Open Access Article
Open Access ArticleEffectiveness of organic solvents for recovering collapsed PDMS micropillar arrays†
Dong Wangabc,
Zhuang Maabcd and
Xinchun Tian *ab
*ab
aSchool of Materials Science and Engineering, Beijing Institute of Technology, Beijing, 100081, China. E-mail: xctian@bit.edu.cn
bNational Key Laboratory of Science and Technology on Material under Shock and Impact, Beijing, 100081, China
cTangshan Research Institute, Beijing Institute of Technology, Tangshan 063000, China
dBeijing Institute of Technology Chongqing Innovation Center, Chongqing, 401120, China
First published on 6th February 2023
Abstract
Polydimethylsiloxane (PDMS) micropillar arrays are widely used in research labs and engineering fields as analytical tools for various purposes. When the micropillar length or density surpasses a critical value, micropillars tend to collapse with each other and become unusable. Restoring collapsed PDMS micropillars typically involves the use of low surface tension solvents and ultrasound sonication, but such approach has received little success to date. In this work, we examined the effectiveness of different types of solvents for restoring collapsed PDMS micropillar arrays and show that the swelling ratio of PDMS in selected solvents constitutes an important factor in the effectiveness of restoring collapsed PDMS micropillars. Our results could be a promoter in recycling PDMS micropillar arrays and achieving economic and social benefits.
Introduction
Elastomeric micropillar arrays are frequently used in areas such as biosensors,1–4 force sensors,5–7 microfluidic devices,8,9 superhydrophobic surfaces,10–12 and liquid-infused surfaces.13–15 Among them, polydimethylsiloxane (PDMS) based micropillar arrays have received the most attention in favor of their optical transparency,16 good mechanical resilience,17 and ease of formation and specific functionalization.18,19 However, due to their small sizes, PDMS micropillars are prone to collapse upon the drying of seating liquid samples which drive micropillars together under capillary force.20,21 The problem becomes more severe for high and dense micropillars which could collapse even during the demolding step.22To reuse micropillar arrays, simple and effective recovering methods are continuously sought. One common approach relies on using organic solvents with low surface tension, such as ethanol, and ultrasound sonication to separate collapsed micropillars. However, this method has not been very successful. Many works have been devoted to the basic understanding of different factors that potentially affect the collapsing and restoring behaviors of PDMS micropillars since then.23–26 There also have been some new methods proposed to deal with the problem, but their applicability is often limited.27–30
In this work, we reexamined the collapsing and recovery processes of PDMS micropillars when using different solvents to wash the micropillars and reveal the effects of two properties of the solvents – surface tension and swelling ratio – on the recovery of PDMS micropillars. Our results find that the solvent-induced polymer swelling, which is sometimes considered as damaging for PDMS integrity, can help the recovery of collapsed micropillars. We further propose a mechanism accounting for the assisted recovery process.
Experimental section
Sample preparation
Silicon mold with microholes was fabricated by etching wafers with SF6/C4F8 plasma. The PDMS micropillar arrays were prepared by one-step replication of the silicon mold.31 To facilitate demolding, the surface of silicon mold was modified with 1H,1H,2H,2H-perfluorooctyltrichlorosilane (Aladdin, >97%) to reduce the surface adhesion. Sylgard 184 (Dow Corning) with part A and part B were mixed in a 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 weight ratio and poured onto the silicon mold and drained the bubbles under vacuum. The PDMS mixture was then cured at 70 °C for 4 hours, after which the silicon mold was successfully removed. The height and width of the obtained PDMS micropillar were 16 μm and 4 μm, respectively, and the spacing between adjacent micropillars was 3 μm.
1 weight ratio and poured onto the silicon mold and drained the bubbles under vacuum. The PDMS mixture was then cured at 70 °C for 4 hours, after which the silicon mold was successfully removed. The height and width of the obtained PDMS micropillar were 16 μm and 4 μm, respectively, and the spacing between adjacent micropillars was 3 μm.
Collapsed PDMS micropillars were obtained using the following steps. Ethanol was first added to the PDMS micropillars and quickly spread out. Then, water was added to the PDMS micropillars to mix with ethanol and infuse the interspaces between micropillars with water. Finally, the PDMS micropillars were dried at 70 °C for 1 hour.
In situ observation of PDMS micropillars in solvents
The micropillars were immersed in one of the solvents listed in Table S1 (ESI†) for 30 minutes each time. Then the geometry of PDMS micropillars when immersed and dried was observed in situ using an optical microscope (KOPPACE, KP-A9510V-1800U). The PDMS micropillars were then thoroughly dried at 70 °C for 30 minutes and checked with the optical microscope again.Results and discussion
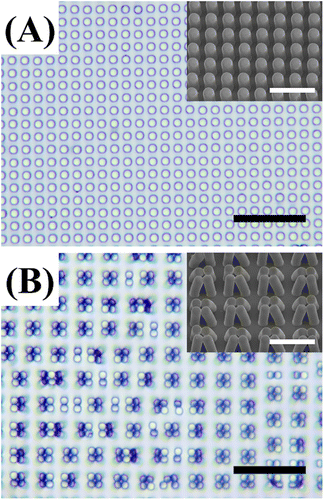
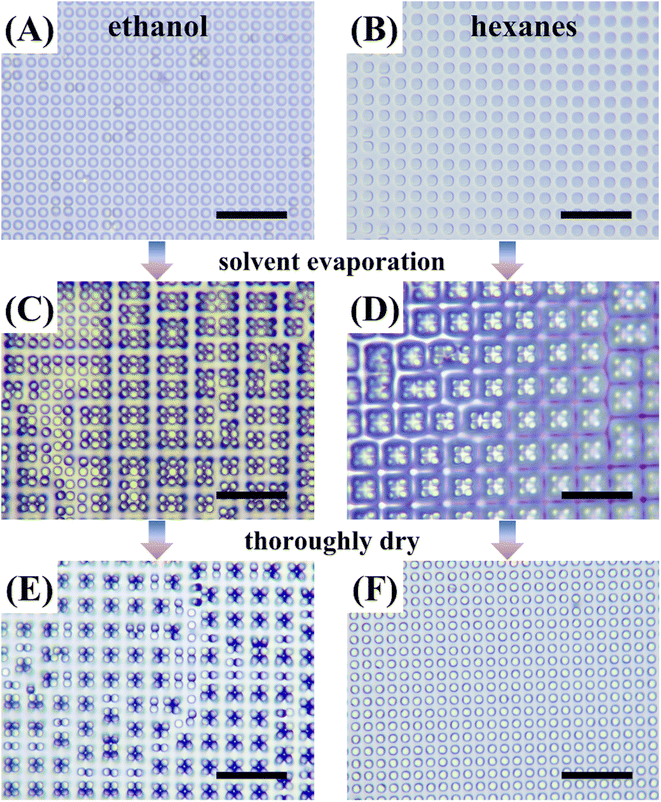
PDMS has a surface tension typically of 22 mN m−1 and Young's modulus typically of 1.7 MPa. The as-prepared PDMS micropillars fabricated in this work have a length-to-radius ratio of 4 and is considered as susceptible to lateral collapsing.32 Before interacting with any liquids, all micropillars stood up straight and showed no sign of obvious tilting or collapsing (Fig. 1A). But after infused with water and subsequent drying, nearly all the micropillars collapsed in groups (Fig. 1B), consistent with expectation. Scanning electron microscope (SEM) micrographs from a glancing view angle show the micropillars to collapse in a head-to-head manner (Fig. 1B, inset).Our first attempt to restore the collapsed micropillars is to use ethanol as the washing solvent with the assistance of ultrasound sonication. Specifically, we immersed the collapsed PDMS micropillars in an ethanol bath for 30 minutes and then sonicated the bath for 2 minutes at 100 W. Observed under an optical microscope, we found most of the micropillars in ethanol recovered from collapsing (Fig. 2A). The micropillars are then dried in air and monitored under the microscope. When most of the solvent has evaporated and some micropillars protruded out, the contour of the solvent/air interface deforms to encage certain numbers of micropillars in groups (Fig. 2C, right part). We also noticed that encaging a large number of micropillars are difficult. This can be explained since large groups require the micropillars at the perimeter to deform significantly, which is difficult.
To dry the solvent thoroughly, we put the sample in an oven at 70 °C for 30 minutes. As shown in Fig. 2E, although a small fraction of micropillars recovered to its original vertical position after oven drying, a considerable amount of revived micropillars collapsed again. We repeated the same process and adjusted the immersion and sonication time length, but no significant improvement in micropillar recovery was observed.
Crosslinked PDMS was known to swell in some organic solvents, such as hexanes and toluene.33,34 The swelling ratio was defined as the length ratio of the PDMS after swelling to that before swelling. Some laboratories have suggested to use solvents with high swelling ratios to clean up PDMS residues on glass slides.35 Here, we used hexanes to immerse the collapsed PDMS micropillars in the same way as used for ethanol but without ultrasound sonication. As shown in Fig. 2B, the collapsed micropillars were recovered soon after solvent immersion. And the micropillar radius was also observed to increase as a result of the swelling. The PDMS sample was then taken out of hexanes and checked under the microscope. Similar formation of grouped micropillars was also observed (Fig. 2D). But as shown in Fig. 2F, the micropillars were all recovered after hexanes dried up thoroughly in oven.
The above observations suggest that swelling ratio of PDMS could be a determinant in using solvent to recover collapsed micropillars. To find out the roles of surface tension and swelling ratio on the recovery of PDMS micropillars, we selected 10 different solvents with known surface tension ranging from 15.4 mN m−1 to 43.7 mN m−1 and swelling ratio from 1.00 to 1.44 (Table S1†).35–37
The same washing procedure was adopted for all solvents, namely, 30 minutes of immersion in solvents first and then 30 minutes of drying in oven at 70 °C. The recovery ratio (R) was defined as the percentage of upright standing micropillars in the arrays relative to the total amount of micropillars counted (Fig. S1, ESI†). Five randomly selected locations in the arrays were used to calculate R for each solvent. The results were then plotted in a 3D table shown in Fig. 3. There is an abrupt increase in R when the swelling ratio exceeds around 1.20, while the increase of R relative to the surface tension is rather gradual. This indicates that the role of swelling ratio in the recovery process is more critical than that of the surface tension. In addition, we also used hexanes to successfully restored the collapsed micropillars after washed and dried by solvents with low swelling ratios, suggesting that the recovery capability of hexanes is independent of the collapsing process (Fig. S2, ESI†).
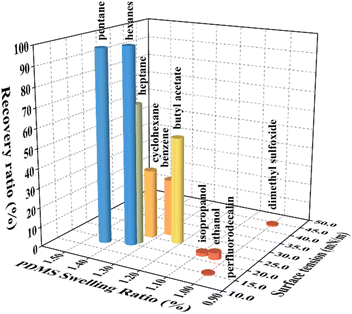 | ||
| Fig. 3 Effects of surface tension and swelling ratio of organic solvents on restoring collapsed PDMS micropillars. The recovery ratio is the number of restored micropillars divided by the total number of micropillars. Details of the solvents can be found in Table S1 (ESI†). | ||
Since the cause of micropillar collapsing is often attributed to capillary force inflicted by drying liquids, it appears straightforward to use low surface tension liquids to reduce the capillary force and thereby avoid micropillar collapsing. However, even after using perfluoroheptane with a surface tension of 11.7 mN m−1, we are still unable to recover the PDMS micropillars fully.
Some references suggested the following relationship for micropillar arrays,24,32
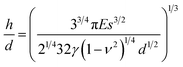 | (1) |
| E ∼ Q−k | (2) |
The discrepancy could originate from the incorrect γ value we put in the above calculation. When using the surface tension of pristine PDMS, it is assumed that the contact interface between collapsed PDMS micropillars remains solid–solid even after swelling. However, since swollen polymer networks allow the penetration of solvent molecules, solvent can accumulate at the contact area between collapsed micropillars to form a “liquid bridge” and change the interface to solid–liquid–solid. Consequently, separation of micropillars involves the interplay between the elastic force of bent PDMS micropillars and the deformation of the liquid bridge. Fig. 4 illustrates the idea of how the existence of liquid bridge allows the easy separation of two collapsed micropillars under their own elastic force. In contrast, when the washing solvent does not induce swelling in micropillars, formation of liquid bridge will be difficult.
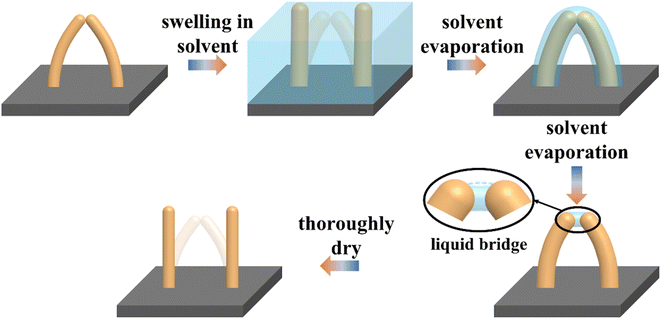 | ||
| Fig. 4 Schematic diagram showing the swelling and recovery of two collapsed micropillars and the presence of liquid bridge. | ||
The existence of liquid bridge is also consistent with the gradual increase of the recovery ratio along with the decrease of surface tension of the washing solvents as observed in Fig. 3. Since the liquid bridge is losing volume during drying, the liquid bridge's contour will deform from convex to concave due to its good wetting property with PDMS.40 The change of contour shape is schematically shown in the black-line circled region in Fig. 4 wherein the cyan dash-line refers to the original convex contour. Once the concave surface is formed, the liquid bridge will generate a Laplace pressure which keeps the two micropillars from breaking and is proportional to the surface tension of the solvent. As a result, a solvent with a lower surface tension will have a larger recovery ratio when the swelling capability of the solvent is unchanged. This explains the lower recovery ratio of cyclohexane compared with that of hexanes. We also find that drying at a temperature slightly higher than the boiling point of the washing solvent will also increase the recovery ratio (Fig. S3†). This may be due to the significant decrease of the surface tension of the solvent at the boiling point.
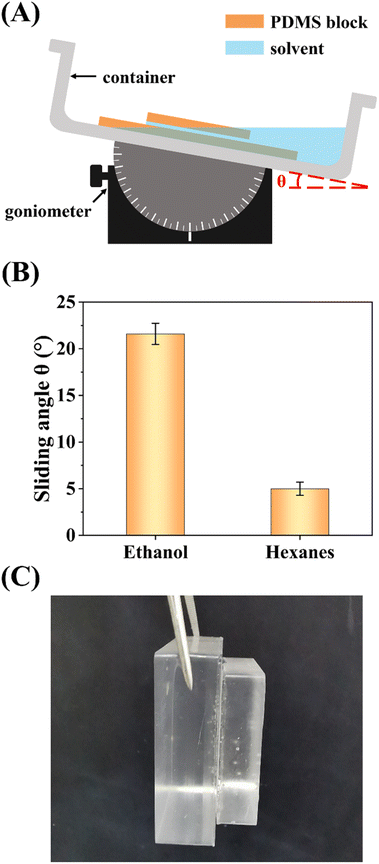
To corroborate the presence of liquid bridge, we conducted two experiments using a large and a small flat PDMS blocks which were placed in a container with the small block located above the large one (schematically shown in Fig. 5A). In the first test, the container was filled with solvent (either ethanol or hexanes) first and the two PDMS blocks were placed into the solvent separately. As the container was gradually tilted, the solvent was drained out of the small block and eventually the small block started to slide on the large one (the corresponding tilting angle is named “sliding angle”). In the case of ethanol, the sliding angle is 22°. While in the case of hexanes, the sliding angle is only 5° (Fig. 5B). Since a liquid layer typically reduce the friction between two solid surfaces, the difference in sliding angle suggests that hexanes can stay between the two PDMS blocks much easier than ethanol does, consistent with our propose that a liquid bridge is present between collapsed PDMS micropillars in hexanes. In the second test, the two blocks were firstly placed into the container and the solvent was added later. In the case of ethanol, the two blocks stick together even after they were taken out and held at 90° (Fig. 5C). In contrast, the small block started to slide on the large one soon after hexanes were added.
Conclusions
In summary, we examined the effectiveness of common solvents on the recovery of PDMS micropillars and reveal that the swelling ratio of PDMS in some organic solvents is an important factor in the recovery of collapsed PDMS micropillars. Our findings suggest that using solvent with high swelling ratio of PDMS could potentially be a simple approach to recycle collapsed PDMS micropillar arrays.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
The authors acknowledge the financial support provided by the National Natural Science Foundation of China (52102060), National Key Laboratory Foundation of Science and Technology on Materials under Shock and Impact (202120943215), and Beijing Institute of Technology Research Fund Program for Young Scholars (XSQD-202108001).References
- T. Steinberg, S. Schulz, J. P. Spatz, N. Grabe, E. Mussig, A. Kohl, G. Komposch and P. Tomakidi, Nano Lett., 2007, 7, 287–294 CrossRef CAS PubMed.
- C. S. Chen, M. Mrksich, S. Huang, G. M. Whitesides and D. E. Ingber, Science, 1997, 276, 1425–1428 CrossRef CAS PubMed.
- K. N. Hass, M. Bao, Q. He, L. Liu, J. He, M. Park, P. Qin and K. Du, ACS Omega, 2020, 5, 27433–27441 CrossRef CAS PubMed.
- X. Liu, R. Liu, Y. Gu and J. Ding, ACS Appl. Mater. Interfaces, 2017, 9, 18521–18530 CrossRef CAS PubMed.
- F. Zhang, S. Anderson, X. Zheng, E. Roberts, Y. Qiu, R. Liao and X. Zhang, Appl. Phys. Lett., 2014, 105, 0337023 Search PubMed.
- S. Johari and L. Y. Shyan, INCAPE2017, 2017, vol. 162, p. 01080 Search PubMed.
- H. Park, Y. R. Jeong, J. Yun, S. Y. Hong, S. Jin, S.-J. Lee, G. Zi and J. S. Ha, ACS Nano, 2015, 9, 9974–9985 CrossRef CAS PubMed.
- D. Wu, S.-z. Wu, Q.-D. Chen, S. Zhao, H. Zhang, J. Jiao, J. A. Piersol, J.-N. Wang, H.-B. Sun and L. Jiang, Lab Chip, 2011, 11, 3873–3879 RSC.
- B. Zhou, W. Xu, A. A. Syed, Y. Chau, L. Chen, B. Chew, O. Yassine, X. Wu, Y. Gao, J. Zhang, X. Xiao, J. Kosel, X.-X. Zhang, Z. Yao and W. Wen, Lab Chip, 2015, 15, 2125–2132 RSC.
- Y. Tan, J. Yang, Y. Li, X. Li, Q. Wu, Y. Fan, F. Yu, J. Cui, L. Chen, D. Wang and X. Deng, Adv. Mater., 2022, 34, 220216730 Search PubMed.
- S. Y. Lee, Y. Rahmawan and S. Yang, ACS Appl. Mater. Interfaces, 2015, 7, 24197–24203 CrossRef CAS PubMed.
- Y. Rahmawan, M.-W. Moon, K.-S. Kim, K.-R. Lee and K.-Y. Suh, Langmuir, 2010, 26, 484–491 CrossRef CAS PubMed.
- C. Chen, Z. Huang, L.-A. Shi, Y. Jiao, S. Zhu, J. Li, Y. Hu, J. Chu, D. Wu and L. Iang, Adv. Funct. Mater., 2019, 29, 190476640 Search PubMed.
- J. Li, E. Ueda, D. Paulssen and P. A. Levkin, Adv. Funct. Mater., 2019, 29, 18023174 Search PubMed.
- X. Tian, S. Banerjee, I. Gonzalez-Alfonzo and L. Cademartiri, Langmuir, 2020, 36, 5106–5111 CrossRef CAS PubMed.
- Y. N. Xia, E. Kim, X. M. Zhao, J. A. Rogers, M. Prentiss and G. M. Whitesides, Science, 1996, 273, 347–349 CrossRef CAS PubMed.
- Z. Wang, A. A. Volinsky and N. D. Gallant, J. Appl. Polym. Sci., 2014, 131, 4105022 CrossRef.
- D. C. Duffy, J. C. McDonald, O. J. A. Schueller and G. M. Whitesides, Anal. Chem., 1998, 70, 4974–4984 CrossRef CAS PubMed.
- Y. N. Xia and G. M. Whitesides, Angew. Chem., Int. Ed., 1998, 37, 550–575 CrossRef CAS PubMed.
- D. Garcia-Gonzalez, J. Snoeijer, M. Kappl and H.-J. Butt, Langmuir, 2020, 36, 11581–11588 CrossRef CAS PubMed.
- S. F. Chini and A. Amirfazli, Langmuir, 2010, 26, 13707–13714 CrossRef CAS PubMed.
- G. Shao, J. Wu, Z. Cai and W. Wang, Sens. Actuators, A, 2012, 178, 230–236 CrossRef CAS.
- K. G. Sharp, G. S. Blackman, N. J. Glassmaker, A. Jagota and C. Y. Hui, Langmuir, 2004, 20, 6430–6438 CrossRef CAS PubMed.
- P. Roca-Cusachs, F. Rico, E. Martinez, J. Toset, R. Farre and D. Navajas, Langmuir, 2005, 21, 5542–5548 CrossRef CAS PubMed.
- D. Chandra and S. Yang, Acc. Chem. Res., 2010, 43, 1080–1091 CrossRef CAS PubMed.
- Y.-H. Yeh, K.-H. Cho and L.-J. Chen, Soft Matter, 2012, 8, 1079–1086 RSC.
- H. Liu, B. Lei, W. Jiang, Y. Li, L. Yin, B. Chen and Y. Shi, RSC Adv., 2016, 6, 16640–16644 RSC.
- J. S. Choi, S. Lim, J. Kim, S. S. Chung, S. E. Moon, J. P. Im, J. H. Kim and S. M. Kang, ACS Appl. Mater. Interfaces, 2021, 13, 58201–58208 CrossRef CAS PubMed.
- M. Matsunaga, M. Aizenberg and J. Aizenberg, J. Am. Chem. Soc., 2011, 133, 5545–5553 CrossRef CAS PubMed.
- S. M. Kim, S. M. Kang, C. Lee, S. Jang, J. Kim, H. Seo, W.-G. Bae, S. Yang and H. Yoon, J. Mater. Chem. C, 2016, 4, 9608–9612 RSC.
- D. Qin, Y. Xia and G. M. Whitesides, Nat. Protoc., 2010, 5, 491–502 CrossRef CAS PubMed.
- N. J. Glassmaker, A. Jagota, C. Y. Hui and J. Kim, J. R. Soc. Interface, 2004, 1, 23–33 CrossRef CAS PubMed.
- K. Urayama and S. Kohjiya, J. Chem. Phys., 1996, 104, 3352–3359 CrossRef CAS.
- X. Xu, X. Yao, F. Chen and Q. Fu, RSC Adv., 2015, 5, 3733–3742 RSC.
- J. N. Lee, C. Park and G. M. Whitesides, Anal. Chem., 2003, 75, 6544–6554 CrossRef CAS PubMed.
- ChemSpider, Royal Society of Chemistry, 2023, http://www.chemspider.com/ Search PubMed.
- Y. Wang, J. Balowski, C. Phillips, R. Phillips, C. E. Sims and N. L. Allbritton, Lab Chip, 2011, 11, 3089–3097 RSC.
- S. H. Yoo, C. Cohen and C.-Y. Hui, Polymer, 2006, 47, 6226–6235 CrossRef CAS.
- K. Sivasailam and C. Cohen, J. Rheol., 2000, 44, 897–915 CrossRef CAS.
- P. Papadopoulos, P. Bat-El, M. Tress, D. Vollmer, M. Kappl and H. Butt, Soft Matter, 2018, 14, 7429–7434 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Values of surface tension and swelling ratio of various solvents and optical images of PDMS micropillar arrays after washing and drying with different solvents after collapsing induced by different solvents. See DOI: https://doi.org/10.1039/d2ra08109a |
| This journal is © The Royal Society of Chemistry 2023 |