Open Access Article
Open Access ArticleLate-stage functionalization of 5-nitrofurans derivatives and their antibacterial activities†
Geshuyi Chena,
Zhe Changb,
Pei Yuana,
Si Wangb,
Yongxiu Yang *acd,
Xiaolei Liang*ac and
Depeng Zhao
*acd,
Xiaolei Liang*ac and
Depeng Zhao *b
*b
aThe First Clinical Medical College, Lanzhou University, Lanzhou, China. E-mail: chengshy20@lzu.edu.cn
bGuangdong Provincial Key Laboratory of Chiral Molecule and Drug Discovery, School of Pharmaceutical Sciences, Sun Yat-Sen University, Guangzhou, China. E-mail: zhaodp5@mail.sysu.edu.cn
cThe First Clinical Medical College, Lanzhou University. Department of Obstetrics and Gynecology, The First Hospital of Lanzhou University, Key Laboratory for Gynecologic Oncology, Lanzhou, 730000, Gansu Province, China. E-mail: yxyanglzu@163.com; liangxl07@lzu.edu.cn
dLead Contact, China
First published on 20th January 2023
Abstract
Structure modification of drugs is a reliable way to optimize lead compounds, among which the most striking and direct method is late-stage functionalization (LSF). Here, we employed the Cu-catalyzed C–H LSF to modify 5-nitrofuran drugs. A series of modifications have been carried out including hydroxylation, methylation, azidination, cyanation, arylation, etc. Antibacterial activities of all compounds in vitro were measured. The results showed that compound 1 and compound 18 were the most active among all compounds. Meanwhile, the cell cytotoxicity assays of potent compounds 1, 3, 4, 5 & 18 and the parent drug FZD were conducted.
Introduction
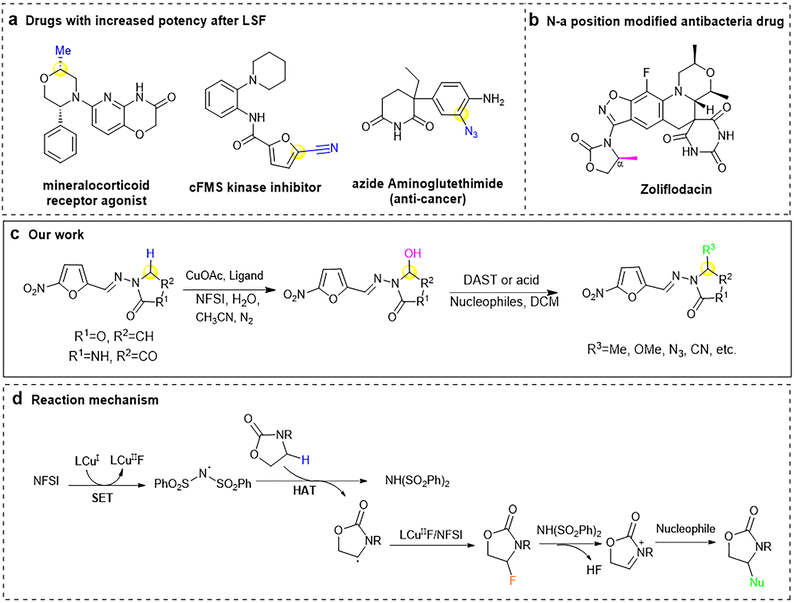
Structural modification of lead molecules is to modify functional groups based on the molecules' original skeleton,1–3 which has the following effects: (a) changes the solubility or pKa of the drug,4 (b) enhances the drug's bioavailability,5 (c) improves the pharmacokinetics and prolongs the action time of the drug,6 (d) maintains excellent metabolic stability, (e) increases the target selectivity of drugs,7 and (f) reduces the toxicity and side effects of drugs.4 Therefore, chemical structure modification of drugs is a reliable method for optimizing lead compounds and is widely used by medicinal chemists all over the world.C–H late-stage functionalization (LSF) is the most direct approach of structural modification, helping to generate new drugs rapidly.8–13 So far, there have been many examples of successfully improving activity after modification such as “magic methyl” effect,14–18 cyanation,19 azidination20 (Fig. 1a). Since the improper use of antibiotics21 and the natural selection of bacteria,22 more and more diseases are difficult to cure by current antibiotics, new strategies must be developed, such as the discovery of derivatives of known antibacterial agents. Nitrogen heterocycles (N-heterocycles) are common structures in antibacterial and anti-inflammatory drugs,23 and their N-α position can be used as a modification object in antibacterial drug Zoliflodacin (Fig. 1b).24–26 It has been reported27 that modifying N-heterocycles can improve their protein-binding ability without influencing the acting mechanism. Furazolidone (FZD, 3-(5-nitrofurfurylideneamino)-2-oxazolidinone) and Nitrofurantoin (NFT, 1-(((5-nitro-2-furanyl) methylene) amino)-2,4-imidazolidinedione) are members of 5-nitrofurans (NFs),28 which have broad-spectrum antibacterial effect against Gram-negative and Gram-positive bacteria.29,30 In particular, FZD was proved to be the only successful NFs against Helicobacter pylori (H. pylori).30,31 Therefore, modification of N-α position of these two antibacterial drugs can afford more active compounds without affecting the target.
Herein, we successfully modified the N-α position of FZD and NFT, and converted the N-α position C–H bond into C–O, C–C, C–N, and C–S bonds by Cu(I) catalyzed C–H LSF (Fig. 1c) and the reaction mechanism is shown in Fig. 1d. 18 analogues including 16 FZD derivatives and 2 NFT derivatives were synthesized and their antibacterial activities against Staphylococcus aureus (S. aureus), Escherichia coli (E. coli), Candida albicans (C. albicans) and Helicobacter pylori (H. pylori) in vitro were measured. Several compounds were superior to parent drug in activity. We hope that this method can provide an idea of LSF at the N-α position to medicinal chemists and help them obtain desired compounds in a fast and low-cost way.
Results and discussion
Synthetic procedures
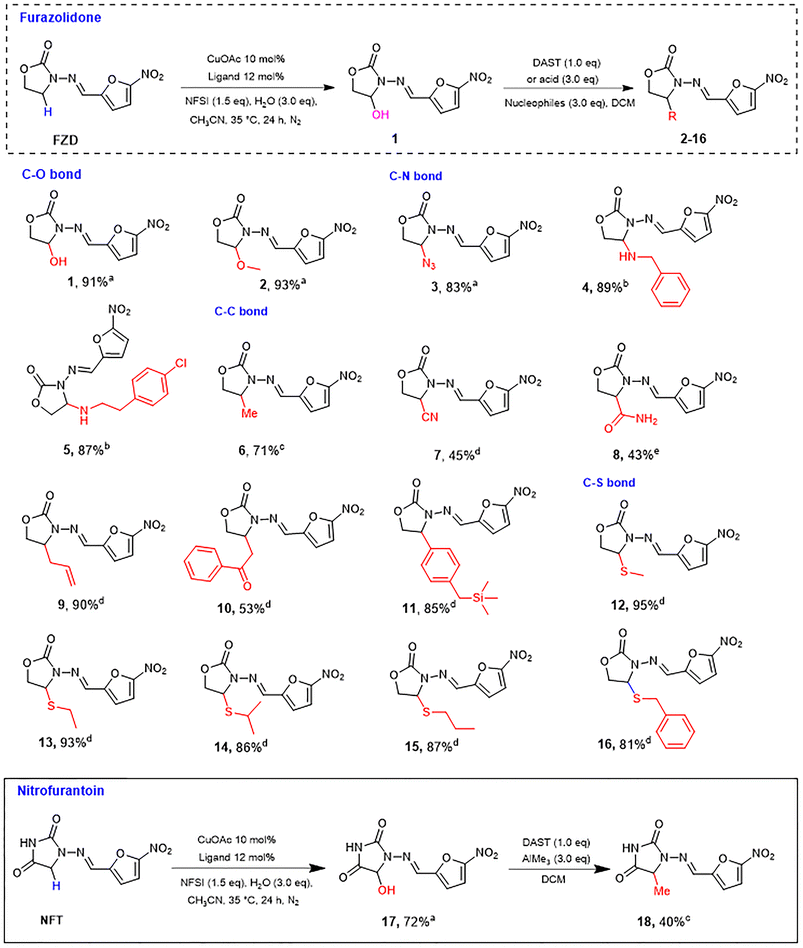
Inspired by our recent advances in copper catalyzed late-stage C–H functionalization of N-heterocycles,32 which can generate hemiaminal intermediate via cross-dehydrogenation coupling reaction after C–H bond oxidation, we attempted to modify the α-position of N-heterocycle-containing drugs. A variety of functional groups, such as those containing O, N, and S, were projected to be introduced in N-α position in order to increase the receptor-binding capacity and activity of the drug. Herein, we successfully converted C–H bonds into C–O and C–C bonds. Since N and S are the most common active atoms in medicinale compounds, we also paid attention to C–N and C–S coupling for the first time. Derivatives of FZD were obtained using copper(I) catalyzed C–H LSF in desired yield (43–95%). A series of groups were added in N-α position of NFs containing –OH, –OCH3, –N3, –CN, etc. The analogues are shown in Fig. 2, including 16 FZD derivatives and 2 NFT derivatives.We initially oxidized FZD with the conditions of CuOAc/Ligand, H2O and N-fluorobenzenesulfonimide (NFSI) in CH3CN, obtained hemiaminal 1 in 91% yield. Replacing water with methanol or azidotrimethylsilan in above system, the corresponding FZD derivatives 2 and 3 were formed in one-step with good yields (93% and 83% respectively).
Influenced by the one-step reaction of trimethylsilane, we proceeded with the C–N coupling. Amines were initially added to our reaction system in order to obtain C–N coupled products in one-step, but the reaction failed. Therefore, a one-pot reaction was performed. After the completion of the reaction of hemiaminal 1, an amine was added to afford 4 and 5 in excellent yields (89% and 87% respectively). It is worth mentioning that chain amines such as dimethylamine, n-propylamine, N-methyl-n-propylamine and ammonium salts including methylamine hydrochloride and ethylamine hydrochloride could react with hemiaminal 1 giving corresponding C–N coupling products. However, these desired products were all unstable, they partially hydrolyzed during purification, back to hemiaminal 1, preventing us from obtaining pure products.
Next, we focused on the C–C coupling at the N-α position of NFs. Methylation of 1 with diethylaminosulfur trifluoride (DAST) afforded 6 in moderate yields (71%). Treatment of 1 with BF3·OEt2 and trimethylsilylcyanide provided products 7 in moderate yield (45%). Moreover, in order to evaluate the effect of active hydrogen, we further synthesized amide 8 with two active hydrogens based on product 7 under the conditions of LiOH and H2O2, the overall yield of two steps was 43%. Compounds 9–10 were products of hemiaminal 1, BF3·OEt2, and corresponding organic nucleophilic reagents (allyltrimethylsilane and trimethyl [(1-phenylethyl) oxy] – silane) in yields 90% and 53% respectively. Interestingly, when benzyltrimethylsilane was used as a nucleophilic reagent, the 4-position of benzene ring was substituted rather than the benzyl position, resulting in product 11 in 85% yield.
In view of the importance of the S atom in pharmaceutical chemistry, we decided to introduce sulfur-containing groups. 1 reacted with sodium methylthiolate and sodium ethanethiolate after adding BF3·OEt2, providing products 12 and 13 in excellent yields, (95% and 93%, respectively). Unexpectedly, when propanethiol, isopropylthiol, and benzylthiol were used as nucleophiles, BF3·OEt2 could not promote the reaction. Other Lewis acids were also used for the same reason but failed. Finally, trifluoroacetic acid (TFA), a type of Brønsted acid, was used to drive the reaction, and products 14–16 were afforded in good yields (81–87%).
NFT derivative heminaminal 17 was obtained in 72% yield under conditions of CuOAc/Ligand, H2O and NFSI, same conditions as hemiaminal 1. Methylation of 17 with DAST afforded 18 in moderate yields (41%).
In vitro biological activity
Antibacterial activity
All synthesized compounds (1–18) were screened in vitro for antibacterial activities (Table 1) against S. aureus strain ATCC 29213, E. coli strain ATCC 25922 and C. albicans strain ATCC14053, which are representative of the Gram-positive bacteria, Gram-negative bacteria and fungi, respectively. Compound 1, 6, and FZD were evaluated the ability against H. pylori strain SS1 additionally. All compounds were assessed by a standard two-fold microdilution assay against these four strains.| Compounds | Mol. weight (g mol−1) | MIC (μg ml−1) | |||
|---|---|---|---|---|---|
| S. Aureus ATCC 29213 | E. coli ATCC 25922 | C. Albicans ATCC14053 | H. pylori SS1 | ||
| FZD | 225 | 3.125 | 0.39065 | >50 | <0.0976 |
| 1 | 241 | 1.5625 | 1.5625 | >50 | <0.0976 |
| 2 | 255 | >50 | >50 | >50 | |
| 3 | 266 | 3.125 | 1.5625 | >50 | |
| 4 | 330 | 3.125 | 3.125 | >50 | |
| 5 | 378 | 3.125 | 3.125 | >50 | |
| 6 | 239 | 25 | 6.25 | >50 | <0.0976 |
| 7 | 250 | 12.5 | 12.5 | >50 | |
| 8 | 268 | 25 | 25 | >50 | |
| 9 | 265 | >50 | >50 | >50 | |
| 10 | 343 | >50 | >50 | >50 | |
| 11 | 387 | >50 | >50 | >50 | |
| 12 | 271 | 25 | 25 | >50 | |
| 13 | 285 | 12.5 | 50 | >50 | |
| 14 | 299 | 12.5 | >50 | >50 | |
| 15 | 299 | 12.5 | >50 | >50 | |
| 16 | 347 | 3.125 | >50 | >50 | |
| NFT | 238 | 25 | 12.5 | >50 | |
| 17 | 254 | 50 | 50 | >50 | |
| 18 | 252 | 25 | 6.25 | >50 | |
Hydroxyl is an important active group in drugs, able to both change physicochemical properties and enhance hydrogen bonding interactions with the target proteins.10 Compared with the parent compound FZD, compound 1 has an additional –OH group at the N-α position, which gives it stronger activity against S. aureus with a MIC value of 1.5625 μg ml−1, 2-fold superior to that of FZD. However, compound 2, with an additional –OCH3, exhibited low activity against these three bacterial strains. We speculated that the enhanced activity of compound 1 is due to the active hydrogen on the hydroxyl group. Compounds 3–5 containing C–N bonds also showed strong inhibition against S. aureus and E. coli. Neither compound 6 nor 7 which containing small groups such as –CN, –CH3 showed better activity. It is worth mentioning that we synthesized compound 8, an amide with two active hydrogens based on compound 7. Intramolecular hydrogen bonds might form in compound 8, but the activity was poor. Antibacterial result showed 8 even had lower activity than compound 7. Coupling with allyl group afforded compound 9, which led to a substantial decrease in activity. Introducing an aromatic moiety into N-α position resulted in analogues 10 and 11, which were found to have a complete loss of activity in comparison to FZD. This may have been because the aromatic moieties of 10 and 11 influenced their binding ability to the target. Furthermore, the introduction of sulfur did not afford better activity for compounds 12–16. In addition, representative compounds 1 (OH-FZD) and 6 (Me-FZD) were chosen and their ability against H. pylori was tested. MICs of FZD, 1, and 6 were all less than 0.0976. It was revealed that our modification maintained their anti-H. pylori efficacy.
LSF of NFT was also performed, and compounds 17 and 18 containing –OH and –CH3 were synthesized, respectively. Unexpectedly, compound 18 with a methyl group showed stronger anti-E. coli activity than its parent drug, with a 2-fold superior efficacy to the MIC of NFT, demonstrating the “magic methyl” effect, which differed from the results from the methylation of FZD.
The results indicated that parent drugs FZD and NFT had very low inhibition on fungal strain. The MIC values were more than 50 μg ml−1 against C. Albicans. Analogues 1–18 after modification didn't afford better activity.
Cytotoxicity analysis
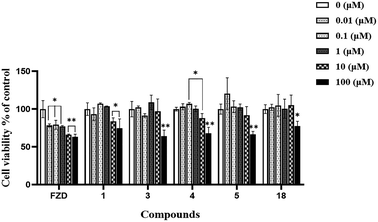
We chose HepaRG cells, which are liver bipotent progenitors,33–35 to assess the hepatotoxicity of the analogues. 5 potent derivatives (1, 3, 4, 5 & 18) and the parent drug FZD were select for cell viability study with MTT assay method. Cells were incubated for 24 h at different concentrations of FZD and derivatives. As shown in Table 2, all compounds have low toxicity, and the values of IC50 are more than 100 μM.| Compounds | Cytotoxicity IC50 (μM) |
|---|---|
| FZD | >100 |
| 1 | >100 |
| 3 | >100 |
| 4 | >100 |
| 5 | >100 |
| 18 | >100 |
As shown in Fig. 3, results from MTT assay showed that after 24 h exposure, FZD exhibited almost 75% cell viability at concentration of 0.01–1 μM (p < 0.05), while all other derivatives exhibited nearly 100% cell viability in the same concentration. Furthermore, all compounds still exhibited more than 50% cell viability even though treated in a high concentration of 100 μM (p < 0.05), indicating a non-cytotoxic against HepaRG cells. Hence, these compounds in future could be used as good candidates for drugs in the field of medicinal chemistry.
Conclusions
In summary, we developed an effective method for the synthesis of substituted FZD and NFT by late-stage C(sp3)–H functionalization, and the modification of the α-position of the N-heterocycles. This method was firstly applied to the coupling of C–N and C–S bonds. A series of functional groups were added including –OH, –OCH3, –N3, –CN, etc. We successfully synthesized 16 FZD derivatives and 2 NFT derivatives in moderate to good yields. Three bacteria strains and one fungi strain were measured, the modified compounds had broad-spectrum antibacterial activity, especially derivatives 1 and 18. Compounds 1, 3, 4, and 5 exhibited identical or better activity than that of the parent compound FZD against S. aureus. Cell cytotoxicity assays of potent compounds 1, 3, 4, 5, and 18 indicated non-cytotoxic against HepaRG cells. This study is an attempt to explore the application of copper(I) catalyzed C–H LSF, which provides good results and proves the practicability of this method. We believe our study could provide a reference for the application of LSF in pharmaceutical chemistry.Author contributions
Y. X. Y. and D. P. Z. designed the study. G. S. Y. C. performed experiments, acquired and analyzed data and wrote this manuscript. Z. C., P. Y. and S. W. assisted with experiments and analyzed data. D. P. Z. and X. L. L. contributed to completing the revision. Y. X. Y. contributed to funding support and submission of the article. All authors have given approval to the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No.81960278), the Outstanding Youth Funds of Science and Technology Department of Gansu Province (No.20JR5RA371).Notes and references
- P. Das, M. D. Delost, M. H. Qureshi, D. T. Smith and J. T. Njardarson, J. Med. Chem., 2019, 62, 4265–4311 CrossRef CAS PubMed.
- B. Das, A. T. K. Baidya, A. T. Mathew, A. K. Yadav and R. Kumar, Bioorg. Med. Chem., 2022, 56, 116614 CrossRef CAS PubMed.
- O. Boutureira and G. J. Bernardes, Chem. Rev., 2015, 115, 2174–2195 CrossRef CAS PubMed.
- Q. Zhao, L. Xin, Y. Liu, C. Liang, J. Li, Y. Jian, H. Li, Z. Shi, H. Liu and W. Cao, J. Med. Chem., 2021, 64, 10557–10580 CrossRef CAS PubMed.
- R. Z. Wu, H. Y. Zhou, J. F. Song, Q. H. Xia, W. Hu, X. D. Mou and X. Li, J. Med. Chem., 2021, 64, 17627–17655 CrossRef CAS PubMed.
- S. Alam Khan and M. Jawaid Akhtar, Bioorg. Chem., 2022, 120, 105599 CrossRef CAS PubMed.
- M. D. Cummings and S. Sekharan, J. Med. Chem., 2019, 62, 6843–6853 CrossRef CAS PubMed.
- K. Korvorapun, R. Kuniyil and L. Ackermann, ACS Catal., 2020, 10, 435–440 CrossRef CAS.
- O. Abdulla, A. D. Clayton, R. A. Faulkner, D. M. Gill, C. R. Rice, S. M. Walton and J. B. Sweeney, Chem. Eur. J., 2017, 23, 1494–1497 CrossRef CAS PubMed.
- B. Hong, T. Luo and X. Lei, ACS Cent. Sci., 2020, 6, 622–635 CrossRef CAS PubMed.
- C. A. Kuttruff, M. Haile, J. Kraml and C. S. Tautermann, ChemMedChem, 2018, 13, 983–987 CrossRef CAS PubMed.
- M. Moir, J. J. Danon, T. A. Reekie and M. Kassiou, Expert Opin. Drug Discovery, 2019, 14, 1137–1149 CrossRef CAS PubMed.
- H. Robinson, S. A. Oatley, J. E. Rowedder, P. Slade, S. J. F. Macdonald, S. P. Argent, J. D. Hirst, T. McInally and C. J. Moody, Chem. Eur. J., 2020, 26, 7678–7684 CrossRef CAS PubMed.
- D. Aynetdinova, M. C. Callens, H. B. Hicks, C. Y. X. Poh, B. D. A. Shennan, A. M. Boyd, Z. H. Lim, J. A. Leitch and D. J. Dixon, Chem. Soc. Rev., 2021, 50, 5517–5563 RSC.
- E. J. Barreiro, A. E. Kümmerle and C. A. Fraga, Chem. Rev., 2011, 111, 5215–5246 CrossRef CAS PubMed.
- C. S. Leung, S. S. Leung, J. Tirado-Rives and W. L. Jorgensen, J. Med. Chem., 2012, 55, 4489–4500 CrossRef CAS PubMed.
- H. Schönherr and T. Cernak, Angew. Chem., Int. Ed., 2013, 52, 12256–12267 CrossRef PubMed.
- A. Vasilopoulos, S. W. Krska and S. S. Stahl, Science, 2021, 372, 398–403 CrossRef CAS PubMed.
- Y. Wang, Y. Du and N. Huang, Future Med. Chem., 2018, 10, 2713–2728 CrossRef CAS PubMed.
- H. Fang, Y. Dou, J. Ge, M. Chhabra, H. Sun, P. Zhang, Y. Zheng and Q. Zhu, J. Org. Chem., 2017, 82, 11212–11217 CrossRef CAS PubMed.
- E. L. Ellsworth, T. P. Tran, H. D. Showalter, J. P. Sanchez, B. M. Watson, M. A. Stier, J. M. Domagala, S. J. Gracheck, E. T. Joannides, M. A. Shapiro, S. A. Dunham, D. L. Hanna, M. D. Huband, J. W. Gage, J. C. Bronstein, J. Y. Liu, D. Q. Nguyen and R. Singh, J. Med. Chem., 2006, 49, 6435–6438 CrossRef CAS PubMed.
- C. Marc, B. Vrignaud, K. Levieux, A. Robine, C. G. Guen and E. Launay, J. Child Health Care, 2016, 20, 530–536 CrossRef PubMed.
- E. Vitaku, D. T. Smith and J. T. Njardarson, J. Med. Chem., 2014, 57, 10257–10274 CrossRef CAS PubMed.
- X. H. Su, B. X. Wang, W. J. Le, Y. R. Liu, C. Wan, S. Li, R. A. Alm, J. P. Mueller and P. A. Rice, Antimicrob. Agents Chemother., 2016, 60, 621–623 CrossRef CAS PubMed.
- C. Shi, Y. Zhang, T. Wang, W. Lu, S. Zhang, B. Guo, Q. Chen, C. Luo, X. Zhou and Y. Yang, J. Med. Chem., 2019, 62, 2950–2973 CrossRef CAS PubMed.
- P. Govender, R. Müller, K. Singh, V. Reddy, C. J. Eyermann, S. Fienberg, S. R. Ghorpade, L. Koekemoer, A. Myrick, D. Schnappinger, C. Engelhart, J. Meshanni, J. A. W. Byl, N. Osheroff, V. Singh, K. Chibale and G. S. Basarab, J. Med. Chem., 2022, 65, 6903–6925 CrossRef CAS PubMed.
- N. H. Zuma, F. J. Smit, R. Seldon, J. Aucamp, A. Jordaan, D. F. Warner and D. D. N'Da, Bioorg. Chem., 2020, 96, 103587 CrossRef PubMed.
- J. C. Spain, Annu. Rev. Microbiol., 1995, 49, 523–555 CrossRef CAS PubMed.
- M. Mohammadi, B. Attaran, R. Malekzadeh and D. Y. Graham, Dig. Dis. Sci., 2017, 62, 1890–1896 CrossRef CAS PubMed.
- N. H. Zuma, J. Aucamp and D. D. N'Da, Eur. J. Pharm. Sci., 2019, 140, 105092 CrossRef CAS PubMed.
- Z. T. Zheng and Y. B. Wang, J. Gastroenterol. Hepatol., 1992, 7, 533–537 CrossRef CAS PubMed.
- Z. Chang, J. Huang, S. Wang, G. Chen, H. Zhao, R. Wang and D. Zhao, Nat. Commun., 2021, 12, 4342 CrossRef CAS PubMed.
- A. M. Timperio, H. A. Kuiper and L. Zolla, Xenobiotica, 2003, 33, 153–167 CrossRef CAS PubMed.
- P. Gripon, S. Rumin, S. Urban, J. Le Seyec, D. Glaise, I. Cannie, C. Guyomard, J. Lucas, C. Trepo and C. Guguen-Guillouzo, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 15655–15660 CrossRef CAS PubMed.
- R. Parent, M. J. Marion, L. Furio, C. Trépo and M. A. Petit, Gastroenterology, 2004, 126, 1147–1156 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ra07676d |
| This journal is © The Royal Society of Chemistry 2023 |