Open Access Article
Open Access ArticleEffects of rootstocks on the flavor quality of huanglongbing-affected sweet orange juices using targeted flavoromics strategy†
Xin Liu ab,
Frederick G. Gmitter Jra,
Jude W. Grossera and
Yu Wang
ab,
Frederick G. Gmitter Jra,
Jude W. Grossera and
Yu Wang *ab
*ab
aCitrus Research and Education Center, University of Florida, Lake Alfred, Florida 33850, USA. E-mail: yu.wang@ufl.edu
bDepartment of Food Science and Human Nutrition, University of Florida, Gainesville, Florida 32611, USA
First published on 15th February 2023
Abstract
Citrus greening disease or Huanglongbing (HLB) is one of the most destructive diseases affecting all varieties of citrus worldwide. Aimed at optimizing the scion/rootstock combination to improve HLB-affected orange juice quality, a flavoromics strategy was used to investigate the effects of six different rootstocks (CH, blue, 1804, FG, SW, and Volk) on flavor quality of HLB affected orange juices. A sensory quality test was conducted by a panel to evaluate the sensory attributes of different orange juices. The orange juice from rootstock CH had the best flavor quality with highest sweetness, low sourness and bitterness, while rootstocks Volk and FG produced the poorest quality orange juices. Chemical profile analysis resulted in semi-quantification of 89 metabolites including 57 nonvolatile compounds and 32 volatile compounds using UHPLC-MS and GC-MS, respectively. Canonical correlation analysis indicated that some specific sugar and sugar alcohols including raffinose, xylose, rhamnose, glucose, sorbitol, and myo-inositol made a strong positive contribution to sweetness. Meanwhile, several amino acids including alanine, glutamic acid, proline, arginine, serine, asparagine, as well as aspartic acid were responsible for positive flavor quality. On the other hand, some nucleotides and limonin increased bitterness. In addition, KEGG pathway enrichment analysis demonstrated different rootstocks could affect aminoacyl-tRNA biosynthesis, ABC transporters, and monoterpenoid biosynthesis. These results indicated different rootstocks can change specific metabolites and thus affect the flavor quality of orange juices. This study also provides reference for optimizing the scion/rootstock combination to improve HLB-affected orange juice quality.
1. Introduction
Citrus greening disease or huanglongbing (HLB) is one of the most destructive diseases affecting all varieties of citrus worldwide. It is caused by unculturable phloem-limited bacteria including Candidatus Liberibacter asiaticus (CLas), Ca. L. africanus (CLaf), and Ca. L. americanus (CLam).1 The Asian citrus psyllid Diaphorina citri, the vector of HLB, can transmit the disease-causing bacterium among trees.2 Orange fruits from HLB-affected trees do not completely mature and possess negative flavor described as bitter-harsh, metallic, and less juicy and fruity.3 Recently, several studies demonstrated that HLB-affected oranges had lower soluble solids content, resulting in the juice being perceived as less sweet and more bitter than the juice from healthy orange trees.4,5 Since HLB has brought large economic damage to the citrus industry worldwide, different strategies including chemical control of the insect vector, supplying trees with enhanced nutrition, and planting of disease-free nursery stock have been developed to prevent or slow down HLB.6 Although other strategies, such as thermotherapy and foliar applications of antibiotics are being employed, they remain far away from large-scale commercial production.The rootstock is the root section of a compound plant, with a portion of the stem to which a scion cultivar bud is grafted.7 The combination between scion and rootstock is important for plant disease prevention. Rootstocks are not only capable of increasing fruit yield and quality but also enable specific scions to be more resistant to abiotic and biotic stresses such as drought, frost, and diseases.8 Therefore rootstock/scion combination trials offer a promising future for HLB tolerance or resistance. During past decades, researchers and growers have been developing effective rootstocks that will allow for sustainable and profitable citriculture with commercial scions, including more HLB-susceptible sweet oranges, grapefruits, and mandarins.9–11 Our previous studies also demonstrated an optimum tolerant scion/rootstock combination was helpful to improve orange juice quality from HLB-affected citrus trees.12
Flavoromics is an approach combining flavor components (profiling of aroma and taste compounds) with chemometrics to conduct food quality assessment.13–16 Flavoromics established relationships between chemical profiling of flavor-impacting compounds and sensory science. Prompted by evolution of modern high-resolution analytical techniques and new data analysis capabilities, this approach deals with large datasets involved in chemical profiling methodologies, with procedures of advanced chemometrics and data mining.
Fruit or orange juice quality can be affected by a specific combination between scion and rootstocks. Therefore, understanding the relationship between fruit or juice flavor quality and different rootstocks is essential for scion/rootstock optimization of HLB tolerance or resistance. In this study, we continue our ongoing study for optimizing the scion/rootstock combination to improve HLB-affected orange juice quality. The objectives are (1) to evaluate the sensory attributes of orange juices from HLB affected Valencia sweet orange scions grafted on six different rootstocks, (2) to detect the chemical composition of the orange juices by targeted flavor profiling analysis, and (3) to investigate the relationship between the sensory attributes and chemical profiles using flavoromics strategy. This study will not only provide reference to flavoromics research on orange juices, but also make a positive contribution to citrus breeding in the HLB era.
2. Materials and methods
2.1. Chemical and reagents
All solvents were LC-MS grade and purchased from Fisher Scientific Co. (Waltham, MA). Reference compounds were purchased from Sigma-Aldrich (St. Louis, MO), Indofine Chemical Company (Hillsborough Township, NJ, USA), Fisher Scientific (Hampton, NH), TCI America (Portland, OR, USA), and Santa Cruz Biotechnology (Santa Cruz, CA, USA).2.2. Plant materials and juice preparation
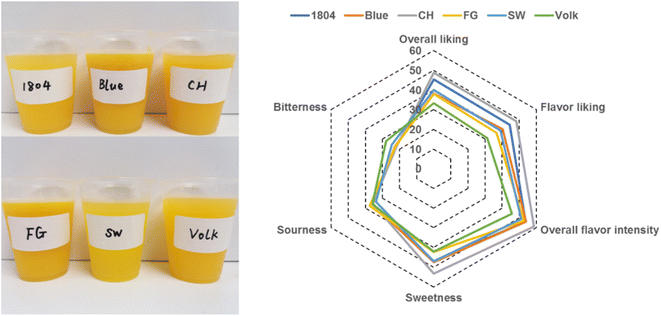
The scion/rootstock combination trials were located in St. Helena, Dundee, and Polk County, Florida, USA. All the trees were planted in 2008 and were clearly affected by HLB by 2012. The scion is Valencia sweet orange SF-14W-62 and trademarked as “Valquarius®”, maturing during January and February. The six rootstocks are as follows: Orange 1804 (1804) is a diploid sexual hybrid of Cleopatra mandarin × trifoliate orange (from the Foguet Rootstock Breeding Program, Argentina); FG1733 (FG) is a diploid sexual hybrid of putative pummelo × mandarin; Changsha + Benton (CH) is a tetraploid somatic hybrid of Changsha mandarin + Benton citrange; Blue 3 (Blue) is a tetraploid sexual hybrid from a cross of two somatic hybrids [(Nova tangelo + Hirado Buntan pink pummelo) × (Sour orange + Palestine sweet lime)]; Swingle (SW) is a diploid citrumelo hybrid from a cross of white grapefruit with trifoliate orange (the most widely planted rootstock in Florida before HLB); Volkamer lemon (Volk) is a vigorous diploid lemon type. These rootstocks were selected based on the tree health of productivity. 1804 and FG were tolerant varieties; SW and Volk were commercial rootstocks; CH and Blue were HLB-sensitive rootstocks. Thus, fruits and juices from six different rootstocks, named 1804, FG, CH, Blue, SW, and Volk, were selected for further investigation. Fruits from each tree were randomly harvested in February 2020. Orange juices were mechanically juiced using an FMC citrus juice extractor (Philadelphia, PA, USA) and delivered in one-gallon jugs. The juices were stored in a refrigerator and used for consumer sensory tests (Fig. 1). The remaining juice of each sample was frozen at −20 °C for subsequent chemical analysis. For each juice sample from different rootstock, six technical replicates were prepared for further instrumental analysis.2.3. Sensory evaluation
The sensory test was conducted by a panel to evaluate the sensory attributes of different orange juices. The panel consisted of a total of 72 consumers (recruited from the Citrus Research and Education Center, University of Florida) between ages of 20 and 70 with 55% female and 45% male. The sensory assessment was performed in individual sensory testing booths under artificial daylight and constant temperature (22 °C). Each orange juice sample was labeled with a random three-digit number and supplied with a cup of water and unsalted crackers as palate cleansers. All the consumers went through a training session to familiarize them with scaling systems and procedures, then the orange juice samples were rated on a scale system using Compusense software (Compusense, Inc., Guelph, Ontario, Canada). The sensory attributes including sweetness, sourness, bitterness, overall liking, flavor liking, overall flavor intensity, and overall appearance were evaluated using the global sensory intensity scale (GSIS), which ranges from 0 to 100 (0 = no sensation, 100 = most intense sensation). Overall flavor liking was rated using the global hedonic intensity scale (GHIS), which ranges from −100 to 100. Compusense20 software was used to record the scores, and the mean values of each sample among 72 consumers were obtained and analyzed. All the procedures involving the human participants were in accordance with the ethical standards of the institutional or national research committees and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study protocol and consent procedure received ethical approval from the Institutional Review Board (IRB) of the University of Florida. Informed consent was obtained from all individual participants included in the study.2.4. Brix and total acidity measurement
Total soluble solids (TSS), expressed in oBx, was detected using a Brix-Acidity Meter (PAL-BXIACID1, ATAGO, Tokyo, Japan). TSS is a parameter reflecting sugar concentration level in orange juice. Meanwhile, the titratable acidity (TA) was determined after dilution (49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, w/w) with water.
1, w/w) with water.
2.5. Analysis of sugars, sugar alcohols, organic acids, amino acids, nucleotides, nucleosides, flavonoids, and limonoid
The orange juice (0.5 mL) was lyophilized, and then mixed with 1.0 mL 90% methanol containing internal standards (hippuric acid-d5 100 ppm, proline-d3 50 ppm, tyrosine-13C6 50 ppm, phenylalanine-13C6 50 ppm). The mixture was sonicated for 15 min at room temperature, and then vortexed using a multi-tube vortex mixer (Fisher Scientific, USA) for 15 min at room temperature. After centrifugation at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 15 min at 4 °C, the supernatant was filtered through 0.22 μm nylon syringe filter (Bonna-Agela Technologies Inc. Wilmington, DE, USA) and then detected using U-HPLC-Q-Exactive Plus HR-ESI-MS (Thermo Fisher Scientific, USA).
000 rpm for 15 min at 4 °C, the supernatant was filtered through 0.22 μm nylon syringe filter (Bonna-Agela Technologies Inc. Wilmington, DE, USA) and then detected using U-HPLC-Q-Exactive Plus HR-ESI-MS (Thermo Fisher Scientific, USA).
The analyte was analyzed on a Poroshell HILIC-Z column (2.1 mm × 100 mm, particle size 2.7 μm, Agilent Technologies, Santa Clara, CA, USA) with a guard column at the column temperature of 40 °C using (A) 10 mM ammonium acetate in water containing 25 mM methylphosphonic acid (MPA), pH 9.0, and (B) acetonitrile-10 mM ammonium acetate (90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, v/v) as the mobile phase. The flow rate was 0.25 mL min−1, the gradient elution was performed as follows: 0–2 min 90% B, 2–8 min 90–70% B, 8–10 min 70–40% B, 10–18 min 40% B, 18–19 min 40–90% B. The injection volume was 3.0 μL. The column was equilibrated for the initial mobile phase for at least one hour before sequence running.
10, v/v) as the mobile phase. The flow rate was 0.25 mL min−1, the gradient elution was performed as follows: 0–2 min 90% B, 2–8 min 90–70% B, 8–10 min 70–40% B, 10–18 min 40% B, 18–19 min 40–90% B. The injection volume was 3.0 μL. The column was equilibrated for the initial mobile phase for at least one hour before sequence running.
The mass spectrometer was operated in positive and negative electrospray ionization mode depending on targeted single ion monitoring mode (SIM). The parameters of ESI source were as follows: spray voltage, 3500 V (positive) and 2500 V (negative), capillary temperature 325 °C, aux gas heater temperature 350 °C, sheath gas flow rate 50, aux gas flow rate 10.
The samples were also analyzed on an Acclaim™ C30 column (2.1 mm × 150 mm, particle size 3.0 μm, Thermo scientific, USA) with a guard column at the column temperature of 40 °C using (A) 0.1% formic acid aqueous solution and (B) 0.1% formic acid in acetonitrile as mobile phase. The flow rate was 0.20 mL min−1, the gradient elution was performed as follows: 0–3 min 2% B, 3–25 min 2–50% B, 25–30 min 50–90% B, 30–35 min 95% B, 35–36 min 95–2% B. The injection volume was 3.0 μL. The mass parameters were identical with those mentioned above for the HILIC column detection conditions. Data analysis was conducted using Xcalibur 4.3 software (Thermo Fisher Scientific, CA, USA). All external standards are listed in ESI.†
2.6. Analysis of volatile compounds
Volatile compound analytes were prepared using headspace solid-phase microextraction (HS-SPME) and determined on gas chromatography-mass spectrometry/olfactometry (GC-MS/O) according to our previous study.17 The GC-MS system included a Clarus 680 GC (PerkinElmer, Inc., Waltham, MA, USA) coupled with a Clarus SQ 8T mass spectrometer. For sample preparation, 5 mL of orange juice was added into a 40 mL headspace vial containing a magnetic stir bar and capped with a PTFE/silicon septum cap. Sodium chloride (1.8 g) and internal standard (5 μL of 4-heptadecanone, 0.4 mg mL−1 in methanol) were added to the juice sample. The juice sample was incubated in a water bath (40 °C) for 20 min followed by exposure a 50/30 μm divinylbenzene/carboxen/polydimethylsiloxane SPME fiber (Supelco Inc., Bellefonte, PA, USA) to the headspace of each vial for 20 min.Volatile compounds adsorbed on the SPME fiber were desorbed for 10 min at 250 °C in the splitless injector of the GC combined with a mass spectrometer operated in electron impact mode (MS-EI, 70 eV ionization energy) with a scan range from m/z 50 to 300 in the positive mode with a 2.0 min solvent delay. Separation was achieved on a TR-FFAP capillary column (30 m × 0.25 mm, 0.25 μm film thickness, Thermo Fisher Scientific, Waltham, MA, USA) with a constant pressure. The column temperature was programmed to rise from 40 °C after 2 min hold, then increased 4 °C min−1 to 230 °C with a 5 min hold. Helium gas was used as the carrier gas at a flow rate of 1.1 mL min−1. A series of n-alkanes (C7–C30) was used to determine linear retention indices (RI) for each compound. Compound identification was compared with reference standards. Relative concentration of volatile compounds was semi-quantified based on the internal standard. Each sample was repeated in sextuplicate.
2.7. Statistical analysis
All sensory and chemical data were analyzed by one-way analysis of variance (ANOVA) followed by Tukey's Honestly Significant Difference (HSD) test to compare sensory and analytical variances of difierent orange juices. The results were considered statistically significant for value of P < 0.05. Metabolome data were processed using the web-based tool MetaboAnalyst 3.0 (https://www.metaboanalyst.ca/).18 Orthogonal partial least-squares-discriminate analysis (OPLS-DA) was performed on datasets using SIMCA (version 17.0; Umetrics, Umea, Sweden). The OPLS-DA model was validated by the R2Y and Q2 from a random permutation test (n = 999) in SIMCA. The Canonical Correlation Analysis (CCA) and heatmap were conducted using R studio software (Boston, MA, USA).3. Results and discussion
3.1. Sensory attributes of different orange juices
In this study, the consumer sensory test was performed to evaluate the sensory quality profiles in terms of overall liking, flavor liking, overall flavor intensity, and sensory attributes including sweetness, sourness, and bitterness. As shown in Fig. 1, the sensory overall liking rating of six different orange juices were ranked in the following manner: CH > 1804 > SW > Blue > FG > Volk. The flavor liking and overall flavor intensity were similar to that of overall liking. Orange juice from rootstock CH showed the best flavor quality with high sweetness (P < 0.05), low sourness, and bitterness compared with the other samples, and led in overall liking, flavor liking, and overall flavor intensity. However, Volk and FG were lowest in the overall liking and flavor liking (P < 0.05). No significant differences in sourness and bitterness were found among six different orange juices, though Volk and FG had the highest score of bitterness and sourness, respectively.Perception of overall liking, overall flavor intensity, and flavor liking are almost consistent with sweetness, which means that overall liking of different orange juices may be primarily affected by sweetness. Basically, CH turned out to be the best rootstock in positive sensory attributes, while Volk showed some negative sensory attributes; the sensory quality of 1804, Blue, FG, and SW were similar. These results indicated the flavor quality of orange juices from HLB affected scions were influenced by different rootstocks.
3.2. Effects of different rootstocks on juices TSS and total acidity measurement
As shown in Table 1, Brix level in orange juice from CH rootstock was significantly higher than those in orange juices from other rootstocks, and its acid level was significantly lower than those from Volk and FG. The levels of sugar and acid are considered the primary flavor drivers. The Brix/acid ratio is very important for juice quality, as it is a measure for the balance between sweetness and sourness. Even though all six juice samples are from HLB affected scions, the Brix/acid ratio value of CH and SW meet the USDA requirement for Grade A orange juice. Significantly, the Brix/acid ratio did not match very well with the sensory attributes; the sensory properties are likely also influenced by aroma and other taste compounds. Therefore, it is necessary to profile aroma and taste compounds to better understand the relationship between sensory attributes and chemical profiles.| TSS (°Brix) | TA (%) | TSS/TA | |
|---|---|---|---|
| a Values with each sample marked by different uppercase letters within the same row are significantly different (P < 0.05). | |||
| 1804 | 11.10 ± 0.08ab | 1.32 ± 0.03a | 8.41b |
| Blue | 11.53 ± 0.05ab | 1.11 ± 0.10ab | 10.38ab |
| CH | 12.55 ± 0.17a | 0.85 ± 0.01b | 14.76a |
| FG | 10.95 ± 0.06ab | 1.36 ± 0.11a | 8.05b |
| SW | 11.88 ± 0.09ab | 0.79 ± 0.02b | 15.04a |
| Volk | 9.80 ± 0.08b | 1.34 ± 0.13a | 7.31b |
3.3. Chemical profiles of different orange juices
To illustrate what chemical constituents are responsible for flavor attributes, chemical profiles of orange juices produced from trees on the six different rootstocks were analyzed. The nonvolatile compounds including sugars, sugar alcohols, organic acids, amino acids, nucleotides, nucleosides, flavonoids, and limonoids were semi-quantified using UHPLC-HRMS, and volatile compounds were detected using SPME-GC-MS. In this study, a total of 57 nonvolatile metabolites were identified and semi-quantified after normalization, as shown in the heatmap (Fig. 2), and their relative concentrations were listed in Table S1.† Six sugars (rhamnose, sucrose, raffinose, xylose, glucose, and fructose), three sugar alcohols (sorbitol, myo-inositol, and arabitol), and one sugar phosphate (glucose 6-phosphate) were identified. Sucrose, glucose, fructose, and myo-inositol were major sugars and sugar alcohol in these samples. A total of 15 organic acids including quinic acid, shikimic acid, malonic acid, ferulic acid, maleic acid, pyruvic acid, abscisic acid, benzoic acid, tartaric acid, ascorbic acid, malic acid, isocitric acid, citric acid, oxaloacetic acid, and uric acid were detected. Citric acid, isocitric acid, malic acid, and ascorbic acid were predominant organic acids. A total amount of 17 amino acids were detected, with phenylalanine, arginine, and proline, the three most abundant amino acids, accounting for 84% of total detected amino acids. Serine, alanine, valine, glutamine, lysine, threonine, and histidine were present at less than 1% of total detected amino acids. In addition, eight flavonoids (rutin, hesperidin, didymin, narirutin, eriocitrin, nobiletin, sinensetin, and tangeretin), six nucleotides (adenine, cytosine, adenosine, guanosine, uridine, and cytidine) as well as limonin were identified and semi-quantified. Hesperidin and adenosine were the major flavonoid and nucleotide, respectively.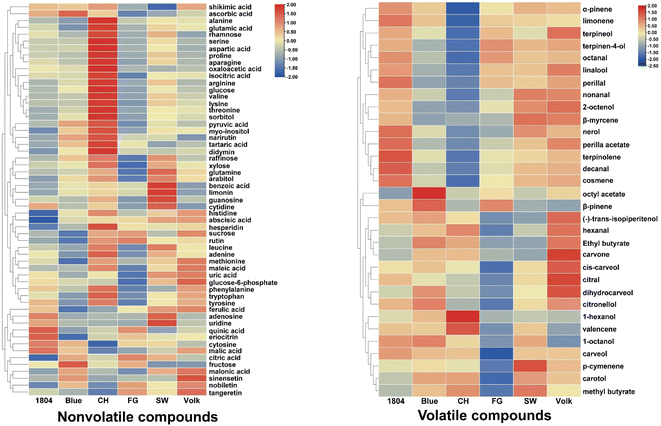 | ||
| Fig. 2 Heatmaps of the relative concentrations of 57 nonvolatile and 32 volatile compounds in orange juices from scions grafted on six different rootstocks. | ||
OPLS-DA was employed as a multivariate data analysis method to display the nonvolatile metabolites pattern of orange juices from different rootstocks. As shown in Fig. 3A, clusters of six different orange juices were separated from each other, indicating that the targeted nonvolatile compound profiles were significantly affected by different rootstocks. R2X, R2Y, and Q2 values were 0.743, 0.982, and 0.836, respectively. The y intercepts of R2 and Q2 in the permutation test (999×) were 0.448 and −0.597, respectively, indicating a valid model. On the basis of OPLS-DA results, 32 significant metabolites with a variable importance for projection (VIP) more than 1.0 were considered as potential markers for distinguishing different orange juices (Table S3†).
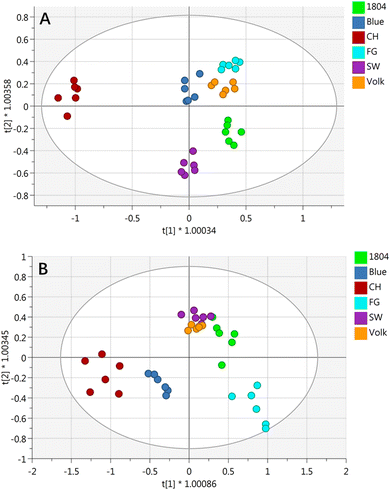 | ||
| Fig. 3 Loading plot of OPLS-DA with the relative concentrations of nonvolatile compounds (A) and volatile compounds (B) in six different orange juices. | ||
A total of 32 volatile compounds were detected and semi-quantified as shown in the heatmap after normalization (Fig. 2), and their relative concentrations were presented in Table S2.† These volatile compounds included 13 alcohols (1-hexanol, linalool, 1-octanol, terpinen-4-ol, 2-octenol, dihydrocarveol, carveol, carotol, terpineol, trans-isopiperitenol, citronellol, nerol, and cis-carveol), eight terpenes (α-pinene, β-pinene, β-myrcene, limonene, cosmene, terpinolene, p-cymenene, and valencene), six aldehydes (hexanal, octanal, nonanal, decanal, perilla aldehyde, and citral), four esters (methyl butyrate, ethyl butyrate, octyl acetate, and perilla acetate), and one ketone (carvone). Limonene, linalool, terpinen-4-ol, terpineol, and octanal were dominant volatile compounds, especially limonene and linalool which accounted for more than 90% of the detected volatile compounds.
The loading plot of OPLS-DA on volatile compounds displayed an obvious separation among six different orange juices based on the first two principal variables with good coefficient (R2X = 0.842, R2Y = 0.867 and Q2 = 0.715) (Fig. 3B). Volk and SW were relatively close. The permutation test results indicated that y intercepts for R2 and Q2 were 0.326 and −0.467, respectively, suggesting a valid model.
3.4. Relationship between sensory attributes and flavor
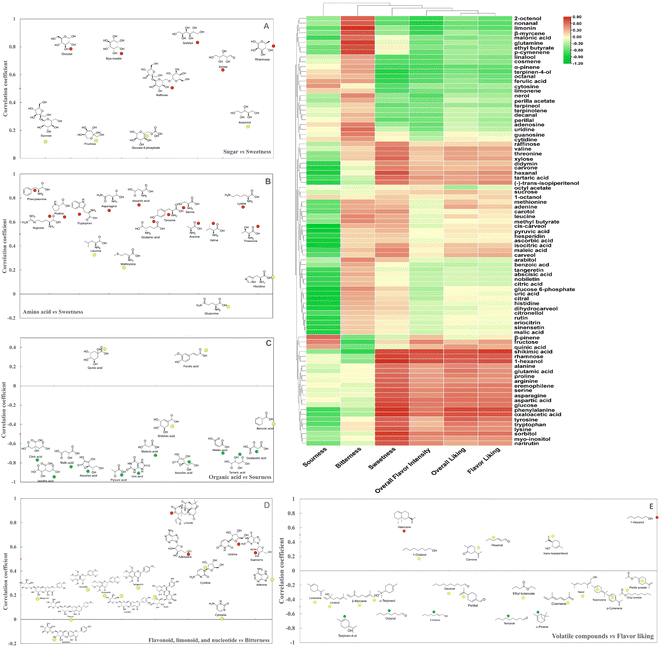
Sensory evaluation results indicated that orange juice from CH was superior to other varieties in terms of sweetness, overall liking, flavor liking, and overall flavor intensity. According to our previous studies, specific rootstock/scion combinations can make a positive contribution toward improved fruit or juice quality from HLB-affected citrus trees.12,19 Sensory quality is not a simple superposition of different tastes, but a comprehensive result from complicated interactions of various chemical components including sugar, organic acid, amino acid, flavonoids, limonoids, and volatile compounds. Canonical correlation analysis (CCA) coupled with cluster analysis was used to elucidate the relationship between sensory attributes and metabolites in six different orange juices. As shown in the heatmap (Fig. 4), sweetness was grouped with overall liking, flavor liking, and overall flavor intensity as one cluster, which was consistent with the sensory test results. Sweetness depends not only on the amounts of various sugars but also on the proportions and types of sugars. Correlation analysis indicated that raffinose, xylose, rhamnose, glucose, sorbitol, and myo-inositol showed strong contribution to sweetness (Fig. 4A). Although sucrose was the most abundant sugar in these orange juices, its concentration did not show any significant difference, indicating that sucrose could not present obvious positive correlation with sweetness. Additionally, the contents of glucose, xylose, sorbitol, and myo-inositol in CH were significantly higher than those in other varieties. Xylose, a five-carbon sugar, presents about 70% sweetness of sucrose. Rhamnose is a naturally occurring six-carbon sugar with around 33% sweetness of sucrose.20 Myo-inositol and sorbitol are two typical sugar alcohols detected in fruits. Myo-inositol with half the sweetness of sucrose provides a mild and pleasant sweetness.21 Sorbitol, perceived 60% sweetness of sucrose, can bring a cool, sweet taste due to its negative heat of solution.22 Therefore, relatively high concentration of glucose, xylose, sorbitol, and myo-inositol in CH might be involved in the chemical stimuli for sweet taste receptors clustered in taste buds on the tongue, and enhance sweetness by providing mild, pleasant, and cool taste.Amino acids are crucial components to influence orange juice sensory quality. As shown in Fig. 4B, most amino acids with the highest concentration in CH juice were closely correlated with sweetness and positive sensory attributes. Nevertheless, glutamine was implied to contribute positively to bitterness. Previous research revealed that free amino acids might improve the fruit taste in tomato and strawberry, by enhancing sweetness with alanine, serine, or proline.23 Phenylalanine, the final product of the shikimate pathway, is known as one of the most important flavor precursors in fruits.24 Even though phenylalanine, the richest amino acid in all juices, is perceived as bitter taste, its metabolism generates floral and fruity volatile compounds, which may contribute to the overall liking and flavor liking.25 Furthermore, phenylalanine, tyrosine, tryptophan, and lysine were clustered into one group with sorbitol and myo-inositol, suggesting that these amino acids might have an additive effect on the sweetness.
Organic acids are mainly involved in sourness. Although some individual organic acids levels significantly varied among six orange juices, the concentrations of major organic acids including citric acid, isocitric acid, malic acid, ascorbic acid, and quinic acid did not display any significant differences. These results were consistent with the similar sourness sensory attribute among six orange juices. Consequently, most of the organic acids did not positively correlate with sourness except for quinic acid and ferulic acid (Fig. 4C). Quinic acid, a well-known degradation product of chlorogenic acid, was reported to stimulate astringent and bitter taste, indicating that it might have a prominent effect on generation of sourness in orange juice.26 Ferulic acid, a bitter organic acid, displayed positive correlation to sourness and negative correlation to sweetness, overall liking, flavor liking, and overall intensity. It is worth mentioning that tartaric acid, shikimic acid, and oxaloacetic acid might contribute to positive sensory attributes such as sweetness, overall liking, flavor liking, and overall intensity, which further affect the consumer liking to a different extent (Fig. 4). Tartaric acid is naturally found in grapes and bananas and has been reported to enhance the beverage flavor.27 Shikimic acid is an important precursor for aromatic amino acid biosynthesis. Therefore, higher concentration of tartaric acid, shikimic acid, and oxaloacetic acid in CH juice might have a potential synergistic effect on its better flavor.
Flavonoids and limonoids are the main bitter components in orange juice according to empirical knowledge. However, in this study, flavonoids were not closely correlated with bitterness, particularly the three richest flavonoids, hesperidin, narirutin, and didymin (Fig. 4D). Previous studies on flavonoid profile affecting bitterness in different citrus species have demonstrated that the bitter species contain most flavanone neohesperidosides, while the non-bitter species contain mostly flavanone rutinosides, which are tasteless. Although both these two flavanone classes are diglycosides consisting of a rhamnose-glucose disaccharide attached at the C-7 position of flavanone. In bitter neohesperidosides, rhamnose is linked with glucose moiety via the hydroxy at the C-2 position, while in the tasteless rutinosides, rhamnose is attached via the hydroxy group at the C-6 position of the glucose unit. The attachment position of rhamnose is the determinant of the bitter flavor in these flavanones.28
Thus, five flavanone rutinosides including hesperidin, didymin, narirutin, eriocitrin and rutin did not positively contribute to bitterness. Moreover, three polymethoxyflavones (nobiletin, sinensetin, and tangeretin) were slightly correlated with bitterness. Studies on the sensation of bitterness in citrus samples demonstrated that the taste of bitterness in orange juice triggered by flavonoids is referred to as primary or immediate bitterness, while the bitterness caused by limonoids is often perceived as delayed bitterness. Limonin is known to be the off-flavor component in HLB-affected fruit or juice.12 Consequently, limonin showed a strong contribution to bitterness in our study. Nucleotides are rarely reported in orange juice, however, in this study, the bitter attribute was closely correlated with uridine, adenosine, guanosine, and cytidine.
The volatile compounds were clustered into different groups and showed complicated pattern correlations to sensory attributes to different extents, as displayed from the heatmap (Fig. 4). Similarly, much lower correlations were observed between the major volatile compounds and flavor liking attribute (Fig. 4E). Several terpenoids including α-pinene, nerol, β-myrcene, perilla acetate, terpineol, terpinolene, perillal, linalool, cosmene, terpinen-4-ol, positively correlated with the bitterness. Terpenes are usually linked with terpene-like attributes, and they enhance bitterness and negatively influence the desirable attributes. These results were consistent with our previous study, which attributed to the congruency effects between aromas and tastants.20 Meanwhile, carvone, hexanal, 1-hexanol (−)-trans-isopiperitenol were grouped with tartaric acid, didymin, shikimic acid, rhamnose, displaying positive contribution to pleasant sensory attributes. Commonly reported in orange juice, hexenal is a grassy aldehyde while 1-hexanol is believed to contribute a fruity-green note. Both carvone and trans-isopiperitenol have the mint odor. However, some volatile compounds such as limonene, linalool, β-myrcene, and α-terpineol manifested poor correlation with sensory attributes, which may be related to their flavor complexity. Further investigation is needed to make a better clarification.
3.5. Effects of different rootstocks on metabolites of orange juices
Based on targeted flavoromics analysis, the total amounts of amino acids, sugars, sugar alcohols, and flavonoids in CH were higher than those in other varieties (Fig. 5). More specifically, the levels of several major metabolites including proline, arginine, tryptophan, glucose, myo-inositol, hesperidin, and didymin were significantly higher in CH than in other samples. CH, Blue, and SW showed higher organic acid concentrations than the other three samples. The content of total nucleotides in SW was higher than the other samples, followed by 1804 (Fig. 5). In addition, the highest limonin concentration was observed in SW.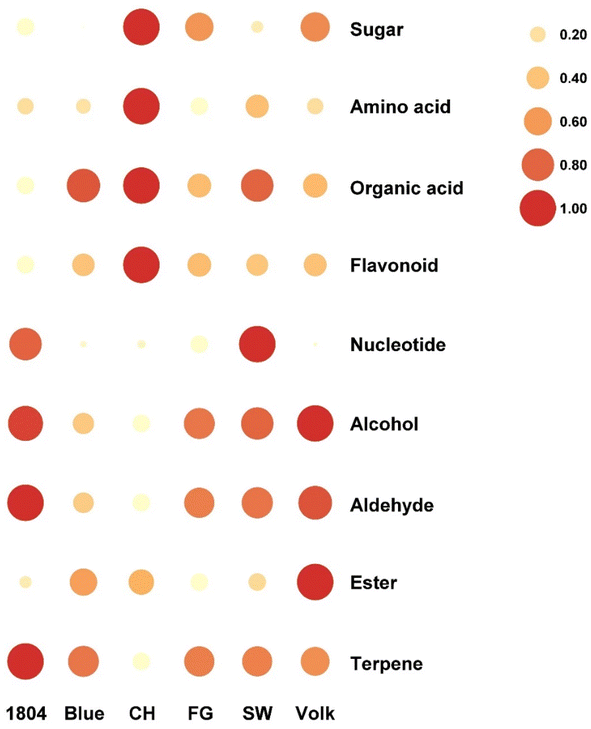 | ||
| Fig. 5 Relative concentrations (after normalization) of different categories metabolites in six orange juices. | ||
As for volatile compounds, the orange juice from rootstock CH contained the lowest volatile compounds concentration, especially the major representative compounds limonene and linalool. Further comparison of different volatile compound categories suggested that 1804 had higher amounts of aldehydes and terpenes than other samples, especially octanal and limonene. Volk showed the highest levels of alcohol and ester. Basically, no obvious correlation between the volatile compound concentration and juice flavor quality was observed. The sensory quality of juice is not dependent on the volatile compound quantity, but rather on the volatile compound quality. For example, esters could enhance the sweetness perception, overall flavor, and overall liking. Therefore, the synergistic effect of the esters in CH cannot be ignored. On the other hand, most terpenes contribute to the grassy or earthy notes which could be negatively correlated with overall liking. Briefly, the above results indicated that different rootstocks could affect the juice quality by altering the content of metabolites. Interestingly, effects of HLB on juice quality were not correlated with effects on overall tree health or productivity, as the most sensitive rootstock in poor health produced the best quality juice.
KEGG enrichment pathway analysis was conducted with the selected marker compounds based on the OPLS-DA (Table S3†). Three pathways including aminoacyl-tRNA biosynthesis, ABC transporters, and monoterpenoid biosynthesis showed high matching with low p-values and FDR correction values, indicating their close relationship with rootstock discrimination (Table 2). Eight amino acids were involved in aminoacyl-tRNA biosynthesis and ABC transporters pathways, respectively. The aminoacyl-tRNA synthesis pathway plays an important role in protein synthesis by linking amino acids to their cognate transfer RNAs.29 ABC transporters are essential for plant development, playing roles in processes such as gametogenesis, seed development, seed germination, organ formation, and secondary growth.30 Both pathways were related to amino acid biosynthesis, and these results were well-matched with our previous study indicating their important role in the effect on metabolism of orange trees by different scion/rootstock combinations.12
4. Conclusion
In this study, the relationship between the sensory attributes and metabolites of orange juices from six different rootstocks were investigated using a flavoromics strategy. CCA indicated that raffinose, xylose, rhamnose, glucose, sorbitol, and myo-inositol showed strong contribution to sweetness, and alanine, glutamic acid, proline, arginine, serine, asparagine, and aspartic acid were also responsible for positive flavor quality. These results showed that rootstock CH could enhance accumulation of some specific amino acids, sugars, and flavonoids, resulting in better sensory quality. The synergistic effect of volatile compounds with low concentrations in CH cannot be ignored. Overall, the effect of different rootstocks on flavor quality of orange juice is a complicated phenomenon that greatly depends on interactions between rootstock and scion. Examining data regarding different rootstock effects on the metabolite profiles and sensory attributes of orange juice, may provide insight into the importance of rootstock selection for HLB resistance or tolerance. Although we focused on the common flavor compounds in this study, the comprehensive profile on the metabolites from different rootstocks is still unknown, especially some specific metabolites involved in HLB. Therefore, untargeted metabolomics will be conducted to detect more metabolites to explore the effects of different rootstocks on fruit quality and plant defense in later studies.Author contributions
Xin Liu: conducted experiments, data analysis, writing – original draft. Frederick G. Gmitter Jr: resources, revision, supervision. Jude W. Grosser: resources, revision, supervision. Yu Wang: designing experiments, revision, supervision.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This work was funded by USDA National Institute of Food and Agriculture (USDA-NIFA 2017-70016-26328). The authors are thankful to Joon Hyuk Suh for assistance with the instrumental analysis.References
- N. Wang, The citrus huanglongbing crisis and potential solutions, Mol. Plant, 2019, 12, 607–609 CrossRef CAS PubMed.
- A. J. F. Diniz, A. G. Garcia, G. R. Alves, C. Reigada, J. M. Vieira and J. R. P. Parra, The enemy is outside: Releasing the parasitoid Tamarixia radiata (Hymenoptera: Eulophidae) in external sources of HLB inocula to control the Asian citrus psyllid Diaphorina citri (Hemiptera: Liviidae), Neotrop. Entomol., 2020, 49, 250–257 CrossRef CAS PubMed.
- A. Plotto, E. Baldwin, G. McCollum, J. Manthey, J. Narciso and M. Irey, Effect of Liberibacter infection (Huanglongbing or “Greening” disease) of citrus on orange juice flavor quality by sensory evaluation, J. Food Sci., 2010, 75, S220–S230 CrossRef CAS PubMed.
- J. Kiefl, B. Kohlenberg, A. Hartmann, K. Obst, S. Paetz, G. Krammer and S. Trautzsch, Investigation on key molecules of Huanglongbing (HLB)-induced orange juice off-flavor, J. Agric. Food Chem., 2017, 66, 2370–2377 CrossRef PubMed.
- W. Hung and Y. Wang, Metabolite profiling of Candidatus Liberibacter infection in Hamlin sweet oranges, J. Agric. Food Chem., 2018, 66, 3983–3991 CrossRef CAS PubMed.
- R. B. Bassanezi, S. A. Lopes, M. P. de Miranda, N. A. Wulff, H. X. L. Volpe and A. J. Ayres, Overview of citrus huanglongbing spread and management strategies in Brazil, Trop. Plant Pathol., 2020, 45, 251–264 CrossRef.
- Z. Tietel, S. Srivastava, A. Fait, N. Tel-Zur, N. Carmi and E. Raveh, Impact of scion/rootstock reciprocal effects on metabolomics of fruit juice and phloem sap in grafted Citrus reticulata, Plos One, 2020, 15, e0227192 CrossRef CAS PubMed.
- J. Syvertsen and Y. Levy, Salinity interactions with other abiotic and biotic stresses in citrus, HortTechnology, 2005, 15, 100–103 Search PubMed.
- H. Shokrollah, T. L. Abdullah, K. Sijam and S. N. A. Abdullah, Potential use of selected citrus rootstocks and interstocks against HLB disease in Malaysia, Crop Prot., 2011, 30, 521–525 CrossRef.
- G. McCollum and K. D. Bowman, Rootstock effects on fruit quality among ‘Ray Ruby’grapefruit trees grown in the Indian River District of Florida, HortScience, 2017, 52, 541–546 Search PubMed.
- F. A. de Azevedo, T. F. Milaneze, P. M. da Conceição, C. de Andrade Pacheco, R. Martinelli and M. Bastianel, Winter pruning: option for management against alternaria brown spot ('Alteraria alternata'f. sp.'citri') in Honey Murcott tangor ['Citrus reticulata' Blanco x 'C. sinensis'(L.) Osbeck], Aust. J. Crop Sci., 2019, 13, 1631–1637 CAS.
- L. Huang, J. Grosser, F. G. Gmitter Jr., C. A. Sims and Y. Wang, Effects of scion/rootstock combination on flavor quality of orange juice from Huanglongbing (HLB)-affected trees: a two-year study of the targeted metabolomics, J. Agric. Food Chem., 2020, 68, 3286–3296 CrossRef CAS PubMed.
- I. Ronningen, M. Miller, Y. Xia and D. G. Peterson, Identification and validation of sensory-active compounds from data-driven research: a flavoromics approach, J. Agric. Food Chem., 2017, 66, 2473–2479 CrossRef PubMed.
- T. Feng, M. Shui, S. Song, H. Zhuang, M. Sun and L. Yao, Characterization of the key aroma compounds in three truffle varieties from China by flavoromics approach, Molecules, 2019, 24, 3305 CrossRef CAS PubMed.
- X. Song, G. Wang, L. Zhu, F. Zheng, J. Ji, J. Sun, H. Li, M. Huang, Q. Zhao, M. Zhao and B. Sun, Comparison of two cooked vegetable aroma compounds, dimethyl disulfide and methional, in Chinese Baijiu by a sensory-guided approach and chemometrics, LWT, 2021, 146, 111427 CrossRef CAS.
- Y. Wang, P. Yu, J. Sun, Y. Jia, C. Wan, Q. Zhou and F. Huang, Investigation of volatile thiol contributions to rapeseed oil by odor active value measurement and perceptual interactions, Food Chem., 2022, 373, 131607 CrossRef PubMed.
- S. Feng, L. Niu, J. H. Suh, W. L. Hung and Y. Wang, Comprehensive metabolomics analysis of mandarins (Citrus reticulata) as a tool for variety, rootstock, and grove discrimination, J. Agric. Food Chem., 2018, 66, 10317–10326 CrossRef CAS PubMed.
- J. Xia and D. S. Wishart, Web-based inference of biological patterns, functions and pathways from metabolomic data using MetaboAnalyst, Nat. Prot., 2011, 6(6), 743–760 CrossRef CAS PubMed.
- L. Reuss, S. Feng, W. L. Hung, Q. Yu, F. G. Gmitter Jr and Y. Wang, Analysis of flavor and other metabolites in lemon juice (Citrus limon) from Huanglongbing-affected trees grafted on different rootstocks, J. Food Drug Anal., 2020, 28, 67–78 Search PubMed.
- S. Feng, F. G. Gmitter Jr, J. W. Grosser and Y. Wang, Identification of key flavor compounds in citrus fruits: a flavoromics approach, ACS Food Sci. Technol., 2021, 1(11), 2076–2085 CrossRef CAS.
- H. S. Lee and G. A. Coates, Quantitative study of free sugars and myo-inositol in citrus juices by HPLC and a literature compilation, J. Liq. Chromtogr. Relat. Technol., 2000, 23, 2123–2141 CrossRef CAS.
- W. Zhang, J. Chen, Q. Chen, H. Wu and W. Mu, Sugar alcohols derived from lactose: lactitol, galactitol, and sorbitol, Appl. Microbiol. Biotechnol., 2020, 104, 9487–9495 CrossRef CAS PubMed.
- A. J. Keutgen and E. Pawelzik, Contribution of amino acids to strawberry fruit quality and their relevance as stress indicators under NaCl salinity, Food Chem., 2008, 111(3), 642–647 CrossRef CAS.
- N. T. Hall, J. M. Smoot, R. J. Knight Jr and S. Nagy, Protein and amino acid compositions of ten tropical fruits by gas-liquid chromatography, J. Agric. Food Chem., 1980, 28, 1217–1221 CrossRef CAS PubMed.
- S. Masuo, L. Osada, S. Zhou, T. Fujita and N. Takaya, Aspergillus oryzae pathways that convert phenylalanine into the flavor volatile 2-phenylethanol, Fungal Genet. Biol., 2015, 77, 22–30 CrossRef CAS PubMed.
- Q. Frank, G. Zehentbauer and T. Hofmann, Bioresponse-guided decomposition of roast coffee beverage and identification of key bitter taste compounds, Eur. Food Res. Technol., 2006, 222, 492–508 CrossRef.
- S. S. Samant, P. G. Crandall, S. E. Jarma Arroyo and H. S. Seo, Dry pet food flavor enhancers and their impact on palatability: a review, Foods, 2021, 10(11), 2599 CrossRef CAS PubMed.
- J. J. Peterson, J. T. Dwyer, G. R. Beecher, S. A. Bhagwat, S. E. Gebhardt, D. B. Haytowitz and J. M. Holden, Flavanones in oranges, tangerines (mandarins), tangors, and tangelos: a compilation and review of the data from the analytical literature, J. Food Compos. Anal., 2006, 19, S66–S73 CrossRef CAS.
- P. B. Moutiez and M. Gondry, Aminoacyl-tRNA-utilizing enzymes in natural product biosynthesis, Chem. Rev., 2017, 117, 5578–5618 CrossRef PubMed.
- T. H. T. Do, E. Martinoia and Y. Lee, Functions of ABC transporters in plant growth and development, Curr. Opin. Plant Biol., 2018, 41, 32–38 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ra08182b |
| This journal is © The Royal Society of Chemistry 2023 |