DOI:
10.1039/D2RA08287J
(Paper)
RSC Adv., 2023,
13, 6954-6965
Hybrid carbonaceous adsorbents based on clay and cellulose for cadmium recovery from aqueous solution
Received
28th December 2022
, Accepted 17th February 2023
First published on 28th February 2023
Abstract
The current work describes the synthesis of carbonaceous composites via pyrolysis, based on CMF, extracted from Alfa fibers, and Moroccan clay ghassoul (Gh), for potential use in heavy metal removal from wastewater. Following synthesis, the carbonaceous ghassoul (ca-Gh) material was characterized using X-ray fluorescence (XRF), Scanning Electron Microscopy coupled with Energy Dispersive X-ray (SEM-EDX), zeta-potential and Brunauer–Emmett–Teller (BET). The material was then used as an adsorbent for the removal of cadmium (Cd2+) from aqueous solutions. Studies were conducted into the effect of adsorbent dosage, kinetic time, initial concentration of Cd2+, temperature and also pH effect. Thermodynamic and kinetic tests demonstrated that the adsorption equilibrium was attained within 60 min allowing the determination of the adsorption capacity of the studied materials. The investigation of the adsorption kinetics also reveals that all the data could be fit by the pseudo-second-order model. The Langmuir isotherm model might fully describe the adsorption isotherms. The experimental maximum adsorption capacity was found to be 20.6 mg g−1 and 261.9 mg g−1 for Gh and ca-Gh, respectively. The thermodynamic parameters show that the adsorption of Cd2+ onto the investigated material is spontaneous and endothermic.
1. Introduction
All life on Earth depends on water, a renewable natural resource; due to overuse and contamination, fresh water is becoming increasingly rare.1 Worldwide, water contamination causes illness and numerous deaths.2 It is an issue in both developed and developing nations. The main threat to water quality are activities like mining, urban development, and agriculture.3 Pollutants can have a variety of sorts and characteristics such as non-biodegradable polymers synthetic chemicals, organic and inorganic dyes, and heavy metals, which have no or very little adsorptive potential.4 These contaminants build up in the environment over time, as their number or quantity grows, so does their harm. It has been reported that oral exposure to heavy metals such as Cd has been found to target organ toxicity, including unfavourable health effects on neurological, reproductive, cardiovascular, gastrointestinal, renal, musculoskeletal, and cutaneous systems.5,6 Cd ions are produced in large quantities by industries such as ceramics, batteries, metallurgy, electroplating as well as mining.7 The concentration of this hazardous heavy metal must therefore be eliminated or reduced in the aquatic environment. Several treatment methods, including adsorption, membrane separation,8 coagulation–flocculation,9 osmosis,10 and chemical precipitation11 have been used to get rid of harmful metal ions. Adsorption is a common treatment method because it is affordable, easy to use, and especially environmentally friendly.12–15 In this context, more efficient approach must be investigated instead of the more popular typically expensive treatment methods. Clay has been the subject of various studies in order to develop potential adsorbents.16–18 One of the clays that caught the interest of researchers was ghassoul. It is an abundant Moroccan stevensite and its use in water treatment applications was the subject of numerous investigations.16,19 It was mentioned that stevensite can exchange the cations stored in its aluminosilicate structure for cations from an external solution through the process of ion exchange.20 The effectiveness of Ghassoul in adsorbing malachite green and basic yellow 28 from an aqueous solution is already discussed in the literature.21 Another study looked at this Moroccan clay's ability to remove cationic dye from aqueous solution.22 Ghassoul has also proved its potential in removing heavy metals.23–25 On the other side, the use of variety of biopolymers for the removal of heavy metals as adsorbent from wastewater has also attracted great attention. Due to their affordability, effectiveness and biodegradability, lignin, cellulose, chitin and alginate-based adsorbents have been employed widely, as it’s the case in those research.26–30 Those biopolymers have a variety of functional groups which improve the effectiveness of removing heavy metals from water samples.31–35 Cellulose can be found in wood and cotton; however, many other resources have been investigated to resolve environmental concerns.28,36,37 Dong Shen Tong et al. reported in their work38 that recently, a new class of adsorbents has emerged, consisting of carbonaceous materials with numerous oxygen-containing functional groups anchored on the surface of solid surface. The challenges produced by excessive levels of pollution demonstrate the necessity of research to reduce the damage. The quest for solids that function as highly polluting heavy metals is an ongoing activity in the context of adsorption. Finding low-cost, abundant, and environmentally friendly adsorbents is essential. To our knowledge, no research has previously been done on the potential for cellulose and ghassoul combined to create a low-cost adsorbent material. Therefore, this combination could be very interesting by providing better adsorbent qualities such as a larger surface area, higher adsorption efficiency. One of the primary goals of investigating a contaminant's adsorption process is to determine the optimum operating parameters. Numerous studies were conducted to identify the quantities or values required for the various factors to achieve optimal removal efficiency. pH, temperature, contact time, adsorbent mass, and adsorbate concentration are among the characteristics considered. In this work, pyrolysis will be used for the synthesis of a novel material based on ghassoul combined with cellulose. The created material will be fully characterized using a variety of techniques. Also understanding the behaviour of the adsorbent toward cadmium ions in aqueous solutions is going to be investigated by varying various experimental conditions.
2. Materials and methods
2.1. Materials
The clay used in this work was a commercial ghassoul (Gh), washed with distilled water, dried, milled and sieved in a stainless-steel sieve of diameters of 300 μm, then it was used for synthesis of the composite materials. Raw Alfa Fibers (Stipa Tenacissima plant) (RF) used in this work were collected from the oriental region of eastern Morocco. Cadmium sulfate (CdSO4) and the chemicals used for the cellulose micro-fibers (CMF) extraction were all purchased from Sigma-Aldrich.
2.2. Extraction of cellulose microfibers and preparation of the carbonaceous composite
CMF were extracted from Raw Alfa fibers (RF) through an alkali treatment followed by a bleaching process. For the alkali treatment, the ratio of fibers to liquor was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 (g mL−1), the grind RF were treated once with 10 wt% NaOH solution at 80 °C for 2 hours under mechanical stirring giving rise to ATF. ATF were followed by a bleaching treatment which was carried out using a solution made up of equal parts (v/v) of acetate buffer (27 g NaOH and 75 mL glacial acetic acid, diluted to 1 L of distilled water) and aqueous sodium chlorite (1.7 wt% NaClO2 in water). This treatment was done once for 2 hours at 80 °C under mechanical stirring, resulting in white CMF. The first step of the synthesis of the carbonaceous composite was the mixing of the two materials, for that we used solution mixing technique. As shown in the Fig. 1, a distinct quantity of 7.5 g of the extracted CMF was well dispersed in distilled water for several minutes, followed by the addition of 22.5 g of Gh to the mixture. After two hours of continuous magnetic stirring, the solution was freeze at −80 °C then dried by a freeze drier for 48 hours. The freeze-drying method has been chosen in order to ensure the adherence and the good dispersion of the fibers with the clay. Right forward, the pyrolysis process was carried out for two hours straight at 700 °C under argon (5°C min−1). 19.39 g of carbonaceous ghassoul (ca-Gh) was obtained after pyrolysis.
20 (g mL−1), the grind RF were treated once with 10 wt% NaOH solution at 80 °C for 2 hours under mechanical stirring giving rise to ATF. ATF were followed by a bleaching treatment which was carried out using a solution made up of equal parts (v/v) of acetate buffer (27 g NaOH and 75 mL glacial acetic acid, diluted to 1 L of distilled water) and aqueous sodium chlorite (1.7 wt% NaClO2 in water). This treatment was done once for 2 hours at 80 °C under mechanical stirring, resulting in white CMF. The first step of the synthesis of the carbonaceous composite was the mixing of the two materials, for that we used solution mixing technique. As shown in the Fig. 1, a distinct quantity of 7.5 g of the extracted CMF was well dispersed in distilled water for several minutes, followed by the addition of 22.5 g of Gh to the mixture. After two hours of continuous magnetic stirring, the solution was freeze at −80 °C then dried by a freeze drier for 48 hours. The freeze-drying method has been chosen in order to ensure the adherence and the good dispersion of the fibers with the clay. Right forward, the pyrolysis process was carried out for two hours straight at 700 °C under argon (5°C min−1). 19.39 g of carbonaceous ghassoul (ca-Gh) was obtained after pyrolysis.
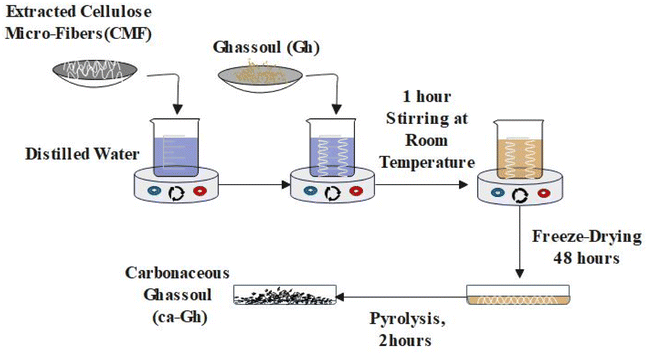 |
| | Fig. 1 Schematic representation of the synthesis of ca-Gh. | |
2.3. Characterization techniques
Fourier transform infrared spectra (FTIR) was used to identify the functional groups present in the samples using PerkinElmer Spectrum 2000 FTIR. The experiments were carried out in the range of 4000 to 600 cm−1 and an accumulation of 16 scans. Bruker diffractometer was used in this work to identify the crystalline structure of the samples using a monochromatic CuKα radiation at λ = 1.54056 Å in the range of 2θ = 5°–40° with a scanning rate of 2° min−1. The crystallinity index (CrI) was calculated using the Segal equation from the XRD data:| |
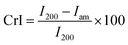 | (1) |
where I200 refers to the principal intensity of the crystalline portion of cellulose and Iam is the intensity of the amorphous portion of cellulose and other constituents.
The basal spacing of the clay was calculated according to the Bragg equation:
| |
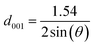 | (2) |
where
θ represents the incident angle and
d001 stands for the clay basal spacing.
The chemical composition of the clay was determined by X-ray fluorescence spectrometry (XRF) using Malvern Panalytical Epsilon 4 dispersive spectrometer. Discovery TGA, TA instruments, was used to evaluate the thermal stability of the materials. 5–10 mg of the samples were heated to 700 °C under a nitrogen atmosphere at a heating rate of 10 °C min−1. Zeiss Evo 10 scanning electron microscopy (SEM) connected to an Energy Dispersive X-ray spectroscope (EDX) was used to examine the morphology of the materials and to determine the elements on the surface of the samples. The sample's surface was coated with a thin conductive gold layer using sputtering apparatus. GEMINI VII 2390 Instrument was used to measure the specific surface area and also adsorption/desorption isotherm (BET) of the samples using the adsorption of N2 at the temperature of liquid nitrogen, the materials were both degassed at 200 °C for 12 hours before the analysis. For the zeta potential measurement, a 1 g L−1 clay suspension after two minutes of sonication was stirred for 1 hour long. After decantation, the zeta potential is determined from the supernatant using Malvern Zetasizer Nano ZS.
2.4. Adsorption study
ca-Gh adsorbent was tested for its adsorption efficiency of cadmium ions (Cd2+) in response to the initial Cd2+ concentration, stirring time, temperature, masse of the adsorbent and pH of the medium effect. For this purpose, 15 mg of the adsorbent was added to a container containing 15 mL of 100 mg L−1 of Cd2+ solution. The containers were stirred at room temperature and then the solution is analysed using an atomic absorption spectrometer (Agilent Technologies 200 Series AA). The amount of Cd2+ ions adsorbed per gram of adsorbent (qe) was calculated using the following equation:| |
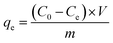 | (3) |
where C0 stands for initial concentration of Cd2+ (mg L−1), Ce: Cd2+ concentration at equilibrium time (mg L−1), m: dry adsorbent's mass (g) and V: total volume of suspension (L).
2.4.1. Temperature effect. Different thermodynamic parameters were investigated in order to analyse and understand the adsorption process. For this study, the temperature of the experience varied from T = 25 °C to T = 40 °C. The Van't Hoff equation was used to determine the thermodynamic parameters, including the changes in standard enthalpy (H), standard entropy (S), and standard free energy (G).39| |
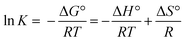 | (4) |
T is the solution's temperature (K), K represents the equilibrium constant or linear adsorption distribution coefficient, and R refers to the gas constant (R = 8.314 J (mol−1 K−1)). ΔH° and ΔS° are calculated from the slope and intercept of the plot of ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K vs. 1/T, respectively.
K vs. 1/T, respectively.
2.4.2. Kinetic and equilibrium modelling. For the kinetic study, the mass of the adsorbent and the concentration of Cd2+ was kept constant, m = 15 mg and Ci = 100 mg L−1, respectively, while varying the time from t = 30 min to t = 360 min. To evaluate the efficiency of ca-Gh in removing Cd2+ ions and to determine the mechanisms of the adsorption process, the experimental data were fitted using two kinetic models: pseudo-first-order (eqn (5)) and pseudo second-order (eqn (6)) models.40 qt (mg g−1) and qe (mg g−1) stand for the amount of Cd2+ adsorbed at t time and equilibrium time, respectively. For pseudo-first-order and pseudo-second-order kinetic models, K1 and K2 are the rate constant, respectively.| |
log(qe − qt) = log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe − k1t qe − k1t
| (5) |
| |
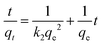 | (6) |
For the isotherm investigation, the only parameter that was varying is the initial concentration of Cd2+, from C = 20 mg L−1 to C = 600 mg L−1. Langmuir (eqn (7)) and Freundlich (eqn (8)) models were used to investigate the isotherms data.41 In terms of adsorption capacity, qe is the maximum Cd2+ uptake per mass of the adsorbent (mg g−1), KL is the Langmuir constant (L mol−1), which is related to the energy of the adsorption, KF is the Freundlich constant, and n describes the adsorption intensity.
| |
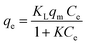 | (7) |
3. Results and discussion
3.1. Characterization
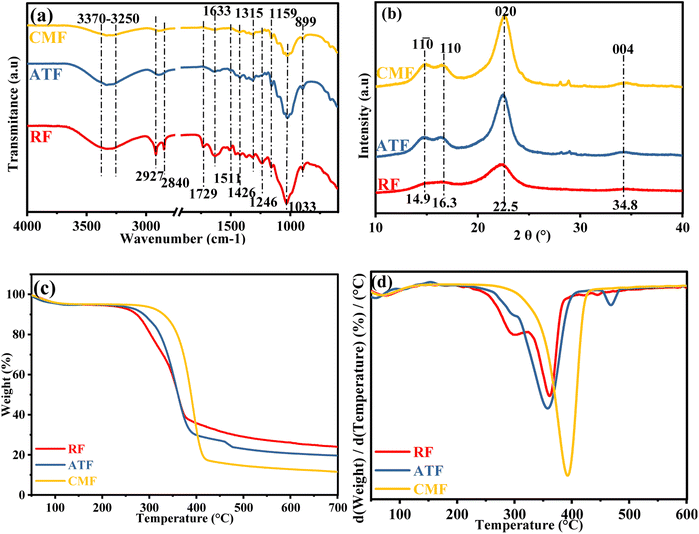
3.1.1. Cellulose microfibers characterization. The extraction yield from RF to ATF was found to be 48.46%, and from ATF to CMF, the yield is 82.51%. The chemical composition and the surface functionality of materials at different phases of the treatment were investigated using FTIR spectroscopic technique. Fig. 2(a) shows the FTIR spectra of RF, ATF, and CMF. The broad peaks observed at 3370–3250 cm−1 are attributed to hydroxyl groups O–H and aliphatic C–H stretching vibrations present in lignocellulosic materials.42,43 The peaks at 1511 cm−1 and 1246 cm−1 correspond to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibration from the aromatic ring and C–O–C out of plane stretching vibration of the aryl group in lignin and hemicelluloses. The peak at 1729 cm−1 can be assigned to the acetyl and uronic ester groups of hemicellulose or the ester linkage of carboxylic group of fluoric and p-coumaric acids of lignin and/or hemicellulose.44 As it can be noticed, the mentioned peaks were not detected in the treated fibers spectra, which can suggest the successful removal of the lignin and hemicellulose after the alkali and bleaching treatment. The peak at 2927 cm−1 and 2840 cm−1 are assigned to methylene group and symmetric stretch of CH3 of methoxyl group respectively. The peak detected at 1633 cm−1 is caused by the bending mode of the adsorbed water.42 The peak at 1159 cm−1 corresponds to C–O–C asymmetric stretching of the cellulose as was reported in this work.42 The gradual increasing intensity of the mentioned peak indicates that the cellulose content was increased after the alkali and bleaching treatment.44
C stretching vibration from the aromatic ring and C–O–C out of plane stretching vibration of the aryl group in lignin and hemicelluloses. The peak at 1729 cm−1 can be assigned to the acetyl and uronic ester groups of hemicellulose or the ester linkage of carboxylic group of fluoric and p-coumaric acids of lignin and/or hemicellulose.44 As it can be noticed, the mentioned peaks were not detected in the treated fibers spectra, which can suggest the successful removal of the lignin and hemicellulose after the alkali and bleaching treatment. The peak at 2927 cm−1 and 2840 cm−1 are assigned to methylene group and symmetric stretch of CH3 of methoxyl group respectively. The peak detected at 1633 cm−1 is caused by the bending mode of the adsorbed water.42 The peak at 1159 cm−1 corresponds to C–O–C asymmetric stretching of the cellulose as was reported in this work.42 The gradual increasing intensity of the mentioned peak indicates that the cellulose content was increased after the alkali and bleaching treatment.44
 |
| | Fig. 2 (a) FTIR spectra, (b) XRD pattern, (c) TGA and (d) DTG curve of RF, ATF and CMF. | |
Fig. 2(b) shows the XRD profile of the raw and treated fibers. From this result, it can be noticed that all cellulosic materials show distinctive peaks of the cellulose crystalline structure type I around 14.9°, 16.3° and 22.5°, corresponding to the crystallographic planes at 1![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0, 110 and 020, respectively.42 As it was expected, after the chemical treatment, the magnitude of the observed crystalline peaks increased, which is due to the removal of amorphous lignin and hemicellulose. It is noteworthy to notice that the crystal structure of the cellulosic component was not altered throughout the alkali and bleaching treatment. The crystallinity index (CrI) was found to be about 58%, 79% and 82% for RF, ATF and CMF samples, respectively. The increase of CrI as the process goes on confirms the elimination of the amorphous phases.45
0, 110 and 020, respectively.42 As it was expected, after the chemical treatment, the magnitude of the observed crystalline peaks increased, which is due to the removal of amorphous lignin and hemicellulose. It is noteworthy to notice that the crystal structure of the cellulosic component was not altered throughout the alkali and bleaching treatment. The crystallinity index (CrI) was found to be about 58%, 79% and 82% for RF, ATF and CMF samples, respectively. The increase of CrI as the process goes on confirms the elimination of the amorphous phases.45
As mentioned in the literature, the thermal decomposition of lignocellulosic materials starts at a lower temperature for hemicellulose in general, followed by an early stage of degradation of lignin molecules, and then by the decomposition of cellulose.46 It can be noticed from the results presented in Fig. 2(c) and (d). Due to the hydrophilic nature of the cellulosic materials, all the samples show a small weight loss of around 100 °C due to the evaporation of absorbed moisture.47 RF undergoes a two-step degradation which is confirmed by the presence of two peaks in the DTG curve, the first degradation occurred at 279 °C and the second one at 340 °C, the first degradation is attributed to the decomposition of hemicellulose and lignin, the second degradation is attributed to the decomposition of cellulose. It's noteworthy to mention that the degradation of the ATF and CMF samples follows an almost identical degradation behaviour, as shown in TGA and DTG curve, indicating that most of the hemicellulose and lignin were removed after the alkali and bleaching treatment.48
SEM was used to analyse the morphology of RF, ATF, and CMF. Fig. 3 demonstrates significant changes in RF after the alkali treatment and bleaching. Untreated RF Fig. 3(a) exhibited a strongly bounded fiber with rough compact surface, whereas ATF appears separated into bundled individual micro-sized structures in Fig. 3(b). In Fig. 3(c), single microfibers with a clean and smooth surface are individualized. The changes in the fiber morphology indicate that the hemicellulose and lignin were successfully removed during the chemical treatments.
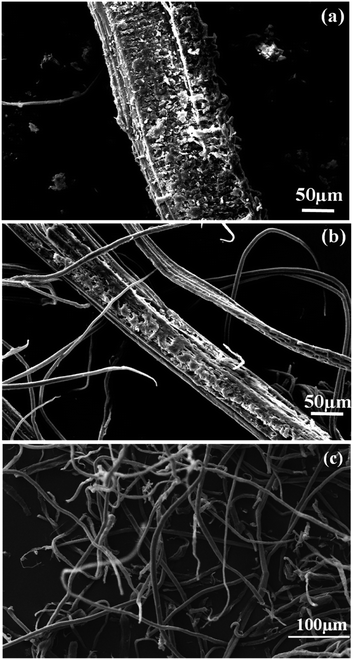 |
| | Fig. 3 SEM images of (a) RF, (b) ATF and (c) CMF. | |
3.1.2. Clay and composite characterization. ca-Gh composite has been prepared simply, which molecular structure was characterized by XRD, zeta-potential, BET, EDX and SEM to provide a theoretical basis for subsequent adsorption experimental results. According to the XRD patterns of Gh and ca-Gh shown in Fig. 4(a), the raw clay appeared to be mainly composed of the clay mineral stevensite (S), with a distinctive peak at d001 = 15.27 Å. dhkl was determined using the Bragg equation. The other peaks are attributed to the associated minerals quartz (Q) and dolomite (D), as previously reported.49 The interfoliar space decrease as it was d001 = 15.27 Å and as a result to the thermal treatment it became d100 = 9.93 Å. The XRD pattern shows few major changes as a result of the heat treatment, such as the disappearance and the weakening of the Q and D peaks, also additional new peaks appear to signify dolomite breakdown. Peaks that correspond to CaCO3 are formed by the carbonisation of CaO, which becomes relatively unstable after MgO is depleted.50 Mohamed et al. also came to the same conclusion that dolomite beaks down into CaCO3 and MgCO3.51
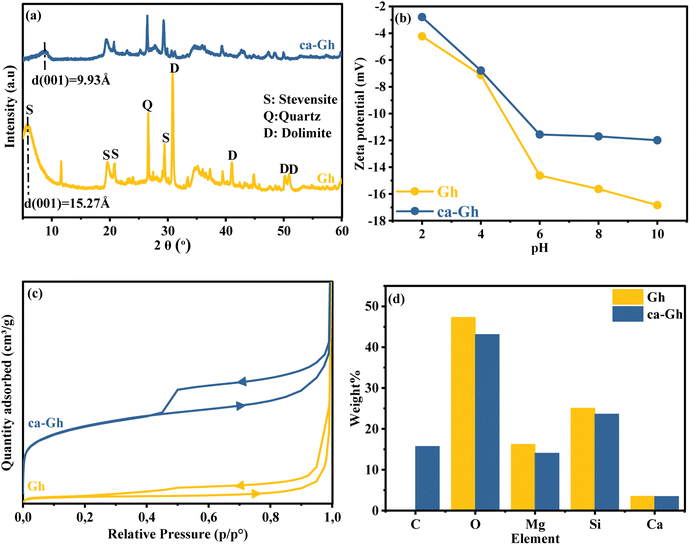 |
| | Fig. 4 (a) XRD pattern, (b) zeta potential, BET adsorption/desorption isotherm of Gh (c) and ca-Gh, and (d) EDX of Gh and ca-Gh. | |
The surface charge of an adsorbent material generally has the strongest effect on its effectiveness.52 Therefore, the zeta potential was measured under various pH levels in order to determine the surface charge properties of Gh and ca-GH, as shown in Fig. 4(b). Due to the presence of SiO2 groups, Gh and ca-Gh, both exhibit a negative charge over the whole pH range studied. The observed results show that the zeta-potential rose with pH value, indicating good electronegativity at higher pHs. Fig. 4(c) show the results of the adsorption/desorption isotherms from the BET analysis, it can be concluded from these results that both materials display the isotherm type II characteristics with hysteresis loops (type H3). This type of isotherm was also observed in other clay-based materials, which is resulted from the aggregates with macro/mesopores, even though only the initial monolayer–multilayer segment of the isotherm is reversible.53 EDX results displayed in Fig. 4(d) reveals the elements present in the surface of Gh and ca-Gh, the oxygen, magnesium, silica and calcium are the main elements in the composition of the clay mineral stevensite, the carbon in ca-Gh is generated from the carbonized fibers of CMF during the pyrolysis process, the initial composition of the CMF was 25%, and considering the weight loss during the thermal treatment, the qualitative value given by the EDX of carbon was 15.72%.
The result of the X-ray fluorescence analysis is in Table 1 reveals the typical composition of clays, with high content of magnesium for Gh which is a stevensite. The high silica's compositional content is a synonym of the presence of free silica and the presence of carbonate is relevant.
Table 1 Chemical composition by XRF of Gh and ca-Gh
| Clay minerals |
Chemical composition (%) |
| SiO2 |
Al2O3 |
SO3 |
CaO |
MgO |
Fe2O3 |
K2O |
Other elements |
| Gh |
62.48 |
1.77 |
2.38 |
17.20 |
10.51 |
2.72 |
1.20 |
1.29 |
According to the SEM images of ca-Gh in Fig. 5(a) and (b), we can notice that the surface of the material is extremely rough, additionally, we can see tiny particles formed which might have resulted from the exfoliation of Gh layers.54 The surface mapping displayed in Fig. 5(c) and (d)–(h) reveals the presence of all the elements and each component (O, Si, Mg, Ca, C) by itself, respectively. The surface mapping allows to observe how these elements are distributed all over the composite's surface, it also enables to confirm that the carbon was successfully deposited on the surface without changing the essential elements of the composition of the clay. After carbonisation, the BET analysis revealed that the surface area of Gh increased significally, going from 88.31 m2 g−1 to 164.97 m2 g−1 for Gh and ca-Gh, respectively.
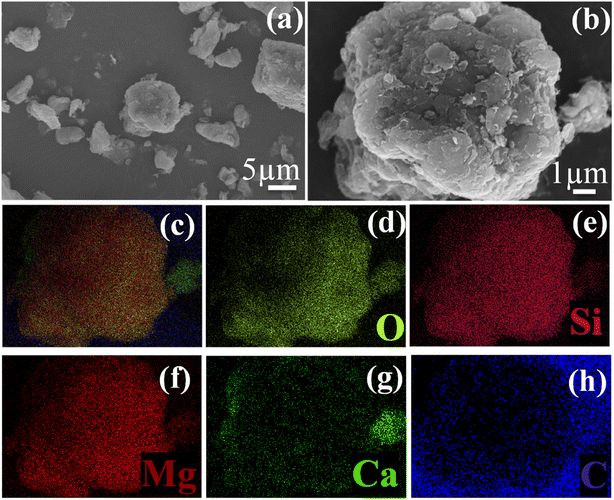 |
| | Fig. 5 (a and b) SEM images and (c–h) surface mapping of ca-Gh. | |
3.2. Adsorption study
3.2.1. pH effect. The pH value of the solution directly influences the adsorption capacity. As it can be noticed from Fig. 6(a), the quantity adsorbed for both adsorbents increased as the pH increased. According to the characterization of the ghassoul clay that have been done in this work, it was revealed that it is mainly composed of stevensite, quarts and dolomite. It is well known that the clay mineral surface has a net positive charge a pH value lower that it pHpzc and a net negative charge at pH levels higher than its pHpzc.55 According to Benhammou's work,49 the pHpzc of Gh is 2, meaning that at pH values higher, the clay's surface is negatively charged. The reduced sorption at low pH is also possibly due to the competition of H+ with the cations for the adsorption sites of the adsorbent, same behaviour was reported in previous studies.49
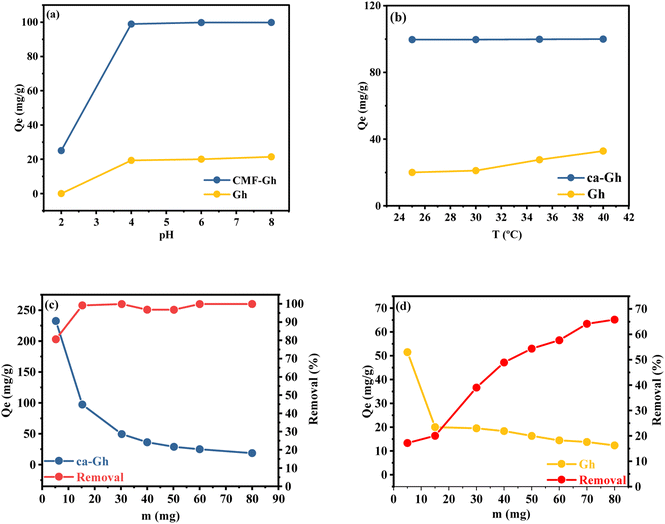 |
| | Fig. 6 (a) Effect of pH, (b) temperature and (c and d) dose of absorption of Cd2+ by Gh and ca-Gh. | |
3.2.2. Temperature effect. The temperature of the solution is one of the factors that strongly influences the adsorption capacity. From Fig. 6(b), for Gh it can be noticed that the elimination rises with temperature as a result of an increase in metal ions mobility. Although for ca-Gh, this is not the case, which could be explained by the fact that the adsorption capacity has already reached its maximum. The values of the thermodynamic parameters are reported in Table 2. The fact that ΔG° is always negative for ca-Gh at all temperatures tested reveals that the Cd2+ adsorption is spontaneous, N. Mohammadi et al. also have found a negative charge.1 The endothermic nature of Cd2+ adsorption is supported by the decreasing ΔG° values with temperature which results in positive values for ΔH°, which is also compatible with the fact that the removal is temperature-dependant. Additionally, the positive values of ΔS° support the adsorption process's favourable and spontaneous nature, which may be connected to an increase in randomness at the solid–liquid surface, M. Qhubu. et al. also have found a positive value of ΔS° which lead them to the same conclusion.2
Table 2 Thermodynamic parameters for Cd2+ adsorption onto Gh and ca-Gh at various temperatures
| Temperature |
ΔG° (J mol−1) |
ΔH° (J mol−1) |
ΔS° (J mol−1) |
| 298.15 K |
303.15 K |
308.15 K |
313.15 K |
| Gh |
412.59 |
399.59 |
297.33 |
224.18 |
0.000024 |
0.000361 |
| ca-Gh |
−1684.14 |
−1759.12 |
−1989.34 |
−2378.19 |
0.000008 |
0.000445 |
3.2.3. Adsorbent masse effect. The outcome of varying the adsorbent mass from 5 to 80 mg were applied to study the effect of adsorbent dosage Fig. 6(c) and (d) while keeping the initial concentration of Cd2+ fixed (100 mg L−1). It was found that when the adsorbent mass increases, the removal percentage considerably improves, going from 17.17% to 65.79% for Gh and from 80.61% to 99.91% for ca-Gh. For Gh, after 40 mg, the adsorption capacity did not significally increase, that can be explained by either the filling of nearly all the sites of the surface of our adsorbent or by inter-particle interactions caused by the agglomeration of the clay particles between them, as it was reported in this work.16 In the case of ca-Gh, the adsorption capacity has almost reached its maximum after 15 mg, which may be concluded from the nearly complete removal of all the cadmium ions from the solution.
3.2.4. Time effect. The variation of adsorption capacity with increasing contact time was investigated. Fig. 7(a) shows that the amount of Cd2+ adsorbed on the two investigated adsorbents improves with increasing time until it reaches a maximum level of approximately 60 minutes. For Gh and ca-Gh respectively, the maximum uptake capacity adsorbed was 20.40 mg g−1 and 99.76 mg g−1. For a better understanding of how the adsorption process takes place on the surface of the adsorbent, numerous kinetic models are available in the literature.56–58 Two kinetic models, pseudo-first-order and pseudo-second-order were used to study the adsorption of Cd2+ ions in this study. Fig. 7(b) and (c) presents the linear plot of the experimental data, and Table 3 provides a summary of the parameter's estimated values. The pseudo-second-order model has a higher correlation coefficient for both adsorbents, according to the kinetic modelling results. The predicted uptake capacities of 20.45 mg g−1 and 100 mg g−1 for Gh and ca-Gh, respectively, were closer to those of the experimental data, with R2 = 0.9995 and 1 for Gh and ca-Gh, respectively. We can assume that chemisorption is the mechanism behind the adsorption phenomenon.59
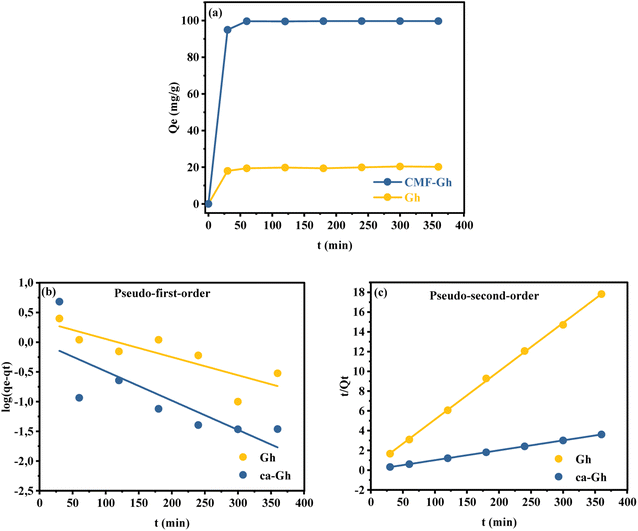 |
| | Fig. 7 (a) Experimental and (b and c) fitted data to two kinetic models for the adsorption of Cd2+ by Gh and ca-Gh. | |
Table 3 Parameters of two kinetic models for Cd2+ adsorption by Gh and ca-GH
| Kinetic model |
Parameters |
Gh |
ca-Gh |
| Pseudo-first-order |
K1 (min−1) |
0.003 |
0.005 |
| qe (mg g−1) |
1.432 |
1.002 |
| R2 |
0.691 |
0.628 |
| Pseudo-second-order |
K2 (g mg−1 min−1) |
0.010 |
0.013 |
| q2 (mg g−1) |
20.449 |
100.000 |
| R2 |
0.999 |
0.999 |
3.2.5. Concentration effect. Cd(II) ions were studied by mixing 15 mg of the adsorbent with 15 mL of Cd2+ solution at concentrations of 20, 40, 80, 100, 120, 160, 200, 500 and 600 mg L−1. Syringe filters were used to filter suspensions once they had reached equilibrium. Fig. 8(a) illustrates how the initial metal concentration affects the efficiency of Gh and ca-Gh to remove Cd2+ ions from an aqueous solution. As it can be noticed the adsorbed quantities rise as the initial concentration rises especially for ca-Gh. This increase could be explained by the fact that the availability of Cd2+ ions and the progressive saturation of the adsorption sites available at the surface of the adsorbent.60,61 The clearance rates of Cd2+ for Gh and ca-Gh were 20.6 mg g−1 and 261.9 mg g−1, respectively. The results of the isotherm Fig. 8(b) were fitted to Langmuir Fig. 8(c) and Freundlich Fig. 8(d) isotherm models. According to the Langmuir adsorption model, there is a saturated monolayer of adsorbate molecules at the surface of the adsorbent, which corresponds to a maximum limiting uptake.
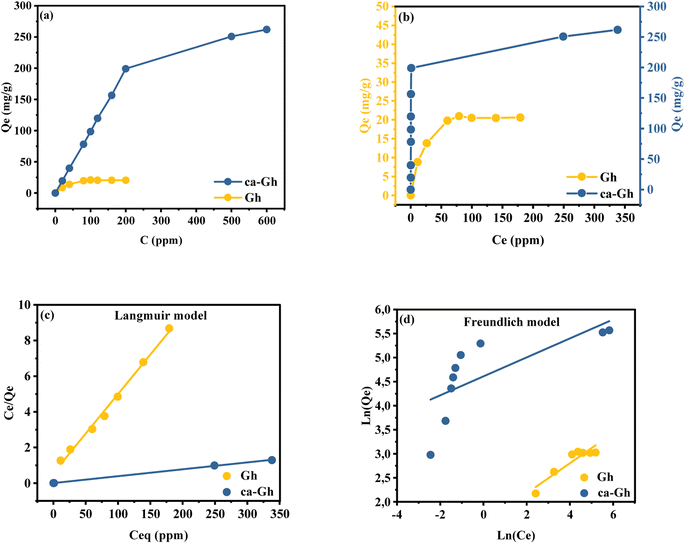 |
| | Fig. 8 (a) Experimental equilibrium, (b) isotherm, (c) linear fitting data to Langmuir and (d) Freundlich isotherm equation of Cd2+ adsorption of by Gh and ca-Gh. | |
On the other side, according to the Freundlich model, adsorption takes place on a heterogeneous surface using a multilayer adsorption mechanism, and the amount that is adsorbed rises in proportion to the concentration of molecules that are being adsorbed.62
As shown in Table 4, the correlation coefficients R2 of the Langmuir model are higher and nearer to 1 for both adsorbents. Also, the fact that the values of n are higher than 1, indicates that the adsorption of Cd2+ is advantageous since this parameter describes the adsorption intensity. Furthermore, the estimated maximum adsorption capacities from Langmuir models are quite similar to those obtained experimentally. In this context, monolayer adsorption on a homogeneous surface is what we might refer to as the cadmium adsorption process into Gh and ca-Gh. The adsorption capacity of various materials described in literature is compiled in Table 5. Compared to the majority of the materials mentioned, the synthetized carbonaceous ghassoul has a high adsorption capacity.
Table 4 Parameters of Langmuir and Freundlich models for Cd2+ adsorption by Gh and ca-GH
| Sample |
Langmuir |
Freundlich |
| Parameters |
Qm (mg g−1) |
KL (L mg−1) |
R2 |
KF (mg g−1) |
n |
R2 |
| Gh |
22.5734 |
0.0781 |
0.9941 |
4.7185 |
3.1949 |
0.8654 |
| ca-Gh |
256.4103 |
1.2581 |
0.9995 |
100.4800 |
5.0709 |
0.5173 |
Table 5 Adsorption capacities for Cd2+ using different adsorbents based on carbonaceous materials
| Adsorbent |
Q (mg g−1) |
Ref. |
| Oxidized multiwalled carbon nanotube |
23.40 |
63 |
| Hydroxyapatite tailored hierarchical porous biochar composite |
88.10 |
64 |
| Carbonaceous material from pyrolyzed sewage sludge |
15.00 |
65 |
| Carbonaceous nanowire membrane |
70.00 |
66 |
| Carbonaceous composite |
256.41 |
This study |
4. Conclusion
In the present study, a natural Moroccan stevensite known as ghassoul, and cellulose microfibers isolated from Alfa fibers were used to synthetize a carbonaceous composite by pyrolysis. By employing alkali and bleaching treatments, pure CMF with a yield of 40% have been obtained from RF. The CMF, Gh and ca-Gh were characterized. Gh appeared to be mainly composed of stevensite. The carbon was deposed successfully and appeared to be well dispersed on the surface of the clay. The material appeared to be a powerful adsorbent for cadmium ions in aqueous media according to the adsorption values. The Langmuir isotherm model was best suited to simulate the cadmium adsorption on the synthetized composite. Monolayer adsorption on homogeneous surfaces appeared to be the adsorption mechanism. The experimentally determined maximal adsorption capacity was 261.9 mg g−1 for ca-Gh. In addition to the strong correlation coefficients, experimental kinetic data showed that the pseudo-second order model provides a good agreement between model fitting and experimentally equilibrium adsorption capacity. It can be concluded that the chemical process seemed to be in control of Cd2+ adsorption. Additionally, the thermodynamic characteristics indicated that the adsorption process is endothermic and spontaneous. In conclusion, the novel carbonaceous composite that was synthetized in this work is very efficient for the recovery of cadmium from aqueous media.
Conflicts of interest
There are no conflicts to declare.
Acknowledgements
The financial assistance of the Office Chérifien des Phosphates (OCP S.A.) in the Moroccan Kingdom toward this research is hereby acknowledged.
References
- M. Thamarai Selvi and A. Zahir Hussain, Mater. Today: Proc., 2022, 59, 1349–1356 CAS.
- Y. Aoulad El Hadj Ali, M. Ahrouch, A. Ait Lahcen, Y. Abdellaoui and M. Stitou, Chem. Afr., 2022, 2022, 1–30 Search PubMed.
- M. Ahrouch, J. M. Gatica, K. Draoui, D. Bellido and H. Vidal, Chemosphere, 2020, 259, 127526 CrossRef CAS.
- R. El Kaim Billah, F. El Bachraoui, B. El Ibrahimi, H. Abou Oualid, Z. Kassab, G. Giácoman-Vallejos, M. Sillanpää, M. Agunaou, A. Soufiane and Y. Abdellaoui, J. Solid State Chem., 2022, 310, 123023 CrossRef CAS.
- G. H. Parker, C. E. Gillie, J. V. Miller, D. E. Badger and M. L. Kreider, Toxicol. Rep., 2022, 9, 238–249 CrossRef CAS PubMed.
- B. A. Fowler, Toxicol. Appl. Pharmacol., 2009, 238, 294–300 CrossRef CAS PubMed.
- M. Ahrouch, J. M. Gatica, K. Draoui, D. Bellido and H. Vidal, Chemosphere, 2020, 259, 127526 CrossRef CAS.
- Q. Lin, G. Zeng, G. Yan, J. Luo, X. Cheng, Z. Zhao and H. Li, Chem. Eng. J., 2022, 427, 131668 CrossRef CAS.
- C. Y. P. Ayekoe, D. Robert and D. G. Lanciné, Catal. Today, 2017, 281, 2–13 CrossRef CAS.
- M. Fasano, M. Morciano, L. Bergamasco, E. Chiavazzo, M. Zampato, S. Carminati and P. Asinari, Appl. Energy, 2021, 304, 117661 CrossRef.
- C. F. Z. Lacson, M. C. Lu and Y. H. Huang, Chem. Eng. J., 2021, 415, 128917 CrossRef CAS.
- M. Ahrouch, J. M. Gatica, K. Draoui, D. Bellido-Milla and H. Vidal, Environ. Technol. Innovation, 2022, 27, 102765 CrossRef CAS.
- L. Bouna, A. A. El Fakir, A. Benlhachemi, K. Draoui, S. Villain and F. Guinneton, Mater. Today: Proc., 2020, 22, 22–27 CAS.
- R. El Kaim Billah, M. Aminul Islam, H. Lgaz, E. C. Lima, Y. Abdellaoui, Y. Rakhila, O. Goudali, H. Majdoubi, A. A. Alrashdi, M. Agunaou and A. Soufiane, Arabian J. Chem., 2022, 15, 104123 CrossRef CAS.
- Y. Abdellaoui, H. Abou Oualid, A. Hsini, B. El Ibrahimi, M. Laabd, M. El Ouardi, G. Giácoman-Vallejos and P. Gamero-Melo, Chem. Eng. J., 2021, 404, 126600 CrossRef CAS.
- A. Naboulsi, M. El Himri, E. K. Gharibi and M. El Haddad, Surf. Interfaces, 2022, 33, 102227 CrossRef CAS.
- Z. Sun, J. Xu, G. Wang, A. Song, C. Li and S. Zheng, Appl. Clay Sci., 2020, 184, 105373 CrossRef CAS.
- Y. Abdellaoui, M. T. Olguín, M. Abatal, B. Ali, S. E. Díaz Méndez and A. A. Santiago, Superlattices Microstruct., 2019, 127, 165–175 CrossRef CAS.
- K. Elass, A. Laachach, A. Alaoui and M. Azzi, Appl. Clay Sci., 2011, 54, 90–96 CrossRef CAS.
- Y. El Mouzdahir, A. Elmchaouri, R. Mahboub, A. ElAnssari, A. Gil, S. A. Korili and M. A. Vicente, Appl. Clay Sci., 2007, 35, 47–58 CrossRef CAS.
- A. Naboulsi, M. El Himri, E. K. Gharibi and M. El Haddad, Surf. Interfaces, 2022, 33, 102227 CrossRef CAS.
- K. Elass, A. Laachach, A. Alaoui and M. Azzi, Appl. Clay Sci., 2011, 54, 90–96 CrossRef CAS.
- M. Ahrouch, J. M. Gatica, K. Draoui, D. Bellido-Milla and H. Vidal, Environ. Technol. Innovation, 2022, 27, 102765 CrossRef CAS.
- A. Benhammou, A. Yaacoubi, L. Nibou and B. Tanouti, J. Colloid Interface Sci., 2005, 282, 320–326 CrossRef CAS PubMed.
- Y. El Mouzdahir, A. Elmchaouri, R. Mahboub, A. ElAnssari, A. Gil, S. A. Korili and M. A. Vicente, Appl. Clay Sci., 2007, 35, 47–58 CrossRef CAS.
- X. Wang, X. Li, L. Peng, S. Han, C. Hao, C. Jiang, H. Wang and X. Fan, Chemosphere, 2021, 279, 130504 CrossRef CAS PubMed.
- S. Chaouf, S. El Barkany, H. Amhamdi, I. Jilal, Y. El Ouardi, M. Abou-salama, M. Loutou, A. El-Houssaine, H. El Ouarghi and A. El Idrissi, Mater. Today: Proc., 2020, 31, S175–S182 CAS.
- M. H. Salim, Z. Kassab, E. Ablouh, H. Sehaqui, A. Aboulkas, R. Bouhfid, A. E. K. Qaiss and M. El Achaby, Int. J. Biol. Macromol., 2022, 200, 182–192 CrossRef CAS PubMed.
- E.-H. Ablouh, Z. Kassab, F. Semlali Aouragh Hassani, M. El Achaby and H. Sehaqui, RSC Adv., 2022, 12, 1084–1094 RSC.
- E.-H. Ablouh, R. Jalal, M. Rhazi and M. Taourirte, Int. J. Biol. Macromol., 2020, 151, 492–498 CrossRef CAS PubMed.
- B. G. Fouda-Mbanga, E. Prabakaran and K. Pillay, Biotechnol. Rep., 2021, 30, e00609 CrossRef CAS PubMed.
- E. Ablouh, A. Essaghraoui, N. Eladlani, M. Rhazi and M. Taourirte, Water Environ. Res., 2019, 91, 239–249 CrossRef CAS PubMed.
- N. Eladlani, E. M. Dahmane, E. Ablouh, A. Ouahrouch, M. Rhazi, M. Taourirte and M. Neffa, J. Water Chem. Technol., 2019, 41, 175–181 CrossRef.
- E. Ablouh, Z. Hanani, N. Eladlani, M. Rhazi and M. Taourirte, Sustainable Environ. Res., 2019, 29, 5 CrossRef CAS.
- S. Chaouf, S. El Barkany, H. Amhamdi, I. Jilal, Y. El Ouardi, M. Abou-salama, M. Loutou, A. El-Houssaine, H. El Ouarghi and A. El Idrissi, Mater. Today: Proc., 2020, 31, S175–S182 CAS.
- Z. Kassab, Y. Abdellaoui, M. H. Salim and M. El Achaby, Mater. Lett., 2020, 280, 128539 CrossRef CAS.
- M. H. Salim, Y. Abdellaoui, A. Ait Benhamou, E. H. Ablouh, M. el Achaby and Z. Kassab, Cellulose, 2022, 29, 5117–5135 CrossRef CAS.
- D. S. Tong, C. W. Wu, M. O. Adebajo, G. C. Jin, W. H. Yu, S. F. Ji and C. H. Zhou, Appl. Clay Sci., 2018, 161, 256–264 CrossRef CAS.
- N. E. Mousa, C. M. Simonescu, R. E. Pătescu, C. Onose, C. Tardei, D. C. Culiţă, O. Oprea, D. Patroi and V. Lavric, React. Funct. Polym., 2016, 109, 137–150 CrossRef CAS.
- K. L. Tan and B. H. Hameed, J. Taiwan Inst. Chem. Eng., 2017, 74, 25–48 CrossRef CAS.
- K. Y. Foo and B. H. Hameed, Chem. Eng. J., 2010, 156, 2–10 CrossRef CAS.
- M. El Achaby, Z. Kassab, A. Barakat and A. Aboulkas, Ind. Crops Prod., 2018, 112, 499–510 CrossRef CAS.
- F.-Z. El Bouchtaoui, E.-H. Ablouh, I. Kassem, Z. Kassab, H. Sehaqui and M. El Achaby, J. Coat. Technol. Res., 2022, 19, 1551–1565 CrossRef CAS.
- Z. Kassab, A. Boujemaoui, H. Ben Youcef, A. Hajlane, H. Hannache and M. El Achaby, Cellulose, 2019, 26, 9567–9581 CrossRef CAS.
- A. Bahloul, Z. Kassab, F. Aziz, H. Hannache, R. Bouhfid, A. E. K. Qaiss, M. Oumam and M. El Achaby, Cellulose, 2021, 28, 4089–4103 CrossRef CAS.
- D. Watkins, M. Nuruddin, M. Hosur, A. Tcherbi-Narteh and S. Jeelani, J. Mater. Res. Technol., 2015, 4, 26–32 CrossRef CAS.
- F.-Z. El Bouchtaoui, E.-H. Ablouh, M. Mhada, I. Kassem, M. H. Salim, S. Mouhib, Z. Kassab, H. Sehaqui and M. El Achaby, Int. J. Biol. Macromol., 2022, 221, 398–415 CrossRef CAS PubMed.
- D. Watkins, M. Nuruddin, M. Hosur, A. Tcherbi-Narteh and S. Jeelani, J. Mater. Res. Technol., 2015, 4, 26–32 CrossRef CAS.
- A. Benhammou, A. Yaacoubi, L. Nibou and B. Tanouti, J. Colloid Interface Sci., 2005, 282, 320–326 CrossRef CAS PubMed.
- Y. T. Algoufi, G. Kabir and B. H. Hameed, J. Taiwan Inst. Chem. Eng., 2017, 70, 179–187 CrossRef CAS.
- M. A. Harech, M. Mesnaoui, Y. Abouliatim, Y. Elhafiane, A. Benhammou, A. Abourriche, A. Smith and L. Nibou, Bol. Soc. Esp. Ceram. Vidrio, 2021, 60, 194–204 CrossRef CAS.
- E. H. Ablouh, Z. Kassab, F. Z. Semlali Aouragh Hassani, M. El Achaby and H. Sehaqui, RSC Adv., 2022, 12, 1084–1094 RSC.
- K. S. W. Sing and R. T. Williams, Adsorpt. Sci. Technol., 2004, 22, 773–782 CrossRef CAS.
- D. S. Tong, C. W. Wu, M. O. Adebajo, G. C. Jin, W. H. Yu, S. F. Ji and C. H. Zhou, Appl. Clay Sci., 2018, 161, 256–264 CrossRef CAS.
- H. Moussout, H. Ahlafi, M. Aazza, R. Chfaira and C. Mounir, Heliyon, 2020, 6(3), e03634 CrossRef CAS PubMed.
- N. Dobe, D. Abia, C. Tcheka, J. P. N. Tejeogue and M. Harouna, Chem. Phys. Lett., 2022, 801, 139707 CrossRef CAS.
- I. M’barek, H. Slimi, A. K. D. AlSukaibi, F. Alimi, R. H. Lajimi, L. Mechi, R. Ben Salem and Y. Moussaoui, Arabian J. Chem., 2022, 15, 103679 CrossRef.
- Y. Abdellaoui, B. El Ibrahimi, H. Abou Oualid, Z. Kassab, C. Quintal-Franco, G. Giácoman-Vallejos and P. Gamero-Melo, Chem. Eng. J., 2021, 421, 129909 CrossRef CAS.
- C. A. Cimá-Mukul, Y. Abdellaoui, M. Abatal, J. Vargas, A. A. Santiago and B. Zambrano, Bioinorg. Chem. Appl., 2019, 1–13 Search PubMed.
- Y. Sun, Y. Yu, S. Zhou, K. J. Shah, W. Sun, J. Zhai and H. Zheng, Sep. Purif. Technol., 2022, 282, 120002 CrossRef CAS.
- N. Dobe, D. Abia, C. Tcheka, J. P. N. Tejeogue and M. Harouna, Chem. Phys. Lett., 2022, 801, 139707 CrossRef CAS.
- I. M’barek, H. Slimi, A. K. D. AlSukaibi, F. Alimi, R. H. Lajimi, L. Mechi, R. Ben Salem and Y. Moussaoui, Arabian J. Chem., 2022, 15, 103679 CrossRef.
- M. Šolić, S. Maletić, M. K. Isakovski, J. Nikić, M. Watson, Z. Kónya and S. Rončević, J Environ. Chem. Eng., 2021, 9, 105402 CrossRef.
- W. Wu, Z. Liu, M. Azeem, Z. Guo, R. Li, Y. Li, Y. Peng, E. F. Ali, H. Wang, S. Wang, J. Rinklebe, S. M. Shaheen and Z. Zhang, J. Hazard. Mater., 2022, 437, 129330 CrossRef CAS PubMed.
- E. Gutiérrez-Segura, M. Solache-Ríos, A. Colín-Cruz and C. Fall, J. Environ. Manage., 2012, 97, 6–13 CrossRef PubMed.
- Y.-J. Zhong, S.-J. You, X.-H. Wang, X. Zhou, Y. Gan and N.-Q. Ren, Chem. Eng. J., 2013, 226, 217–226 CrossRef CAS.
|
| This journal is © The Royal Society of Chemistry 2023 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article *a,
El-houssaine Ablouh
*a,
El-houssaine Ablouh *a,
Adil Aboulkas
*a,
Adil Aboulkas b,
Mounir El Achaby
b,
Mounir El Achaby a and
Khalid Draoui*c
a and
Khalid Draoui*c
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 (g mL−1), the grind RF were treated once with 10 wt% NaOH solution at 80 °C for 2 hours under mechanical stirring giving rise to ATF. ATF were followed by a bleaching treatment which was carried out using a solution made up of equal parts (v/v) of acetate buffer (27 g NaOH and 75 mL glacial acetic acid, diluted to 1 L of distilled water) and aqueous sodium chlorite (1.7 wt% NaClO2 in water). This treatment was done once for 2 hours at 80 °C under mechanical stirring, resulting in white CMF. The first step of the synthesis of the carbonaceous composite was the mixing of the two materials, for that we used solution mixing technique. As shown in the Fig. 1, a distinct quantity of 7.5 g of the extracted CMF was well dispersed in distilled water for several minutes, followed by the addition of 22.5 g of Gh to the mixture. After two hours of continuous magnetic stirring, the solution was freeze at −80 °C then dried by a freeze drier for 48 hours. The freeze-drying method has been chosen in order to ensure the adherence and the good dispersion of the fibers with the clay. Right forward, the pyrolysis process was carried out for two hours straight at 700 °C under argon (5°C min−1). 19.39 g of carbonaceous ghassoul (ca-Gh) was obtained after pyrolysis.
20 (g mL−1), the grind RF were treated once with 10 wt% NaOH solution at 80 °C for 2 hours under mechanical stirring giving rise to ATF. ATF were followed by a bleaching treatment which was carried out using a solution made up of equal parts (v/v) of acetate buffer (27 g NaOH and 75 mL glacial acetic acid, diluted to 1 L of distilled water) and aqueous sodium chlorite (1.7 wt% NaClO2 in water). This treatment was done once for 2 hours at 80 °C under mechanical stirring, resulting in white CMF. The first step of the synthesis of the carbonaceous composite was the mixing of the two materials, for that we used solution mixing technique. As shown in the Fig. 1, a distinct quantity of 7.5 g of the extracted CMF was well dispersed in distilled water for several minutes, followed by the addition of 22.5 g of Gh to the mixture. After two hours of continuous magnetic stirring, the solution was freeze at −80 °C then dried by a freeze drier for 48 hours. The freeze-drying method has been chosen in order to ensure the adherence and the good dispersion of the fibers with the clay. Right forward, the pyrolysis process was carried out for two hours straight at 700 °C under argon (5°C min−1). 19.39 g of carbonaceous ghassoul (ca-Gh) was obtained after pyrolysis.
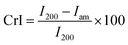
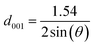
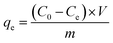
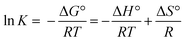
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K vs. 1/T, respectively.
K vs. 1/T, respectively.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe − k1t
qe − k1t
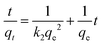
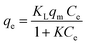
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibration from the aromatic ring and C–O–C out of plane stretching vibration of the aryl group in lignin and hemicelluloses. The peak at 1729 cm−1 can be assigned to the acetyl and uronic ester groups of hemicellulose or the ester linkage of carboxylic group of fluoric and p-coumaric acids of lignin and/or hemicellulose.44 As it can be noticed, the mentioned peaks were not detected in the treated fibers spectra, which can suggest the successful removal of the lignin and hemicellulose after the alkali and bleaching treatment. The peak at 2927 cm−1 and 2840 cm−1 are assigned to methylene group and symmetric stretch of CH3 of methoxyl group respectively. The peak detected at 1633 cm−1 is caused by the bending mode of the adsorbed water.42 The peak at 1159 cm−1 corresponds to C–O–C asymmetric stretching of the cellulose as was reported in this work.42 The gradual increasing intensity of the mentioned peak indicates that the cellulose content was increased after the alkali and bleaching treatment.44
C stretching vibration from the aromatic ring and C–O–C out of plane stretching vibration of the aryl group in lignin and hemicelluloses. The peak at 1729 cm−1 can be assigned to the acetyl and uronic ester groups of hemicellulose or the ester linkage of carboxylic group of fluoric and p-coumaric acids of lignin and/or hemicellulose.44 As it can be noticed, the mentioned peaks were not detected in the treated fibers spectra, which can suggest the successful removal of the lignin and hemicellulose after the alkali and bleaching treatment. The peak at 2927 cm−1 and 2840 cm−1 are assigned to methylene group and symmetric stretch of CH3 of methoxyl group respectively. The peak detected at 1633 cm−1 is caused by the bending mode of the adsorbed water.42 The peak at 1159 cm−1 corresponds to C–O–C asymmetric stretching of the cellulose as was reported in this work.42 The gradual increasing intensity of the mentioned peak indicates that the cellulose content was increased after the alkali and bleaching treatment.44
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0, 110 and 020, respectively.42 As it was expected, after the chemical treatment, the magnitude of the observed crystalline peaks increased, which is due to the removal of amorphous lignin and hemicellulose. It is noteworthy to notice that the crystal structure of the cellulosic component was not altered throughout the alkali and bleaching treatment. The crystallinity index (CrI) was found to be about 58%, 79% and 82% for RF, ATF and CMF samples, respectively. The increase of CrI as the process goes on confirms the elimination of the amorphous phases.45
0, 110 and 020, respectively.42 As it was expected, after the chemical treatment, the magnitude of the observed crystalline peaks increased, which is due to the removal of amorphous lignin and hemicellulose. It is noteworthy to notice that the crystal structure of the cellulosic component was not altered throughout the alkali and bleaching treatment. The crystallinity index (CrI) was found to be about 58%, 79% and 82% for RF, ATF and CMF samples, respectively. The increase of CrI as the process goes on confirms the elimination of the amorphous phases.45







