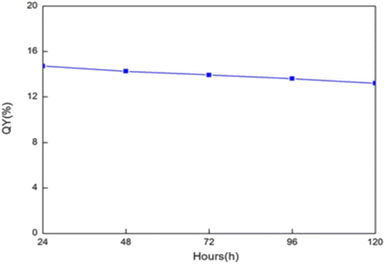 | ||
| Fig. 9 Quantum yield of the CQDs inside the thin film under continuous irradiation (this figure has been adapted/reproduced from ref. 31 with permission from Wiley, copyright 2017). | ||
In 2016, Y. Liu et al. prepared highly fluorescent nitrogen-doped carbon dots (N-CDs) using precursors such as citric acid (CA) and tris(hydroxymethyl)methyl aminomethane (Tris) via one-step hydrothermal route. The mixture solution of N-CDs underwent a hydrothermal reaction for 6 hours in an autoclave at 200 °C. As a nitrogen-doping agent, Tris was used along with a structure resembling a dendron and abundance of hydroxyl groups (–OH). The synthesised N-CDs were amorphous in nature and had an average diameter of 3.45 nm with a QY of 75%.
N-CDs were exposed to UV irradiation (365 nm, 250 W) from mercury lamp with radiant intensity 20 mW cm−2 in order to evaluate the photostability and no appreciable change in the fluorescence intensity of N-CDs was seen as the testing time was increased by 30 minutes. A 96% retention of fluorescence intensity was seen even after a 360 minutes exposure (Fig. 10).32
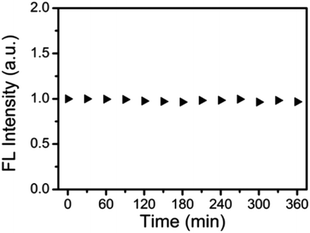 | ||
| Fig. 10 Fluorescence intensity of N-CDs exposed to 20 mW cm−2 UV irradiation from a 250 W mercury lamp at various time intervals (this figure has been adapted/reproduced from ref. 32 with permission from Royal Society of Chemistry, copyright 2017). | ||
In 2018 B. Ju et al., synthesised excitation-independent yellow-green emission carbon dots (CDs) from chloroform and o-phenylenediamine using a facile solvothermal method in which prepared mixtures of precursors were heated at 160 °C for 12 hours in an autoclave. The prepared CDs were ∼5–30 nm size and exhibited a PL QY of 13.20%. It was found that with continuous UV light illumination (365 nm, 12 W) on CDs for 8 hours, only slight attenuations were recorded (Fig. 11) indicating excellent photostability of CDs. These CDs exhibited highly selective detection toward explosive 2,4,6-trinitrophenol (TNP) in environmental monitoring.33
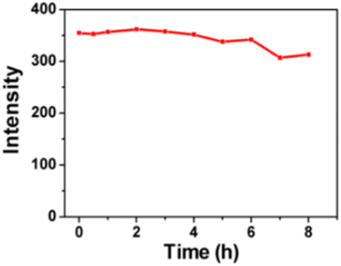 | ||
| Fig. 11 Photostability measurements of CDs solution (this figure has been adapted/reproduced from ref. 33 with permission from American Chemical Society, copyright 2018). | ||
In 2018, W. U. Khan et al., synthesized extremely emissive green Nitrogen-Doped Carbon Dots (N-CDs) by heating the precursors (ammonium citrate and urea) in an oven at 200 °C for 1 hour. The generated N-CDs had an average size of roughly 9.3 nm and a QY of 13.4% and 50.3% upon optimal excitation at 400 nm in deionized water and ethanol, respectively.
As shown in Fig. 12(a), very minor attenuations were seen after three hours of continuous UV lamp illumination (365 nm, 8 W) on N-CDs, and 96% of the original intensity remained after 180 minutes of UV lamp irradiation, demonstrating the N-CDs' remarkable photobleaching resistance. On contrary, when these N-CDs were integrated with PVA matrix to form flexible and transparent N-CD/PVA composite film and exposed to UV light illumination (365 nm, 8 W), the emission intensity of the N-CDs/PVA composite decreased to 5% of their initial intensity after 120 minutes continuous UV irradiation as shown in Fig. 12(b).34
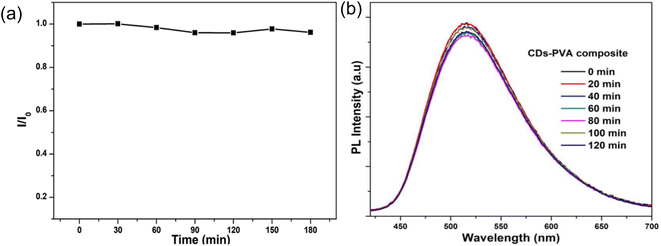 | ||
| Fig. 12 (a) N-doped CDs' photochemical stability under 365 nm UV light; (b) photo-stability of CDs-PVA composite under UV (365 nm) lamp irradiation at various intervals of time (this figure has been adapted/reproduced from ref. 34 with permission from American Chemical Society, copyright 2018). | ||
In 2019 A. Dager et al. synthesised crystalline CQDs using fennel seeds as a natural carbon source by a single-step pyrolysis route. In this method, fennel seeds were crushed to obtain greenish fennel powder which was then heated for 3 hours at a steady 500 °C. With a QY of 9.5% and an average size diameter of 3.90 ± 0.91 nm, the CQDs that were prepared were extremely mono-disperse.
In order to assess the photoirradiation stability of CQDs, a continuous 4 hours Xe lamp (150 W) illumination of the CQDs was done. No drop in the PL intensity indicated the excellent photostability of prepared CQDs as shown in Fig. 13. Enhanced photostability of synthesised CQDs may be attributed to extensive carbonization and improved crystalline structure.35
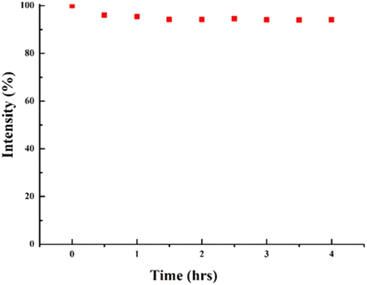 | ||
| Fig. 13 Continual exposure to a 150 W Xe lamp for 4 hours results in nearly no detectable loss in PL intensity (this figure has been adapted/reproduced from ref. 35 with permission from Nature, copyright 2019). | ||
In 2021, W. U. Khan et al., synthesised green emissive carbon nanodots (CNDs) using ascorbic acid and p-phenylenediamine via a facile hydrothermal method. The obtained CNDs had an average size of 1.9 nm and a size distribution between 1 and 3 nm. In response to a 400 nm excitation, the produced CNDs had QY of up to 10.1%.
The photostability of CNDs were examined by continuous irradiating the CNDs with UV irradiation (8 W) for 90 min and PL measurements were taken as shown in Fig. 14. The normalized PL intensity was almost constant as shown in Fig. 14 inducing the excellent photostability of prepared CNDs.36
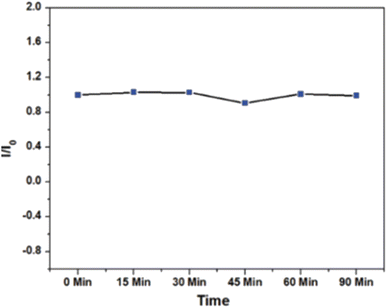 | ||
| Fig. 14 Normalized fluorescence intensity versus time for photostability test under UV exposure (90 min) (this figure has been adapted/reproduced from ref. 36 with permission from Royal Society of Chemistry, copyright 2021). | ||
2.2. Thermal stability of CQDs
Thermal stability is the property of a material which indicates the stability of material under the thermal condition. Usually, fluorescent material's chemical structure changes at elevated temperatures and consequently the emission decays. Therefore, carbon dots must possess high thermal photostability in order to withstand high temperature applications.In 2014, T. H. Kim et al. tested the synthesised carbon dots (CDs) at 80 °C for thermal stability. During the 264 hours testing period, CDs made of silica exhibited less PL intensity degradation when subjected to heat than when exposed to UV light. For CDs in silica, a loss of 20% of the initial PL intensity was seen in less than an hour, but it took over 6 hours for KBr S-CDs (the most thermally stable) to degrade to the same level.
At the end of the thermal stability assessment, it was observed that CDs in silica, S-CDs in KCl, S-CDs in NaCl retained 20%, 40% and 60%, respectively, of the initial PL intensity whereas the best result was observed for S-CDs in KBr in around 80% of the original PL intensity was retained. All samples showed highly rapid PL intensity degradation during the first 4 hours of temperature exposure, which may have been caused by retained oxygen and water (Fig. 15).27 However, once the water was removed from the crystals through evaporation, the degradation either slowed down or stabilised.
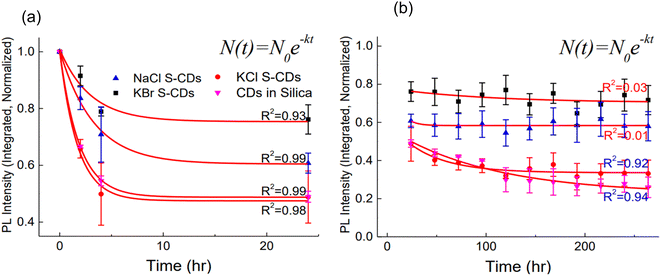 | ||
| Fig. 15 Decay fittings of the thermal stability data (a) first order fitting for the first 24 hours and (b) first order fitting for the remaining hours (this figure has been adapted/reproduced from ref. 27 with permission from Elsevier, copyright 2014). | ||
In 2014, Chuanxi Wang et al., also noticed that emission intensity of the as-prepared carbon dots (CDs) strongly affected by temperature but the emission spectra of the CDs do not shift with temperature as shown in Fig. 16(a). The intensity decreased by 52% as temperature rose from 15 to 90 °C. When the temperature increases from 15 to 60 °C, the PL intensity of the obtained CDs changes somewhat linearly (Fig. 16(b)).The fact that this temperature range exceeds the physiological temperature makes these CDs interesting in vivo temperature monitoring applications.28
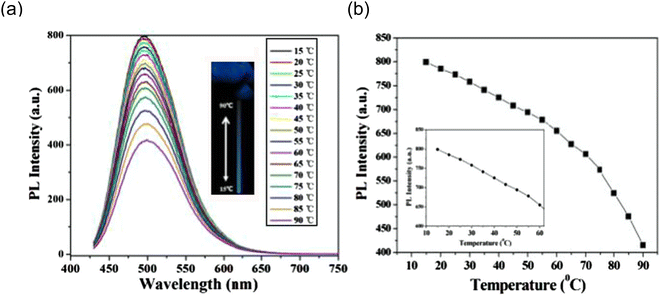 | ||
| Fig. 16 (a) Temperature-dependent fluorescence emission spectra (excitation 400 nm) for the range of 15 to 90 °C (b) fluorescence emission versus temperature (this figure has been adapted/reproduced from ref. 28 with permission from Elsevier, copyright 2015). | ||
In 2016, the thermal stability of N-CDs was tested by Yingbo Liu et al. under two different scenarios: (1) heating test over a broad range of temperature; and (2) heating test at a constant temperature for a predetermined amount of time. Observations revealed that the initial fluorescence intensity of N-CDs was preserved during 25 °C to 65 °C and as temperature was further increased from 75 °C to 95 °C, a small 2% increment in fluorescence intensity was noticed as shown in Fig. 17(a). By doing a heating test for 360 minutes at fixed temperatures of 70 °C, 80 °C, and 90 °C, the thermal stability of the N-CDs was further examined (Fig. 17(b)). No decrease in fluorescence intensity was observed for any of the fixed temperatures over the course of the entire heating process in comparison to the fluorescence intensity at the beginning, though after 360 minutes, increase in fluorescence intensity of 2%, 7%, and 5%, respectively, were noted at temperatures of 70 °C, 80 °C, and 90 °C. The constitution and steric effects of Tris, which contains a lot of dendritic hydroxylmethyl groups, are responsible for its exceptional stability performance.
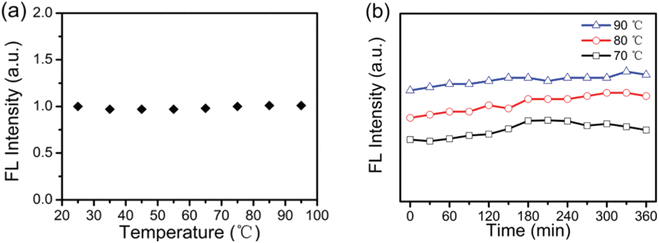 | ||
| Fig. 17 (a) Fluorescence intensity of the N-CDs at temperatures ranging from 25 to 95 °C (b) fluorescence intensity of the N-CDs at fixed temperatures of 70, 80, and 90 °C at various time intervals (this figure has been adapted/reproduced from ref. 32 with permission from Royal Society of Chemistry, copyright 2017). | ||
The N-CDs were used as invisible ink for loading crucial data for confidential communication due to their transparency (90% in the whole visible spectrum), high QY, and outstanding stability against UV irradiation and heating. They also observed that the N-CDs/PVA flexible composite film emits strong blue fluorescence when exposed to UV light and that the QY of the N-CDs/PVA film was found to be 92%, which was significantly higher than the QY of N-CDs in an aqueous solution. The numerous hydroxyl groups on the N-CDs surface and the hydrogen bonds that develop between them may be responsible for this improvement in QY. These hydrogen bonds stabilise the electrons and holes, allowing for more effective radiative recombination.32
In 2018, Waheed Ullah Khan et al., performed various experiments to test the thermal stability of as prepared N-CDs. (1) When the temperature was increased from 20–90 °C, it was found that the intensity of the N-CDs also increased by 9% as shown in Fig. 18(a). (2) No decrease in luminescence was seen at any stage during the entire heating procedure even though temperatures were kept constant (70, 80, and 90 °C) for 120 minutes at each temperatures shown in Fig. 18(b). (3) The as-prepared CQDs' UV-vis absorption spectra were recorded at both room temperature and a high temperature (90 °C), and both overlapped, showing that the chemical structure of the synthesised CQDs is stable at high temperatures (Fig. 18(c)). (4) The remarkable thermal stability of N-CDs was further confirmed by the lack of a noticeable change in the decay curves at high temperatures between 60 and 90 °C (Fig. 18(d)).34
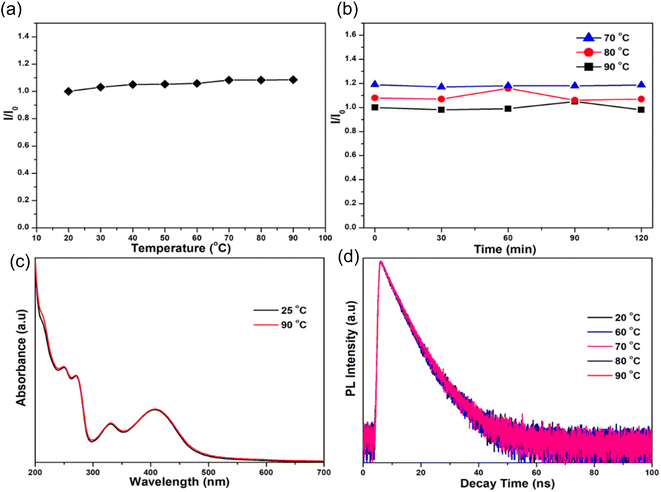 | ||
| Fig. 18 (a) The PL intensity of N-CDs as a function of temperature in the range of 20 to 90 °C; (b) the distribution of PL intensity as a function of fixed temperature (70, 80, 90 °C) at various time intervals; (c) the UV-vis spectra of N-CDs at 25 and 90 °C; and (d) the decay curves of N-CDs at 20, 60, 70, 80 and 90 °C (this figure has been adapted/reproduced from ref. 34 with permission from American Chemical Society, copyright 2018). | ||
In 2019, V. Rimal and colleagues synthesised bright green luminous CDs by pyrolyzing oleic acid as an organic substrate at 230–260 °C for a short period of time while stirring continuously between 180–260 rpm. This method produces two principal products, namely aqueous CDs and solid CDs, as well as a secondary product (anhydrous CDs), which was extracted from the aqueous CDs. The prepared CQDs were of the order of 10 nm and had a QY of 50%.
According to the TGA investigation, solid CDs are stable up to 400 °C, and the weight (%) loss was less than 1% for temperatures below 100 °C. However, above this point, the weight (%) loss becomes abrupt with a consistent degradation curve, as shown in Fig. 19(a). In contrast, aqueous CDs have less thermal stability than oleic acid, yet a constant mass of 10% remains unaltered after 200 °C. TGA graph of aqueous CDs clearly indicate that the thermal stability of bare oleic acid was effectively increased by synthesizing it to CDs. The synthesis of CDs containing oleic acid improved thermal stability for the first time in this study. In order to produce anhydrous CDs, the aqueous CDs were subsequently dehydrated under the influence of heat. At 100 °C, these anhydrous CDs show a slight bulge that might be caused by the evaporation of water that has been adsorbed because of the environment. The anhydrous CDs show a total weight decrease of 30%, indicating a novel class of functional nanoparticles with excellent heat stability. Compared to their counterparts, anhydrous CDs can operate at much higher temperatures, up to orders of magnitude above 800 °C. This suggests that they may have potential applications in high temperature environments, including drug delivery, optical chemo/biosensors, optoelectronic devices, bio-imaging, catalysis and white light-emitting diodes, among others.
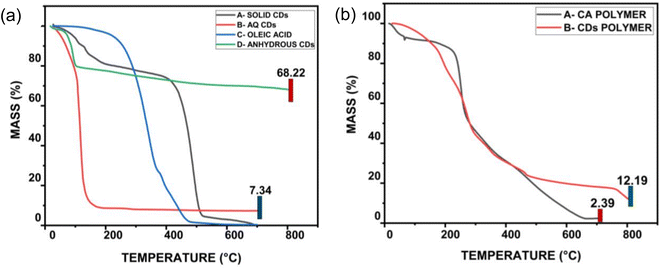 | ||
| Fig. 19 (a) TGA study of A-solid portion of CDs, B-aqueous CDs, C-oleic acid and D-anhydrous CDs (b) nanocomposite made of A-cellulose acetate polymer and B-CDs polymer that underwent TGA analysis (this figure has been adapted/reproduced from ref. 37 with permission from Springer, copyright 2019). | ||
A cellulose acetate polymer matrix served as a host for the CDs as well. At first, this polymer nanocomposite showed a steady weight loss curve, however as illustrated in Fig. 19(b), the curve exhibits constant weight at orders larger than 500 °C. The existence of the synthesised CDs, which are unaffected at high temperatures, is the major reason for the composite's increased thermal stability. Additionally, data demonstrates that the characteristics of oleic acid CDs retains even when embedded in a polymer matrix.37
In 2021, Waheed Ullah Khan et al., test the thermal stability of prepared green emitting CNDs in two ways: (1) the temperature was increased from 10 °C to 80 °C and it was observed that PL remains unchanged till 30 °C and it decreases by 8% (of its maximum value) when temperature is raise to 80 °C as shown in Fig. 20(a). (2). No luminescence loss was ever noticed while the produced aqueous solution of CNDs was maintained at a constant high temperature (80 °C) for 30 minutes as shown in Fig. 20(b). Results show that the synthesised CNDs exhibit high thermal stability even in harsh thermal conditions.36
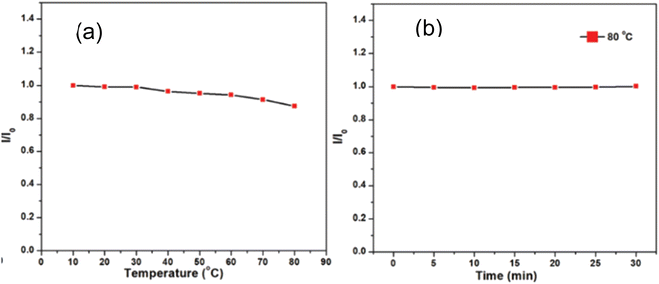 | ||
| Fig. 20 Thermal stability test (a) fluorescence intensity versus temperature (b) fluorescence intensity versus time at 80 °C (this figure has been adapted/reproduced from ref. 36 with permission from Royal Society of Chemistry, copyright 2021). | ||
2.3. Ion or pH or time stability of CQDs
Ion or pH or time stability is the important factor in ensuring CQDs to realize practical applications. The interference of certain common cations (at various concentrations) with the synthesised fluorescent CQDs was used to investigate the ion stability. Likewise, the pH stability was assessed by observing the quenching of PL under different pH conditions. Time stability signifies the time span for which the properties of CQDs are retained. This is a very crucial parameter from application perspective as the life of the CQDs-integrated systems will directly depend on the time stability of CQDs.In 2014, Chuanxi Wang et al., investigated the ion stability of as prepared carbon dots (CDs). In this investigation, 20 mM solutions of NaCl, KNO3, NH4Cl, ZnCl2, Ba(NO3)2, FeCl2, Ca(NO3)2, MgSO4, Cu(NO3)2, Ni(NO3)2, (CH3COO)2Mn, Co(NO3)2, Cd(NO3)2, PbCl2, and (CH3COO)3Cr were added to 1.0 ml produced CDs and mixed for 3 min. In this approach, the fluorescence spectrophotometer was used to measure the impact of the cations such as Na+, K+, NH4+, Zn2+, Ba2+, Fe2+, Ca2+, Mg2+, Cu2+, Ni2+, Mn2+, Co2+, Cd2+, Pb2+, and Cr3+on the emission response of the synthesized CDs. By comparing the fluorescence intensities of CDs solution in the presence and absence of the interfering ions, the relative fluorescence intensity was calculated. It was observed that most of the common cations show either no or negligible interference (Fig. 21) which shows that the fluorescence intensity of the prepared CDs was not significantly hampered by these cations.
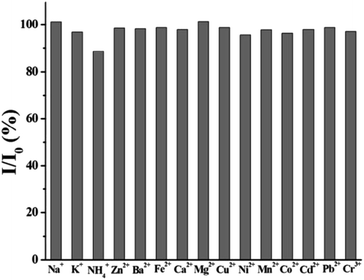 | ||
| Fig. 21 Stability of CDs in presence of various metal ions (concentration of 10−2 M) (this figure has been adapted/reproduced from ref. 28 with permission from Elsevier, copyright 2015). | ||
An optical response with pH change was also observed when the prepared CDs were assessed at different pH values. A spectrofluorometer was used to measure the fluorescence intensity of a series of the prepared CDs solutions, whose pH ranged from 1 to 13.5, in order to observe the effect. As can be seen in Fig. 22(a), the maximum emission intensity was seen at pH = 3, although there was a significant pH drop from pH = 3 to pH = 10, and there was only a small shift in intensity from pH 10 to 13.
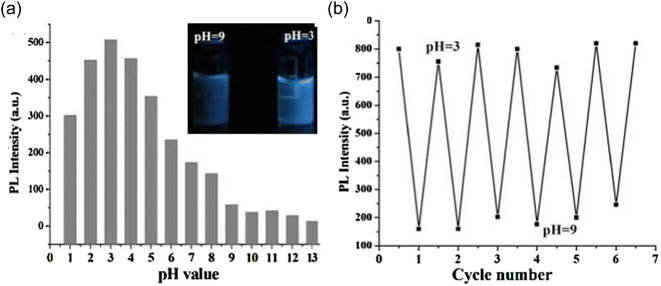 | ||
| Fig. 22 (a) PL intensity of CDs at different pH values; (b) PL intensity of CDs during cyclic pH switching between 3 and 9 (this figure has been adapted/reproduced from ref. 28 with permission from Elsevier, copyright 2015). | ||
On the basis of pH fluctuation, the reversibility of CD switching action was also assessed. When manufactured CDs were cycled between pH values of 3 and 9, utilising acid and base as modulators, PL intensity was noticed. Without tiring, the switching procedure was carried out six times in a row, demonstrating the good reversibility of two-way switching operations (Fig. 22(b)). The findings suggested that the as prepared CDs might be used for liquid temperature and pH monitoring. Their group also accessed the time stability of CQDs and they discovered no appreciable variation in the fluorescence intensity by preserving the synthesized CDs for one month under normal conditions as shown in Fig. 23.28
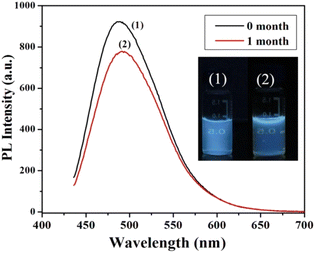 | ||
| Fig. 23 The fluorescence spectra of as-prepared CDs (under 365 nm UV light illumination) (1) in 0 month and (2) after 1 month when stored under normal condition (this figure has been adapted/reproduced from ref. 28 with permission from Elsevier, copyright 2015). | ||
In 2016, Yongming Guo et al., observed that as prepared CQD exhibited good stability under high-salt circumstances (1.0 M NaCl) as almost no change in fluorescence intensity was observed as shown in Fig. 24. In addition, the fluorescence intensity of the prepared CQDs dropped by 18% in NaClO (100 μM) solution, indicating that the CQDs had good antioxidant action.
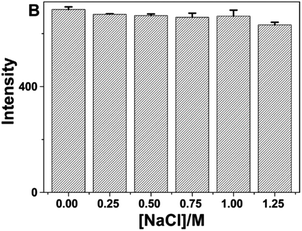 | ||
| Fig. 24 Fluorescence intensity of CQDs in the presence of various NaCl concentrations (conducted in a pH 5.0 solution with 50 mM NaAc-HAc buffer) upon UV (330 nm) irradiation (this figure has been adapted/reproduced from ref. 30 with permission from Nature, copyright 2016). | ||
Additionally, they investigated how pH affected CQD fluorescence intensity over a broad pH range (pH 1–14). Because several acidic functional groups were found to be present on the surface of CQDs, it was discovered that the fluorescence intensity of these materials was quite low between pH 1–4. However, in an aqueous solution with a wide pH range (pH 5–11), the fluorescence intensity remained practically constant, indicating the significant potential for CQDs in a variety of fields. Fig. 25(a) illustrates the decrease in fluorescence intensity at pH values higher than 11.
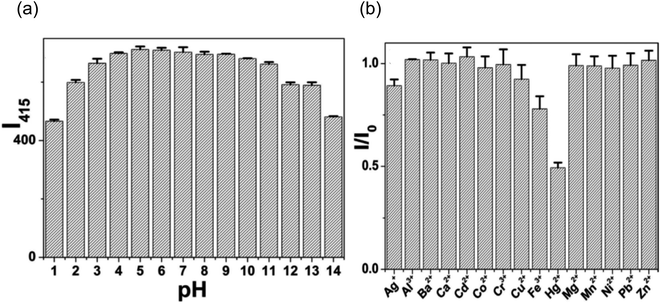 | ||
| Fig. 25 (a) Effect of pH on CQDs fluorescence intensity (b) comparison of CQD fluorescence intensity when various ions are present at excitation wavelength of 330 nm (this figure has been adapted/reproduced from ref. 30 with permission from Nature, copyright 2016). | ||
In addition, they also looked at how selective CQDs were for various metal ions, such asAg+, Al3+, Ba2+, Ca2+, Cd2+, Co2+, Cr3+, Cu2+, Fe3+, Hg2+, Mg2+, Mn2+, Ni2+, Pb2+ and Zn2+. The fluorescence response of these metal ions was measured after being introduced to the CQDs solution at a concentration of 100 μM as shown in Fig. 25(b). Fluorescence quenching in the obtained CQDs was observed by metal ions such as Hg2+ and the smallest observable amount of Hg2+ in the CQDs solution was found to be 10 nM. This confirmed the viability of building a nanosensor for the detection of metal ions using prepared CQDs. This confirmed that prepared CQDs can be used to construct a nanosensor for metal ion detection since they have some functional groups on their surface.30
In 2016, researchers led by Yingbo Liu and colleagues used a conventional pen with an ink made of N-CDs that was invisible to write data on readily available filter paper. The information was “CDs” and “Changchun Institute of Applied Chemistry Chinese Academy” which can be clearly seen in Fig. 26(a) under the irradiation of a 365 nm UV lamp while blank filter paper was seen in daylight (Fig. 26(b)). The data on filter paper was reproducible when seen under 365 nm UV lamp after 90 days storage under ambient air (Fig. 26(c)). This proves that as prepared N-CDs exhibit good time stability under ambient air.32
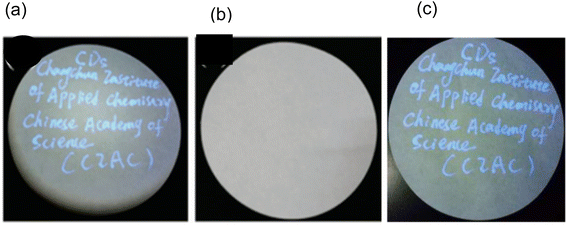 | ||
| Fig. 26 Data written on a filter paper using the N-CDs invisible ink under (a) 365 nm UV lamp, (b) daylight and (c) reproducibility of the data written on filter paper under 365 nm UV lamp for 90 days storage under ambient air (this figure has been adapted/reproduced from ref. 32 with permission from Royal Society of Chemistry, copyright 2017). | ||
In 2018, Waheed Ullah Khan et al. observed that obtained N-CDs were reasonably constant over the pH range of 1 to 9.5, as shown in Fig. 27(a), and there was no notable change in fluorescence intensity when the N-CDs were stored for 15 days, as shown in Fig. 27(b), indicating that the N-CDs were stable even after 15 days with minimal variation. The synthesised CQDs were utilised in bio-imaging applications and solid-state LED manufacturing.34
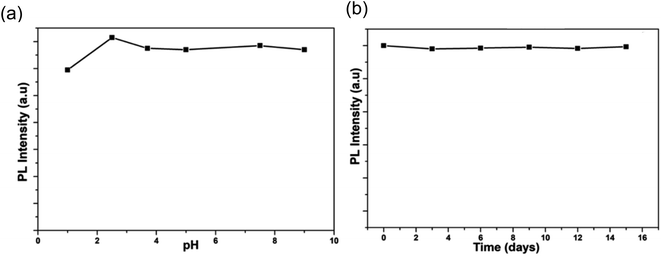 | ||
| Fig. 27 (a) PL intensity of N-CDs across a range of pH values (b) PL intensity of N-CDs as a function of time (this figure has been adapted/reproduced from ref. 34 with permission from American Chemical Society, copyright 2018). | ||
In 2020, M. Perikala et al., synthesised white-light-emitting CDs by utilising citric acid as a carbon source, octadecene (ODE) as a high boiling-point solvent and hexadecyl amine (HDA) as the surface-functionalizing agent via a colloidal synthesis technique. Bare CDs and Surface-functionalized CDs (SFCDs) were synthesized using this method. The SFCDs were made with HDA concentrations ranging from 0.1 to 0.4 M. The bare CDs possessed an average size of approximately 4 nm with QY of 4% and QY of 31% was reported for SFCDs.
The research group tested the time stability of both bare CDS and SFCDs. They observed that the emission spectrum of CQDs was highly stable when exposed to an open atmosphere. The maximal emission count for bare CDs when excited at 250 nm was of the order of 105, whereas that for SFCDs was of the order of 106 as shown in Fig. 28(a) and (c). However, when the excitation wavelength was altered to 350 nm, the maximum emission intensity was increased by the order of 10 for both types of CQDs as shown in Fig. 28(b) and (d). It was noticed that emission intensity decreased with exposure time in all cases mentioned above except for SFCDs (when excited at 350 nm). In this case, the emission intensity was decreased during early exposure and then increased with exposure time (Fig. 28(d)) but the reason for such behaviour was not clear. Interestingly, the normalised emission spectra did not appear to be changed at all by exposure to the open atmosphere for as long as 216 hours.38
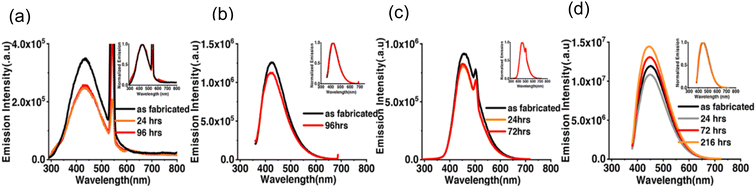 | ||
| Fig. 28 Emission spectra for varied durations of exposure to an open atmosphere for (a) bare CDs excited at 250 nm, (b) bare CDs excited at 350 nm, (c) SFCDs excited at 250 nm, and (d) SFCDs excited at 350 nm (this figure has been adapted/reproduced from ref. 38 with permission from American Chemical Society, copyright 2019). | ||
In 2021, Waheed Ullah Khan et al., tested the stability of prepared green emitting CNDs in a high ionic environment using KCl salt. The concentration of KCl varied from 0 to 1.0 M and corresponding PL were recorded as indicated in Fig. 29. The nearly constant PL intensity indicates the great stability of CQDs in an environment with a high ionic concentration.36 The comparative study of stability of carbon quantum dots synthesised by various researchers are summarized (Table 1).
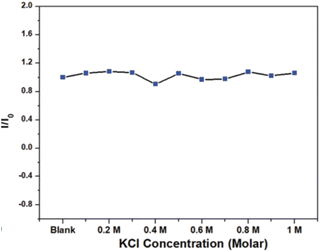 | ||
| Fig. 29 Normalized PL intensity under different KCl concentration (this figure has been adapted/reproduced from ref. 36 with permission from Royal Society of Chemistry, copyright 2021). | ||
| Year | Research group | Performed stability test | Exposure condition | Stability response | CQD size (nm) | Quantum yield | Application of as prepared CQDs |
|---|---|---|---|---|---|---|---|
| 2014 | Tak H. Kim et al.27 | Photostability | UV exposure (365 nm, 5 W) for 264 hours | Poor | ∼0.9 | 47% | Salt-embedded carbon nanodots as a fluorophore for GaN UV-LEDs |
| Thermal stability | 80 °C | Poor | |||||
| 2014 | Chuanxi Wang et al.28 | Photostability | Xenon exposure (450 W) for 2 hours | Excellent | 2.6 ± 0.2 | 7.20% | Nanoprobes for pH and temperature sensing in liquids |
| Thermal stability | 90 °C | Poor | |||||
| Time stability | 30 days | Good | |||||
| 2015 | Wenshuo Wang et al.29 | Photostability | UV exposure (365 nm, 8 W) for 17 hours NIR exposure (800 nm) for 17 hours | Poor | 0.921 ± 0.005 | 35% | Mechanisms to avoid photobleaching of CQDs |
| 2016 | Yongming Guo et al.30 | Photostability | UV exposure (365 nm, 16 W) for 1 hour 20 minutes | Excellent | 2–8 | 10.75% | Sensing Hg2+ |
| Thermal stability | 95 °C | Excellent | |||||
| Ion stability | NaCl (1.25 M) Hg2+ (10 nM) | Good sensitive to Hg2+ | |||||
| 2016 | Jinxing Zhao et al.31 | Photostability | UV exposure for 120 hours | Excellent | 3–5 | ∼15% | Flexible devices |
| 2016 | Yingbo Liu et al.32 | Photostability | UV exposure (365 nm, 250 W) for 6 hours | Excellent | 2–5 | 75% | Invisible ink for encoding confidential information and enhanced anti-counterfeiting measures |
| Time stability | 90 days | Excellent | |||||
| 2018 | Bo Ju et al.33 | Photostability | UV exposure (365 nm, 12 W) for 8 hours | Good | 5–30 | 13.20% | Highly selective detection of explosive 2,4,6-trinitrophenol (TNP) |
| Thermal stability | 90 °C | Excellent | |||||
| 2018 | Waheed Ullah Khan et al.34 | Photostability | UV exposure (365 nm, 8 W)for 3 hours | Good | 9.3 | 13.4% (DI water) 50.3% (ethanol) | Bioimaging and fabrication of solid-state LED |
| Time stability | 15 days | Excellent | |||||
| 2019 | Vishal Rimal et al.37 | Thermal stability | 800 °C | Good | 10 | 50% | Development of polymer nano composites |
| 2019 | Manasa Perikala et al.38 | Time stability | 9 days | Good | ∼4 | 4% (bare CQDs) 31% (SFCD) | Fabrication of photonic devices |
| 2019 | Akansha Dager et al.8 | Photostability | Xenon exposure (150 W) for 4 hours | Good | 3.90 ± 0.91 | 9.50% | Bio-sensing, cellular imaging, solar cell etc. |
| 2021 | Waheed Ullah Khan et al.36 | Photostability | UV exposure (8 W), 1.5 hours | Excellent | 13 | 10.10% | Incubation in T-ca cancer cells for bio-imaging applications |
| Thermal stability | 80 °C | Good | |||||
| Ion stability | KCl (1.0 M) | Excellent |
3. Conclusion, challenges, and future prospects
Since Xu et al. discovered CQDs in 2004 while separating single-walled carbon nanotubes, CQDs have garnered a great deal of attention due to their distinctive emission features, superior biocompatibility and minimal biological toxicity. In recent years, CQD applications have expanded fast beyond materials science to healthcare, catalysis, energy, and the environment, among other domains. In spite of fast development of CQDs, its various aspects of research are still in its infancy.In this review article, we have portrayed the stability of CQDs synthesised by different methods and different precursors. The stability of CQDs is a prerequisite for its application in various fields. It is distinctly shown in the article that the stability of CQDs is dependent on the ambient conditions like impact of UV exposure, temperature, salt, pH, time and other parameters.
Photostability tests revealed that the emission yield of CQDs were almost unchanged in most of the reports under continuous UV exposure for some hours but in few cases as reported by Bo Ju et al.33 the emission intensity falls down. Stable emission is due to the stable structure of CQDs under exposure to UV light. Similarly, CQDs possess good thermal stability for temperatures less than 100 °C but it decreased too in few cases as reported by W. U. Khan et al.36 However, CQDs synthesised by V. Rimal et al.37 exhibit highest thermal stability up to 800 °C temperature which has ever been reported till date. This has unfolded the possibilities of applications of CQDs at high temperature.
As per the reports, CQDs has also shown long term stability up to 1 month under ambient condition indicating CQDs storage is not critical and they can be preserved for a long period of time before its use.28 However, a decreased quantum yield was observed after 6 months. On the other hand, the CQDs emission is very sensitive to the pH of the medium. The emission intensity has dropped in highly acidic (pH < 3) and highly basic (pH > 11) medium whereas emission intensity is almost constant for pH values 3–10 in most of the cases.30 Still, the dependence of CQDs emission on pH is not consistent in all reports.
It was observed that stability was enhanced when (1) salts (like NaCl, KCl, etc.) were incorporated in CQDs, and (2) CQDs were incorporated in the polymer matrix. Enhanced stability of CQDs in polymer matrices is a key for its application in flexible devices.
Stability of CQDs depends primarily on its structure and composition which in turn depends on various parameters including precursors, solvent, synthesis technique, synthesis condition (pH, duration, etc.). In addition, the ambient conditions also affect the stability of the synthesised CQDs. Based on the type of surface functional groups the CQDs exhibits sensitivity towards ambient parameters like temperature, concentration, etc. Stability can be modified either after synthesis or during synthesis of CQDs though the former is most commonly reported. Post treatment method involves the surface group modification or surface passivation through polymers, salts, etc.
Oxygen-containing groups (such as hydroxyl, etc.) on CQDs surface are generally known as the luminescent quenching centers which provided non-radiative pathways. The oxidation of unstable surface groups in the open air led to the generation of non-radiative defect states which suppress the radiative emission and plenty of energy is dissipated in this way thus overall fluorescence intensity of CQDs decreases. Establishing a chemical bonding between CQDs (utilizing the oxygen-related groups) and a polymer matrix modifies the surface functional groups of CQDs and passivates the CQDs surface. As a consequent, non-radiative recombination centres are effectively eliminated and emission becomes more effective from the radiative surface states. This chemical bonding proves to be an effective way to enhance emission intensity but also improves the stability of CQDs. By the surface passivation, the functional groups on the surface get modified and hence the CQDs can exhibit greater stability with minimal fluorescence quenching over a long span.
In order to accept CQDs as a very stable contestant for applications, it is highly demanded that future research must carry out stability tests under stressful conditions. For example, longer duration (days instead of a few hours) UV exposure, exposure with high optical power, high temperature (above 100 °C) and long term stability or lifetime test (in years). In addition, research must concentrate on designing CQD structures according to application needs. These studies are very important as this will give the true picture of stability of CQDs and will greatly unfold many commercial applications of CQDs.
Large absorption coefficients, broad absorption spectra, good electron transfer and charge separation ability of CQDs make them suitable for the photovoltaic applications. CQDs can cover multiple roles in photovoltaic devices going from energy shift material to sensitizers to charge carrier layers to dopant to donor/acceptor material and counter electrodes.
The broad emission characteristics (full width half maxima >80 nm) of CQDs due to strong electron-phonon coupling and as well as highly dispersed particle size makes CQDs a useful alternative for the white LEDs (WLEDs).39 Although Red-CQDs have been reported by some researchers, long-wavelength emission and high QYs of these CQDs are difficult to realize, which are necessary for achieving warm WLEDs of high color quality. Translucent or transparent nature of CQDs solution makes its useful for invisible data transfer, labels or tags but lowering of QY with time makes it challenging to retrieve the invisible data after longer duration of time. In vivo diagnostic applications of CQDs are well known due to their biocompatible nature but the required high QY emission in NIR biological window-I (650–950 nm) is rarely observed. The significant advantages of low cytotoxicity and cost make CQDs an ideal raw material for constructing effective sensing devices for food toxin detection. High surface area, low toxicity, low cost, and chemical stability possessed by CQDs made them a suitable material for rechargeable batteries. CQDs based composites are employed to build cathode/anode materials and electrolytes in lithium ion batteries (LiBs) and superior electrochemical performance, including outstanding ultrafast energy storage capability and negligible capacity fading has been observed.
Despite of the intense research conducted in the last fifteen years on CQDs, many challenges need to be resolved for the widespread adoption of CQDs. (1) It is critical to synthesize CQDs of desired structure and size because precise control over various synthesis parameters is required, which is hardly available to date with a few exceptions. As such, there is a need to develop the manufacturing processes to efficiently control the core structure or the chemical composition of the emitting surface centres. (2) The enormous degree of inconsistency within the literature, related to the material's classification, synthesis approach, purification, and subsequent characterizations of the CQDs properties hinders the in depth investigation of CQDs and the applications of CQDs in the applied research. Consistency in the said areas is a must for true comparative study and reasonable conclusions. (3) The CQDs have low values of QY except for some limited cases and the QY are even lower or compromised when measured at longer wavelengths. Efforts must be made to extend the spectrum of CQDs, especially in the near-IR region for widespread applications of CQDs including organic bioelectronics. (4) Very few researches discusses about the stability and stability enhancement of the synthesised CQDs, although stability is the most important parameter from the application perspective. There is a wide scope of research on the stability analysis of the CQDs and to investigate the correlation between the structure and the stability of the CQDs if any exists.
With the development of sophisticated technologies and characterizations, we expect controllable synthetic procedures, large-scale production, and a deeper understanding of the structure–stability connection. Once this is achieved, the stability can be controlled by precisely controlling the structure. Regardless of the challenges associated with CQDs, its versatile nature, capacity to surface modifications and tuneable optical properties exhibit tremendous potential for a broad range of applications which ensures that CQDs have a bright future.
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
The authors are grateful to the University of Delhi and Vice Chancellor, Professor Yogesh Singh, for encouraging and facilitating faculty research. The authors would also like to express their gratitude to Bhaskaracharya College of Applied Sciences, University of Delhi, for providing a suitable environment for research endeavours.References
- V. Magesh and K. Ashok, Sundramoorthy, Dhanraj Ganapathy, Recent Advances on Synthesis and Potential Applications of Carbon Quantum Dots, Front. Mater., 2022, 9, 1–27 CrossRef.
- N. Azam, M. N. Ali and T. J. Khan, Carbon Quantum Dots for Biomedical Applications: Review and Analysis, Front. Mater., 2021, 8, 1–21 CrossRef.
- H. Jung, V. S. Sapner, A. Adhikari, B. R. Sathe and R. Patel, Recent Progress on Carbon Quantum Dots Based Photocatalysis, Front. Chem., 2022, 10, 1–28 CrossRef PubMed.
- W. Zhang, S. F. Yu, L. F. Fei, L. Jin, S. Pan and P. Lin, Large area color controllable remote carbon white-light light emitting diodes, Carbon, 2015, 85, 344–350 CrossRef CAS.
- L. Tang, R. Ji, X. Cao, J. Lin, H. Jiang, X. Li, K. S. Teng, C. M. Luk, S. Zeng, J. Hao and S. P. Lau, Deep ultraviolet photoluminescence of water-soluble self-passivated graphene quantum dots, ACS Nano, 2012, 6, 5102–5110 CrossRef CAS PubMed.
- K. Hola, A. B. Bourlinos, O. Kozak, K. Berka, K. M. Siskova, M. Havrdova, J. Tucek, K. Safarova, M. Otyepka, E. P. Giannelis and R. Zboril, Photoluminescence effects of graphitic core size and surface functional groups in carbon dots: COO- induced red-shi emission, Carbon, 2014, 70, 279–286 CrossRef CAS.
- M. Ganesan, K. D. Ramadhass, H. C. Chuang and G. Gopalakrishnan, Synthesis of nitrogen-doped carbon quantum dots@ Fe2O3/multiwall carbon nanotubes ternary nanocomposite for the simultaneous electrochemical detection of 5-fluorouracil, uric acid, and xanthine, J. Mol. Liq., 2021, 331, 115768 CrossRef CAS.
- A. Dager, T. Uchida, T. Maekawa and M. Tachibana, Synthesis and characterization of Mono-disperse Carbon Quantum Dots from Fennel Seeds: Photoluminescence analysis using Machine Learning, Sci. Rep., 2019, 9, 14004 CrossRef PubMed.
- P. Kumar, S. Dua, R. Kaur, M. Kumar and G. Bhatt, A review on advancements in carbon quantum dots and their application in photovoltaics, RSC Adv., 2022, 12, 4714–4759 RSC.
- A. Zhang, C. Y. Liu and Y. Liu, A Novel One-Step Approach to Synthesize Fluorescent Carbon Nanoparticles, Eur. J. Inorg. Chem., 2010, 4411–4414 CrossRef.
- X. Xu, R. Ray, Y. Gu, H. J. Ploehn, L. Gearheart, K. Raker and W. A. Scrivens, Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments, J. Am. Chem. Soc., 2004, 126(40), 12736–12737 CrossRef CAS PubMed.
- I. Singh and R. Arora, Hardik Dhiman, Rakesh Pahwa, Carbon Quantum Dots: Synthesis, Characterization and Biomedical Applications, Turk. J. Pharm. Sci., 2018, 15(2), 219–230 CrossRef CAS PubMed.
- Y. Li, F. Liu, J. Cai, X. Huang, L. Lin, Y. Lin, H. Yang and S. Li, Nitrogen and sulfur co-doped carbon dots synthesis via one step hydrothermal carbonization of green alga and their multifunctional applications, Microchem. J., 2019, 147, 1038–1047 CrossRef CAS.
- J. M. Keenan, Y. Zhou and R. M. Leblanc, Recent Development of Carbon Quantum Dots Regarding their Optical Properties, Photoluminescence Mechanism, and Core Structure, Nanoscale, 2019, 11, 4634–4652 RSC.
- C. M. Carbonaro, R. Corpino, M. Salis, F. Mocci, S. V. Thakkar, C. Olla and P. C. Ricci, On the Emission Properties of Carbon Dots: Reviewing Data and Discussing Models, C--Open Access Carbon Res. J., 2019, 5, 2–15 Search PubMed.
- L. Li and T. Dong, Photoluminescence Tuning in Carbon Dots: Surface Passivation or/and Functionalization, Heteroatom Doping, J. Mater. Chem. C, 2018, 6, 7944–7970 RSC.
- B. Zhi, X. X. Yao, Y. Cui, G. Orr and C. L. Haynes, Synthesis, applications and potential photoluminescence mechanism of spectrally tunable carbon dots, Nanoscale, 2019, 11, 20411–20428 RSC.
- A. Sciortino, E. Marino, B. Dam, P. Schall, M. Cannas and F. Messina, Solvatochromism Unravels the Emission Mechanism of Carbon Nanodots, J. Phys. Chem. Lett., 2016, 7, 3419–3423 CrossRef CAS PubMed.
- Y. P. Sun, B. Zhou, Y. Lin, W. Wang, K. A. S. Fernando, P. Pathak, M. J. Meziani, B. A. Harruff, X. Wang, H. Wang, P. G. Lu, H. Yang, M. E. Kose, B. Chen, L. M. Veca and S. Y. Xie, Quantum-Sized Carbon Dots for Bright and Colorful Photoluminescence, J. Am. Chem. Soc., 2006, 128, 7756–7757 CrossRef CAS PubMed.
- M. P. Sk and A. Dutta, New-generation quantum dots as contrast agent in imaging, Nanomaterials in Diagnostic Tools and Devices, Elsevier, 2020, pp. 525–556 Search PubMed.
- J. B. Essner and G. A. Baker, The emerging roles of carbon dots in solar photovoltaics: a critical review, Environ. Sci. Nano, 2017, 4, 1216–1263 RSC.
- P. Kumar, G. Bhatt, R. Kaur, S. Dua and A. Kapoor, Synthesis and modulation of the optical properties of carbon quantum dots using microwave radiation, Fuller. Nanotub. Carbon Nanostructures, 2020, 28(9), 724–731 CrossRef CAS.
- C. M. Martindale, G. A. M. Hutton, C. A. Caputo, S. Prantl, R. Godin, J. R. Durrant and E. Reisner, Enhancing Light Absorption and Charge Transfer Efficiency in Carbon Dots through Graphitization and Core Nitrogen Doping, Angew. Chem., Int. Ed., 2017, 56, 1–6 CrossRef PubMed.
- T. Yu, H. Wang, C. Guo, Y. Zhai, J. Yang and J. Yuan, A rapid microwave synthesis of green-emissive carbon dots with solid-state fluorescence and pH-sensitive properties, R. Soc. Open Sci., 2018, 5, 1–15 Search PubMed.
- Y. Wang and A. Hu, Carbon quantum dots: synthesis, properties and applications, J. Mater. Chem. C, 2014, 2, 6921–6939 RSC.
- L. Cui, X. Ren, J. Wang and M. Sun, Synthesis of homogeneous carbon quantum dots by ultrafast dual-beam pulsed laser ablation for bioimaging, Mater. Today Nano, 2020, 12, 100091 CrossRef.
- T. H. Kim, F. Wang, P. McCormick, L. Wang, C. Brown and L. Qin, Salt-embedded carbon nanodots as a UV and thermal stable fluorophore for light-emitting diodes, J. Lumin., 2014, 154, 1–7 CrossRef CAS.
- C. Wang, Z. Xu, H. Cheng, H. Lin, M. G. Humphrey and C. Zhang, A hydrothermal route to water-stable luminescent carbon dots as nanosensors for pH and temperature, Carbon, 2015, 82, 87–95 CrossRef CAS.
- W. Wang, C. Damm, J. Walter, T. J. Nacken and W. Peuker, Photobleaching and stabilization of carbon nanodots produced by solvothermal synthesis, Phys. Chem. Chem. Phys., 2016, 18, 466–475 RSC.
- Y. Guo, L. Zhang, F. Cao and Y. Leng, Thermal treatment of hair for the synthesis of sustainable carbon quantum dots and the applications for sensing Hg2+, Sci. Rep., 2016, 6, 35795 CrossRef CAS PubMed.
- J. Zhao, C. Liu, Y. Li, J. Liang, J. Liu, T. Qian, J. Ding and Y. C. Cao, Preparation of carbon quantum dots based high photostability luminescent membranes, Luminescence, 2017, 32, 625–630 CrossRef CAS PubMed.
- Y. Liu, L. Zhou, Y. Li, R. Deng and H. Zhang, Highly fluorescent nitrogen-doped carbon dots with excellent thermal and photo stability applied as invisible ink for loading important information and anti-counterfeiting, Nanoscale, 2017, 9, 491–496 RSC.
- B. Ju, Y. Wang, Y.-M. Zhang, T. Zhang, Z. Liu, M. Li and S. X.-A. Zhang, Photostable and Low-Toxic Yellow-Green Carbon Dots for Highly Selective Detection of Explosive 2,4,6-Trinitrophenol Based on the Dual Electron Transfer Mechanism, ACS Appl. Mater. Interfaces, 2018, 10, 13040–13047 CrossRef CAS PubMed.
- W. U. Khan, D. Wang and Y. Wang, Highly Green Emissive Nitrogen-Doped Carbon Dots with Excellent Thermal Stability for Bioimaging and Solid-State LED, Inorg. Chem., 2018, 57, 15229–15239 CrossRef CAS PubMed.
- A. Dager, T. Uchida, T. Maekawa and M. Tachibana, Synthesis and characterization of Mono-disperse Carbon Quantum Dots from Fennel Seeds: Photoluminescence analysis using Machine Learning, Sci. Rep., 2019, 9, 14004 CrossRef PubMed.
- W. U. Khan, L. Qin, A. Alam, P. Zhou, Y. Peng and Y. Wang, Water-soluble green-emitting carbon nanodots with enhanced thermal stability for biological applications, Nanoscale, 2021, 13, 4301–4307 RSC.
- V. Rimal, S. Shishodia and P. K. Srivastava, Novel synthesis of high thermal stability carbon dots and nanocomposites from Oleic acid as an organic substrate, Appl. Nanosci., 2019, 10, 455–464 CrossRef.
- M. Perikala and A. Bhardwaj, Highly Stable White-Light-Emitting Carbon Dot Synthesis Using a Non-coordinating Solvent, ACS Omega, 2019, 4(25), 21223–21229 CrossRef CAS PubMed.
- P. Kumar, S. Dua, B. Pani and G. Bhatt, Application of Fluorescent CQDs for Enhancing the Performance of Solar Cells and WLEDs, Intech Open, 2022 Search PubMed.
Footnote |
| † Present address: Ramjas College, University of Delhi, India. |
| This journal is © The Royal Society of Chemistry 2023 |
