Open Access Article
Open Access ArticleTwo-photon absorption behavior of conjugated oligomers suitable for low colour temperature LEDs†
Tianhao Huang *a,
Chengzi Jiangb,
Tianning Xu*a,
Jinhui Yinga,
Ran Lu
*a,
Chengzi Jiangb,
Tianning Xu*a,
Jinhui Yinga,
Ran Lu c and
Huipeng Zhou*d
c and
Huipeng Zhou*d
aDepartment of Science, Zhijiang College of Zhejiang University of Technology, Shaoxing 312030, China. E-mail: hth@zzjc.edu.cn; xutianning@zjut.edu.cn
bGriffith Business School, Griffith University, Parklands Dr, Southport, QLD 4215, Australia
cCollege of Chemistry, Jilin University, Changchun 130012, China
dJiangsu Sino-tech Polym. New Materials Industry Technology Research Institute Co., Ltd., Changzhou 213000, China. E-mail: zhouhuipeng0318@163.com
First published on 10th February 2023
Abstract
Light emitting diodes (LEDs) with low colour temperatures (CTs) have been proved to be physiologically-friendly light sources. However, there are few reports on the photophysical properties of luminescent materials with CTs lower than candlelight. Herein, one- and two-photon optical properties of four fluorenone-based conjugated oligomers have been systemically investigated. By using a sum-over-essential states (SOS) approach, we obtained the transition dipole moments and two-photon absorption (TPA) cross sections. The triphenylamine end-capped oligomer exhibits reddish orange luminescence with extremely low colour temperature of 1686 K, which is much lower than that of candlelight. Fluorene–ethylene units serving as π-spacers could weaken the role of electron-donating units and effectively enhance the TPA performance of oligomers. Our results provide an effective way for the design and optimization of universal light-emitting material candidates in optoelectronic devices.
Introduction
Colour temperature (CT) is a characteristic of visible light that has important applications in lighting, photography, manufacturing and other fields.1 Interestingly, higher colour temperatures (CTs) exhibit cooler colours, while lower CTs exhibit warmer colours. Therefore, different CTs produce different effects on human physiology and psychology, which may be harmful or beneficial.2,3 Among many types of light sources, light emitting diodes (LEDs) are attracting considerable attention because their luminescence is similar to natural light sources. LEDs with low CTs are proven to be helpful in protecting the ocular surface, ameliorating insomnia, promoting the secretion of melatonin and glutamate, accelerating wound healing and hair regeneration, and are very suitable as healthy indoor light sources.4,5With the continuous improvement of people's requirements for life quality, creating human-friendly LEDs with low CTs like sunset or candlelight is highly desirable. In recent years, Jou et al.,6 Gong et al.7 and Korshunov et al.8 reported candle light-style organic LEDs with CTs as low as 1900 K, 1843 K and 1722 K, respectively. However, there are few reports on the photophysical properties of luminescent materials with CTs lower than candlelight, so it is necessary to further strengthen the basic research on the preparation strategies and photophysical mechanisms of such materials. More noteworthy, if such materials also have good two-photon absorption (TPA) characteristics, they will have wider potentials in the fields of two-photon fluorescence microscopy, optical data storage and thermodynamic therapy.
Herein, we compare the photophysical properties of four fluorenone-based conjugated oligomers: 2,7-di(4-(diphenylamino)styryl)-9-fluorenone (#1), 2,7-di((E)-2-(10-octyl-10H-phenothiazin-3-yl)vinyl)-9-fluorenone (#2), 2,7-di((E)-2-(7-(4-(diphenylamino)styryl)-9,9-dioctyl-9H-fluoren-2-yl)vinyl)-9-fluorenone (#3) and 2,7-di((E)-2-(9,9-dioctyl-7-((E)-2-(10-octyl-10H-phenothiazin-3-yl)vinyl)-9H-fluoren-2-yl)vinyl)-9-fluorenone (#4). The structure–property relationships are analyzed by performing a variety of steady-state and transient spectral measurements. Meanwhile, the transition dipole moments and TPA cross sections are simulated by using sum-over-essential states (SOS) approach. We observe that the electron-donating and withdrawing groups directly linked oligomers present CTs lower than 1700 K, while the incorporation of spacer units could weaken the role of electron-donating groups and effectively enhance the TPA performance of oligomers. It is hoped that our research will provide suitable candidates for the design and fabrication of light-emitting materials in photoelectric devices with low CTs and good two-photon performance.
Experimental
Materials
The conjugated oligomers #1, #2, #3 and #4 were synthesized following Heck reaction,9 and the synthetic procedures have been described in detail previously.10–12 As seen in Fig. 1, for #1 and #2, triphenylamine and phenothiazine serve as the electron-donating groups, are linked to fluorenone directly. While for #3 and #4, fluorene–ethylene units are inserted between electron-donating and withdrawing groups. Toluene solutions with concentrations at 5 × 10−5 and 5 × 10−4 M were prepared for one- and two-photon measurements, respectively. All the measurements were carried out at room temperature, with the samples placed in 3 mm path length quartz cells.Measurements
Mass spectra were performed on Agilent 1100 MS series and AXIMA CFR matrix assisted laser desorption ionization/time of flight (MALDI/TOF) mass spectrometer. The 1H-NMR spectra were recorded in CDCl3 on a 500 MHz NMR spectrometer (JEOL, JNM-500EX). C, H and N elemental analyses were taken on a elemental analyzer (PerkinElmer, 240C). The infrared (IR) spectra were measured using a FT-IR spectrometer (Bruker, VERTEX 80v) by incorporating samples in KBr disks. The steady-state absorption spectra were measured by a bi-pass fiber spectrometer (Avantes, AvaSpec-2048). One-photon fluorescence (OPF) measurements were carried out by use of a fiber optic spectrometer (Ocean Optics 4000). Two-photon fluorescence (TPF) and two-colour pump–probe experiments were carried out with Ti:sapphire mode-lock femtosecond laser system (Coherent), which consists of pumping source (Verdi-V5), oscillator stage (Mira 900), amplifier stage (Legend Elite) and optical parametric amplifier (Topas). The centered wavelength of the pulses from the oscillator stage is 800 nm, with average power of 600 mW, repetition rate of 76 MHz, and pulse width of 110 fs. When the pulses passed through the amplifier stage, the repetition rate becomes 1 kHz, and the single pulse energy becomes 3.5 mJ.Results and discussion
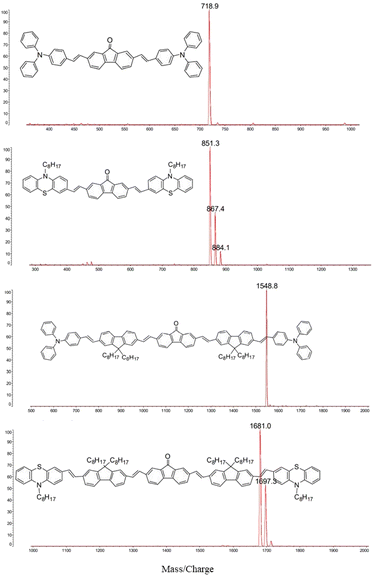
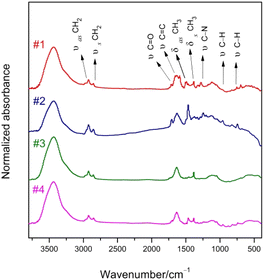
The structures of the conjugated oligomers were characterized by MALDI/TOF mass spectroscopy, 1H-NMR, elemental analysis and IR spectra. By utilizing 1,8,9-trihydroxyanthracene as a matrix, molecular ion peaks of target molecules could be clearly observed, as shown in Fig. 1. However, due to the existence of oxygen free radicals in the environment, which are easy to bind with lone-pair electrons on phenothiazine units, regular oxygen-rich molecular ion peaks could be observed in #2 and #4. The data of 1H-NMR and elemental analysis are available in the ESI (S1†). The 500 MHz 1H-NMR spectra exhibit that no signal corresponding to the protons in cis-double bonds at 6.5 ppm appeared in all the target compounds (Fig. S1, ESI†).Fig. 2 shows the IR spectra of compound #1–#4. The frequencies and assignments of characteristic vibrational peaks are listed in Table 1. The oligomers exhibit obvious CH2 asymmetrical stretching (υas CH2) and symmetrical stretching (υs CH2) vibration intensity due to their massive methylene groups. When fluorene–ethylene units are introduced into #1 and #2, the characteristic peaks of keto-carbonyl (υ C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)13 are weakened obviously, implying that spacer groups inhibited the vibration ability of the electron-withdrawing groups. Besides, the stretching vibration peaks of C
O)13 are weakened obviously, implying that spacer groups inhibited the vibration ability of the electron-withdrawing groups. Besides, the stretching vibration peaks of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C (υ C
C (υ C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) are enhanced with C
C) are enhanced with C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C prolonging. The characteristic C–N stretching (υ C–N) frequencies show a red-shift with the electron-donating groups change from triphenylamine to phenothiazine, and the peak intensities are strongly weakened by the introduction of spacer groups. The characteristic band of thiophene is located around 600–800 cm−1. It is noteworthy that the oligomers exhibit IR absorption bands near 960 cm−1 arising from the wagging vibration of the trans-double bond (CH
C prolonging. The characteristic C–N stretching (υ C–N) frequencies show a red-shift with the electron-donating groups change from triphenylamine to phenothiazine, and the peak intensities are strongly weakened by the introduction of spacer groups. The characteristic band of thiophene is located around 600–800 cm−1. It is noteworthy that the oligomers exhibit IR absorption bands near 960 cm−1 arising from the wagging vibration of the trans-double bond (CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH),14 but no characteristic absorption was observed at the vibration position of the cis-double bond at 830 cm−1. Therefore, we confirm that all the double bonds in the synthesized oligomers are trans configurations. The trans-form compounds have good planarity, which is beneficial to the improvement of the fluorescence property.
CH),14 but no characteristic absorption was observed at the vibration position of the cis-double bond at 830 cm−1. Therefore, we confirm that all the double bonds in the synthesized oligomers are trans configurations. The trans-form compounds have good planarity, which is beneficial to the improvement of the fluorescence property.
| Assignment | Vibrational frequencies/cm−1 (relative strength/%) | |||
|---|---|---|---|---|
| #1 | #2 | #3 | #4 | |
| υas CH2 | 2923 (9.9) | 2925 (20.6) | 2925 (13.6) | 2924 (12.5) |
| υs CH2 | 2855 (7.4) | 2851 (11.0) | 2854 (8.5) | 2851 (8.2) |
υ C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
1715 (10.1) | 1707 (15.8) | 1719 (6.1) | 1703 (6.1) |
υ C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C C |
1635 (27.4) | 1630 (24.2) | 1635 (29.4) | 1634 (27.9) |
| δas CH3 | 1463 (8.3) | 1467 (43.4) | 1457 (3.3) | 1461 (11.9) |
| δs CH3 | 1385 (14.3) | 1385 (6.4) | 1385 (14.3) | 1382 (11.1) |
δ ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H C–H |
964 (5.5) | 958 (8.4) | 960 (2.5) | 960 (5.1) |
δ ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H C–H |
754 (5.9) | 739 (8.6) | 753 (1.9) | 741 (5.4) |
| υ C–N | 1281 (9.5) | 1247 (10.3) | 1270 (1.3) | 1246 (2.6) |
The steady-state absorption spectra of oligomers dissolved in toluene (5 × 10−5 M) are shown in Fig. 3. Absorption bands in the ultraviolet region around 320 nm should be ascribed to the n–π* transitions of heteroatoms in triphenylamine and phenothiazine moieties, the high intensity peaks near 420 nm can be assigned to π–π* electronic transitions of the conjugated molecules,15 and the 480–500 nm shoulder bands could be attributed to intramolecular charge transfer (ICT) from electron-donating to withdrawing groups.16,17 Spectral parameters obtained by employing multi-peaks Gaussian fit are summarized in Table S1 (ESI†). The n–π* band of #2 possesses larger percentage owing to the electron-rich nitrogen and sulfur heteroatoms which favor n–π* transition. It is worth noting that #2 also exhibits larger area percentage of ICT, as compared with #1, indicating the electron-donating ability of phenothiazine is stronger than that of triphenylamine, due to its electron-rich tricyclic heteroarene with nonplanar butterfly structure.18 However, the insertion of fluorene–ethylene units resulted in a much smaller ICT area percentage for #3 and #4, because of the spacer groups effectively increase the conjugated length of the molecule, making the distance between the electron-donating and withdrawing groups obviously larger. The similar spectral parameters between #3 and #4 also indicate that the fluorene–ethylene spacer groups can eliminate the difference between the spectral behaviors of oligomers with different electron-donating groups. The normalized steady-state absorption and OPF spectra are together shown in Fig. S2 (ESI†) for comparison, with the corresponding linear optical parameters summarized in Table S2 (ESI†). Compared to #1 and #2, the absorption maximum of #3 and #4 shows a red-shift of ∼15 nm, indicating a more conjugated feature. The OPF spectra of #1 and #2 exhibit structureless band, and there is a 10 nm bathochromic shift with the electron-donating groups change from triphenylamine to phenothiazine, which could be attributed to the enhancement of the ICT. When it comes to #3 and #4, introducing of spacer groups resulted in blue-shift of the emission peak, in accompany with a new emerging shoulder band at ∼610 nm, which may originate from the fluorene-based characteristic emission. The Stokes shift presents a trend of #2 > #1 > #3 ≈ #4, indicating an enhanced ICT character19 occurs in phenothiazine end-capped oligomer #2, and the ICT between electron-donating and withdrawing groups could be inhibited by the inserted π-spacer groups. The optical bandgap Eoptg shows opposite trend of #2 < #1 < #3 = #4, which further validates the above inference.
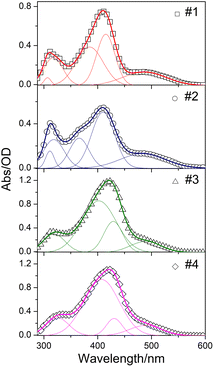 | ||
| Fig. 3 Steady-state absorption spectra of compound #1–#4. The solid lines represent the fitted Gaussian line-shape. | ||
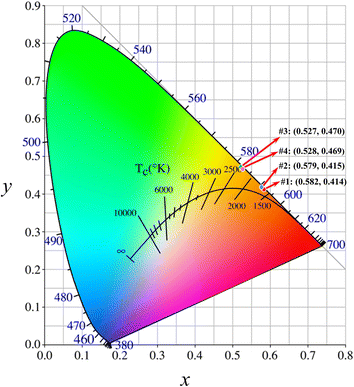
#1 and #2 exhibited reddish orange luminescence with spectral maxima at 597 and 607 nm, respectively. With the incorporation of fluorene–ethylene spacers, the emission maxima of #3 and #4 were hypsochromic shifted by 24 and 36 nm respectively relative to #1 and #2, and the luminescence colour became yellowish orange. The colour coordinates of oligomers in International Commission on Illumination (CIE) are presented in Fig. 4. The colour coordinates are located near the border of CIE, which is indicative of high colour saturation. Through the coordinates, correlated CTs of #1–#4 are calculated to be 1686, 1699, 2350 and 2331 K. #1 and #2 present extremely low CTs, which are even lower than those of matchstick flame, candlelight and sunset/sunrise (1700–1800 K). Although the CTs of #3 and #4 are higher as compared with #1 and #2, they are still lower than those of incandescent light bulb, studio lamps and photofloods (2700–3300 K). Meanwhile, #3 and #4 exhibit about 2.5–2.6 times enhancement in the whole emission intensity owing to the incorporation of π-spacer units.
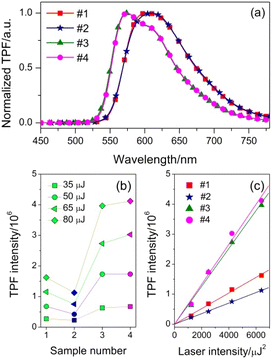
We have demonstrated that these oligomers are suitable candidates for low CT optoelectronic devices. Furthermore, if these materials also have excellent TPA properties, they will have wider applications in many fields. To investigate the nonlinear optical properties, we measure the TPF spectra at different laser intensities from 35 to 80 μJ under the femtosecond laser pulses of 800 nm. Fig. 5(a) shows the normalized TPF spectra of oligomers in toluene (5 × 10−4 M). The TPF maxima are located at 605, 610, 574 and 574 nm for #1, #2, #3 and #4 respectively, which are slightly red-shifted relative to those of OPF, because of the reabsorption effect.20 The spectral line shapes of TPF are the same as those of the OPF, suggesting that the emission state does not change and that we can simulate the nonlinear optical parameters by linear optical spectra. The plots of integrated TPF intensity versus sample number are depicted in Fig. 5(b). For all the oligomers, the TPF intensities are gradually increased with increasing laser intensity. At the same laser intensity, the stronger ICT character of #2 leads to fluorescence quenching compared to #1, due to the stronger electron-donating capacity of phenothiazine units. However, the difference between the TPF intensity of #3 and #4 is much smaller than that between #1 and #2 when the fluorene–ethylene spacers were introduced. More importantly, the TPF intensity could be significantly enhanced with the increase of conjugated length. The laser intensity-dependent integrated TPF intensity of oligomers is shown in Fig. 5(c). A clear linear dependence of the TPF intensity on the square of laser intensity is strong indication that the 800 nm excited fluorescence could be assigned to TPA process. According to the slopes of linear fitted lines, the TPF efficiency of #3 and #4 increases about 2.4- and 3.8-fold compared to their counterparts without fluorene–ethylene spacers. Meanwhile, the difference of TPF efficiency between triphenylamine and phenothiazine end-capped oligomers is significantly reduced, and the ratios before and after the introduction of π-spacers are 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (#1
2 (#1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) #2) and 10
#2) and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 (#3
11 (#3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) #4), respectively.
#4), respectively.
TPA cross section is an important parameter in quantifying the TPA abilities of compounds.21,22 Since TPF and OPF come from the same emission state as mentioned above, linear absorption spectra can be used to simulate the TPA cross section and other corresponding parameters of oligomers by exploiting SOS approach derived from semi-classical time-dependent perturbation theory.23 All calculations are performed in centimeter-gram-second system of electromagnetic units. Assuming that the absorption spectra exhibit Gaussian line-shape24,25
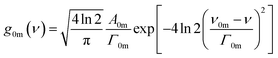 | (1) |
Assuming the excitation light is linearly polarized and therefore the dipole moments are parallel, the transition dipole moment of transition from the ground to m state can be calculated using:26
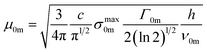 | (2) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10/NA, where ε is the molar absorption coefficient, NA is the Avogadro's constant.
10/NA, where ε is the molar absorption coefficient, NA is the Avogadro's constant.
The absorption spectra are well fitted with sum of multi-Gaussian, as shown by the solid lines in Fig. 3. According to the results of Gaussian fitting, we assume that the oligomers whose electron-donating and withdrawing moiety connected directly have one initial state (|S0〉), one intermediate state (|S1〉) and four final states (|S2〉–|S5〉), while the spacer introduced counterparts have one initial state (|S0〉), one intermediate state (|S1〉) and three final states (|S2〉–|S4〉). Then the TPA cross section can be expressed as:27
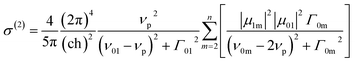 | (3) |
| TPA parameters | #1 | #2 | #3 | #4 |
|---|---|---|---|---|
| υ01/cm−1 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 553 553 |
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 684 684 |
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 462 462 |
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 467 467 |
| υ02/cm−1 | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 113 113 |
24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 416 416 |
23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 330 330 |
23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 236 236 |
| υ03/cm−1 | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 990 990 |
27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 368 368 |
24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 885 885 |
24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 577 577 |
| υ04/cm−1 | 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 526 526 |
31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 437 437 |
31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 399 399 |
30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 778 778 |
| υ05/cm−1 | 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 625 625 |
32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 162 162 |
||
| Γ01/cm−1 | 3437 | 3594 | 2404 | 2534 |
| Γ02/cm−1 | 1987 | 2554 | 1905 | 1577 |
| Γ03/cm−1 | 3414 | 2707 | 3979 | 3998 |
| Γ04/cm−1 | 3589 | 3817 | 4083 | 4095 |
| Γ05/cm−1 | 1390 | 1635 | ||
| μ01/debye | 9.8 | 9.8 | 7.4 | 7.3 |
| μ12/debye | 3.6 | 5.0 | 2.9 | 0.3 |
| μ13/debye | 4.8 | 0.8 | 11.1 | 12.2 |
| μ14/debye | 1.8 | 1.6 | 2.6 | 2.7 |
| μ15/debye | 6.0 | 4.1 | ||
| σT(2)/GM | 986 | 754 | 1658 | 1766 |
| σE(2)/GM | 884 | 781 | 1759 | 1743 |
The multi-energy-level diagrams for compound #1–#4 under the SOS approach are depicted in Fig. 6. Consistent with the absorption spectra, the |S1〉 state represents the ICT state, while the higher states represent the localized-excited states. The transition dipole moments between the |S0〉 and |S1〉 (μ01) are lower for #3 and #4, probably due to the introduction of fluorene–ethylene spacer, whose electron-donating ability is weaker than that of triphenylamine and phenothiazine, thus inhibiting the interaction between electron-donating and withdrawing groups. However, the μ13 and μ14 are higher for #3 and #4, because of their larger conjugated length.23 The values of theoretical TPA cross section (σT(2)) obtained using eqn (3) are 986, 754, 1658, 1766 GM, the experimental TPA cross section (σE(2)) values achieved by z-scan technique in previous studies11,12 are also listed in Table 2 for comparison. It is obvious that the incorporating of fluorene–ethylene units could lead to 170–230% enhancement of TPA cross section.
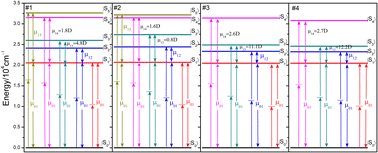 | ||
| Fig. 6 Multi-energy-level diagrams for compound #1–#4 used to describe the TPA process under the SOS approach. | ||
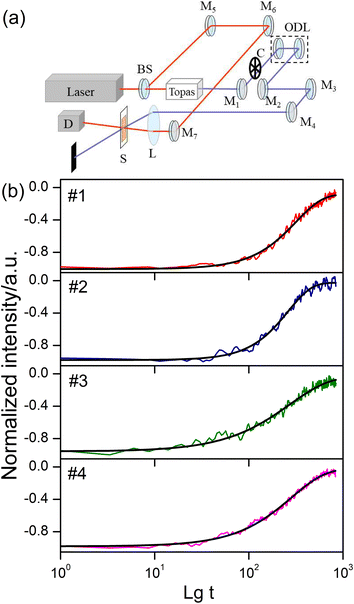
After a molecule is in thermodynamic equilibrium with the solvent, it will relax to an ICT state. The relaxation process of ICT state is accompanied by fluorescence emission and usually completed in hundreds of picoseconds. To better understand the ICT dynamics influenced by the electron-donating capacity and π-spacer units, two-color pump–probe experiment has been performed. Schematic illustration of the two-color pump–probe system is shown in Fig. 7(a). The amplified 800 nm laser beam is separated into two beams. The stronger one pumps the Topas which serves as a tunable source, and the other one is used as the probe beam. The pump beam is chopped with a chopper at a rate of 410 Hz and focused onto the sample cuvette after passing through an optical delay line (ODL), the probe beam is also focused and overlapped with the pump beam. The signal of transmittance change is collected by a photodiode connected with a lock-in amplifier (SR830 DSP) and finally recorded on a computer. Photoexcitation is carried out at 410 nm, corresponding the lowest energy π–π* transition.28 The absorption decay traces were measured with a probe wavelength at 800 nm. The results of two-color pump–probe dynamics together with fitting curves are shown in Fig. 7(b). Logarithm of the decay time is taken as the abscissa. Considering the multiformity of transient decay processes, we exploit the stretched exponential,29 which can be expressed as:
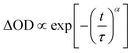 | (4) |
Conclusions
In summary, one- and two-photon optical properties of four fluorenone-based conjugated oligomers have been experimentally and theoretically analysed in detail. The emission of triphenylamine and phenothiazine end-capped oligomers could present extremely low CTs, which are even lower than 1700 K. The spectral line-shape, peak position, TPF efficiency and ICT lifetime are all modulated by electron-donating capacity and introduction of π-spacer units. The TPA cross section and other corresponding parameters of oligomers have been calculated exploiting SOS approach. The results suggest that phenothiazine groups with stronger electron-donating ability could increase the ICT behavior of oligomers in which the electron-donating and withdrawing groups are directly linked. However, the introduction of fluorene–ethylene spacers could inhibit the interaction between electron-donating and withdrawing groups. Because of the larger conjugated length, fluorene–ethylene spacers could also effectively enhance the TPA performance of oligomers. This study would be helpful for the design and optimization of photoelectric materials with both low colour temperature and excellent two-photon optical properties.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by Zhejiang Provincial Natural Science Foundation of China (LQ19F050004).Notes and references
- A. K. R. Choudhury, Principles of Colour and Appearance Measurement, Woodhead Publishing Limited, Cambridge, 2014 Search PubMed.
- K. Choi, C. Shin, T. Kim, H. J. Chung and H.-J. Suk, Sci. Rep., 2019, 9, 345 CrossRef PubMed.
- P. R. Mills, S. C. Tomkins and L. J. M. Schlangen, J. Circadian Rhythms, 2007, 5, 2 CrossRef PubMed.
- M. Jin, X. F. Li, F. Yan, W. X. Chen, L. Jiang and X. Zhang, J. Photochem. Photobiol., B, 2021, 214, 112099 CrossRef CAS PubMed.
- J. Q. Lin, X. W. Ding, C. Hong, Y. L. Pang, L. M. Chen, Q. W. Liu, X. Zhang, H. B. Xin and X. L. Wang, Sci. Rep., 2019, 9, 7560 CrossRef PubMed.
- J.-H. Jou, C.-Y. Hsieh, J.-R. Tseng, S.-H. Peng, Y.-C. Jou, J. H. Hong, S.-M. Shen, M.-C. Tang, P.-C. Chen and C.-H. Lin, Adv. Funct. Mater., 2013, 23, 2750–2757 CrossRef CAS.
- Y. Y. Gong, J. Liu, Y. R. Zhang, G. F. He, Y. Lu, W. B. Fan, W. Z. Yuan, J. Z. Sun and Y. M. Zhang, J. Mater. Chem. C, 2014, 2, 7552–7560 RSC.
- V. M. Korshunov, T. N. Chmovzh, E. A. Knyazeva, I. V. Taydakov, L. V. Mikhalchenko, E. A. Varaksina, R. S. Saifutyarov, I. C. Avetissov and O. A. Rakitin, Chem. Commun., 2019, 55, 13354–13357 RSC.
- R. F. Heck and J. P. Nolley, J. Org. Chem., 1972, 37, 2320 CrossRef CAS.
- T.-H. Huang, D. Yang, Z.-H. Kang, E.-L. Miao, R. Lu, H.-P. Zhou, F. Wang, G.-W. Wang, P.-F. Cheng, Y.-H. Wang and H.-Z. Zhang, Opt. Mater., 2013, 35, 467–471 CrossRef CAS.
- T.-H. Huang, X.-C. Li, Y.-H. Wang, Z.-H. Kang, R. Lu, E.-L. Miao, F. Wang, G.-W. Wang and H.-Z. Zhang, Opt. Mater., 2013, 35, 1373–1377 CrossRef CAS.
- T.-H. Huang, J.-Q. Hou, Z.-H. Kang, Y.-H. Wang, R. Lu, H.-P. Zhou, X. Zhao, Y.-G. Ma and H.-Z. Zhang, J. Photochem. Photobiol., A, 2013, 261, 41–45 CrossRef CAS.
- W.-Y. Wong, G.-L. Lu, K.-H. Choi and Z. Y. Lin, Eur. J. Org. Chem., 2003, 2003, 356–373 CrossRef.
- X. P. Qiu, R. Lu, H. P. Zhou, X. F. Zhang, T. H. Xu, X. L. Liu and Y. Y. Zhao, Tetrahedron Lett., 2007, 48, 7582–7585 CrossRef CAS.
- H. Hoppe and N. S. Sariciftci, J. Mater. Res., 2004, 19, 1924–1945 CrossRef CAS.
- J. L. Segura, N. Martín and D. M. Guldi, Chem. Soc. Rev., 2005, 34, 31–37 RSC.
- F. Garnier, Acc. Chem. Res., 1999, 32, 209–215 CrossRef CAS.
- Y. Rout, C. Montanari, E. Pasciucco, R. Misra and B. Carlotti, J. Am. Chem. Soc., 2021, 143, 9933–9943 CrossRef CAS PubMed.
- A. Bhaskar, G. Ramakrishna, Z. K. Lu, R. Twieg, J. M. Hales, D. J. Hagan, E. Van Stryland and T. Goodson, J. Am. Chem. Soc., 2006, 128, 11840–11849 CrossRef CAS PubMed.
- L. Z. Wu, X. J. Tang, M. H. Jiang and C. H. Tung, Chem. Phys. Lett., 1999, 315, 379–382 CrossRef CAS.
- Y. C. Wang, Y. Z. Wang, G. Q. Wang and D. J. Liu, Optik, 2018, 172, 186–190 CrossRef CAS.
- M. Sheik-Bahae, A. A. Said, T.-H. Wei, D. J. Hagan and E. W. Van Stryland, IEEE J. Quantum Electron., 1990, 26, 760–769 CrossRef CAS.
- M. G. Vivas, S. L. Nogueira, H. Santos Silva, N. M. Barbosa Neto, A. Marletta, F. Serein-Spirau, S. Lois, T. Jarrosson, L. De Boni, R. A. Silva and C. R. Mendonca, J. Phys. Chem. B, 2011, 115, 12687–12693 CrossRef CAS PubMed.
- L.-J. Gong, Y.-H. Wang, Z.-H. Kang, T.-H. Huang, R. Lu and H.-Z. Zhang, Chin. J. Chem. Phys., 2012, 25, 643–648 CrossRef.
- T.-H. Huang, Y.-H. Wang, T.-N. Xu, F.-Y. Yu, H.-Z. Zhang and Y.-R. Wang, Chin. J. Chem. Phys., 2015, 28, 557–562 CrossRef CAS.
- P. N. Day, K. A. Nguyen and R. Pachter, J. Phys. Chem. B, 2005, 109, 1803–1814 CrossRef CAS PubMed.
- K. Kamada, K. Ohta, Y. Iwase and K. Kondo, Chem. Phys. Lett., 2003, 372, 386–393 CrossRef CAS.
- R. Siebert, D. Akimov, M. Schmitt, A. Winter, U. S. Schubert, B. Dietzek and J. Popp, ChemPhysChem, 2009, 10, 910–919 CrossRef CAS PubMed.
- D. C. Elton, Stretched Exponential Relaxation, arXiv, 2018, preprint, arXiv:1808.00881, DOI:10.13140/RG.2.1.5109.5287.
- W. Freeden and M. Gutting, Special Functions of Mathematical (Geo-)Physics, Springer, Basel, 2013 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra00083d |
| This journal is © The Royal Society of Chemistry 2023 |