Open Access Article
Open Access ArticleRationally tailored redox ability of Sn/γ-Al2O3 with Ag for enhancing the selective catalytic reduction of NOx with propene
Ning Lia,
Tiantian Zhangb,
Zuliang Wubc,
Jing Libc,
Wei Wangbc,
Jiali Zhubc,
Shuiliang Yao*bc and
Erhao Gao *bc
*bc
aSchool of Petrochemical Engineering, Changzhou University, Jiangsu 213164, China
bSchool of Environmental Science and Engineering, Changzhou University, Jiangsu 213164, China. E-mail: yaos@cczu.edu.cn
cAdvanced Plasma Catalysis Engineering Laboratory for China Petrochemical Industry, Changzhou University, Jiangsu 213164, China. E-mail: gaoerhao@cczu.edu.cn
First published on 11th January 2023
Abstract
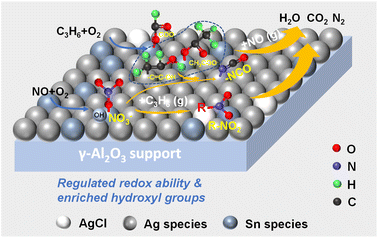
The development of excellent selective catalytic reduction (SCR) catalysts with hydrocarbons for lean-burn diesel engines is of great significance, and a range of novel catalysts loaded with Sn and Ag were studied in this work. It was found that the synergistic effects of Sn and Ag enabled the 1Sn5Ag/γ-Al2O3 (1 wt% Sn and 5wt% Ag) to exhibit superior C3H6-SCR performance. The de-NOx efficiency was maintained above 80% between 336 and 448 °C. The characterization results showed that the presence of AgCl crystallites in the 1Sn5Ag/γ-Al2O3 catalyst helped its redox ability maintain an appropriate level, which suppressed the over-oxidation of C3H6. Besides, the number of surface adsorbed oxygen (Oα) and hydroxyl groups (Oγ) were enriched, and their reactivity was greatly enhanced due to the coexistence of Ag and Sn. The ratio of Ag0/Ag+ was increased to 3.68 due to the electron transfer effects, much higher than that of Ag/γ-Al2O3 (2.15). Lewis acid sites dominated the C3H6-SCR reaction over the 1Sn5Ag/γ-Al2O3 catalyst. The synergistic effects of Sn and Ag facilitated the formation of intermediates such as acetates, enolic species, and nitrates, and inhibited the deep oxidation of C3H6 into CO2, and the C3H6-SCR mechanism was carefully proposed.
1. Introduction
Nitrogen oxides (NOx) are one of the main pollutants that cause atmospheric pollution such as acid rain, photochemical smog, and global warming, and cause serious harm to the ecological environment and human health.1–3 Automotive exhaust is an important source of NOx emissions, of which diesel vehicle exhaust contributes around 90%. The large amount of NOx emissions has been one of the main reasons for the ozone pollution in many urban areas in China in recent years.4 At present, the selective catalytic reduction technology (SCR) using NH3 as a reducing agent has been commercially applied for diesel exhaust gas denitrification under lean-burn conditions.5 However, NH3-SCR technology requires addition of urea to generate ammonia, which increases operating costs, and NH3 slip usually causes secondary pollution.As an alternative, the technology of removing NOx by hydrocarbons (HCs) has attracted extensive attention, because no extra reducing agent needs to be supplemented, and the HC pollutants could be removed simultaneously. The saturated hydrocarbons would preferentially react with oxygen and showed poor activity, by contrast, the unsaturated hydrocarbons, such as propene could preferentially react with NOx under certain conditions.6–8 However, C3H6-SCR technology generally has problems such as poor low-temperature activity and narrow temperature window, and the core of this problem lies in the design of catalysts. Optimizing the acidity and redox properties of the catalyst through bimetallic active components has been proved to be an effective method to improve the low-temperature de-NOx activity. More et al.9 reported that Au adding onto Ag/Al2O3 effectively lowered the temperature window of C3H6-SCR, and the de-NOx efficiency reached nearly 100% at 350 °C. However, the high cost of the noble metals as active components restrains its application. Besides, it was also found that 7% Mg-doped Ag/Al2O3 could effectively improve the C3H6-SCR efficiency below 400 °C,10 which suggests that the co-loading of noble metal and base metal is a feasible approach for C3H6-SCR activity promotion. The rational design of superior bi-metal supported catalysts for C3H6-SCR and the structure–performance relationships and reaction mechanism of the catalyst are worthy of being further explored.
Due to the abundant reserves and low price, transitional metal-based catalysts such as Cu, Sn, Fe, Co, and Mn have been widely studied for C3H6-SCR.11–19 In addition, due to the strong oxidizing ability of precious metals and excellent NOx removal performance, Ag, Pt, Pd and other precious metals are also widely used in various industrial flue gases purification.20,21 Among them, SnO2 has been found to be rich in surface oxygen vacancies and acidic sites and showed great potential. Kung et al.22 and Liu et al.23 studied the C3H6-SCR performance of SnO2/Al2O3 catalysts and found that the Sn loading has a significant effect on the de-NOx efficiency, and it reached a maximum value of 83% at 450 °C. Lai et al.24 prepared a single-layer supported SnO2/beta catalyst and the de-NOx efficiency reached 85% at 500 °C. It was found that the number of beneficial surface oxygen vacancies increased due to the electron transfer and interfacial interaction between Sn4+ and H-beta support. Besides, Zhang et al.11 studied the C3H6-SCR activity of SnO2/ZSM-5 catalyst, which reached the highest de-NOx efficiency of ca. 80% at around 450 °C. The inferior catalytic activity in the low-temperature range is mainly due to its weak oxidation ability for C3H6, leading to its limited practical application. As one of the relatively cheap noble metals, supported Ag-based catalysts have also been extensively studied for C3H6-SCR. Chaieb et al.25 and Wang et al.26 studied the C3H6-SCR performance of Ag/Al2O3, and found that the de-NOx efficiency reached about 70% at 450 °C. He et al.27,28 found that 4 wt% Ag loading amounts improved the de-NOx efficiency, reaching about 90% at 450 °C. However, unlike Sn-based catalysts, the main factor that restrains the SCR activity of Ag-based catalysts in the low-temperature range results from its strong oxidizing ability, which facilitates C3H6 to deeply oxidized into CO2 instead of reactive organic intermediates to react with NOx. Therefore, a combination of Sn and Ag is possibly conducive to a broadened temperature window. In addition, Wang et al.29 reported that 2% AgCl/Al2O3 catalyst achieved almost 100% de-NOx efficiency between 300 and 500 °C with the reducing gases of C3H6 and H2. They found that AgCl nanoparticles could promote the production of formats and enolic species. Due to the fact that the oxidation ability of AgCl nanoparticles was much weaker than that of Ag2O, the presence of small amounts of AgCl particles might be conducive to suppressing the deep oxidation process of C3H6. Inspired by this, in addition to the preparation of Ag and Sn bimetallic supported catalysts, the present study will also introduce AgCl nanoparticles through using tin chloride salts to optimize the acidity and redox properties of the catalysts, thus further widening the low-temperature window of C3H6-SCR.
Herein, a series of γ-Al2O3 supported catalysts with different Sn/Ag ratios were prepared and their activity was tested in this work. Furthermore, the physicochemical properties of the catalysts were characterized by N2 adsorption–desorption, scanning electron microscope (SEM), high resolution-transmission electron microscope (HR-TEM), X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), pyridine infrared (Py-IR), H2-temperature programmed reduction (H2-TPR), and O2-temperature programmed desorption (O2-TPD). Besides, in situ diffuse reflectance Fourier transform infrared spectroscopy (in situ DRIFTS) was carried out to explore the catalytic reaction mechanism. The structure–activity relationships of the 1Sn5Ag/γ-Al2O3 catalyst and its C3H6-SCR mechanism were fully explored.
2. Experimental
2.1 Catalyst preparation
The catalysts tested in this work were prepared by wet impregnation method. As an example, 1Sn5Ag/γ-Al2O3 catalyst was prepared as follows: an appropriate amount of fresh γ-Al2O3 was dried in an oven at 120 °C for 6 h and then calcined in a muffle furnace at 500 °C for 2 h for subsequent use. 0.0234 g SnCl4·5H2O and 888 μL standard Ag(NO)3 solution (1.022 mol L−1) were dissolved in 20 mL deionized water to prepare the impregnation solution, and then 1 g pre-treated γ-Al2O3 powder was add, and stir vigorously in a water bath at 80 °C for about 2 h until it is perfectly dry. The catalyst was then transferred into the oven and dried at 80 °C for 6 h. Finally, the catalyst was placed in a tube furnace and calcined at a temperature of 600 °C under air atmosphere for 6 h to obtain 1Sn/γ-Al2O3. After that, a certain amount of AgNO3 was dissolved in deionized water, then 1Sn/γ-Al2O3 was dosed, and the above procedures were repeated to obtain the 1Sn5Ag/γ-Al2O3 catalyst.2.2 Catalyst characterization
N2 adsorption–desorption was carried out with an automatic specific surface and porosity analysers (Micromeritics TriStar II 3020) at −196 °C. SEM was performed with a Zeiss SUPRA-55 field emission scanning electron microscope. HR-TEM was tested with the American FEI Talos F200S transmission electron microscope. XRD was measured using an X-ray diffractometer (Rigaku Ultima IV). The incident ray light source is a copper target, the test angle is 5–120°, and the step size is 0.02°. XPS was carried out with an X-ray photoelectron spectrometer (Thermo Scientific K-Alpha) equipment with monochromatic Al–K X-ray radiation at 250 W. H2-TPR was carried out on the gas chromatography equipped with a thermal conductivity detector (TCD). 200 mg of the catalysts were placed in a U-type reaction tube, and the temperature was from room temperature to 300 °C at a ramp of 10 °C min−1 under O2 flow for 30 min to remove surface adsorbed species. Then 30 mL per min N2 was purged for 30 min and cooled to 50 °C. The temperature was increased to 750 °C at a heating rate of 5 °C min−1 in 5% H2/N2, and the signals were continuously recorded. O2-TPD was also performed similarly. 200 mg of the catalysts were placed in a U-type reaction tube, and the temperature was from room temperature to 300 °C at a ramp of 10 °C min−1 under O2 flow for 30 min to remove surface adsorbed species. Subsequently, 30 mL per min N2 was purged for 30 min to remove unstable surface adsorption of O2 and cooled to 50 °C. Finally, the sample was heated from 30 °C to 600 °C at a rate of 5 °C min−1 under an N2 atmosphere. Py-IR and in situ DRIFTS were obtained on a Nicolet iS50 spectrometer equipped with in situ reaction cell (Harrick HVC-DRP-5), and the scan resolution was 4 cm−1. After pre-treatment in the Ar atmosphere at 400 °C for 1 h, the catalyst was reacted with pyridine vapor at 300 °C for 30 min, and the corresponding IR spectrum was recorded after stabilizing for 10 min. Prior to the in situ DRIFTS tests, the catalyst was pre-treated in the in situ cell at 300 °C for 30 min under air, and then cooled to ambient temperature. Subsequently, the temperature was increased to 300 °C at 10 °C min−1, and the corresponding components of NO/N2, C3H6/N2, and O2 were purged as needed, and the IR spectrum was recorded continuously.2.3 Catalytic performance evaluation
The catalyst was sieved to 40–60 mesh and packed in quartz tubes (4 mm i.d.). The model flus gas was composed of 0.05 vol% NO, 0.3 vol% C3H6, 5 vol% O2, and balanced with N2. The NOx and C3H6 conversion were calculated in terms of the eqn (1) and (2), respectively, and the COx selectivity was calculated in terms of the eqn (3):
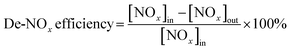 | (1) |
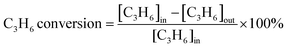 | (2) |
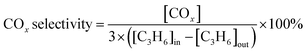 | (3) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mL g−1 h−1. NOx concentrations were measured with an online NOx analyzer (Thermo Fisher, 42i-LH), and C3H6, CO and CO2 concentrations were measured by gas chromatography (Fuli, GC 9790II).
000 mL g−1 h−1. NOx concentrations were measured with an online NOx analyzer (Thermo Fisher, 42i-LH), and C3H6, CO and CO2 concentrations were measured by gas chromatography (Fuli, GC 9790II).
3. Results and discussion
3.1 Texture properties
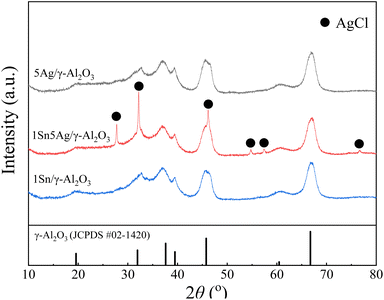
XRD patterns for 1Sn/γ-Al2O3, 5Ag/γ-Al2O3, 1Sn5Ag/γ-Al2O3 catalysts are presented in Fig. 1, where diffraction peaks observed for all the catalysts at 2θ = 19.6°, 32.2°, 37.5°, 39.6°, 46.0°, 60.1°, and 66.7° were ascribed to γ-Al2O3 (JCPDS #02-1420), indexed to (111), (220), (311), (222), (400), (511), and (440) crystal planes, respectively. This also suggests that the loading of Ag and Sn active components did not disrupt the crystalline structure of the support. In addition, no diffraction peaks belonging to the corresponding metals or oxides of Ag and Sn were detected on any of the catalysts, indicating that Ag and Sn species remained in the amorphous state on the γ-Al2O3 support. It was noted that the 1Sn5Ag/γ-Al2O3 catalyst showed significantly different diffraction peaks at 2θ = 27.6°, 32.1°, 46.2°, 54.8°, 57.5°, and 76.8° from the other two catalysts, which were all ascribed to the diffraction peaks of AgCl crystalline. This should be related to the fact that the precursor of tin used for catalyst preparation was SnCl4 as mentioned in Section 2.1. The specific surface area loss for 1Sn5Ag/γ-Al2O3 catalyst might be related to the formation of AgCl crystallites as well.N2 adsorption–desorption was carried out to obtain the specific surface area and pore structure of the catalysts, the results of which are shown in Table 1 and Fig. 2. It can be seen that the loading of Sn or Ag had little effect on the specific surface area, and the values of 1Sn/γ-Al2O3 and 5Ag/γ-Al2O3 were very close to the γ-Al2O3 support (180.10 m2 g−1). However, the catalysts loaded with both Sn and Ag (1Sn5Ag/γ-Al2O3) were found to show an obvious surface area loss, and it exhibited the lowest specific surface area (172.2 m2 g−1). This is possibly due to the precipitation of Ag and Sn species into the channel of γ-Al2O3, resulting in a reduction in the specific surface area of the catalysts.30 The change in pore volume and pore size of the catalysts showed a slight decrease in pore volume and pore size after loading. As shown in Fig. 2a, the adsorption–desorption isotherms of the 1Sn/γ-Al2O3, 5Ag/γ-Al2O3, and 1Sn5Ag/γ-Al2O3 catalysts were all of type IV, and the hysteresis loops were of type H2b. This suggests the mesoporous structure of the catalysts, and the loading of Sn and Ag had little effect on the pore structure. The pore size distributions of the three catalysts were also similar, with the pore sizes mainly in the mesoporous range of 2–30 nm (Fig. 2b).
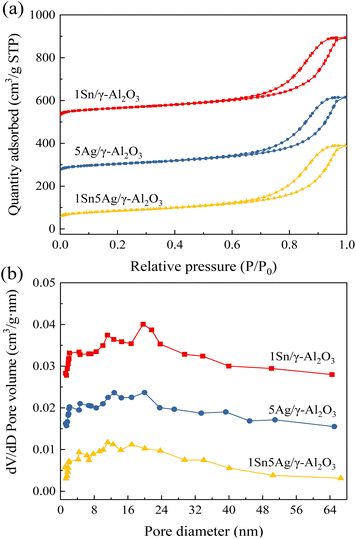 | ||
| Fig. 2 N2 adsorption–desorption isotherms (a) and pore diameter distribution (b) of γ-Al2O3, 1Sn/γ-Al2O3, 5Ag/γ-Al2O3, and 1Sn5Ag/γ-Al2O3 catalysts. | ||
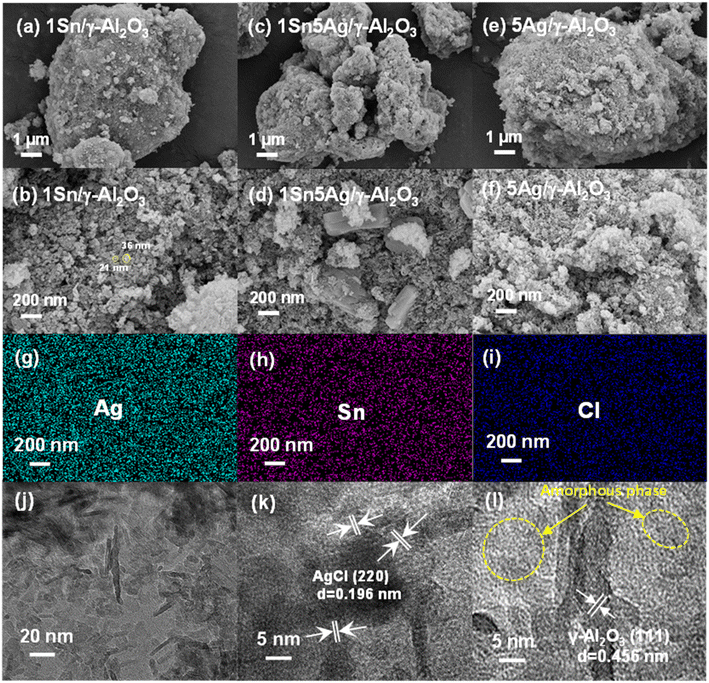
Fig. 3 demonstrates the micrographs of 1Sn/γ-Al2O3, 5Ag/γ-Al2O3, and 1Sn5Ag/γ-Al2O3, and energy dispersive spectrometer (EDS) mapping of 1Sn5Ag/γ-Al2O3. Small particles belonging to Sn and Ag species were observed, and the particle sizes mainly fell into the range of 20–50 nm (Fig. 3a–f). EDS mapping of 1Sn5Ag/γ-Al2O3 detected a uniform distribution of Ag, Sn, and Cl elements on the catalyst surface (Fig. 3g–i), indicating a high dispersion of Ag and Sn species on the surface of 1Sn5Ag/γ-Al2O3 catalyst. Besides, it was noted that the 1Sn5Ag/γ-Al2O3 had rod-like components with larger size that were distinct from 1Sn/γ-Al2O3 and 5Ag/γ-Al2O3. In combination with the XRD results, it was speculated that these rod-like components were probably AgCl species. Furthermore, the states of Ag and Sn species of the 1Sn5Ag/γ-Al2O3 catalyst were determined by HR-TEM, and the representative micrographs are shown in Fig. 3j–l. Both γ-Al2O3 support exposing the (111) crystal plane and AgCl crystallites exposing the (220) crystal plane could be clearly observed, which corresponded well to the XRD patterns presented in Fig. 1. Besides, many areas with instinct lattice fringe were also found. This implies that a mass of amorphous phases also existed in the catalyst. Combined with the catalyst preparation and XRD results, it was deduced that the amorphous phases were highly dispersed oxides of Sn and Ag. To sum up, the pore structure and morphological characteristics of the catalysts were little affected by Sn and Ag loading, indicating that the increased de-NOx activity of 1Sn5Ag/γ-Al2O3 might be mainly due to changes in its chemical properties.
3.2 Chemical states of surface elements
High-resolution XPS was performed to determine the chemical states of the elements on the surface of catalysts. The spectra of O 1s, Sn 3d, and Ag 3d of 1Sn/γ-Al2O3, 5Ag/γ-Al2O3, and 1Sn5Ag/γ-Al2O3 catalysts, and their deconvolution results are presented in Fig. 4. The Sn 3d spectrum on 1Sn/γ-Al2O3 catalyst (Fig. 4a), where the binding energies at 495.35 and 495.35 eV are 3d5/2 and 3d3/2 resulting from Sn 3d spin–orbit coupling, respectively. This indicates that the Sn species mainly exists in the form of Sn4+,24 which were active sites in C3H6-SCR reaction.31 However, when Ag and Sn were co-loaded, the binding energies of Sn 3d3/2 and Sn 3d5/2 of 1Sn5Ag/γ-Al2O3 shifted from 495.35 eV to 495.5 eV and 486.65 eV to 486.8 eV, respectively. This indicated that the Sn 3d electron cloud density of 1Sn5Ag/γ-Al2O3 catalyst decreased, which was possibly due to the electron transfer from Sn to Ag species, and the strong electronegativity of Cl− might contribute as well.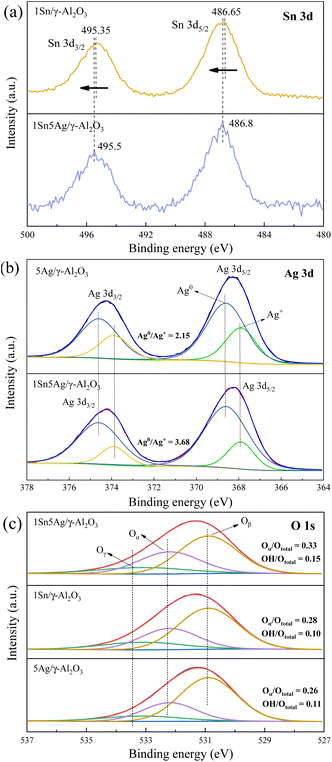 | ||
| Fig. 4 XPS spectra of Sn 3d (a), Ag 3d (b), and O 1s (c) of 1Sn/γ-Al2O3, 5Ag/γ-Al2O3, and 1Sn5Ag/γ-Al2O3 catalysts. | ||
Two XPS characteristic peaks of Ag 3d, 3d5/2 and 3d3/2, were observed, and they could be deconvoluted into Ag+ and Ag0 (ref. 29) (Fig. 4b). The ratio of Ag0/Ag+ was calculated to be 2.15 for 5Ag/γ-Al2O3 and 3.68 for 1Sn5Ag/γ-Al2O3, respectively. The coexistence of Ag and Sn species on the surface of the 1Sn5Ag/γ-Al2O3 resulted in a significant increase in the relative Ag0 content, which was deduced to be the electron cloud transfer from Sn to Ag species. The existence of Ag0 on the surface of 5Ag/γ-Al2O3 catalyst was resulted from the strong metal-support interaction (SMSI) effects. In combination with the XRD results, it was concluded that the presence of Ag on the surface of the 5Ag/Al2O3 was in the amorphous states of metallic Ag0 and Ag2O. The presence of Ag on the surface of the 1Sn5Ag/γ-Al2O3 was in the amorphous states of metallic Ag0, Ag2O and AgCl crystalline. The highly dispersed state of Ag0 species had a positive effect on the SCR activity. Xu et al. found that metallic silver species were conducive to the partial oxidation of hydrocarbons and form active enolic species, thus the low-temperature catalytic efficiency was improved.32 More et al.9 also reported similar positive effects of Ag0 species for NOx removal.
O 1s was deconvoluted into three peaks at 530.9, 533.1, and 532.2 eV, corresponding to surface lattice oxygen (denoted as Oβ), surface adsorbed oxygen (denoted as Oα), and surface hydroxyl (denoted as Oγ), respectively.33 It is found that the relative ratios of Oα (0.33) and Oγ (0.15) for 1Sn5Ag/γ-Al2O3 were greatly increased compared to that of 1Sn/γ-Al2O3 and 5Ag/γ-Al2O3. It is well known that Oα species with high mobility are more favorable for the SCR process.34 Besides, the Oγ species were also important active sites for the adsorption and activation of C3H6 and NO, especially in the low temperature range.13 The increased relative ratios of Oα and Oγ species on the surface of 1Sn5Ag/γ-Al2O3 made great contributions to its de-NOx activity improvement.
3.3 The mobility of surface oxygen species
The mobility of surface oxygen species on the surface of 1Sn/γ-Al2O3, 5Ag/γ-Al2O3 and 1Sn5Ag/γ-Al2O3 catalysts were further studied by H2-TPR and O2-TPD, and the results are shown in Fig. 5. Due to the high temperature pretreatment of the Al2O3 support and its strong Al–O bond, no significant reduction peaks should be observed in the temperature range tested. As presented in Fig. 5a, 1Sn/γ-Al2O3 showed only one H2 reduction peak at 200 °C, which could be ascribed to Sn4+ to Sn2+ reduction peak. Two reduction peaks were observed at 91 °C and 123 °C for 5Ag/γ-Al2O3, probably corresponding to the Ag+ reduction of AgCl and Ag+ reduction of Ag2O, respectively. In contrast, a broad reduction peak at 187 °C was observed for the 1Sn5Ag/γ-Al2O3, as well as a reduction peak at 533 °C. The peak at 187 °C should be an overlap of the Sn4+ reduction peak and the Ag+ reduction peak, while the peak at 533 °C was ascribed to the Sn2+ to Sn0 reduction peak.23 Compared with 5Aag/γ-Al2O3, there was a significant increase in the number of reducible species for 1Sn5Ag/γ-Al2O3, but the reducibility was weakened. This indicates that a higher number of surface oxygen species on 1Sn5Ag/γ-Al2O3 could participate in the SCR reaction, but with a weaker mobility.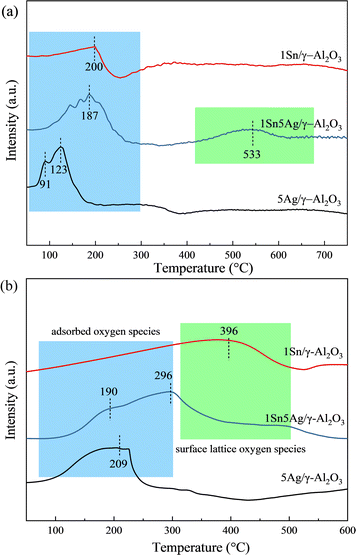 | ||
| Fig. 5 H2-TPR (a) and O2-TPD (b) profiles of 1Sn/γ-Al2O3, 5Ag/γ-Al2O3, and 1Sn5Ag/γ-Al2O3 catalysts. | ||
As shown in Fig. 5b, several O2 desorption bands were clearly observed for the catalysts tested. It is generally considered that the desorption bands between 50 and 300 °C corresponded to surface adsorption oxygen species, and the bands between 300 and 600 °C corresponded to surface lattice oxygen species.35 There was a broad band centered at 396 °C of 1Sn/γ-Al2O3, with the highest desorption temperature among the three catalysts, and this suggests that the surface oxygen species mobility of 1Sn/γ-Al2O3 was inferior to that of 5Ag/γ-Al2O3 and 1Sn5Ag/γ-Al2O3. By contrast, there was a broad desorption band centered at 209 °C of 5Ag/γ-Al2O3, indicating the high mobility of its surface adsorbed oxygen species. This also explains why it has the strongest ability to deeply oxidize C3H6 into CO2. Besides, no desorption peaks attributed to lattice oxygen were detected for 5Ag/γ-Al2O3, indicating the inferior mobility of surface lattice oxygen species and they were difficult to participate in the SCR reaction. In comparison to the 5Ag/γ-Al2O3, the amount of adsorbed oxygen species on the surface of 1Sn5Ag/γ-Al2O3 increased, and the amount of total oxygen species desorbed was the largest. Besides, it is noted that the surface adsorbed oxygen desorption temperature of 1Sn5Ag/γ-Al2O3 shifted to 190 °C and became weaker, but a new surface lattice oxygen desorption band (296 °C) appeared. The decrease in the number of adsorbed oxygen species on the surface of 1Sn5Ag/γ-Al2O3 inhibited the over-oxidation of C3H6, while the high mobility of surface lattice oxygen was conducive to partial oxidation of C3H6.36 Therefore, 1Sn5Ag/γ-Al2O3 catalyst showed better de-NOx efficiency. The combined results of O2-TPD and H2-TPR showed that the redox ability of the catalyst was effectively regulated by co-loading Sn and Ag. The coexistence of Sn and Ag promoted the oxidation of C3H6 and NO to acetates, nitrates and other important intermediates on 1Sn5Ag/γ-Al2O3, but inhibited the over-oxidation of C3H6 to CO2, thus the de-NOx efficiency was improved.
3.4 Surface acidity properties
Py-IR was carried out to identify the nature of surface acid sites, which is another determining factor for C3H6-SCR performance, and the results of 1Sn5Ag/γ-Al2O3 catalyst are shown in Fig. 6. The bands at 1450 and 1575 cm−1 were assigned to the pyridine molecule (Py-L)37 adsorbed on the Lewis acid sites, and the band at 1612 cm−1 was assigned to Lewis–Brønsted acid complex,18,37 the band at 1612 cm−1 was assigned to the adsorbed pyridine ion (Py-H+).38 It is worth noting that compared with the number of Lewis acid sites, Brønsted acid sites were extremely lacking. There were only a few Brønsted acid sites on the surface of the 5Ag/γ-Al2O3 catalyst, and none for 1Sn5Ag/γ-Al2O3. Some researchers reported that the Brønsted acid sites were indispensable for C3H6-SCR reaction, because they played a key role in C3H6 activation and forming related intermediates.36,39 However, Lewis acids were found to be essential for NO adsorption, which was a key step in C3H6-SCR,40 and the adsorption strength was stronger than on Brønsted acid sites.41 Hence, it was deduced that for the 1Sn5Ag/γ-Al2O3 catalyst, the adsorption and activation of C3H6 and NO were mainly achieved at the Lewis acid sites, i.e., the Lewis acids dominated C3H6-SCR reaction. Besides, Lin et al.42 reported that Lewis acid sites were responsible for deep oxidation of C3H6. The 5Ag/γ-Al2O3 catalyst had the largest number of Lewis acid sites, which explains why it exhibited the highest C3H6 conversion. In comparison, total acid content and acid strength of 1Sn5Ag/γ-Al2O3 greatly decreased, thus inhibited C3H6 over-oxidation into CO2, which was beneficial to the improvement of the de-NOx efficiency. A similar phenomenon was also observed for C3H6-SCR over Mg–Ag/Al2O3 catalyst.10 The above studies revealed that the improved de-NOx efficiency for 1Sn5Ag/γ-Al2O3 catalyst was largely attributed to the synergistic effect between Sn and Ag species, which positively changed their chemical states, the mobility of surface oxygen species and surface acidity for C3H6-SCR.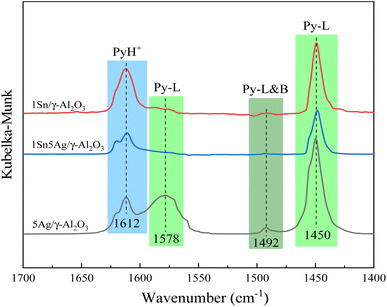 | ||
| Fig. 6 DRIFTS of pyridine adsorption on 5Ag/γ-Al2O3, 1Sn/γ-Al2O3, and 1Sn5Ag/γ-Al2O3 catalysts at 300 °C. | ||
3.5 Catalytic activity
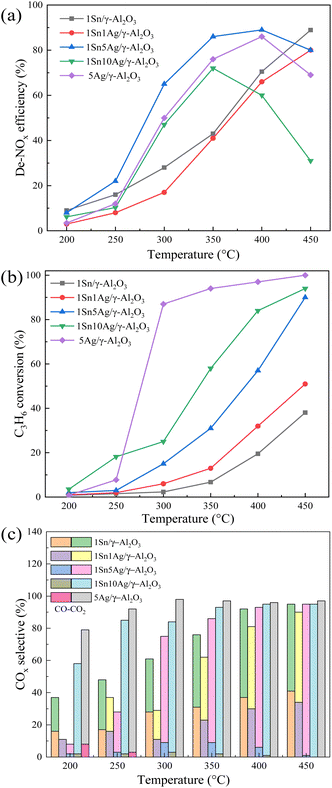
Ag species with various amounts were loaded onto 1Sn/γ-Al2O3, and the results are shown in Fig. 7a. It is seen that the de-NOx efficiency was somewhat suppressed after the introduction of 1 wt% Ag component onto 1Sn/γ-Al2O3. As the Ag loading was increased 5 wt%, the overall de-NOx efficiency reached the highest level. The 1Sn5Ag/γ-Al2O3 catalyst showed the widest temperature window of T80 (the temperature at which 80% de-NOx efficiency was achieved), which was 336–448 °C, and it reached a maximum de-NOx efficiency of 88% at 400 °C. At 350 °C, the 1Sn5Ag/γ-Al2O3 catalyst achieved ca. 50% higher de-NOx activity than 1Sn/γ-Al2O3, and ca. 15% higher than 5Ag/γ-Al2O3. Several representative metal oxides supported catalysts were compiled in Table 2 and the de-NOx efficiency at 350 °C was compared with 1Sn5Ag/γ-Al2O3 catalyst. However, a further increase of Ag loading to 10 wt% resulted in a significant decrease in de-NOx efficiency. This might be attributed to the high Ag loading resulting in a large amount of C3H6 being over-oxidized into CO2 and thus less involved in NOx reduction. Based on the above results, there were obvious synergistic effects between the Sn and Ag components supported on γ-Al2O3, and the best de-NOx efficiency was achieved when the loading amount of Sn and Ag was 1 wt% and 5 wt%, respectively.| Catalysts | Reaction conditions/vol% | WHSV/mL g−1 h−1 | T80/°C | De-NOx efficiency at 350 °C/% | Ref. |
|---|---|---|---|---|---|
| SnO2/Beta | 0.05% NO, 0.05% C3H6, 5% O2 | 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
470 | 13 | 24 |
| SnO2/ZSM-5 | 0.05% NO, 0.05% C3H6, 5% O2 | 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
446 | 35 | 11 |
| SnO2/Al2O3 | 0.05% NO, 0.05% C3H6, 5% O2 | 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
448 | 32 | 23 |
| 5%Ag/Al2O3 | 0.08% NO, 0.1714% C3H6, 10% O2, 10% H2O | 20,0000 | 425 | 23 | 26 |
| 1.6%Au/Al2O3 | 0.21% H2, 0.0385% NO, 0.04% C3H6, 8% O2 | 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
— | 40 | 44 |
| 2%Ag–CeZr | 0.2% NO, 0.2% C3H6, 10% O2, 10% H2 | 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
300 | 83 | 45 |
| Cu0.71Fe0.29-600c | 0.05% NO, 0.1% C3H6, 2% O2 | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
— | 60 | 18 |
| Cu/SO42−/Al–Ce-PILC | 0.22% NO, 0.12% C3H6, 2% O2 | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
— | 47 | 42 |
| This work | 0.05% NO, 0.3% C3H6, 5% O2 | 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
336 | 85 | — |
60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
— | 77 |
Fig. 7b and c demonstrate the C3H6 conversion and COx selectivity. It is seen that the 5Ag/γ-Al2O3 catalyst achieved the highest conversion for the catalytic oxidation of C3H6, and the CO2 selectivity was close to 100% at 300 °C. However, the deep oxidation of C3H6 was unfavorable for its participation in the SCR process. In the C3H6-SCR process, the reaction of C3H6 with NOx and the direct oxidation of C3H6 are in competition, and the NOx reduction mainly depends on the intermediate species such as acetates generated by the partial oxidation of C3H6.43 Higher ratio of C3H6 being deeply oxidized to CO2 resulted in less C3H6 for NOx reduction and thus deteriorated the de-NOx efficiency. It is seen that the C3H6 conversion of 1Sn5Ag/γ-Al2O3 catalyst at 300 °C was reduced by ca. 70% compared to that of 5Ag/γ-Al2O3 (Fig. 7b). The total COx selectivity was ca. 75%, indicating that a large number of organic intermediates might have been generated on the surface of 1Sn5Ag/γ-Al2O3 catalyst. For the 1Sn1Ag/γ-Al2O3 catalyst, the COx selectivity was the lowest overall among all samples tested (Fig. 7c), although its C3H6 conversion was slightly higher than that of 1Sn/γ-Al2O3. Moreover, the de-NOx efficiency was also lower than that of 1Sn/γ-Al2O3. This suggests that the oxidation of C3H6 possibly generated a certain number of intermediates over 1Sn1Ag/γ-Al2O3, and they exhibited inferior reactivity with NOx. As for 1Sn10Ag/γ-Al2O3 catalyst, the decrease in de-NOx efficiency might be due to the higher amount of C3H6 over-oxidation after the Ag loading increases. The above results suggest that the synergistic effects between a certain proportion of Ag and Sn components maintained the oxidation of C3H6 at a suitable level, so that more reactive organic intermediates would be generated to participate in the SCR reaction, thus effectively improving the de-NOx efficiency.
3.6 Reaction mechanism
Firstly, the adsorption characteristics of C3H6 and NO molecules on the 1Sn5Ag/γ-Al2O3 catalyst were studied, and the corresponding FTIR bands at 30 °C are presented in Fig. 8. When C3H6 and O2 were introduced, five bands emerged between 1200 and 1800 cm−1. The band observed at 1383 cm−1 was assigned to formate species.15 The band at 1406 cm−1 was assigned to enolic species.29 The bands at 1456 and 1593 cm−1 were assigned to acetate species.46 The band at 1654 cm−1 was assigned to the δ(OH) hydroxyl group of surface adsorbed water.47 Besides, the broad band between 3000 and 3200 cm−1 was ascribed to ν(C–H) of C3H6, and 3200–3500 cm−1 was ascribed to hydrogen-bonded OH groups.36 The above results indicate that C3H6 could be easily oxidized over 1Sn5Ag/γ-Al2O3 catalyst, and acetates were the main intermediates.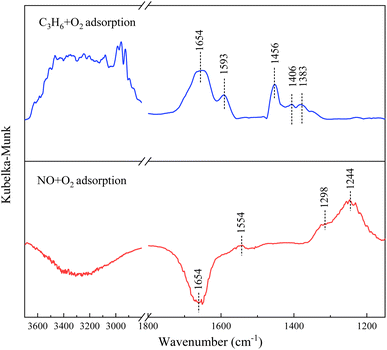 | ||
| Fig. 8 C3H6 + O2 adsorption at 30 °C and NO + O2 adsorption at 30 °C over 1Sn5Ag/γ-Al2O3 catalyst. Reaction conditions: 0.05% NO, 0.3% C3H6, 5% O2, and N2 balance. | ||
When NO and O2 were introduced, four bands were observed between 1200 and 1800 cm−1. The band at 1244 cm−1 was assigned to bridged nitrates. The bands at 1298 and 1554 cm−1 were assigned to bidentate nitrates.16 The bands at 1654 cm−1 and 3200–3500 cm−1 were assigned to surface hydroxyl groups. This indicates that NO could be easily oxidized over 1Sn5Ag/γ-Al2O3 catalyst to generate bridged nitrate and bidentate nitrate. In addition, it was noted that the surface hydroxyl groups (originated from the synergistic effects between Sn and Ag based on the XPS results) were largely consumed after the introduction of NO. Therefore, the presence of surface hydroxyl groups helped to promote the formation of key intermediate nitrates, and then effectively improve the low-temperature de-NOx activity.
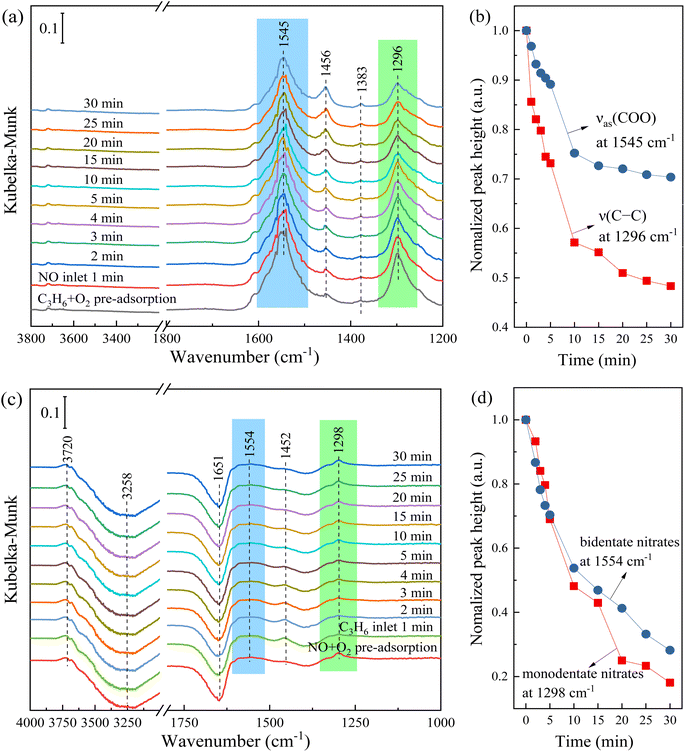
The transient reaction experiments were carried out to further explore the C3H6-SCR mechanism of 1Sn5Ag/γ-Al2O3 catalyst. At first, C3H6 and O2 were pre-adsorbed on the surface of 1Sn5Ag/γ-Al2O3 catalyst at 300 °C, and then NO was introduced. The results of the bands changes at different time durations are shown in Fig. 9a. The bands at 1296, 1456, 1545 cm−1 were assigned to ν(C–C), νs(COO), and νas(COO) of acetates.26,27,48 Comparing with the FTIR spectra at 30 °C, it was found that the thermal stability of formates was quite high (1383 cm−1), but the acetates would partially disappear (1456 and 1593 cm−1) as the temperature was increased to 300 °C. Besides, it was noted that the bands at 1296 and 1545 cm−1 were continuously consumed after NO was introduced, following the E-R mechanism. In contrast, formates were almost inert and did not react with NO. Fig. 9b show normalized peak height changes of the band at 1296 and 1545 cm−1 with the time of NO feeding. It is seen that the acetates were quickly consumed within 10 min, indicating that acetates were key active intermediates involved in the C3H6-SCR reaction over 1Sn5Ag/γ-Al2O3 catalyst. Acetates with high reactivity were also detected over Ag/Al2O3 and Cu/Al2O3 catalysts, and being consumed at a similar rate to NOx.49–51 The band at 1456 cm−1 was possibly ascribed to the overlapping of acetates and monodentate nitrates, because monodentate nitrates would accumulate with the continuous flow of NO while acetates should be consumed.
Subsequently, NO and O2 were pre-adsorbed on the surface of 1Sn5Ag/γ-Al2O3 catalyst at 300 °C, and then C3H6 was introduced. The results of the bands changes at different time durations are shown in Fig. 9c. The bands at 1298 and 1456 cm−1 were assigned to monodentate nitrates, and the band at 1554 cm−1 was assigned to bidentate nitrates. Comparing with the FTIR spectra at 30 °C, it was found that the bridge nitrates was not thermally stable, and it could be converted into monodentate and bidentate nitrates at elevated temperature. It is also noted in the FTIR bands belonging to monodentate nitrate (1456 cm−1) and bidentate nitric acid (1554 cm−1) were gradually consumed as C3H6 was introduced, following the E-R mechanism. The negative band assigned to adsorbed water at 1654 cm−1 was gradually weakened with the introduction of C3H6, which was related to the formation of the R–NO2 intermediates (1651 cm−1).14 Fig. 9d demonstrate the consumption rates of monodentate nitrates and bidentate nitrates as C3H6 was introduced, and both were consumed ca. 80% within 30 min. Besides, no accumulation of acetates was observed. Combined with the results in Fig. 9a, it suggests that the reactivity between gaseous C3H6 and adsorbed nitrates was higher than that of gaseous NO and adsorbed acetates. This is also consistent with the Py-IR results that the Lewis acid sites dominate nitrate species formation and activation. Besides, it is speculated that the nitrate species adsorbed on the hydroxyl group on the surface of the catalyst also participates in the SCR reaction, because with the introduction of C3H6, the band at 3258 cm−1 assigned to the surface hydroxyl group weakened gradually, and water (3720 cm−1) was formed at the same time.
Besides, NO + C3H6 + O2 was introduced simultaneously between 200 and 500 °C, and the results are shown in Fig. 10. A new band at 2325 cm−1 emerged, and it was assigned to NCO species.52 NCO was also a key active intermediate for Ag/Al2O3 catalysts,52 which was formed through the reaction between acetates and nitrates, and was easily further oxidized into CO2 and N2. Xu et al.46 and Guo et al.12 also reported that NCO species played an important role in improving C3H6-SCR process. The band assigned to NCO did not appear under the pre-adsorption reaction conditions, indicating that the SCR reaction of the 1Sn5Ag/γ-Al2O3 catalyst not only followed the E-R mechanism, but also followed the L-H mechanism. Besides, the band assigned to NCO at 300 °C and below was very weak. With the increase of the reaction temperature, the amount of NCO generated increases, indicating that the formation of NCO was quite limited by the reaction kinetics at low temperature. The low amount NCO produced was responsible for the inferior de-NOx efficiency. With the further increase of the reaction temperature, the monodentate and bidentate nitrates accumulated and could not be further converted, thus deteriorated the de-NOx efficiency. The band assigned to water at 3545 and 3720 cm−1 became stronger with the increase of temperature, which might be related to the SCR reaction and the direct oxidation of C3H6.
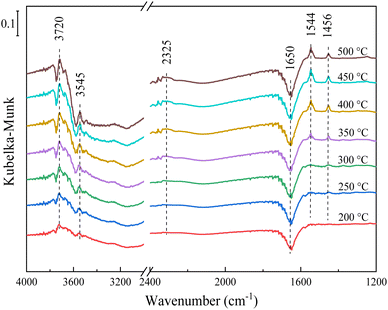 | ||
| Fig. 10 In situ DRIFTS spectra of 1Sn5Ag/γ-Al2O3 catalyst in a flow of NO + C3H6 + O2 from 200 to 500 °C. Reaction conditions: 0.05% NO, 0.3% C3H6, 5% O2, and N2 balance. | ||
Based on the results of catalyst characterizations and in situ DRIFTS, the C3H6-SCR reaction mechanism over 1Sn5Ag/γ-Al2O3 catalyst was proposed and depicted in Scheme 1. The Lewis acid sites were mainly responsible for the C3H6-SCR reaction. A majority of C3H6 would therefore be partially oxidized into adsorbed formates, acetates and enolic species, and NO would be oxidized into monodentate and bidentate nitrates, on 1Sn5Ag/γ-Al2O3 catalyst. The presence of AgCl particles and hydroxyl species helped to promote the reactions. The reaction mainly occurred between adsorbed acetates and nitrates to form NCO species, and gaseous C3H6 with adsorbed monodentate and bidentate to form R–NO2 species, and CO2 and N2 would be finally generated.
4. Conclusion
In this work, a series of Sn/γ-Al2O3 and SnAg/γ-Al2O3 catalysts with different loadings were prepared and tested, and it was found that the 1Sn5Ag/γ-Al2O3 catalyst exhibited the best C3H6-SCR activity, which maintained above 80% de-NOx efficiency between 336 and 448 °C. The synergistic effects of Sn and Ag improved the C3H6-SCR activity effectively. The characterizations found that the improvement of the de-NOx activity of 1Sn5Ag/γ-Al2O3 was closely related to the formation of AgCl nanoparticles, the change of Ag valence state, and the improvement of the mobility of surface oxygen species. The surface oxygen species contributed to the adsorption and activation of gaseous C3H6 and NO, and the highly dispersed metallic Ag species could promote the partial oxidation of C3H6 to enolic species with high reactivity. The presence of AgCl nanoparticles adjusted the redox ability of 1Sn5Ag/γ-Al2O3 to a moderate level and inhibited the deep oxidation of C3H6, which was essential for C3H6-SCR. The Lewis acid sites were mainly responsible for C3H6-SCR over 1Sn5Ag/γ-Al2O3 catalyst, and the reaction followed both E-R and L-H mechanism. The main reactions occurred between adsorbed monodentate/bidentate nitrates and gaseous C3H6, as well as acetates and nitrates, then key intermediates such as NCO and R–NO2 would be generated. Finally, they would convert into harmless N2 and CO2. This work reveals the synergistic effects of noble and base metals (Ag and Sn) for promoting C3H6-SCR performance, which provided new theoretical ideas and guidance for developing new HC-SCR catalysts.Author contributions
Ning Li: investigation, writing – original draft, data curation; Tiantian Zhang: formal analysis; Zuliang Wu, Jing Li, Wei Wang, and Jiali Zhu: investigation; Shuiliang Yao: supervision; Erhao Gao: conceptualization, writing – review & editing, funding acquisition.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This work was funded by Natural Science Foundation of Jiangsu Province, China (Grant No. BK20210857), Scientific Research Foundation of Jiangsu Provincial Education Department, China (Grant No. 21KJB610006), and Leading Innovative Talent Introduction and Cultivation Project of Changzhou City, China (Grant No. CQ20210083). The authors also would like to thank shiyanjia lab for the support of XPS test.References
- X. Wang, X. Li, J. Mu, S. Fan, X. Chen and L. Wang, et al., Oxygen Vacancy-rich porous Co3O4 Nanosheets toward boosted NO reduction by CO and CO oxidation: insights into the structure-activity relationship and performance enhancement mechanism, ACS Appl. Mater. Interfaces, 2019, 11(45), 41988–41999 CrossRef CAS PubMed.
- H. Asakura, M. Kirihara, K. Fujita, S. Hosokawa, S. Kikkawa and K. Teramura, et al., Fe-modified CuNi Alloy catalyst as a nonprecious metal catalyst for three-way catalysis, Ind. Eng. Chem. Res., 2020, 59(45), 19907–19917 CrossRef CAS.
- T. Liu, J. Qian, Y. Yao, Z. Shi, L. Han and C. Liang, et al., Research on SCR of NO with CO over the Cu0.1La0.1Ce0.8O mixed-oxide catalysts: effect of the grinding, Mol. Catal., 2017, 430, 43–53 CrossRef CAS.
- Ministry of Ecology and Environment of the People’s Republic of China, China mobile source environmental management annual report (Excerpt 2), Environ. Protect., 2021, 49(19), 60–70 Search PubMed.
- Y. Yu, X. Yi, J. Zhang, Z. Tong, C. Chen and M. Ma, et al., Application of ReOx/TiO2 catalysts with excellent SO2 tolerance for the selective catalytic reduction of NOx by NH3, Catal. Sci. Technol., 2021, 11(15), 5125–5134 RSC.
- H. Zhou, Y. Su, W. Liao, W. Deng and F. Zhong, NO reduction by propane over monolithic cordierite-based Fe/Al2O3 catalyst: Reaction mechanism and effect of H2O/SO2, Fuel, 2016, 182, 352–360 CrossRef CAS.
- R. Raj, M. P. Harold and V. Balakotaiah, Kinetic modeling of NO selective reduction with C3H6 over Cu-SSZ13 monolithic catalyst, Chem. Eng. J., 2014, 254, 452–462 CrossRef CAS.
- G. Liu, D. Han, J. Cheng, Y. Feng, W. Quan and L. Yang, et al., Performance of C2H4 Reductant in activated-carbon-supported MnOx-based SCR catalyst at low temperatures, Energies, 2019, 12, 123 CrossRef CAS.
- P. M. More, D. L. Nguyen, P. Granger, C. Dujardin, M. K. Dongare and S. B. Umbarkar, Activation by pretreatment of Ag-Au/Al2O3 bimetallic catalyst to improve low temperature HC-SCR of NOx for lean burn engine exhaust, Appl. Catal., B, 2015, 174, 145–156 CrossRef.
- P. M. More, N. Jagtap, A. B. Kulal, M. K. Dongare and S. B. Umbarkar, Magnesia doped Ag/Al2O3-sulfur tolerant catalyst for low temperature HC-SCR of NOx, Appl. Catal., B, 2014, 144, 408–415 CrossRef CAS.
- L. Zhang, Q. Lai, Y. Liu, X. Li, W. Zhang and X. Xu, et al., Study on the monolayer dispersion behavior of SnO2 on ZSM-5 for NOx-SCR by C3H6: the remarkable promotional effects of air plasma treatment, Phys. Chem. Chem. Phys., 2022, 24(7), 4212–4225 RSC.
- J. Xu, T. Tang, Q. Zhang, C. Zhang and F. Guo, Remarkable low temperature catalytic activity for SCR of NO with propylene under oxygen-rich conditions over Mn0.2La0.07Ce0.05Ox/ZSM-5 catalyst, Vacuum, 2021, 188, 110174 CrossRef CAS.
- J. Xu, T. Tang, X. Sheng, Y. Zhang, Q. Zhang and F. Guo, Excellent activity caused by dielectric barrier discharge (DBD) plasma activation for selective catalytic reduction with propylene (C3H6-SCR): Insight into the low temperature catalytic behavior of Mn/ZSM-5 catalysts, J. Environ. Chem. Eng., 2022, 10(2), 107009 CrossRef CAS.
- N. Wen, Y. Su, W. Deng, H. Zhou, M. Hu and B. Zhao, Synergy of CuNiFe-LDH based catalysts for enhancing low-temperature SCR-C3H6 performance: surface properties and reaction mechanism, Chem. Eng. J., 2022, 438, 135570 CrossRef CAS.
- Y. Su, N. Wen, J. Cheng, W. Deng, H. Zhou and B. Zhao, Experimental study on SCR-C3H6 over Cu-Fe/Al-PILC catalysts: catalytic performance, characterization, and mechanism, Ind. Eng. Chem. Res., 2020, 59(33), 14776–14788 CrossRef CAS.
- L. Ma, C. Y. Seo, X. Chen, K. Sun and J. W. Schwank, Indium-doped Co3O4 nanorods for catalytic oxidation of CO and C3H6 towards diesel exhaust, Appl. Catal., B, 2018, 222, 44–58 CrossRef CAS.
- M. Kashif, M. N. Khan, Y. Su and P. M. Heynderickx, Most efficient mesoporous Mn/Ga-PCH catalyst for low-temperature selective catalytic reduction of NO with C3H6, Vacuum, 2022, 198, 110879 CrossRef CAS.
- N. Wen, Y. Su, W. Deng, H. Zhou and B. Zhao, Selective catalytic reduction of NO with C3H6 over CuFe-containing catalysts derived from layered double hydroxides, Fuel, 2021, 283, 119296 CrossRef CAS.
- L. F. Liotta, G. Pantaleo, A. Macaluso, G. Di Carlo and G. Deganello, CoOx catalysts supported on alumina and alumina-baria: influence of the support on the cobalt species and their activity in NO reduction by C3H6 in lean conditions, Appl. Catal., A, 2003, 245(1), 167–177 CrossRef CAS.
- T. Thiruppathiraja, A. L. Arokiyanathan and S. Lakshmipathi, Pyrrolic, pyridinic, and graphitic sumanene as metal-free catalyst for oxygen reduction reaction – A density functional theory study, Fuel Cells, 2021, 21(6), 490–501 CrossRef CAS.
- N. Rajkoomar, A. Murugesan, S. Prabu and R. M. Gengan, Synthesis of methyl piperazinyl-quinolinyl alpha-aminophosphonates derivatives under microwave irradiation with Pd-SrTiO(3)catalyst and their antibacterial and antioxidant activities, Phosphorus Sulfur Silicon Relat. Elem, 2020, 195(12), 1031–1038 CrossRef CAS.
- P. W. Park, H. H. Kung, D. W. Kim and M. C. Kung, Characterization of SnO2/Al2O3 lean NOx catalysts, J. Catal., 1999, 184(2), 440–454 CrossRef CAS.
- Y. Liu, Q. Lai, Y. Sun, X. Xu, X. Fang and Y. Liu, et al., SnO2/Al2O3 catalysts for selective reduction of NOx by propylene: on the promotional effects of plasma treatment in air atmosphere, Catal. Today, 2019, 337, 171–181 CrossRef CAS.
- Q. Lai, Y. Liu, L. Zhang, X. Li, Z. Qiu and X. Xu, et al., Expounding the monolayer dispersion threshold effect of SnO2/Beta catalysts on the selective catalytic reduction of NOx (NOx-SCR) by C3H6, Mol. Catal., 2021, 504, 111464 CrossRef CAS.
- T. Chaieb, L. Delannoy, C. Louis and C. Thomas, On the origin of the optimum loading of Ag on Al2O3 in the C3H6-SCR of NOx, Appl. Catal., B, 2013, 142, 780–784 CrossRef.
- J. Wang, H. He, Q. C. Feng, Y. B. Yu and K. Yoshida, Selective catalytic reduction of NOx with C3H6 over an Ag/Al2O3 catalyst with a small quantity of noble metal, Catal. Today, 2004, 93–5, 783–789 CrossRef.
- H. He, C. B. Zhang and Y. B. Yu, A comparative study of Ag/Al2O3 and Cu/Al2O3 catalysts for the selective catalytic reduction of NO by C3H6, Catal. Today, 2004, 90, 191–197 CrossRef CAS.
- H. He, X. Zhang, Q. Wu, C. Zhang and Y. Yu, Review of Ag/Al2O3-Reductant system in the selective catalytic reduction of NOx, Catal. Surv. Asia, 2008, 12(1), 38–55 CrossRef CAS.
- Z. Wang, G. Xu, X. Liu, T. Wei, Y. Yu and H. He, Investigation of water and sulfur tolerance of precipitable silver compound Ag/Al2O3 catalysts in H2-assisted C3H6-SCR of NOx, ACS Omega, 2020, 5(45), 29593–29600 CrossRef CAS PubMed.
- G. Xu, J. Ma, L. Wang, W. Xie, J. Liu and Y. Yu, et al., Insight into the origin of sulfur tolerance of Ag/Al2O3 in the H2-C3H6-SCR of NOx, Appl. Catal., B, 2019, 244, 909–918 CrossRef CAS.
- Z. Liu, J. Li and J. Hao, Selective catalytic reduction of NOx with propene over SnO2/Al2O3 catalyst, Chem. Eng. J., 2010, 165(2), 420–425 CrossRef CAS.
- G. Xu, Y. Yu and H. He, Silver valence state determines the water tolerance of Ag/Al2O3 for the H2-C3H6-SCR of NOx, J. Phys. Chem. C, 2018, 122(1), 670–680 CrossRef CAS.
- Z. Wen, B. Huang, Z. Shi, Z. Yang, M. Dai and W. Li, et al., Mechanism of Zn salt-induced deactivation of a Cu/activated carbon catalyst for low-temperature denitration via CO-SCR, RSC Adv., 2022, 12(24), 14964–14975 RSC.
- H. Hu, S. Cai, H. Li, L. Huang, L. Shi and D. Zhang, In Situ DRIFTs investigation of the low-temperature reaction mechanism over Mn-Doped Co3O4 for the Selective catalytic reduction of NOx with NH3, J. Phys. Chem. C, 2015, 119(40), 22924–22933 CrossRef CAS.
- Y. Zheng, Y. Yu, H. Zhou, W. Huang and Z. Pu, Combustion of lean methane over Co3O4 catalysts prepared with different cobalt precursors, RSC Adv., 2020, 10(8), 4490–4498 RSC.
- M. Yuan, Y. Su, W. Deng and H. Zhou, Porous clay heterostructures (PCHs) modified with copper ferrite spinel as catalyst for SCR of NO with C3H6, Chem. Eng. J., 2019, 375, 122091 CrossRef CAS.
- J. Datka, A. M. Turek, J. M. Jehng and I. E. Wachs, Acidic properties of supported niobium oxide catalysts: an infrared spectroscopy investigation, J. Catal., 1992, 135, 186–199 CrossRef CAS.
- A. Sultana, M. Haneda, T. Fujitani and H. Hamada, Influence of Al2O3 support on the activity of Ag/Al2O3 catalysts for SCR of NO with decane, Catal. Lett., 2007, 114(1–2), 96–102 CrossRef CAS.
- M. Yuan, W. Deng, S. Dong, Q. Li, B. Zhao and Y. Su, Montmorillonite based porous clay heterostructures modified with Fe as catalysts for selective catalytic reduction of NO with propylene, Chem. Eng. J., 2018, 353, 839–848 CrossRef CAS.
- D. Yuan, X. Li, Q. Zhao, J. Zhao, S. Liu and M. Tade, Effect of surface lewis acidity on selective catalytic reduction of NO by C3H6 over calcined hydrotalcite, Appl. Catal., A, 2013, 451, 176–183 CrossRef CAS.
- Y. Wang, Z. Lei, B. Chen, Q. Guo and N. Liu, Adsorption of NO and N2O on Fe-BEA and H-BEA zeolites, Appl. Surf. Sci., 2010, 256(12), 4042–4047 CrossRef CAS.
- Q. Lin, J. Hao, J. Li, Z. Ma and W. Lin, Copper-impregnated Al-Ce-pillared clay for selective catalytic reduction of NO by C3H6, Catal. Today, 2007, 126(3–4), 351–358 CrossRef CAS.
- Y. Yu, X. Zhang and H. He, Evidence for the formation, isomerization and decomposition of organo-nitrite and -nitro species during the NOx reduction by C3H6 on Ag/Al2O3, Appl. Catal., B, 2007, 75(3–4), 298–302 CrossRef CAS.
- T. Chaieb, L. Delannoy, S. Casale, C. Louis and C. Thomas, Evidence for an H2 promoting effect in the selective catalytic reduction of NOx by propene on Au/Al2O3, Chem. Commun., 2015, 51(4), 796–799 RSC.
- J. Duan, L. Zhao, S. Gao and X. Li, New aspects on a low-medium temperature mechanism of H2-assisted C3H6-SCR over xAg-CeZr catalyst, Fuel, 2021, 305, 121574 CrossRef CAS.
- G. Xu, J. Ma, G. He, Y. Yu and H. He, An alumina-supported silver catalyst with high water tolerance for H2 assisted C3H6-SCR of NOx, Appl. Catal., B, 2017, 207, 60–71 CrossRef CAS.
- J. Ji, X. Lu, C. Chen, M. He and H. Huang, Potassium-modulated delta-MnO2 as robust catalysts for formaldehyde oxidation at room temperature, Appl. Catal., B, 2020, 260, 118210 CrossRef CAS.
- L. Q. Nguyen, C. Salim and H. Hinode, Roles of nano-sized Au in the reduction of NOx by propene over Au/TiO2: an in situ DRIFTS study, Appl. Catal., B, 2010, 96(3–4), 299–306 CrossRef CAS.
- K. Shimizu, J. Shibata, H. Yoshida, A. Satsuma and T. Hattori, Silver-alumina catalysts for selective reduction of NO by higher hydrocarbons: structure of active sites and reaction mechanism, Appl. Catal., B, 2001, 30(1–2), 151–162 CrossRef CAS.
- K. Shimizu, J. Shibata, A. Satsuma and T. Hattori, Mechanistic causes of the hydrocarbon effect on the activity of Ag-Al2O3 catalyst for the selective reduction of NO, Phys. Chem. Chem. Phys., 2001, 3(5), 880–884 RSC.
- K. Shimizu, H. Kawabata, A. Satsuma and T. Hattori, Role of acetate and nitrates in the selective catalytic reduction of NO by propene over alumina catalyst as investigated by FTIR, J. Phys. Chem. B, 1999, 103(25), 5240–5245 CrossRef CAS.
- J. Li, R. Ke, W. Li and J. Hao, Mechanism of selective catalytic reduction of NO over Ag/Al2O3 with the aid of non-thermal plasma, Catal. Today, 2008, 139(1–2), 49–58 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2023 |