Open Access Article
Open Access ArticleGreen synthesis of silica and silicon from agricultural residue sugarcane bagasse ash – a mini review
Lyle A. Septembera,
Ntombizonke Kheswab,
Ntalane S. Seroka *a and
Lindiwe Khotseng
*a and
Lindiwe Khotseng *a
*a
aDepartment of Chemistry, University of the Western Cape, Robert Sobukwe Rd, Private Bag X17, Bellville 7535, South Africa. E-mail: lkhotseng@uwc.ac.za; 3727392@myuwc.ac.za; 3754640@myuwc.ac.za
bTandetron Laboratory, Research Institute, Ithemba Labs, Old Faure Road, Eerste River, Faure 7131, South Africa. E-mail: ny.kheswa@ilabs.nrf.ac.za
First published on 5th January 2023
Abstract
Silicon dioxide (SiO2), also known as silica, has received attention in recent years due to wide range of capable applications including biomedical/pharmaceutical, energy, food, and personal care products. This has accelerated research in the extraction of materials from various agricultural wastes; this review investigates the extraction of silica and silicon nanoparticles from sugarcane bagasse ash with potential applications in electronic devices. Specific properties of silica have attracted the interest of researchers, which include surface area, size, biocompatibility, and high functionality. The production of silica from industrial agricultural waste exhibits sustainability and potential reduction in waste production. Bagasse is sustainable and environmentally friendly; though considered waste, it could be a helpful component for sustainable progress and further technological advancement. The chemical, biogenic and green synthesis are discussed in detail for the production of silica. In green synthesis, notable attempts have been made to replace toxic counterparts and decrease energy usage with the same quantity and quality of silica obtained. Methods of reducing silica to silicon are also discussed with the potential application-specific properties in electronic devices, and modern technological applications, such as batteries, supercapacitors, and solar cells.
1. Introduction
Green synthesis has received a lot of attention in nanotechnology as a reliable, sustainable, and environmentally friendly protocol for synthesis of a wide range of nanomaterials for the development of nanoelectronics.1,2 In essence, green synthesis is viewed as an important route for mitigating the negative effects associated with traditional methods of synthesizing nanoparticles for the preparation of nano electrodes. It is well known that the four most important parameters for the synthesis of nanoparticles using the green protocol are the selection of an environmentally friendly solvent, a source of nanomaterials, a reducing agent, and a harmless material for stabilization.1–3The synthesis of nanomaterials from agricultural waste is considered green because agricultural waste is an environmental hazard if not handled properly. For instance, sugarcane bagasse dumps attract many insects that harm the health of the population and cause many diseases. Production of agricultural foods is related to the formation of a considerable amount of waste and the rise in human population has demanded higher agricultural productivity. Most agricultural operations lead to waste creation, which is produced in vast numbers in many countries, generating serious environmental issues as the majority of agricultural waste ends up in landfills.2 In landfills, organic wastes generate carbon dioxide (CO2), nitrous oxide (N2O) and mainly methane (CH4) due to anaerobic decay, which actively contribute majorly from fossil fuels to greenhouse gas emissions. Additionally, during agricultural production mainly CH4 and N2O greenhouse gases are emitted, while a minor amount of CO2 emissions is released.3 The reuse of agricultural solid waste is considered a vital strategy to reduce waste and meets the requirements of a holistic sustainable waste management system; the reuse of these wastes can actively contribute to the establishment of new green technologies, bio-energy generation and bio-conversion to nanomaterials.1,2
Numerous types of nanomaterials have been extracted from agricultural wastes using biogenic syntheses; these synthesis methods are an appealing alternative to conventional synthesis methods as they are green and environmentally favourable. Nanotechnology has evolved as one of the most important branches of science with several applications in industries, such as the conversion of biomass into valuable materials in niche applications.4 An excellent example of this is the green synthesis of silver nanoparticles (AgNPs); this synthesis method makes use of biomolecules present in plants and agricultural wastes, which function as reducing and stabilizing agents.5 Moreover, other notable examples of biogenic methods include a green synthesis method for the production of degradable polyurethane utilizing castor oil6 and the synthesis of doped zinc oxide (ZnO) nanoparticles derived from Synadenium grantii leaf extract with concentrations of copper-dopant.7 Furthermore, silicon dioxide (SiO2), also known as silica, can be extracted from agricultural waste, such as sugarcane bagasse ash (SCBA) and rice husk (RH), by using a sustainable-green synthesis method.
At present, silica is extracted from sand or quartz, which is done by smelting with sodium carbonate (Na2CO3) to produce sodium silicate (Na2SiO3), a silica precursor.8 From this method, silica has succeeded in applications such as components in electronics, but high volumes of energy are required, making it less feasible. Silica can also be extracted from agricultural wastes; the production of silica from quartz sand is rarely compared to silica produced from agricultural wastes though it can be utilized to fulfil commercial requirements.9 In previous research on biosynthesis, strong acids and bases are generally used for the extraction of silica from agricultural wastes. A strong acid is utilized as a leaching agent to remove metallic impurities from the composition; this treatment is not only harmful to the environment but also poses an economical challenge as it requires the use of expanding materials that are resistant to strong acid corrosion and needs specific disposal methods.10 Thus, researchers are seeking alternative routes for sustainable production of silica nanoparticles to solve current concerns.
Green methods for the production of silica and silicon nanoparticles (SiNPs) are widely researched and make use of agricultural wastes. Conventional methods make use of harmful procedures and toxic substances; this review set out to investigate methods that are green and sustainable by making use of organic acids and decreased energy usage during the synthesis of silica and silicon extracted from SCBA. Green nanotechnology of silica from SCBA presents a window of industry applications, which synthesizes nanoparticles using environmentally nonthreatening processes and bio-sources. Sustainable approaches provide unique advantages for producing silica and SiNPs with desired qualities and have received a great deal of interest because of their favourably reactive surface area-to-volume ratio, physical and chemical stability, low toxicity, and straightforward surface chemistry.11 Other benefits of a green synthesis include a feasible methodology, non-hazardous and viable procedures with a wide range of applications in nanotechnology, biomedicine and nano-optoelectronics, among others.12 Silica is extensively used in industry due to it being chemically inert with a high melting point, making it highly functionable with control over specific characteristics. The green synthesis of silica and SiNPs appears to be an important field of research with a lot of room for growth in the future.
2. Background
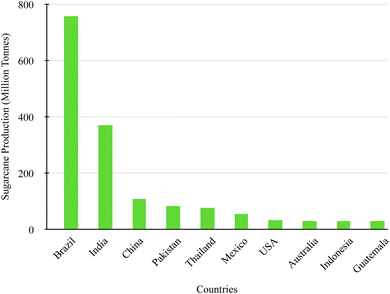
Considering that silica is a vital material for industrial application, much research has been conducted to extract it from various sources, including plants, rocks, clay, and agricultural wastes. Waste from agricultural activities is produced daily and can result in a significant amount. Wastes such as rice husk, rice straw, bamboo leaves, corn cob, wheat straw and sugarcane bagasse have been utilized for the production of silica due to the high content of silica in the composition (Table 1). Traditionally, agro-wastes such as rice husk and sugarcane bagasse have been used due to their high silica content.11,13 Rice husk is commonly mentioned in research, while sugarcane bagasse has a higher potential to be exploited as a viable source of silica.Sugarcane commonly known as “Saccharum officinarum” is a commercially grown crop in the harvesting season under the climate conditions of slightly sunny and colder environments, cultivated in tropical and subtropical countries. The United Nations Food and Agriculture Organization (FAO) in 2020 estimated sugarcane production to annually yield 1.89 billion metric tonnes; countries with the highest-produced sugarcane crop include Brazil, India, and China, illustrated in Fig. 1. Sugarcane is an essential agricultural crop that is grown to produce sugar and alcohol; with the high demand for this crop a significant amount of agricultural waste, bagasse, is produced each year. Bagasse production is estimated to generate millions of tonnes per year, which is estimated to produce 279 million metric tonnes, which can amount to a large amount of waste annually.22 These figures are expected to increase in the coming years as a result of economic initiatives encouraging the production of bioethanol from sugarcane to decrease the use of fossil fuels. Furthermore, the increase in the demand for sugar and bioethanol internationally stimulates the rise in the availability of SCBA, driving its use for sustainable materials as well as meeting sustainable development goals.
Sugarcane bagasse has the potential to be a viable renewable energy supply and silica source, illustrated in Fig. 2. Bagasse is generally employed as a fuel source to power sugar mills as an alternate energy source, resulting in the production of ash, SCBA, as a by-product of combustion.23 SCBA has a high content of carbon and silica, carbon stemming from the incomplete burning of bagasse and silica originating from the soil where the plant was cultivated. Monosilicic acid (H4SiO4) is absorbed by the roots of the plant where it is delivered as amorphous silica; the silica composition in the plant is dependent on the concentration of H4SiO4 in the soil.24
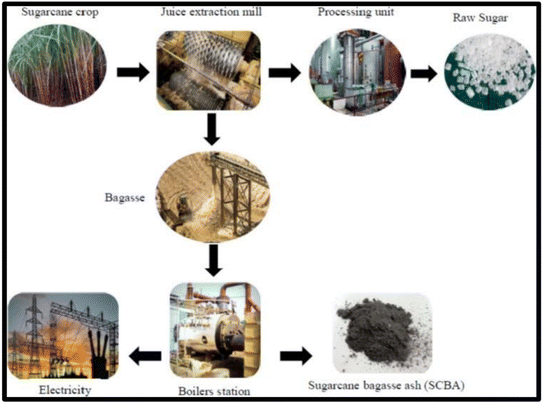 | ||
| Fig. 2 Production pathway for the production of sugar, energy and SCBA. Reproduced with permission from MDPI @2022.25 | ||
The key factors for sustainable waste management are dependent on the production of less waste, recycling and the recovery of waste produced. Notably, waste recovery and the production of high-value added materials via a green synthesis method reduce over-reliance on hazardous materials, resulting in improved efficiency through waste reduction and the use of natural and sustainable resources.26 The primary benefit of employing agricultural waste is that it is a sustainable method for the production of nanomaterials and secondly that it is readily present at the end of every harvest season in large quantities.
The green synthesis approach of materials has gained a considerable amount of attention due to numerous key features such as safety and environmentally friendly procedures with non-toxic by-products.27 Most research efforts are primarily focused on the end product, ignoring by-product production, and failing to account for the proportions of other counterparts during synthesis. Bio-inspired methods are emerging as viable options for producing high-value materials while remaining sustainable. At present, there is a need for procedures that are entirely green throughout the synthesis of nanomaterials.
3. Synthesis of silica
Silica has been synthesized using several methods over time, including the reverse microemulsion process, Stober's method, flame synthesis, combustion synthesis, microwave method and sol–gel approach, which is the most commonly used. Since then, various novel methods for synthesizing silica have emerged that focus to control the size, morphology, and surface reactivity. The synthesis of silica can be divided into two groups, namely chemical and biogenic synthesis. The chemical synthesis methods utilize high energy usage and different precursors such as tetraethyl orthosilicate (TEOS) and sodium silicate (NaSiO4), which are toxic.28,29 These precursors are generally used to synthesize mesoporous silica such as Santa Barbara Amorphous15 (SBA-15) and Mobil Composition of Matter-41 (MCM-41), which are widely applied in industry. In the industrial synthesis of silica, sodium silicate is used as a silicon source; however, sodium silicate, which is produced by smelting quartz sand and sodium carbonate at 1300 °C, not only consumes a lot of energy but also requires further purification29 and is not environmentally viable. Given that TEOS is not the greatest commercial source of silica because of its high prices, flammability, handling and storage challenges, and the production costs,30 silica obtained from a bio-source like sugarcane bagasse has attracted researchers as it is environmentally friendly and cost effective.Biogenic synthesis includes the use of different plants and organisms with the main advantages of being green, low energy usage and cost-effective compared to chemical synthesis, and the biosynthesis of nanomaterials through exploiting agricultural wastes is rational when compared. There are two basic methods for converting biomass to bio-products; conversion pathways are bio-chemical and thermochemical. While the former involves using microbes and enzymes to break down biomass into gaseous and/or liquid fuels, the latter involves using heat to break the complex chemical structure of biomass into a variety of products such as power, materials and chemicals,31 where the thermochemical conversion path is ideal for extracting silica from SCBA.
The extraction of silica from agricultural wastes is not commonly agreed upon according to research, though the pretreatment of the biomass is often utilized. Acid leaching (pretreatment) is a crucial step in removing residual metallic impurities, which is due to it being cost-effective and results in the efficient and faster breakdown of complex compositions. High impurity content, such as metal oxides like iron oxide (Fe2O3), obstructs the process of obtaining silica with an acceptable high purity;32 thus, acid leaching is required to retain the biogenic structure of silica while removing impurities. The most common leaching agent is H2SO4, although other acids have been explored, such as hydrochloric acid (HCl), hydrogen fluoride acid (HF), nitric acid (HNO3) and phosphoric acid (H3PO4). According to Norsuraya et al.,33 the silica content in the composition of SCBA was 53.1% before acid leaching and 88.13% after leaching with HCl. Table 2 illustrates the leaching efficiency of HCl and demonstrates the decrease in impurities. High content of impurities, such as metal oxides, obstructs the process of obtaining silica with high purity. Additional purification procedures are required to increase purity, such as chemical and thermal treatments. Furthermore, the extracted sodium silicate through the use of NaOH resulted in a similar infrared spectrum when compared to that in industry (shown in Fig. 3).
| Compound | Raw sample (wt%) | After acid treatment sample (wt%) |
|---|---|---|
| SiO2 | 53.10 | 88.13 |
| SO3 | 11.20 | 4.69 |
| MgO | 20.72 | 3.04 |
| P2O5 | 7.36 | 1.15 |
| Fe2O3 | 0.78 | 0.94 |
| MnO | 1.45 | 0.62 |
| CaO | 3.77 | 0.57 |
| K2O | 1.26 | 0.50 |
| Other | 0.36 | 0.32 |
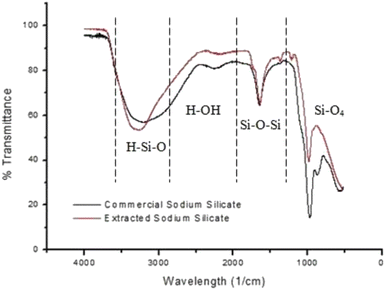 | ||
| Fig. 3 FTIR analysis of the commercial sodium silicate and extracted sodium silicate from SCBA, reproduced with permission from © 2016 Elsevier Ltd.33 | ||
To extract silica from agricultural wastes while maintaining the material's biogenic structure, thermal treatment is utilized, which promotes the removal of organic components. In thermal treatment, through the use of calcination, the temperature affects the silica produced as the calcination temperature and duration have a substantial impact on the morphology. Controlling the temperature and duration of thermal treatment increases the possibility of achieving the desired pozzolanic activity of the bagasse ash by keeping the silica in a non-crystalline state.34 According to research on an acceptable temperature for extracting amorphous silica, the ideal calcination temperature is 600 °C. Higher temperatures result in pore structure densification, which produces a decreased surface area, pore diameter and pore volume.
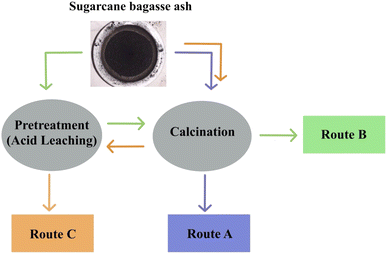
Bortolotto Teixeira et al.32 investigated different routes of synthesis, demonstrated in Fig. 4. Route A illustrates the direct calcination (with temperatures ranging between 500 and 700 °C) of SCBA without pretreatment, thus resulting in the purity of silica ranging between 43.6 and 52.1% as the temperature increased. Furthermore, routes B and C illustrate the synthesis pathways of initially using acid leaching or thermal treatment, route B showing a higher purity of silica when compared to route C (99.9% and 95.3, respectively). This illustrates that a higher silica purity will be achieved via route B, the initial acid leaching followed by thermal treatment.
The synthesis of silica nanoparticles, via the sol–gel process, using SCBA as a source of silica. To recover NaSiO4 from SCBA, alkaline extraction is the preferred approach making use of a base, such as NaOH. eqn (1) illustrates the addition of NaOH to the pre-treated SCBA, with eqn (2) showing the addition of an acid, H2SO4, and the formation of silica. Falk et al.35 synthesized silica using the sol–gel process, with the leached SCBA and further use of NaOH and HCl for the formation of silica gel. In this study, the sol–gel process was compared to acid leaching followed by calcination; the sol–gel method resulted in 96.8% purity, while the silica produced from calcination resulted in a slightly lower purity of 95.2%. The synthesis of silica through the extraction of sodium silicate via the sol–gel method results in higher purity, making it more appealing for application.
| SiO2 + 2NaOH → Na2SiO3 + H2O | (1) |
| Na2SiO3 + H2SO4 → SiO2 + NaSO4 + H2O | (2) |
In other relevant research, Rovani et al.13 effectively synthesized silica nanoparticles from SCBA and yielded nanoparticles with excellent purity (>99%). As a stabilizer and size controller, cetyltrimethylammonium bromide (CTAB) was used in the formation after pretreatment with HCl and NaOH was used to produce a Na2SiO3 solution. The resulting high purity of silica exhibited a size ranging from several micrometers to less than 20 nm and a specific surface area of 131 m2 g−1. The use of CTAB resulted in a controlled size distribution of the silica nanoparticles produced with an increase in specific surface area when compared to the SCBA. Additionally, Rahmat et al.36 extracted Na2SiO2 from SCBA for use as a precursor for the synthesis of mesoporous silica, SBA-15. The sodium silicate produced showed a similar spectrum to the commercial pattern used in industry, making SCBA a suitable source for the synthesis of mesoporous materials. In industry, the sodium silicate synthesized can be utilized in various applications.
Currently, chemicals employed in the synthesis of silica by various traditional procedures with the use of toxic counterparts are not environmentally viable. Despite their good technical performance and ability to remove heavy metals and inorganics, it is feasible to replace toxic reagents in these procedures with green alternatives for future progress in this sector.
4. Green synthesis of silica
Green and sustainable approaches to obtaining advanced materials for industry application can help avoid complicated procedures while also reducing environmental toxins. Methods of green synthesis have been researched for the extraction of silica from various agricultural biomass sources. Extraction of silica from biomass using organic acids, alkalis and solvents is one of the most promising methods for green synthesis due to them being capable of decomposing more complex-structured substrates.37 Chemical and thermal treatment of biomass by replacing toxic reactants and decreasing temperatures is one of the most promising methods for the green synthesis of silica and silicon nanoparticles.Leaching using an organic acid, namely citric acid (CA, C6H8O7), as an alternative for the pretreatment of SCBA. This organic acid has the potential of being a chelating agent as well as a mild triprotic acid.38 CA has the advantage of being an organic and bio-based acid, which has an impact on the materials synthesized and the effluent treatment costs and when compared to strong mineral acids like HCl and H2SO4 CA has the benefit of being an eco-friendly leachate.39 In research, Rodríguez-Machín et al.38 studied the effect of leaching using CA for the pretreatment of SCBA with variations of thermal degradation and its behaviour. In this study, as illustrated in Table 3, CA was compared to well-known conventional leaching agents, namely HCl and H2SO4, in terms of their impact on the chemical, structural and thermal characteristics of leached ash. Depending on leaching duration and temperature, inorganic element removal ranged from 39.9% to 54.1%, and CA functioned similarly to the strong mineral acids. This investigation shows that CA is a functional leaching agent when compared to traditional acids for the pretreatment of SCBA.
| Element | Raw SCBA | HCl | H2SO4 | C6H8O7 |
|---|---|---|---|---|
| K | 1800 | 17.5 | 15.7 | 17 |
| Al | 279 | 110 | 122 | 131 |
| Fe | 327 | 110 | 101 | 123 |
| Si | 8600 | 3600 | 4400 | 4400 |
| Mg | 287 | 25.1 | 26.4 | 68.1 |
| Na | 32.1 | 18.6 | 8.02 | <5 |
Similarly, Maseko et al.40 investigated biogenic amorphous silica that was extracted from different agricultural wastes using the thermo-chemical treatment process. Biomass samples were leached using 7% CA and 7% H2SO4 before thermal treatment in a furnace; CA pretreated was slightly less effective at removing inorganic impurities when compared to H2SO4 pretreated SCBA (silica content of 95.4% and 99.3%, respectively). The two leaching agents resulted in similar characteristics in the synthesized amorphous biogenic silica, with textual qualities of 323 m2 g−1 surface area, 5.0 nm average pore diameter, and 0.41 cm3 g−1 pore volume. The leaching efficacy of CA is slightly less effective than H2SO4 but it is still capable of functioning as a leaching agent as it results in similar characteristics in the silica synthesized.
A notable attempt at a green synthesis of crystalline structured silica has been made through a novel synthesis method making use of organic solvents. Seroka et al.41 reported the use of organic chemicals, namely CA and L-cysteine hydrochloride monohydrate (L-Cys), in the leaching of SCBA, which resulted in crystalline silica with average sizes of 26 nm for CA and 29 nm for L-Cys. Furthermore, tetrapropylammonium hydroxide (TPAH) was utilized as an extraction solvent for further purification by eliminating excess residuals and impurities in the synthesized silica. The successful use of organic chemicals for the extraction of silica from SCBA in a green synthesis method, L-Cys as a leaching agent, was effectively shown in this study along with the use of TPAH as an extraction solvent, summarized in Table 4.
| Leaching agent | Pretreatment conditions | Weight loss after pretreatment (wt%) | Calcination temperature (°C) | Extraction solvent | Silica purity (wt%) | Reference |
|---|---|---|---|---|---|---|
| Citric acid, C6H8O7 | 1 hour, 50 °C | 38.9–54.1 | — | — | — | 38 |
| Citric acid, C6H8O7 | 2 hours, 80 °C | 29.9 | 300–600 | — | 95.4 | 40 |
| Citric acid, C6H8O7 | 24 hours, 70 °C | — | 700 | TPAH | 79.85 | 41 |
| L-cysteine hydrochloride | 24 hours, 70 °C | — | 700 | TPAH | 89.75 | 41 |
5. Thermal reduction
Silicon (Si) is an abundantly important functional material with a wide range of applications due to its chemical and physical properties; its applications include electronics, metallurgy and energy. Si is an earth-abundant element but exists as silicon oxides, such as SiO2. Furthermore, SiO2 can be reduced to Si by thermal reduction, which has been extensively investigated, and current pathways are shown in Fig. 5. For the reduction of SiO2, high volumes of energy are required to produce Si; this is due to the high bonding energy linking between silicon and oxygen. There are many methods of reduction and for a green synthesis, the need for minimal energy usage is vital. Methods of reduction will be discussed, namely carbothermic reduction, metallothermic reduction and electrochemical reduction processes, to determine the least amount of energy required for a green synthesis.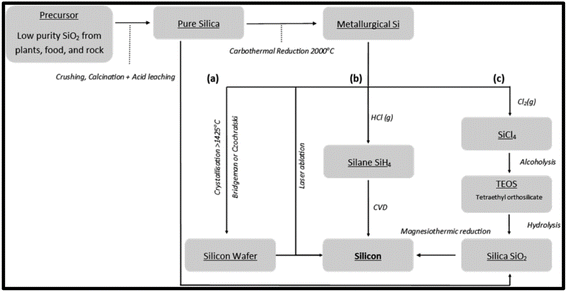 | ||
| Fig. 5 Schematic outline for the production of silica and silicon, along with reduction processes of silica to silicon via carbothermic and magnesiothermic reduction. Reproduced with permission from MDPI@2022.25 | ||
5.1 Carbothermal reduction
Carbothermal reduction is widely utilized for the reduction of SiO2 to Si in industry, which uses an electric arc furnace (EAF) operating at 2000 °C. This method has been investigated to the fullest extent as it is considered the best method for large-scale production despite that it requires stabilizing procedures for the process. Stabilizing processes are required due to high temperatures to achieve high thermal efficiency and large-scale production of silicon.42 High-temperature operations are required for the method; however, this procedure consumes a lot of energy and liquefies the silicon, obliterating any original SiO2 morphology.A small number of attempts have been made for novel approaches for alternate methods of carbothermal reduction, such as the use of concentrated solar energy as an energy source by vacuum carbothermal reduction of silica,43 which is a notable attempt to decrease energy usage. Furthermore, Maeng et al.44 reduced silica to crystalline silicon through an ultrafast carbothermal reduction using a CO2 laser beam as an energy source, a heat source in the form of intensified heat flux, the CO2 laser beam, with a mixture of silica and carbon black. Through this novel method of reduction, the reaction time was faster than that of traditional carbothermic reduction and resulted in a decrease in energy. Generally, this process is very endothermic, which results in enormous energy consumption and substantial CO2 emissions. As a result, this procedure ultimately results in high manufacturing costs and poses a threat to the environment.
5.2 Metallothermic reduction
Methods of metallic thermal reduction include magnesiothermic, aluminothermic, and calciothermic reduction, which can all be used to reduce SiO2 to Si. Mg, Ca, Al, and Ti are metallic metals that can reduce SiO2 at a lower temperature and produce condensed phase products. Magnesium as a reducing agent for the reduction of silica is more environmentally viable due to its lower working temperature (650 °C); magnesiothermic reduction has recently attracted a lot of attention.45,46 Magnesiothermic silica reduction can generate porous silicon products at lower temperatures than traditional silica reduction processes, carbothermic reduction. Considering that silicon has a melting temperature of 1414 °C, carbothermal reduction at 2000 °C is ineffective for preserving the silica structure. Magnesiothermic reduction has been shown to create silicon structures from silica at temperatures ranging from 500 to 950 °C, allowing for template-assisted formation of silicon nanostructures.47| SiO2 + 2Mg → Si + 2MgO | (3) |
By using SCBA and magnesiothermic processes, Falk et al.35 were able to produce silica and silicon nanoparticles. Magnesiothermic reaction was used to produce the nano-Si, illustrated in eqn (3). To eliminate the magnesium oxide and/or magnesium silicate due to side reactions, the powder was acid-leached with HCl. Finally, the silicon was centrifuged, rinsed with distilled water and absolute ethanol, and dried. The results demonstrate that nano-Si was efficiently manufactured utilizing sustainable silica sources via a magnesiothermic process. Furthermore, the synthesized material has a shape comparable to that of the silica precursor, along with high crystallinity and compact size, giving it a performance advantage over previous materials.35
5.3 Electrochemical reduction
There have been reports of silica being successfully reduced to silicon electrochemically. With the help of this electrochemical reduction technology, it has been made feasible for the reaction to start right when the electrode comes into contact with the material and for the process temperature to be lower than 1000 °C. As a novel method for producing high-purity silicon from SiO2, the electrochemical reduction of SiO2 in high-temperature molten salts has received a lot of attention. As an electrolyte in these procedures, molten CaCl2, which has a high O2− ion solubility, is frequently employed.48 Eqn (4) displays the solid-state reaction which allows for the reduction of SiO2 to Si when an electric conductor comes into direct contact with molten salt bound SiO2.| SiO2 + 4e− → Si + 2O2− | (4) |
The operating temperature was investigated for the electrochemical reduction of SiO2 in molten CaCl2, which is reported to work at 850 °C. Due to the formation of intermediates on the electrodes, namely Ca2SiO4, reduction of silica via the electrochemical route is rarely used in industry given that it has lower operating temperatures than carbothermal reduction.49 Compared to the present carbothermic technique, this is thought to be a low-cost approach that uses less energy.
6. Application of bio-silica and silicon nanoparticles
Silica and silicon nanoparticles are becoming more promising for application in electronic devices. Mesoporous silica is an excellent example of a material that uses nano-structuring, and it has several applications in electronic devices due to its ideal qualities. Because of their size-dependent optoelectronic capabilities, silica particles are a promising material for biomedical, photovoltaic, and energy storage applications.50The reduction of silica to silicon can open a wide range of capable applications. In traditional applications of silicon, such as metallurgy and the semiconductor industry, Si possesses a wide range of applications, with the unique properties and nanoparticles of Si resulting in new applications in new technologies, including nanoelectronics, energy harvesting and storage. A semiconductor material is essential for electronic devices, and these devices make our lives more convenient; for example, silicon wafers are used in numerous electronic devices for integrated circuit (IC) chips. Researchers have investigated advanced energy storage devices and energy harvesting in response to environmental concerns and the desire for sustainable energy.
6.1 Supercapacitors
Supercapacitors have been at the forefront of energy storage technology in recent years and with the use of mesoporous silica, advances can be made. Due to their role in the storage of charges, mesoporous properties such as specific surface area, and charge transport available at the electrode are crucial to the effectiveness of supercapacitors. Mesoporous silica, which has consistent pore size, hydrophilic surface properties and a large surface area, has electrochemically active centres, which improve electron transport and promote electrolyte penetration, meeting the supercapacitor application. Examples include supercapacitors, low-k materials, and sensors. Porous silica is a noticeable material for fabricating electrodes or interlevel dielectrics in this case. There is a direct correlation between specific capacitance and pore diameter; silica with smaller or bigger pores has much lower capacitance when compared to a pore size of 5.24 nm.51 Silica has a severe disadvantage: it is non-conductive. Pores in porous silica can be lined with a conductive substance like carbon to ensure conductivity.52 A method for making porous silica conductive is to combine it with a conductive polymer like polyaniline. Zhongkai et al.53 have developed a unique mesoporous SBA-15 nanocomposite electrode material for supercapacitors with excellent electrochemical performance.Supercapacitors are among the most well-known electrical devices that use porous silica-based materials. For supercapacitor capacity, the active electrode surface is important since the properties of these devices are highly dependent on the surface.54 Araichimani et al.55 investigated the use of biosilica nanoparticles derived from RH with a large surface area and mesoporous structure. The supercapacitor resulted in a capacitance of 448 F g−1 at 1 A g−1 current density with the use of SnO2 nanoparticles. The bio-silica resulted in impurities along with the large surface area, which allowed for better redox reaction at the electrode surface, improving the overall supercapacitive performance. In this regard, biosilica nanomaterials possessing a highly ordered nanostructure appear to be more viable than other alternatives for supercapacitors.56
6.2 Battery storage
Silicon has a high specific capacity, making it one of the most attractive options for new-generation negative electrode materials in LIBs.57 It was extracted for lithium-ion battery anodes using a low-cost, energy-efficient microwave-aided sustainable technique.58 Utilizing nano-Si as an anode material in next-generation lithium-ion (Li-ion) batteries for electronics as a means of energy storage has received enormous attention. The increased interest in Si is due to a specific charge storage capacity of 4200 mA h g−1, which is 10 times greater than the theoretical capacity of conventional graphite anodes when compared, thus making Si anodes more favourable, but due to issues such as pulverization, which is known to result in volume changes in Li insertion and extraction, graphite anodes are generally selected over Si anodes. Current research shows that Si with decreased size to the nanoscale can effectively solve these issues and perform greater when compared to graphite anodes.SiNPs extracted from biomass can effectively be used in energy storage, resulting in decreased cost and increased performance. Liu et al.59 synthesized nano-structured silicon from RH for application in Li-ion batteries, resulting in higher performance of the battery anodes. In this study, biosilica was reduced to nano-Si using magnesiothermic reduction with the outcome of a high purity of 99.6% with traces of metal concentrations, high enough for application in Li-ion battery anodes. SiNPs extracted from biomass consist of a distinctive and intrinsic nanostructure, which enables the decrease in pulverization and increased battery performance. Furthermore, for greater improvement in the performance of nano-Si anodes, surface coatings, conducting polymers and the use of sodium alginate as a binder for increased functioning are proposed.
6.3 Solar cells
Silicon nanoparticles are promising materials that may be used in solar cells and have benefits over other materials, which include flexibility in shape, ease of installation, and the use of a manufacturing method similar to printing to increase conversion efficiency.46 In the photovoltaic industry, the demand for lower costs of silicon is becoming increasingly important for the commercialization of silicon solar cells. The use of silicon wafers is increasing due to them playing a major role in crystalline silicon solar cells, where the use of silicon derived from agricultural waste is cheaper and can result in high purity.Converting agro-waste into low-cost SoG-Si nanoparticles for the fabrication of low-cost solar cells might be a way to address environmental issues such as ineffective waste management and energy scarcity.60 To reduce material prices, the silicon photovoltaic sector is moving to new technologies like thin film or thin wafer technologies, but the options for lowering production costs are limited. Marchal and colleagues61 synthesized solar-grade silicon from an agricultural waste, namely RH, which resulted in a purity of ≈99.9999%. In this synthesis for the reduction to silicon, the use of a submicron for the mixture of carbon and SiO2 for carbothermic reduction resulted in a higher yield of product and a faster rate of reaction. On an industrial scale, this could be advantageous due to faster silicon production and decreased costs of energy usage. Hybrid organic-silicon solar cells have recently emerged as a viable option for lowering production costs by using less complicated, scalable, and solution-processed conjugated polymers to produce a heterojunction with silicon at the interface. The utilization of a textured Si substrate surface in organic-silicon hybrid solar cell devices with excellent conversion efficiency has been researched.62
7. Limitations
There are a lot of uncertainties, even if turning agricultural waste into sophisticated materials for electronic applications may seem like a great circular economy. Traditional ways of nanoparticle production and a wide range of applications of silica and silicon nanoparticles have resulted in the development of numerous ways of production using biological methods. However, the current ‘green’ synthesis of nanoparticles has drawbacks such as the involvement of toxic chemicals and the high-energy requirements of production, which make it difficult for them to be widely implemented in industry. Currently, the chemicals and energy usage employed in these new ‘green’ methods of nanoparticle synthesis are the same ones used in more traditional methods. Future developments in this area may allow for the employment of eco-friendly substitutes for the dangerous chemicals employed in current contemporary methods. Furthermore, notable attempts have been made for a holistic green synthesis of silica and silicon from SCBA but there are still possibilities for improvement and additional research is required.The main limitation gathered from this review is the application of silica and silicon derived from SCBA and the lack of research thereof. Additionally, silicon purity improvement and post-reduction processing of silicon nanoparticles produced are required for scale-up of silicon for application. In order to refine silicon further for solar cells, additional work would be required for scale-up, which also enables the fabrication of Si wafers and actual application in solar cells of silicon derived from SCBA. Specifically, scaling up silicon from SCBA for application should result in design changes that enable production with eco-friendly counterparts at low temperatures.
8. Conclusion
The green synthesis of silica nanoparticles produced with the use of organic chemicals and decreased energy usage is possible and allows for precise control of particle shape, size and morphology. This has led to new innovative strategies and more research conducted to improve already existing green technologies and methodologies for future preparation of silica. The use of toxic chemicals for the processing of SCBA to produce silica is extremely dangerous to both the environment and individuals.A lot of research is being done to develop nano-based systems and nanocarriers using silicon and silica nanomaterials. Various properties can be obtained depending on the starting materials, which can affect the final features of the prepared NPs. Greener and more sustainable synthetic methods with the benefits of eco-friendliness, low cost, low energy, low or non-toxicity, and simple procedures are very promising in this regard and should be prioritized by researchers. The use of biomolecules in the fabrication of multifunctional silicon and silica nanomaterials has enormous economic and environmental implications. To avoid high temperatures, energy, pressure, and the use of toxic and/or hazardous agents and conditions, sustainable and eco-friendly silicon and silicon NP production procedures with appealing advantages over conventional methods are being developed.
In summary, the research gathered here is promising and illustrates the possibility of obtaining silica from sustainable sources and its use as a precursor for the preparation of nanostructured silicon from SCBA and demonstrates a path of green synthesis by using eco-friendly solvents. This is exhibited by using organic chemicals, and lower operating temperatures for thermal treatment and thermal reduction. This review concludes that additional research is required in the green synthesis of silica and silicon nanoparticles from SCBA for support in electronic applications.
Conflicts of interest
There are no conflicts to declare.References
- N. Ferronato and V. Torretta, Waste Mismanagement in Developing Countries: A Review of Global Issues, Int. J. Environ. Res. Public Health, 2019, 16(6) DOI:10.3390/ijerph16061060.
- M. Duque-Acevedo, L. J. Belmonte-Ureña, F. J. Cortés-García and F. Camacho-Ferre, Agricultural waste: Review of the evolution, approaches and perspectives on alternative uses, Glob. Ecol. Conserv., 2020, 22, e00902, DOI:10.1016/j.gecco.2020.e00902.
- J. Lynch, M. Cain, D. Frame and R. Pierrehumbert, Agriculture’s Contribution to Climate Change and Role in Mitigation Is Distinct From Predominantly Fossil CO2-Emitting Sectors, Front. Sustain. Food Syst., 2021, 4, 300, DOI:10.3389/fsufs.2020.518039.
- S. A. Mazari, et al., Nanomaterials: applications, waste-handling, environmental toxicities, and future challenges – a review, J. Environ. Chem. Eng., 2021, 9(2), 105028, DOI:10.1016/j.jece.2021.105028.
- F. Rodríguez-Félix, et al., Trends in Sustainable Green Synthesis of Silver Nanoparticles Using Agri-Food Waste Extracts and Their Applications in Health, J. Nanomater., 2022, 2022, 8874003, DOI:10.1155/2022/8874003.
- A. Zuliani, M. Rapisarda, D. Chelazzi, P. Baglioni and P. Rizzarelli, Synthesis, Characterization, and Soil Burial Degradation of Biobased Polyurethanes, Polymers, 2022, 14(22), 4948, DOI:10.3390/polym14224948.
- K. V. Karthik, et al., Green synthesis of Cu-doped ZnO nanoparticles and its application for the photocatalytic degradation of hazardous organic pollutants, Chemosphere, 2022, 287, 132081, DOI:10.1016/j.chemosphere.2021.132081.
- X. Dong, et al., A facile route to synthesize mesoporous SBA-15 silica spheres from powder quartz, Mater. Lett., 2017, 204, 97–100, DOI:10.1016/j.matlet.2017.05.115.
- B. H. Jo, C. S. Kim, Y. K. Jo, H. Cheong and H. J. Cha, Recent developments and applications of bioinspired silicification, Korean J. Chem. Eng., 2016, 33(4), 1125–1133, DOI:10.1007/s11814-016-0003-z.
- C. P. Faizul, C. Abdullah, M. Bari and N. Jamil, Extraction of Silica from Palm Ash Using Organic Acid Leaching Treatment, Key Eng. Mater., 2014, 594–595, 329–333, DOI:10.4028/www.scientific.net/KEM.594-595.329.
- F. Ghorbani, A. M. Sanati and M. Maleki, Production of Silica Nanoparticles from Rice Husk as Agricultural Waste by Environmental Friendly Technique, Environ. Stud. Persian Gulf, 2015, 2(1), 56–65 Search PubMed.
- B. Bhardwaj, P. Singh, A. Kumar, S. Kumar and V. Budhwar, Eco-Friendly Greener Synthesis of Nanoparticles, Adv. Pharm. Bull., 2020, 10(4), 566–576, DOI:10.34172/apb.2020.067.
- S. Rovani, J. J. Santos, P. Corio and D. A. Fungaro, Highly Pure Silica Nanoparticles with High Adsorption Capacity Obtained from Sugarcane Waste Ash, ACS Omega, 2018, 3(3), 2618–2627, DOI:10.1021/acsomega.8b00092.
- P. Chindaprasirt and U. Rattanasak, Eco-production of silica from sugarcane bagasse ash for use as a photochromic pigment filler, Sci. Rep., 2020, 10(1), 9890, DOI:10.1038/s41598-020-66885-y.
- S. Rangaraj and R. Venkatachalam, A lucrative chemical processing of bamboo leaf biomass to synthesize biocompatible amorphous silica nanoparticles of biomedical importance, Appl. Nanosci., 2017, 7(5), 145–153, DOI:10.1007/s13204-017-0557-z.
- G. M. Faé Gomes, C. Philipssen, E. K. Bard, L. D. Zen and G. de Souza, Rice husk bubbling fluidized bed combustion for amorphous silica synthesis, J. Environ. Chem. Eng., 2016, 4(2), 2278–2290, DOI:10.1016/j.jece.2016.03.049.
- V. H. Le, C. N. H. Thuc and H. H. Thuc, Synthesis of silica nanoparticles from Vietnamese rice husk by sol–gel method, Nanoscale Res. Lett., 2013, 8(1), 58, DOI:10.1186/1556-276X-8-58.
- A. B. D. Nandiyanto, T. Rahman, M. A. Fadhlulloh, A. G. Abdullah, I. Hamidah and B. Mulyanti, Synthesis of silica particles from rice straw waste using a simple extraction method, IOP Conf. Ser.: Mater. Sci. Eng., 2016, 128, 12040, DOI:10.1088/1757-899x/128/1/012040.
- H. Ahmad Alyosef, et al., Meso/Macroporous Silica from Miscanthus, Cereal Remnant Pellets, and Wheat Straw, ACS Sustain. Chem. Eng., 2015, 3(9), 2012–2021, DOI:10.1021/acssuschemeng.5b00275.
- N. J. Shamle, C. J. Dados, S. E. Iwoh, J. G. Nangbes and A. U. Awode, Comparative Assessment of the Yields of Silica from Husk Ashes of Digitaria exilis (acha), Wheat and Rice, IOSR J. Appl. Chem., 2014, 7(7), 01–04, DOI:10.9790/5736-07730104.
- F. C. Pa, A. Chik and M. F. Bari, Palm Ash as an Alternative Source for Silica Production, MATEC Web Conf., 2016, 78, 01062, DOI:10.1051/matecconf/20167801062.
- R. Melati, A. Schmatz, F. Pagnocca, J. Contiero and M. Brienzo, Sugarcane bagasse: Production, composition, properties, and feedstock potential, in Sugarcane: Production Systems, Uses and Economic Importance, 2017, pp. 1–38 Search PubMed.
- M. A. Amezcua-Allieri, et al., Techno-economic analysis and life cycle assessment for energy generation from sugarcane bagasse: case study for a sugar mill in Mexico, Food Bioprod. Process., 2019, 118, 281–292, DOI:10.1016/j.fbp.2019.09.014.
- M. Sahebi, et al., Importance of silicon and mechanisms of biosilica formation in plants, Biomed Res. Int., 2015, 2015, 396010, DOI:10.1155/2015/396010.
- N. S. Seroka, R. T. Taziwa and L. Khotseng, Extraction and Synthesis of Silicon Nanoparticles (SiNPs) from Sugarcane Bagasse Ash : A Mini-Review, Appl. Sci., 2022, 12(5), 2310, DOI:10.3390/app12052310.
- A. K. Jassim, Sustainable Solid Waste Recycling, in Skills Development for Sustainable Manufacturing, ed. C. O. Ijagbemi and H. M. Campbell, IntechOpen, Rijeka, 2017 Search PubMed.
- Z. Usmani, et al., Bioprocessing of waste biomass for sustainable product development and minimizing environmental impact, Bioresour. Technol., 2021, 322, 124548, DOI:10.1016/j.biortech.2020.124548.
- I. A. Rahman and V. Padavettan, Synthesis of Silica Nanoparticles by Sol–Gel: Size-Dependent Properties, Surface Modification, and Applications in Silica-Polymer Nanocomposites—A Review, J. Nanomater., 2012, 2012, 132424, DOI:10.1155/2012/132424.
- S. Affandi, H. Setyawan, S. Winardi, A. Purwanto and R. Balgis, A facile method for production of high-purity silica xerogels from bagasse ash, Adv. Powder Technol., 2009, 20, 468–472 CrossRef CAS.
- H. Purwaningsih, Y. Ervianto, V. M. Pratiwi, D. Susanti and A. Purniawan, Effect of Cetyl Trimethyl Ammonium Bromide as Template of Mesoporous Silica MCM-41 from Rice Husk by Sol-Gel Method, IOP Conf. Ser. Mater. Sci. Eng., 2019, 515(1), 1–10, DOI:10.1088/1757-899X/515/1/012051.
- A. Anukam and J. Berghel, Biomass Pretreatment and Characterization: A Review, in Biotechnological Applications of Biomass, ed. T. P. Basso, T. O. Basso and L. C. Basso, IntechOpen, Rijeka, 2021 Search PubMed.
- L. Bortolotto Teixeira, E. Guzi de Moraes, G. Paolinelli Shinhe, G. Falk and A. P. Novaes de Oliveira, Obtaining Biogenic Silica from Sugarcane Bagasse and Leaf Ash, Waste Biomass Valorization, 2021, 12(6), 3205–3221, DOI:10.1007/s12649-020-01230-y.
- S. Norsuraya, H. Fazlena and R. Norhasyimi, Sugarcane Bagasse as a Renewable Source of Silica to Synthesize Santa Barbara Amorphous-15 (SBA-15), Procedia Eng., 2016, 148, 839–846, DOI:10.1016/j.proeng.2016.06.627.
- N. Rahmat, A. Sabali, N. Sahiron and G. Sandu, Study of Calcination Temperature and Concentration of NaOH Effect on Crystallinity of Silica from Sugarcane Bagasse Ash (SCBA), Rev. Chim., 2016, 67, 1872 CAS.
- G. Falk, G. P. Shinhe, L. B. Teixeira, E. G. Moraes and A. P. N. de Oliveira, Synthesis of silica nanoparticles from sugarcane bagasse ash and nano-silicon via magnesiothermic reactions, Ceram. Int., 2019, 45(17), 21618–21624, DOI:10.1016/j.ceramint.2019.07.157.
- N. Rahmat, Characterization of sodium silicate derived from sugarcane bagasse ash, Malaysian J. Anal. Sci., 2016, 21, 512, DOI:10.17576/mjas-2017-2102-26.
- J. U. Hernández-Beltrán, I. Hernández-De Lira, M. Cruz-Santos, A. Saucedo-Luevanos, F. Hernández-Terán and N. Balagurusamy, Insight into pretreatment methods of lignocellulosic, Appl. Sci., 2019, 9(18), 3721 CrossRef.
- L. Rodríguez-Machín, L. E. Arteaga-Pérez, R. A. Pérez-Bermúdez, Y. Casas-Ledón, W. Prins and F. Ronsse, Effect of citric acid leaching on the demineralization and thermal degradation behavior of sugarcane trash and bagasse, Biomass and Bioenergy, 2018, 108, 371–380, DOI:10.1016/j.biombioe.2017.11.001.
- W. Astuti, T. Hirajima, K. Sasaki and N. Okibe, Comparison of effectiveness of citric acid and other acids in leaching of low-grade Indonesian saprolitic ores, Miner. Eng., 2016, 85, 1–16, DOI:10.1016/j.mineng.2015.10.001.
- N. N. Maseko, et al., The Production of Biogenic Silica from Different South African Agricultural Residues through a Thermo-Chemical Treatment Method, Sustainability, 2021, 13(2), 577, DOI:10.3390/su13020577.
- N. S. Seroka, R. Taziwa and L. Khotseng, Green Synthesis of Crystalline Silica from Sugarcane Bagasse Ash: Physico-Chemical Properties, Nanomaterials, 2022, 12(13), 2184, DOI:10.3390/nano12132184.
- Y. Liu, J. Kong, Y. Zhuang, P. Xing, H. Yin and X. Luo, Recycling high purity silicon from solar grade silicon cutting slurry waste by carbothermic reduction in the electric arc furnace, J. Clean. Prod., 2019, 224, 709–718, DOI:10.1016/j.jclepro.2019.03.187.
- P. G. Loutzenhiser, O. Tuerk and A. Steinfeld, Production of Si by vacuum carbothermal reduction of SiO2 using concentrated solar energy, JOM, 2010, 62(9), 49–54, DOI:10.1007/s11837-010-0137-0.
- S.-H. Maeng, H. Lee, M. S. Park, S. Park, J. Jeong and S. Kim, Ultrafast carbothermal reduction of silica to silicon using a CO2 laser beam, Sci. Rep., 2020, 10(1), 21730, DOI:10.1038/s41598-020-78562-1.
- Y. Shen, Rice Husk Silica-Derived Nanomaterials for Battery Applications: A Literature Review, J. Agric. Food Chem., 2017, 65(5), 995–1004, DOI:10.1021/acs.jafc.6b04777.
- F. Farirai, et al., Methods of extracting silica and silicon from agricultural waste ashes and application of the produced silicon in solar cells: a mini-review, Int. J. Sustain. Eng., 2021, 14(1), 57–78, DOI:10.1080/19397038.2020.1720854.
- Z. Bao, et al., Chemical reduction of three-dimensional silica micro-assemblies into microporous silicon replicas, Nature, 2007, 446(7132), 172–175, DOI:10.1038/nature05570.
- M. M. Islam, et al., Electrodeposition and characterization of silicon films obtained through electrochemical reduction of SiO2 nanoparticles, Thin Solid Films, 2018, 654, 1–10, DOI:10.1016/j.tsf.2018.03.072.
- Y. Katasho, Y. Norikawa, T. Yamamoto, K. Yasuda and T. Nohira, In situ synchrotron X-ray diffraction study of the electrochemical reduction of SiO2 in molten CaCl2, Electrochem. commun., 2020, 115, 106740, DOI:10.1016/j.elecom.2020.106740.
- S. Prabha, D. Durgalakshmi, S. Rajendran and E. Lichtfouse, Plant-derived silica nanoparticles and composites for biosensors, bioimaging, drug delivery and supercapacitors: a review, Environ. Chem. Lett., 2021, 19(2), 1667–1691, DOI:10.1007/s10311-020-01123-5.
- Ł. Laskowski, M. Laskowska, N. Vila, M. Schabikowski and A. Walcarius, Mesoporous Silica-Based Materials for Electronics-Oriented Applications, Molecules, 2019, 24(13), 2395, DOI:10.3390/molecules24132395.
- J. Zhi, S. Deng, Y. Wang and A. Hu, Highly Ordered Metal Oxide Nanorods inside Mesoporous Silica Supported Carbon Nanomembranes: High Performance Electrode Materials for Symmetrical Supercapacitor Devices, J. Phys. Chem. C, 2015, 119(16), 8530–8536, DOI:10.1021/acs.jpcc.5b01230.
- Z. Hu, Z. Lei, J. Yanhua, L. Huiqin, L. Yang, X. Wang and C. Xiuguo, High performance nanocomposite electrodes of mesoporous silica platelet-polyaniline synthesized via impregnation polymerization, Polym. Compos., 2017, 38(8), 1616–1623, DOI:10.1002/pc.23729.
- K. Cendrowski, W. Kukułka and E. Mijowska, Silica nanospheres as a key process element in the Green engineering for the synthesis of carbon nanotubes as a supercapacitors additives, Mater. Res. Bull., 2022, 146, 111620, DOI:10.1016/j.materresbull.2021.111620.
- P. Araichimani, et al., Rice Husk-Derived Mesoporous Silica Nanostructure for Supercapacitors Application: a Possible Approach for Recycling Bio-Waste into a Value-Added Product, Silicon, 2022, 14(15), 10129–10135, DOI:10.1007/s12633-022-01699-3.
- A. Divyashree and G. Hegde, Activated carbon nanospheres derived from bio-waste materials for supercapacitor applications – a review, RSC Adv., 2015, 5(107), 88339–88352, 10.1039/C5RA19392C.
- Y. Tan, T. Jiang and G. Z. Chen, Mechanisms and Product Options of Magnesiothermic Reduction of Silica to Silicon for Lithium-Ion Battery Applications, Front. Energy Res., 2021, 9, 651386, DOI:10.3389/fenrg.2021.651386.
- S. Praneetha and A. V. Murugan, Development of Sustainable Rapid Microwave Assisted Process for Extracting Nanoporous Si from Earth Abundant Agricultural Residues and Their Carbon-based Nanohybrids for Lithium Energy Storage, ACS Sustain. Chem. Eng., 2015, 3(2), 224–236, DOI:10.1021/sc500735a.
- N. Liu, K. Huo, M. T. McDowell, J. Zhao and Y. Cui, Rice husks as a sustainable source of nanostructured silicon for high performance Li-ion battery anodes, Sci. Rep., 2013, 3(1), 1919, DOI:10.1038/srep01919.
- J. A. Adebisi, J. O. Agunsoye, S. A. Bello, I. I. Ahmed, O. A. Ojo and S. B. Hassan, Potential of producing solar grade silicon nanoparticles from selected agro-wastes: A review, Sol. Energy, 2017, 142, 68–86, DOI:10.1016/j.solener.2016.12.001.
- F. P. McDonnell, K. Sun, D. Krug and R. Laine, A Low Cost, Low Energy Route to Solar Grade Silicon from Rice Hull Ash (RHA), a Sustainable Source, Green Chem., 2015, 17 10.1039/C5GC00622H.
- S. Iqbal, et al., Fabrication of an efficient planar organic-silicon hybrid solar cell with a 150 nm thick film of PEDOT: PSS, Micromachines, 2019, 10(10), 1–7, DOI:10.3390/mi10100648.
| This journal is © The Royal Society of Chemistry 2023 |